Abstract
Background
It has been known that positive end-expiratory pressure (PEEP) increases the vasoconstriction threshold by baroreceptor unloading. We compared the effect on the thermoregulatory responses according to anesthetic techniques between an inhalation anesthesia with desflurane and a total intravenous anesthesia (TIVA) with propofol and reminfentanil when PEEP was applied in patients undergoing tympanoplasty.
Methods
Forty-six patients with a scheduled tympanoplasty were enrolled and the patients were divided in two study groups. Desflurane was used as an inhalation anesthetic in group 1 (n = 22), while TIVA with propofol and remifentanil was used in group 2 (n = 24). PEEP was applied by 5 cmH2O in both groups and an ambient temperature was maintained at 22-24℃ during surgery. The core temperature and the difference of skin temperature between forearm and fingertip were monitored for about 180 minutes before and after the induction of general anesthesia.
Results
The final core temperature was significantly higher in group 2 (35.4 ± 0.7℃) than in group 1 (34.9 ± 0.5℃). Peripheral thermoregulatory vasoconstriction was found in 5 subjects (23%) in group 1 and in 21 subjects (88%) in group 2. The time taken for reaching the thermoregulatory vasoconstriction threshold was 151.4 ± 19.7 minutes in group 1 and 88.9 ± 14.4 minutes in group 2.
Conclusions
When PEEP will be applied, anesthesia with TIVA may have more advantages in core temperature preservation than an inhalation anesthesia with desflurane.
Keywords: Desflurane, Inhalation anesthesia, Intravenous anesthesia, Positive end expiratory pressure, Thermoregulation, Vasoconstriction
Introduction
Thermoregulatory response, which maintains the body temperature by controlling of vasodilation or vasoconstriction, occurs as a response according to the core temperature threshold [1]. The interthreshold range of thermoregulation is known as about 0.2-0.3℃; thus body temperature is tightly regulated in the normal state. However, general anesthesia interferes in thermoregulatory responses against cold environment such as heat production, activation of autonomic nervous system and active body response [1]. Thus, the interthreshold range during anesthesia increases ten-times larger by reducing the cold-response threshold.
It has been shown that thermoregulatory responses after general anesthesia can be changed according to various conditions. The degree of decrease in core temperature after general anesthesia varies with the anesthetic agents used for induction and maintenance of anesthesia [2,3,4]. In general, a high concentration of inhalation anesthetics inhibits vasoconstriction more than intravenous anesthetics during anesthesia [1]. Moreover, baroreceptor unloading by positive end-expiratory pressure (PEEP) provokes vasoconstriction, which delays hypothermia induced by general anesthesia [5,6]. The effect of PEEP on thermoregulatory responses has been known in general anesthesia with inhalation anesthetics and TIVA [5,6]. However, there is no comparative study yet about the effect of anesthetic methods (inhalation anesthesia vs. TIVA) on the thermoregulatory responses when PEEP is applied. Thus, we compared the effect on the thermoregulatory responses during PEEP according to the anesthetic techniques in patients undergoing a tympanoplasty.
Materials and Methods
The study was approved by the Institutional Review Board. A total number of 50 patients with a physical status I-II according to the American Society of Anesthesiologists, an age between 20 and 60 years and a scheduled elective tympanoplasty were enrolled. Patients with thyroid disease, Raynaud syndrome, diabetes, hypertension, obesity, or concomitant medication for cardiovascular diseases were excluded. An informed consent form was obtained from each patient after careful explanation of object and procedure of the study.
No premedication was administered before anesthesia. For temperature monitoring, the arms of patients were spared from intravenous line insertion and intravenous catheters were inserted into a vein of legs of patients instead. After arrival at the operation room, monitoring devices (Anesthetic Monitoring System S/5TM, Datex-Ohmeda Inc., Helsinki, Finland) such as electrocardiogram, pulse oximetry, end tidal carbon dioxide (ETCO2) monitor, non-invasive blood pressure (NIBP), and the bispectral index (BIS) monitor (BIS monitor A-2000; Aspect Medical Systems, Norwood, MA, USA) were attached to the patients. Neuromuscular monitor (888418 M-NMT Mechano-Sensor, Datex-Ohmeda Inc., Helsinki, Finland) was attached on the same side arm as for the NIBP monitor to monitor an appropriate degree of muscle relaxation. Lactated Ringer's solution at ambient temperature was used during the operation.
