Abstract
Background
Incretin–based therapies which include glucagon-like peptide-1 (GLP-1) receptor agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors are recommended by several practice guidelines as second-line agents for add-on therapy to metformin in patients with type 2 diabetes (T2DM) who do not achieve glycemic control with metformin plus lifestyle interventions alone. The purpose of this study is to perform a systematic review with meta-analysis of existing head to head studies to compare the efficacy and safety of GLP-1 analogues with DPP-4 inhibitors.
Methods
We performed a systematic review and meta-analysis of head-to-head studies to compare GLP-1 analogues with DPP-4 inhibitors in the management of type 2 diabetes. A random effects model was selected to perform the meta-analyses, results were expressed as weighted mean differences for continuous outcomes and relative risks for dichotomous outcomes, both with 95% confidence intervals, and with I2 values and P values as markers of heterogeneity.
Results
Four head-to-head randomized controlled studies with 1755 patients were included. Compared to sitagliptin, GLP-1 analogues are more effective in reducing HbA1C (weight mean difference −0.41%, 95% CI −0.51 to −0.31) and body weight (weight mean difference −1.55 kg, 95% CI −1.98 to −1.12). Conversely, GLP-1 analogues are associated with a higher incidence of gastrointestinal adverse events compared to sitagliptin: nausea (relative risk 3.14, 95% CI 2.15 to 4.59), vomiting (relative risk 2.60, 95% CI 1.48 to 4.56), diarrhea (relative risk 1.82, 95% CI 1.24 to 2.69), and constipation (relative risk 2.50, 95% CI 1.33 to 4.70).
Conclusions
The result of this meta-analysis demonstrates that compared to sitagliptin, GLP-1 analogues are more effective for glycemic control and weight loss, but have similar efficacy in reducing blood pressure and lipid parameters, however, GLP-1 analogues are associated with a higher incidence of gastrointestinal adverse events and a similar incidence of hypoglycemia compared to sitagliptin.
Introduction
In patients with T2DM, the incretin effect is reduced or in some cases, absent [1]. Targeting the incretin system has become an important therapeutic approach to lowering elevated plasma glucose levels in type 2 diabetes. Incretin hormones are intestinally derived peptides that play a role in the maintenance of glycemic control. There are two naturally occurring incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which are responsible for insulin release in a glucose-dependent manner, however, other physiological effects between these two hormones differ significantly in regards to glucagon suppression and effects on satiety and body weight. Both GLP-1 and GIP have a short half-life because of their rapid inactivation by DPP-4 enzyme. GLP-1 has multiple physiological effects that make it a more attractive candidate for treatment of T2DM. Administration of pharmacological levels of GLP-1 analogues resistant to DPP-4, not only increases insulin secretion while inhibiting glucagon release in a glucose-dependent fashion, but also delays gastric emptying and suppresses food intake [1]–[3].
Current GLP-1 analogues approved for use in the United States and the European Union include: exenatide twice daily [4], exenatide once weekly [5], liraglutide once daily [6], lixisenatide once daily (not approved in the U.S.) [7] and albiglutide once weekly [8], which are all delivered through subcutaneous injection and initial dose titration is required to improve gastrointestinal tolerance. The DPP-4 inhibitors reduce endogenous GLP-1 degradation, by inhibiting DPP-4 enzyme, thereby providing physiological levels of GLP-1 [9]. Currently available DPP-4 inhibitors include sitagliptin [10], saxagliptin [11], linagliptin [12], vildagliptin (not approved in the U.S.) [13], and alogliptin [14]. DPP-4 inhibitors are available orally and there is no need for dose titration when initiating treatment [15].
GLP-1 receptor agonists and DPP-4 inhibitors are included in the 2012 American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) and 2013 American Association of Clinical Endocrinologists (AACE) guidelines as second-line therapy for patients who do not achieve glycemic control with metformin therapy and lifestyle modifications alone. The National Institute for Health and Clinical Excellence (NICE) clinical guideline for T2DM recommends adding a DPP-4 inhibitor instead of a sulfonylurea as second line treatment to first line metformin when there is a considerable hypoglycemia risk or a sulfonylurea is contraindicated or not tolerated [16]. As both GLP-1 analogues and DPP-4 inhibitors are increasingly used in the management of T2DM (more often in combination therapy with metformin) [17], one important question that may arise is which one of the two drug classes is more favorable as a second-line treatment of T2DM [18], [19].
A meta-analysis of placebo-controlled clinical trials assessing the safety and efficacy of incretin-based therapy showed that the GLP-1 analogues are more effective in lowering blood glucose and weight loss, whereas sitagliptin lowers blood glucose levels to a lesser degree and are weight neutral [20]: the results showed that unadjusted HbA1c changes for exenatide, liraglutide, and sitagliptin are −0.75% (−0.83, −0.67), −1.03% (−1.16, −0.90), and −0.79% (−0.93, −0.65), respectively; and unadjusted weight changes for exenatide, liraglutide, and sitagliptin are −1.10 kg (−1.32, −0.88), −0.82 kg (−1.92, 0.27), and 0.60 kg (0.33, 0.87), respectively. However, a major potential pitfall of this meta-analysis was the use of unadjusted data which introduces confounding factors that may affect the end outcomes of the study [18]. Therefore, head-to-head comparative studies are needed to compare the efficacy and safety of GLP-1 analogues and DPP-4 inhibitors directly and accurately. Pinelli et al., performed a meta-analysis to compare long acting GLP-1 analogues with short acting exenatide and sitagliptin [21]; only one study included in this meta-analysis directly compared the 2 classes of incretin-based therapies. Other reviews in the literature have reported on the efficacy and safety of GLP-1 analogues and DPP-4 inhibitors [22]–[27]; however, to our know knowledge, no meta-analysis of head to head studies comparing the 2 classes of incretin therapy has been published. Thus we performed a systematic review with meta-analysis of existing head-to-head studies comparing the efficacy and safety of GLP-1 analogues with the DPP-4 inhibitors [28]–[37] to provide a more accurate and rigorous statistical analysis.
