Abstract
The Vitamin E family consists of four tocopherols and four tocotrienols. α-Tocopherol (αT) is the predominant form of vitamin E in tissues and its deficiency leads to ataxia in humans. However, results from many clinical studies do not support protective roles of αT in disease prevention in people with adequate nutrient status. On the other hand, recent mechanistic studies indicate that other forms of vitamin E such as γ-tocopherol (γT), δ-tocopherol (δT) and γ-tocotrienol (γTE) have unique antioxidant and anti-inflammatory properties that are superior to αT in prevention and therapy against chronic diseases. These vitamin E forms scavenge reactive nitrogen species, inhibit cyclooxygenase- and 5-lipoxygenase-catalyzed eicosanoids and suppress pro-inflammatory signaling such as NF-κB and STAT3/6. Unlike αT, other vitamin E forms are significantly metabolized to carboxychromanols via cytochrome P-450 (CYP4F2)-initiated side-chain ω-oxidation. Long-chain carboxychromanols, esp.13’-carboxychromanols, are shown to have stronger anti-inflammatory effects than un-metabolized vitamins and may therefore contribute to beneficial effects of vitamin E forms in vivo. Consistent with mechanistic findings, animal and human studies show that γT and tocotrienols may be useful against inflammation-associated diseases. This review focuses on non-αT forms of vitamin E with respect to their metabolism, anti-inflammatory effects and mechanisms and in vivo efficacy in preclinical models as well as human clinical intervention studies.
Keywords: tocopherol, tocotrienol, cyclooxygenase, 5-lipoxygenase, cancer, inflammation, asthma, lung injury, long-chain carboxychromanol
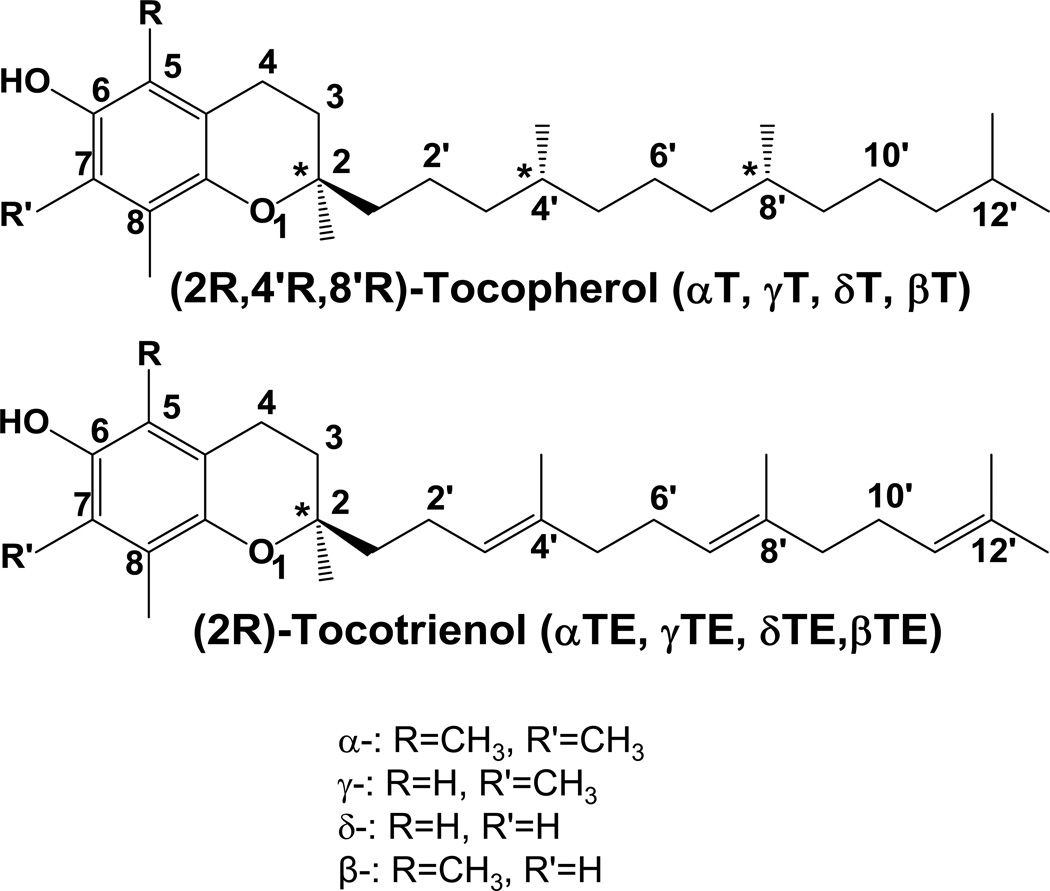
Naturally-occurring vitamin E forms are eight lipophilic molecules, which include α-, β-, γ-, δ-tocopherol (αT, βT, γT, δT) and α-, β-, γ-, δ-tocotrienol (αTE, βTE, γTE, δTE) (Figure 1). All vitamin E forms have a chromanol ring and a 16-carbon phytyl-like side chain, in which tocopherols are saturated and tocotrienols have three double bonds (Figure 1). Different isoforms of tocopherols and tocotrienols differ at 5- or 7-position of chromanol ring with either -H or -CH3 group. Natural tocopherols have RRR configuration at 2, 4’ and 8’-position, and tocotrienols have R-configuration at 2-position (Figure 1).
Figure 1. Natural forms of vitamin E.
All tocopherols and tocotrienols are potent antioxidants with lipoperoxyl radical scavenging activities. Only until recently, most research on vitamin E has primarily focused on αT [1], because αT is the predominant form of vitamin E in tissues and low intake of this form results in vitamin E deficiency associated ataxia [2]. However, many human and animal studies on αT supplementation have yielded disappointing outcomes regarding its protective role in prevention or treatment of chronic diseases including cardiovascular diseases and cancer [3, 4]. On the other hand, recent mechanistic studies combined with preclinical animal models have indicated that compared with αT, other forms of vitamin E appear to have different and superior biological properties that may be useful for prevention and therapy against chronic diseases. Furthermore, emerging evidence suggest that some long-chain vitamin E metabolites have even stronger anti-inflammatory effects than their vitamin precursors. These metabolites may be novel anti-inflammatory agents, and may contribute to beneficial effects of vitamin E forms in vivo. Here we discuss recent development in the field of non-αT forms of vitamin E with respect to their metabolism, antioxidant and anti-inflammatory effects.
1. FOOD SOURCES AND BIOAVAILABILITY
Natural forms of vitamin E are made by plants. Although αT exists in some fruits and vegetables [5], plant seeds including commonly-used nuts are rich sources of αT and γT. For instance, αT is predominantly found in peanuts, almonds and sunflower seeds, while γT is the major vitamin E in walnuts, pecans, pistachios and sesame seeds [6, 7]. As a result, αT and γT are found in many food oils like corn, soybean and peanut oil (Table 1) [6, 7]. Due to the widespread use of corn and soybean oil, γT represents ~60–70 % vitamin E consumed in the typical US diet, while αT accounts for 20–25% [6]. Good sources of δT include tomato seeds, rice germ and soybean oil [6]. Tocotrienols are much less prevalent than tocopherols in commonly-used nuts; and they are mostly found in palm oil, barley, annatto and some cereal grains [6, 8].
Table 1. Tocopherols and fatty acids in commonly-used vegetable oils.
PUFA: polyunsaturated fatty acid; MUFA: monounsaturated fatty acid; ref [6, 158] and www.Veganhealth.org.
| Vegetable oils | αT; γT; δT (mg/100g oil) | PUFA in % (ratio of n-6/n-3) |
MUFA (%) |
|---|---|---|---|
| Corn oil | 14.3; 64.9; 2.8 | 61 (80/1) | 25 |
| Soybean | 10.99; 62.4; 20.38 | 61 (7.5/1) | 24 |
| Flaxseed oil | 0.31; 19.95 | 71 (0.25/1) | 21 |
| Canola oil | 23; 40 | 31 (2/1) | 63 |
| Peanut oil | 11.62; 12.98 | 34 (only n-6) | 49 |
| Olive oil | 11.92; 0.72 | 9 (13.2/1) | 77 |
| Almond oil | 39.2; 0.92 | 18 (only n-6) | 73 |
Vitamin E forms coexist with fats in many dietary sources. Therefore, high intake of these vitamers is associated with enhanced consumption of certain types of fatty acids found in the same diet. Interestingly, γT-rich nuts or oils often contain high levels of polyunsaturated fatty acids (PUFA), while many αT-rich plant oils tend to have more monounsaturated fatty acids (MUFA) than PUFA (Table 1). With only a few exceptions, large majority of PUFA in plant oils are n-6 fatty acids. These nutrient data suggest that it may be necessary to consider confounding factors from different types of fat when potential association of vitamin E forms with diseases is considered in epidemiological cohort and case/control studies. Considering that γT-rich diets often predominantly contain n-6 PUFA, plasma γT may be a marker of high PUFA intake which plays complicated roles in diseases [9]. In contrast, high αT may in part reflect favorable MUFA intake.
As the major vitamin E forms in the food, αT and γT are more abundant than other tocopherols or tocotrienols in tissues. On the other hand, despite variations in food sources, it has long been observed that αT is the predominant form of vitamin E in the body. For instance, plasma concentrations of αT in non-supplemented individuals range from 20–30µM, whereas γT, despite higher than αT in the US diets, is often 5–10 times lower than αT in the blood [1]. The preferential retention of αT over other vitamin E including γT is rooted in distinct binding affinity and activity toward γT vs. other vitamers by liver proteins that are important for transport and metabolism of tocopherols and tocotrienols.
2. METABOLISM AND METABOLTIES
2.1. Overview of Absorption and Metabolism
In the intestine, dietary tocopherols and tocotrienols appear to be similarly absorbed along with dietary fat and are secreted in chylomicron particles together with triacylglycerol, phospholipids and cholesterol [1, 10–12]. The chylomicron-bound vitamin E forms are transported via lymphatic system to the peripheral tissues, including muscle, bone marrow, adipose, skin and possibly brain. In these tissues, vitamin E forms are picked up by lipoprotein receptor-mediated process, which is not-well understood [1, 12, 13]. Chylomicron-associated tissue uptake of vitamin E may contribute to the accumulation of non-αT forms of vitamin E such as γT in human skin, adipose and muscle, where unexpectedly high concentrations of γT were observed, in contrast to its low levels in the plasma [14]. The resulting chylomicron remnants are subsequently taken up by the liver.
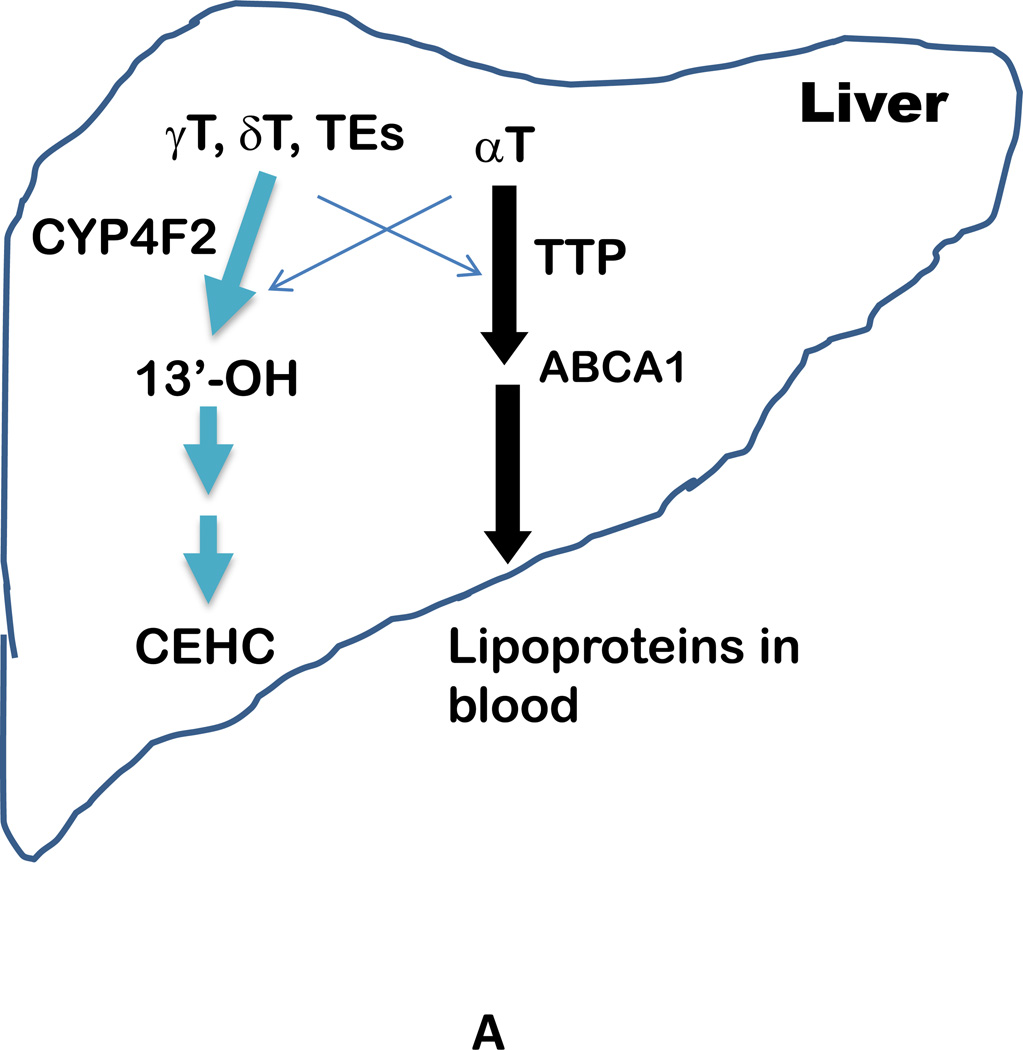
In the liver, αT is preferentially bound to α-tocopherol-transfer protein (α-TTP). α-TTP, together with ATP-binding cassette transporter A1 (ABCA1) [15], incorporates αT into lipoproteins, which transport vitamin E to other tissues via circulation [1, 2, 12] (Figure 2). In contrast to high affinity to αT (100%), α-TTP has much lower affinity toward other vitamin E forms, e.g. 50%, 10–30%, or 1% affinity to βT, γT and δT, respectively. Unlike αT which is bound and thus protected by α-TTP, large portions of non-αT forms of vitamin E are catabolized in the liver via cytochrome P450 (CYP4F2) initiated ω-hydroxylation and oxidation followed by β-oxidation of the phytyl chain to generate 13’-hydroxychromanol (13’-OH), various carboxychromanols and terminal metabolite 3’-carboxychromanol (3’-COOH) or (2’-carboxyethyl)-6-hydroxychromans (CEHCs) (Figure 2). Conjugation such as sulfation and glucuronidation of the phenolic on the chromanol may take place in parallel with β-oxidation when there is high intake of vitamin E forms (Figure 2).
