Abstract
Background
Concurrent administration of dopamine and serotonin reuptake inhibitors reduces cocaine self-administration in monkeys. Consonant with this, clinical trials assessing modafinil and selective serotonin reuptake inhibitors alone show some efficacy as potential pharmacotherapies for cocaine dependence. We hypothesized that combining modafinil with escitalopram would attenuate the euphoric effects of cocaine to a greater degree than modafinil alone.
Methods
In a randomized, double blind, parallel groups design participants received either placebo (0 mg/day; n = 16), modafinil (200 mg/day; n = 16), escitalopram (20 mg/day; n = 17), or modafinil+escitalopram (200+20 mg/day; n = 15) for 5 days. On day 5, during separate sessions participants received an intravenous sample of cocaine (0 or 20mg; randomized) and five $1 bills. Participants rated the subjective effects of the infusions and subsequently made choices to either return $1 and receive another infusion or keep $1 and receive no infusion.
Results
Compared to saline, cocaine (20mg) significantly (p ≤ 0.008) increased most ratings, including “Good Effects”, “Stimulated”, and “High”. Relative to placebo, modafinil significantly (p ≤ 0.007) attenuated subject-rated increases of “Any Drug Effect”, “High”, “Good Effects”, and “Stimulated” produced by cocaine. Compared to saline, participants chose cocaine infusions significantly more; however, no treatment significantly reduced choices for cocaine infusions. Escitalopram did not enhance the efficacy of modafinil to reduce any measure.
Conclusions
Modafinil attenuated many positive subjective effects produced by cocaine; however, escitalopram combined with modafinil did not enhance the efficacy of modafinil to reduce cocaine effects.
Keywords: Cocaine, Modafinil, Escitalopram, Subjective Effects
1. INTRODUCTION
According to the World Health Organization report, an estimated 17 million people worldwide had used cocaine at least once in 2008 (UNODC, 2010). This is consistent with the 2012 National Survey on Drug Use and Health (NSDUH) report, which estimated that 9.2% of Americans aged 12 or older (23.9 million) were current (past month) illicit drug users and 8.5% met criteria for a substance use disorder (SUD; Substance Abuse and Mental Health Services Administration, 2013a). Cocaine users accounted for 1.1 million of all those classified with a SUD in 2012, an increase of nearly 34% since 2011 (Substance Abuse and Mental Health Services Administration, 2013a). Estimates from the Treatment Episode Data Set and NSDUH indicate that 13.1–15.6% of all Americans who reported receiving treatment for illicit substance use received treatment for their cocaine use (Substance Abuse and Mental Health Services Administration, 2013a, 2013c) and 70.5% of cocaine user admissions reported having at least one prior treatment episode (Substance Abuse and Mental Health Services Administration, 2013c). Further, out of the 1.25 million emergency department visits made by patients in 2011 that involved illicit drug use, cocaine was the most commonly involved illicit drug, accounting for over 40% of all illicit drug use visits (Substance Abuse and Mental Health Services Administration, 2013b). Unfortunately, despite extensive efforts to identify potential pharmacotherapies for cocaine dependence, none exists.
Efforts to identify pharmacotherapies have concentrated heavily on medications that influence dopamine (DA; Verrico et al., 2013). This approach was based logically on the ability of cocaine to acutely increase intrasynaptic DA to supraphysiological levels, via blockade of the DA transporter (DAT; Volkow et al., 2002), which correlates with both the positive subjective effects (Volkow et al., 1997) as well as the reinforcing effects (Hart et al, 2008) produced by cocaine. Further, long-term cocaine use appears to cause neuroadaptations within the mesocorticolimbic system (Verrico et al., 2013), including cortical hypofrontality and reduced dopaminergic neurotransmission, which have been associated with deficits in executive functioning (Groman and Jentsch, 2012; Kalechstein et al, 2003), poor treatment response (Martinez et al., 2011), and an increased vulnerability to relapse (Sofuoglu, 2010).
The wake-promoting medication modafinil targets the DAT, although it also produces appreciable effects on noradrenergic, GABAergic, and glutamatergic neurotransmission (Ferraro et al., 1998; Madras et al., 2006; Volkow et al., 2009). In preclinical models, modafinil attenuated reinstatement of cocaine self-administration (Mahler et al., 2012), as well as methamphetamine self-administration (Reichel and See, 2010). In human laboratory studies, modafinil attenuated the positive subjective effects of intravenous (IV) cocaine (Dackis et al., 2003; Malcolm et al., 2006) and decreased choices to smoke cocaine (Hart et al, 2008). Further, modafinil significantly reduced cocaine-positive urines in an outpatient clinical trial (Dackis et al., 2005), and while two larger subsequent clinical trials of modafinil were negative (considering group means), modafinil reduced cocaine-positive urines in subsets of participants - specifically in males, but not females (Dackis et al., 2012), and in those without a history of alcohol dependence (Anderson et al., 2009).
In addition to DA, studies implicate a role for serotonin in mediating the positive subjective and reinforcing effects produced by cocaine (Filip et al., 2010; Walsh and Cunningham, 1997). Consistent with this interpretation, non-human primate studies have revealed that increasing synaptic serotonin levels reduces the reinforcing effects (Howell, 2008; Rothman et al., 2006) and self-administration (Czoty et al., 2002; Spealman, 1993) of cocaine. In humans, treatment with the selective serotonin reuptake inhibitor (SSRI) fluoxetine significantly reduced the positive subjective effects produced by cocaine (Walsh et al., 1994). In outpatient trials, the SSRI citalopram significantly reduced cocaine-positive urines (Moeller et al., 2007), while the SSRI sertraline delayed relapse in recently abstinent individuals presenting with depressive symptoms (Oliveto et al., 2012). However, a review by Pani et al. (2011) found that SSRIs did not support abstinence from cocaine use over placebo.
