Abstract
Bone marrow-derived endothelial progenitor cells (EPCs) infiltrate into sites of neovascularization in adult tissues and mature into functional blood endothelial cells (BECs) during a process called vasculogenesis. Human marrow-derived EPCs have recently been reported to display a mixed myeloid and lymphatic endothelial cell (LEC) phenotype during inflammation-induced angiogenesis; however, their role in cancer remains poorly understood. We report the in vitro differentiation of human cord blood CD133+CD34+ progenitors into podoplanin+ cells expressing both myeloid markers (CD11b, CD14) and the canonical LEC markers vascular endothelium growth factor receptor 3 (VEGFR-3), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), and prospero homeobox 1 (PROX-1). These podoplanin+ cells displayed sprouting behavior comparable to that of LECs in vitro and a dual hemangiogenic and lymphangiogenic activity in vivo in an endothelial cell sprouting assay and corneal vascularization assay, respectively. Furthermore, these cells expressed vascular endothelium growth factor (VEGF) family members A, -C, and -D. Thus, bone-marrow derived EPCs stimulate hemangiogenesis and lymphangiogenesis through their ability to differentiate into LECs and to produce angiogenic factors. Importantly, plasma from patients with breast cancer induced differentiation of CD34+ cord blood progenitors into hemangiogenic and lymphangiogenic CD11b+ myeloid cells, whereas plasma from healthy women did not have this effect. Consistent with these findings, circulating CD11b+ cells from breast cancer patients, but not from healthy women, displayed a similar dual angiogenic activity. Taken together, our results show that marrow-derived EPCs become hemangiogenic and lymphangiogenic upon exposure to cancer plasma. These newly identified functions of bone-marrow derived EPCs are expected to influence the diagnosis and treatment of breast cancer.
Keywords: lymphatic endothelial cells, angiogenesis, lymphangiogenesis, podoplanin, vasculogenesis, bone-marrow derived cells, breast cancer
Introduction
The lymphatic system ensures tissue homeostasis through the transport of nutrients and tissue wastes, absorbs lipids from the intestinal tract, serves as a conduit for leukocyte trafficking to regional lymph nodes, and modulates the immune response.1 A role for vascular endothelium growth factors (VEGFs) and VEGF receptor (VEGFR) tyrosine kinases in the development and maintenance of blood and lymphatic vessels has been established in a spectrum of genetic mouse models.2 In mammals, the lymphatic endothelial system appears to be derived from the blood endothelial compartment and lymphatic progenitor cells,1 whereas most types of leukocytes appear to be dispensable for the development of a functional blood and lymphatic vascular system.3 However, recent data suggest that postnatal neovascularization is not restricted to angiogenesis (i.e., neovessel formation within a pre-existing mature vascular network), but also involves vasculogenesis, a process requiring the differentiation of progenitor cells and their participation in the development of a de novo vascular network.4,5 Bone marrow-derived cells (BMDCs), including endothelial progenitor cells (EPCs), have been suggested to contribute to neo-lymphangiogenesis through two distinct mechanisms: (1) through their incorporation into growing lymphatic vessels in association with vasculogenesis; and (2) via the secretion of lymphangiogenic growth factors. In mouse experimental models, bone-marrow derived EPCs have been reported to display a mixed myeloid/endothelial phenotype,3 to produce the lymphangiogenic growth factors VEGF-A, VEGF-C, and VEGF-D, and to be involved in the neovascularization of adult tissues.4,5 Consistent with these findings, depletion of BMDC in murine models using Clodrolip (a liposomal drug that depletes macrophages) or genetic approaches severely impairs lymphatic vessel functions.3,6 In mice, the contribution of BMDC to inflammatory lymphangiogenesis has been demonstrated in response to various environmental stimuli, including lipopolysaccharide (LPS) infection,7-9 skin-wounding in diabetes,10 skin homeostasis in hypertension,11 and cancer.12-15 In contrast, two studies have demonstrated that lymphatic endothelial cells (LECs) arise independently of the myeloid lineage during both embryogenesis and tumor-stimulated angiogenesis.16,17
In humans, a contribution of myeloid cells to lymphangiogenesis has been experimentally evinced in the context of pathophysiologic conditions such as cancer,18,19 kidney transplantation,20 nematode infection,21 and pulmonary fibrosis.22 Hence, inflammation seems to be a prerequisite for the transdifferentiation of myeloid cells into LECs. Human endothelial progenitor cells (EPCs) have been isolated from peripheral blood,18,19,23-25 cord blood,23,26 fetal liver,24 or bone marrow,24 and found to reside within a subset of CD34+CD133+VEGFR3+,24 CD34+CD11b+,26 or CD14+/CD34low25 cells. To date, EPCs have been identified on the basis of their proliferative potential and the phenotype of their progeny,18,24-26 rather than by their vasculogenic potential.26 Moreover, to our knowledge, the lymphvasculogenic potential of human myeloid progenitors has not yet been demonstrated, although it has been suggested. Indeed, there is evidence for the incorporation of macrophages expressing LEC markers into the lymphatic vasculature of inflamed tissues20,21 and the formation in vitro of lymphatic-like vessels induced by CD11b+ macrophages isolated from patients with pulmonary fibrosis.22 Furthermore, correlations have been reported between lymph node metastasis and the frequency of circulating bone marrow-derived EPCs in small cell lung cancer19 and the density of tumor-associated macrophages expressing VEGFR-3, VEGF-C, and VEGF-D in cervical squamous carcinoma.18
Herein, we report the dual hemangiogenic and lymphangiogenic activity of myeloid CD11b+ podoplanin+ cells differentiated in vitro from CD133+CD34+ human cord blood progenitors after 15 d of exposure to a cocktail of interleukin-6 (IL-6), thrombopoietin, stem cell factor/KIT ligand (SCF/KITL), Fms-related tyrosine kinase 3 (FLT-3) ligand, and human plasma. Importantly, both hemangiogenic and lymphangiogenic activities of these CD11b+podoplanin+ cells dramatically increased during differentiation in the presence of plasma from breast cancer patients compared with plasma from healthy women. In vitro differentiated CD11b+ podoplanin+ cells expressed both myeloid markers and the canonical LEC markers VEGFR-3, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), and prospero homeobox 1 (PROX-1). In response to VEGF, podoplanin+ cells proliferated and formed sprouts in vitro. Furthermore, CD11b+ podoplanin+ cells induced the growth of blood and lymphatic vessels in the cornea of immunocompromised mice and consistently produced VEGF-A, -C, and -D. Thus, our data show that human bone marrow-derived EPCs contribute to lymphangiogenesis through differentiation into LECs and secretion of VEGFs.