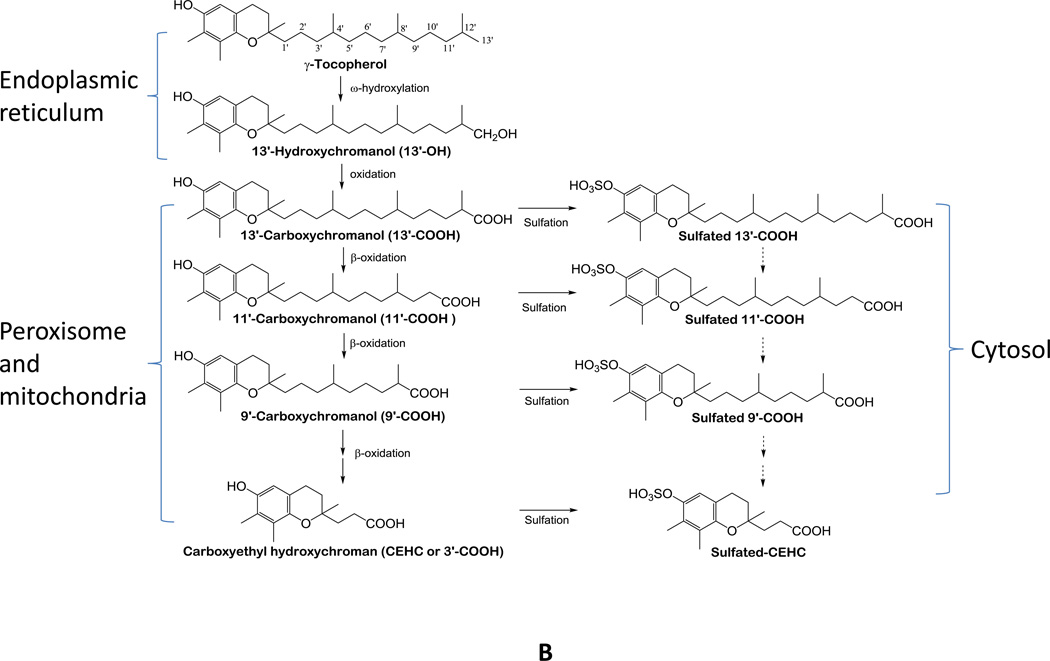
Figure 2.
A - Transport and metabolism of vitamin E forms in the liver. With exception of αT, large portions of other vitamin E forms such as γT, δT and γTE are metabolized by CYP4F2-initiated ω-oxidation to form terminal metabolite CEHCs. In contrast, αT and small amounts of other vitamin E forms are incorporated into lipoproteins by α-TTP with assistance of ABCA1 before being transported to other tissues via circulation. The crisscross arrows (light blue) indicate relatively minor events taking place for αT (catabolism) and other forms of vitamin E (binding to α-TTP) in the liver. B – Molecular mechanism of vitamin E metabolism (representatively shown by γT). Vitamin E forms are metabolized by CYP4F2-mediated ω-hydroxylation and ω-oxidation in endoplasmic reticulum. 13’-COOHs are then further metabolized via β-oxidation in peroxisome and mitochondria to generate series of shorter-chain carboxychromanols. Under the condition of high vitamin E intake, sulfation of carboxychromanols in the cytosol may take place in parallel with β-oxidation. It is currently not clear whether sulfated forms can be further metabolized via β-oxidation (dash arrows).
2.2. Mechanism of vitamin E catabolism
The terminal metabolite CEHC from δT was first identified from rats’ urine in 1984 [16]. Similar end metabolites derived from αT and γT were subsequently found in human plasma and urine [17–21]. The structural characteristic of CEHCs suggests that vitamin E catabolism involves oxidation of the hydrophobic side chain via cytochrome P450-catalyzed reactions. It is not until 2002 that the mechanism of how vitamin E forms are metabolized was unequivocally elucidated. Sontage and Parker [22] and Birringer et al [23] showed that cultured hepatic HepG2 cells metabolize γT, δT and γTE to 13’-hydroxychromanol, long-chain carboxychromanols including 13’-, 11’ and 9’-carboxychromanol (13’-COOH, 11’-COOH, 9’-COOH) and shorter side chain carboxychromanols (7’-COOH, 5’-COOH and 3’-COOH) (Figure 2). The identification of these intermediate metabolites in cell culture media provides direct evidence that vitamin E forms are metabolized via cytochrome P-450 mediated ω-hydroxylation and oxidation of 13’-carbon, followed by stepwise β-oxidation to cut off a two- or three carbon moiety each cycle from the side chain.
Conjugation including sulfation also plays a role in tocopherol metabolism. In human A549 cells, γT, δT and γTE are catabolized to sulfated long-chain carboxychromanols, i.e., SO3-13’-COOH, SO3-11’-COOH and SO3-9’-COOH, (Figure 2), in addition to unconjugated carboxychromanols [24]. Although conjugated CEHCs have previously been reported to be excreted to the urine, the discovery of conjugated long-chain carboxychromanols indicates that sulfation occurs simultaneously with β-oxidation. Interestingly, sulfated 13’-COOH, 11’-COOH and 9’-COOH as well as 13’-OH and 13’-COOH were detected in the plasma of rats which were supplemented with γT, δT and γTE [24–26]. Furthermore, the majority of plasma carboxychromanols were found to be in the conjugated forms in rats supplemented with γTE [25]. These observations indicate that under supplementation condition, sulfation takes place in parallel with β-oxidation in the body (Figure 2) [24, 25]. These in vivo data confirm that vitamin E forms are metabolized via ω-hydroxylation and β-oxidation as well as sulfation in a whole body environment. Consistently, high levels of long-chain carboxychromanols including 13’-COOH were found in feces in mice supplemented with γT, δT or mixed tocopherols [27–29], although Zhao et al [30] reported relatively high fecal excretion of short-chain carboxychromanols.
To illustrate which subcellular compartment hosts different steps of vitamin E metabolism, Mustacich et al. [31] analyzed subcellular contents of αT and metabolites in the liver of rats injected with mega doses of αT. They observed much greater levels of αT and 13’-OH in the microsomes which contain endoplasmic reticulum membranes than those in the mitochondria and peroxisomes. On the other hand, α-CEHC was almost exclusively detected in the mitochondria. These data indicate that like other CYP enzymes, ω-hydroxylation and β-oxidation of 13’-carbon (by CYP4F2) take place in hepatic endoplasmic reticulum, while subsequent β-oxidation of long-chain and short-chain carboxychromanols occurs in the peroxisomes and mitochondria, respectively [31]. The differential localization of ω-oxidation and subsequent β-oxidation helps explain the observation that sulfated long-chain carboxychromanols are detected in rats supplemented with γT or γTE [24–26]; Specifically, when long-chain carboxychromanols are transported from microsomes to peroxisomes and/or mitochondria for further β-oxidation, cytoplasmic sulfotransferases may catalyze sulfation of these intermediate metabolites. Hashiguchi et al. [32] showed that sulfotransferases in SULT1 family were effective in sulfation of carboxychromanols.
Besides ω-hydroxylase mediated ω-hydroxylation of 13’-carbon, 11’- and 12’-OH-tocopherols were recently identified from the feces of mice that were supplemented with γT and δT, indicating ω-1 and ω-2 hydroxylase activity [27, 28]. It was estimated that under a moderate supplementation dose, ω-hydroxylation appears to be the predominant path of vitamin E metabolism, accounting for more than 80% fecal excreted metabolites [28].
2.3. Regulation of vitamin E retention, metabolism and excretion
It is becoming clear that both α-TTP and vitamin E ω-hydroxylase play critical roles in controlling bioavailability and metabolism of vitamin E. α-TTP, which preferentially binds to αT over other vitamers, functions to secrete vitamin E forms and facilitate transportation of them among intracellular organelles [15]. As a result, α-TTP prevents αT and other bound vitamin E forms from being catabolized in the liver. On the other hand, vitamin E ω-hydroxylase, which is in charge of catabolizing unbound vitamin E forms, has stronger activities toward non-αT forms than αT. Because of these two opposite interactions, αT is predominantly accumulated in body tissues, whereas γT and other vitamin E forms are preferentially metabolized to hydroxycarboxychromanol, carboxychromanols and their conjugated counterparts (Figure 2A). In addition, proteins controlling vitamin E absorption and excretion may also play a role in its bioavailability.
2.3.1. α-TTP
α-TTP is a member of the CARL-TRIO family, which consists of lipid-binding proteins in control of intracellular trafficking of hydrophobic molecules ([15] and references therein). α-TTP has strong affinity for αT with K(d) of 25nM, but shows much weaker affinity to other vitamin E forms (K(d) =124, 266, 586 nM for βT, γT and δT, respectively) [33]. Qian et al [34] showed that α-TTP is necessary for transporting tocopherols from the lysosome to the plasma membrane prior to vitamin E secretion from hepatocytes. Cell-based studies further indicate that optimal liver secretion of vitamin E requires not only α-TTP but also ABCA1, a membrane protein known for cholesterol transport [34–36]. As a result, knockout of ABCA1 led to lowered plasma levels of tocopherols and cholesterol compared with those in the wild-type mice [36].
α-TTP is primarily responsible for maintaining high tissue contents of αT and preferentially enriching αT over other vitamin E forms in the body. This notion is strongly supported by the fact that knockout of α-TTP gene leads to severe deficiency of αT in mice [37, 38]. Familial vitamin E deficiency, which stems from genetic mutations of α-TTP in human, is associated with very low levels of αT in the plasma and develops ataxia [39]. Compared with healthy controls, patients with genetic defects in α-TTP also have impaired selectivity between αT and γT [40] and enhanced urinary excretion of α-CEHC despite much reduced plasma αT concentrations [41]. α-TTP also distinguishes naturally-occurring RRR-αT vs. synthetic stereoisomers, which leads favorable retention of naturally occurring RRR- than synthetic αT [10]. Interestingly, α-TTP appears to even play a role in retaining non-αT forms of vitamin E as α-TTP knockout mice have not only lowered αT but also decreased γT in tissues compared with controls [37].
Despite its critical role in preserving vitamin E forms in tissues and selectivity among the different vitamers, α-TTP is not the only protein responsible for favorable retention of αT over other vitamin E forms. This is because mice or Drosophila without TTP (TTP−/−) still maintain higher levels of αT than γT in tissues [37, 42]. This observation suggests that catabolic enzymes may also have selection in tocopherols and tocotrienols.
2.3.2. Tocopherol ω-hydroxylase (cytochrome P450 4F2)
Cytochrome P450 mediated hydroxylation and oxidation of the side chain is responsible for initiating vitamin E catabolism. Sontag and Parker [22] demonstrated that tocopherol-ω-hydroxylase activity is associated with cytochrome P450 4F2 (CYP4F2) but not other isoforms including CYP3A. CYP4F2, a microsomal ω-hydroxylase, was initially identified to metabolize leukotriene B4 via ω-hydroxylation [43]. Sontag and Parker [44] further demonstrated that ω-hydroxylase has higher activities toward vitamin E forms with unsubstituted C5 in the chromanol ring such as γT and δT than αT which has methylated C5. The Vmax of ω-hydroxylase toward tocotrienols is much higher than that to their tocopherol counterparts. Interestingly, αT is a positive effector of ω-hydroxylation as it stimulates metabolism of other vitamers [44]. Consistent with the key role of CYP4F2 enzyme in vitamin E catabolism, mice with knockout of Cyp4f14, a murine ortholog of human CYP4F2, has greatly enhanced tissue retention of non-αT forms of vitamin E such as γT and δT and decreased urinary and fecal excretion of metabolites [28]. Nevertheless, Cyp4f14 knockout did not completely abolish omega-series metabolites, suggesting involvement of other ω-hydroxylase(s) in mice. Interestingly, αT levels were not affected in the absence of Cyp4f14, which is in agreement with the notion that CYP4F2 has low catabolic activity of this vitamin E form [28].
CYP4F2-initiated ω-oxidation metabolism is estimated to account for generation of >70% whole body vitamin E metabolites [28]. Besides CYP4F2-mediated ω-hydroxylation of 13’-carbon, ω-1 and ω-2 hydroxylase-catalyzed reactions have been reported as significant amounts of 12’- and 11’-hydroxychromanol (12’-OH, 11’-OH) were found to be excreted in mouse feces [27, 28]. It remains to be determined which cytochrome P450 enzymes has ω-1 and ω-2 hydroxylase activities. In addition, extra-hepatic metabolism is evident by the observation that mice with liver-specific knockout of cytochrome P-450 activity have reduced body metabolic capacity by 70% but not 100% [27]. To this end, intestine has been shown to have ω-hydroxylase activity, which nevertheless is much less effective than that in the liver [27].
The revelation of executive functions of α-TTP and tocopherol ω-hydroxylase helps explain some well-documented phenomena related to vitamin E bioavailability. For instance, it is well known that αT supplementation depletes plasma and tissue γT [1]. This observation is likely partially rooted in α-TTP’s preferential affinity for αT as increased αT may compete with γT in TTP binding and therefore leaves more γT for catabolism by hydroxylases. Meanwhile, increased intake of αT may stimulate γT catabolism due to its effector activity to CYP4F2 [44]. As a result, αT supplementation increased secretion of γ-CEHC [45]. In addition, it has been reported that supplementation of sesame seed or sesamin, a lignin in sesame seeds, result in enhanced plasma and tissue levels of γT [46, 47]. This is because sesamin is a potent CYP4F inhibitor [48], and therefore blocks γT from degradation and enhances its tissue retention. Recently, two common single nucleotide polymorphisms in the CYP4F2 gene, i.e. W12G and V433M (which have a minor allele frequency of 6–21% and 9–26%, respectively), have been shown to have differential catalytic activity [49]. This observation suggests that these genetic variants may contribute to alterations of tissue status of vitamin E forms and their metabolites, which warrants further investigation in human.
2.3.3. Other vitamin E binding proteins
In addition to TTP and CYP4F2, several tocopherol associated proteins (TAPs) such as SEC14p like proteins and supernatant protein factor (SPF) have been reported to be able to bind tocopherols [33, 50, 51]. SPF appears to have stronger affinity to γT (Kd ~268nM) than αT (Kd at 615nM) [33], although its binding affinity is much weaker than that of TTP toward αT (Kd at ~25nM). Recently, a ubiquitous cytosolic protein saposin B is reported to bind to γT more potently than αT or Coenzyme Q [52]. Despite preference of γT over αT, these proteins may not play critical roles in maintaining γT due to relatively low affinity. Interestingly, Ulatowski et al. [53] showed that αT status was perturbed in the brain and liver of Niemann-Pick type C (NPC) gene (NPC1 and NPC2)-knockout mice, while α-TTP status and plasma αT levels were not affected by NPC gene knockout. These data indicate that NPC1/2 proteins, which regulate transport of lipid including cholesterol through the endocytic pathway, play a role in vitamin E transport and status. Nevertheless, more research is needed to determine how these proteins regulate vitamin E transport and metabolism.
