Abstract
Background
Gamma band oscillations and their synchronization have been implicated in the coordination of activity between distributed neuronal assemblies in the service of sensory registration of stimuli and perceptual binding of their features. Prior electroencephalographic (EEG) studies of chronic schizophrenia patients have documented deficits in the magnitude and/or phase synchrony of stimulus-evoked gamma oscillations, findings that have been linked to neurotransmission abnormalities involving GABA and NMDA-glutamate receptors. However, it remains unclear whether these abnormalities are present at the onset of the illness, or indeed, whether they are present during the prodromal period preceding illness onset. Accordingly, we examined the magnitude and phase-synchrony of the transient gamma band response (GBR) elicited by an auditory stimulus in young patients with schizophrenia and in patients at clinical high risk for psychosis based on their manifestation of putatively prodromal symptoms.
Methods
EEG was recorded during an auditory oddball target detection task in 3 groups: young schizophrenia patients early in their illness (YSZ; n=19), patients at clinical high risk for psychosis (CHR; n=43), and healthy controls (HC; n=42). Single trial EEG epochs and the average event-related potential time-locked to standard tones from the oddball task were subjected to time-frequency decomposition using Morlet wavelet transformations. The GBR between 50-100 msec following the tone onset was quantified in terms of evoked power, total power, and the phase-locking factor (PLF) reflecting cross-trial phase synchrony.
Results
GBR evoked power was significantly reduced in YSZ (p<.01) and CHR (p<.05) patients, relative to HC. Similarly, GBR PLF was significantly reduced in YSZ (p<.01) and showed a marginal reduction in CHR patients (p=.057), relative to HC. GBR total power was not reduced in CHR patients (p=.68) and showed only a trend level reduction in YSZ (p=.072). Within the CHR group, there were no significant GBR differences between the patients who converted to a psychotic disorder and those who did not convert to psychosis during a 12-month follow-up period.
Conclusion
Reductions in the transient auditory GBR, as reflected by evoked power and phase synchrony, are evident in the early stages of schizophrenia and appear to precede psychosis onset. However, the absence of total power GBR abnormalities in CHR patients, with only a trend toward reduction in YSZ patients, suggests that the magnitude of the GBR is intact early in the course of schizophrenia, whereas the phase synchrony of this response is deficient. Given that the GBR failed to distinguish the CHR patients who converted to psychosis from those who did not convert, the role of GBR disruption in the emergence of psychosis warrants further investigation. Asynchronous gamma activity may represent an elemental neurobiological abnormality in schizophrenia that is also evident in patients at high clinical risk for psychosis regardless of their longer-term clinical outcomes.
Keywords: schizophrenia, clinical high-risk, auditory, gamma, total power, evoked power, PLF
1. Introduction
Synchronization of neural activity in the gamma range (30-80 Hz) is thought to be a mechanism for integrating sensory information across different modalities and cortical areas, thereby creating a coherent cortical representation of complex external sensory stimuli (Basar et al., 2000; Engel et al., 2001; Singer, 1999). The coordination of sensory gamma oscillations has been shown to contribute to sensory registration and perceptual processes (Clementz et al., 1997; Hong et al., 2004a), as well as higher order cognitive operations such as attention and expectation (Basar-Eroglu and Basar, 1991; Debener et al., 2003; Gurtubay et al., 2004; Gurtubay et al., 2001; Tallon-Baudry et al., 1997; Tiitinen et al., 1993). It has been hypothesized that the widespread cognitive deficits consistently observed in schizophrenia may be attributable to core abnormalities in the timing, synchronization, and efficiency of neuro-oscillatory activity, particularly in the gamma band, that subserves the binding and integration of information processed across different brain regions (see Uhlhaas and Singer, 2010).
Patients with schizophrenia have been shown to have abnormal electroencephalographic (EEG) gamma-band responses (GBRs) associated with sensory (Gallinat et al., 2004; Haig et al., 2000b; Hall et al., 2011a; Hall et al., 2011b; Kwon et al., 1999; Leicht et al., 2010b; Light et al., 2006; Roach and Mathalon, 2008; Spencer et al., 2008a), perceptual (Spencer et al., 2003), attentional (Haig et al., 2000a; Symond et al., 2005), and cognitive control (Cho et al., 2006) processes. We and others have recently reported decreased early gamma activity in response to auditory stimuli in patients with schizophrenia, as reflected by evoked power (Hall et al., 2011a; Hall et al., 2011b; Leicht et al., 2010b) and phase-locking factor (Hall et al., 2011b; Leicht et al., 2010b; Roach and Mathalon, 2008; Roach, this issue). In auditory oddball paradigms, schizophrenia has been associated with an abnormal reduction of GBRs to target (Haig et al., 2000b; Symond et al., 2005) and non-target (Haig et al., 2000b; Hall et al., 2011b; Roach and Mathalon, 2008) auditory tones, suggesting that the early-evoked GBRs to auditory stimuli are either compromised by a lack of phase consistency across trials, reduced response magnitude, or some combination of the two. While gamma oscillation abnormalities are evident in chronic schizophrenia patients (Gallinat et al., 2004; Hall et al., 2011a; Hall et al., 2011b; Kwon et al., 1999; Light et al., 2006; e.g., Roach and Mathalon, 2008; Roach, this issue), in first-episode psychosis (Spencer et al., 2008b; Symond et al., 2005; Williams et al., 2009), and to a lesser degree, in unaffected relatives (Hall et al., 2011b; Hong et al., 2004b; Leicht et al., 2010a), but see (Hall et al., 2011a), it remains unclear whether they are present in individuals at clinical high risk for the development of psychosis.
2. Mechanisms of Gamma-band Oscillations
Fast-spiking inhibitory γ-aminobutyric acid (GABA) interneurons expressing the calcium-binding protein parvalbumin have been implicated in the mediation of gamma oscillatory activity (Carlen et al., 2011; Gonzalez-Burgos et al., 2010; Lewis et al., 2011; Sohal et al., 2009). The inhibition of pyramidal cell and interneuron networks by GABAergic interneurons produces gamma band oscillations through an inhibition and rebound excitation cycle that is modulated by GABAA receptors (Sohal et al., 2009; Whittington et al., 1995). Moreover, glutamatergic neurotransmission at NMDA receptors provides excitatory regulation of parvalbumin fast-spiking interneurons, contributing to the generation of gamma oscillations in pyramidal cell networks (Carlen et al., 2011; Doheny et al., 2000; Roopun et al., 2008). Gamma oscillatory activity non-invasively measured by EEG in patients with schizophrenia is of interest because disrupted GABAergic and glutamatergic cortical activity have been implicated in the pathophysiology of the illness (Benes, 2000; Gonzalez-Burgos and Lewis, 2008; Lewis et al., 2005; Lewis et al., 2011; Lewis et al., 2008; Roopun et al., 2008).
