Abstract
The last decade has seen a growing interest in adjuvant treatments that synergistically influence mechanisms underlying rehabilitation of paretic upper limb in stroke. One such approach is invasive neurostimulation of spared cortices at the periphery of a lesion. Studies in animals have shown that during training of paretic limb, adjuvant stimulation targeting the peri-infarct circuitry enhances mechanisms of its reorganization, generating functional advantage. Success of early animal studies and clinical reports, however, failed to translate to a phase III clinical trial. As lesions in humans are diffuse, unlike many animal models, peri-infarct circuitry may not be a feasible, or consistent target across most. Instead, alternate mechanisms, such as changing transcallosal inhibition between hemispheres, or reorganization of other viable regions in motor control, may hold greater potential. Here, we review comprehensive mechanisms of clinical recovery and factors that govern which mechanism(s) become operative when. We suggest novel approaches that take into account a patient’s initial clinical–functional state, and findings from neuroimaging and neurophysiology to guide to their most suitable mechanism for ideal targeting. Further, we suggest new localization schemes, and bypass strategies that indirectly target peri-lesional circuitry, and methods that serve to counter technical and theoretical challenge in identifying and stimulating such targets at the periphery of infarcts in humans. Last, we describe how stimulation may modulate mechanisms differentially across varying phases of recovery- a temporal effect that may explain missed advantage in clinical trials and help plan for the next stage. With information presented here, future trials would effectively be able to target patient’s specific mechanism(s) with invasive (or noninvasive) neurostimulation for the greatest, most consistent benefit.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0245-y) contains supplementary material, which is available to authorized users.
Keywords: Stroke rehabilitation, Peri-infarct, Plasticity, Cortical stimulation, Function, Neuromodulation, Stimulation, M1, Motor cortex, Premotor cortex, fMRI, DTI, Corticospinal, Epidural stimulation, Transcranial magnetic stimulation, Transcranial direct current stimulation, Reorganization, Dentatothalamocortical pathway
Stroke is the leading cause of long-term adult disability [1]. More than 60 % of survivors experience residual deficits of the paretic upper limb [2], which remains one of the most important predictors of disability and poor quality of life [3]. Disability adds to the costs of disease management that total $74 billion/year [4]. Developing methods to effectively alleviate residual deficits will reduce health and economic burden of stroke.
Several evidence-based rehabilitative approaches have been developed with prominent ones, including neuromuscular electrical stimulation [5], motor learning [6], robotic training [7] constraint-induced movement therapy [8], and bilateral arm training [9], etc. In spite of extensive therapy, recovery is frequently incomplete [10]. There is a critical need for adjuvant strategies that augment rehabilitative outcomes of paretic hand.
The last decade has seen a growing interest in the promise of adjuvant treatments that synergistically influence mechanisms associated with rehabilitation. One such approach is invasive neuromodulation, involving electrical stimulation applied to cortical or subcortical targets spared by stroke. By directly stimulating viable targets, it is believed that potential for plasticity underlying rehabilitation can be enhanced to further recovery [11–16]. Evidence for such emerges from pioneering work in animals [11–16] and clinical trials [17–21].
State of Evidence in Invasive Neuromodulation in Stroke Rehabilitation
The general approach involves stimulating the peri-infarct cortices concurrently with rehabilitative training of the paretic limb. In animals, first, representations of upper limb within the primary motor cortex (M1) are mapped using intracortical microstimulation or electrical stimulation-evoked potentials. Artificial infarcts are created to destroy parts of representation devoted to distal forelimb, offering well-defined, residual peri-lesional representations for tailored stimulation. Subthreshold (40–70 % of motor evoked potential threshold), variable frequency (50–250 Hz), and monopolar or bipolar stimulation has typically been employed. The nature of paired training has varied from skilled reaching or grasping [12–16] to precise manipulation of wrist/digits [11]. In training of paretic limb, paired stimulation of peri-lesional M1 has invariably been advantageous in animals [11–16].
Animal studies have evidenced that the synergistic benefit of stimulation in training involves evolutionarily conserved fundamental neural mechanisms [15]. Stimulating peri-infarct areas initiates large-scale reorganization of spared motor representations. For instance, representations devoted to paretic distal forelimb can re-emerge adjacent to the lesion or, at times, at remote distances [11]. Morphologic and physiologic plasticity underlies gross reorganization; regions in the vicinity of the lesion express higher dendritic density [14], and, ultimately, greater synaptogenesis and enhanced synaptic efficacy [13, 16]. The edge offered by concurrent stimulation in rehabilitation thus appears to involve re-mapping of function to targeted and interconnected regions [11, 15], potentially mediated by long-term potentiation-like synaptic plasticity [16].
Success of animal models translated positively to preliminary clinical [19] and phase I/II clinical trials [17, 18, 20] (see Table 1 for details of all clinical studies). In prospective, open-label, randomized controlled studies, patients with chronic stroke were assigned to either an investigational or a control group. The investigational group received implanted stimulation of M1 in the stroke/ipsilesional hemisphere during rehabilitative therapy, while the control group received rehabilitation alone without implantation. For the investigational group, electrodes were implanted with guidance from functional magnetic resonance imaging (fMRI) [17–21]. The voxel with highest activation in M1 that was localized with frameless stereotaxis [22] served as the center for electrode implantation. Stimulation (50 or 100 Hz) was delivered at a subthreshold level (50 % of movement threshold). For both groups, rehabilitation included occupational therapy to improve shoulder/forearm and hand function, over 3–6 weeks. Phase I [20] and II trials [17, 18] witnessed greater benefit for paretic upper limb in the investigational group than the control group at both, short- (4 weeks) and long-term (24 weeks) follow-up. Patients in the investigational group were explanted a few weeks after the end of rehabilitation protocol.
Table 1.