Results
Breast cancer patient plasma promotes differentiation of CD34+ progenitor cells into hemangiogenic and lymphangiogenic CD11b+ cells in vitro
We have previously reported that conditioned media of human breast cancer cell lines induces the commitment of CD34+ progenitors into hemangiogenic CD11b+ cells in a placenta growth factor (PLGF)-dependent manner.26 Here, we examined the contribution of plasma from breast cancer patients to the differentiation of CD34+ progenitors into hemangiogenic and lymphangiogenic CD11b+ cells. To this end, we isolated CD34+ cells from human cord blood (purity >95%, Fig. S1) and cultured them in the presence of IL-6, thrombopoietin, SCF, and FLT-3 ligand to achieve continuous production of hematopoietic progenitor cells.27,28 Additionally, we supplemented the culture with 10% plasma isolated from the peripheral blood of either healthy women or untreated breast cancer patients. Ten days later, the resultant CD11b+ cells (differentiated from CD34+ progenitors) were purified from the culture by positive immunomagnetic selection and assessed according to their hemangiogenic and lymphangiogenic activities via their ability to induce sprouting of human umbilical vein endothelial cells (HUVECs) and LECs grown in vitro on microcarrier beads embedded in a 3D fibrin gel. CD11b+ cells that differentiated in the presence of plasma from breast cancer patients induced dramatic sprouting of human HUVECs and LECs as compared with their counterparts differentiated in the presence of plasma from healthy women (Fig. 1A). Consistent with these findings, CD11b+ cells isolated from the peripheral blood of breast cancer patients showed much stronger hemangiogenic and lymphangiogenic activities than those isolated from the blood of healthy women (Fig. 1B).
Figure 1. Increased hemangiogenic and lymphangiogenic activities of CD11b+ cells differentiated in vitro from CD34+ progenitors in the presence of plasma from breast cancer patients. (A–D) The hemangiogenic and lymphangiogenic activities of CD11b+ cells were assessed in vitro by measuring their ability to induce sprouting of human umbilical vein endothelial cells (HUVECs) and lymphatic endothelial cells (LECs) grown on microcarrier beads and embedded in a 3D fibrin gel. (A) Cumulative sprout length for HUVEC and LEC beads following exposure for 10 d to purified CD11b+ cells differentiated in vitro from CD34+ progenitors in the presence of plasma from healthy (H) individuals or breast cancer (BC) patients (n = 10–12). (B) Cumulative sprout length for CD11b+ cells isolated from peripheral blood of healthy individuals (H) and breast cancer patients (BC). (C–D) CD11b+ cells were differentiated in vitro from CD34+ progenitors following exposure to healthy plasma and the kinetics of hemangiogenic and lymphangiogenic activities (C) or differentiation into podoplanin+ cells (D) were measured. Background sprouting measured with coated HUVEC and LEC beads alone is shown (none). Data in (C) show cumulated sprout length normalized to that measured in the absence of bone marrow-derived cells (BMDCs) and in the presence of 50 ng/mL VEGF-A. Statistical significance was determined by Student’s t test and differences are indicated for all panels (*P < 0.05; **P < 0.005; ***P < 0.001).
In vitro differentiation of podoplanin+ cells from cord blood CD34+ progenitors
Differentiation of CD34+ progenitors into angiogenic CD11b+ cells was induced by plasma from healthy individuals after a long exposure time of 20 d (Fig. 1C). However, plasma from breast cancer patients increased both the kinetics of differentiation of CD34+ progenitors into angiogenic CD11b+ cells (Fig. 1C) and the angiogenic potential of these cells (Fig. 1A and C).
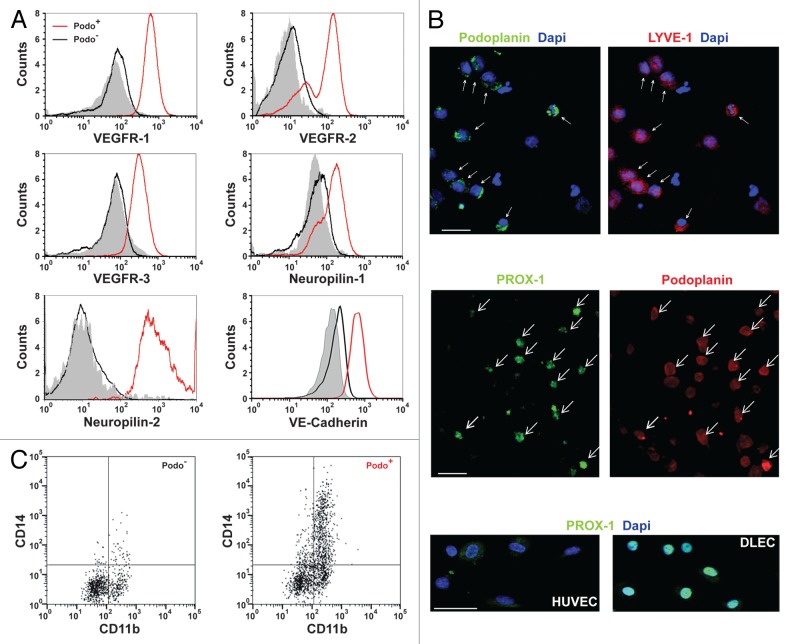
These observations suggest that under the culture conditions used in this experiment, BMDCs stimulate hemangiogenesis and lymphangiogenesis through their ability to differentiate into endothelial cells and/or by providing a source of pro-angiogenic factors. To address these hypotheses, we performed longitudinal monitoring of the cell phenotype over 35 d of culture. First, using flow cytometry we examined the expression of neuropilin-1 and 2, which function in angiogenesis as co-receptors stabilizing the VEGF/VEGFR complex.2 At day 0, all CD34+ cord blood progenitors expressed CD45, but not the VEGF co-receptor neuropilin-1. A small but detectable fraction of CD34+ cells (≤5%) expressed the stem cell marker CD133 as well as neuropilin-2 and VEGFR-3 (Fig. S1), consistent with a previous report.24 After 10 d in culture, a fraction of the purified CD34+ cord blood progenitors had differentiated into a population of adherent podoplanin+ cells (Fig. 1D) that persisted, matured, and expanded over a 5-wk period. After 3 wk in culture, these podoplanin+ cells were detectable in all experiments with an average frequency of 10.75% ± 6.95% (range 5–23%, n = 10). At day 20 to 35, the podoplanin+ cells consisted of two populations according to CD31 expression (Fig. 2A). The podoplanin+CD31− population was predominantly CD34low and CD45low, whereas the podoplanin+CD31+ subset expressed intermediate levels of these markers and retained 19% CD34+ progenitor cells. The culture also contained a large proportion of podoplanin+CD31+ cells that heterogeneously expressed CD34 and CD45 (Fig. 2A). Finally, differentiation of CD34+ cord blood progenitor cells into podoplanin+ cells was not significantly enhanced by growing the cells on surfaces coated with collagen, gelatin, or fibronectin, displaying an average increase in podoplanin+ cell frequency relative to the untreated surface of 0–12% ± 4%, P > 0.05, n = 4). CD31+podoplanin− cell populations that express CD34 but not CD45 may be mature endothelial cells whereas their CD34− counterparts are likely to be fibroblasts (Fig. 2A).29 Moreover, CD31+podoplanin−CD34−CD45+ cells are candidate myeloid cells because a fraction of them express CD11b.29