Collective evidence to date suggests that as monotherapies neither modafinil (Dackis et al., 2012; Anderson et al., 2009) nor SSRIs (Pani et al., 2011) consistently reduce cocaine use; however, preclinical data suggests that serotonin and DA act together to produce important effects of cocaine in brain. For example, in non-human primates increasing synaptic serotonin levels reduced the reinforcing effects and self-administration of cocaine, as well as cocaine-induced DA release (Czoty et al., 2002; Howell, 2008; Rothman et al., 2006; Spealman, 1993). In fact, administration of an SSRI in combination with the experimental compound RTI-336, which is a highly selective DAT inhibitor (Howell et al., 2007), or administration of a dual DA/serotonin releaser (Rothman et al., 2007, 2008), significantly suppressed cocaine self-administration in monkeys, suggesting this might be a useful therapeutic strategy in humans (Rothman et al., 2008).
We hypothesized that combining a DAT inhibitor (i.e., modafinil; Andersen et al., 2010; Kim et al., 2014; Schmitt and Reith, 2011; Zolkowska et al., 2009) with a SSRI may more robustly reduce the euphoric and reinforcing effects produced by cocaine in humans. To test this hypothesis, we conducted a human laboratory study in non-treatment seeking volunteers who met cocaine dependence criteria to determine whether relative to modafinil alone, escitalopram would enhance the efficacy of modafinil to attenuate the effects produced by cocaine, and choices for IV cocaine over an alternative reinforcer (i.e., money).
2. METHODS
The Baylor College of Medicine (BCM) and Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) Institutional Review Boards reviewed and approved this study.
2.1 Trial Design
This randomized, double blind, placebo-controlled, parallel groups study allocated participants (1:1:1:1) to a treatment group. The MEDVAMC Research Pharmacy used a randomization generated by www.randomization.comto maintain the blind.
2.2 Participants
Potential participants completed an initial telephone screen to assess basic eligibility before completing in-person assessments. Prior to obtaining written informed consent and determining eligibility at the in-person interview, potential risks associated with the study were fully described. Eligibility assessments included medical and drug use histories, urine screens for illicit drugs, electrocardiograms, and vital sign assessments. Exclusion criteria included any psychiatric or medical illnesses, serious neurological or seizure disorders, use of any psychoactive medications, and drug or alcohol dependence, excluding cocaine and nicotine. Women were excluded if they were pregnant, breast-feeding, or not using a reliable form of birth control. To meet inclusion criteria, subjects had to report recent cocaine use, provide a benzoylecgonine-positive urine sample for cocaine during the in-person screening process, meet DSM-IV diagnostic criteria for cocaine dependence or abuse, and not be actively seeking substance abuse treatment. All participants received compensation and were in good health with no contraindications to study participation.
2.3. Study Procedures
Eligible participants resided at the MEDVAMC Research Commons for 6 nights. Upon arrival, participants were randomly allocated to one of four treatment groups: 1) placebo + placebo, 2) placebo + modafinil (200 mg/day), 3) placebo + escitalopram (20 mg/day), or 4) modafinil (200 mg/day) + escitalopram (20 mg/day). All treatments (Section 2.4) were over-encapsulated in two tablets to maintain the blind, and taken orally at 08:00 on Days 1–5. On Day 6, participants were discharged once deemed medically stable by a physician. The procedures described below were identical for each group. Sample size was calculated based on a power analysis performed on data obtained by Hart and colleagues (Hart et al, 2008).
2.4 Study Medications
The FDA provided an IND for the use of modafinil and escitalopram in this study. Greenpark Compounding Pharmacy (Houston, TX) provided over-encapsulated modafinil, escitalopram, and a matched placebo. A NIDA Drug Supply Program (RTI International, Durham, NC) contractor provided cocaine HCl for human use. The MEDVAMC Research Pharmacy prepared the sterile cocaine and saline solutions.
Treatment with 200 mg of modafinil was equipotent to 400 mg at significantly attenuating the subjective and reinforcing effects produced by smoked cocaine (Hart et al., 2008). In addition, treatment with 200 mg modafinil has been found to attenuate the subjective effects produced by methamphetamine (De La Garza et al., 2010) and to improve cognitive function in both healthy participants (Turner et al., 2003) and in cocaine-dependent individuals (Kalechstein et al., 2013). Absorption of modafinil is rapid, with peak plasma concentrations occurring at ~2–4 hours. The effective elimination half-life of modafinil after multiple doses is ~15 hours, and steady state plasma concentrations are achieved after 2–4 days of dosing. Treatment with 20 mg of escitalopram is routinely used to treat major depressive disorder and generalized anxiety disorder in humans. Following a single oral dose, peak levels occur in ~5 hours. Escitalopram has a mean terminal half-life of ~27–32 hours and with once daily dosing, steady state concentrations in plasma are achieved within one week. Thus, treatment with modafinil in the presence or absence of escitalopram for five days assured that both compounds reached physiologically and behaviorally relevant steady state levels in human research volunteers.