Design and parameters of clinical studies of invasive cortical stimulation in stroke
| Study [ref.] | Design | Lesion | Baseline function | Localization | Intra-cortical mapping | Parameters | Duration of rehabilitation | Endpoint | Efficacy results |
|---|---|---|---|---|---|---|---|---|---|
| Brown et al. [19] | Case study | Subcortical | Fugl–Meyer = 36 | fMRI activation in peri-rolandic M1 | Intraoperative confirmation of functioning pathways from fMRI target | 3 × 3 array, 50 Hz, 100-ms pulses current ~4.5 mA | 3 wks | Case report | ∆ Fugl–Meyer = 10 |
| Brown et al. [20] | Unblinded, phase I, multicenter clinical trial. Investigational and control groups |
Cortical or subcortical | Fugl–Meyer: investigational = 36 ± 9 Fugl–Meyer: control = 41 ± 5 |
fMRI activation in peri-rolandic M1 | Intraoperative confirmation of functioning pathways from fMRI target in 83 % of patients in investigational group | 3 × 3 array, 50 Hz, 50 % movement threshold. Biphasic pulses. Outer rows anode and cathode |
2 × 1.5-h sessions, daily × 3 wks | Safety | ∆ Fugl–Meyer = 10 in investigational vs 1.9 in control group |
| Levy et al. [18] Huang et al. [17] | Unblinded, phase II, randomized 7-center trial. Investigational and control groups |
Cortical, capsular, or pontine | Fugl–Meyer: investigational = 34.4 ± 8.9 Fugl–Meyer: control 30.5 ± 7.3 |
fMRI activation in peri-rolandic M1 | Intraoperative confirmation not discussed, but during thresholding in rehabilitation, stimulation elicited contralateral response in 42 % of patients in investigational group | 2 × 3 array, 50 or 101 Hz, 50 % movement threshold. Outer rows anode and cathode. 2.7 × 2.6 cm2 area | 2.5 h daily for 6 wks | Efficacy = ∆ Fugl–Meyer = 3.5 | ∆ Fugl– Meyer = 5.5 in investigational vs 1.9 in control. 67 % had clinically meaningful ∆ in investigational group |
| Harvey et al. (2009) [21] (results in press release Northstar Neuroscience Inc.) | Single-blinded, randomized multicenter phase III trial. Investigational and control groups |
Cortical or subcortical above midbrain | Fugl–Meyer: investigational = 37.6 Fugl–Meyer: control 37.6 |
fMRI activation in peri-rolandic M1 | Intraoperative confirmation not discussed, but during thresholding in rehabilitation, stimulation elicited contralateral response in 15.3 % of patients in investigational group | 2 × 3 array, 50 Hz, 50 % movement threshold. Outer rows anode and cathode | 2.5 h daily for 6 wks | Efficacy = ∆ Fugl–Meyer = 4.5 points + ∆ Arm Motor Ability Test of 0.21 | ∆ Fugl–Meyer + Arm Motor Ability = 4.3 and 0.37 in investigational group vs = 4. 0 and 0.26 in control group. 30.8 % had clinically meaningful ∆ in investigational group |
fMRI functional magnetic resonance
The advantage of invasive cortical stimulation for rehabilitation witnessed in early clinical trials, however, failed to generalize to a subsequent pivotal phase III clinical trial [21, 23–25] (Table 1). The study failed to meet its primary composite end-point of efficacy at 4-week follow-up [23, 25], achieving less than the required 20 % difference between investigational and control groups [21]. Atypical findings of phase III, compared to phase I/II and animal studies, are now attributed to numerous factors [23–26], some of which we list briefly in Table 2 and in detail below.
Table 2.
Theoretical-, methodological-, and measurement-related factors implicated in failed phase III trial of invasive stimulation versus early clinical and animal studies
| Factors | Description | |
|---|---|---|
| Theoretical | Types of lesions: animals vs humans | Well-defined cortical lesions in animals, but diffuse, cortical/subcortical/cortical + subcortical lesions in humans |
| Mechanism of reorganization targeted by stimulation | Residual representations in peri-infarct M1 circumscribed in animals—consistent potential for reorganization with stimulation. Poorly-defined lesions of M1 and pathways in humans create inconsistent potential for peri-infarct M1 to reorganize. Other patient-specific neural mechanisms should instead be stimulated |
|
| Stimulation in patients: is it generalizable? | Best effects achieved only in patients with functioning corticospinal pathways. Refining patient selection in future studies. | |
| Methodological | Localization and its confirmation | fMRI activation localized implants in trials, but only phase I confirmed the site elicited contralateral response—signifying functioning pathways |
| Functionality of descending pathways | 83 % and 42 % of investigational group possessed functioning pathways in phase I and II, unlike 15 % in phase III | |
| Focality of stimulation | 2.7 × 2.6 cm2 area may be too focal for humans. Animal studies note greater benefit when stimulation is distributed at periphery of motor targets than in interior of M1. Less-focal, noninvasive cortical stimulation achieves greater ∆ (> 5 points on Fugl–Meyer) with comparable or shorter paired rehabilitation |
|
| % Volume of tissue activated | Volume of human precentral gyrus is 100 times than that in animals; however, stimulation contact area is only 4–7 times larger | |
| Task-specificity in rehabilitation | Animal studies note best effects for task paired with stimulation. Analogously, is stimulation in humans best for laboratory-based tasks or varied types of training used in clinical rehabilitative practice? | |
| Measurement | Study design | Blinded raters in phase III vs early trials, which were open-label. Phase III: large, multicenter set-up vs early trials employed smaller set-up across fewer centers |
| Differences in control | Control group in phase II apparently more impaired (Fugl–Meyer = 30.5 ±7.3; range = 20–44) than in phase III (Fugl–Meyer = 37.6, range = 29–50). So, more benefit for controls in phase III vs II (∆ Fugl–Meyer: 4 vs 1.9) | |
| Defining endpoint | Stricter criteria of efficacy in phase III vs phase II., so fewer % in investigational group achieved clinically meaningful ∆ in phase III |
fMRI functional magnetic resonance imaging; M1 primary motor cortex
Theoretical Factors
Lesions in animals are cortical [12], affecting layers I–VI, unlike in humans where they are diffuse, affecting cortical and subcortical regions. Thus, the probability of damage to corticospinal pathways is exaggerated in humans and the potential of surviving M1 to support recovery alone becomes inconsistent. Although clinical studies choose fMRI activation in peri-infarct M1, its activation alone, as we [5, 27] and others [28] have shown, is not completely predictive of its role in recovery; other regions could partake in mechanisms instead.
