Abstract
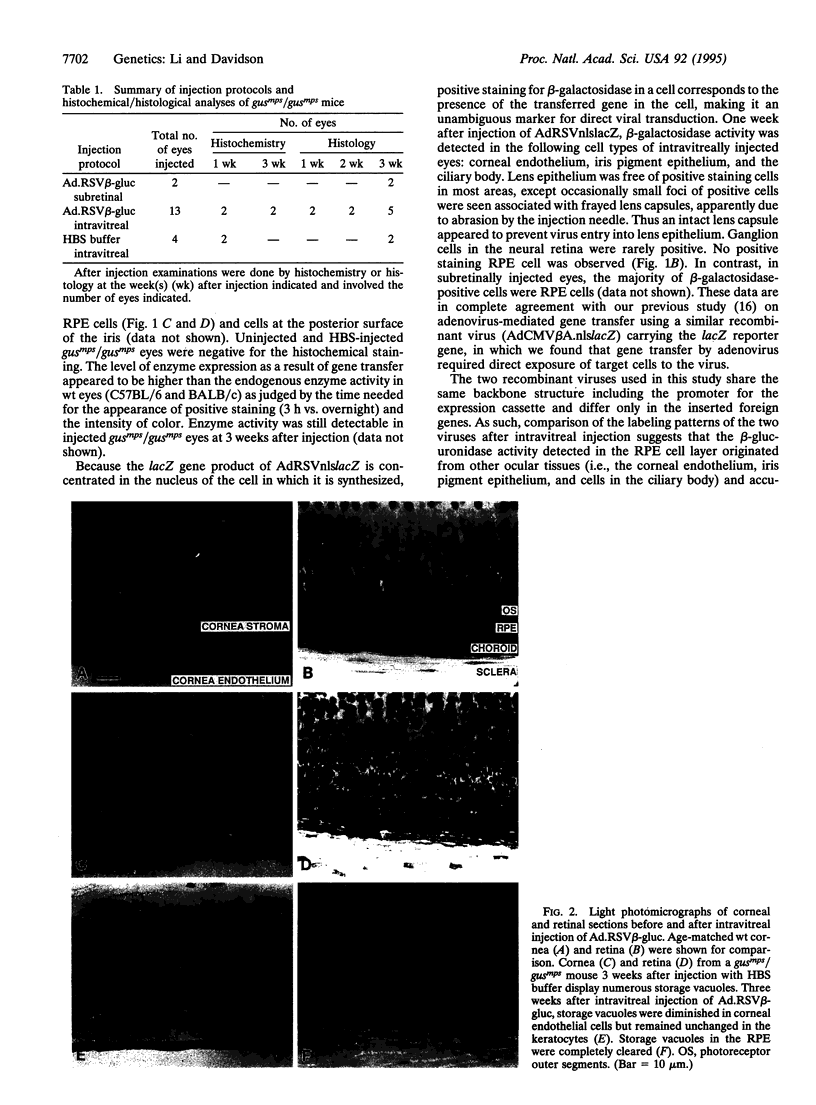
We have studied the use of adenovirus-mediated gene transfer to reverse the pathologic changes of lysosomal storage disease caused by beta-glucuronidase deficiency in the eyes of mice with mucopolysaccharidosis VII. A recombinant adenovirus carrying the human beta-glucuronidase cDNA coding region under the control of a non-tissue-specific promoter was injected intravitreally or subretinally into the eyes of mice with mucopolysaccharidosis VII. At 1-3 weeks after injection, the treated and control eyes were examined histochemically for beta-glucuronidase expression and histologically for phenotypic correction of the lysosomal storage defect. Enzymatic expression was detected 1-3 weeks after injection. Storage vacuoles in the retinal pigment epithelium (RPE) were still present 1 week after gene transfer but were reduced to undetectable levels by 3 weeks in both intravitreally and subretinally injected eyes. There was minimal evidence of ocular pathology associated with the viral injection. These data indicate that adenovirus-mediated gene transfer to the eye may provide for adjunctive therapy for lysosomal storage diseases affecting the RPE in conjunction with enzyme replacement and/or gene therapies for correction of systemic disease manifestations. The data also support the view that recombinant adenovirus may be useful as a gene therapy vector for retinal degenerations that result from a primary genetic defect in the RPE cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akli S., Caillaud C., Vigne E., Stratford-Perricaudet L. D., Poenaru L., Perricaudet M., Kahn A., Peschanski M. R. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993 Mar;3(3):224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- Bennett J., Wilson J., Sun D., Forbes B., Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2535–2542. [PubMed] [Google Scholar]
- Birkenmeier E. H., Barker J. E., Vogler C. A., Kyle J. W., Sly W. S., Gwynn B., Levy B., Pegors C. Increased life span and correction of metabolic defects in murine mucopolysaccharidosis type VII after syngeneic bone marrow transplantation. Blood. 1991 Dec 1;78(11):3081–3092. [PubMed] [Google Scholar]
- Birkenmeier E. H. Correction of murine mucopolysaccharidosis type VII (MPS VII) by bone marrow transplantation and gene transfer therapy. Hum Gene Ther. 1991 Summer;2(2):113–113. doi: 10.1089/hum.1991.2.2-113. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Davisson M. T., Beamer W. G., Ganschow R. E., Vogler C. A., Gwynn B., Lyford K. A., Maltais L. M., Wawrzyniak C. J. Murine mucopolysaccharidosis type VII. Characterization of a mouse with beta-glucuronidase deficiency. J Clin Invest. 1989 Apr;83(4):1258–1266. doi: 10.1172/JCI114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B. L., Allen E. D., Kozarsky K. F., Wilson J. M., Roessler B. J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993 Mar;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Davidson B. L., Doran S. E., Shewach D. S., Latta J. M., Hartman J. W., Roessler B. J. Expression of Escherichia coli beta-galactosidase and rat HPRT in the CNS of Macaca mulatta following adenoviral mediated gene transfer. Exp Neurol. 1994 Feb;125(2):258–267. doi: 10.1006/exnr.1994.1028. [DOI] [PubMed] [Google Scholar]
- Dorey C. K., Wu G., Ebenstein D., Garsd A., Weiter J. J. Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci. 1989 Aug;30(8):1691–1699. [PubMed] [Google Scholar]