For the peripheral temperature measurement, two thermometers were attached to the patient forearms on the opposite side of blood pressure manometer and wrapped with Tegaderm™ (3M healthcare, Borken, Germany). A skin temperature thermometer was installed on the middle part of an inside forearm and the other one was installed on the index finger tip. Before anesthesia induction, initial core temperature was measured with a tympanic thermometer (Thermoscan IRT 4020, Braun, Kronberg, Germany). An esophageal stethoscope was inserted after induction of anesthesia for the intraoperative monitoring of core temperature. After initial monitoring, the patients were covered with a surgical drape. No other body heating was performed during anesthesia. Ambient temperature was monitored with an indoor thermometer (SH-104S, Saehan, Busan, Korea) near the patient's head and maintained at 22-24℃.
Patients were randomly divided into two groups using computerized random number generation. Patients of group 1 have been administered with propofol 2 mg/kg and rocuronium 1.0 mg/kg intravenously for the induction of anesthesia. After endotracheal intubation, anesthesia was maintained with inhalation anesthetics using desflurane 6 ± 1.0 vol% and 50% oxygen-air mixture. The patients of group 2 have been anesthetized with TIVA using propofol and remifentanil. Minto and Marsh pharmacokinetic models were used for the TIVA with a TCI device (Orchestra® Base Primea, Fresenius Vial, Brézins, France). The targeted effect-site concentrations (Ce) of propofol and remifentanil for induction were 3 µg/ml and 2.5 ng/ml, respectively. When the appropriate neuromuscular blocking effect was achieved after an intravenous injection of rocuronium 1.0 mg/kg, an endotracheal intubation was done. Thereafter, the Ce of propofol and remifentanil were adjusted to 3 ± 0.5 µg/ml and 2.5 ± 2.0 ng/ml, respectively. During anesthesia, BIS score was maintained within the range of 40-60 and the changes of mean blood pressure and heart rate were maintained below 20% of variation. Ephedrine or nicardipine were used to maintain the vital signs if they were not controlled by the adjustment of anesthetics. However, if vasoactive drugs were used, the patient dropped out from the study.
Mechanical ventilation was controlled by tidal volume and respiratory rate to maintain an ETCO2 between 35 and 40 mmHg and the PEEP was applied with 5 cmH2O. After induction of anesthesia, mean blood pressure, heart rate, core temperature, forearm skin temperature, and finger skin temperature were measured with an interval of 15 minutes for 3 hours. The difference between forearm and finger skin temperature was calculated at each moment. If the forearm-finger skin temperature difference became less than 0℃, it was assumed that the peripheral vasodilation has developed. When the forearm-finger skin temperature difference became 0℃, it was considered that the thermoregulatory vasoconstriction has occurred. The core temperature at this point was regarded as the thermoregulatory vasoconstriction threshold. The core temperature at this time and the time taken for reaching the peripheral vasoconstriction threshold were recorded [2,4,5,6]. The total amounts of fluid administration and urinary output were also measured during surgery.
Sample size was calculated with a 0.05 level of significance and 85% power. Effect size was 0.85 which was based on the vasoconstriction threshold of previous study [5,6]. Required sample size was 21 patients in each group. A dropout rate of 20% was assumed for each group and thus 25 patients were enrolled.
All results are presented as mean ± standard deviation. Data were analyzed using SPSS (Windows ver. 12.0, SPSS Inc., Chicago, IL, USA). A t-test was performed to evaluate statistical significance between the two groups for age, weight, height, total amounts of fluid administration, urinary output, ambient temperature, initial core temperature, final core temperature, vasoconstriction threshold, and the time taken for reaching the vasoconstriction threshold. Sex and vasoconstriction number, which were nonparametrically distributed, were analyzed by the Mann-Whitney U test. Repeated measures two-way ANOVA was used to analyze the statistical differences between two groups for heart rate, mean blood pressure, core temperature gradient, and forearm-finger skin temperature difference. Thereafter, t-test was performed to compare the statistical significances between the groups at each time point. Values with P < 0.05 were considered statistically significant.
Results
A total of 50 patients were recruited and 46 patients were enrolled. Two patients in group 1 were excluded because the surgery ended before 180 minutes. From each study group, one patient was excluded because of the movement of the arm where the temperature probe was attached for surgical reasons.