3. Early Gamma-band Responses in Schizophrenia
The auditory GBR has been quantified with different methods, typically involving the transformation of the time-voltage domain EEG or event-related potential (ERP) signal into the time-frequency domain. Repeated applications of Fourier, Hilbert, or wavelet transformations produce time-frequency decompositions of EEG or ERP signals. Our analysis focuses on measures of total power, evoked power, and phase-locking factor (PLF), and typically these effects are fronto-centrally distributed across EEG channels. Total power (or event-related spectral perturbation (Delorme and Makeig, 2004) is a measure of the event-related change in power across individual trials, including both phase synchronous and asynchronous activity. Evoked power describes only phase-locked event-related changes in power because the time-frequency transformation is derived from the time-domain averaged ERP waveform. Finally, PLF (also called intertrial phase coherence (Delorme and Makeig, 2004) is a measure of phase consistency across trials from a single electrode or source.
Several studies have investigated the early, evoked auditory GBR associated with sensory registration of auditory stimuli (Pantev et al., 1991) using evoked power or PLF measures in schizophrenia. Clementz (Clementz et al., 1997) initially observed early abnormalities in auditory evoked gamma power, and multiple subsequent studies (Basar-Eroglu et al., 2009; Hall et al., 2011a; Hall et al., 2011b; Hirano et al., 2008; Krishnan et al., 2009; Leicht et al., 2010a; Leicht et al., 2010b; Lenz et al., 2011; Roach and Mathalon, 2008; Teale et al., 2008), but not all (Blumenfeld and Clementz, 2001; Brenner et al., 2009; Brockhaus-Dumke et al., 2008a; Spencer et al., 2008a), have reported reduced evoked power or PLF in the gamma band. However, the selective examination of these two measures limits our ability to adequately describe the stimulus-driven activity of neuronal assemblies. Evoked power reflects the amplitude of the oscillations that are phase-locked to a stimulus event, since averaging across trials tends to cancel out non-phase locked oscillatory activity. Accordingly, because the evoked power measure initially requires some consistency of phase across single trials, and then depends on the average magnitude of the phase-locked signal, evoked power deficits could be due to decreased phase-locking or to reduced single trial amplitude. Given that many previous studies have acknowledged the increase in phase variance (i.e., latency jitter) in measures of neural activity in patients with schizophrenia (Ford et al., 1994), it is surprising that very few studies (Krishnan et al., 2009) have assessed total power of the early auditory GBR in this population to separate magnitude from phase-locking abnormalities.
Furthermore, it is unknown whether abnormalities in transient GBRs observed in schizophrenia patients predate the onset of psychosis, or co-occur with illness onset. This question can be addressed by studying patients at clinical high risk (CHR) for developing psychosis. Being at “clinical high risk” is defined by the Criteria of Prodromal Syndromes (COPS; Miller et al., 2002) and the similar criteria for At Risk Mental States (ARMS; Yung and McGorry, 1996). The North American Prodromal Longitudinal Study (NAPLS) consortium reported that 35% of patients meeting COPS criteria converted to a psychotic disorder within a 2.5 year follow-up period (Cannon et al., 2008). An increasing number of studies are finding that electrophysiological abnormalities associated with schizophrenia are evident in clinical high risk patients (Bodatsch et al., 2011; Brockhaus-Dumke et al., 2008b; Jahshan et al., 2011; Perez et al., 2011a; Perez et al., 2011b; Shin et al., 2009; van Tricht et al., 2010); however, none has assessed whether GBRs are abnormal in these patients. GBR abnormalities in CHR patients may reflect vulnerability to the disorder and/or abnormal neurodevelopment, particularly in connection with abnormal function of NMDA and GABAA receptors. A major motivation for identifying neurophysiological abnormalities in a CHR sample is to enhance the accuracy of the prediction of which at risk patients will convert to psychosis, setting the stage for development of targeted preventive interventions aimed at those patients at greatest risk.
4. Design of Present Study
In order to assess whether transient GBRs quantified with total power, evoked power, and PLF time-frequency measures are compromised early in the course of schizophrenia, we compared young schizophrenia patients, clinical high risk patients, and healthy controls on these measures. Based on previous studies reporting reduced gamma band evoked power and PLF in schizophrenia, we predicted that young schizophrenia patients, still early in their illness course, would show diminished evoked power and PLF in the GBR. Based on the hypothesis that abnormal GBRs may be physiological risk markers for the development of psychosis, we predicted that patients at clinical high risk for psychosis would also be abnormal on these measures. However, given the heterogeneous nature of clinical high risk samples, with only a minority of patients destined to convert to schizophrenia, we predicted that, as a group, their abnormalities would be intermediate relative to early illness schizophrenia patients and healthy controls. Finally, we predicted that clinical high risk patients who subsequently converted to psychosis would have greater GBR abnormalities.
5. Method
5.1. Participants
Study participants (Table 1) included 43 patients at clinical high risk (CHR) for psychosis based on the Structured Interview for Prodromal Syndromes (SIPS; Miller et al., 2002), 19 young patients with DSM-IV schizophrenia (YSZ) based on the Structured Clinical Interview for DSM-IV (SCID), and 42 healthy control (HC) subjects. CHR patients met criteria for at least one of the three sub-syndromes defined by the COPS (Miller et al., 2002): 1) Attenuated Positive Symptoms (APS), 2) Brief Intermittent Psychotic States (BIPS), and/or 3) Genetic Risk with Deterioration in social/occupational functioning (GRD). Interviews were conducted by a trained research assistant, psychiatrist, or clinical psychologist.
Table 1.