Figure 2. In vitro differentiation of CD34+ cord blood precursors into podoplanin+ cells. (A–B) CD34+ hematopoietic progenitors from cord blood were isolated by immunomagnetic selection and cultured in vitro. Resultant cells were characterized by immunostaining and cytofluorimetric analysis 15 to 35 d after the isolation of CD34+ cord blood precursors. Representative flow cytometry dot plots of the expression profiles of podoplanin, CD31, CD34, and CD45 (panel A) and podoplanin, CD133, and neuropilin-1 (panel B) are shown from 10 distinct cultures.
At days 20 to 35, a subset of the podoplanin+ cells (6.5%) co-expressed neuropilin-1 but not CD133, whereas CD133 progenitors represented more than 15% of cells in the culture (Fig. 2B). A vast majority of the CD133+ progenitor cells was podoplanin− and neuropilin-1− (>85%, Fig. 2B). To assess whether these progenitor cells had matured into podoplanin+ cells, we isolated CD133+ cells from a 20-d-old culture by immunomagnetic selection, stained them with carboxyfluorescein diacetate succinimidyl ester (CFSE), and returned them to the original culture. Over the first 4 d, the CD133 progenitor cells differentiated into podoplanin+ cells and expanded more than 16-fold (Fig. S2) indicating that in our cell culture system, and similar to other reports,24,26 EPCs reside within the subset of CD34+CD133+ cells. Furthermore, this cell culture system allows continuous in vitro maintenance and renewal of a significant proportion (>10%) of CD34+ progenitors for over a year,28,30 as well as sustained differentiation of podoplanin+ cells from CD34+ progenitors over several months (Fig. 2). Interestingly, the podoplanin+ cells retained a significant fraction (approximately 20%) of CD34+ progenitor cells (Fig. 2A).
Angiogenic CD11b+ cells from the peripheral blood of cancer patients showed reduced expression levels of CD31, podoplanin, and neuropilin-1 and 2 relative to podoplanin+ cells differentiated in vitro. A significant increase in the frequency of CD11b+CD14+neuropilin+ cells was observed in the peripheral blood of breast cancer patients relative to blood from healthy donors (31% ± 13 vs. 15% ± 6, P < 0.05).
In vitro differentiated podoplanin+ cells express markers of myeloid and lymphatic endothelial cells
The expression of LECs and myeloid cell markers was examined by both flow cytometry and confocal microscopy 22 to 35 d after isolation of the CD34+ precursors from cord blood. Although VEGFR-1 is known to be involved in hemangiogenesis,2 VEGFR-3, a receptor for VEGF-C and VEGF-D, has been shown to be required for LEC function and lymphatic development.1 Similarly, VEGFR-2 has been implicated in lymphangiogenesis, possibly through binding of VEGF-A, C, and D. VEGFR-3, neuropilin-2, the transcription factor PROX-1, and podoplanin serve as LEC signature markers because their genetic deletion in mice has been shown to compromise the development of the lymphatic system.1 However, despite the predominant and continuous expression of podoplanin in LECs and absence in blood endothelial cells (BECs), podoplanin is diversely expressed in a broad range of other cell types. Similarly, LYVE-1 has been identified as a LEC marker, but is also reportedly expressed in activated tissue macrophages.1 We observed that podoplanin+ cells differentiated in vitro from CD34+ cord blood progenitors expressed VEGFR-1, VEGFR-2, neuropilin-1, and the LEC markers VEGFR-3, neuropilin-2, and LYVE-1 (Fig. 3A). In contrast, podoplanin− cells exhibited weaker (VEGFR-1, VEGFR-2, neuropilin-1) or undetectable (VEGFR-3, neuropilin-2, and LYVE-1) expression of these markers (Fig. 3A). In contrast to podoplanin− cells, podoplanin+ cells showed elevated expression of VE-cadherin, a marker of blood endothelial cells (BEC) and LECs (Fig. 3A). PROX-1 activity is required not only for LEC specification but also to maintain the mature differentiated LEC fate.31-35 Therefore, as shown in Figure 3B, we used confocal microscopy to examine PROX-1 and found that most (>80%) of the podoplanin+ cells displayed intermediate PROX-1 expression levels relative to HUVECs (negative) and LECs (high). In addition, we compared the expression of CD31 and canonical lymphatic markers in HUVECs, mature LECs, and podoplanin+ cells by flow cytometry. Podoplanin and neuropilin-2 were expressed by both podoplanin+ cells and LECs, but not by HUVECs (Table 1). Podoplanin+ cells displayed intermediate expression levels of LYVE-1 and VEGFR-3 relative to HUVECs and LECs, and heterogeneous expression of CD31 (Table 1). Taken together, these results indicate that podoplanin+ cells display a phenotype of maturing LECs. In addition, in contrast to HUVECs and LECs, most of the podoplanin+ cells expressed CD11b, consistent with their myeloid origin (Fig. 3C). Approximately 30% of podoplanin+CD11b+ cells expressed the lipopolysaccharide receptor CD14, but not CD11c, indicating that this cell population encompassed a monocytic cell subset (Fig. 3C). Finally, the presence of hematopoietic progenitors was examined by a methylcellulose-based colony assay (StemCell Technologies, Inc.). In contrast to podoplanin− cells, podoplanin+ cells could not form colonies consistent with the expression of a maturing LEC phenotype (data not shown).