Methods
The main objective of this meta-analysis was to assess the efficacy and safety of GLP-1 analogues compared to the DPP-4 inhibitors in the management of patients with T2DM. Outcome measures included glycemic control, weight loss, changes in blood pressure, lipid profile, and common adverse events. We followed the methods specified in the Cochrane Handbook for Reviews on Interventions [38].
Data sources
Eligible trials were identified through electronic and manual searches. Electronic searches were performed in Medline, Embase, Cochrane Library, and Clinicaltrials.gov from its inception until January 2014. The search was limited to English articles. In the Medline database, we used the search strategy for “exenatide”, “liraglutide”, “lixisenatide” or “glucagon-like peptide-1”; and “dipeptidyl peptidase-4” or “sitagliptin” or “saxagliptin” or “linagliptin” or “alogliptin” or “vildagliptin”; and “Randomized Controlled Trial” or “RCT” or “random”. These terms were adjusted to fit the requirements specified in the remaining databases. Manual searches included scanning of reference lists in relevant papers, conference proceedings. Literature search was performed by two independent reviewers (ZG and TW).
Study selection
Electronic searching results were imported in a reference management software (Mendeley Desktop 1.10.1). After deleting the duplicate results, two reviewers (TW and ZG) independently screened all titles and abstracts and investigated full texts for eligible studies. Studies were included if they met the following inclusion criteria: (1) designed as randomized controlled trials; (2) head-to-head trials comparing GLP-1 analogues and DPP-4 inhibitors as monotherapy or add-on therapy to metformin; (3) Enrollment of patients with type 2 diabetes only; (4) duration of intervention of at least 12 weeks.
Data extraction
Two authors extracted data independently (TW, ZG) and any discrepancies were resolved by consensus. From each study we extracted study characteristics (author identification, year of publication, National Clinical Trial (NCT) number, name of the trial if applicable, study location, sample size for each group, duration of intervention); participants’ baseline characteristics (age, sex, race, duration of type 2 diabetes, HbA1C, body weight, body mass index (BMI)); and pre-specified outcomes of efficacy and safety. Our primary outcome was glycemic control as measured by the change in HbA1C from baseline to end of study. Secondary efficacy outcomes included changes in body weight, fasting and postprandial plasma glucose values, percentage of patients achieving a HbA1C <7%, blood pressure (systolic and diastolic) and lipid parameters (total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride levels). Safety outcomes extracted included withdrawal rates from any adverse events that documented incidence of hypoglycemia, nausea, vomiting, diarrhea, constipation, urinary tract infection (UTI), upper respiratory infection (URTI), nasopharyngitis, and headache based on their clinical relevance or relatively high frequency in previous studies [24], [26], [39]. An attempt to contact the investigators was made to clarify or request additional information if appropriate.
Quality assessment
Cochrane Collaboration’s risk of bias tool was used to assess risk of bias in randomization methods (allocation sequence generation and allocation concealment), blinding (of participants, personnel and investigators), completeness of outcome data, reporting of data and other biases [40]. We summarized the risk of bias of all six domains to produce an overall risk of bias. The following judgments were used: low risk, high risk, or unclear (either lack of information or uncertainty over the potential for bias). Two authors (TW, ZG) independently assessed the risk of bias and resolved disagreements by consensus with a third author (SZ) to resolve disagreements if necessary.
Data analysis
Meta-analysis was conducted with the Review Manager (Revman Version 5.2, Copenhagen, Denmark). The Cochran Q χ2 test and I2 statistic were used to assess heterogeneity among studies. As the observed effect estimates can vary across studies because of real differences in the treatment effect in each study as well as sampling variability, random effects model was selected. Results of the meta-analysis were expressed as weighted mean differences for continuous outcomes and relative risks for dichotomous outcomes, both with 95% confidence intervals, and with I2 values and P values as markers of heterogeneity. I2 values of 30–60% and over 75% represent moderate and considerable heterogeneity, respectively [41]. If a standard deviation was not provided in a study, this was calculated from the sample size and the standard error or the 95% confidence interval (CI); when calculation was not feasible, standard deviation was imputed from other studies [42]. Data for intention to treat (all participants randomized) or modified intention to treat (all randomized participants who received intervention and had at least one measurement after baseline) populations were used when these were available either in a published paper or trial registries (www.clinicaltrial.gov).
We performed subgroup analyses to examine different interventions (exenatide, liraglutide). The mean difference or relative risk was further evaluated by classifying each study into one of these categories. Initial sensitivity analyses included repeating all meta-analyses using fixed effect models. The results of these analyses were only reported if the conclusions differed. A sensitivity analyses was performed to evaluate the influence of each study in each main analysis through omitting one study at a time to assess whether the pooled estimates were excessively influenced by any single study. Publication bias was examined by Egger’s test if >10 studies were included in the analysis of the primary outcomes [43]. Meta-regression was performed to investigate the characteristics of different studies if >10 studies were included [40].