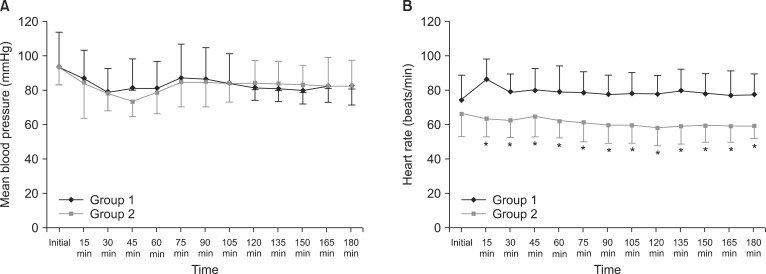
There were no significant differences in demographic data, total fluid volume, urinary output, operating room temperature, and pre-anesthetic core temperature between the two groups (Tables 1 and 2). The heart rate significantly decreased from 15 minutes until 180 minutes after induction in group 2, while the mean blood pressure showed no significant differences during anesthesia (Fig. 1).
Table 1.
Demographic Data, Fluid Intake, and Urinary Output during Anesthesia

Values are mean ± SD. There were no statistical differences between two groups. Group 1: inhalation anesthesia using desflurane with positive end-expiratory pressure (PEEP) 5 cmH2O, Group 2: total intravenous anesthesia (TIVA) with propofol and remifentanil and with PEEP 5 cmH2O.
Table 2.
Intraoperative Thermoregulatory Responses
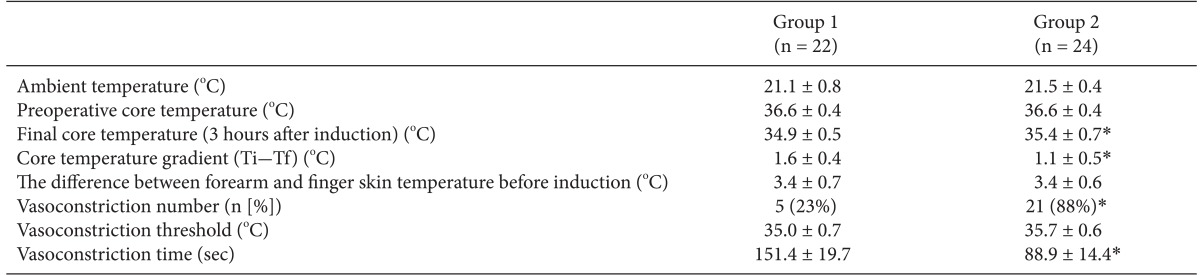
Values are mean ± SD. Ti-Tf: initial core temperature-final core temperature. Group 1: inhalation anesthesia using desflurane with positive end-expiratory pressure (PEEP) 5 cmH2O, Group 2: total intravenous anesthesia (TIVA) with propofol and remifentanil with PEEP 5 cmH2O. *P < 0.05 compared with group 1.
Fig. 1.
Changes in mean blood pressure and heart rate during anesthesia. All data are shown as mean ± SD. There was no significant difference in mean blood pressure between the two groups (A). Heart rate was significantly lower from 15 minutes after induction of anesthesia until the end of the anesthesia in group 2 (B). Group 1: inhalation anesthesia using desflurane with positive end-expiratory pressure (PEEP) 5 cmH2O, Group 2: total intravenous anesthesia (TIVA) using propofol and remifentanil with PEEP 5 cmH2O. *P < 0.05 compared with group 1.
Core temperature went down after induction in both groups and was, in group 2, significantly higher from 15 minutes after induction of anesthesia until 180 minutes after induction (Fig. 2). Final core temperature was significantly higher in group 2 (35.4 ± 0.7℃) than in group 1 (34.9 ± 0.5℃) (Table 2). The core temperature gradient (initial core temperature - final core temperature) was smaller in group 2 (1.6 ± 0.4℃ vs. 1.1 ± 0.5℃) (P < 0.05) (Table 2).
Fig. 2.