2.3.4. Excretion
Besides catabolism, excretion of vitamin E and their metabolites is another factor influencing tissue retention of these compounds. Short chain carboxychromanols and their sulfated or glucuronidated counterparts are excreted via urination, while unconjugated carboxychromanols are primarily found in feces [27, 28, 30]. Bardowell et al. [28] estimated that as much as 80% of total metabolites were excreted via feces, in contrast to the previous assumption that vitamin E metabolites are primarily excreted in the urine. Unmetabolized tocopherols and tocotrienols are also discarded via biliary excretion. Liver seems to preferentially excrete γT compared with αT [54–56]. Excretion of excess hepatic vitamin E into the bile is thought to be mediated by the ABC transporter, P-glycoprotein (MDR2) [57]. Injection of large amounts of αT leads to modulation of genes involved in hepatic xenobiotic metabolism such as CYP3 [58] and ABC transporters [59], which may be resultant from its activation of the pregnane X receptor [60]. Interestingly, fecal excretion of βT and αT is markedly enhanced in response to their supplementation, which likely prevents excess accumulation of these tocopherols in tissues [27, 28]. However, it is not clear whether enhanced excretion is partially caused by decreased absorption. A recent study using Caco-2 monolayers suggested that apolipoprotein B-dependent pathway and ABCA1 play important roles in vitamin E secretion from intestine and ABCA1-dependent secretion shows favorable selectivity of αT and γT over δT [61]. Factors that determine vitamin E absorption, secretion and excretion warrant further investigation.
3. ANTIOXIDANT ACTIVITIES OF VITAMIN FORMS AND CARBOXYCHROMANOLS
All vitamin E forms are potent antioxidants as they scavenge lipid peroxyl radicals by donating hydrogen from the phenolic group on the chromanol ring (Figure 3). Because of possessing similar phenolic moiety, all vitamin E forms are considered to have potent antioxidant activities [1]. On the other hand, tocotrienols have been suggested to be better than αT in scavenging peroxyl radicals due to more even distribution of tocotrieonls in the phospholipid bilayer and more effective interaction with lipid peroxyl radicals than tocopherols in membrane environments [62, 63].
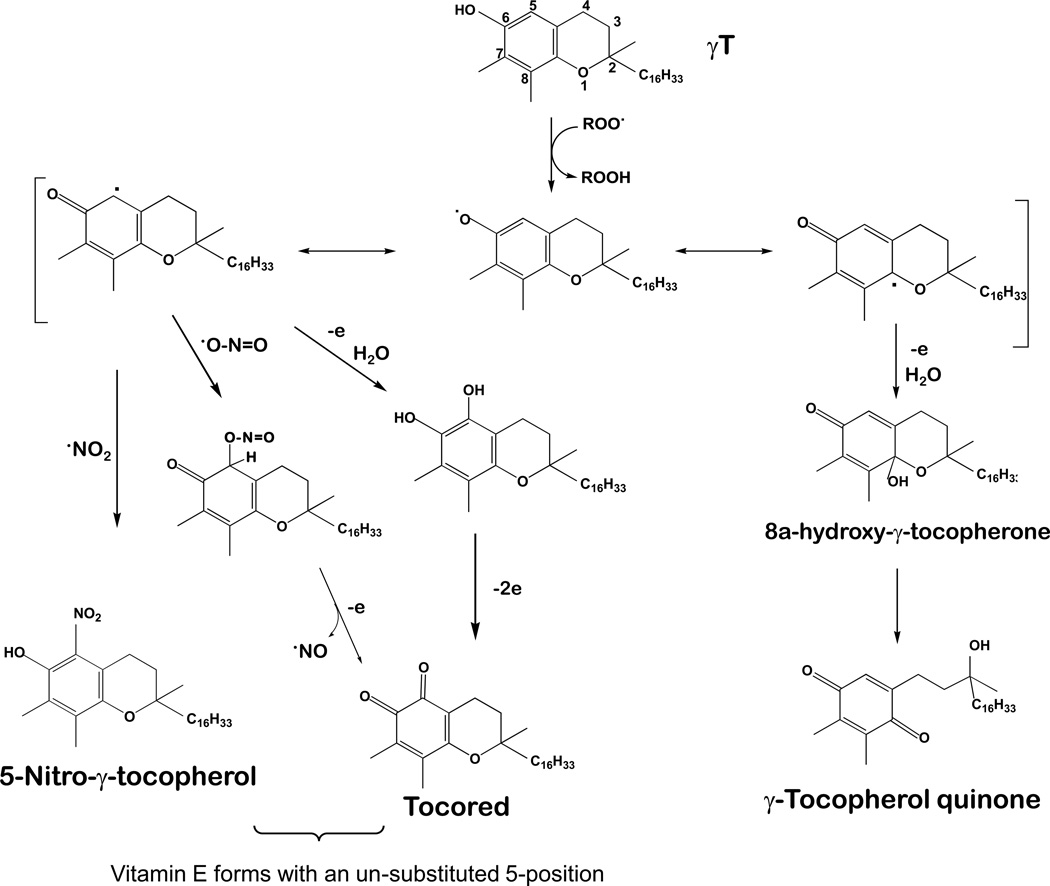
Figure 3. Antioxidant activities of vitamin E forms (representatively shown by γT).
Tocopherols and tocotrienols are potent lipophilic antioxidants by scavenging lipid peroxyl radicals via donating hydrogen from the phenolic group on the chromanol ring. Vitamin E forms with an un-substituted 5-position including γT may trap electrophiles such as NO2 or peroxynitrite to form 5-nitro-γ-tocopherol (5-NγT). This figure is modified based on ref [1].
In contrast to the similar hydrogen donating capability, natural vitamin E forms possessing an un-substituted 5-position such as γT are able to trap electrophils including reactive nitrogen species (Figure 3), which are enhanced during inflammation. On the other hand, this activity is not possessed by vitamin E with a methyl group at the 5-position, such as αT [1]. As a result, γT is shown to be superior to αT in detoxifying NO2 and peroxynitrite via formation of 5-nitro-γT [1, 64–66]. Consistently, 5-nitro-γT was elevated in zymosan induced-peritonitis in rats [67] and during FeCl3 patch-induced occlusive thrombus formation in rats [68].
In addition to tocopherols and tocotrienols, 13’-hydroxychromanol or 13’-carboxychromanol from δT or δTE have been shown to have potent antioxidant activities by preventing lipid peroxidation in vitro. Compared with αT, these compounds exhibit slightly stronger radical scavenging activity [69].
4. ANTI-INFLAMMATORY ACTIVITIES AND MECHANISMS
4.1. Overview of pro-inflammatory pathways and anti-inflammatory mechanisms by vitamin E forms and metabolites
Inflammatory diseases such as rheumatoid arthritis and asthma are among the leading causes of disability worldwide. Chronic inflammation contributes significantly to the development of chronic diseases including cardiovascular diseases and cancer [70–72]. Inflammation results from over-reacting immune response and is characterized by a plethora of production of reactive oxygen/nitrogen species and pro-inflammatory mediators including lipid mediators, notably prostaglandins and leukotriences, and cytokines like TNF-alpha and IL-6, which in turn aggravate inflammation and lead to excessive damage to host tissues [73–75].
Prostaglandins and leukotriences are synthesized from arachidonic acid (AA) and play important roles in mediating inflammatory response [74, 75]. For instance, prostaglandin E2 (PGE2), which is produced from COX-1 and COX-2-catalyzed oxidation of AA, is believed to cause pain and fever [74, 76], as well as activate cytokine formation [77]. Leukotriene B4 (LTB4), another lipid mediator derived from AA via the 5-lipoxygenase (5-LOX)-catalyzed reaction in neutrophils, is one of the most potent chemotactic agents [75]. Leukotriene C4 and D4, which are also generated by 5-LOX in eosinophils and mast cells, play key roles in allergic inflammatory diseases and asthma [78]. COXs- and 5-LOX-catalyzed eicosanoids are known to promote different types of cancer [79]. Because of the central roles of PGE2 and LTB4 in inflammation, COXs and 5-LOX have been recognized as key targets for drug therapy against chronic diseases. In particular, COX inhibitors, which are non-steroidal anti-inflammatory drugs (NSAIDs), have proven effective in attenuating inflammatory response, treatment of inflammatory diseases and prevention against cancer [79–81]. 5-LOX inhibitor Zileuton has clinically been used to treat asthma [82].
Cytokines are critical in regulation of inflammation and pathogenesis of inflammation-associated diseases. For instance, interleukin-6 (IL-6) secreted from stimulated macrophages is a pro-inflammatory cytokine and contributes to arthritis, cancer and obesity-related promotion of carcinogenesis [83, 84]. Anti-IL-6 antibody is clinically used to treat anti-TNFα nonresponsive arthritis [85, 86]. Nuclear factor (NF)- κB and JAK-STAT6/3 (signal transducer and activator of transcription) are central transcriptional factors in mediating response and expression of cytokines and chemokines. Activated by receptor-mediated signaling in immune and other types of cells, NF-κB and STATs binds to consensus target sequences in various promoters and induce expression of a large amount of genes including proinflammatory cytokines.
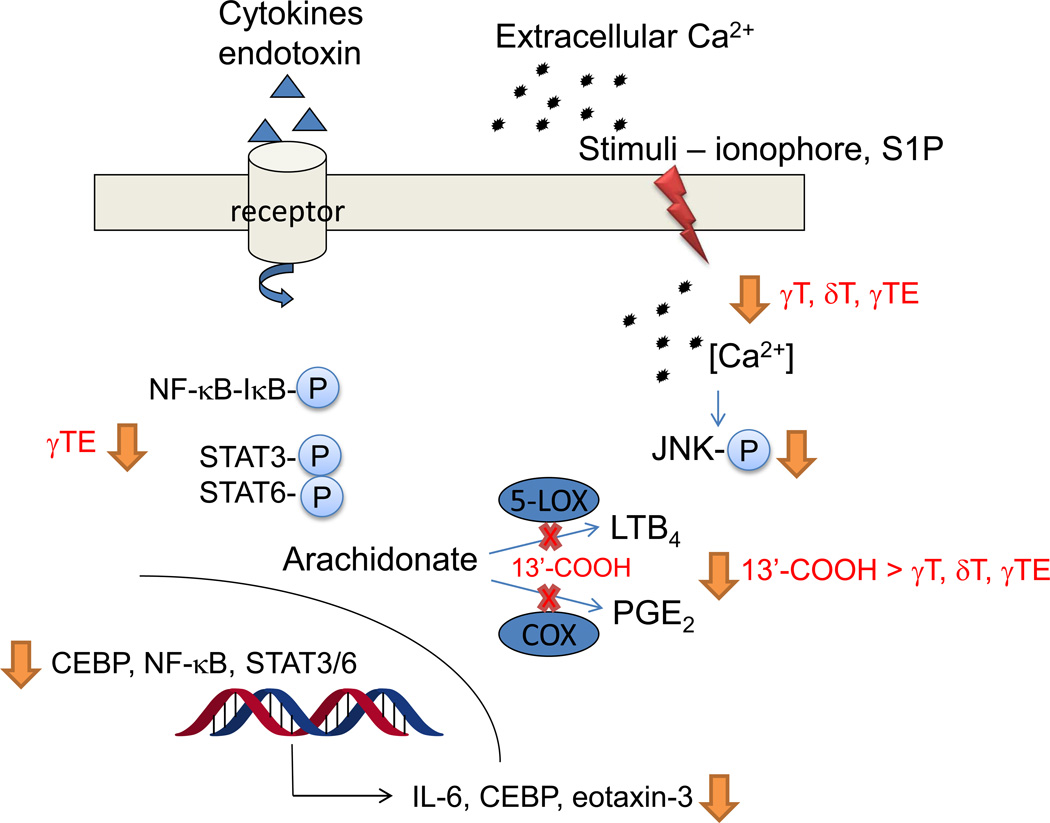
Mechanistic studies have demonstrated that specific forms of vitamin E such as γT, δT and tocotrienols (esp. γTE) have anti-inflammatory effects by inhibiting COX-2- and 5-LOX mediated eicosanoids, and suppressing NF-κB and JAK-STAT6 or JAK-STAT3 signaling pathways in various types of cells (summarized in Figure 4). Long-chain carboxychromanols esp. 13’-COOHs have been shown to inhibit COXs and 5-LOX more strongly than un-metabolized vitamin E forms.
Figure 4. Anti-inflammatory activities and mechanisms of vitamin E forms and long-chain carboxychromanols.
In epithelial cells, macrophages and neutrophils, γT, δT and γTE modestly inhibit PGE2 and LTB4 without inhibiting COXs and 5-LOX activity. 13’-COOHs potently inhibit COX-1/COX-2 and 5-LOX enzyme activity (red cross marks). In neutrophils, vitamin E forms suppress ionophore- or S1P (sphingosine 1-phosphare)-stimulated calcium influx and its downstream signaling. In lung epithelial cells, macrophages and some cancer cells, γTE inhibits activation of NF-κB and STAT6/3 as well as their regulated genes including cytokines and chemokines.
4.2. Vitamin E forms suppress generation of prostaglandins and leukotrienes in cellular environments, but do not inhibit the COX-2 or 5-LOX activity
Jiang et al [87, 88] have shown that vitamin E forms differentially inhibited COX-2-mediated PGD2 and PGE2 formation in LPS-stimulated RAW264.7 macrophages and IL-1β-activated lung epithelial cells, respectively, with relative potency of δT ≈ γTE > γT >> αT. Although γT, δT and γTE had no effects on endotoxin- or cytokine-stimulated COX-2 up-regulation, they suppressed COX-2 activity in cellular environments [87, 88]. On the other hand, none of these vitamin E forms appeared to inhibit the activity of purified COX-2 [87, 88]. These results support the notion that vitamin E forms are weak COX-2 inhibitors like sallysalic acid and acetomanophen, both of which inhibit PGE2 in intact cells but show weak inhibition of the purified COXs [89, 90].