Results
Literature searches and study inclusion
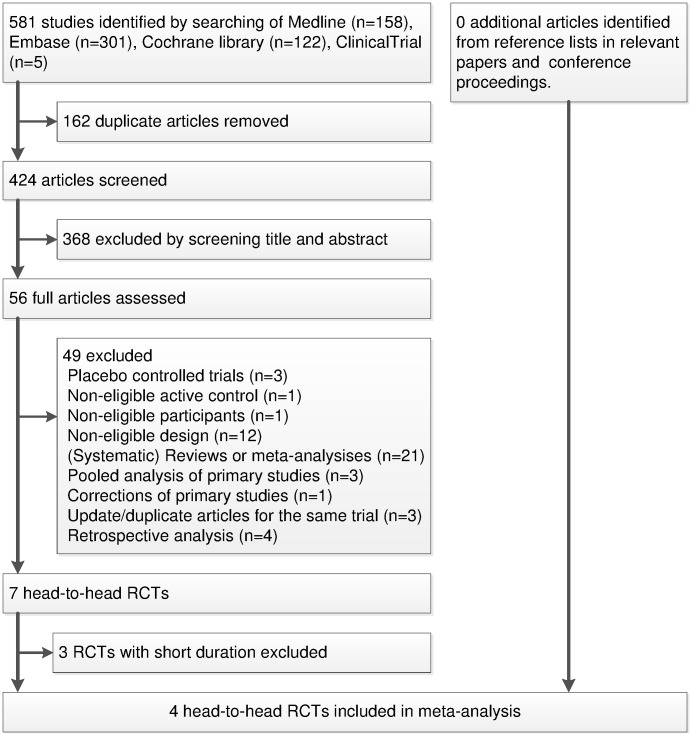
The electronic searches identified 581 potentially relevant articles. After excluding duplicates and studies that did not meet our inclusion criteria, 7 head to head RCTs were identified [28]–[30], [32]–[34], [36] and 4 RCTs were included in the meta-analysis [30], [32], [34], [36] (Figure 1). All 4 trials were published as full paper articles between 2010 and 2013 (Table 1). We did not obtain any eligible studies through manual searches. The patient characteristics at baseline were similar across trials. Mean body mass index ranged from 31.4 to 32.7 kg/m2 in the GLP-1 groups and 31.8 to 32.6 kg/m2 in the sitagliptin groups. Mean values of HbA1C at baseline ranged from 8.1% to 8.5% for GLP-1 analogues and 8.2% to 8.5% for sitagliptin groups, respectively. All 4 RCTs were multicentered (mean number of clinical sites 50) and multinational (most were done in US and Europe). Three RCTs were of short duration ranging from 12 to 26 weeks and one RCT had a 26-week main study phase [34] with a 26-week extension phase [35], thus the data for the main phase of the study was used for meta-analysis to minimize the heterogeneity. One trial intensified therapy by increasing the dose for GLP-1 group and adding glimepiride to sitagliptin groups after 12 weeks [32], so only data at 12 weeks were included for meta-analysis, and standard deviation of this trial was imputed from the other trial assessing liraglutide [34] with a reasonably high standard deviation [42].
Figure 1. Article selection diagram for meta-analysis.
Table 1. Characteristics of head to head RCT studies of GLP-1 analogues and Sitagliptin included in the meta-analysis.
| Source(NCT number) | Location | Study type | StudyDuration,(primarystudy +extension)weeks | Backgroundtherapy | Treatment | No. ofpatient | Woman(%) | White(%)I/C | BaselineHbA1Clevel (%)I/C | Age (years)I/C | Duration ofT2DM I/C | Weight(kg) I/C | BMI (kg/m2)I/C |
| Bergenstal 201030 (NCT00637273) | 72 hospitals andclinics in theUSA,India, andMexico | double-blind,double-dummyRCT | 26 | Metformin | I: Exenatide2 mg QW | I: 160 | I: 44 | I: 33 | I: 8.3 (1.0) | I: 52 (10) | I: 6 (5) | I: 89 (20) | I: 32 (5) |
| Metformin | C: Sitagliptin100 mg QD | C: 166 | C: 48 | C: 30 | C: 8.3 (1.1) | C: 52 (11) | C: 5 (4) | C: 87 (20) | C: 32 (5) | ||||
| Charbonel 201332 (NCT01296412) | 111 clinical sitesin 21 countries | Open-label RCT | 12a+14 | Metformin | I: Liraglutide1.2 mg QD | I: 327 | I: 45 | I: 84 | I: 8.1 (0.9) | I: 57.6 (10.8) | I: 7.6 (4.8) | I: 92.1 (20.4) | I: 32.7 (6.1) |
| Metformin | C: Sitgaliptin100 mg QD | C: 326 | C: 45 | C: 86 | C: 8.2 (1.1) | C: 56.9 (10.0) | C: 8.2 (6.2) | C: 91.0 (20.5) | C: 32.6 (5.9) | ||||
| Pratley 201034 (NCT00700817) | 83 clinical sitesin 14 countries | parallel-group,open-label RCT | 26b | Metformin | I1: Liraglutide1.2 mg QD | I1: 221 | I1: 48.4 | NR | I1: 8.4 (0.8) | I1: 55.9 (9.6) | I1: 6.0 (4.5) | NR | I1: 32.6 (5.2) |
| Metformin | I2: Liraglutide1.8 mg QD | I2: 218 | I2: 47.5 | NR | I2: 8.4 (0.7) | I2: 55.0 (9.1) | I2: 6.4 (5.4) | NR | I2: 33.31 (5.1) | ||||
| Metformin | C: Sitagliptin100 mg QD | C: 219 | C: 45.2 | NR | C: 8.5 (0.7) | C: 55.0 (9.0) | C: 6.3 (5.4) | NR | C: 32.6 (5.4) | ||||
| Russell-Jones 2012360 (NCT00676338) | 62 clinical sitesin 22 countries | Double-blindRCT | 26 | None | I: Exenatide2 mg QW | I: 248 | I: 44 | I: 68.1 | I: 8.5 (1.2) | I: 54 (11) | I: 2.7 (3.2) | I: 87.5 (18.9) | I: 31.4 (5.3) |
| None | C: Sitgaliptin100 mg QD | C: 163 | C: 42.3 | C: 69.3 | C: 8.5 (1.3) | C: 52 (11) | C: 2.7 (3.7) | C: 88.7 (18.7) | C: 31.8 (5.4) |
I, intervention; C, control; BMI, body mass index; FPG, fasting plasma glucose; EXE, exenatide; SIT, sitagliptin; GLI, glimepiride, QW, every week, QD, every day; NR, not reported.