Core temperature changes during anesthesia. Core temperature was significantly higher from 15 minutes after induction of anesthesia until 180 minutes in group 2. Group 1: inhalation anesthesia using desflurane with positive end-expiratory pressure (PEEP) 5 cmH2O, Group 2: total intravenous anesthesia (TIVA) with propofol and remifentanil with PEEP 5 cmH2O. *P < 0.05 compared with group 1.
A peripheral thermoregulatory vasoconstriction was found in 5 subjects (23%) in group 1 and in 21 subjects (88%) in group 2. The time taken for reaching the thermoregulatory vasoconstriction threshold was 151.4 ± 19.7 minutes in group 1 and 88.9 ± 14.4 minutes in group 2 (Table 2). This means peripheral vasoconstriction occurred more frequently and shortly in group 2. However, there was no difference in the vasoconstriction threshold between the groups.
After the induction of anesthesia, the difference between forearm and finger skin temperature fell abruptly to less than 0℃, after and then, gradually decreased. In group 2, the difference (Tforearm - Tfingertip) was significantly greater from 90 minutes after induction of anesthesia until 180 minutes after induction (Fig. 3).
Fig. 3.
Difference between forearm and finger skin temperature (Tforearm - Tfingertip). The difference (Tforearm - Tfingertip) was significantly greater from 90 minutes after induction of anesthesia until 180 in group 2. Group 1: inhalation anesthesia using desflurane with positive end-expiratory pressure (PEEP) 5 cmH2O, Group 2: total intravenous anesthesia (TIVA) using propofol and remifentanil with PEEP 5 cmH2O. *P < 0.05 compared with group 1.
Discussion
The aim of the study was to compare thermoregulatory responses between inhalation anesthesia using desflurane with PEEP and TIVA using propofol-remifentanil with PEEP. The results showed that peripheral thermoregulatory vasoconstriction developed more frequently and rapidly during TIVA with PEEP. It seems that the core temperature was more preserved during TIVA with PEEP than during inhalation anesthesia with PEEP. Thus, TIVA was the more effective method in core temperature preservation than the inhalation anesthesia with desflurane when PEEP was applied.
Hypothermia causes many complications such as coagulation disorder, platelet dysfunction, infection, delayed wound healing, delayed recovery, postoperative heart complications, etc. [1,7]. It is known that general anesthesia causes hypothermia mainly by redistribution of heat from the core to the peripheral regions of the body [3]. The flow of arterio-venous shunts, which are located only in acral regions such as fingers, toes, nose, etc., became minimized by tonic sympathetic stimulation in cold environment [1]. Arterio-venous shunt was known to be controlled by alpha adrenergics through norepinephrine which is released from sympathetic nerves. However, anesthetic agents decrease cold-response thresholds and increase the interthreshold range. The flow of arterio-venous shunts cannot be minimized until the core body temperature reached to decreased vasoconstriction threshold by anesthetic agents [1].
Previous studies showed that intravenous anesthetics such as propofol or opiods decreased vasoconstriction threshold markedly and linearly by its vasodilative effect and inhibitory effect on autonomic nervous system [5,7,8,9]. On the contrary, an inhalation anesthetic agent such as desflurane decreases the threshold slightly and non-linearly at low concentration [2,10], and reduces vasoconstriction threshold substantially at high concentration above 1 minimum alveolar concentration (MAC) as much as propofol [1,11].
The degree of reduction in vasoconstriction threshold is various during anesthesia according to the anesthetic agents that were used for the induction of anesthesia or the concentration of inhalation anesthetics [1,4,12]. Even though low concentrations of inhalation anesthetics below 1 MAC are used, propofol which was used during induction of anesthesia can accelerate the decrease of the vasoconstriction threshold. A similar level of decrease in the vasoconstriction threshold could be developed compared to TIVA eventually [4,12]. When a higher concentration of inhalation anesthetics is used during anesthesia, the vasoconstriction threshold decreases more than when propofol is used during TIVA [1].
PEEP increases the vasoconstriction threshold through baroreceptor unloading which augments the peripheral vasoconstriction and catecholamine response to core hypothermia. Those effects were identified in both inhalation anesthesia and TIVA [5,6]. Thus, PEEP has a core temperature preserving effect during anesthesia [1,5,6].