Group Demographic Dataa
| Clinical High Risk (CHR) | Young Schizophrenia (YSZ) | Healthy
Controls (HC) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Patients | ||||||||
| n=43 | n = 19 | n = 42 | χ 2 | p-value | |||||
| N | % | N | % | N | % | ||||
| Gender | 2.0 | 0.363 | |||||||
| female | 17 | 39.5 | 4 | 21.1 | 15 | 35.7 | |||
| male | 26 | 60.5 | 15 | 78.9 | 27 | 64.3 | |||
| Handednessb | 1.7 | 0.787 | |||||||
| right | 36 | 83.7 | 16 | 84.2 | 35 | 83.3 | |||
| left | 3 | 7.0 | 1 | 5.3 | 5 | 11.9 | |||
| both | 4 | 9.3 | 2 | 10.5 | 2 | 4.8 | |||
| Diagnostic Subtype | |||||||||
| paranoid | 12 | 63.2 | |||||||
| disorganized | 1 | 5.3 | |||||||
| undifferentiated | 2 | 10.5 | |||||||
| residual | 1 | 5.3 | |||||||
| schizoaffective | 3 | 15.7 | |||||||
| schizophreniform | |||||||||
| Clinical High Risk Criteriac | |||||||||
| APS | 41 | 95.3 | |||||||
| BIPS | 1 | 2.3 | |||||||
| GRD | 1 | 2.3 | |||||||
| Antipsychotic type | |||||||||
| atypical | 10 | 23.3 | 14 | 73.7 | |||||
| typical | 0 | 0.0 | 0 | 0.0 | |||||
| both | 0 | 0.0 | 3 | 15.8 | |||||
| none | 33 | 76.7 | 2 | 10.5 | |||||
| M | SD | M | SD | M | SD | F | p-value |
Post hoc contrasts |
|
| Age, years | 16.86 | 3.5 | 23.91 | 6.2 | 19.89 | 5.5 | 13.93 | <.001 | YSZ>HC>CHR |
| Parental Socioeconomic Statusd | 36.9 | 14.8 | 40.44 | 9.1 | 27.61 | 13.0 | 7.89 | <.001 | HC<CHR=YSZ |
| PANSS Symptom Totale | 70.14 | 15.50 | |||||||
| SOPS Symptom Total | 37.40 | 15.60 | |||||||
Note. Values are given as number and percentage of subjects for gender, handedness, diagnostic subtype, clinical high risk criteria, and antipsychotic type. Group means with the standard deviation for age, parental socioeconomic status, PANSS, and SOPS are reported. Gender and handedness were analyzed with Pearson chi-square tests. Age and parental socioeconomic status were analyzed with one-way ANOVA and Tukey-Kramer post hoc tests.
Abbreviations: CHR, clinical high risk patients; YSZ, young schizophrenia patients; HC, healthy controls; APS, Attenuated Positive Symptoms; BIPS, Brief Intermittent Psychotic Symptoms; GRD, Genetic Risk and Deterioration; PANSS, Positive and Negative Syndrome Scale; SOPS, Scale of Prodromal Symptoms.
The Crovitz-Zener (1962) questionnaire was used to measure handedness.
Clinical High Risk criteria APS, BIPS, and GRD are not mutually exclusive.
The Hollingshead (1975) four-factor index of parental socioeconomic status (SES) is based on a composite of maternal education, paternal education, maternal occupational status, and paternal occupational status. Lower scores represent higher SES. d, SES values are missing from 2 YSZ patients (n=2).
PANSS scores are missing from 5 YSZ patients (n=5).
HC were recruited by advertisements and word-of-mouth. Exclusion criteria for HC included a past or current DSM-IV Axis I disorder based on a SCID interview or having a first-degree relative with a psychotic disorder. Exclusion criteria for all groups included history of substance dependence or abuse within the past year, a history of a significant medical or neurological illness, or a history of head injury resulting in loss of consciousness. The study was approved by the Yale University institutional review board, and adult participants provided written informed consent. In the case of minors, parents provided written informed consent; youths provided written informed assent.
5.2. Clinical Ratings
Within 1 month of EEG assessment (M=9.8, SD=22.99 days), a clinically trained research assistant, psychiatrist, or clinical psychologist rated YSZ symptoms using the Positive and Negative Syndrome Scale (PANSS; Kay, 1987) and CHR symptoms using the Scale of Prodromal Symptoms (SOPS; Miller et al., 2002).
5.3. Conversion to Psychosis
Of the 43 CHR patients, 15 converted to a psychotic disorder within 2.5 years of study participation. Conversion to psychosis was defined by a rating of 6 on at least one of the positive symptom items from the SIPS, indicating the presence of a full-blown psychotic syndrome. A positive symptom is given a rating of 6 if it has reached a high intensity level (e.g., delusional conviction) and exceeds a frequency or duration threshold (≥1 hour/day for ≥4 days/week during the past month), or if it has a seriously disorganizing or dangerous impact on the patient (Cannon, 2008). Only CHR patients who were clinically followed for a minimum of twelve months (n=16) and had not yet converted to a psychotic disorder were included in the non-conversion group.
5.4. Gamma Band Response Paradigm
Subjects listened to a random series of infrequent (n=45, 10%) high tones (1000 Hz), infrequent novel sounds (n=45, 10%), and frequent (n=360, 80%) low tones (500 Hz). Subjects were asked to press a response key to each occurrence of the infrequent, high tone (i.e., target stimulus). Transient GBRs were assessed from the frequent tones exclusively. These tones were 80 dB SPL and 50 ms in duration presented with a stimulus-onset asynchrony of 1.25 sec. Sounds were delivered via Etymotic ER-3A insert earphones at 80dB SPL through a STIM audio box (Compumedics Neuroscan).
5.5. EEG Acquisition
EEG data were acquired at 1000 Hz from 20 sites (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, Oz, O2), bandpass filtered between 0.05 Hz and 100 Hz, and referenced to linked ears. Additional electrodes were placed on the outer canthi of both eyes and above and below the left eye to record eye movements and blinks (vertical and horizontal electro-oculogram; VEOG, HEOG). All impedances were maintained at or below 10 kOhm throughout the recording session with most EEG sites below 5 kOhm.
Single trial EEG epochs were stimulus-locked to the onset of each tone, including data from 250 ms before the tone onset and 750 ms after its onset. Individual trials were baseline corrected using the 100 ms period preceding the tone onset after correcting for eye movements and blinks using EOG data (Gratton et al., 1983). Finally, trials containing artifacts (voltages exceeding ±100 μV) in any of the central 9 electrodes (F3, Fz, F4, C3, Cz, C4, P3, Pz, or P4) were rejected.
5.6. EEG Time-Frequency Analysis
Time-frequency analysis of the EEG gamma-activity was based on Morlet wavelets using the freely distributed FieldTrip (http://fieldtrip.fcdonders.nl/) software in Matlab (http://www.mathworks.com/products/matlab/), as in our prior work (Roach and Mathalon, 2008). The Morlet wavelet has a Gaussian shape that is defined by a ratio (σf = f/C) and a wavelet duration (6σt), where f is the center frequency and σt = 1/(2πσf). In a classic wavelet analysis, C is a constant (e.g., 7), ensuring an equal number of cycles in the mother wavelet for each frequency. In this approach, as the frequency (f) increases, the spectral bandwidth (6σf) increases. This method was used to decompose single trial time-frequency values between 20 and 60 Hz for the central nine electrodes.