*the number is obtained by contacting the author;
Charbonel et al 2013 [32], only data from 1st 12 weeks were used to because after 12 weeks, patients on sitagliptin with an HbA1c ≥7.0% and FFG >6.1 mmol/l had glimepiride added to their treatment regimen for an additional 14 weeks, and patients on liraglutide 1.2 mg/d with an HbA1c ≥7.0% had the liraglutide dose, as per label, titrated up to 1.8 mg/day.
Data of week 26 is obtained from Prateley 2010 [34].
All 4 trials directly compared GLP-1 analogues groups with sitagliptin. Oral sitagliptin 100 mg daily was the only dose assessed in the control groups. The GLP-1 analogues were given as once weekly exenatide (2 mg) in 2 RCTs, and once daily liraglutide 1.2 mg in one RCT (Table 1); another RCT compared liraglutide 1.2 and 1.8 mg/day with sitagliptin, respectively [34], the outcome data of liraglutide 1.2 mg was used for major meta-analysis to minimize heterogeneity; we also repeated analysis with the data of liraglutide 1.8 mg and reported the results if they differed from the major meta-analysis. The efficacy and safety data were shown in Table S1 and Table S2, respectively.
Quality of bias control
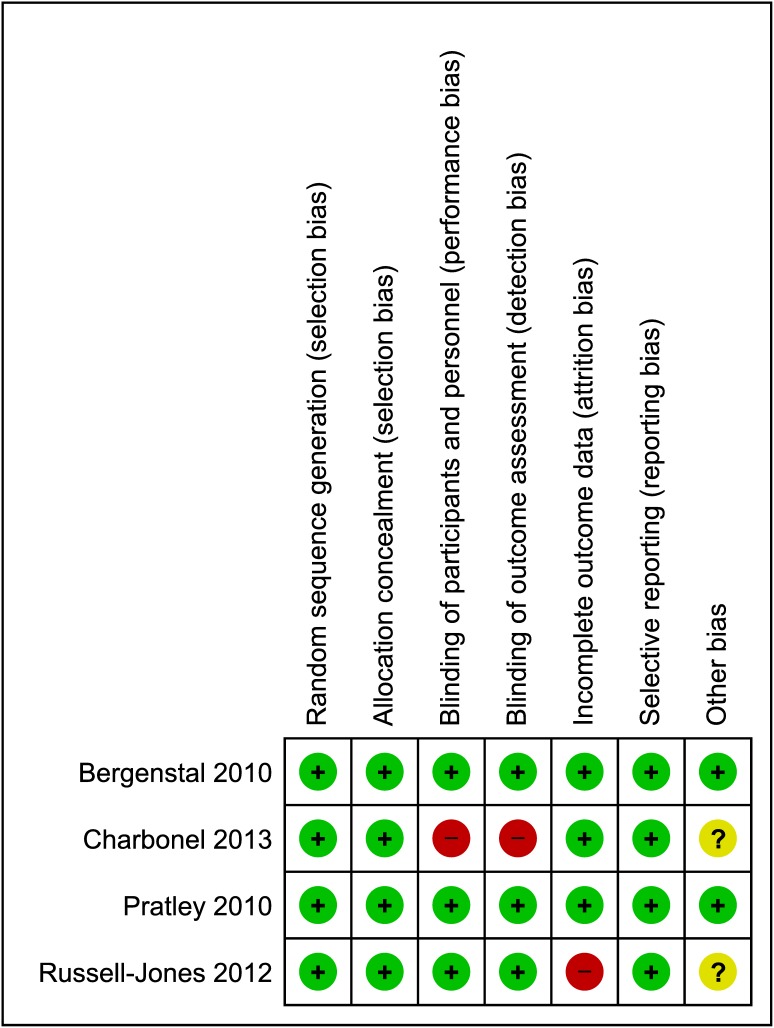
The randomization methods were described as adequate in 4 trials (Figure 2). None of the trials found differences in the baseline characteristics of participants between the GLP-1 analogue and sitagliptin groups. All 4 trials described random sequence generation and allocation concealment, reported clinically relevant outcome measures, and undertook sample size calculations [30], [32], [34], [36]. Three trials provided a clear description of losses to follow-up and accounted for patients with missing data in the analyses [30], [32], [34]. None of the included trials were terminated prematurely.
Figure 2. Risk of bias summary.
+, Low risk of bias; − high risk of bias; ?, unknown risk of bias. Risk of bias assessment for random sequence generation and allocation concealment is performed at the study level. Risk of bias assessment for blinding of participants and personnel, incomplete outcome data, selective reporting, and overall risk of bias are for the primary outcome (change in HbA1c).