2.5 Outcome Measures
Data collection occurred on Day 5, during two choice sessions conducted at 10:00 and 14:00. During both sessions, participants received five $1 bills, before receiving the sample dose of cocaine (0 or 20 mg; randomized between sessions) that could subsequently be purchased during that session. Following the sample dose (infused over 2 minutes), participants had five opportunities, at 13-min intervals, to either purchase another infusion (the same as the sample dose) or keep $1 for that choice opportunity.
2.5.1 Subjective Effects
Subjective effects were measured on Day 5 after a non-contingent “sample” infusion of cocaine or saline. Participants completed visual analog scale (VAS) forms during both sessions 15 minutes before (baseline), and 5 and 10 minutes after the infusions. VAS ratings, recorded on a scale from 0 to 100, included ratings of “Any Drug Effect”, “High”, “Good Effects”, “Stimulated”, “Drug Liking”, “Desire”, “Likely to Use if Given Access”, “Bad Effects”, “Depressed”, “Anxious”, and “Pay”, asking participants ’How much would you “Pay” for the dose you just received?
2.5.2 Reinforcing Effects
Reinforcement was assessed as the number of “Choices” made to receive infusions during each infusion session on Day 5.
2.5.3 Cardiovascular
Cardiovascular measures included 1) heart rate (HR), 2) systolic blood pressure (BP), and 3) diastolic BP. A GE Dash-3000 blood pressure monitor and pulse oximetry attachment (GE Medical Systems, Tampa, FL) recorded these measures throughout the infusion session. Infusions did not occur if cardiovascular measures exceeded preset safety limits.
2.6 Data Analysis
Demographic and baseline drug use characteristics were compared using analysis of variance (ANOVA) and chi-square tests for continuous and categorical variables, respectively. VAS and cardiovascular data, presented as change from baseline, were averaged across the 5- and 10-minute time points before analyses. Primary analyses included 4 × 2 ANOVAs with Treatment (placebo, modafinil, escitalopram, and modafinil+escitalopram) as a between-subject factor and Cocaine Dose (0 and 20 mg) as a within-subjects factor. To test a priori hypotheses we conducted pairwise comparisons within the 20 mg cocaine dose using the Holm-Sidak method, including 1) placebo vs. modafinil to confirm efficacy of modafinil alone as described in the literature, 2) modafinil vs. modafinil+escitalopram to test our primary hypothesis, and for completeness 3) placebo vs. escitalopram and 4) placebo vs. modafinil+escitalopram. We analyzed the data using SigmaPlot 12.0.
3. RESULTS
3.1 Participants
Sixty-four participants were randomized to medication conditions [placebo (n = 16), modafinil (n = 16), escitalopram (n = 17), and modafinil+escitalopram (n = 15)]; however, three subjects did not complete the study due to cardiovascular measures exceeding preset safety limits (n = 1), voluntary withdrawal (n = 1), or protocol violations (n = 1) and technical issues precluded data from one participant in the escitalopram group (missing all baseline (pre-infusion) data) from being included in the analyses. Of the remaining 60, one participant in the modafinil group was missing cardiovascular and “Pay” data). Data analyses occurred on all the remaining data from the 60 study completers.
3.2 Demographics
On average, the 60 participants were 44 years old (SD; ± 6.2), African American (n = 44), and male (n = 51) with 12 years (SD; ± 1.4) of education. While all participants had reported previously using cocaine via the IV and/or smoked route, the majority of participants usually smoked (n = 59) cocaine, although one participant usually snorted it. On average, participants used 2 grams (SD; ± 2.0) of cocaine/day and used on 18 of the last 30 days (SD; ± 7.8). Most participants also drank alcohol (n = 50) and smoked cigarettes (n = 48), while about half (n = 29) also smoked cannabis. Analyses revealed no significant differences across demographic and drug use variables between groups (Table 1).
Table 1.
Demographics and Drug Use
| Treatment Groups | Analyses | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Modafinil | Modafinil Escitalopram | Modafinil+Escitalopram | Placebo | p = | ||