Figure 3.
In vitro differentiated podoplanin+ cells display markers of lymphatic endothelial cells and myeloid cells.(A–C)CD34+ hematopoietic progenitors from cord blood were isolated by immunomagnetic selection and cultured in vitro. Expression of the lymphatic endothelial cell (LEC) markers vascular endothelium growth factor receptors (VEGFRs), neuropilins, prospero-related homeobox 1 (PROX-1), and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) and of VE-cadherin was assessed by immunostaining and fluorescence cytometry (A) and confocal microscopy (B) in podoplanin+ and podoplanin− cell populations. A. Flow cytometry histograms of the expression level of the indicated marker in each of the two cell types, with filled histograms depicting labeling with isotype control antibodies. (B) All podoplanin+ cells (arrows) expressed LYVE-1 and most (> 80%) showed intermediate PROX-1 expression relative to human umbilical vein endothelial cells (HUVECs) and human dermal lymphatic microvascular endothelial cells (DLECs). (C) Podoplanin+ cells expression of the myeloid cell markers CD11b and CD14. Representative images from three to five experiments are shown. Cell characterizations were performed in 22- to 35-d-old cultures. Bar, 25 µm.
Table 1. Expression of LEC markers (LYVE-1, podoplanin, and VEGFR-3), CD31, and neuropilin-2 on the surface of HUVECs, DLECs, and in vitro differentiated podoplanin+ cells.
| CD31 | LYVE-1 | Neuropilin-2 | Podoplanin | VEGFR-3 | |
|---|---|---|---|---|---|
| HUVEC | 4380 | 200 | 13 | 36 | 830 |
| DLEC | 6858 | 748 | 729 | 47 000 | 17 231 |
| Culture 1 Podo+ cells | 3200 | 458 | 500 | 500 | 4317 |
| Culture 2 Podo+ cells | 4079 | 386 | 610 | 157 | 6450 |
| Culture 3 Podo+ cells | 7043 | 1007 | 528 | 243 | 3613 |
Data shown are the mean fluorescence intensity determined by cytofluorimetric analysis, corrected for background staining. Expression was measured in the podoplanin+ (Podo+) cell fraction of three distinct differentiated cultures (1–3). DLEC, dermal lymphatic endothelial cell; LEC, lymphatic endothelial cell; HUVEC, human umbilical vein endothelial cell; LYVE-1, lymphatic vessel endothelial hyaluronan receptor 1; VEGFR, vascular endothelium growth factor receptor.
Podoplanin+ cells and mature LECs display a comparable aptitude to form sprouts in vitro in response to VEGF
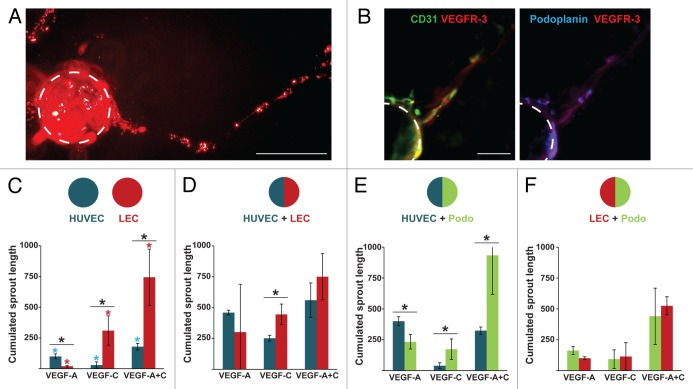
We observed that podoplanin+ cells expanded in response to VEGF stimulation (Fig. S3A) and showed a transient increase in surface expression of neuropilin-1 and continuous increase in expression of podoplanin (Fig. S3B and C). We next examined the ability of podoplanin+ cells to form sprouts in response to VEGF treatment. Podoplanin+ cells that were positively selected from 25-d-old cultures were grown on collagen-coated microcarrier beads and embedded in a 3D fibrin gel. We observed that podoplanin+ cells adhered weakly to collagen microcarrier beads compared with HUVECs and LECs and failed to form the cohesive spheroids in methylcellulose that are usually generated in a spheroid sprouting assay. Nevertheless, in response to VEGF-A and -C podoplanin+ cells formed sprouts (Fig. 4A) composed of cells expressing podoplanin, VEGFR-3, and CD31 (Fig. 4B). However, the weak adhesion of podoplanin+ cells to the microcarrier beads precluded accurate quantification of the sprouting of podoplanin+ cells and prompted us to generate chimeric HUVEC/podoplanin+ cells or LEC/podoplanin+ cell beads that showed sustained and reproducible coverage. Sprouts emerging from HUVEC, LEC, or podoplanin+ cells were identified by incorporation of CFSE in one of the cell types before beading. We observed that CFSE incorporation had a weak effect on the sprouting ability of endothelial cells of lymphatic origin but a moderate effect on those of blood origin, therefore CFSE was preferentially used to label the lymphatic cells.