Glycemic control
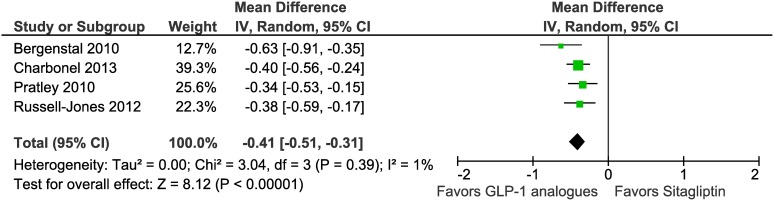
All 4 trials reported change in HbA1C from baseline to end of study period. We performed a random effects meta-analysis that included 915 participants assigned to GLP-1 analogue groups and 840 patients assigned to the sitagliptin groups (Table 2). The weighted mean reduction in HbA1C was larger for patients in GLP-1 analogues groups than for those in the sitagliptin groups (mean difference –0.41%, 95% CI –0.51 to –0.31) (Figure 3,Table 2). We found no evidence of significant heterogeneity in the analysis (I2 = 1%, P = 0.39). Subgroup analyses showed an HbA1C reduction in trials assessing exenatide (–0.49%, –0.73 to −0.25, I2 = 50%, P = 0.16) and liraglutide (–0.38%, –0.50 to –0.26, I2 = 0%, P = 0.64). The proportion of participants who achieved the HbA1C target (<7%) was higher in the GLP-1 analogues groups than in sitagliptin groups (relative risk 2.63, 95% CI 2.05 to 3.37, I2 = 0%, P = 0.45) (Table 2). The corresponding number needed to treat using the pooled odds ratio from the meta-analysis would be 5 (95% CI 4 to 6) [44].
Table 2. Summary of Meta-analyses of Outcomes in Patients with Type 2 Diabetes treated With GLP-1 analogues vs Sitagliptin.
| Outcome | No. ofStudiesContributingData | Risk Ratio(95% CI),GLP-1analogues vsSitagliptin | Weighted MeanDifference(95% CI) inChange FromBaseline, GLP-1analogues vsSitagliptin | I2Heterogeneity,% | No. ofParticipantsWith DataAnalyzed forGLP-1analogues groups | No. of ParticipantsWith DataAnalyzed forSitagliptin groups |
| HbA1C | 4 | −0.41 (−0.51, −0.31) | 0 | 915 | 840 | |
| Percentage ofpatientsachieved HbA1c<7% | 3 | 2.63 (2.05, 3.37) | 0 | 607 | 528 | |
| Fasting plasmaglucose level | 4 | −1.10 (–1.31, −0.89) | 0 | 890 | 817 | |
| Weight loss | 3 | −1.55 (−1.98, −1.12) | 0 | 590 | 521 | |
| Systolic bloodpressure | 4 | −0.83 (−3.00, 1.34) | 71 | 588 | 517 | |
| Diastolic bloodpressure | 4 | 0.07 (−1.29, 1.44) | 57 | 629 | 559 | |
| Total cholesterol | 3 | −0.10 (−0.23, 0.02) | 33 | 539 | 468 | |
| HDL | 3 | −0.01 (−0.03, 0.01) | 0 | 539 | 468 | |
| LDL | 1 | −0.05 (−0.19, 0.09) | N/A | 194 | 200 | |
| Triglyceride | 1 | 0.21 (−0.05, 0.47) | N/A | 191 | 198 |
N/A, not applicable; CI, confidence interval.
Figure 3. Meta-analysis of change in HbA1C (%) in included trials using random effects model.
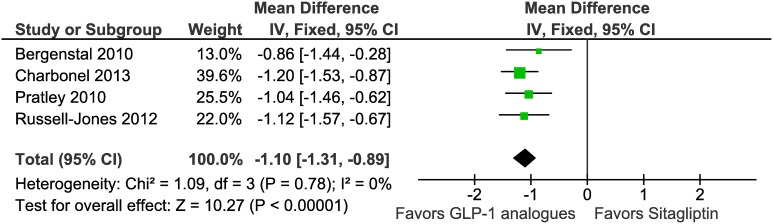
All 4 trials reported fasting plasma glucose (FPG). Random effects meta-analysis showed that there is a significant difference for reduction in FPG between patients in GLP-1 analogues groups and those in the sitagliptin groups (mean difference –1.10 mmol/L, 95% CI –1.31 to −0.89, I2 = 0%, P = 0.78) (Figure 4, Table 2). Subgroup analyses showed significant difference in FPG reduction between the exenatide group (−1.02 mmol/L, −1.38 to 0.67, I2 = 0%, P = 0.49) and sitagliptin group, and a significant reduction in liraglutide group compared to sitagliptin groups (–1.14 mmol/L, –1.40 to –0.88, I2 = 0%, P = 0.56) as well. None of the studies reported postprandial plasma glucose.
Figure 4. Meta-analysis of change in Fasting Plasma Glucose (mmol/L) in included trials using random effects model.