Jung et al. [2] reported that the final core temperature dropped to 33.4 ± 0.3℃ and a vasoconstriction threshold was shown with 33.6 ± 0.4℃ during inhalation anesthesia with desflurane. On the contrary, the final core temperature and vasoconstriction threshold in the present study were 34.4 ± 0.5℃ and 35.0 ± 0.7℃ during an inhalation anesthesia with desflurane and PEEP. Also An and Yang [5] reported that the final core temperature and vasoconstriction threshold were 34.7 ± 0.3℃ and 35.0 ± 0.4℃ respectively, during the TIVA with propofol and remifentanil. However, in the current study, the final core temperature and vasoconstriction threshold were 35.4 ± 0.7℃ and 35.7 ± 0.7℃ during TIVA using propofol and remifentanil with PEEP. Therefore, it seemed that PEEP increased core temperature and vasoconstriction threshold by baroreceptor unloading in both, inhalation anesthesia and TIVA. Additionally the core temperature was higher during TIVA if compared to inhalation anesthesia and the peripheral vasoconstriction occurred more frequently and shortly during TIVA than during inhalation anesthesia in the current study.
We hypothesized that the difference of increase degree in core temperature and vasoconstriction threshold is related to the differences of hemodynamics during inhalation anesthesia and TIVA. Changes of hemodynamics such as blood pressure, heart rate or vascular resistance are detected by baroreceptor. Those changes are regulated by the autonomic nervous system to maintain homeostasis [13]. However, the autonomic nervous system was inhibited and sympathetic nervous modulation to peripheral vasculature was also decreased during anesthesia. For a while, arterial blood pressure increases when the thermoregulatory vasoconstriction has developed [14]. These changes were greater during anesthesia with inhalation anesthetics than with TIVA [14]. Systemic vascular resistance (SVR) is proportional to blood pressure and is inversely proportional to stroke volume and heart rate [13]. In the current study, blood pressures were similar between the groups, while heart rates were significantly lower during TIVA. If the stroke volume is regarded to be under the same condition, SVR is thought to be higher during TIVA because the heart rate was significantly lower. Moreover, it was reported in previous studies that significant reductions in blood pressure and forearm vascular resistance can occur during steady-state anesthesia with desflurane. It seems it has direct relaxing effects on vascular smooth muscles [15], while remifentanil may have effect to increase SVR during continuous intravenous administration [16]. Thus, vasodilation may occur more largely in the initial stage after an inhalation anesthesia with desflurane and vasoconstriction according to thermoregulatory response may develop faster during TIVA. Therefore, we assumed that the arterio-venous shunts flow would be minimized and core temperature could be preserved more effectively during TIVA than during inhalation anesthesia with desflurane. However, the SVR was not directly measured in the current study.
Several limitations were found in our study. First, the hypothesis about the differences in the temperature preserving effect between the inhalation anesthesia and a TIVA during PEEP is theoretical only. Further studies to verify such hypothesis such as an evaluation of autonomic nervous functions, a direct measurement of hemodynamic parameters such as SVR, and blood flow measuring by Doppler ultrasound should be conducted. Second, we assumed the equivalent anesthetic depth has accomplished according to the BIS monitoring during anesthesia. However, it is difficult to find out equally effective anesthetic concentrations that produce equal BIS values when different anesthetics with different mechanisms are used. Third, a BIS range between 40 and 60 is wide enough to influence the anesthetic concentrations. Although we tightly monitored the BIS score and vital signs and adjusted the concentrations of anesthetic (desflurane, 6.0 ± 1.0 vol%; Ce of propofol, 3.0 ± 0.5 µg/ml; Ce of remifentanil, 2.5 ± 2.0 ng/ml), the possibility that thermoregulatory response has been influenced by a subtle distinction of anesthetic concentration cannot be excluded.
Our study was conducted to compare the effects on the thermoregulatory responses in patients with tympanoplasty according to the anesthetic techniques such as inhalation anesthesia with desflurane and TIVA with PEEP application. Due to PEEP, core temperature and vasoconstriction threshold were increased in both, inhalation anesthesia and TIVA. However, the core temperature was higher during TIVA compared to the inhalation anesthesia and peripheral vasoconstriction occurred more frequently and shortly during TIVA than during inhalation anesthesia. In conclusion, when the PEEP has applied, TIVA with propofol and remifentanil may increase the vasoconstrictive threshold and has more advantages to preserve the core temperature than an inhalation anesthesia.
Acknowledgments
This study was supported by research fund from Chosun University, 2012.