Figure 4. Podoplanin+ cells and LECs show similar abilities to form sprouts in vitro in response to vascular endothelium growth factor (VEGF). In vitro sprouting of endothelial cells cultured 25 d using podoplanin+ cells positively selected using anti–podoplanin-PE antibody and immunomagnetic separation. Podoplanin+ cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CSFE) and cultured on collagen-coated microcarrier beads embedded in a 3D fibrin gel, as described below. (A) Epifluorescence microscopy image of CFSE-labeled sprouts (dashed line) of podoplanin+ cells after 5 d of stimulation with vascular endothelium growth factor (VEGF)-A and -C. (B) Confocal microscopy images of sprouts of podoplanin+ cells in 3D fibrin gel 3 d post-stimulation. Green, CD31; blue, podoplanin; red, VEGF receptor (VEGFR). Bar, 75 µm. (C–F) Sprouting capacity of endothelial cells in response to VEGF was evaluated in vitro by measuring the mean cumulative sprout length. (C) Beads were coated exclusively with human umbilical vein endothelial cells (HUVECs) or lymphatic microvascular endothelial cells (LECs) (D–F) Chimeric beads were coated with both HUVECs and LECs (D), HUVEC and podoplanin+ cells (E), or LECs and podoplanin+ cells in a 2:1 ratio (F). Sprouts of lymphatic origin were identified by incorporation of CFSE into LECs or podoplanin+ cells. Cumulative data from a minimum of three experiments are shown. Background sprouting (96.8 ± 60 pixels) measured with coated beads alone was subtracted from all data. Significant differences between cell types in response to the same treatment were measured by Student’s t test; *P < 0.05 in panels C–F; in panel C, significant differences among the treatments for HUVECs and LECs are indicated by blue and red asterisks, respectively.
Beads covered exclusively with HUVECs showed strong and weak sprouting capacity in response to VEGF-A and VEGF-C, respectively. Beads covered exclusively with LEC showed a clear opposite trend (Fig. 4C). A combination of VEGF-A and VEGF-C had a significant synergistic effect on the sprouting capacity of both HUVECs and LECs, with a much stronger effect on LECs (Fig. 4C). These differences tended to be much less pronounced on chimeric HUVEC/LEC beads (Fig. 4D), suggesting that HUVECs and LECs influence each other. More specifically, LECs displayed a highly variable response to VEGF-A on chimeric HUVEC/LEC beads (compare panels C and D). Compared with LECs, podoplanin+ cells showed similar responsiveness to VEGF-A and VEGF-C when mixed with HUVECs on beads (compare panels D and E). Furthermore, in chimeric LEC/podoplanin+ cell beads, podoplanin+ cells and LECs showed comparable responses to VEGF treatment (Fig. 4F). Thus, a hallmark of lymphatic cells (i.e., LECs and podoplanin+ cells) is a robust synergistic stimulatory effect of combined VEGF-A and VEGF-C on their sprouting capacity (Fig. 4C and F). Finally, comparable results were obtained using CD11b+ positively selected cells (data not shown).
In vitro differentiated podoplanin+ cells are lymphangiogenic
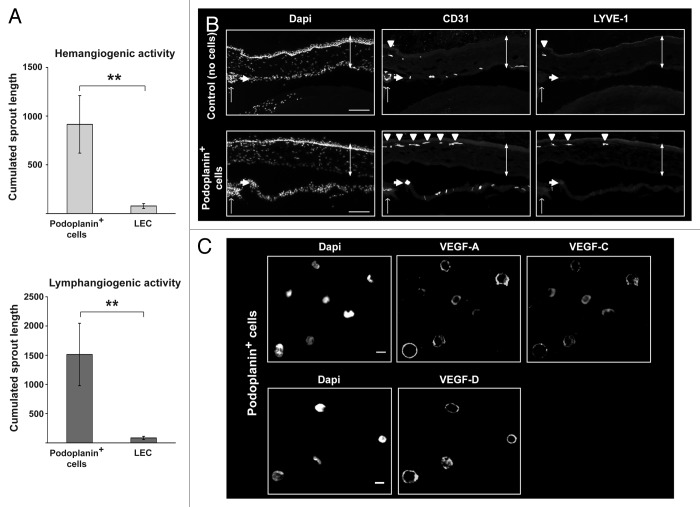
Like BMDCs, podoplanin+ cells share markers with both LECs and myeloid cells (Fig. 3). Because some mouse BMDCs have been reported to contribute to lymphangiogenesis,14 we compared the angiogenic potential of podoplanin+ cells and mature LECs. To this end, we applied podoplanin+ cells or LECs to microcarrier beads that were coated with LECs or HUVECs and embedded in a 3D fibrin gel. Podoplanin+ cells induced robust sprouting of HUVEC and LEC cells, whereas LECs failed to do so (Fig. 5A). These results indicate that, in contrast to LECs, podoplanin+ cells are both hemangiogenic and lymphangiogenic in vitro. Moreover, the hemangiogenic and lymphangiogenic activities showed a 6.5-fold enrichment in the podoplanin+ cell fraction relative to the podoplanin− cell fraction. We further examined the angiogenic activity of podoplanin+ cells by injecting them into the cornea of mice. The cornea is avascular and any growth of new vessels from the peripheral limbal vasculature reflects angiogenic activity of the injected podoplanin+ cells.36 Podoplanin+ cells induced the growth of blood and lymphatic vessels in the cornea from pre-existing limbal BECs and LECs, as shown by CD31 and LYVE-1 immunostaining of sagittal sections of the eyes after 30 d (Fig. 5B, arrow heads). Taken together these results indicate that, like BMDCs and in contrast to mature LECs, podoplanin+ cells enable both hemangiogenesis and lymphangiogenesis in vivo. Consistent with these findings, we observed that podoplanin+ cells expressed VEGF-A and -C by confocal microscopy (Fig. 5C).
Figure 5. Podoplanin+ cells are hemangiogenic and lymphangiogenic in vitro and in vivo. The hemangiogenic and lymphangiogenic activities of podoplanin+ cells (derived from cultured cord blood CD34+ precursor cells) and microvascular lymphatic endothelial cells (LECs) were assessed in vitro by measuring their aptitude to induce sprouting of human umbilical vein endothelial cells (HUVECs) and LECs grown on microcarrier beads and embedded in a 3D fibrin gel. (A) Cumulated sprout length for HUVEC (left panel) and LEC (right panel) beads following exposure to podoplanin+ cells or LECs. Background sprouting (71.4 ± 57 pixels) measured with HUVEC and LEC beads alone was subtracted. Significant differences were determined by Student’s t test, *P < 0.05; **P < 0.005). (B) Corneal vascularization assay to assess the in vivo hemangiogenic and lymphangiogenic activity of podoplanin+ cells was performed by transplantation of 30 000 podoplanin+ cells into the corneal stroma of anesthetized NOD-SCID/IL2Rγnull mice. Fluorescent microscopy images of sagittal sections of mouse eyes using antibodies specific for CD31 and lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) revealed de novo formation of blood and lymphatic vessels emerging from the peripheral limbal vasculature (arrow heads) and expanding into the cornea (vertical double-headed arrow). Single arrow depicts the iris. Bar, 100 μm. Cumulative data of three independent experiments are shown in (A) and a representative experiment among three is shown in (B). (C) Expression of vascular endothelium growth factor (VEGF)-A and -C in the podoplanin+ cell fraction detected by confocal microscopy. Bar, 12 µm.