Body weight
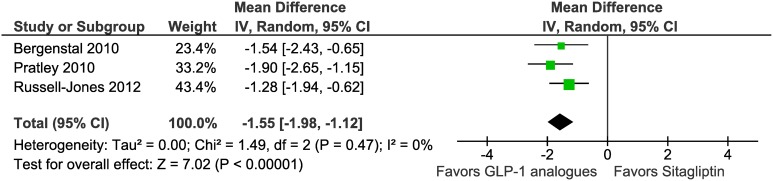
Three trials reported weight loss. We did a random effects meta-analysis including 590 participants assigned to GLP-1 analogues groups and 521 assigned to the sitagliptin groups. The intervention groups in the analysis received exenatide or liraglutide (Table 1). The weighted mean change in body weight was larger for patients in GLP-1 analogues groups than for those in the sitagliptin groups (mean difference –1.55 kg, 95% CI –1.98 to –1.12, I2 = 0%, P = 0.47) (Figure 5, Table 2). Subgroup analyses showed a weight reduction in trials assessing exenatide (–1.37 kg, –1.90 to –0.84, I2 = 0%, P = 0.65).
Figure 5. Meta-analysis of change in body weight (kg) of included trials using random effects model.
Blood pressure and Lipid
Random effects meta-analysis didn’t show significant difference in reduction in blood pressure or lipid profile between GLP-1 analogues groups and Sitagliptin groups: mean difference for systolic blood pressure and diastolic blood pressure was –0.91 mmHg (–3.63 to 1.82) and −0.34 mmHg (−1.66 to 0.98) respectively (Table 2); and mean difference for total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL), and triglyceride were –0.10 mmol/L (–0.23 to 0.02), −0.01 mmol/L (−0.03 to 0.01), −0.05 mmol/L (−0.19 to 0.09) and 0.21 mmol/L (−0.05 to 0.47), respectively (Table 2). A repeat of meta-analysis using a high dose of a GLP-1 analogue (liraglutide 1.8 mg) showed a significant reduction in total cholesterol compared to sitagliptin: mean difference –0.16 mmol/L (–0.25 to −0.06, I2 = 0%, P = 0.63). Subgroup analyses showed that compared to sitagliptin, the exenatide group had a significant reduction in total cholesterol (–0.16 mmol/L, –0.29 to −0.03, I2 = 0%, P = 0.34) and no significant increase in HDL cholesterol (–0.02 mmol/L, –0.05 to 0.02, I2 = 0%, P = 0.68).
Adverse events
Major hypoglycemia (Table S2) was reported in only 2 patients receiving GLP-1 analogues (liraglutide 1.2 mg QD); and there was no difference in reported minor to moderate hypoglycemia (Table S2) between GLP-1 analogues and sitagliptin (relative risk 1.35, 95% CI 0.71–2.58) (Table 3). Treatment with sitagliptin resulted in lower discontinuation rates (relative risk 2.89, 95% CI 1.42–5.87); nausea, vomiting, diarrhea, and constipation were also more common in patients receiving GLP-1 analogues than sitagliptin (Table 3). No difference in the incidence of urinary tract infection (UTI), upper respiratory tract infection (URTI), nasopharyngitis, and headache was evident between GLP-1 analogues and sitagliptin. Overall, sitagliptin were better tolerated, with lower absolute rates of adverse effects. Table 3 summarizes the findings of the main analyses for safety outcomes.
Table 3. Summary of meta-analyses of adverse events in patients with type 2 diabetes treated with GLP-1 analogues vs Sitagliptin.
| Adverse event | No. of studiescontributing data | Relativerisk (95% CI) | I2Heterogeneity, % | Comparatorgroup (Event/Total) | |
| GLP-1analogues | Sitagliptin | ||||
| Withdrawal | 3 | 2.89 (1.42 to 5.87) | 0 | 31/629 | 10/548 |
| Hypoglycemia | 4 | 1.35 (0.71 to 2.58) | 16 | 33/956 | 22/874 |
| Nausea | 3 | 3.14 (2.15 to 4.59) | 1 | 112/629 | 32/548 |
| Vomiting | 3 | 2.60 (1.48 to 4.56) | 0 | 47/629 | 16/548 |
| Diarrhea | 3 | 1.82 (1.24 to 2.69) | 0 | 72/629 | 35/548 |
| Constipation | 3 | 2.50 (1.33 to 4.70) | 0 | 40/629 | 13/548 |
| Urinary tract infection | 1 | 1.15 (0.48 to 2.76) | N/A | 10/160 | 9/166 |
| Upper respiratory tract infection | 1 | 0.41 (0.17 to 1.04) | N/A | 6/160 | 15/166 |
| Nasopharyngitis | 2 | 0.83 (0.57 to 1.22) | 0 | 46/469 | 47/382 |
| Headache | 3 | 0.87 (0.61 to 1.23) | 0 | 56/629 | 57/548 |
N/A, not applicable; CI, confidence interval.
Discussion
Explanation for findings
In this meta-analysis we assessed the efficacy and safety of incretin therapies using data from 4 trials comparing sitagliptin with GLP-1 analogue. The results demonstrate that compared to sitagliptin, the GLP-1 analogues, exenatide and liraglutide, are more efficacious in reducing HbA1C and body weight with similar efficacy in reducing blood pressure and changes in lipid parameters compared to sitagliptin. In terms of adverse effects, sitagliptin is better tolerated and has a lower incidence of gastrointestinal adverse events compared to GLP-1 analogues. Compared to GLP-1 analogues, sitagliptin treatment did not seem to increase the risk of hypoglycemia, although a previous meta-analysis showed that GLP-1 analogues did not similarly increase the risk of hypoglycemia [24]. In addition, sitagliptin did not appear to increase the risk of developing UTI, URTI, nasopharyngitis, and headache. Most trials lasted less than 26 weeks, limiting our assessment of long-term efficacy and safety. Serious or rare adverse events such as pancreatitis are not addressed in this meta-analysis because they are hard to detect from RCTs with a relatively small sample size. The included trials did not provide enough data to compare GLP-1 analogues and sitagliptin regarding major cardiovascular events. Only ongoing prospective head to head clinical trials specifically designed to study the effects of cardiovascular events will provide further information in this respect.