After applying this method, PLF was calculated as 1-minus the circular phase angle variance, as described by Tallon-Baudry et al. (Tallon-Baudry et al., 1997). PLF provides a measure of the phase consistency of frequency-specific oscillations with respect to stimulus onset across trials on a millisecond basis. In addition, event-related total power was calculated by averaging the squared single trial magnitude values in each 1 Hz frequency bin on a millisecond basis. The average total power values were 10log10 transformed and then baseline corrected by subtracting the average of the pre-stimulus baseline (−100 to 0 ms) from each time point separately for every frequency. The resulting event-related change in total power values (relative to baseline) are in decibels (dB).
5.7. ERP Time-Frequency Analysis
To quantify evoked power, single trial EEG epochs were averaged to create event-related potentials (ERPs). ERP data were subjected to the wavelet decomposition described above, and evoked power was calculated by squaring the output. The evoked power values were 10log10 transformed and then baseline corrected by subtracting the average of the pre-stimulus baseline (−100 to 0 ms) from each time point separately for every frequency. The resulting event-related change in evoked power values (relative to baseline) are in decibels (dB).
5.8. Principal Components Analysis of Time-Frequency Data
To capture and quantify the GBR, a time-frequency principal components analysis (TF-PCA) approach similar to that implemented by others (Bernat et al., 2005) was adopted. In particular, total power, evoked power, and PLF measures were down-sampled to reduce the number of points (originally 1ms X 1Hz) in the TF matricies by sampling every 2 Hz (e.g., 20, 22, 24 Hz …) between 20 and 60 Hz and every 5 ms (e.g., −50, −45, −40 ms…) between −50 and 150ms. The TF-PCA was then calculated separately for total power, evoked power, and PLF by rearranging the 2-D TF data into 1-D by transposing the frequency data at the first time point (−50 ms) and concatenating it with similarly transposed vectors at all other time points. This results in 861 sample row vectors for each electrode (n=9) and each subject (n=104), which were submitted to a covariance matrix PCA implemented in Matlab (Kayser and Tenke, 2003). All components were retained and subjected to a varimax rotation, yielding orthogonal factors corresponding to major TF components. To produce interpretable TF factor loadings, 1-D factor loadings were rearranged back into the original order of the 2-D TF measures, and individual subject and electrode factor scores corresponding to the transient GBR component were saved for subsequent analyses.
5.9. Statistical Correction for Normal Aging Effects
To control for the effects of normal aging, we derived a single age-corrected value for each subject for total power, evoked power, and PLF scores. First, total power PCA factor scores were regressed on age in the HC group (age range 12-37 years). Next, the resulting regression equation was used to derive age-specific predicted values that were subtracted from the observed values and divided by the standard error of regression, yielding age-corrected total power factor z-scores for subjects across all groups. The resulting age-corrected z-scores reflected deviations in standard units from the values expected for a normal healthy subject of a given age. This method has been used previously to correct for normal aging effects in structural MRI data (Pfefferbaum et al., 1992). The method is preferable to using age as a covariate in an ANCOVA because it removes normal aging effects while preserving any pathological aging effects present in the patient data. This procedure was repeated to acquire age-corrected evoked power factor z-scores and PLF factor z-scores for all subjects across groups.
5.10. Statistical Analysis
Separate 3 Group (HC, CHR, YSZ) X 2 site (Fz, Cz) repeated measures analysis of variance (ANOVA) models were performed on each of the age-corrected GBR dependent variables (total power, evoked power, PLF). Significant Group effects were further parsed with a priori planned contrasts. Converters and non-converters were compared on total power, evoked power, and PLF, using a 2 Group (converters, non-converters) X 2 site (Fz, Cz) repeated measures ANOVA.
To assess the relationship between symptom severity and total power, evoked power, and PLF in the YSZ group, GBR factor scores from each measure were correlated with positive (sum of PANSS positive symptom ratings) and negative (sum of PANSS negative symptom ratings) symptom summary scores. Correlations with SOPS positive and negative symptom summary scores were performed in the CHR group. A Bonferonni correction was applied for the number of correlations performed within each patient group, maintaining the corrected alpha level at p=.05.
6. RESULTS
6.1. GBR PCA Factor Loadings
Inspection of the factor loadings for total power (Figure 1a) revealed that the first factor peaked at ~40 Hz and 50 ms, corresponding to the GBR, and accounted for 12.59% of the variance. Inspection of the factor loadings for evoked power (Figure 1b) revealed that the first factor peaked at ~40 Hz and 50 ms, corresponding to the GBR, and accounted for 7.62% of the variance. Inspection of the factor loadings for PLF (Figure 1c) revealed that the third factor peaked at ~40 Hz and 50 ms, corresponding to the GBR, and accounted for 14.96% of the variance.
Figure 1.