| Demographics | Gende | Male | 13 | 12 | 14 | 12 | 0.731 |
| Female | 3 | 3 | 1 | 2 | |||
| Ethnicity | African American | 10 | 10 | 11 | 13 | 0.426 | |
| Caucasian | 3 | 3 | 4 | 1 | |||
| Hispanic | 1 | 0 | 0 | 0 | |||
| Multi-Ethnic | 2 | 2 | 0 | 0 | |||
| Other | Age | 42.88 ± 6.77 | 42.80 ± 7.19 | 44.47 ± 5.17 | 46.14 ± 5.17 | 0.419 | |
| Education (years) | 12.31 ± 1.40 | 12.33 ± 1.35 | 12.13 ± 1.64 | 12.82 ± 1.38 | 0.621 | ||
| Cocaine | Use | Years | 18.63 ± 8.16 | 17.33 ± 7.82 | 21.53 ± 6.64 | 19.21 ± 4.39 | 0.421 |
| Last 30 days | 17.94 ± 7.87 | 15.67 ± 9.98 | 20.73 ± 4.98 | 18.57 ± 7.58 | 0.370 | ||
| Grams/day | 2.15 ± 1.81 | 1.76 ± 0.81 | 2.88 ± 3.59 | 1.31 ± 0.41 | 0.252 | ||
| Primary Route | Smoke | 13 | 14 | 15 | 13 | 0.559 | |
| Smoke and Nasal | 1 | 1 | 0 | 1 | |||
| Smoke and Oral | 1 | 0 | 0 | 0 | |||
| Nasal | 1 | 0 | 0 | 0 | |||
| Nicotine | Current Users | 14/16 | 11/15 | 10/15 | 13/14 | 0.263 | |
| Years | 23.57 ± 7.76 | 19.18 ± 8.07 | 20.80 ± 6.44 | 22.69 ± 9.33 | 0.134 | ||
| Days used of past 30 | 28.93 ± 4.01 | 24.36 ± 9.83 | 27.50 ± 7.91 | 25.69 ± 8.09 | 0.257 | ||
| Cigarettes per day | 14.93 ± 9.26 | 11.27 ± 7.68 | 12.70 ± 8.68 | 7.54 ± 5.32 | 0.225 | ||
| Alcohol | Current Users | 12/16 | 13/15 | 11/15 | 14/14 | 0.194 | |
| Years | 18.75 ± 9.71 | 19.73 ± 8.84 | 18.60 ± 11.36 | 20.36 ± 9.09 | 0.956 | ||
| Days used of past 30 | 13.38 ± 9.98 | 7.53 ± 7.85 | 7.60 ± 8.92 | 12.43 ± 9.20 | 0.159 | ||
| Drinks per day | 2.44 ± 1.97 | 2.07 ± 1.75 | 2.40 ± 2.72 | 3.29 ± 2.09 | 0.484 | ||
| Cannabis | Current Users | 11/16 | 5/15 | 6/15 | 7/14 | 0.225 | |
| Years | 15.20 ± 12.64 | 10.20 ± 12.02 | 14.53 ± 15.20 | 10.36 ± 9.24 | 0.574 | ||
| Days used of past 30 | 7.75 ± 10.21 | 1.93 ± 5.18 | 2.73 ± 7.65 | 1.29 ± 1.64 | 0.055 | ||
| Times per day | 1.50 ± 1.67 | 0.53 ± 1.06 | 1.00 ± 1.73 | 0.71 ± 0.83 | 0.247 | ||
Data presented as means ± standard deviations.
3.3 Subjective Effects
Analyses revealed that compared to saline, cocaine (20 mg) significantly increased all ratings (p ≤ 0.008) except “Depressed” (p = 0.503) and “Anxious” (p = 0.051); however, analyses did not reveal any significant cocaine×treatment interactions (p ≥ 0.053) for any rating.
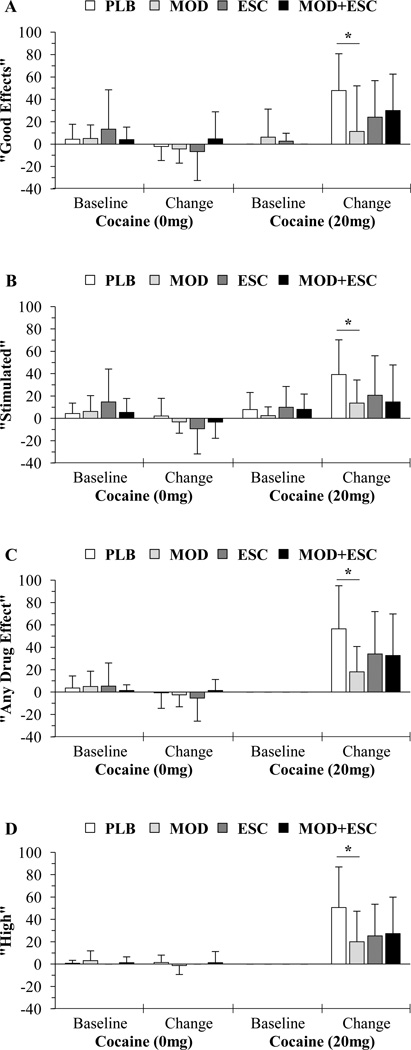
For “Good Effects” and “Stimulated” (Figure 1A, 1B) analyses revealed significant treatment effects (p ≤ 0.030). Pairwise comparisons within the 20 mg cocaine dose revealed significant differences for both ratings between placebo vs. modafinil (p ≤ 0.007), but not between modafinil vs. modafinil+escitalopram (p ≥ 0.134), placebo vs. escitalopram (p ≥ 0.074), or placebo vs. modafinil+escitalopram (p ≥ 0.169).
Figure 1.
Average baseline VAS subjective ratings and change from baseline means (of the 5 and 10 minute ratings following infusions) for (A) “Good Effects”, (B) “Stimulated”, (C) “Any Drug Effect”, and (D) “High” after administration of cocaine (0 and 20 mg) for the placebo (PLB; n = 14), modafinil (MOD; n = 16), escitalopram (ESC; n = 15), and modafinil+escitalopram (MOD+ESC; n = 15) groups. An asterisk (*) indicates a significant (p < 0.05) difference between placebo and modafinil groups following the 20 mg cocaine infusions. Mean baseline values provided for illustrative purposes. Data presented as means ± standard deviations.
For “Any Drug Effect” and “High” (Figure 1C, 1D), analyses revealed no significant treatment effects (p ≥ 0.058). Pairwise comparisons within the 20 mg cocaine dose revealed significant differences for both ratings between placebo vs. modafinil (p = 0.003), but not between modafinil vs. modafinil+escitalopram (p ≥ 0.408), placebo vs. escitalopram (p ≥ 0.105), or placebo vs. modafinil+escitalopram (p ≥ 0.090).
For “Drug Liking”, “Desire”, “Likely to Use if Given Access”, “Bad Effects”, and “Pay”, analyses revealed no significant treatment effects (p ≥ 0.329) and pairwise comparisons within the 20 mg cocaine dose revealed no significant differences between placebo vs. modafinil (p ≥ 0.165), modafinil vs. modafinil+escitalopram (p ≥ 0.134), placebo vs. escitalopram (p ≥ 0.188), or placebo vs. modafinil+escitalopram (p ≥ 0.087).