γT, δT and γTE strongly inhibited A23187-stimulated LTB4 and LTC4 with IC50 of ~5 µM in neutrophil- and eosinophil-like HL60 cells as well as human neutrophils isolated from peripheral blood, while αT was much less effective with IC50s of 40–60 µM [91]. However, these vitamin E forms failed to inhibit human recombinant 5-LOX at physiologically relevant concentrations (IC50 > 200µM) [91]. Instead, δT and γT potently suppressed A23187-stimulated ERK phosphorylation and 5-LOX translocation from cytosol to the nucleus, which are key events for activation of 5-LOX to generate LTB4 in neutrophils [91]. Mechanistic investigation revealed that vitamin E forms reversed ionophore-triggered membrane perturbation and consequently suppressed calcium influx, and thus leading to inhibition of Ca2+ activated downstream signaling [91]. It is interesting to note that the inhibitory effect of vitamin E forms on calcium influx and LTB4 depends upon specific stimuli; for instance, δT inhibits LTB4 stimulated by calcium ionophores (A24187, ionomycin), sphingosine-1-phosphate and lysophosphatic acid, but not by fMLP or thapsigagin [91]. These observations are consistent with the lack of direct inhibition of 5-LOX enzyme activity by vitamin E forms and suggest that their suppressive effects of leukotrienes likely vary upon the nature of inflammation under whole body environments.
4.3. 13’-COOHs are dual inhibitors of COXs and 5-LOX
In contrast to unmetabolized vitamin E forms, long-chain carboxychromanols like 13’-COOHs are able to suppress PGE2 in cellular environments and directly inhibit COX-1 and COX-2 enzyme activity [88]. In these activities, 13’-COOH from δT (δT-13’-COOH) exhibits similar potency to ibuprofen and is much stronger than tocopherols or shorter-chain carboxychromanols including 9’-COOH, 5’-COOH or 3’-COOH (α-CEHC and γ-CEHC) [87, 92]. Mechanistic studies indicate that δT-13’-COOH inhibits the cyclooxygenase but not the peroxidase activity of COX-1 and COX-2 [88]. Enzyme kinetic data reveal that 13’-COOH is a competitive inhibitor of COX-1 and COX-2 with Ki at 3.9 and 10.7 µM, respectively [88], indicating that 13’-COOH competes with arachidonic acid at the substrate-binding site of the enzymes. Consistently, computer simulation confirms that 13’-COOHs are capable of binding to the substrate binding site of COX and like arachidonic aicd, the carboxylic acid group of 13’-COOH appears to form hydrogen-bonds with Tyr355 and Arg120. Moreover, Phe209, Phe381 and His226 are thought to provide extra interaction with the chromanol of 13’-COOH via hydrophobic interaction and hydrogen bond formation [88].
δT-13’-COOH also inhibits ionophore-stimulated LTB4 in HL-60 cells or isolated human neutrophils. Unlike un-metabolized vitamin E forms, δT-13’-COOH directly inhibits human 5-LOX activity with IC50 of 1–2µM and effectively decreases LTB4 in neutrophils regardless of the type of stimuli used for cell stimulation [91]. Our unpublished data indicate that 13’-COOH is much stronger than shorter chain carboxychromanols in inhibition of the activity of 5-LOX (Park N et al, unpublished observations). The nature of how 13’-COOH inhibits 5-LOX is currently under investigation.
4.4. The effect of vitamin E forms on pro-inflammatory cytokines via modulating key transcription factors C/EBPβ (CCAAT-enhancer binding protein β), NF-κB and STAT6/STAT3
Several studies including ours [93–95] have shown that vitamin E forms inhibited LPS-stimulated IL-6 in macrophages. Among different forms of vitamin E, γTE is the strongest in this activity. On the other hand, vitamin E forms did not have significant impact on IL-10, an anti-inflammatory cytokine [93, 94]. Mechanistic studies demonstrate that γTE inhibited IL-6 by suppressing LPS-induced activation of NF-κB and up-regulation of C/EBPβ and C/EBPδ [93]. γTE also inhibits NF-κB activation in cancer cell lines [96]. Consistent with its inhibition of C/EBPs, γTE decreases LPS-stimulated granulocyte-colony stimulating factor (G-CSF), a C/EBPβ target gene. Compared with RAW264.7 cells, γTE shows similar or stronger inhibitory effects on LPS-triggered activation of NF-κB, C/EPBβ and C/EBPδ, and more potently suppresses IL-6 and G-CSF in primary bone marrow-derived macrophages [93].
γTE is stronger than other vitamin E forms in inhibition of interleukin-13 (IL-13)-stimulated generation of eotaxin-3 via blocking phosphorylation of STAT6 and DNA-binding of STAT6 in human lung epithelial A549 cells [97]. Eotaxins-3 (CCL26) is a key chemokine in pathogenesis of asthma due to its capability of inducing airway eosinophilia, a hallmark of asthma. Mechanistic investigation revealed that γ-TE inhibited IL-13/STAT6-activated eotaxin via up-regulation of PAR4 (prostate-apoptosis-response 4), which consequently suppresses atypical protein kinase C (aPKC)-mediated STAT6 activation. In addition, γTE is shown to block JAK1-STAT3 signaling via induction of protein-tyrosine phosphatase SHP-1 in various types of cancer cells [98].
4.5. Effects on gene expression in activated T cells
Zingg et al [99] examined potential effect of αT or γT on CD3/CD28-stimulated gene expression in spleen T cells isolated from old mice supplemented with these tocopherols at a low (30mg/kg) or high dose (500mg/kg). Compared with high-dose αT, γT supplementation led to suppression of CD3/CD28-induced cytokines, chemokines and signaling lymphocytic activation molecules. As a result, γT appears to more strongly prevent gene up-regulation upon T cell activation than αT [99]. On the other hand, αT seems to support T cell activation. Despite these interesting results, the role of αT or γT in T cell activation is not clear because comparisons were made between low and high doses of αT and γT without including the control diet. Furthermore, it is not clear if similar results can be seen with young animals.
5. ANTI-INFLAMMATORY AND IMMUNE MODULATORY ACTIVITIES IN PRECLINICAL DISEASE MODELS
Overview
Animal studies in preclinical models have been conducted to examine potential benefits of γT and other vitamin E forms under pathological conditions associated with inflammation and oxidative stress (Summarized in Tables 2 and 3). Many of these studies have confirmed antioxidant and antiinflammatory mechanisms discovered in cell-based studies (Figure 4). Among these preclinical studies, γT shows protection against lung injury, modest colitis and tumorigenesis. The modulatory effect of γT on allergic inflammation is evident, although contradictory outcomes have been reported possibly because of different routes of drug administration. As to tocotrienols, beneficial results have been observed in immune response, radiation protection and anticancer activities. It should be noted that this review includes the studies where anticancer effects of tocopherols and tocotrienols are partially rooted in anti-inflammatory mechanisms, while anticancer outcomes due to other mechanisms have been reviewed elsewhere [3].
Table 2. Anti-inflammatory and antioxidant effects of γT and mixed tocopherols in animal preclinical models.
| Animal model | Vitamin E forms and doses | Major outcomes and references |
|---|---|---|
| Acute inflammation and tissue-injury models | ||
| Zymosan-induced peritonitis in F344 rats | γT at 100mg/kg in chow diet for four weeks before induction of peritonitis | γT decreased 3-nitrotyrosine and ascorbate oxidation, and spared starvation-induced loss of vitamin C [106]. |
| Carrageenan-induced airpouch inflammation model in Wistar rats | Daily oral γT (33, 100mg/kg bw) or αT 33 mg/kg (in core oil) for 3 days prior to induction of inflammation | γT but not αT inhibited inflammation-induced PGE2, LTB4, and 8-isoprostane. γT attenuated tissue damage and partial loss of food consumption [100]. |
| Combinations of aspirin (150mg/kg) with γT (33mg/kg) or αT (33mg/kg) for 3 days via gavage (in corn oil) | γT+aspirin showed prolonged anti-inflammatory effects compared with aspirin. γT+aspirin attenuated, but Aspirin+αT worsened, aspirin-caused gastric injury [101]. | |
| Acute lung injury caused by burn and smoke inhalation in sheep | γT administered one hour after injury via lipid aerosolization (51mg/ml, 24mL for 47h in flaxseed oil) or in ethanol (3–48 h) | γT nebulization attenuated burn- and smoke-induced acute lung injury, improved pulmonary function, decreased nitrotyrosine and cytokines and ameliorated collagen deposition [107–109]. |
| Vascular injury in rats with high fructose-induced insulin resistance | Oral αT or γT at 100mg/kg for three days prior to induction of the vascular injury by a balloon catheter | γT but not αT reduced vascular injury and attenuated 3-nitrotyrosine. Both tocopherols decreased lipid peroxidation in the plasma, but neither had effects on superoxide production in the carotid arteries [110]. |
| Airway inflammation and allergic asthma models | ||
| Airway inflammation caused by intranasal LPS in male F344/N rats | Oral gavage of γT at 30mg/kg bw daily prior and during intranasal LPS (5 or 20µg) challenge | γT decreased neutrophil infiltration, BALF PGE2, secreted mucins, intraepithelial mucosubstances as well as chemokines and mucus-production cytokines, while enhanced IL-10 and IFNγ [104, 115] |
| Allergy airway inflammation and asthma models in ovalbumin (OVA)-sensitized and challenged rats or mice | Oral γT (100mg/kg bw daily) before [102] or after [103] intranasal antigen challenge in Brown Norway rats | γT suppressed eosinophila, cys-leukotrienes and cytokines (MCP-1, IL-6) in BALF and attenuated ozone-enhanced intraepithelial mucosubstances [102, 103]. |
| Subcutaneous injection of αT or γT (100mg/kg bw) prior to and during antigen challenge in Balb/c mice [113, 116] | In contrast to αT, γT elevated airway inflammation and blocked αT-related anti-inflammatory effects partially by modulating endothelial cell signaling and PKCα activation [113, 116] | |
| Colitis, colon inflammation and carcinogenesis | ||
| Colitis induced by dextran sodium sulfate (DSS) in mice | γT or mixed tocopherols (45% γT, 45% δT and 10% αT) at 0.1%-diet, a week before DSS (1.5% or 2.5% in water) in BALB/c mice | γT but not mixed tocopherols attenuated moderate colitis induced by one cycle of 1.5% DSS, while neither was protective to severe colitis induced by 3 cycles of 2.5% DSS. [29] |
| γT-rich mixed tocopherols (57% γT, 24% δT and 14% αT) at 0.1, 0.17, or 0.3% diet, DSS at 1.5% in CF-1 or 1% in C57BL/SV129 mice. | Mixed tocopherols mitigated AOM/DSS-induced colon inflammation but had no effect on 1.5%-DSS induced colitis [119], while dose-dependently attenuated 1%-DSS induced colitis [105]. | |
| Inflammation-promoted tumorigenesis induced by AOM-DSS in mice | γT or mixed tocopherols (45% γT, 45% δT and 10% αT) at 0.1%-diet, AOM (10mg/kg)-DSS (1.5% or 2.5% for 3 cycles) in BALB/c mice. | γT but not mixed tocophcerols suppressed AOM-DSS (1.5%)-promoted colon tumorigenesis, but was ineffective to severe inflammation (3 cycles of 2.5% DSS) promoted carcinogenesis [29]. |
| γT-rich mixed tocopherols (57% γT, 24% δT and 14% αT) at 0.17 or 0.3% in AOM (5 or 10mg/kg) and DSS induced colon cancer in CF-1 mice. | Mixed tocopherols suppressed AOM-DSS induced tumorigenesis [119] | |
Table 3. Protective effects of tocotrienols in various inflammation-associated disease models.
| Animal model | Vitamin E forms and doses | Major outcomes and references |
|---|---|---|
| UVB-induced inflammation in HR-1 hairless mice | γTE-rich mixture (2.3 mg/d), oral in corn oil | γTE attenuated UVB-induced skin damage and increased thickness as well as up-regulation of COX-2 [122]. |
| Gamma irradiation in CD2F1 mice | δTE (400 mg/kg bw) subcutaneously injected to mice 24h before and 6h after total body irradiation at 5 or 8.75 Gy at 0.6Gy/min. | δTE not only protected irradiation-induced death, but also promoted cell survival and regeneration of hematopoietic microfoci, stem and progenitor cells in irradiated mouse bone marrow [124]. |
| Pancreatitis induced by repeated arginine injection in male Wistar rats | Tocotrienol-rich fraction from palm oil (TRF) (100mg/kg bw) by gavage 1wk before and day 5 after arginine administration | TRF blunted arginine-induced pancreatic atrophy, activation of stellate cell, protease and Smad 3, and increased hydroxyproline and TGF-β1 [126]. |
| PiCl-induced allergic dermatitis in NC/Nga mice | Oral γTE-rich tocotrienols containing 89.9% γTE, 1 mg per mouse in corn oil | Tocotrienols suppressed PiCl-induced allergic dermatitis and serum histamine secretion [125]. |
| Tetanus-toxoid (TT) immunezation in Balb/c mice | Oral tocotrienol-rich fraction (TRF), or αT or δTE, 1 mg daily for two weeks before TT vaccination | TRF and δTE treatment led to increase of anti-TT antibody and promoted Th1 cytokines like IFN-γ and IL-4 [129] |
| Pancreatic cancer cells implanted in nude mice | Oral γTE (400 mg/kg bw daily) with or w/o gemcitabine (25mg/kg) in MIA PaCa-2 cells orthotopically implanted model | γTE inhibited tumor growth and enhanced antitumor effects of gemcitabine probably via suppression of NF-κB [130] and/or STAT3 [98]. |
| Oral δTE (200 mg/kg bw) or with gemcitabine in AsPc-1 implanted to both flanks of nude mice | δTE was stronger than other tocotrienols in suppression of pancreatic tumor growth and inhibited NF-κB activation, and augmented anticancer effects of gemcitabine [131]. |
5.1. Anti-inflammatory and antioxidant effects in acute inflammation and oxidative stress models
Potential inhibition of proinflammatory eicosanoids by γT has been examined in acute inflammation models. Specifically, when pre-administered for three days prior to the induction of inflammation, γT but not αT significantly inhibited pro-inflammatory PGE2, LTB4 and 8-isoprostane and attenuated inflammation-associated damage in the rats’ carrageenan-induced airpouch inflammation model, which is believed to mimic joint diseases [87, 100]. In this model, a combination of γT and aspirin was better than aspirin in suppression of inflammation and attenuated aspirin-induced stomach lesions. In contrast, aspirin combined with αT unexpectedly worsened aspirin-caused gastric injury [101]. In addition, supplementation with γT or γT-rich mixed tocopherols inhibited PGE2 and/or leukotrienes (LTB2, LTC4) in airway allergic inflammation [102–104] and colitis models [105] (below).