References
- 1.Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–338. doi: 10.1097/ALN.0b013e31817f6d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung JD, An TH, Song HS. Thermoregulatory responses of sevoflurane, desflurane, and isoflurane during gynecologic laparoscopic surgery. Korean J Anesthesiol. 2009;56:525–530. doi: 10.4097/kjae.2009.56.5.525. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda T, Sessler DI, Kikura M, Kazama T, Ikeda K, Sato S. Less core hypothermia when anesthesia is induced with inhaled sevoflurane than with intravenous propofol. Anesth Analg. 1999;88:921–924. doi: 10.1097/00000539-199904000-00044. [DOI] [PubMed] [Google Scholar]
- 4.Im UJ, Lee DJ, Kim MC, Lee JS, Lee SJ. Difference in core temperature in response to propofol-remifentanil anesthesia and sevoflurane-remifentanil anesthesia. Korean J Anesthesiol. 2009;57:704–708. doi: 10.4097/kjae.2009.57.6.704. [DOI] [PubMed] [Google Scholar]
- 5.An TH, Yang JW. Effects of PEEP on the thermoregulatory responses during TIVA in patients undergoing tympanoplasty. Korean J Anesthesiol. 2011;61:302–307. doi: 10.4097/kjae.2011.61.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizobe T, Nakajima Y, Sunaguchi M, Ueno H, Sessler DI. Clonidine produces a dose-dependent impairment of baroreflex-mediated thermoregulatory responses to positive end-expiratory pressure in anaesthetized humans. Br J Anaesth. 2005;94:536–541. doi: 10.1093/bja/aei086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurz A, Go JC, Sessler DI, Kaer K, Larson MD, Bjorksten AR. Alfentanil slightly increases the sweating threshold and markedly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;83:293–299. doi: 10.1097/00000542-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Matsukawa T, Kurz A, Sessler DI, Bjorksten AR, Merrifield B, Cheng C. Propofol linearly reduces the vasoconstriction and shivering thresholds. Anesthesiology. 1995;82:1169–1180. doi: 10.1097/00000542-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Morgan G, Mikhail M, Murray M. Clinical anesthesiology. 4th ed. New York: McGraw-Hill; 2006. pp. 192–197. [Google Scholar]
- 10.Annadata R, Sessler DI, Tayefeh F, Kurz A, Dechert M. Desflurane slightly increases the sweating threshold but produces marked, nonlinear decreases in the vasoconstriction and shivering thresholds. Anesthesiology. 1995;83:1205–1211. doi: 10.1097/00000542-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Kurz A. Physiology of thermoregulation. Best Pract Res Clin Anaesthesiol. 2008;22:627–644. doi: 10.1016/j.bpa.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Iwata T, Inoue S, Kawaguchi M, Takahashi M, Sakamoto T, Kitaguchi K, et al. Comparison of the effects of sevoflurane and propofol on cooling and rewarming during deliberate mild hypothermia for neurosurgery. Br J Anaesth. 2003;90:32–38. [PubMed] [Google Scholar]
- 13.Fadel PJ. Arterial baroreflex control of the peripheral vasculature in humans: rest and exercise. Med Sci Sports Exerc. 2008;40:2055–2062. doi: 10.1249/MSS.0b013e318180bc80. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa Y, Iwasaki K, Shibata S, Kato J, Ogawa S, Oi Y. Different effects on circulatory control during volatile induction and maintenance of anesthesia and total intravenous anesthesia: autonomic nervous activity and arterial cardiac baroreflex function evaluated by blood pressure and heart rate variability analysis. J Clin Anesth. 2006;18:87–95. doi: 10.1016/j.jclinane.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Rödig G, Wild K, Behr R, Hobbhahn J. Effects of desflurane and isoflurane on systemic vascular resistance during hypothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:54–57. doi: 10.1016/s1053-0770(97)90253-6. [DOI] [PubMed] [Google Scholar]
- 16.Chanavaz C, Tirel O, Wodey E, Bansard JY, Senhadji L, Robert JC, et al. Haemodynamic effects of remifentanil in children with and without intravenous atropine. An echocardiographic study. Br J Anaesth. 2005;94:74–79. doi: 10.1093/bja/aeh293. [DOI] [PMC free article] [PubMed] [Google Scholar]