Methodological
Although all clinical studies used fMRI to localize stimulation, only phase I used intra-operative stimulation in conjunction. Although phase II/III did not discuss intraoperative mapping, they noted later that contralateral response was elicited in 42 % [18] and 15 % [25] during thresholding. If, in fact, their implantation had been guided by physiologic mapping, using intraoperative [20] or transcranial stimulation [29] besides fMRI, the probability of localizing to sites of functioning pathways could have been high, as in phase I.
Indeed, one of the most cited reasons for differential success between late versus early trials is the variance in distribution of functioning corticospinal pathways—the most important substrate for recovery (Fig. 1) [29]. Success of invasive stimulation diminishes almost linearly from phase I to III trials (Fig. 1). This, we conjecture, is partly attributable to the fact that 83 % of the investigational group in phase I had functioning pathways, so they noted greatest success—a 10-point gain in Fugl–Meyer assessment. However, as this proportion reduced in phase II (42 %) and III (15.3 %), the potency of stimulation also diminished, with a 5.5- and 4.3-point gain, respectively. In phase III, when patients with demonstrated functioning pathways (15 %) in the investigational group alone were examined, gains with stimulation became remarkable and significant [25].
Fig. 1.
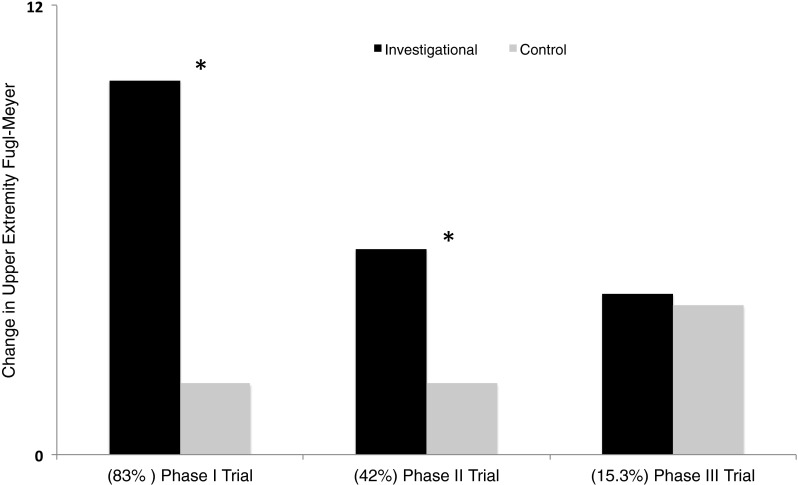
Representation of data from early clinical trials and latest phase III clinical trial of invasive cortical stimulation in stroke. All trials compared an investigational group that received stimulation of peri-lesional M1 during rehabilitation to a control group that received rehabilitation alone. Black bars represent percentage of patients in investigational group who achieved a clinically meaningful change in upper extremity Fugl–Meyer score; gray bars show percentage of patients in the control group who achieved such a meaningful effect. In parentheses below, we list the percentage of patients in the investigational group in respective trials who were deemed to possess functioning corticospinal tracts. These values are derived from Brown et al. [20] (phase I), Levy et al. [18], Huang et al. [17] (phase II), and Nouri and Cramer [25] (phase III trial). Asterisk (*) denotes reported statistical significance between investigational and control groups. One can note a qualitatively inverse relationship between advantage of invasive cortical stimulation and stage of clinical trials. An important reason for such a relation may be the decreasing percentage of patients with functioning corticospinal tracts from early to late clinical trials
Another methodological issue is whether we are even affecting all of the peri-infarct potential in humans. For example, although cytoarchitectonic analysis of human precentral gyrus suggests its volume is 100 times larger than that of rodents, the stimulation contact area in humans is only 4–7 times greater [30]. Is the dramatically variable success of animal versus clinical studies simply a function of a disproportionately smaller percentage volume targeted in humans? Our speculation aligns with the success of noninvasive cortical stimulation that is less focal. Using transcranial magnetic stimulation (TMS) [31], or transcranial direct current stimulation (tDCS), as in Bolognini et al. [32], or in our ongoing study [33], a > 5-point gain in Fugl–Meyer assessment is noted within a few weeks. Thus, it is important to realize the perfect balance between focality and potency of stimulation, as well as the drawbacks of the surgical process itself, which can take a toll on patients’ function, attenuating gains in rehabilitation.
Last, the type of training paired with stimulation is critical. Effects of cortical stimulation in animals are driven in a task-specific manner; improvement is specific to the task that the animal learns during cortical stimulation [12]. By adopting a variety of techniques in rehabilitation in pairing with stimulation, are we diluting its effect? But, then, how else do we generalize benefit?
Measurement
Whereas phase I/II trials adopted an unblinded design across few centers, the phase III trial was a single-blinded, nationwide multicenter study. Potential biases in evaluation, compounded by the inhomogeneity of subject selection and study execution, could have led to variable success between phase III versus earlier, smaller trials. Further, clinically meaningful change was set as an increase of 4.5 on Fugl–Meyer assessment and 0.21 on arm motor ability in phase III; however, in phase II, success was defined as gain of 3.5 on Fugl–Meyer assessment. Thus, even though a change of 4.3 in phase III was not too different from a change of 5.5 in phase II, the proportion of patients in the investigational group that reached the clinical criterion of success was much less in phase III (30.8 %) than in phase II (67 %). Success may also have differed owing to varying baseline characteristics. Patients in the control group in the phase II trial were seemingly more impaired (baseline Fugl–Meyer score: 32.4, range 19–49) than those in phase III (baseline Fugl–Meyer score: 37.6, range 28.5–49.5). This may explain why, in phase II, the control group achieved a rather small gain with rehabilitation—1.9 points versus the corresponding group in phase III, which showed almost as prominent a gain (4 points) as the investigational group. In the end, success of stimulation in early versus pivotal trial may be related to differences in experimental blinding, study structure, subject inhomogeneity, and varying endpoint of success. In building upon these factors, we suggest features in designing future clinical studies that are critical to consider.