Protective effects of γT via anti-inflammatory and scavenging reactive nitrogen species have been demonstrated in different disease models. In zymosan-induced peritonitis, supplementation of γT in αT-sufficient chow diet significantly reduced formation of protein bound 3-nitrotyrosine and ascorbate oxidation in the kidney and prevented starvation-induced ascorbate decrease [106]. To effectively increase γT in the lung, Hamahata and colleagues [107] designed a lipid aerosolization device for delivering γT in flaxseed oil via nebulization. They found that γT nebulization improved pulmonary function in sheep suffering from 40% total body surface area burn and smoke-inhalation injury. Specifically, γT nebulization prevented burn and smoke inhalation injury-caused fall in oxygenation, reduced the obstruction score and edema and decreased nitrotyrosine and pro-inflammatory cytokines IL-6 and IL-8. In a ovine similar model, Yamamoto et al. [108, 109] showed that nebulization with γT attenuated oxidative stress and lung injury after burn and smoke inhalation. In addition, γT but not αT attenuated balloon catheter-induced increase of neointima/media ratio (a vascular injury marker) and 3-nitrotyrosine in insulin resistant rats. Interestingly, both tocopherols reduced lipid peroxidation in the plasma, but neither showed effects on superoxide production in the carotid arteries [110]. Saldeen et al [111] reported that γT was more potent than αT in attenuating FeCl3-induced platelet aggregation, superoxide production and occlusive thrombus in rats.
5.2 – Asthma and allergic airway inflammation
Asthma is a chronic airway inflammatory disease. Vitamin E and C have been reported to decrease in airway fluids of asthmatics [112]. Therefore, it is proposed that supplementation of these antioxidants may be helpful in asthma treatment. Potential benefits of αT to asthma have been examined in allergic airway models, but these studies revealed inconclusive outcomes [113, 114]. Meanwhile, inhibition of 5-LOX including clinical use of zileuton (a 5-LOX inhibitor) is recognized as an effective strategy in asthma treatment [82]. Because γT and long-chain carboxychromanols inhibited 5-LOX-catalyzed leukotrienes in neutrophils [91], these compounds may be useful anti-asthmatic agents. To this end, several studies have been conducted to investigate the role of γT in airway inflammation in various animal models.
Wagner et al [102] showed that oral administration of γT before or after antigen nasal challenge led to suppression of airway eosinophilia and mucous cell hyperplasia in ovalbumin (OVA)-induced allergic rhinitis and asthma in rats. γT supplementation decreased pulmonary production of PGE2, LTB4, cysteinyl leukotrienes and nasal expression of cytokines. In a similar airway allergic inflammation model, γT attenuated OVA- and ozone-stimulated eosinophilic infiltration and decreased bronchoalveolar lavage fluid (BALF) cys-leukotriene and cytokines [103]. γT also suppressed ozone-enhanced intraepithelial mucosubstances in main axial airway [103]. Furthermore, in intranasal LPS-challenge induced airway inflammation model, γT significantly mitigated neutrophil infiltration to the lung tissue and neutrophil accumulation in the BALF fluid [104, 115]. γT also inhibited LPS-stimulated BALF PGE2, mucin secretion, and cytokines including neutrophil-chemotactic cytokines (MIP-2 and GRO-KC) and mucus-production cytokines (Muc5AC) [104].
In contrast to the observed asthma-dampening effects, when γT was given via subcutaneous injection prior to antigen challenge, it enhanced airway inflammation and abolished αT-exerted anti-inflammatory effects possibly by modulating endothelial cell signaling and PKCα activation [113, 116]. The reason for this discrepancy with other above-mentioned studies is not clear but may be in part due to different routes of drug administration. This is because subcutaneous injection, which bypasses liver metabolism, likely leads to much higher levels of γT but lower metabolites in the blood and tissues than oral gavage. Given that vitamin E metabolites esp. long-chain carboxychromanol inhibit 5-LOX, whereas the suppressive effect of tocopherols on leukotrienes is contingent upon specific stimuli [91], it is reasonable to assume that oral administration of γT may yield more favorable outcomes than subcutaneous injection, as substantiated in above-cited literature. Further investigation is necessary to confirm the adverse effects from subcutaneous γT administration, which should probably not be recommended in clinical studies.
5.3. Anticancer effects via antioxidant and anti-inflammatory mechanisms
Inflammatory bowel diseases including colitis are known to dramatically increase the risk of colon cancer [72]. Eicosanoids from COXs and 5-LOX pathways are recognized to contribute to cancer development [79, 80, 117, 118]. Because of their inhibitory effects on COX-2 and 5-LOX catalyzed generation of eicosanoids [87, 88, 91], γT and δT have been proposed to be potentially useful chemoprevention agents against cancer. To this end, Ju et al. [119] reported that γT-rich mixed tocopherols suppressed colon tumorigenesis, inflammation and eicosanoids (PGE2 and LTB4) induced by azoxymethane (AOM) and dextran sodium sulfate (DSS) in mice, which is an experimental cancer model mimicking colitis-promoted colon cancer. In a subsequent study, the same group of investigators observed that γT-rich mixed tocopherols alleviated DSS (1%)-induced oxidative damage, PGE2 and leukocyte infiltration in colon tissues and these protective effects were independent of the nuclear factor (erythroid-derived 2)-like 2 (NFE2L2 or Nrf2)[105]. Jiang et al [29] showed that γT attenuated moderate (induced by one cycle of DSS) but not severe colitis induced by three cycles of DSS. γT also suppressed moderate colitis-promoted colon carcinogenesis in the AOM-DSS model, while was not effective toward severe colitis-promoted tumorigenesis [29].
Besides colon cancer, Sanches LD et al [120] recently reported that γT-enriched diet decreased ventral prostate epithelial dysplasia and attenuated upregulation of COX-2 and matrix metalloproteinase (MMP-9) activity induced by N-methyl-N-nitrosourea (MNU) in rats. Barve et al. [121] showed that γT-rich mixed tocopherols suppressed the incidence of palpable tumor and maintained redox sensitive transcription factor Nrf2 as well as Nrf2-regulated antioxidant genes in the murine prostate cancer TRAMP model.
5.4. Anti-inflammatory and immune modulatory effects by tocotrienols
Besides tocopherols, recent literatures have documented anti-inflammatory and immune-modulatory effects of γTE and δ-tocotrienol (δTE) in various disease models (Table 3). Shibata et al [122] showed that γTE but not αT suppressed UVB-induced PGE2 and cytokines via blocking pro-inflammatory signaling in keratinocytes and attenuated UVB-caused increase of skin thickness and COX-2 induction in HR-1 hairless mice. Dietary supplementation of tocotrienol mixtures enhanced lymphocyte proliferation without affecting major cytokines in old but not young C57BL/6 mice [123]. In CD2F1 mice, subcutaneous injection of δTE prevented whole body irradiation-induced mortality as indicated by 30-day post irradiation survival, promoted bone barrow stem and progenitor cell regeneration and attenuated irradiation-caused damage [124]. This protective effect appeared to stem from activation of extracellular signaling-related kinase and rapamycin signaling in mouse bone marrow cells [124]. Tsuduki et al [125] reported that γTE-rich tocotrienols attenuated allergic dermatitis in mice and suppressed degranulation and histamine release in mast cells. In addition, tocotrienol rich fraction from palm oil mitigated chronic pancreatitis [126], showed protection against potassium dichromate-caused acute renal injury [127], and attenuated the increase of plasma liver enzyme and inflammatory cell infiltration to the liver in high carbohydrate plus high fat diet-induced chronic disease model [128].
Radhakrishnan AK et al [129] examined potential effects of αT, δTE and mixed tocotrienols on tetanus toxoid immunization in mice. These vitamin E forms were capable of enhancing production of antibodies against tetanus toxoid with relative effectiveness of δTE > mixed tocotrienols > αT. Interestingly, while these vitamin E forms increased interferon-γ and interleukin-4, they decreased TNF-α in stimulated splenocytes.
Based on the inhibitory effect on NF-κB and STAT3, γTE and δTE have been proposed to be useful in chemoprevention or adjuvant chemotherapy for cancer. To this end, γTE and δTE are shown to inhibit pancreatic tumor growth and sensitized cancer cells to gemcitabine treatment by inhibition of NF-κB in vivo [130, 131]. As a result, γTE or its combination with gemcitabine led to down-regulation of NF-κB regulated gene products such as cyclin D1, MMP-9 and CXCR4. Moreover, γTE also suppressed pancreatic tumor growth by blocking STAT3 signaling and down-regulating the expression of STAT3-regulated anti-apoptotic genes such as Bcl-2 and Bcl-xL in the orthotopic model [98].
6. HEALTH BENEFITS IN HUMAN CLINICAL STUDIES
6.1. Observational studies
Many epidemiological studies have observed inverse association between cardiovascular diseases (CVD) and dietary intake of vitamin E which contains mainly γT and αT. Association of vitamin E supplementation which primarily includes just αT with CVD has been inconclusive. Meanwhile, both positive and negative association of αT and γT with cancer risk has been reported [3]. Due to their well-recognized limitations, observational studies need to be interpreted with caution. It is worth mentioning that since diet with γT and αT often contains high levels of distinct fatty acids, e.g., PUFA vs. MUFA (Table 1), the type and amount of dietary fat should be considered as potential confounding factors in association studies.
6.2. Clinical intervention studies
Unlike αT which has been investigated in many large clinical trials, only a handful of small-scale clinical studies have been conducted to investigate potential beneficial effects γT in humans (Table 4). In hemodialysis patients, supplementation of γT but not αT decreased pro-inflammatory IL-6 and C-reactive protein (CRP) [45, 132] and a combination of γT and DHA decreased IL-6 without influencing CRP [133]. Meanwhile, αT or γT supplementation attenuated contrast-induced kidney injury in patients with chronic kidney disease [134]. It is recently reported that γT combined with high n-3 fatty acids significantly reduced relapse of multiple sclerosis and decreased the risk of sustained progression of disability in multiple sclerosis patients [135]. However, in diabetic patients, mixed results have been observed regarding the effect of γT and αT; Specifically, γT or its combination with αT suppressed CRP and attenuated oxidative stress [136, 137], whereas in another study, αT or mixed tocopherols increased blood pressure without affecting cytokines or endothelium-dependent and independent vasodilation [138]
Table 4. Human intervention studies regarding beneficial effects of γT or mixed tocopherols.
| Subjects / design (References) | Vitamin E forms; duration | Major outcomes |
|---|---|---|
| Patients with renal/kidney diseases and multiple sclerosis | ||
| Hemodialysis patients with end-stage renal disease / [45, 132] | 300 mg αT or 300 mg γT-rich mixed toocpherols (60% γT, 28% δT and 10% αT), for 14 d, n=15 per group | Supplementation of γT-rich mixed tocopherols decreased CRP and IL-6 in the plasma, whereas αT alone increased IL-6 without affecting CRP. |
| Hemodialysis maintenance patients / randomized, double-blinded, placebo-controlled [133] | 308 mg γT plus 800 mg DHA (docosahexaenoic acid) (n=31) vs. placebo (n=30) for 4 and 8 wk | Compared with placeboes, γT plus DHA decreased white blood cells, neutrophils and erythropoietin index as well as IL-6, but did not affect plasma CRP, F2-isoprostane or carbonyls. |
| Patients with chronic kidney disease undergoing coronary procedures / prospective, double-blind, randomized and placebo-controlled trial [134] | 350 mg αT, or 300 mg γT, or placebo (n=101–102 per group) for 5 days prior to coronary procedure and 2 days afterwards | Prophylaxis administration with αT or γT decreased the risk of contrast-induced acute kidney injury in chronic kidney disease patients. |
| Patients with relapsing-remitting multiple sclerosis / randomized, blind, placebo-controlled [135] | A: n-3 (EPA+DHA)/n-6 fatty acids at 1:1 with αT (22mg); B: A plus γT (760mg); C: γT (760mg); placebo, for 30 months. n=20 per group | The combination of n-3/n-6 (1:1) fatty acids and γT (B) significantly reduced relapse rate of multiple sclerosis by 64%, delayed disability progression by 72% and decreased the risk of the sustained progression disability by 85%. |
| Diabetic patients and CVD | ||
| Type 2 diabetic patients / double-blind, placebo-controlled [137, 138] | 500 mg of RRR-αT, or γT-rich mixed tocopherols (75mg αT, 315mg γT and 110mg δT), and placebo (n=18–19), for 6 wk. | αT or γT-rich mixed tocopherols decreased plasma F2-isoprostane but increased blood pressure without affecting inflammation markers. γT-rich mixed tocopherols but not αT reduced LTB4 from stimulated neutrophils. |
| Participants with metabolic syndromes / randomized, placebo-controlled double-blind trial [136] | 800 mg αT, or 800 mg γT, or their combination, or placebo (n=20 per group) for 6 wk | The combination of αT and γT decreased CRP, nitrotyrosine and oxidation markers, while αT and γT alone showed partial benefits regarding these markers. |
| Healthy subjects | ||
| Healthy volunteers / platelet aggregation after tocopherol supplementation in placebo controlled study [140]. | Mixed tocopherols (100mg γT, 40mg δT and 20mg αT) (n=18) or all-rac-αT acetate at 100mg (n=18), or placebo (n=10) for 8 wk | Mixed tocopherols were better than αT in suppression of ADP-induced platelet aggregation and in induction of nitric oxide release and endothelial constitutive nitric oxide synthase, while showed similar effect on SOD and PKC. |
| Healthy sedentary subjects in strenuous exercise / randomized placebo controlled [139] | 300 mg γT, or 400IU αT, every day or every other day for 6 wk (n=36) | γT but not αT ameliorated exercise-induced decrease of APTT (activated partial thromboplastin time) and exercise-increased platelet aggregation induced by collagen. |
| Healthy volunteers challenged by intranasal endotoxin (LPS) / double-blind, randomized, placebo controlled, crossover study [115] | γT-enriched tocopherols (540, 50 and 240 mg of γT, αT, δT) twice a day for a week before endotoxin inhalation challenge (n=13) | γT-rich tocopherols attenuated intranasal LPS-induced tissue infiltration and accumulation of airway neutrophils, reduced % eosinophils in sputum and neutralized LPS-induced increase of IL-β. |
| Healthy men with oral glucose tolerance test following overnight fasting / randomized, crossover, single-blind design [142, 143] | Mixed tocopherols (500mg γT, 60mg αT, 170mg δT and 9mg βT) daily for 5 days (n = 15) | γT-rich tocopherols attenuated glucose-induced decrease of brachial artery flow-mediated dilation, lipid peroxidation and disruption in NO homeostasis as well as dicarbonyl methylglyoxal. |
Potential modulatory effects of γT on inflammation and oxidative stress have been investigated in healthy subjects. For instance, supplementation with γT or γT-rich mixed tocopherols but not αT is reported to attenuate strenuous exercise-increased platelet coagulation [139] or ADP-induced platelet aggregation [140]. γT-rich tocopherols significantly attenuated endotoxin inhalation-induced increase of airway neutrophils [115], suppressed ex vivo LPS-enhanced pro-inflammatory cytokines from peripheral blood mononuclear cells [141], and alleviated postprandial hyperglycemia-caused impairment of endothelial function, enhancement of lipid peroxidation and disruption in NO homeostasis [142, 143].