Based upon our study, GLP-1 analogues demonstrate superiority in clinical efficacy (−0.41%) and weight loss (−1.55 kg) but have a higher incidence of gastrointestinal events and require delivery by subcutaneous injection. On the other hand, sitagliptin is less efficacious but has fewer gastrointestinal side effects and is available by oral administration [18]. Incretin therapies will play an increasing role in management of patients with T2DM as add-on agents to metformin therapy or as recommended options for three drug combinations that includes basal insulin. Sitagliptin might be considered as a more favorable option for early intervention in T2DM management as the initial add on therapy to metformin in patients whose glycemic control is closer to target goals and GLP-1 analogues might be preferred in over-weight or obese patients who require better glycemic control.
Assessment of quality of included studies
Inadequate randomization and attrition bias could result in overestimating effects of an intervention. The study by Charbonel et al., did not blind the participants or account for the intention to treat population in their results and analyses [32]. These aspects weakened the internal validity of our findings. Since only trials that used clinically relevant doses given for clinically relevant treatment periods were included in our meta-analysis, the results can be extrapolated to clinical practice.
Strengths and Limitations
The strengths of this meta-analysis are related to the incorporation of direct evidence from recently published head to head trials, the variety of outcomes assessed, and the investigation of plausible clauses of heterogeneity by sensitivity analyses. However, some limitations should also be recognized.
First, we only included 4 head to head trials based on our rigorous inclusion criteria. Three head to head studies were excluded due to their short study period of 2, 4, and 8 weeks, respectively [28], [29], [33]. Furthermore, intervention effects of different doses and formulations were not examined in subgroup analysis due to the paucity of available data, and our conclusions regarding exenatide or liraglutide interventions compared to sitagliptin in subgroup analysis were not robust enough because of the small number of relevant trials. Secondly, there was considerable variation in the risk of bias across the included studies, although exclusion of trials at high risk of bias in a sensitivity analysis did not alter the results of the main analysis. Third, long term efficacy and safety of GLP-1 analogues and sitagliptin was not compared; and rare, serious adverse events such as pancreatitis or renal failure [22] were not evaluated. Lastly, head-to-head studies of DPP-4 inhibitors in the literature included primarily sitagliptin as the comparator. Other DPP4 inhibitors were not included in this meta-analysis.
Conclusion
This meta-analysis included 4 head-to-head studies comparing the short-term efficacy and safety of GLP-1 analogues and sitagliptin. The results demonstrate that compared to sitagliptin, GLP-1 analogues are more efficacious for glycemic control and weight loss, but not better in reducing blood pressure and lipid profile; and GLP-1 analogues have a higher incidence of gastrointestinal adverse events and similar hypoglycemic events compared to sitagliptin. For less common adverse events, GLP-1 analogues and sitagliptin have a similar incidence of headache, UTI, URTI, and nasopharyngitis. If weight loss is not a particular concern and only a small decrease in A1C is required, a DPP-4 inhibitor may be better choice. Future long-term head-to-head RCTs assessing the GLP-1 analogues versus sitagliptin should be designed to provide a definitive answer regarding the place of the two classes of agents in the treatment algorithm.
Supporting Information
PRISMA checklist.
(DOC)
Summary of efficacy from included studies.
(DOCX)
Summary of adverse events from included studies.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the paper and its Supporting Information files.
Funding Statement
These authors have no support or funding to report.
References
- 1. Nauck M, Stöckmann F, Ebert R, Creutzfeldt W (1986) Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 29 (1): 46–52. [DOI] [PubMed] [Google Scholar]
- 2. De Fronzo RA (2009) Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58 (4): 773–95 10.2337/db09-9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696–705 10.1016/S0140-6736(06)69705-5 [DOI] [PubMed] [Google Scholar]
- 4.Byetta® (2011) [package insert] San Diego, CA: Amylin Pharmaceuticals.
- 5.Bydureon® (2012) [package insert] San Diego, CA: Amylin Pharmaceuticals.
- 6.Victoza® (2010) [package insert] Princeton, NJ: Novo Nordisk.
- 7.European Medicines Agency lixisenatide: summary of product characteristics. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf. Assessed 2014 April 15.
- 8.FDA news release: FDA approves Tanzeum to treat type 2 diabetes. Available: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm393289.htm. Assessed 2014 April 15.
- 9. Deacon CF, Johnsen AH, Holst JJ (1995) Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 80(3): 952–957. [DOI] [PubMed] [Google Scholar]
- 10.Januvia (2007) [package insert] Whitehouse Station, NJ: Merck and Co, Inc.
- 11.Onglyza (2011) [package insert] Princeton, NJ; Bristol-Myers Squibb Company.
- 12.Tradjenta (2011) [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.
- 13.European Medicines Agency Vildagliptin: summary of product characteristics. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf. Assessed 2014 April 15.
- 14.Nesina (2013) [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc.