Theoretical: What are the Candidate Mechanism(s) to Target?
Are Peri-infarct Processes Targeted in Animal Studies Translatable to Humans?
Even though restoration of peri-infarct circuitry may represent the best basis for stroke motor recovery in animal models with well-defined lesions [11–16], this prospect is not feasible for many patients. Motor representations in peri-infarct cortices are mostly spared in the subset of patients who experience small, focal infarcts in M1 or sensory cortex [34, 35], similar to experimental models of stroke. Even when large peri-lesional representations are spared, they tend to be inconsistent [6, 34, 35] and bear little relation to motor function [36, 37]. Thus, peri-infarct activation may not represent the quintessential mechanism to feasibly target in clinical stroke. Studies in animals even question its sensitivity. Adkins-Muir and Jones [14] discuss the fact that both cathodal and anodal stimulation offer behavioral advantages, but only cathodal stimulation is associated with enhanced neuronal density in peri-infarct M1. As a converse, Kleim et al. [15] report that peri-infarct representations re-map with both bipolar and monopolar stimulation, but behavior is accentuated with monopolar stimulation. Do peri-infarct movement representations even linearly relate to recovery?
Is peri-infarct cortical reorganization sufficient to effectuate recovery? Even though they experience key peri-infarct reorganization, animals with severe impairments do not show benefits of adjunctive stimulation [13]. It may be that their descending pathways are so compromised that stimulation of peri-infarct mechanisms can still not enhance use of the impaired limb. A threshold level of functioning pathways may thus be necessary to realize the benefits of peri-infarct stimulation [13].
Are There Other Processes that can be Effective to Modulate in Humans?
A more commonly evidenced process in clinical recovery, which is relatively underinvestigated in animal models, is the normalization of interhemispheric balance. Following stroke, an imbalance emerges between ipsilesional and contralesional activity due to abnormalities of transcallosal inhibition [8, 38–40]. Stroke reduces inhibition exerted by ipsilesional upon contralesional M1, leading to unabated activation of latter in movement of the paretic hand [41, 42]. Overly-active contralesional M1 exercises greater than typical inhibition upon ipsilesional M1 [8]. As ipsilesional M1 is unable to counter exaggerated inhibition prior to and during movement of paretic hand [38], patients switch to compensating with the nonparetic hand, reinforcing learned non-use of the paretic hand [8].
An important strategy to normalize interhemispheric balance involves neurostimulation, mainly using noninvasive methods such as repetitive TMS (rTMS) or tDCS. Depending on the frequency, low or high rTMS can suppress or enhance excitability of targeted cortices. tDCS, depending on the polarity, can also enhance (anodal) or suppress (cathodal) excitability of the targeted cortex [43]. Studies have generally downregulated contralesional activity using low-frequency rTMS [44] or cathodal tDCS [45], or upregulated ipsilesional activity using high-frequency rTMS [46] or anodal tDCS [47], or simultaneously applied both [48, 49].
Although its simplicity of application, cost-effectiveness, and excellent safety profile is appealing, noninvasive neuromodulation still presents limited (ranging between 8 and 30 %) and transient gains [50]. Its low precision requiring labor-intensive manipulation to ensure consistent targeting presents practical hurdles in stimulating chronically over-repeated outpatient visits [21]. However, as it offers the advantage of promptly elucidating the effects of stimulation in safe and outpatient paradigms, it could serve as a precursor to implanted stimulation, which could strongly restore interhemispheric balance and permanently reverse learned non-use of the paretic hand.
Alternate Substrates and Their Role in Recovery: Cortical Surrogates
Besides interhemispheric balance, ipsilesional areas, remote from the infarct rim such as supplementary motor area and premotor cortices, may vicariate in recovery, that is, assume the role of damaged M1 [24]. In a primate model, axons from the ventral premotor area bypassed the lesioned site [51] to establish atypical remote cortico-cortical connections [52]. Similarly, in humans, higher motor areas, such as dorsal premotor, may act vicariously [5, 53]. Their reversible inhibition, rather than that of ipsilesional M1 [54], can reinstate deficits in recovered animals [54] and humans [55]. Their contribution evidently emerges from substantial (~60 %) and independent projections to corticospinal pathways [56], extensive reciprocal connections with M1 [57], and dense transcallosal connections with their homologues [57]. Although we currently explore how stimulating higher motor areas potentiates vicariation [33], a recent rodent model by Boychuk et al. [30] may offer some translational insight. They have discussed that stimulating distributed regions in the periphery of ipsilesional motor cortices is more efficacious than focal stimulation in the interior of M1.
Alternate Substrates and Their Role in Recovery: Contralesional Hemisphere
Although we have emphasized the importance of mitigating interhemispheric competition as one of the first alternate mechanisms in recovery, we cannot be dismissive of the adaptive role of contralesional hemisphere. There is compelling evidence that contralesional motor cortices play an important role in recovery [42]. Transiently inactivating them completely abolishes the reaching ability of the affected limb in animals with large infarcts [58], and slows response times in patients with severe impairments [59]. Thus, contralesional activity may not, invariably, be overly competitive. Instead, it may be partially compensatory, especially in those who have such extensive damage to corticospinal pathways that stimulation of neither peri-infarct nor alternate motor substrates in ipsilesional hemisphere may afford any benefit [59]. To resolve seeming controversies regarding disparate roles of contralesional motor cortices, it is important to understand where the critical tipping point lies: Where is it that the activity of contralesional motor cortices ceases to be competitive to instead become compensatory?
In summary, it appears that to rectify theoretical factors implicated in the failure of phase III clinical trial of invasive stimulation, novel approaches may have to address interhemispheric balance, or target ipsilesional surrogates, in expanding from the scope offered by peri-infarct M1. In the absence of any resources on the paretic side, however, where ipsilesional motor systems are damaged beyond threshold level, harnessing contralesional activity may be partially adaptive through formation of atypical connections [60–62].