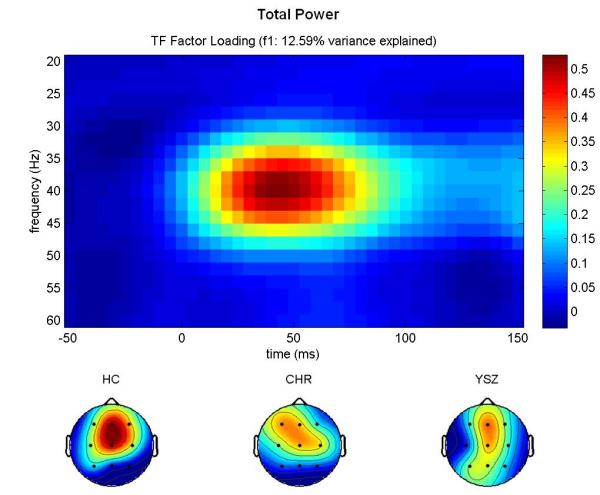
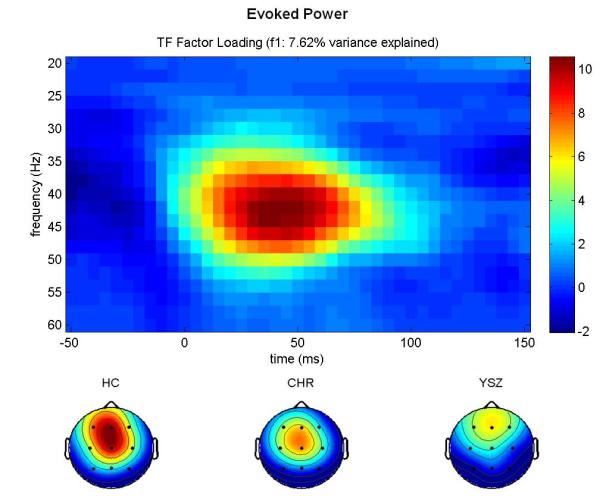
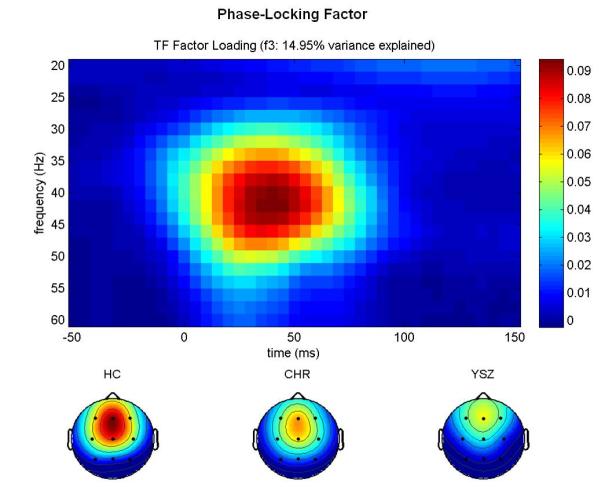
GBR Factor Loadings.
Time-frequency (TF) factor loadings are plotted from a principal components analysis (PCA) with varimax rotation for (a) total power, (b) evoked power, and (c) phase-locking factor gamma-band responses (GBR). Time (ms) is plotted on the x-axis; EEG frequency (Hz) is plotted on the y-axis. Stimulus onset occurred at 0 ms. Below each TF plot, scalp topography maps for each group (Healthy Control (HC), Clinical High-Risk (CHR), and Young Schizophrenia (YSZ)) show a fronto-central distribution for each GBR across groups. YSZ show reduced GBRs relative to HC. CHR are intermediate to the HC and YSZ groups. Scaling was uniform across groups. Greater total power and evoked power GBRs, and greater phase-locking consistency across trials, is shown in hot colors (red), as indicated on the color scale to the right of each TF plot.
Furthermore, scalp topographies of the extracted PCA factor scores are consistent with the expected fronto-central distribution of the auditory gamma band response, as shown in Figures 1a-c.
6.2. Group Differences in GBR PCA Factor Scores
The factor scores corresponding to the GBR components shown in Figure 1 were extracted for electrode sites Fz and Cz, subjected to age-correction procedures described previously, and used in the group analyses (see Table 2). Age-corrected mean total power, evoked power, and PLF z-scores (+/- standard error) are plotted for each group in Figure 2. Note that because z-scores are normed relative to the HC group data, the HC group has mean z-score values of zero for each measure.
Table 2.
Group Differences on Measures of Gamma-band Responsea
| Group Analyses for Age-Corrected Total Power Gamma-band Response. | |||||
|
| |||||
| ANOVA Results (HC, CHR, YSZ) | Cohen’s d | df | F | p-value | |
|
| |||||
| Group | 2, 101 | 1.72 | 0.19 | ||
| Site (Fz, Cz) | 1, 101 | 0.00 | 0.98 | ||
| Group X Site | 2, 101 | 1.1 | 0.34 | ||
|
| |||||
| Planned Group Comparisons: | |||||
| HC vs. YSZ | 0.431 | 0.072 | |||
| HC vs. CHR | 0.088 | 0.687 | |||
|
| |||||
| Group Analyses for Age-Corrected Evoked Power Gamma-band Response. | |||||
|
| |||||
| ANOVA Results (HC, CHR, YSZ) | Cohen’s d | df | F | p-value | |
|
| |||||
| Group | 2, 101 | 5.45 | 0.006 | ||
| Site (Fz, Cz) | 1, 101 | 0.001 | 0.97 | ||
| Group X Site | 2, 101 | 0.901 | 0.41 | ||
|
| |||||
| Planned Group Comparisons: | |||||
| HC vs. YSZ | 0.688 | 0.004 | |||
| HC vs. CHR | 0.503 | 0.011 | |||
|
| |||||
| Group Analyses for Age-Corrected Phase-locking Factor of the Gamma-band Response. | |||||
|
| |||||
| ANOVA Results (HC, CHR, YSZ) | Cohen’s d | df | F | p-value | |
|
| |||||
| Group | 2, 101 | 4.51 | 0.013 | ||
| Site (Fz, Cz) | 1, 101 | 0.00 | 0.99 | ||
| Group X Site | 2, 101 | 1.36 | 0.26 | ||
|
| |||||
| Planned Group Comparisons: | |||||
| HC vs. YSZ | 0.791 | 0.005 | |||
| HC vs. CHR | 0.372 | 0.057 | |||
One-way ANOVA comparing Young Schizophrenia (YSZ), Clinical High Risk (CHR), and Healthy Control (HC) group on age-corrected total power, evoked power, and phase-locking factor principal components analysis (PCA) z-scores in gamma-band responses at Fz and Cz. Simple planned contrasts compared HC to patient groups.
Significance based on alpha=0.05, two-tailed.
Figure 2.
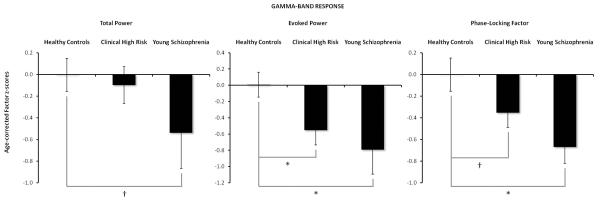
Group Differences in Age-Corrected GBR PCA Factor Scores.
Mean (± standard error) age-corrected PCA factor z-scores for the GBR in the Healthy Control (HC) group, Clinical High-Risk (CHR), and Young Schizophrenia (YSZ) patients to frequent stimuli during the auditory oddball task. In (a), marginal group differences between the YSZ and HC groups for Total Power are plotted. CHR did not show a difference in Total Power from HC. In (b), significant CHR and YSZ deviations from HC Evoked Power are plotted. In (c), YSZ show reductions in Phase-Locking Factor relative to HC, with a trend for CHR to have reduced values relative to HC. [* p<.02, † p<0.08]
6.3. Group Differences in Age-Corrected PCA Total Power GBR
The repeated measures ANOVA revealed an absence of any significant group differences (HC, CHR, YSZ) in the total power GBR (Figure 1a, 2a). Furthermore, the Site and Group X Site interaction effects were not significant. A priori pairwise comparisons showed that age-corrected total power GBR factor z-scores were marginally reduced in YSZ relative to HC (Cohen’s d=.43; p=0.072). No reduction in total power GBR was observed in the CHR group relative to the HC group (Cohen’s d=.09; p=.687).