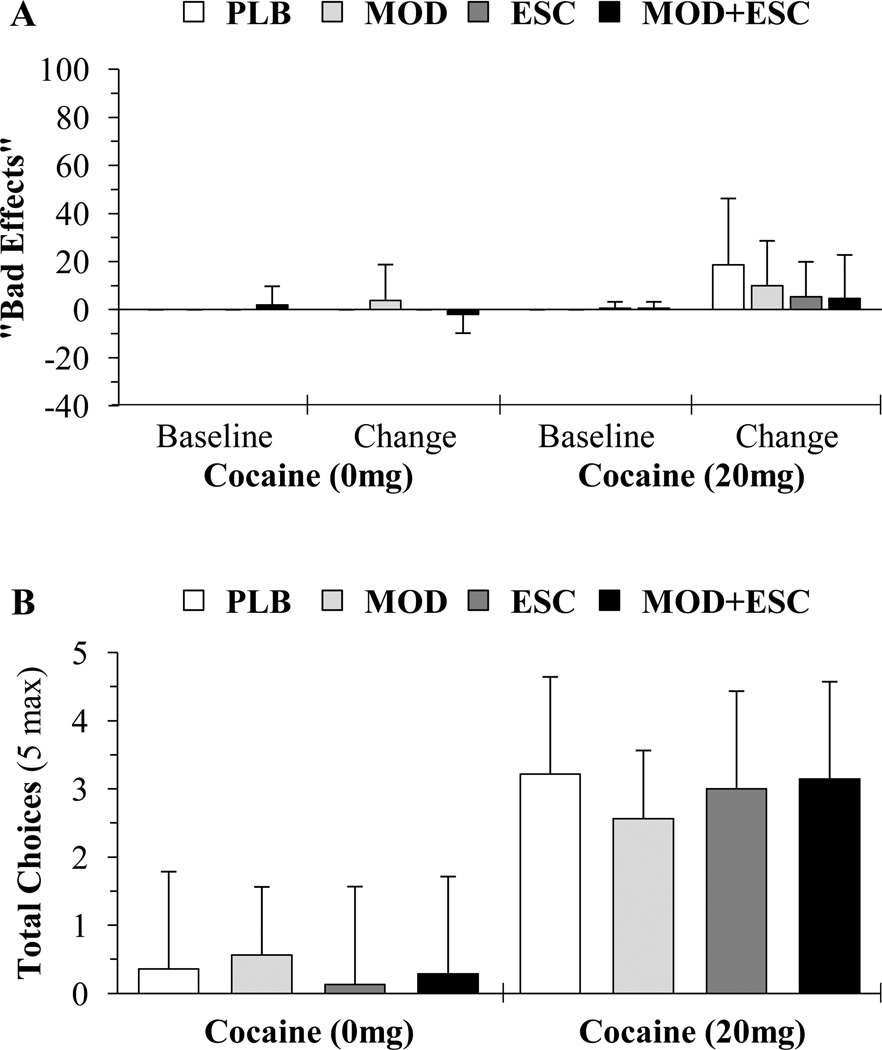
It is interesting that cocaine significantly increased “Bad Effects” ratings, and while not statistically significant, increased ratings were more likely to be reported by participants receiving modafinil (Figure 2A).
Figure 2.
(A) Average baseline VAS subjective ratings and change from baseline mean (of the 5 and 10 minute ratings following infusions) for “Bad Effects” and (B) average number of Choices made to receive 0 and 20 mg cocaine infusions for the placebo (PLB; n = 14), modafinil (MOD; n = 16), escitalopram (ESC; n = 15), and modafinil+escitalopram (MOD+ESC; n = 15), groups. Mean baseline values provided for illustrative purposes. Data presented as means ± standard deviations.
3.4 Reinforcing Effects
For “Choices” (Figure 2B), analyses revealed that compared to saline, cocaine (20 mg) significantly increased the number of infusions received (F(1,112) = 95.225; p < 0.001); however, analyses did not reveal significant treatment effects (F(3,112) = 0.198; p = 0.897) or significant cocaine×treatment interactions (F(3,119) = 0.729; p = 0.537). Pairwise comparisons within the 20 mg cocaine dose revealed no significant differences between placebo vs. modafinil (p = 0.744), modafinil vs. modafinil+escitalopram (p = 0.728), placebo vs. escitalopram (p = 0.911), or placebo vs. modafinil+escitalopram (p = 0.925). Overall, 20% of the participants chose at least one infusion of saline [placebo (29%), modafinil (31%), escitalopram (20%), and modafinil+escitalopram (20%)] and 80% of the participants chose at least one infusion of cocaine [placebo (86%), modafinil (75%), escitalopram (87%), and modafinil+escitalopram (80%)].