- Engelhardt J. F., Litzky L., Wilson J. M. Prolonged transgene expression in cotton rat lung with recombinant adenoviruses defective in E2a. Hum Gene Ther. 1994 Oct;5(10):1217–1229. doi: 10.1089/hum.1994.5.10-1217. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Ye X., Doranz B., Wilson J. M. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haskins M. E., Desnick R. J., DiFerrante N., Jezyk P. F., Patterson D. F. Beta-glucuronidase deficiency in a dog: a model of human mucopolysaccharidosis VII. Pediatr Res. 1984 Oct;18(10):980–984. doi: 10.1203/00006450-198410000-00014. [DOI] [PubMed] [Google Scholar]
- Hoogerbrugge P. M., Poorthuis B. J., Mulder A. H., Wagemaker G., Dooren L. J., Vossen J. M., van Bekkum D. W. Correction of lysosomal enzyme deficiency in various organs of beta-glucuronidase-deficient mice by allogeneic bone marrow transplantation. Transplantation. 1987 May;43(5):609–614. doi: 10.1097/00007890-198705000-00001. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarsky K. F., Wilson J. M. Gene therapy: adenovirus vectors. Curr Opin Genet Dev. 1993 Jun;3(3):499–503. doi: 10.1016/0959-437x(93)90126-a. [DOI] [PubMed] [Google Scholar]
- Kyle J. W., Birkenmeier E. H., Gwynn B., Vogler C., Hoppe P. C., Hoffmann J. W., Sly W. S. Correction of murine mucopolysaccharidosis VII by a human beta-glucuronidase transgene. Proc Natl Acad Sci U S A. 1990 May;87(10):3914–3918. doi: 10.1073/pnas.87.10.3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus H. S., Sly W. S., Kyle J. W., Hageman G. S. Photoreceptor degeneration and altered distribution of interphotoreceptor matrix proteoglycans in the mucopolysaccharidosis VII mouse. Exp Eye Res. 1993 May;56(5):531–541. doi: 10.1006/exer.1993.1067. [DOI] [PubMed] [Google Scholar]
- Li T., Adamian M., Roof D. J., Berson E. L., Dryja T. P., Roessler B. J., Davidson B. L. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest Ophthalmol Vis Sci. 1994 Apr;35(5):2543–2549. [PubMed] [Google Scholar]
- Moullier P., Bohl D., Heard J. M., Danos O. Correction of lysosomal storage in the liver and spleen of MPS VII mice by implantation of genetically modified skin fibroblasts. Nat Genet. 1993 Jun;4(2):154–159. doi: 10.1038/ng0693-154. [DOI] [PubMed] [Google Scholar]
- Roessler B. J., Davidson B. L. Direct plasmid mediated transfection of adult murine brain cells in vivo using cationic liposomes. Neurosci Lett. 1994 Feb 14;167(1-2):5–10. doi: 10.1016/0304-3940(94)91015-4. [DOI] [PubMed] [Google Scholar]
- Sands M. S., Birkenmeier E. H. A single-base-pair deletion in the beta-glucuronidase gene accounts for the phenotype of murine mucopolysaccharidosis type VII. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6567–6571. doi: 10.1073/pnas.90.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewach D. S., Zerbe L. K., Hughes T. L., Roessler B. J., Breakefield X. O., Davidson B. L. Enhanced cytotoxicity of antiviral drugs mediated by adenovirus directed transfer of the herpes simplex virus thymidine kinase gene in rat glioma cells. Cancer Gene Ther. 1994 Jun;1(2):107–112. [PubMed] [Google Scholar]
- Sly W. S., Quinton B. A., McAlister W. H., Rimoin D. L. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973 Feb;82(2):249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- Stramm L. E., Wolfe J. H., Schuchman E. H., Haskins M. E., Patterson D. F., Aguirre G. D. Beta-glucuronidase mediated pathway essential for retinal pigment epithelial degradation of glycosaminoglycans. Disease expression and in vitro disease correction using retroviral mediated cDNA transfer. Exp Eye Res. 1990 May;50(5):521–532. doi: 10.1016/0014-4835(90)90041-r. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Gönczöl E., Engelhardt J. F., Wilson J. M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994 Jul;7(3):362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- Yatziv S., Weiss L., Morecki S., Fuks Z., Slavin S. Long-term enzyme replacement therapy in beta-glucuronidase--deficient mice by allogeneic bone marrow transplantation. J Lab Clin Med. 1982 Jun;99(6):792–797. [PubMed] [Google Scholar]