Discussion
In the present study we report that human CD34+CD133+ bone-marrow derived EPCs possess the ability to differentiate in vitro into myeloid LECs expressing VEGF-A, -C, and -D that consistently display hemangiogenic and lymphangiogenic activities. Our findings are consistent with other in vitro studies that identified EPCs within CD34+ populations of various origins.21-24 Nevertheless, although two of these studies reported that BECs derived from CD34+ cells formed capillary-like structures after plating in Matrigel,21,24 only one reported the differentiation of human CD34+ progenitors into ECs expressing VE-cadherin, CD51/61, CD105, LYVE-1, and podoplanin.22 However, in these studies, authors did not examine the function of these cells or expression of PROX-1, a binary switch whose expression is constantly required to maintain a LEC fate.31-35
Here, we report that in vitro differentiated podoplanin+ cells expressed PROX-1, proliferated, and formed sprouts in response to VEGF-C stimulation. Additionally, we show that a strong synergistic effect of VEGF-A and -C is a common feature of the sprouting behavior of these in vitro-derived podoplanin+ cells and mature LECs. Relative to HUVECs and mature LECs, podoplanin+ cells displayed a decreased aptitude to adhere to extracellular matrix components such as collagen microcarrier beads. However, similar to mature LECs and in marked contrast to HUVECs, podoplanin+ cells failed to form cohesive spheroids in vitro. Furthermore, consistent with their CD45 intermediate hematopoietic phenotype, podoplanin+ cells remained impaired in their ability to form colonies compared with podoplanin− cells, but expanded 16-fold from the initial CD133+ precursors over 4 d of culture. Hence, CD34+CD133+ cells could be viewed as bone marrow-derived EPCs and podoplanin+ cells as BMDCs that have acquired a LEC-like phenotype in vitro (refer to Fig. 3). This plasticity is not surprising given that EC and BMDC cells have been previously shown to exhibit relatively fluid cell fates.37 Finally, in marked contrast to mature LECs, podoplanin+ cells displayed hemangiogenic and lymphangiogenic activities (refer to Fig. 5), suggesting that these functions might be specific to BMDCs. Although we observed that podoplanin+ cells can insert into LEC sprouts, this insertion did not seem to be linked to their lymphangiogenic activity because sprouting was also observed in the absence of insertion.
Importantly, the dual hemangiogenic and lymphangiogenic activity of myeloid cells was enhanced by plasma from breast cancer patients. It is unclear why the BMDC and LEC phenotypes are so closely related. However, in settings of tumor-induced lymphangiogenesis the frequency of CD34+ bone-marrow derived EPCs expressing VEGFR-3 was found to be increased in patients with small cell lung cancer correlating with lymph node metastasis and poor survival.17 Moreover, the density of macrophages expressing VEGF-C and -D was shown to correlate with lymph node metastasis in cervical cancer patients.16 These observations are particularly relevant in solid cancers, such as breast cancer and melanoma, since 80% of metastatic cells are known to disseminate through the lymphatic system38 and their detection in regional lymph nodes is an excellent prognostic indicator.39 Hence, while the role of BMDC in lymphangiogenesis within human breast tumors remains poorly understood, the observation that lymphangiogenesis within the sentinel lymph node precedes the arrival of metastatic cells39,40 is highly suggestive of a contribution of lymphangiogenic BMDCs to lymph node metastatic spread in breast cancer.
Taken together, our results suggest a plausible mechanism accounting for the apparent contribution of marrow-derived EPCs and BMDCs to human breast cancer angiogenesis in which soluble plasma factors induce the differentiation of bone marrow-derived EPCs into hemangiogenic and lymphangiogenic CD11b+ BMDCs. In the case of breast cancer, our data indicate that this differentiation occurs before BMDCs reach the tumor microenvironment and most likely while they are residing in the bone marrow where CD34+ progenitor cells are exposed to unknown plasma factors. Identification of these factors is currently underway using the cell culture systems described in this study. Delineation of the altered signals and pathways driving the differentiation of bone marrow-derived EPC into hemangiogenic and lymphangiogenic BMDCs is expected to have a critical impact on the diagnosis and treatment of breast cancer.
Materials and Methods
Cells and tissue specimens
Human umbilical vein endothelial cells (HUVECs) and adult human dermal lymphatic microvascular endothelial cells (DLECs) were >90% pure and were purchased from Lonza. Cord blood, peripheral blood, and plasma were obtained according to the Declaration of Helsinki and upon written informed consent. This study was approved by the ethics committee of the University Hospital of Lausanne. Breast cancer patients were diagnosed with primary invasive breast carcinoma (T1-T2 < 3 cm, positive lymph node status for 25% of the patients, Grade I-III)41 and untreated.
Confocal microscopy
Cells were processed by cytospin or adhered to Cell-Tak coated slides (BD Biosciences, hereafter referred to as BD), fixed with 10% formalin for 10 min at 4 °C, permeabilized for 20 min at RT with PBS containing 0.05% Triton X-100 and blocked for 40 min with PBS containing 1% bovine serum albumin (BSA) and 5% fetal bovine serum (FBS). Slides were incubated with primary antibodies for 20 h at 4 °C, washed, incubated with secondary antibodies for 1 h at RT, and mounted in Mowiol (Sigma). Gel-embedded beads coated with endothelial cells were dried on glass slides, rehydrated in PBS containing 100 mM Tris pH 8.3, fixed, and blocked, as described above. Staining with primary antibodies was performed for 20 h at 4 °C in blocking buffer containing 0.1% Triton X-100. Incubation with secondary antibodies and mounting were performed as described above. Primary antibodies specific for LYVE-1 (ABBIOTEC, rabbit), VEGFR-3 (ABBIOTEC, rabbit), podoplanin (ABBIOTEC, rabbit), PROX-1 (R&D, goat), neuropilin-1, VEGF-D (GeneTex, rabbit), neuropilin-2 (R&D, goat), VEGF-A (R&D, mouse), CD31 (Abcam, mouse), and VEGF-C (Abcam, mouse) were used. Appropriate secondary antibodies prepared in donkey and coupled to Alexa Fluor-647, -488, or -594 were purchased from Invitrogen. Alternatively, a Zenon Alexa Fluor 647 rabbit IgG labeling kit from Invitrogen was used for fluorescence labeling. Fluorescent images were taken using a Zeiss LSM 510 META confocal microscope in the Cellular Imaging Facility.41 Each image is the result of a maximum intensity projection of six optical sections (optical slice < 1 μm).