Methodological: Localizing to Substrates and Determining Functionality of Corticospinal Pathways in Stroke
In animal models, stimulation is targeted based on detailed mapping of peri-infarct cortex or based on the expected size of experimental injury. In humans, fMRI activation in peri-lesional region is used instead [22]. Although fMRI is one of the most critical assays of reorganization in stroke [5, 6, 42], its ability to localize stimulation in the peri-infarct region is questionable. This is because its perfusion-based contrast is contorted in areas of vascular compromise in stroke [37]. Thus, sites evoking potentials with intraoperative cortical stimulation imperfectly align with fMRI [22], which means that it is now considered less accurate and reliable in presurgical mapping in functional neurosurgery [63]. Next, fMRI activation in stroke is ultimately dictated by functionality of emergent corticospinal pathways [64]. If compromised, targeting activation with cortical stimulation may be less effective in modulating mechanisms of recovery.
Thus, the functioning and integrity of corticospinal pathways is important to study. To accurately map corticospinal pathways from peri-lesional cortex, several techniques are available for humans, including TMS, or epidural or cortical stimulation [65], and an MRI method called diffusion tensor imaging (DTI) [66]. TMS is becoming a noninvasive proxy for invasive cortical stimulation in functional neurosurgery [63], while DTI is gaining popularity to view cortical terminals of corticospinal tracts [66, 67]. In future studies, TMS and DTI could complement fMRI in identifying eloquent cortices that also project most functioning corticospinal pathways, a feat being explored in our preliminary work [68].
Methodological: Targeting Peri-lesional Substrates Even While Bypassing Them
Alternate strategies to bypass practical limitations of mapping/targeting peri-infarct areas in humans would be valuable. One such method, validated in experimental models in stroke, involves facilitating peri- and ipsi-lesional excitability by stimulating an alternate, intact motor pathway that is remote, yet connected to M1—the dentato-thalamocortical pathway. By targeting it at its node of origin in the dentate nucleus or the ascending pathway, the majority of the tract can be activated with a single implanted multipolar electrode [69]. Invasive, subthreshold stimulation, delivered chronically at lower frequencies (in the beta range) can generate sustained facilitatory effects upon excitability of M1 [70] and promote recovery of the impaired forelimb to a significantly greater degree than in a control [71, 72].
Targeting the dentato-thalamocortical pathway carries potential advantages compared with peri-infarct cortical stimulation. As the pathway projects to cortical areas extending from premotor to parietal, stimulation via this natural output may deliver therapeutic effects to a much larger cortical area than only the cortical arrays. As the effects are mediated by thalamocortical connections, stimulation can also potentially activate cortical areas embedded in deep sulci that are likely to be missed with epidural cortical stimulation [69]. As effects of epidural cortical stimulation depend upon the orientation of neurons to the stimulating electrode [73], effects achieved with deep brain stimulation of the dentato-thalamocortical pathway will likely be independent of gyral anatomy and microstructural variations. Last, targeting the dentato-thalamocortical pathway would obviate the need for mapping peri-infarct cortices in stroke as, by orienting the surgical technique and implant to an anatomically intact, subcortical target, consistent application could be achieved across all patients, reducing inhomogeneity in an already heterogeneous population.
Measurement: Designing for the Future
Invasive neurostimulation trials in stroke have often been performed without sham surgery [21], carrying greater confound of placebo for the investigational group. The risk is only compounded when no experimental blinding is introduced, such as in phase I/II. The ethical dilemma of introducing sham surgery is intense in neurosurgery, given the risk of interventions to the brain. Nevertheless, the importance of generating rigorous empirical evidence cannot be undermined. To balance the ethical–scientific dilemma, a prospect may be to choose randomized, controlled, crossover designs as in neurosurgical trials of neurostimulation for movement disorders [74].
Patient inhomogeneity becomes difficult to control across multiple centers. Although clinical trials set specific criteria (Fugl–Meyer score between 20 and 50), a minimal score of 20 [17–20] can be achieved if patients only possess proximal upper limb control without much hand function or vice versa. Specifying criteria for wrist/hand (as in [29]) or extension (such as in [75] and in our work [5, 33]) may increase the chances that patients with viable ipsilesional motor systems would be enrolled. This is because finger extension is the most important predictor of dexterity [76], and is strongly indicative of functioning corticospinal pathways [29]. Still, while emphasizing the importance of “residual” hand function in predicting success of cortical stimulation, we are cautious of the flip, and unfortunate, side to this argument—that patients with the most severe deficits are likely to be the ones with the greatest need for novel, particularly invasive, and riskier therapies.
Prospects for the Future: Revealing Lesser Known Benefits of Stimulation
Differentiating Between Short- and Long-term Mechanisms of Cortical Stimulation
The temporal profile of advantage of cortical stimulation varies with early versus late period of training, which can be harnessed differentially in rehabilitation. For instance, Teskey et al. [16] and Adkins-Muir and Jones [14] show dramatic differences between groups receiving cortical stimulation versus no stimulation early in training. Similarly, preliminary clinical studies [17, 18, 20] also show greater separation between investigational and control groups early on. We show that adjunctive noninvasive cortical stimulation similarly primes early recovery [77]. This accelerative effect of cortical stimulation may relate to the basis of early learning, such as long-term potentiation or improved synaptic efficacy in superficial layers of M1 [78]. Incorporating serial assessments would confirm the temporal advantage and create opportunities for the use of cortical stimulation as catalysts in rehabilitation.
Besides early benefits, cortical stimulation may facilitate long-term retention of rehabilitative benefit. In phase I and II clinical trials, Brown et al. [20] and Huang et al. [17] note that even after the end of the treatment phase, the investigational group continues to show greater alleviation of impairments compared with the control group. An important caveat is that at delayed follow-up, the group receiving rehabilitation witnesses a regression of function, but this effect is attenuated in the investigational group [17, 20]. Thus, stimulation may promote a myriad of mechanisms that are neuro-restorative, potentially differing from its early accelerative effects. A short bout of fastigial nucleus stimulation in the cerebellum reduces the volume of lesion by putatively protecting the vulnerable penumbra from depolarizing waves [79]. Striatal stimulation also offers neuroprotection in cerebral ischemia, but via alternate mechanisms involving neural repair. Striatal stimulation enhances migration of neural progenitor cells towards the penumbra and their subsequent differentiation into neurons [80]. In acute models, cortical stimulation may operate by upregulating neurotrophic and angiogenic factors in tandem with suppression of apoptotic cell death [81]. The ability of stimulation to induce neuroprotection may emerge from its capacity to salvage ischemic tissue, promote neural repair, and/or lower cell death to help sustain gains from rehabilitation—a promise that remains to be substantiated in future.