- 15. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35(6): 1364–1379 10.2337/dc12-0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Clinical Excellence. Type 2 diabetes: newer agents (2009) Available: www.nice.org.uk/nicemedia/live/12165/44318/44318.pdf.Assessed 2014 April 15. [PubMed]
- 17. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, et al. (2013) AACE comprehensive diabetes management algorithm. Endocr Pract 19 (2): 327–336. [DOI] [PubMed] [Google Scholar]
- 18. Scheen AJ (2012) Dipeptidylpeptidase-4 (DPP-4) inhibitors are favourable to glucagon-like peptide-1 (GLP-1) receptor agonists: yes. Eur J Intern Med 23(2): 126–131 10.1016/j.ejim.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 19. Madsbad S (2012) Dipeptidyl peptidase-4 (DPP-4) inhibitors are favourable to glucagon-like peptide-1 (GLP-1) agonists: no. Eur J Intern Med. 23(2): 132–136 10.1016/j.ejim.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 20. Fakhoury WK, Lereun C, Wright D (2010) A meta-analysis of placebo-controlled clinical trials assessing the efficacy and safety of incretin-based medications in patients with type 2 diabetes. Pharmacology 86: 44–57 10.1159/000314690 [DOI] [PubMed] [Google Scholar]
- 21. Pinelli NR, Kathryn MH (2011) Efficacy and safety of long-acting glucagon-like peptide-1 receptor agonists compared with exenatide twice daily and sitagliptin in type 2 diabetes mellitus: a systematic review and meta-analysis. Ann Pharmacother 45 (7–8): 850–860 10.1345/aph.1Q024 [DOI] [PubMed] [Google Scholar]
- 22. Brunton S (2014) GLP-1 receptor agonists vs. DPP-4 inhibitors for type 2 diabetes: is one approach more successful or preferable than the other? Int J Clin Pract 68(5): 557–567 10.1111/ijcp.12361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A (2012) Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ 344: e1369 10.1136/bmj.e1369 [DOI] [PubMed] [Google Scholar]
- 24. Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL (2012) Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344: d7771 10.1136/bmj.d7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monami M, Iacomelli I, Marchionni N, Mannucci E (2010) Dipeptydil peptidase-4 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc 20: 224–35 10.1016/j.numecd.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 26. Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL (2008) Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev 2: CD006739 10.1002/14651858.CD006739.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Esposito K, Cozzolino D, Bellastella G, Maiorino MI, Chiodini P, et al. (2011) Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes Metab 13: 594–603 10.1111/j.1463-1326.2011.01380.x [DOI] [PubMed] [Google Scholar]
- 28. Arnolds S, Dellweg S, Clair J, Dain MP, Nauck MA, et al. (2010) Further Improvement in Postprandial Glucose Control With Addition of Exenatide or Sitagliptin to Combination Therapy With Insulin Glargine and Metformin A proof-of-concept study. Diabetes Care 33(7): 1509–1515 10.2337/dc09-2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berg JK, Shenouda SK, Heilmann CR, Gray AL, Holcombe JH (2011) Effects of exenatide twice daily versus sitagliptin on 24-h glucose, glucoregulatory and hormonal measures: a randomized, double-blind, crossover study. Diabetes Obes Metab 13(11): 982–989 10.1111/j.1463-1326.2011.01428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bergenstal RM, Wysham C, MacConell L, Malloy J, Walsh B, et al. (2010) Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. The Lancet 376(9739): 431–439 10.1016/S0140-6736(10)60590-9 [DOI] [PubMed] [Google Scholar]
- 31. Best JH, Rubin RR, Peyrot M, Li Y, Yan P, et al. (2011) Weight-related quality of life, health utility, psychological well-being, and satisfaction with exenatide once weekly compared with sitagliptin or pioglitazone after 26 weeks of treatment. Diabetes Care 34(2): 314–319 10.2337/dc10-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charbonnel B, Steinberg H, Eymard E, Xu L, Thakkar P, et al. (2013) Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia 56(7): 1503–1511 10.1007/s00125-013-2905-1 [DOI] [PubMed] [Google Scholar]
- 33. DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, et al. (2008) Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 24(10): 2943–2952 10.1185/03007990802418851 [DOI] [PubMed] [Google Scholar]
- 34. Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, et al. (2010) Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. The Lancet 375(9724): 1447–1456 10.1016/S0140-6736(10)60307-8 [DOI] [PubMed] [Google Scholar]
- 35. Pratley R, Nauck M, Bailey T, Montanya E, Cuddihy R, et al. (2011) One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract 65(4): 397–407 10.1111/j.1742-1241.2011.02656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russell-Jones D, Cuddihy RM, Hanefeld M, Kumar A, González JG, et al. (2012) Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naïve patients with Type 2 diabetes (DURATION-4) a 26-week double-blind study. Diabetes Care 35(2): 252–258 10.2337/dc11-1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wysham C, Bergenstal R, Malloy J, Yan P, Walsh B, et al. (2011) DURATION-2: efficacy and safety of switching from maximum daily sitagliptin or pioglitazone to once-weekly exenatide. Diabet Med 28(6): 705–714 10.2337/dc13-2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins JP, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 Available: http://handbook.cochrane.org. Accessed 2014 April 15.
- 39. Amori RE, Lau J, Pittas AG (2007) Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298: 194–206 10.1001/jama.298.2.194 [DOI] [PubMed] [Google Scholar]
- 40.Sterne J, Egger M, Moher D (2011) Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Ch10_Reporting.pdf. Accessed 15 April 2014.
- 41.Deeks J, Higgins J, Altman D (2011) Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Ch09_Analysing.pdf. Accessed 15 April 2014.
- 42.Higgins J, Deeks J, Altman D (2011) Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Ch16_Specialstatistics.pdf. Accessed 15 April 2014.
- 43. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cates CJ (2002) Simpson’s paradox and calculation of number needed to treat from meta-analysis. BMC Med Res Methodol 2: 1 10.1186/1471-2288-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist.
(DOC)
Summary of efficacy from included studies.
(DOCX)
Summary of adverse events from included studies.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are included within the paper and its Supporting Information files.