In vitro cell differentiation, isolation, and activation
CD34+ hematopoietic progenitors from cord blood were isolated by immunomagnetic selection (StemCell Technologies Inc.) and cultured as previously described.27,28 At 15 to 25 d after isolation, the cells were used for isolation of podoplanin+ cells or stimulated by addition of recombinant VEGF at 50 ng/mL unless stated otherwise. The cell linker dyes PKH2, PKH67 (Sigma-Aldrich) or CFSE (carboxyfluorescein diacetate succinimidyl ester, Invitrogen) were incorporated into precursor cells, and cell proliferation and differentiation was monitored by flow cytometry (see below).
Analysis of cell phenotype by fluorescence cytometry
Following blockade of Fc receptors (FcR blocking reagent, Myltenyi Biotec), cells were labeled with fluorophore conjugated antibodies to the following proteins: human CD31-FITC (BD, mouse), CD34-PerCP (BD, mouse), CD45-Pacific Orange (Invitrogen, mouse), CD11b-PE-Cy7 (BD, mouse), human neuropilin-1-PE (Miltenyi Biotec, mouse), CD133-APC (Miltenyi Biotec, mouse), human VEGFR-1 (Abcam, rabbit), VEGFR-2-PE (R&D, goat), VEGFR-3 (ABBIOTEC, rabbit), CD11c-Pacific Blue (Biolegend, mouse), CD14-PerCP-Cy5.5 (Biolegend, mouse), and CD14-APC (BD, mouse). Unlabeled antibodies used included anti-podoplanin (ABBIOTEC, rabbit followed by a Cy5 goat anti-rabbit or Dylight-488 donkey anti-rabbit, both from Jackson Immunoresearch),and mouse VE-Cadherin (R&D, goat followed by an Alexa-647 donkey anti-goat from Invitrogen). Labeled cells were analyzed using a LSRII flow cytometer (BD Biosciences) equipped with a 610/20 nm filter on the violet detector.
In vitro and in vivo angiogenesis assay
After 15 to 25 d in culture, podoplanin+ cells were isolated by immunomagnetic selection (StemCell Technologies Inc.) using anti–podoplanin-PE antibody (Biolegend). The cell-tracing reagent CFSE was incorporated into endothelial cells to discriminate and quantify sprouts of blood and lymphatic origins. Monolayers of HUVECs and podoplanin+ cells were co-cultured on microcarrier beads (2:1, HUVEC: podoplanin+ cells) and embedded in a three-dimensional fibrin gel42 overlaid with normal human dermal fibroblasts using a modified procedure.43,44 The sprouting capacity of HUVECs and podoplanin+ cells in response to VEGF was evaluated by measuring the mean cumulated sprout length per bead on 10 spheroids or beads.28
Mouse experiments were approved by the cantonal veterinary service (Vaud). For the in vivo corneal vascularization assay, 30,000 podoplanin+ cells were isolated by positive immunomagnetic selection (purity > 95%, Stem Cell Technologies) and injected in a 5-μL volume into the stromal part of the corneas of anesthetized NOD-SCID/IL2Rγnull mice36 using a 35-gauge nanofil injection kit (WPI). Blood and lymphatic vessels were detectable in the mouse cornea from days 15 and 22 post-injection, respectively. Mice were euthanized at 30 d post-injection and isolated eyes were fixed in 4% paraformaldehyde, cryoprotected in a 30% sucrose solution and embedded in Yazulla medium (30% egg albumin, 3% gelatin). In contrast to blood vessels, lymphatics are transparent and vascularization was assessed by immunostaining of 10-µm sagittal sections with antibodies specific for CD31 and LYVE-1 using a Zeiss Axio Imager M1 fluorescent microscope. The retina was used as a positive control for CD31 staining.
Reagents
All chemicals were from Sigma-Aldrich unless indicated otherwise. Human recombinant cytokines were purchased from PeproTech and R&D Systems.
Statistical analyses
The Student t test statistical analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software).
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported grants from Oncosuisse (M.-A.D. project 02069-04-2007), the Swiss National Foundation (M.-A.D. project 310030-120473 and J.-F.D. project CR32I3_135073), and the Medic Foundation (M.-A.D. Project). We thank Pr T. Petrova (University of Lausanne) for providing antibodies specific for Prox1 and VE-Cadherin.
Authorship contributions
E.F. designed the study, performed research, and analyzed and interpreted the data. S.B. and R.T. performed research and analyzed the data. L.H. and G.C. interpreted the data and wrote the manuscript. J.-F.D. and A.I.T. managed patients, contributed human specimens, and interpreted data. M.-A.D. designed and performed research, interpreted the data, and wrote the manuscript.