Beyond Upper Limb Rehabilitation
Although we started this discussion by focusing on the most common reason for persistent disability in stroke—upper limb deficit—the story of invasive neurostimulation will remain incomplete if we do not outline ongoing investigations in other domains. For the last 2 years, the promise of epidural cortical stimulation is being realized for aphasia therapy. Studies are promising at an early clinical stage, but in learning carefully from the strengths and shortcomings of trials in upper limb rehabilitation, groups are already envisioning customized stimulation for individual patient profiles [82, 83]. Another new dimension for neurostimulation lies in the field of executive planning and memory, impaired not only in stroke, but also in traumatic brain injury, autism, cerebral palsy, and Alzheimer’s disease [84]. The prospect of neurostimulation is still only experimental, but ideas of frequency-contingent learning, where, through a theta rhythm, hippocampal plasticity can be induced, are now showing promise in experimental models [85]. Further, inspired by the vivid percept of memory upon cortical microstimulation [86], new opportunities now await where memory prostheses can be created by use of widespread neurostimulation.
Other directions that would improve generalizability include treatment of neuropathic pain. Pain is an often under-recognized comorbidity after stroke that can significantly limit rehabilitative efforts and hamper quality of life, as well as independent living. In particular, post-stroke central pain syndromes, such as thalamic pain syndrome, can be severely debilitating and recalcitrant to conventional medical management. Traditional cerebral neurostimulation techniques for pain, such as motor cortex stimulation [65] and deep brain stimulation [87], have proven to be of limited benefit in this patient population. To address the needs of this population, we have recently proposed a departure from targeting the somatosensory neural networks to produce analgesia. Instead, we have proposed targeting the neural networks that mediate the affective sphere of chronic pain, aiming to reduce the “suffering” associated with chronic pain, reduce pain-related disability, and facilitate multi-domain facilitation [88]. To empirically test this approach, we are currently conducting a prospective, controlled, randomized clinical trial of deep brain stimulation of the ventral striatum and ventral capsular area in patients with central pain syndrome [89].
Conclusions
Here, we have discussed that relying on a singular mechanism of stroke recovery, that is, peri-infarct reorganization, may have limited translation of success of invasive motor cortical stimulation to clinical care. Nevertheless, the lack of remarkable benefits has created numerous opportunities for learning of various other features influencing stroke recovery. In translating concepts from noninvasive neuromodulation, for instance, it is possible that stronger, more consistent benefits may be achieved with renormalizing interhemispheric balance. Discovery of the potential of alternate pathways and cortical targets that help bypass limitations of peri-infarct stimulation generate promising ideas for translational investigations. Developing strategic predictive algorithms that include the clinico-pathologic state of individual, and hierarchically-arranged logical information from imaging and cortical physiology, would help identify what mechanism best suits an individual and why. Regardless, the promise of cortical stimulation in influencing differing phases of rehabilitation cannot be overlooked; its early accelerative and lasting neuroprotective effects need further exploration.
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
Funding sources include grants from the National Institutes of Health, including NIH R01-HD061363 (AM) and K01HD069504 (EP), and American Heart Association’s 13BGIA17120055 (EP).
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Desrosiers J, Noreau L, Rochette A, Bourbonnais D, Bravo G, Bourget A. Predictors of long-term participation after stroke. Disabil Rehabil. 2006;28:221–230. doi: 10.1080/09638280500158372. [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson PR, Wolfe CD, Warburton FG, et al. A long-term follow-up of stroke patients. Stroke. 1997;28:507–512. doi: 10.1161/01.STR.28.3.507. [DOI] [PubMed] [Google Scholar]
- 3.Ones K, Yilmaz E, Cetinkaya B, Caglar N. Quality of life for patients poststroke and the factors affecting it. J Stroke Cerebrovasc Dis. 2005;14:261–266. doi: 10.1016/j.jstrokecerebrovasdis.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Bakhai A. The burden of coronary, cerebrovascular and peripheral arterial disease. Pharmacoeconomics. 2004;22(Suppl. 4):11–18. doi: 10.2165/00019053-200422004-00004. [DOI] [PubMed] [Google Scholar]
- 5.Plow (Bhatt) E, Nagpal A, Greer KH, et al. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Exp Brain Res. 2007;182:435–447. doi: 10.1007/s00221-007-1001-5. [DOI] [PubMed] [Google Scholar]
- 6.Carey JR, Kimberley TJ, Lewis SM, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- 7.Volpe BT, Lynch D, Rykman-Berland A, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:305–310. doi: 10.1177/1545968307311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taub E, Uswatte G, Morris DM. Improved motor recovery after stroke and massive cortical reorganization following Constraint-Induced Movement therapy. Phys Med Rehabil Clin N Am. 2003;14:S77–S91. doi: 10.1016/S1047-9651(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 9.Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paula Caleffi Segura A, Veloso Fontes S, Maiumi Fukujima M, de Andrade Matas SL. The impact evaluation of physical therapy on the quality of life of cerebrovascular stroke patients. Int J Rehabil Res. 2006;29:243–246. doi: 10.1097/01.mrr.0000230053.08981.48. [DOI] [PubMed] [Google Scholar]
- 11.Plautz EJ, Barbay S, Frost SB, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 12.Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol. 2006;200:356–370. doi: 10.1016/j.expneurol.2006.02.131. [DOI] [PubMed] [Google Scholar]
- 13.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol. 2008;212:14–28. doi: 10.1016/j.expneurol.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003;25:780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- 15.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25:789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- 16.Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003;25:794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- 17.Huang M, Harvey RL, Stoykov ME, et al. Cortical stimulation for upper limb recovery following ischemic stroke: a small phase II pilot study of a fully implanted stimulator. Top Stroke Rehabil. 2008;15:160–172. doi: 10.1310/tsr1502-160. [DOI] [PubMed] [Google Scholar]
- 18.Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J Neurosurg. 2008;108:707–714. doi: 10.3171/JNS/2008/108/4/0707. [DOI] [PubMed] [Google Scholar]
- 19.Brown JA, Lutsep H, Cramer SC, Weinand M. Motor cortex stimulation for enhancement of recovery after stroke: case report. Neurol Res. 2003;25:815–818. doi: 10.1179/016164103771953907. [DOI] [PubMed] [Google Scholar]