6.4. Group Differences in Age-Corrected PCA Evoked Power GBR
The repeated measures ANOVA showed a significant main effect of Group (HC, CHR, YSZ) for the evoked power GBR (Figure 1b, 2b). The Site and Group X Site interaction effects were not significant. As reported in Table 2, age-corrected evoked power GBR factor z-scores were reduced in the YSZ and CHR groups relative to the HC group (YSZ vs. HC: Cohen’s d=.69; p=0.004; CHR vs. HC: Cohen’s d=.50; p=0.011).
6.5. Group Differences in Age-Corrected PCA Phase-locking Factor GBR
The repeated measures ANOVA showed a significant main effect of Group (HC, CHR, YSZ) for the PLF of the GBR (Figure 1c, 2c). Neither the Site effect nor the Group X Site interaction was significant. Group analyses are presented in Table 2, where age-corrected PLF z-scores were reduced in the YSZ relative to the HC group (Cohen’s d=.79; p=0.005). Analyses also revealed marginally reduced age-corrected PLF scores in the CHR relative to the HC group (Cohen’s d=.37; p=0.057).
6.6. Converters vs. Non-converters
There were no differences between converters and non-converters for total power (p=.83), evoked power (p=.92), or PLF (p=.26).
6.7. Demographic Differences between Groups
Pearson chi-square analysis showed that HC had significantly higher parental socioeconomic status (SES) than both patient groups, which did not differ from each other (p=.62). Thus, after ruling out group differences in the slopes of the relationships between parental SES and each of the GBR measures (Group x parental SES interaction: total power: p=.75; evoked power: p=0.79; PLF p=0.76) and dropping the interaction terms, Group x Site ANCOVAs were performed on each of the GBR measures using parental SES as a covariate. The parental SES effect was not significant for any of the GBR measures (total power: p=.29; evoked power: p=0.65; PLF: p=0.92), and the Group effects were essentially the same as those resulting from the ANOVA models described above.
6.8. Correlational Analyses with Clinical Ratings
PANSS positive and negative symptom subscales were not significantly correlated with total power, evoked power or PLF scores in the YSZ sample. Similarly in the CHR patients, SOPS positive and negative symptom subscales were not significantly correlated with total power, evoked power or PLF scores.
7. DISCUSSION
We examined the early auditory gamma band response evoked by frequently occurring standard tones from an oddball task, a signal that reflects mechanisms of sensory registration of auditory stimuli (Gurtubay et al., 2004; Gurtubay et al., 2001). Previously, we showed that the early-evoked gamma band response to tones is poorly synchronized in schizophrenia (Roach and Mathalon, 2008) consistent with other reports of abnormalities in the early auditory GBR in chronic schizophrenia patients (for review, see Gandal et al., 2012), first episode patients (Symond et al., 2005; Williams et al., 2009), and unaffected relatives (Hall et al., 2011b; Hong et al., 2004b; Leicht et al., 2010a). Here, we replicated earlier studies (Spencer et al., 2008b; Symond et al., 2005) showing that young schizophrenia patients demonstrate significantly decreased evoked power in the gamma-band. Furthermore, patients at clinical high risk for psychosis demonstrated significantly decreased evoked power in the gamma-band relative to healthy controls, although the magnitude of the abnormality in gamma-band evoked power was less pronounced in the CHR patients than in the young schizophrenia patients, as quantified by the effect sizes. These findings are consistent with other findings of reduced evoked power GBRs in unaffected co-twins of patients with schizophrenia (Hall et al., 2011b) and suggest that reduced auditory evoked gamma power may reflect the risk for developing schizophrenia.
Another aim of this study was to assess patients on the phase synchrony of the GBR using PLF. Consistent with other studies of older chronic patients (Mulert et al., 2011; Roach and Mathalon, 2008; Roach, this issue; Slewa-Younan et al., 2004; Spencer et al., 2009), the current study showed significantly diminished PLF of the GBR in young schizophrenia patients. Interestingly, marginal deficits in GBR phase consistency were present in CHR patients, where the degree of reduction in gamma synchrony was intermediate to healthy controls and young schizophrenia patients. Our findings of an intermediate effect in the at-risk group relative to healthy controls and schizophrenia patients are similar to findings in the literature (e.g., Brockhaus-Dumke et al., 2005; Brockhaus-Dumke et al., 2008b; Perez et al., 2011a; van der Stelt et al., 2005) reporting that CHR groups demonstrate intermediate effects that are not statistically distinguishable from either comparison group (i.e., the HC or SZ groups). In light of this, the nearly significant reduction of gamma PLF in the CHR patients suggests that deficient early auditory gamma-band phase synchrony predates the onset of schizophrenia.
Very few of the previously published GBR studies of patients with schizophrenia (Krishnan et al., 2009) examined the combination of synchronous and asynchronous neural activity by reporting total power. Accordingly, our study, which includes the total power GBR in schizophrenia patients and patients at risk for developing psychosis, represents a significant extension of prior studies. The fact that total gamma power was not reduced in CHR patients, and only showed a trend toward reduction in our young schizophrenia patients, suggests that the magnitude of early auditory gamma band activity is relatively spared early in the course of schizophrenia. Moreover, this lack of total power deficits suggests that the significant evoked gamma power reduction in the patients mainly resulted from poor gamma phase synchrony across trials rather than from reduced magnitude of the gamma oscillations.
Unlike the results of the current study, a significant reduction of early auditory gamma total power was observed in a sample of chronic schizophrenia patients, along with significant reductions in phase synchrony (Roach, this issue). Many factors may contribute to this apparent discrepancy between the findings from these two studies from our laboratory, including the fact that total power measurements in the gamma range are hampered by a relatively poor signal-to-noise ratio compared to evoked power measurements. This is due to the inherently greater noise present in single trial EEG epochs relative to the ERP derived by averaging them. However, another possibility is that reduction in the magnitude of early auditory gamma oscillations is a late developing abnormality that is only evident robustly in chronic schizophrenia patients, whereas reduced gamma phase synchrony and associated reductions in evoked gamma power are present early in the illness course, possibly even before illness onset during the prodromal period. While speculative, this hypothesis is consistent with the idea that the mechanisms subserving the phase synchronization of gamma oscillations with respect to an auditory stimulus are dissociable from the mechanisms governing the magnitude of these oscillations. Moreover, to the extent that the magnitude of gamma oscillations depends on the quantity or extent of underlying neural networks in the cortex, the emergence of deficient gamma oscillation magnitudes in chronic patients is consistent with accumulating longitudinal MRI evidence of progressive gray matter volume loss over the illness course of schizophrenia (e.g., Andreasen et al., 2011; DeLisi, 1999; Hulshoff Pol and Kahn, 2008; Mathalon et al., 2001).