3.5 Cardiovascular Effects
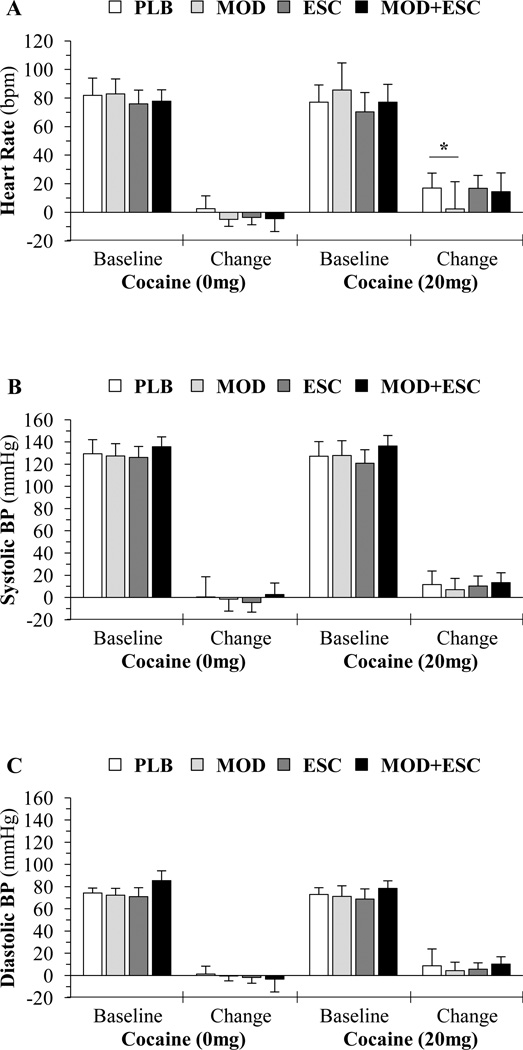
Analyses revealed that compared to saline, cocaine (20 mg) significantly increased (p < 0.001) all cardiovascular measures (Figure 3); however, analyses did not reveal any significant cocaine×treatment interactions (p ≥ 0.180) for any measure.
Figure 3.
Average baseline cardiovascular measures and change from baseline means (of the 5 and 10 minute ratings following infusions) for (A) heart rate [beats per minute (bpm)], (B) systolic blood pressure [millimeters of mercury (mmHg)], and (C) diastolic blood pressure (mmHg) after administration of cocaine (0 and 20 mg) for the placebo (PLB; n = 14), modafinil (MOD; n = 15), escitalopram (ESC; n = 15), and modafinil+escitalopram (MOD+ESC; n = 15) groups. An asterisk (*) indicates a significant (p < 0.05) difference between placebo and modafinil groups following the 20 mg cocaine infusions. Mean baseline values provided for illustrative purposes. Data presented as means ± standard deviations.
For HR (Figure 3A), analyses revealed a significant treatment effect (F(3,117) = 5.442; p = 0.002). Pairwise comparisons within the 20 mg cocaine dose revealed significant differences between placebo vs. modafinil (p = 0.005), but not between modafinil vs. modafinil+escitalopram (p = 0.197), placebo vs. escitalopram (p = 0.766), or placebo vs. modafinil+escitalopram (p = 0.214).
For systolic (Figure 3B) and diastolic BP (Figure 3C), analyses revealed no significant treatment effects (p ≥ 0.120). Pairwise comparisons within the 20 mg cocaine dose revealed no significant differences between placebo vs. modafinil (p ≥ 0.676), modafinil vs. modafinil+escitalopram (p ≥ 0.181), placebo vs. escitalopram (p ≥ 0.677), or placebo vs. modafinil+escitalopram (p ≥ 0.714).
4. DISCUSSION
The present study explored whether, relative to treatment with modafinil, treatment with modafinil+escitalopram would further reduce the subjective and reinforcing effects produced by cocaine. The data revealed that relative to placebo, modafinil monotherapy significantly attenuated several subjective effects produced by cocaine, as well as cocaine-induced increases of heart rate; however, combining modafinil+escitalopram did not enhance any of these effects. In fact, adding escitalopram appeared to reduce modafinil’s ability to block cocaine-induced subjective and cardiovascular effects.
Both clinical and preclinical studies suggested that modafinil+escitalopram polytherapy might more effectively reduce the subjective and reinforcing effects produced by cocaine. In contrast to our hypothesis, we found that modafinil in combination with escitalopram did not more effectively attenuate the effects produced by cocaine, compared to modafinil monotherapy. This finding could be due to the short duration (5 days) of treatment used for the current study. Indeed, a recent imaging study demonstrated that acute dosing with escitalopram (20 mg) administered orally to humans decreased serotonin concentrations in serotonergic projection areas (Nord et al., 2013). Alternatively, the lack of effects observed for the modafinil+escitalopram group may have resulted from increased levels of serotonin (produced by escitalopram) negatively affecting the ability of modafinil to interact with the DAT and increase DA levels. While speculative, the latter explanation is concordant with data showing that the SSRI alaproclate attenuates cocaine-induced increases of DA in monkeys (Czoty et al., 2002). Nonetheless, results from two 12-week outpatient clinical trials of fluoxetine - Schmitz et al. (2001) evaluated 40 mg/day in cocaine-dependent patients with major depressive disorder (n = 68), while Grabowski et al. (1995) evaluated 20 and 40 mg/day in cocaine-dependent patients (n = 155) - demonstrated that fluoxetine is ineffective in reducing cocaine use. These studies imply that even if we had extended modafinil+escitalopram treatment beyond 5 days, it may not have affected the outcomes. Thus, while serotonin modulates the reinforcing and subjective effects produced by cocaine in preclinical studies (Filip et al., 2010; Walsh and Cunningham, 1997), the current results suggest that increasing serotonin levels may offset the ability of modafinil to attenuate the subjective effects produced by cocaine in humans.