- 20.Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006;58:464–473. doi: 10.1227/01.NEU.0000197100.63931.04. [DOI] [PubMed] [Google Scholar]
- 21.Harvey RL, Winstein CJ. Design for the everest randomized trial of cortical stimulation and rehabilitation for arm function following stroke. Neurorehabil Neural Repair. 2009;23:32–44. doi: 10.1177/1545968308317532. [DOI] [PubMed] [Google Scholar]
- 22.Cramer SC, Benson RR, Himes DM, et al. Use of functional MRI to guide decisions in a clinical stroke trial. Stroke. 2005;36:e50–52. doi: 10.1161/01.STR.0000163109.67851.a0. [DOI] [PubMed] [Google Scholar]
- 23.Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res. 2011;192:273–295. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011;77:1076–1083. doi: 10.1212/WNL.0b013e31822e1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hummel FC, Celnik P, Pascual-Leone A, et al. Controversy: Noninvasive and invasive cortical stimulation show efficacy in treating stroke patients. Brain Stimul. 2008;1:370–382. doi: 10.1016/j.brs.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Carey JR, Durfee WK. Plow (Bhatt) E, et al. Comparison of finger tracking versus simple movement training via telerehabilitation to alter hand function and cortical reorganization after stroke. Neurorehabil Neural Repair. 2007;21:216–232. doi: 10.1177/1545968306292381. [DOI] [PubMed] [Google Scholar]
- 28.Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 30.Boychuk JA, Adkins DL, Kleim JA. Distributed versus focal cortical stimulation to enhance motor function and motor map plasticity in a rodent model of ischemia. Neurorehabil Neural Repair. 2011;25:88–97. doi: 10.1177/1545968310385126. [DOI] [PubMed] [Google Scholar]
- 31.Conforto AB, Anjos SM, Saposnik G, et al. Transcranial magnetic stimulation in mild to severe hemiparesis early after stroke: a proof of principle and novel approach to improve motor function. J Neurol. 2012;259:1399–1405. doi: 10.1007/s00415-011-6364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolognini N, Vallar G, Casati C, et al. Neurophysiological and behavioral effects of tDCS combined with constraint-induced movement therapy in poststroke patients. Neurorehabil Neural Repair. 2011;25:819–829. doi: 10.1177/1545968311411056. [DOI] [PubMed] [Google Scholar]
- 33.Plow EB, Cunningham DA, Beall E, et al. Effectiveness and neural mechanisms associated with tDCS delivered to premotor cortex in stroke rehabilitation: study protocol for a randomized controlled trial. Trials. 2013;14:331. doi: 10.1186/1745-6215-14-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer SC, Moore CI, Finklestein SP, Rosen BR. A pilot study of somatotopic mapping after cortical infarct. Stroke. 2000;31:668–671. doi: 10.1161/01.STR.31.3.668. [DOI] [PubMed] [Google Scholar]
- 35.Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–2527. doi: 10.1161/01.STR.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 36.Cramer SC, Shah R, Juranek J, Crafton KR, Le V. Activity in the peri-infarct rim in relation to recovery from stroke. Stroke. 2006;37:111–115. doi: 10.1161/01.STR.0000195135.70379.1f. [DOI] [PubMed] [Google Scholar]
- 37.Binkofski F, Seitz RJ. Modulation of the BOLD-response in early recovery from sensorimotor stroke. Neurology. 2004;63:1223–1229. doi: 10.1212/01.WNL.0000140468.92212.BE. [DOI] [PubMed] [Google Scholar]
- 38.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 39.Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp Neurol. 2008;210:172–181. doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allred RP, Cappellini CH, Jones TA. The “good” limb makes the “bad” limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci. 2010;124:124–132. doi: 10.1037/a0018457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.STR.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 43.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37:2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- 45.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci. 2007;25:123–129. [PubMed] [Google Scholar]
- 46.Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37:1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- 47.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 48.Takeuchi N, Tada T, Toshima M, Matsuo Y, Ikoma K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med. 2009;41:1049–1054. doi: 10.2340/16501977-0454. [DOI] [PubMed] [Google Scholar]
- 49.Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hummel FC, Cohen LG. Non-invasive brain stimulation. Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 51.Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005;25:10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dancause N, Barbay S, Frost SB, et al. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96:3506–3511. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- 53.Ward NS, Newton JM, Swayne OB, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci. 2007;25:1865–1873. doi: 10.1111/j.1460-9568.2007.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128:149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- 55.Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127:747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- 56.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11:667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dancause N, Barbay S, Frost SB, Mahnken JD, Nudo RJ. Interhemispheric connections of the ventral premotor cortex in a new world primate. J Comp Neurol. 2007;505:701–715. doi: 10.1002/cne.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci. 2005;21:989–999. doi: 10.1111/j.1460-9568.2005.03899.x. [DOI] [PubMed] [Google Scholar]
- 59.Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Granziera C, Daducci A, Meskaldji DE, et al. A new early and automated MRI-based predictor of motor improvement after stroke. Neurology. 2012;79:39–46. doi: 10.1212/WNL.0b013e31825f25e7. [DOI] [PubMed] [Google Scholar]
- 61.Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krieg SM, Shiban E, Buchmann N, et al. Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg. 2012;116:994–1001. doi: 10.3171/2011.12.JNS111524. [DOI] [PubMed] [Google Scholar]
- 64.Qiu M, Darling WG, Morecraft RJ, Ni CC, Rajendra J, Butler AJ. White matter integrity is a stronger predictor of motor function than BOLD response in patients with stroke. Neurorehabil Neural Repair. 2011;25:275–284. doi: 10.1177/1545968310389183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Machado A, Azmi H, Rezai AR. Motor cortex stimulation for refractory benign pain. Clin Neurosurg. 2007;54:70–77. [PubMed] [Google Scholar]
- 66.Frey D, Strack V, Wiener E, Jussen D, Vajkoczy P, Picht T. A new approach for corticospinal tract reconstruction based on navigated transcranial stimulation and standardized fractional anisotropy values. Neuroimage. 2012;62:1600–1609. doi: 10.1016/j.neuroimage.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 67.Ellmore TM, Beauchamp MS, O'Neill TJ, Dreyer S, Tandon N. Relationships between essential cortical language sites and subcortical pathways. J Neurosurg. 2009;111:755–766. doi: 10.3171/2009.3.JNS081427. [DOI] [PubMed] [Google Scholar]
- 68.Cunningham DA, Machado A, Rajagopalan V et al. DTI versus fMRI: accuracy and reliability in predicting response to TMS in Stroke. Annals of Neurology: Special Issue 2013 Annual Meeting. 2013;S17:S101.