Attenuated GBRs were not related to positive or negative symptoms in young schizophrenia or CHR patients. This finding is consistent with some (Haig et al., 2000b), but not all (Hall et al., 2011b; Leicht et al., 2010b), prior efforts to relate abnormal oscillations in the gamma frequency range to symptoms. It is worth noting, however, that some studies have found relationships between diminished gamma response and “treatment-resistant” symptoms including disorganization (Gordon, 2001) and psychomotor poverty (Gordon, 2001; Lee, 2003). Additional studies have reported associations between reductions in gamma and disrupted perceptual (Johannesen et al., 2008) and cognitive ability (Uhlhaas et al., 2006), including impaired working memory (Light et al., 2006; Winterer et al., 2004). However, other studies have reported unexpected correlations between enlarged gamma-band response and positive symptoms (Hirano et al., 2008; Lee, 2003; Spencer et al., 2008a; Spencer et al., 2009; Spencer et al., 2004; Uhlhaas et al., 2006). Further clarification is needed about whether the same clinical and cognitive abnormalities are associated with different gamma measures (e.g., total power vs. evoked power vs. intertrial phase coherence). Thus, whether the gamma-band abnormalities are a general characteristic of schizophrenia or are more specifically associated with certain symptom domains remains unclear.
The present findings of abnormal gamma-band responses in CHR patients that are intermediate between young schizophrenia patients and healthy controls indicate that these abnormalities predate psychosis onset, at least in a subset of a CHR patients. While the pathophysiological mechanisms giving rise to these gamma band abnormalities remain to be elucidated, it is reasonable to hypothesize that these mechanisms involve dysfunction of the GABAergic and NMDA glutamatergic micro-circuitry known to subserve gamma oscillations (Carlen et al., 2011; Sohal et al., 2009) that have already been implicated in the pathophysiology of schizophrenia (Benes, 2000; Doheny et al., 2000; Gonzalez-Burgos and Lewis, 2008; Gonzalez-Burgos et al., 2010; Hashimoto et al., 2003; Lewis et al., 2005; Lewis et al., 2011; Lewis et al., 2008; Roopun et al., 2008). In line with previous studies reporting impaired GBR measures in unaffected siblings (Leicht et al., 2010a) and unaffected co-twins of schizophrenia patients (Hall et al., 2011b), the current findings suggest that abnormal early auditory gamma-band response may be a biomarker reflecting the clinical risk for the development of psychosis. However, we did not find abnormal gamma band responses to predict conversion to psychosis among CHR patients. While the lack of a converter vs. non-converter effect may indicate that abnormal gamma band responses reflect clinical vulnerability for psychosis, with other factors determining whether a psychotic disorder subsequently develops, larger sample sizes are needed to address this question definitively. Such large sample CHR studies are currently underway (e.g., Cannon et al., 2008).
References
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672–9. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E. A compound P300-40 Hz response of the cat hippocampus. Int J Neurosci. 1991;60:227–37. doi: 10.3109/00207459109167035. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Schmiedt-Fehr C, Mathes B, Zimmermann J, Brand A. Are oscillatory brain responses generally reduced in schizophrenia during long sustained attentional processing? Int J Psychophysiol. 2009;71:75–83. doi: 10.1016/j.ijpsycho.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35:95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–69. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Williams WJ, Gehring WJ. Decomposing ERP time-frequency energy using PCA. Clin Neurophysiol. 2005;116:1314–34. doi: 10.1016/j.clinph.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Blumenfeld LD, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG. Clin Neurophysiol. 2001;112:1650–9. doi: 10.1016/s1388-2457(01)00604-6. [DOI] [PubMed] [Google Scholar]
- Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I, Brinkmeyer J, Gaebel W, Maier W, Klosterkotter J, Brockhaus-Dumke A. Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011;69:959–66. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Kieffaber PD, Clementz BA, Johannesen JK, Shekhar A, O’Donnell BF, Hetrick WP. Event-related potential abnormalities in schizophrenia: a failure to “gate in” salient information? Schizophr Res. 2009;113:332–8. doi: 10.1016/j.schres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophr Res. 2008a;99:238–49. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkotter J, Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res. 2005;73:297–310. doi: 10.1016/j.schres.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, Klosterkoetter J, Ruhrmann S. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008b;64:376–84. doi: 10.1016/j.biopsych.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Cannon TD. Neurodevelopment and the transition from schizophrenia prodrome to schizophrenia: research imperatives. Biol Psychiatry. 2008;64:737–8. doi: 10.1016/j.biopsych.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–83. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–93. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK. Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport. 2003;14:683–6. doi: 10.1097/00001756-200304150-00005. [DOI] [PubMed] [Google Scholar]
- DeLisi LE. Regional brain volume change over the life-time course of schizophrenia. J Psychiatr Res. 1999;33:535–41. doi: 10.1016/s0022-3956(99)00028-x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Doheny HC, Faulkner HJ, Gruzelier JH, Baldeweg T, Whittington MA. Pathway-specific habituation of induced gamma oscillations in the hippocampal slice. Neuroreport. 2000;11:2629–33. doi: 10.1097/00001756-200008210-00005. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol Psychiatry. 1994;35:96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–74. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Edgar JC, Klook K, Siegel SJ. Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62:1504–18. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–61. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–44. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E, Williams LM, Haig A, Wright J, Meares RA. Symptom profile and “gamma” processing in schizophrenia. Cogn Neuropsychiatry. 2001;6:7–19. [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gurtubay IG, Alegre M, Labarga A, Malanda A, Artieda J. Gamma band responses to target and non-target auditory stimuli in humans. Neurosci Lett. 2004;367:6–9. doi: 10.1016/j.neulet.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Gurtubay IG, Alegre M, Labarga A, Malanda A, Iriarte J, Artieda J. Gamma band activity in an auditory oddball paradigm studied with the wavelet transform. Clin Neurophysiol. 2001;112:1219–28. doi: 10.1016/s1388-2457(01)00557-0. [DOI] [PubMed] [Google Scholar]
- Haig AR, Gordon E, Wright JJ, Meares RA, Bahramali H. Synchronous cortical gamma-band activity in task-relevant cognition. Neuroreport. 2000a;11:669–75. doi: 10.1097/00001756-200003200-00004. [DOI] [PubMed] [Google Scholar]