4.1 Limitations
These findings should be interpreted within the study limitations. We assessed the effects of IV cocaine in participants who typically smoked cocaine. This, as well as the use of a single dose of cocaine, may limit interpretability since pharmacodynamic effects are higher following the smoked vs. the IV route at equivalent plasma concentrations of cocaine (Cone, 1995). However, compared to smoking cocaine, the IV route has the advantage of ensuring greater consistency of doses received.
4.2 Future Directions
The current findings lend further support to the potential efficacy of modafinil as a treatment for cocaine use, though novel drug combinations should be evaluated as a means to enhance the therapeutic effects of modafinil. Repeated cocaine use has been associated with a down-regulation of monoaminergic systems (Pani et al., 2011); thus, combining modafinil with medications that also target the noradrenergic system may prove useful for reducing cocaine use (Haile et al., 2012; Newton et al., 2012). Dextroamphetamine, which is more potent at inducing norepinephrine release than DA release (Rothman et al., 2001), significantly reduced cocaine-positive urines in two outpatient clinical trials (Grabowski et al., 2001, 2004). Interestingly, however, the combination of modafinil and dextroamphetamine tended to increase cocaine use in an outpatient clinical trial (Schmitz et al., 2012). Although poor retention and medication compliance were major limitations of the Schmitz et al. combination study, the use of an immediate release formulation of amphetamine also may have contributed to the poor outcomes. That is, sustained release amphetamines, including meth-amphetamine as reported by Mooney et al. (2009), may be superior to immediate release preparations for reducing cocaine use and craving. Sustained release amphetamines also have lower abuse liability and a better therapeutic window than immediate release formulations in preclinical studies (Heal et al., 2013; Rowley et al., 2012). Thus, evaluation of modafinil combined with a sustained release amphetamine for cocaine dependence appears warranted.
In rodents, repeated cocaine administration reduced both basal glutamate levels (Hotsenpiller et al., 2001), and glutamate immunolabeling in the nucleus accumbens following extended withdrawal (Keys et al., 1998). Further, repeated cocaine administration decreased the synaptic strength of glutamatergic afferents from prefrontal cortex to the nucleus accumbens (Thomas et al., 2001), and increased the capacity of a subsequent acute cocaine injections to elevate extracellular glutamate levels in both the ventral tegmental area (Kalivas and Duffy, 1998) and the nucleus accumbens of rats (Pierce et al., 1996; Reid and Berger, 1996). These studies suggest that combining modafinil with a glutamate-enhancing agent may be effective for reducing the effects produced by cocaine, especially since the glutamate-enhancing agent N-acetylcysteine appears to normalize glutamatergic abnormalities in humans with cocaine dependence (Schmaal et al., 2012).
4.3 Conclusion
This human laboratory study found that short-term treatment with modafinil attenuated subjective effects produced by cocaine in non-treatment seeking cocaine users, but escitalopram failed to enhance the effects of modafinil. While these findings further support the potential efficacy of modafinil as a treatment for cocaine dependence, they do not support the use of SSRIs as a means to enhance the therapeutic effects of modafinil.
Acknowledgements
We acknowledge the BCM General Clinical Research Center nursing staff for their expert assistance. In addition, this material is the result of work supported with resources from, and the use of facilities at, the Michael E. DeBakey VA Medical Center, Houston, TX.
Author Disclosures
Role of Funding Source
The authors declare that Dr. De La Garza received funding for these studies from the National Institutes of Health (DA028387).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Richard De La Garza conceived and, along with Dr. Thomas Newton and Dr. Colin Halie, designed the study and wrote the protocol that led to the submission. James Mahoney and Daisy Thompson-Lake acquired data and played an important role in interpreting the results. Dr. Chris Verrico managed the literature searches, summaries of previous related work, undertook the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors declare no conflicts of interest.
REFERENCES
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, 3rd, Elkashef AM. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ML, Kessler E, Murnane KS, McClung JC, Tufik S, Howell LL. Dopamine transporter-related effects of modafinil in rhesus monkeys. Psychopharmacol. 2010;210:439–448. doi: 10.1007/s00213-010-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone EJ. Pharmacokinetics and pharmacodynamics of cocaine. J. Anal. Toxicol. 1995;19:459–478. doi: 10.1093/jat/19.6.459. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J. Pharmacol. Exp. Ther. 2002;300:831–837. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Zorick T, London ED, Newton TF. Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend. 2010;106:173–180. doi: 10.1016/j.drugalcdep.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW. Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend. 2003;70:29–37. doi: 10.1016/s0376-8716(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacol. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J. Subst. Abuse Treat. 2012;43:303–312. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, O'Connor WT, Tanganelli S, Rambert FA, Fuxe K. The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neurosci. Lett. 1998;253:135–138. doi: 10.1016/s0304-3940(98)00629-6. [DOI] [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegalinski E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict. Biol. 2010;15:227–249. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, Davis C, Creson D, Kirby K. Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled double-blind trials. J. Clin. Psychopharmacol. 1995;15:163–174. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J. Clin. Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict. Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Cognitive control and the dopamine D(2)-like receptor: a dimensional understanding of addiction. Depress. Anxiety. 2012;29:295–306. doi: 10.1002/da.20897. [DOI] [PubMed] [Google Scholar]