In addition to γT, potential benefits from tocotrienol-rich fractions (extracted from palm oil) have been investigated in some human studies. In a double-blinded, placebo-controlled clinical trial, healthy volunteers supplemented with 400mg of tocotrienol-rich fraction had increased production of anti-tetanus toxoid antibody, IL-4 and interferon-gamma induced by tetanus toxoid vaccine challenge but reduced IL-6, compared with placebos [144]. A topical formulation containing tocopherols and tocotrienols showed photoprotective effect in photosensitive subjects [145]. Supplementation of tocotrienol-rich fraction (74% tocotrienol and 26% tocopherols) modulated plasma proteins including CRP in healthy female subjects [146]. Patel et al. [147] reported that tocotrienol mixtures (200mg, n=14) exhibited stronger protective effect than αT (n=5) on end-stage liver disease in cirrhosis patients with hepatitis B and C. In addition, Tamoxifen combined with γTE showed non-significant decrease of breast cancer mortality compared with tamoxifen alone controls [148].
7. SUMMARY AND CONCLUSION REMARKS
During the last 15–20 years, basic research on vitamin E has expanded from primarily focusing on αT and its antioxidant effect to investigation of different tocopherols and tocotrienols, their metabolism and non-antioxidant activities including anti-inflammatory properties. Despite well-documented antioxidant and other beneficial effects [2, 149] as well as negative association between αT intake and chronic diseases, supplementation of αT has failed to offer consistent benefits to prevention of chronic diseases including cancer and cardiovascular diseases in many large clinical intervention studies [3, 4, 150, 151]. One explanation is that αT may be beneficial to individuals with deficiency in αT and/or other micronutrients [149], which can be caused by low dietary intake of this vitamin E, depletion of αT due to pathological situation or malnutrition associated with smoking, alcoholism and mal-absorption. Under these subclinical conditions, αT supplementation is likely beneficial, as indicated in the LinXian study in a population with deficiencies of micronutrients [152] and the ATBC study including heavy smokers [153]. On the other hand, αT supplementation did not show beneficial effects in people with adequate nutrient status [3, 4]. In contrast to αT, despite no evidence that deficiency of other vitamin E forms would result in obvious clinical symptoms, accumulating evidence suggests that γT, δT and tocotrienols appear to have unique properties that are superior to αT and relevant to prevention and therapy against chronic diseases even under conditions with adequate αT status. It is noteworthy that these bioactivities of tocopherols and tocotrienols including anti-inflammatory properties have been identified by mechanistic studies and subsequently substantiated in some preclinical models as well as clinical studies.
One of the most significant differences between αT and other forms of vitamin E is that in contrast to αT that is mostly retained in tissues due to preferential binding by α-TTP, large quantities of other forms of vitamin E are readily metabolized by CYP4F2-initiated ω-oxidation of the side chain to generate carboxychromanols and conjugated counterparts. Short-chain carboxychromanols like CEHCs are excreted in the urine and γ-CEHC has been shown to have natriuretic activities [18]. Long-chain carboxychromamols especially 13’-COOHs are found in tissues and feces in animals supplemented with γT, δT and γTE [25, 27–29]. The discovery of potent anti-inflammatory [88, 91] and anticancer [154] effect of long-chain carboxychromanols represents exciting research direction and provides new insights into physiological role of less tissue-preserved forms of vitamin E.
Vitamin E forms and metabolites appear to have impact on multiple regulatory pathways (Figure 4), and may therefore offer unique opportunities and advantage for prevention and treatment of diseases, compared with pharmaceutical drugs targeting specific proteins. For instance, γTE is effective in suppression of key regulatory transcription factors such as NF-κB and STAT3/6. Unlike NSAIDs that inhibit COXs, γT, δT and γTE suppress both COXs and 5-LOX mediated eicosanoids in cellular environments. 13’-COOHs are more effective than un-metabolized vitamers in dual inhibition of these enzymes and therefore likely contribute to anti-inflammatory activities of vitamin E forms in vivo. Dual inhibition of COXs and 5-LOX has advantage over COXs inhibitors in that blocking both prostaglandin and leukotriene formation likely not only results in more potent anti-inflammatory effects than inhibition of either pro-inflammatory pathway, but also reduces adverse effects compared with commonly-used NSAIDs. This is because a selective shutdown of COX pathway (by COX inhibitors) causes alternative metabolism of arachidonic acid via the 5-LOX pathway to increase leukotrienes, which are pro-inflammatory and promote gastrotoxicity, tumorigenesis and atherogenesis [155–157].
Despite great advance in our understanding of different forms of vitamin E, many important questions still remain and need to be addressed before we know how to utilize them in disease prevention and even treatment. First, more preclinical and clinical studies should be conducted to investigate potential use of γT, mixed tocopherols and tocotrienols as alternative or adjuvant therapy for prevention or treatment of chronic diseases including asthma, multiple sclerosis, kidney diseases and certain types of cancer. Meanwhile, besides efficacy, the safety of long-term use of these vitamin E terms should be thoroughly evaluated before they can be recommended to the public. Secondly, the role of metabolism in vitamin E forms-mediated health benefits should be further addressed. Although high levels of 13’-COOHs are found in feces of animals supplemented with γT or δT [27–29], whether these carboxychromanols directly contribute to in vivo beneficial effects from vitamin E supplementation remains unclear. It is necessary to conduct pharmacokinetic studies to characterize the bioavailability of these metabolites in different tissues. Utilization of CYP4F2 knockout mice in disease models may help evaluate potential contribution of metabolite formation to the protective effect of tocopherols and tocotrienols. In addition, given their unique dual inhibition of COX and 5-LOX, long-chain carboxychromanols and analogs may be a new class of anti-inflammatory and anti-cancer agents. More research is needed to identify other molecular targets of these vitamin E metabolites, test efficacy in preclinical models and evaluate their general safety.
This is a comprehensive review on different forms of vitamin E.
Gamma-, delta-tocopherol and tocotrienols inhibit multiple pro-inflammatory pathways.
Long-chain vitamin E metabolites have unique anti-inflammatory effects.
Gamma-, delta-tocopherol and tocotrienols are beneficial to disease prevention/treatment.
Acknowledgments
FUNDING
This work was supported in part by grants R21 CA152588 and R01AT006882 from National Institutes of Health.
Abbreviations
- αT, βT, γT and δT
α-, β-, γ- and δ-tocopherol
- αTE, βTE, γTE and δTE
α-, γ-, γ- and δ-tocotrienol
- AA
arachidonic acid
- CEHC
carboxyethyl-hydroxychromans
- 13’-COOH
13’-carboxychromanol
- 5-LOX
5-lipoxygenase
- COX-1/-2
cyclooxygenase-1/-2
- NF-κB
nuclear factor kappa B
- STAT
signal transducer and activator of transcription
- JNK
c-Jun N-terminal kinase
- CEPBβ
CCAAT-enhancer binding protein β
- PGE2
prostaglandin E2
- LTB4
leukotriene B4
- BALF
bronchoalveolar lavage fluid
- NSAIDs
non-steroidal anti-inflammatory drugs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. The American journal of clinical nutrition. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 2.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. Faseb J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 3.Moya-Camarena SY, Jiang Q. Sarkar Fazlul H., editor. Chapter 15-The role of vitamin E forms in cancer prevention and therapy-Studies in human intervention trials and animal models. Nutraceuticals and Cancer. 2011:323–354. [Google Scholar]
- 4.Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, Park BJ. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346:f10. doi: 10.1136/bmj.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun J, Lee J, Ye H, Exler J, Eitenmiller RR. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. Journal of Food Composition and Analysis. 2006;19:196–204. [Google Scholar]
- 6.McLaughlin PJ, Weihrauch JL. Vitamin E content of foods. J Am Diet Assoc. 1979;75:647–665. [PubMed] [Google Scholar]
- 7.Dreher ML. Pistachio nuts: composition and potential health benefits. Nutrition reviews. 2012;70:234–240. doi: 10.1111/j.1753-4887.2011.00467.x. [DOI] [PubMed] [Google Scholar]
- 8.Theriault A, Chao JT, Wang Q, Gapor A, Adeli K. Tocotrienol: a review of its therapeutic potential. Clin Biochem. 1999;32:309–319. doi: 10.1016/s0009-9120(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 9.Lecerf JM. Fatty acids and cardiovascular disease. Nutrition reviews. 2009;67:273–283. doi: 10.1111/j.1753-4887.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- 10.Traber MG, Burton GW, Ingold KU, Kayden HJ. RRR-and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. Journal of lipid research. 1990;31:675–685. [PubMed] [Google Scholar]
- 11.Traber MG, Burton GW, Hughes L, Ingold KU, Hidaka H, Malloy M, Kane J, Hyams J, Kayden HJ. Discrimination between forms of vitamin E by humans with and without genetic abnormalities of lipoprotein metabolism. Journal of lipid research. 1992;33:1171–1182. [PubMed] [Google Scholar]
- 12.Traber MG. Vitamin E regulatory mechanisms. Annual review of nutrition. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 13.Traber MG, Olivecrona T, Kayden HJ. Bovine milk lipoprotein lipase transfers tocopherol to human fibroblasts during triglyceride hydrolysis in vitro. The Journal of clinical investigation. 1985;75:1729–1734. doi: 10.1172/JCI111883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. The American journal of clinical nutrition. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [see comments]. [DOI] [PubMed] [Google Scholar]
- 15.Manor D, Morley S. The alpha-tocopherol transfer protein. Vitamins and hormones. 2007;76:45–65. doi: 10.1016/S0083-6729(07)76003-X. [DOI] [PubMed] [Google Scholar]
- 16.Chiku S, Hamamura K, Nakamura T. Novel urinary metabolite of d-delta-tocopherol in rats. Journal of lipid research. 1984;25:40–48. [PubMed] [Google Scholar]
- 17.Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohe R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2'-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am J Clin Nutr. 1995;62:1527S–1534S. doi: 10.1093/ajcn/62.6.1527S. [DOI] [PubMed] [Google Scholar]
- 18.Wechter WJ, Kantoci D, Murray ED, Jr, D'Amico DC, Jung ME, Wang WH. A new endogenous natriuretic factor: LLU-α. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6002–6007. doi: 10.1073/pnas.93.12.6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traber MG, Elsner A, Brigelius-Flohe R. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 1998;437:145–148. doi: 10.1016/s0014-5793(98)01210-1. [DOI] [PubMed] [Google Scholar]
- 20.Swanson JE, Ben RN, Burton GW, Parker RS. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. Journal of lipid research. 1999;40:665–671. [PubMed] [Google Scholar]
- 21.Stahl W, Graf P, Brigelius-Flohe R, Wechter W, Sies H. Quantification of the alpha- and gamma-tocopherol metabolites 2,5,7,8-tetramethyl-2 (2'-carboxyethyl)-6-hydroxychroman and 2,7, 8-trimethyl 2-(2'-carboxyethyl)-6-hydroxychroman in human serum. Anal Biochem. 1999;275:254–259. doi: 10.1006/abio.1999.4312. [DOI] [PubMed] [Google Scholar]
- 22.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. The Journal of biological chemistry. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 23.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132:3113–3118. doi: 10.1093/jn/131.10.3113. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. Journal of lipid research. 2007;48:1221–1230. doi: 10.1194/jlr.D700001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freiser H, Jiang Q. Gamma-tocotrienol and gamma-tocopherol are primarily metabolized to conjugated 2-(beta-carboxyethyl)-6-hydroxy-2,7,8-trimethylchroman and sulfated long-chain carboxychromanols in rats. The Journal of nutrition. 2009;139:884–889. doi: 10.3945/jn.108.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freiser H, Jiang Q. Optimization of the enzymatic hydrolysis and analysis of plasma conjugated gamma CEHC and sulfated long-chain carboxychromanols, metabolites of vitamin E. Analytical biochemistry. 2009;388:260–265. doi: 10.1016/j.ab.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardowell SA, Ding X, Parker RS. Disruption of P450-mediated vitamin E hydroxylase activities alters vitamin E status in tocopherol supplemented mice and reveals extra-hepatic vitamin E metabolism. Journal of lipid research. 2012;53:2667–2676. doi: 10.1194/jlr.M030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bardowell SA, Duan F, Manor D, Swanson JE, Parker RS. Disruption of mouse cytochrome p450 4f14 (Cyp4f14 gene) causes severe perturbations in vitamin E metabolism. The Journal of biological chemistry. 2012;287:26077–26086. doi: 10.1074/jbc.M112.373597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Q, Jiang Z, Hall YJ, Jang Y, Snyder PW, Bain C, Huang J, Jannasch A, Cooper B, Wang Y, Moreland M. Gamma-tocopherol attenuates moderate but not severe colitis and suppresses moderate colitis-promoted colon tumorigenesis in mice. Free radical biology & medicine. 2013;65:1069–1077. doi: 10.1016/j.freeradbiomed.2013.08.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Lee MJ, Cheung C, Ju JH, Chen YK, Liu B, Hu LQ, Yang CS. Analysis of multiple metabolites of tocopherols and tocotrienols in mice and humans. Journal of agricultural and food chemistry. 2010;58:4844–4852. doi: 10.1021/jf904464u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mustacich DJ, Leonard SW, Patel NK, Traber MG. Alpha-tocopherol beta-oxidation localized to rat liver mitochondria. Free radical biology & medicine. 2010;48:73–81. doi: 10.1016/j.freeradbiomed.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashiguchi T, Kurogi K, Sakakibara Y, Yamasaki M, Nishiyama K, Yasuda S, Liu MC, Suiko M. Enzymatic sulfation of tocopherols and tocopherol metabolites by human cytosolic sulfotransferases. Bioscience, biotechnology, and biochemistry. 2011;75:1951–1956. doi: 10.1271/bbb.110352. [DOI] [PubMed] [Google Scholar]