- Haig AR, Gordon E, De Pascalis V, Meares RA, Bahramali H, Harris A. Gamma activity in schizophrenia: evidence of impaired network binding? Clin Neurophysiol. 2000b;111:1461–8. doi: 10.1016/s1388-2457(00)00347-3. [DOI] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Salisbury DF, Levy DL. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophr Bull. 2011a;37:1187–99. doi: 10.1093/schbul/sbq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Toulopoulou T, Ettinger U, Bramon E, Murray RM, Salisbury DF. The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr Bull. 2011b;37:778–87. doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–26. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Hirano Y, Maekawa T, Obayashi C, Oribe N, Kuroki T, Kanba S, Onitsuka T. Abnormal neural oscillatory activity to speech sounds in schizophrenia: a magnetoencephalography study. J Neurosci. 2008;28:4897–903. doi: 10.1523/JNEUROSCI.5031-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW. Gamma/beta oscillation and sensory gating deficit in schizophrenia. Neuroreport. 2004a;15:155–9. doi: 10.1097/00001756-200401190-00030. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Buchanan RW, Thaker GK. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004b;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–66. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2011:1–13. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesen JK, Bodkins M, O’Donnell BF, Shekhar A, Hetrick WP. Perceptual anomalies in schizophrenia co-occur with selective impairments in the gamma frequency component of midlatency auditory ERPs. J Abnorm Psychol. 2008;117:106–18. doi: 10.1037/0021-843X.117.1.106. [DOI] [PubMed] [Google Scholar]
- Kay S, Opler L. The positive-negative dimension in schizophrenia: its validity and significance. Psychiatr Dev. 1987;5:79–103. [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Optimizing PCA methodology for ERP component identification and measurement: theoretical rationale and empirical evaluation. Clin Neurophysiol. 2003;114:2307–25. doi: 10.1016/s1388-2457(03)00241-4. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–9. doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–5. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Haig A, Gordon E. “Gamma (40 Hz) phase synchronicity” and symptom dimensions in schizophrenia. Cogn Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- Leicht G, Karch S, Karamatskos E, Giegling I, Moller HJ, Hegerl U, Pogarell O, Rujescu D, Mulert C. Alterations of the early auditory evoked gamma-band response in first-degree relatives of patients with schizophrenia: Hints to a new intermediate phenotype. J Psychiatr Res. 2010a doi: 10.1016/j.jpsychires.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Leicht G, Kirsch V, Giegling I, Karch S, Hantschk I, Moller HJ, Pogarell O, Hegerl U, Rujescu D, Mulert C. Reduced early auditory evoked gamma-band response in patients with schizophrenia. Biol Psychiatry. 2010b;67:224–31. doi: 10.1016/j.biopsych.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Lenz D, Fischer S, Schadow J, Bogerts B, Herrmann CS. Altered evoked gamma-band responses as a neurophysiological marker of schizophrenia? Int J Psychophysiol. 2011;79:25–31. doi: 10.1016/j.ijpsycho.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Fish KN, Arion D, Gonzalez-Burgos G. Perisomatic inhibition and cortical circuit dysfunction in schizophrenia. Curr Opin Neurobiol. 2011;21:866–72. doi: 10.1016/j.conb.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–93. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–40. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–57. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–5. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol. 2011;79:55–63. doi: 10.1016/j.ijpsycho.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C. Human auditory evoked gamma-band magnetic fields. Proc Natl Acad Sci U S A. 1991;88:8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Loewy RL, Stuart BK, Vinogradov S, Mathalon DH. Auditory Cortex Responsiveness During Talking and Listening: Early Illness Schizophrenia and Patients at Clinical High-Risk for Psychosis. Schizophr Bull. 2011a doi: 10.1093/schbul/sbr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VB, Ford JM, Roach BJ, Woods SW, McGlashan TH, Srihari VH, Loewy RL, Vinogradov S, Mathalon DH. Error monitoring dysfunction across the illness course of schizophrenia. J Abnorm Psychol. 2011b doi: 10.1037/a0025487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res. 1992;16:1078–89. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–26. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Ford JM, Hoffman RE, Mathalon DH. Converging evidence for gamma synchrony deficits in schizophrenia. Suppl Clin Neurophysiol. (this issue) doi: 10.1016/b978-0-7020-5307-8.00011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–73. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin KS, Kim JS, Kang DH, Koh Y, Choi JS, O’Donnell BF, Chung CK, Kwon JS. Pre-attentive auditory processing in ultra-high-risk for schizophrenia with magnetoencephalography. Biol Psychiatry. 2009;65:1071–8. doi: 10.1016/j.biopsych.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–25. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Slewa-Younan S, Gordon E, Harris AW, Haig AR, Brown KJ, Flor-Henry P, Williams LM. Sex differences in functional connectivity in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2004;161:1595–602. doi: 10.1176/appi.ajp.161.9.1595. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2008a;63:744–7. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry. 2008b;64:369–75. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–11. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–93. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–65. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30-70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. NeuroImage. 2008;42:1481–9. doi: 10.1016/j.neuroimage.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Naatanen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–13. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–75. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt O, Lieberman JA, Belger A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr Res. 2005;77:309–20. doi: 10.1016/j.schres.2005.04.024. [DOI] [PubMed] [Google Scholar]
- van Tricht MJ, Nieman DH, Koelman JH, van der Meer JN, Bour LJ, de Haan L, Linszen DH. Reduced parietal P300 amplitude is associated with an increased risk for a first psychotic episode. Biol Psychiatry. 2010;68:642–8. doi: 10.1016/j.biopsych.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–5. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Williams LM, Whitford TJ, Gordon E, Gomes L, Brown KJ, Harris AW. Neural synchrony in patients with a first episode of schizophrenia: tracking relations with grey matter and symptom profile. J Psychiatry Neurosci. 2009;34:21–9. [PMC free article] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. Am J Psychiatry. 2004;161:490–500. doi: 10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Yung AR, McGorry PD. The initial prodrome in psychosis: descriptive and qualitative aspects. Aust N Z J Psychiatry. 1996;30:587–99. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]