- 69.Machado A, Baker KB. Upside down crossed cerebellar diaschisis: proposing chronic stimulation of the dentatothalamocortical pathway for post-stroke motor recovery. Front Integr Neurosci. 2012;6:20. doi: 10.3389/fnint.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baker KB, Schuster D, Cooperrider J, Machado AG. Deep brain stimulation of the lateral cerebellar nucleus produces frequency-specific alterations in motor evoked potentials in the rat in vivo. Exp Neurol. 2010;226:259–264. doi: 10.1016/j.expneurol.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Machado AG, Baker KB, Schuster D, Butler RS, Rezai A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res. 2009;1280:107–116. doi: 10.1016/j.brainres.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Machado AG, Cooperrider J, Furmaga HT, et al. Chronic 30-Hz deep cerebellar stimulation coupled with training enhances post-ischemia motor recovery and peri-infarct synaptophysin expression in rodents. Neurosurgery. 2013;73:344–353. doi: 10.1227/01.neu.0000430766.80102.ac. [DOI] [PubMed] [Google Scholar]
- 73.Manola L, Holsheimer J, Veltink P, Buitenweg JR. Anodal vs cathodal stimulation of motor cortex: a modeling study. Clin Neurophysiol. 2007;118:464–474. doi: 10.1016/j.clinph.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 74.Benabid AL, Koudsie A, Benazzouz A, et al. Subthalamic stimulation for Parkinson's disease. Arch Med Res. 2000;31:282–289. doi: 10.1016/S0188-4409(00)00077-1. [DOI] [PubMed] [Google Scholar]
- 75.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 76.Smania N, Paolucci S, Tinazzi M, et al. Active finger extension: a simple movement predicting recovery of arm function in patients with acute stroke. Stroke. 2007;38:1088–1090. doi: 10.1161/01.STR.0000258077.88064.a3. [DOI] [PubMed] [Google Scholar]
- 77.Plow EB, Obretenova SN, Fregni F, Pascual-Leone A, Merabet LB. Comparison of visual field training for hemianopia with active versus sham transcranial direct cortical stimulation. Neurorehabil Neural Repair. 2012;26:616–626. doi: 10.1177/1545968311431963. [DOI] [PubMed] [Google Scholar]
- 78.Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation in the motor cortex. Science. 1989;245:1385–1387. doi: 10.1126/science.2551038. [DOI] [PubMed] [Google Scholar]
- 79.Golanov EV, Zhou P. Neurogenic neuroprotection. Cell Mol Neurobiol. 2003;23:651–663. doi: 10.1023/A:1025088516742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morimoto T, Yasuhara T, Kameda M, et al. Striatal stimulation nurtures endogenous neurogenesis and angiogenesis in chronic-phase ischemic stroke rats. Cell Transplant. 2011;20:1049–1064. doi: 10.3727/096368910X544915. [DOI] [PubMed] [Google Scholar]
- 81.Baba T, Kameda M, Yasuhara T, et al. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/Akt signaling pathway. Stroke. 2009;40:e598–e605. doi: 10.1161/STROKEAHA.109.563627. [DOI] [PubMed] [Google Scholar]
- 82.Cherney LR, Harvey RL, Babbitt EM, et al. Epidural cortical stimulation and aphasia therapy. Aphasiology. 2012;26:1192–1217. doi: 10.1080/02687038.2011.603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cherney LR, Erickson RK, Small SL. Epidural cortical stimulation as adjunctive treatment for non-fluent aphasia: preliminary findings. J Neurol Neurosurg Psychiatry. 2010;81:1014–1021. doi: 10.1136/jnnp.2009.184036. [DOI] [PubMed] [Google Scholar]
- 84.Serruya MD, Kahana MJ. Techniques and devices to restore cognition. Behav Brain Res. 2008;192:149–165. doi: 10.1016/j.bbr.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.La Corte G, Wei Y, Chernyy N, Gluckman BJ, Schiff SJ. Frequency dependence of behavioral modulation by hippocampal electrical stimulation. J Neurophysiol 2013 Nov 6. [DOI] [PMC free article] [PubMed]
- 86.Moriarity JL, Boatman D, Krauss GL, Storm PB, Lenz FA. Human “memories” can be evoked by stimulation of the lateral temporal cortex after ipsilateral medial temporal lobe resection. J Neurol Neurosurg Psychiatry. 2001;71:549–551. doi: 10.1136/jnnp.71.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plow EB, Pascual-Leone A, Machado A. Brain stimulation in the treatment of chronic neuropathic and non-cancerous pain. J Pain. 2012;13:411–424. doi: 10.1016/j.jpain.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Machado AG, Baker KB, Plow E, Malone DA. Cerebral stimulation for the affective component of neuropathic pain. Neuromodulation 2012 Oct 24. [DOI] [PMC free article] [PubMed]
- 89.Plow EB, Malone DA, Jr, Machado A. Deep brain stimulation of the ventral striatum/anterior limb of the internal capsule in thalamic pain syndrome: study protocol for a pilot randomized controlled trial. Trials. 2013;14:241. doi: 10.1186/1745-6215-14-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)