- 33.Panagabko C, Morley S, Hernandez M, Cassolato P, Gordon H, Parsons R, Manor D, Atkinson J. Ligand specificity in the CRAL TRIO protein family. Biochemistry. 2003;42:6467–6474. doi: 10.1021/bi034086v. [DOI] [PubMed] [Google Scholar]
- 34.Qian J, Morley S, Wilson K, Nava P, Atkinson J, Manor D. Intracellular trafficking of vitamin E in hepatocytes: the role of tocopherol transfer protein. Journal of lipid research. 2005;46:2072–2082. doi: 10.1194/jlr.M500143-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Oram JF, Vaughan AM, Stocker R. ATP-binding cassette transporter A1 mediates cellular secretion of alpha-tocopherol. The Journal of biological chemistry. 2001;276:39898–39902. doi: 10.1074/jbc.M106984200. [DOI] [PubMed] [Google Scholar]
- 36.Reboul E, Trompier D, Moussa M, Klein A, Landrier JF, Chimini G, Borel P. ATP-binding cassette transporter A1 is significantly involved in the intestinal absorption of alpha- and gamma-tocopherol but not in that of retinyl palmitate in mice. The American journal of clinical nutrition. 2009;89:177–184. doi: 10.3945/ajcn.2008.26559. [DOI] [PubMed] [Google Scholar]
- 37.Terasawa Y, Ladha Z, Leonard SW, Morrow JD, Newland D, Sanan D, Packer L, Traber MG, Farese RV., Jr Increased atherosclerosis in hyperlipidemic mice deficient in alpha - tocopherol transfer protein and vitamin E. Proc Natl Acad Sci U S A. 2000;97:13830–13834. doi: 10.1073/pnas.240462697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jishage K, Arita M, Igarashi K, Iwata T, Watanabe M, Ogawa M, Ueda O, Kamada N, Inoue K, Arai H, Suzuki H. Alpha-tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. The Journal of biological chemistry. 2001;276:1669–1672. doi: 10.1074/jbc.C000676200. [DOI] [PubMed] [Google Scholar]
- 39.Traber MG, Sies H. Vitamin E in humans: demand and delivery. Annual review of nutrition. 1996;16:321–347. doi: 10.1146/annurev.nu.16.070196.001541. [DOI] [PubMed] [Google Scholar]
- 40.Kayden HJ, Traber MG. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. Journal of lipid research. 1993;34:343–358. [PubMed] [Google Scholar]
- 41.Schuelke M, Elsner A, Finckh B, Kohlschutter A, Hubner C, Brigelius-Flohe R. Urinary alpha-tocopherol metabolites in alpha-tocopherol transfer protein deficient patients. Journal of lipid research. 2000;41:1543–1551. [PubMed] [Google Scholar]
- 42.Parker RS, McCormick CC. Selective accumulation of alpha-tocopherol in Drosophila is associated with cytochrome P450 tocopherol-omega-hydroxylase activity but not alpha-tocopherol transfer protein. Biochem Biophys Res Commun. 2005;338:1537–1541. doi: 10.1016/j.bbrc.2005.10.124. [DOI] [PubMed] [Google Scholar]
- 43.Jin R, Koop DR, Raucy JL, Lasker JM. Role of human CYP4F2 in hepatic catabolism of the proinflammatory agent leukotriene B4. Archives of biochemistry and biophysics. 1998;359:89–98. doi: 10.1006/abbi.1998.0880. [DOI] [PubMed] [Google Scholar]
- 44.Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their {omega}-oxidation by tocopherol-{omega}-hydroxylase. Journal of lipid research. 2007;48:1090–1098. doi: 10.1194/jlr.M600514-JLR200. [DOI] [PubMed] [Google Scholar]
- 45.Smith KS, Lee CL, Ridlington JW, Leonard SW, Devaraj S, Traber MG. Vitamin E supplementation increases circulating vitamin E metabolites tenfold in end stage renal disease patients. Lipids. 2003;38:813–819. doi: 10.1007/s11745-003-1130-9. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda S, Toyoshima K, Yamashita K. Dietary sesame seeds elevate alpha- and gamma-tocotrienol concentrations in skin and adipose tissue of rats fed the tocotrienol-rich fraction extracted from palm oil. The Journal of nutrition. 2001;131:2892–2897. doi: 10.1093/jn/131.11.2892. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita K, Ikeda S, Iizuka Y, Ikeda I. Effect of sesaminol on plasma and tissue alpha-tocopherol and alpha-tocotrienol concentrations in rats fed a vitamin E concentrate rich in tocotrienols. Lipids. 2002;37:351–358. doi: 10.1007/s11745-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 48.Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochemical and biophysical research communications. 2000;277:531–534. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 49.Bardowell SA, Stec DE, Parker RS. Common variants of cytochrome P450 4F2 exhibit altered vitamin E-{omega}-hydroxylase specific activity. The Journal of nutrition. 2010;140:1901–1906. doi: 10.3945/jn.110.128579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmer S, Stocker A, Sarbolouki MN, Spycher SE, Sassoon J, Azzi A. A novel human tocopherol-associated protein: cloning, in vitro expression, and characterization. The Journal of biological chemistry. 2000;275:25672–25680. doi: 10.1074/jbc.M000851200. [DOI] [PubMed] [Google Scholar]
- 51.Kempna P, Zingg JM, Ricciarelli R, Hierl M, Saxena S, Azzi A. Cloning of novel human SEC14p-like proteins: ligand binding and functional properties. Free Radic Biol Med. 2003;34:1458–1472. doi: 10.1016/s0891-5849(03)00173-4. [DOI] [PubMed] [Google Scholar]
- 52.Jin G, Horinouchi R, Sagawa T, Orimo N, Kubo H, Yoshimura S, Fujisawa A, Kashiba M, Yamamoto Y. Coenzyme Q10-Binding/Transfer Protein Saposin B also Binds gamma-Tocopherol. Journal of clinical biochemistry and nutrition. 2008;43:95–100. doi: 10.3164/jcbn.2008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulatowski L, Parker R, Davidson C, Yanjanin N, Kelley TJ, Corey D, Atkinson J, Porter F, Arai H, Walkley SU, Manor D. Altered vitamin E status in Niemann-Pick type C disease. Journal of lipid research. 2011;52:1400–1410. doi: 10.1194/jlr.M015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone WL, Papas AM, LeClair IO, Min Q, Ponder T. The influence of dietary iron and tocopherols on oxidative stress in the colon. Cancer Detection and Prevention. 1998;22:S110. doi: 10.1016/s0361-090x(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 55.Traber MG, Kayden HJ. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am J Clin Nutr. 1989;49:517–526. doi: 10.1093/ajcn/49.3.517. [DOI] [PubMed] [Google Scholar]
- 56.Yamashita K, Takeda N, Ikeda S. Effects of various tocopherol-containing diets on tocopherol secretion into bile. Lipids. 2000;35:163–170. doi: 10.1007/BF02664766. [DOI] [PubMed] [Google Scholar]
- 57.Mustacich DJ, Shields J, Horton RA, Brown MK, Reed DJ. Biliary secretion of alpha-tocopherol and the role of the mdr2 P-glycoprotein in rats and mice. Archives of biochemistry and biophysics. 1998;350:183–192. doi: 10.1006/abbi.1997.0529. [DOI] [PubMed] [Google Scholar]
- 58.Mustacich DJ, Leonard SW, Devereaux MW, Sokol RJ, Traber MG. Alpha-tocopherol regulation of hepatic cytochrome P450s and ABC transporters in rats. Free radical biology & medicine. 2006;41:1069–1078. doi: 10.1016/j.freeradbiomed.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 59.Traber MG, Labut EM, Leonard SW, Lebold KM. alpha-Tocopherol injections in rats up-regulate hepatic ABC transporters, but not cytochrome P450 enzymes. Free radical biology & medicine. 2011;51:2031–2040. doi: 10.1016/j.freeradbiomed.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gohil K, Godzdanker R, O'Roark E, Schock BC, Kaini RR, Packer L, Cross CE, Traber MG. Alpha-tocopherol transfer protein deficiency in mice causes multi-organ deregulation of gene networks and behavioral deficits with age. Ann N Y Acad Sci. 2004;1031:109–126. doi: 10.1196/annals.1331.012. [DOI] [PubMed] [Google Scholar]
- 61.Nicod N, Parker RS. Vitamin E secretion by Caco-2 monolayers to APOA1, but not to HDL, is vitamer selective. The Journal of nutrition. 2013;143:1565–1572. doi: 10.3945/jn.113.176834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Packer L, Weber SU, Rimbach G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. The Journal of nutrition. 2001;131:369S–373S. doi: 10.1093/jn/131.2.369S. [DOI] [PubMed] [Google Scholar]
- 63.Wong RS, Radhakrishnan AK. Tocotrienol research: past into present. Nutrition reviews. 2012;70:483–490. doi: 10.1111/j.1753-4887.2012.00512.x. [DOI] [PubMed] [Google Scholar]
- 64.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. γ-Tocopherol detoxification of nitrogen dioxide: Superiority to tocopherol. Proc. Natl. Acad. Sci. USA. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cooney RV, Harwood PJ, Franke AA, Narala K, Sundström A-K, Berggren P-O, Mordan LJ. Products of γ-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Rad. Biol. Med. 1995;19:259–269. doi: 10.1016/0891-5849(95)00019-t. [DOI] [PubMed] [Google Scholar]
- 67.Christen S, Jiang Q, Shigenaga MK, Ames BN. Analysis of plasma tocopherols alpha, gamma, and 5-nitro-gamma in rats with inflammation by HPLC coulometric detection. Journal of lipid research. 2002;43:1978–1985. doi: 10.1194/jlr.d200023-jlr200. [DOI] [PubMed] [Google Scholar]
- 68.Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, Williamson KS, West M, Wechter WJ, Floyd RA. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free radical biology & medicine. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 69.Terashima K, Takaya Y, Niwa M. Powerful antioxidative agents based on garcinoic acid from Garcinia kola. Bioorganic & medicinal chemistry. 2002;10:1619–1625. doi: 10.1016/s0968-0896(01)00428-x. [DOI] [PubMed] [Google Scholar]
- 70.McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 71.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 72.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 73.Belardelli F. Role of interferons and other cytokines in the regulation of the immune response. Apmis. 1995;103:161–179. doi: 10.1111/j.1699-0463.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 74.Vane JR. Prostaglandins as mediators of inflammation. Adv Prostaglandin Thromboxane Res. 1976;2:791–801. [PubMed] [Google Scholar]
- 75.Yokomizo T, Izumi T, Shimizu T. Leukotriene B4: metabolism and signal transduction. Archives of biochemistry and biophysics. 2001;385:231–241. doi: 10.1006/abbi.2000.2168. [DOI] [PubMed] [Google Scholar]
- 76.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 77.Williams JA, Shacter E. Regulation of macrophage cytokine production by prostaglandin E2. Distinct roles of cyclooxygenase-1 and -2. The Journal of biological chemistry. 1997;272:25693–25699. doi: 10.1074/jbc.272.41.25693. [DOI] [PubMed] [Google Scholar]
- 78.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 79.Wang D, Dubois RN. Eicosanoids and cancer. Nature reviews. Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 81.Wynne HA, Campbell M. Pharmacoeconomics of nonsteroidal anti-inflammatory drugs (NSAIDs) Pharmacoeconomics. 1993;3:107–123. doi: 10.2165/00019053-199303020-00004. [DOI] [PubMed] [Google Scholar]
- 82.Fanning LB, Boyce JA. Lipid mediators and allergic diseases. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2013;111:155–162. doi: 10.1016/j.anai.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Woodrick R, Ruderman EM. Anti-interleukin-6 therapy in rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2010;68:211–217. [PubMed] [Google Scholar]
- 86.Bannwarth B, Richez C. Clinical safety of tocilizumab in rheumatoid arthritis. Expert Opin Drug Saf. 2011;10:123–131. doi: 10.1517/14740338.2011.537256. [DOI] [PubMed] [Google Scholar]
- 87.Jiang Q, Elson Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20464–20469. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H(2) synthases. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:7130–7135. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mitchell JA, Saunders M, Barnes PJ, Newton R, Belvisi MG. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid. Molecular pharmacology. 1997;51:907–912. doi: 10.1124/mol.51.6.907. [DOI] [PubMed] [Google Scholar]
- 91.Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13'-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J Immunology. 2011;186:1173–1179. doi: 10.4049/jimmunol.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grammas P, Hamdheydari L, Benaksas EJ, Mou S, Pye QN, Wechter WJ, Floyd RA, Stewart C, Hensley K. Anti-inflammatory effects of tocopherol metabolites. Biochemical and biophysical research communications. 2004;319:1047–1052. doi: 10.1016/j.bbrc.2004.05.082. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Jiang Q. gamma-Tocotrienol inhibits lipopolysaccharide-induced interlukin-6 and granulocyte colony-stimulating factor by suppressing C/EBPbeta and NF-kappaB in macrophages. The Journal of nutritional biochemistry. 2013;24:1146–1152. doi: 10.1016/j.jnutbio.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yam ML, Abdul Hafid SR, Cheng HM, Nesaretnam K. Tocotrienols suppress proinflammatory markers and cyclooxygenase-2 expression in RAW264.7 macrophages. Lipids. 2009;44:787–797. doi: 10.1007/s11745-009-3326-2. [DOI] [PubMed] [Google Scholar]
- 95.Qureshi AA, Reis JC, Papasian CJ, Morrison DC, Qureshi N. Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids in health and disease. 2010;9:143. doi: 10.1186/1476-511X-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282:809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Moreland M, Wagner JG, Ames BN, Illek B, Peden DB, Jiang Q. Vitamin E forms inhibit IL-13/STAT6-induced eotaxin-3 secretion by up-regulation of PAR4, an endogenous inhibitor of atypical PKC in human lung epithelial cells. The Journal of nutritional biochemistry. 2012;23:602–608. doi: 10.1016/j.jnutbio.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kannappan R, Yadav VR, Aggarwal BB. gamma-Tocotrienol but not gamma-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents. The Journal of biological chemistry. 2010;285:33520–33528. doi: 10.1074/jbc.M110.158378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Zingg JM, Han SN, Pang E, Meydani M, Meydani SN, Azzi A. In vivo regulation of gene transcription by alpha- and gamma-tocopherol in murine T lymphocytes. Archives of biochemistry and biophysics. 2013;538:111–119. doi: 10.1016/j.abb.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 100.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 101.Jiang Q, Moreland M, Ames BN, Yin X. A combination of aspirin and gamma-tocopherol is superior to that of aspirin and alpha-tocopherol in anti-inflammatory action and attenuation of aspirin-induced adverse effects. The Journal of nutritional biochemistry. 2009;20:894–900. doi: 10.1016/j.jnutbio.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2008;38:501–511. doi: 10.1111/j.1365-2222.2007.02855.x. [DOI] [PubMed] [Google Scholar]