Glossary
Abbreviations:
- BEC
blood endothelial cell
- BMDC
bone marrow-derived cell
- EPC
endothelial progenitor cell
- FLT-3
Fms-related tyrosine kinase 3
- HUVEC
human umbilical vein endothelial cells
- IL-6
Interleukin-6
- LEC
lymphatic endothelial cell
- LPS
lipopolysaccharide
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- PLGF
placenta growth factor
- PROX-1
prospero homeobox 1
- SCF/KITL
stem cell factor
- VEGF
vascular endothelium growth factor
- VEGFR
vascular endothelium growth factor receptor
References
- 1.Choi I, Lee S, Hong YK. The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med. 2012;2:a006445. doi: 10.1101/cshperspect.a006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–71. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 3.Zumsteg A, Christofori G. Myeloid cells and lymphangiogenesis. Cold Spring Harb Perspect Med. 2012;2:a006494. doi: 10.1101/cshperspect.a006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287:C572–9. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 5.Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45(Suppl A):A39–47. doi: 10.1016/j.jvs.2007.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–64. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–9. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 8.Kang S, Lee SP, Kim KE, Kim HZ, Mémet S, Koh GY. Toll-like receptor 4 in lymphatic endothelial cells contributes to LPS-induced lymphangiogenesis by chemotactic recruitment of macrophages. Blood. 2009;113:2605–13. doi: 10.1182/blood-2008-07-166934. [DOI] [PubMed] [Google Scholar]
- 9.Hall KL, Volk-Draper LD, Flister MJ, Ran S. New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One. 2012;7:e31794. doi: 10.1371/journal.pone.0031794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saaristo A, Tammela T, Farkkilā A, Kärkkäinen M, Suominen E, Yla-Herttuala S, Alitalo K. Vascular endothelial growth factor-C accelerates diabetic wound healing. Am J Pathol. 2006;169:1080–7. doi: 10.2353/ajpath.2006.051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi TA, Rebulla P, Armitage S, van Beckhoven J, Eichler H, Kekomäki R, Letowska M, Wahab F, Moroff G. Multi-laboratory evaluation of procedures for reducing the volume of cord blood: influence on cell recoveries. Cytotherapy. 2006;8:254–64. doi: 10.1080/14653240600735677. [DOI] [PubMed] [Google Scholar]
- 12.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–90. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 13.Jiang S, Bailey AS, Goldman DC, Swain JR, Wong MH, Streeter PR, Fleming WH. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One. 2008;3:e3812. doi: 10.1371/journal.pone.0003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Rüegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PLoS One. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon EJ, Rao S, Pollard JW, Nutt SL, Lang RA, Harvey NL. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development. 2010;137:3899–910. doi: 10.1242/dev.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–40. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 18.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–56. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogos K, Renyi-Vamos F, Dobos J, Kenessey I, Tovari J, Timar J, Strausz J, Ostoros G, Klepetko W, Ankersmit HJ, et al. High VEGFR-3-positive circulating lymphatic/vascular endothelial progenitor cell level is associated with poor prognosis in human small cell lung cancer. Clin Cancer Res. 2009;15:1741–6. doi: 10.1158/1078-0432.CCR-08-1372. [DOI] [PubMed] [Google Scholar]
- 20.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Kröber SM, Greinix H, Rosenmaier A, Karlhofer F, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–4. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 21.Attout T, Hoerauf A, Dénécé G, Debrah AY, Marfo-Debrekyei Y, Boussinesq M, Wanji S, Martinez V, Mand S, Adjei O, et al. Lymphatic vascularisation and involvement of Lyve-1+ macrophages in the human onchocerca nodule. PLoS One. 2009;4:e8234. doi: 10.1371/journal.pone.0008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Chemaly S, Malide D, Zudaire E, Ikeda Y, Weinberg BA, Pacheco-Rodriguez G, Rosas IO, Aparicio M, Ren P, MacDonald SD, et al. Abnormal lymphangiogenesis in idiopathic pulmonary fibrosis with insights into cellular and molecular mechanisms. Proc Natl Acad Sci U S A. 2009;106:3958–63. doi: 10.1073/pnas.0813368106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 24.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–72. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 25.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, et al. CD14+CD34low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res. 2005;97:314–22. doi: 10.1161/01.RES.0000177670.72216.9b. [DOI] [PubMed] [Google Scholar]
- 26.Hildbrand P, Cirulli V, Prinsen RC, Smith KA, Torbett BE, Salomon DR, Crisa L. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;104:2010–9. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- 27.Niu XL, Peters KG, Kontos CD. Deletion of the carboxyl terminus of Tie2 enhances kinase activity, signaling, and function. Evidence for an autoinhibitory mechanism. J Biol Chem. 2002;277:31768–73. doi: 10.1074/jbc.M203995200. [DOI] [PubMed] [Google Scholar]
- 28.Laurent J, Hull EF, Touvrey C, Kuonen F, Lan Q, Lorusso G, Doucey MA, Ciarloni L, Imaizumi N, Alghisi GC, et al. Proangiogenic factor PlGF programs CD11b(+) myelomonocytes in breast cancer during differentiation of their hematopoietic progenitors. Cancer Res. 2011;71:3781–91. doi: 10.1158/0008-5472.CAN-10-3684. [DOI] [PubMed] [Google Scholar]
- 29.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters R, Leyvraz S, Faes-Van’t Hull E, Jaunin P, Gerber S, Rollini P. Long-term ex vivo expansion of human fetal liver primitive haematopoietic progenitor cells in stroma-free cultures. Br J Haematol. 2002;119:792–802. doi: 10.1046/j.1365-2141.2002.03873.x. [DOI] [PubMed] [Google Scholar]
- 31.Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn. 2002;225:351–7. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 32.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22:3282–91. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–83. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 34.Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Ylä-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002;21:4593–9. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–13. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gimbrone MA, Jr., Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J Natl Cancer Inst. 1974;52:413–27. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- 37.Oliver G, Srinivasan RS. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development. 2010;137:363–72. doi: 10.1242/dev.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31:4499–508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 39.Christiansen A, Detmar M. Lymphangiogenesis and cancer. Genes Cancer. 2011;2:1146–58. doi: 10.1177/1947601911423028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian CN, Berghuis B, Tsarfaty G, Bruch M, Kort EJ, Ditlev J, Tsarfaty I, Hudson E, Jackson DG, Petillo D, et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365–76. doi: 10.1158/0008-5472.CAN-06-2977. [DOI] [PubMed] [Google Scholar]
- 41.Ibberson M, Bron S, Guex N, Faes-van’t Hull E, Ifticene-Treboux A, Henry L, Lehr HA, Delaloye JF, Coukos G, Xenarios I, et al. TIE-2 and VEGFR kinase activities drive immunosuppressive function of TIE-2-expressing monocytes in human breast tumors. Clin Cancer Res. 2013;19:3439–49. doi: 10.1158/1078-0432.CCR-12-3181. [DOI] [PubMed] [Google Scholar]
- 42.Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–32. doi: 10.1016/S0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- 43.Nehls V, Drenckhahn D. A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res. 1995;50:311–22. doi: 10.1006/mvre.1995.1061. [DOI] [PubMed] [Google Scholar]
- 44.Nakatsu MN, Davis J, Hughes CC. Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp. 2007:186. doi: 10.3791/186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.