- Haile CN, Mahoney JJ, 3rd, Newton TF, De La Garza R., 2nd Pharmacotherapeutics directed at deficiencies associated with cocaine dependence: focus on dopamine, norepinephrine and glutamate. Pharmacol. Ther. 2012;134:260–277. doi: 10.1016/j.pharmthera.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacol. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D. A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to D-amfetamine, methylphenidate and modafinil. Neuropharmacology. 2013;73:348–358. doi: 10.1016/j.neuropharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J. Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Howell LL. Nonhuman primate neuroimaging and cocaine medication development. Exp. Clin. Psychopharmacol. 2008;16:446–457. doi: 10.1037/a0014196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J. Pharmacol. Exp. Ther. 2007;320:757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Mahoney JJ, 3rd, Yoon JH, Bennett R, De la Garza R., 2nd Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacol. 2013;64:472–478. doi: 10.1016/j.neuropharm.2012.06.064. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J. Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Keys AS, Mark GP, Emre N, Meshul CK. Reduced glutamate immunolabeling in the nucleus accumbens following extended withdrawal from self-administered cocaine. Synapse. 1998;30:393–401. doi: 10.1002/(SICI)1098-2396(199812)30:4<393::AID-SYN6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kim W, Tateno A, Arakawa R, Sakayori T, Ikeda Y, Suzuki H, Okubo Y. In vivo activity of modafinil on dopamine transporter measured with positron emission tomography and [18F]FE-PE2I. Int. J. Neuropsychopharmacol. 2014;17:697–703. doi: 10.1017/S1461145713001612. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J. Pharmacol. Exp. Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Hensley-Simon M, Tahsili-Fahadan P, Lalumiere RT, Thomas C, Fallon RV, Kalivas PW, Aston-Jones G. Modafinil attenuates reinstatement of cocaine seeking: role for cystine-glutamate exchange and metabotropic glutamate receptors. Addict. Biol. 2012;19:49–60. doi: 10.1111/j.1369-1600.2012.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm R, Swayngim K, Donovan JL, Devane CL, Elkashef A, Chiang N. Modafinil and cocaine interactions. Am. J. Drug Alcohol Abuse. 2006;32:577–587. doi: 10.1080/00952990600920425. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am. J. Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, Swann AC, Grabowski J. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am. J. Drug Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, Brown G, II, Kosten TR, Mahoney JJ, III, Haile CN. Noradrenergic α1 receptor antagonist treatment attenuates positive subjective effects of cocaine in humans: a randomized trial. PLoS One. 2012;7:e30854. doi: 10.1371/journal.pone.0030854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord M, Finnema SJ, Halldin C, Farde L. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int. J. Neuropsychopharmacol. 2013:1–10. doi: 10.1017/S1461145712001617. [DOI] [PubMed] [Google Scholar]
- Oliveto A, Poling J, Mancino MJ, Williams DK, Thostenson J, Pruzinsky R, Gonsai K, Sofuoglu M, Gonzalez G, Tripathi S, Kosten TR. Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction. 2012;107:131–141. doi: 10.1111/j.1360-0443.2011.03552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani PP, Trogu E, Vecchi S, Amato L. Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database Syst. Rev. 2011:CD002950. doi: 10.1002/14651858.CD002950.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, See RE. Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacol. 2010;210:337–346. doi: 10.1007/s00213-010-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Berger SP. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine 5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol. Sci. 2006;27:612–618. doi: 10.1016/j.tips.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J. 2007;9:E1–E10. doi: 10.1208/aapsj0901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers: potential treatment agents for stimulant addiction. Exp. Clin. Psychopharmacol. 2008;16:458–474. doi: 10.1037/a0014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley HL, Kulkarni R, Gosden J, Brammer R, Hackett D, Heal DJ. Lisdexamfetamine and immediate release d-amfetamine - differences in pharmacokinetic/pharmacodynamic relationships revealed by striatal microdialysis in freely-moving rats with simultaneous determination of plasma drug concentrations and locomotor activity. Neuropharmacol. 2012;63:1064–1074. doi: 10.1016/j.neuropharm.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropharmacol. 2012;37:2143–2152. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt KC, Reith ME. The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. P.Lo.S. One. 2011;6:e25790. doi: 10.1371/journal.pone.0025790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63:207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Rathnayaka N, Green CE, Moeller FG, Dougherty AE, Grabowski J. Combination of modafinil and d-amphetamine for the treatment of cocaine dependence: a preliminary investigation. Front. Psychiatry. 2012;3:77. doi: 10.3389/fpsyt.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spealman RD. Modification of behavioral effects of cocaine by selective serotonin and dopamine uptake inhibitors in squirrel monkeys. Psychopharmacol. 1993;112:93–99. doi: 10.1007/BF02247368. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: 2013a. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-46, HHS Publication No (SMA) 13-4795. [Google Scholar]
- Substance Abuse and Mental Health Services Administration C. f.B.H.S.a.Q. Rockville, MD: 2013b. The DAWN Report: Highlights of the 2011 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration C.f.B.H.S.a.Q. National Admissions to Substance Abuse Treatment Services. Rockville, MD: 2013c. Treatment Episode Data Set (TEDS). 2001–2011. HHS Publication No (SMA) 13-4772, BHSIS Series S-65. [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacol. 2003;165:260–269. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC) United Nations Publication, Sales No. E.10.XI.13; 2010. World Drug Report. [Google Scholar]
- Verrico CD, Haile CN, Newton TF, Kosten TR, De La Garza R. Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin. Investig. Drugs. 2013;22:1549–1568. doi: 10.1517/13543784.2013.836488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol. Learn. Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacol. 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Sullivan JT, Fromme R, Bigelow GE. Fluoxetine alters the effects of intravenous cocaine in humans. J. Clin. Psychopharmacol. 1994;14:396–407. [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J. Pharmacol. Exp. Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]