- 103.Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free radical biology & medicine. 2007;43:1176–1188. doi: 10.1016/j.freeradbiomed.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wagner JG, Birmingham NP, Jackson-Humbles D, Jiang Q, Harkema JR, Peden DB. Supplementation with gamma-tocopherol attenuates endotoxin-induced airway neutrophil and mucous cell responses in rats. Free radical biology & medicine. 2013;68C:101–109. doi: 10.1016/j.freeradbiomed.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li G, Lee MJ, Liu AB, Yang Z, Lin Y, Shih WJ, Yang CS. The antioxidant and anti-inflammatory activities of tocopherols are independent of Nrf2 in mice. Free radical biology & medicine. 2012;52:1151–1158. doi: 10.1016/j.freeradbiomed.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free radical biology & medicine. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 107.Hamahata A, Enkhbaatar P, Kraft ER, Lange M, Leonard SW, Traber MG, Cox RA, Schmalstieg FC, Hawkins HK, Whorton EB, Horvath EM, Szabo C, Traber LD, Herndon DN, Traber DL. gamma-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free radical biology & medicine. 2008;45:425–433. doi: 10.1016/j.freeradbiomed.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamamoto Y, Sousse LE, Enkhbaatar P, Kraft ER, Deyo DJ, Wright CL, Taylor A, Traber MG, Cox RA, Hawkins HK, Rehberg SW, Traber LD, Herndon DN, Traber DL. gamma-tocopherol nebulization decreases oxidative stress, arginase activity, and collagen deposition after burn and smoke inhalation in the ovine model. Shock. 2012;38:671–676. doi: 10.1097/SHK.0b013e3182758759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamamoto Y, Enkhbaatar P, Sousse LE, Sakurai H, Rehberg SW, Asmussen S, Kraft ER, Wright CL, Bartha E, Cox RA, Hawkins HK, Traber LD, Traber MG, Szabo C, Herndon DN, Traber DL. Nebulization with gamma-tocopherol ameliorates acute lung injury after burn and smoke inhalation in the ovine model. Shock. 2012;37:408–414. doi: 10.1097/SHK.0b013e3182459482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takahashi K, Komaru T, Takeda S, Takeda M, Koshida R, Nakayama M, Kokusho Y, Kawakami Y, Yamaguchi N, Miyazawa T, Shimokawa H, Shirato K. gamma-tocopherol, but not alpha-tocopherol, potently inhibits neointimal formation induced by vascular injury in insulin resistant rats. Journal of molecular and cellular cardiology. 2006;41:544–554. doi: 10.1016/j.yjmcc.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 111.Saldeen T, Li D, Mehta JL. Differential effects of alpha- and gamma-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis [see comments] [published erratum appears in J Am Coll Cardiol 2000 Jan;35(1):263] J Am Coll Cardiol. 1999;34:1208–1215. doi: 10.1016/s0735-1097(99)00333-2. [DOI] [PubMed] [Google Scholar]
- 112.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma. Lancet. 1999;354:482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 113.McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of alpha-tocopherol and gamma-tocopherol's effects. J Immunol. 2011;186:3674–3685. doi: 10.4049/jimmunol.1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suchankova J, Voprsalova M, Kottova M, Semecky V, Visnovsky P. Effects of oral alpha-tocopherol on lung response in rat model of allergic asthma. Respirology. 2006;11:414–421. doi: 10.1111/j.1440-1843.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 115.Hernandez ML, Wagner JG, Kala A, Mills K, Wells HB, Alexis NE, Lay JC, Jiang Q, Zhang H, Zhou H, Peden DB. Vitamin E, gamma-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free radical biology & medicine. 2013;60C:56–62. doi: 10.1016/j.freeradbiomed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills JM. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marks F, Muller-Decker K, Furstenberger G. A causal relationship between unscheduled eicosanoid signaling and tumor development: cancer chemoprevention by inhibitors of arachidonic acid metabolism. Toxicology. 2000;153:11–26. doi: 10.1016/s0300-483x(00)00301-2. [DOI] [PubMed] [Google Scholar]
- 118.Marnett LJ. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992;52:5575–5589. [PubMed] [Google Scholar]
- 119.Ju J, Hao X, Lee MJ, Lambert JD, Lu G, Xiao H, Newmark HL, Yang CS . A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev Res (Phila) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sanches LD, Santos SA, Carvalho JR, Jeronimo GD, Favaro WJ, Reis MD, Felisbino SL, Justulin LA., Jr Protective effect of gamma-tocopherol-enriched diet on N-methyl-N-nitrosourea-induced epithelial dysplasia in rat ventral prostate. International journal of experimental pathology. 2013;94:362–372. doi: 10.1111/iep.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barve A, Khor TO, Nair S, Reuhl K, Suh N, Reddy B, Newmark H, Kong AN. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. International journal of cancer. Journal international du cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shibata A, Nakagawa K, Kawakami Y, Tsuzuki T, Miyazawa T. Suppression of gamma-tocotrienol on UVB induced inflammation in HaCaT keratinocytes and HR-1 hairless mice via inflammatory mediators multiple signaling. J Agric Food Chem. 2010;58:7013–7020. doi: 10.1021/jf100691g. [DOI] [PubMed] [Google Scholar]
- 123.Ren Z, Pae M, Dao MC, Smith D, Meydani SN, Wu D. Dietary supplementation with tocotrienols enhances immune function in C57BL/6 mice. The Journal of nutrition. 2010;140:1335–1341. doi: 10.3945/jn.110.121434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li XH, Fu D, Latif NH, Mullaney CP, Ney PH, Mog SR, Whitnall MH, Srinivasan V, Xiao M. Delta-tocotrienol protects mouse and human hematopoietic progenitors from gamma-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica. 2010;95:1996–2004. doi: 10.3324/haematol.2010.026492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsuduki T, Kuriyama K, Nakagawa K, Miyazawa T. Tocotrienol (unsaturated vitamin E) suppresses degranulation of mast cells and reduces allergic dermatitis in mice. Journal of oleo science. 2013;62:825–834. doi: 10.5650/jos.62.825. [DOI] [PubMed] [Google Scholar]
- 126.Gonzalez AM, Garcia T, Samper E, Rickmann M, Vaquero EC, Molero X. Assessment of the protective effects of oral tocotrienols in arginine chronic-like pancreatitis. American journal of physiology. Gastrointestinal and liver physiology. 2011;301:G846–855. doi: 10.1152/ajpgi.00485.2010. [DOI] [PubMed] [Google Scholar]
- 127.Khan MR, Siddiqui S, Parveen K, Javed S, Diwakar S, Siddiqui WA. Nephroprotective action of tocotrienol-rich fraction (TRF) from palm oil against potassium dichromate (K 2 Cr 2 O 7)-induced acute renal injury in rats. Chemico-biological interactions. 2010;186:228–238. doi: 10.1016/j.cbi.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 128.Wong WY, Poudyal H, Ward LC, Brown L. Tocotrienols reverse cardiovascular, metabolic and liver changes in high carbohydrate, high fat diet fed rats. Nutrients. 2012;4:1527–1541. doi: 10.3390/nu4101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Radhakrishnan AK, Mahalingam D, Selvaduray KR, Nesaretnam K. Supplementation with natural forms of vitamin E augments antigen-specific TH1-type immune response to tetanus toxoid. BioMed research international. 2013;2013:78–2067. doi: 10.1155/2013/782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey S, Koca C, Yadav VR, Tong Z, Gelovani JG, Guha S, Krishnan S, Aggarwal BB. {Gamma}-tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer research. 2010;70:8695–8705. doi: 10.1158/0008-5472.CAN-10-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Husain K, Francois RA, Yamauchi T, Perez M, Sebti SM, Malafa MP. Vitamin E delta tocotrienol augments the antitumor activity of gemcitabine and suppresses constitutive NF-kappaB activation in pancreatic cancer. Molecular cancer therapeutics. 2011;10:2363–2372. doi: 10.1158/1535-7163.MCT-11-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978–991. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 133.Himmelfarb J, Phinney S, Ikizler TA, Kane J, McMonagle E, Miller G. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2007;17:296–304. doi: 10.1053/j.jrn.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 134.Tasanarong A, Vohakiat A, Hutayanon P, Piyayotai D. New strategy of alpha- and gamma-tocopherol to prevent contrast-induced acute kidney injury in chronic kidney disease patients undergoing elective coronary procedures. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28:337–344. doi: 10.1093/ndt/gfs525. [DOI] [PubMed] [Google Scholar]
- 135.Pantzaris MC, Loukaides GN, Ntzani EE, Patrikios IS. A novel oral nutraceutical formula of omega-3 and omega-6 fatty acids with vitamins (PLP10) in relapsing remitting multiple sclerosis: a randomised, double-blind, placebo-controlled proof-of-concept clinical trial. BMJ open. 2013;3 doi: 10.1136/bmjopen-2012-002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Devaraj S, Leonard S, Traber MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free radical biology & medicine. 2008;44:1203–1208. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu JH, Ward NC, Indrawan AP, Almeida CA, Hodgson JM, Proudfoot JM, Puddey IB, Croft KD. Effects of alpha-tocopherol and mixed tocopherol supplementation on markers of oxidative stress and inflammation in type 2 diabetes. Clinical chemistry. 2007;53:511–519. doi: 10.1373/clinchem.2006.076992. [DOI] [PubMed] [Google Scholar]
- 138.Ward NC, Wu JH, Clarke MW, Puddey IB, Burke V, Croft KD, Hodgson JM. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo controlled trial. Journal of hypertension. 2007;25:227–234. doi: 10.1097/01.hjh.0000254373.96111.43. [DOI] [PubMed] [Google Scholar]
- 139.Vucinic L, Singh I, Spargo FJ, Hawley JA, Linden MD. Gamma tocopherol supplementation prevents exercise induced coagulation and platelet aggregation. Thrombosis research. 2010;125:196–199. doi: 10.1016/j.thromres.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 140.Liu M, Wallmon A, Olsson-Mortlock C, Wallin R, Saldeen T. Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms. Am J Clin Nutr. 2003;77:700–706. doi: 10.1093/ajcn/77.3.700. [DOI] [PubMed] [Google Scholar]
- 141.Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, Peden DB. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free radical biology & medicine. 2008;45:40–49. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mah E, Noh SK, Ballard KD, Park HJ, Volek JS, Bruno RS. Supplementation of a gamma-tocopherol-rich mixture of tocopherols in healthy men protects against vascular endothelial dysfunction induced by postprandial hyperglycemia. The Journal of nutritional biochemistry. 2013;24:196–203. doi: 10.1016/j.jnutbio.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 143.Masterjohn C, Mah E, Guo Y, Koo SI, Bruno RS. gamma-Tocopherol abolishes postprandial increases in plasma methylglyoxal following an oral dose of glucose in healthy, college-aged men. The Journal of nutritional biochemistry. 2012;23:292–298. doi: 10.1016/j.jnutbio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 144.Mahalingam D, Radhakrishnan AK, Amom Z, Ibrahim N, Nesaretnam K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. European journal of clinical nutrition. 2011;65:63–69. doi: 10.1038/ejcn.2010.184. [DOI] [PubMed] [Google Scholar]
- 145.Pedrelli VF, Lauriola MM, Pigatto PD. Clinical evaluation of photoprotective effect by a topical antioxidants combination (tocopherols and tocotrienols) Journal of the European Academy of Dermatology and Venereology : JEADV. 2012;26:1449–1453. doi: 10.1111/j.1468-3083.2011.04219.x. [DOI] [PubMed] [Google Scholar]
- 146.Heng EC, Karsani SA, Abdul Rahman M, Abdul Hamid NA, Hamid Z, Wan Ngah WZ. Supplementation with tocotrienol-rich fraction alters the plasma levels of Apolipoprotein A-I precursor, Apolipoprotein E precursor, and C reactive protein precursor from young and old individuals. European journal of nutrition. 2013 doi: 10.1007/s00394-012-0485-3. [DOI] [PubMed] [Google Scholar]
- 147.Patel V, Rink C, Gordillo GM, Khanna S, Gnyawali U, Roy S, Shneker B, Ganesh K, Phillips G, More JL, Sarkar A, Kirkpatrick R, Elkhammas EA, Klatte E, Miller M, Firstenberg MS, Chiocca EA, Nesaretnam K, Sen CK. Oral tocotrienols are transported to human tissues and delay the progression of the model for end stage liver disease score in patients. The Journal of nutrition. 2012;142:513–519. doi: 10.3945/jn.111.151902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nesaretnam K, Selvaduray KR, Abdul Razak G, Veerasenan SD, Gomez PA. Effectiveness of tocotrienol-rich fraction combined with tamoxifen in the management of women with early breast cancer: a pilot clinical trial. Breast Cancer Res. 2010;12:R81. doi: 10.1186/bcr2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Molecular aspects of medicine. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Papaioannou D, Cooper KL, Carroll C, Hind D, Squires H, Tappenden P, Logan RF. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta analysis. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2011;13:1085–1099. doi: 10.1111/j.1463-1318.2010.02289.x. [DOI] [PubMed] [Google Scholar]
- 151.Dolara P, Bigagli E, Collins A. Antioxidant vitamins and mineral supplementation, life span expansion and cancer incidence: a critical commentary. Eur J Nutr. 2013;51:769–781. doi: 10.1007/s00394-012-0389-2. [DOI] [PubMed] [Google Scholar]
- 152.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. Journal of the National Cancer Institute. 1993;85:1483–1492. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 153.MRC/BHF Heart Protection Study of cholesterol-lowering therapy and of antioxidant vitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. Eur Heart J. 1999;20:725–741. doi: 10.1053/euhj.1998.1350. [DOI] [PubMed] [Google Scholar]
- 154.Birringer M, Lington D, Vertuani S, Manfredini S, Scharlau D, Glei M, Ristow M. Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free radical biology & medicine. 2010;49:1315–1322. doi: 10.1016/j.freeradbiomed.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 155.Di Gennaro A, Haeggstrom JZ. Targeting leukotriene B in inflammation. Expert opinion on therapeutic targets. 2013 Oct 4;:1–15. doi: 10.1517/14728222.2013.843671. [Epub ahead of print]: 2013. [DOI] [PubMed] [Google Scholar]
- 156.Rainsford KD. Inhibition by leukotriene inhibitors, and calcium and platelet activating factor antagonists, of acute gastric and intestinal damage in arthritic rats and in cholinomimetic-treated mice. J Pharm Pharmacol. 1999;51:331–339. doi: 10.1211/0022357991772330. [DOI] [PubMed] [Google Scholar]
- 157.Rainsford KD. The ever-emerging anti-inflammatories. Have there been any real advances? J Physiol Paris. 2001;95:11–19. doi: 10.1016/s0928-4257(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 158.Kamal-Eldin A, Andersson R. A Multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. JAOCS. 1997;74:375–380. [Google Scholar]