Abstract
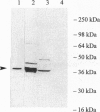
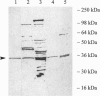
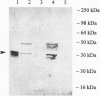
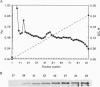
In Escherichia coli and Salmonella typhimurium it has been shown that selenophosphate serves as the selenium donor for the conversion of seryl-tRNA to selenocysteyl-tRNA and for the synthesis of 2-selenouridine, a modified nucleoside present in tRNAs. Although selenocysteyl-tRNA also is formed in eukaryotes and is used for the specific insertion of selenocysteine into proteins, the precise mechanism of its biosynthesis from seryl-tRNA in these systems is not known. Because selenophosphate is extremely oxygen labile and difficult to identify in biological systems, we used an immunological approach to detect the possible presence of selenophosphate synthetase in mammalian tissues. With antibodies elicited to E. coli selenophosphate synthetase the enzyme was detected in extracts of rat brain, liver, kidney, and lung by immunoblotting. Especially high levels were detected in Methanococcus vannielii, a member of the domain Archaea, and the enzyme was partially purified from this source. It seems likely that the use of selenophosphate as a selenium donor is widespread in biological systems.
Full text
PDF
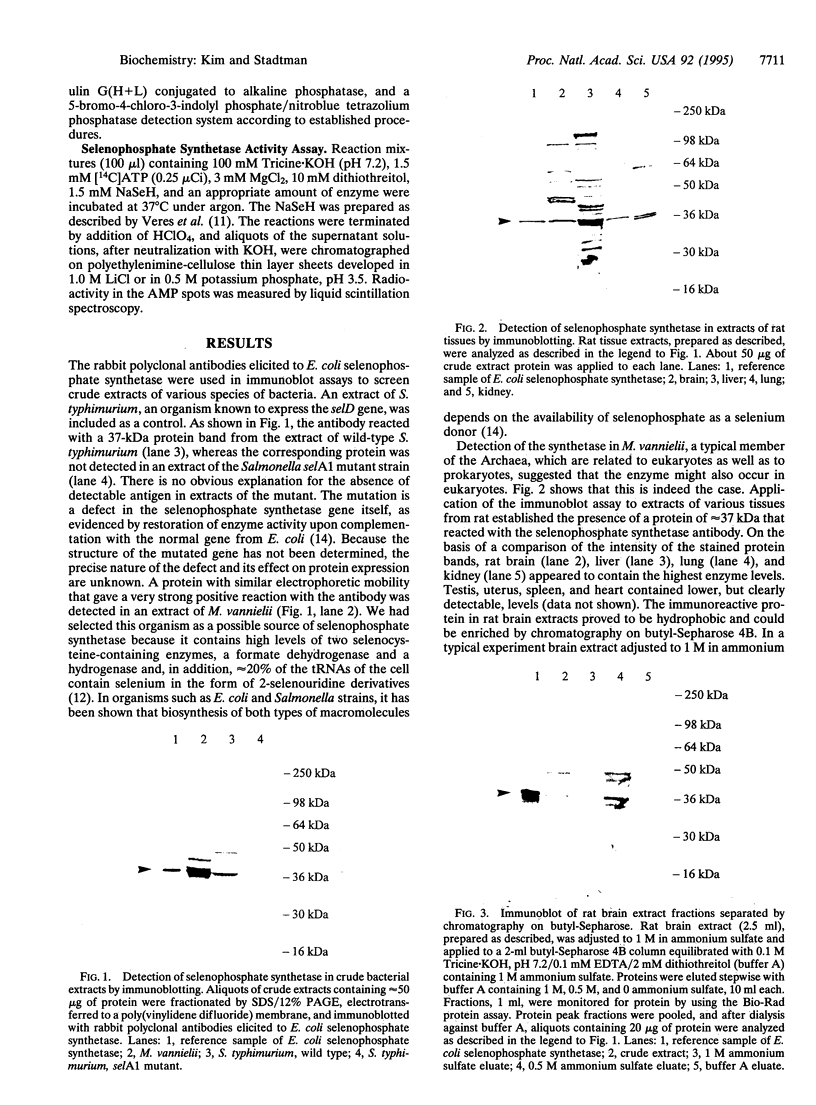
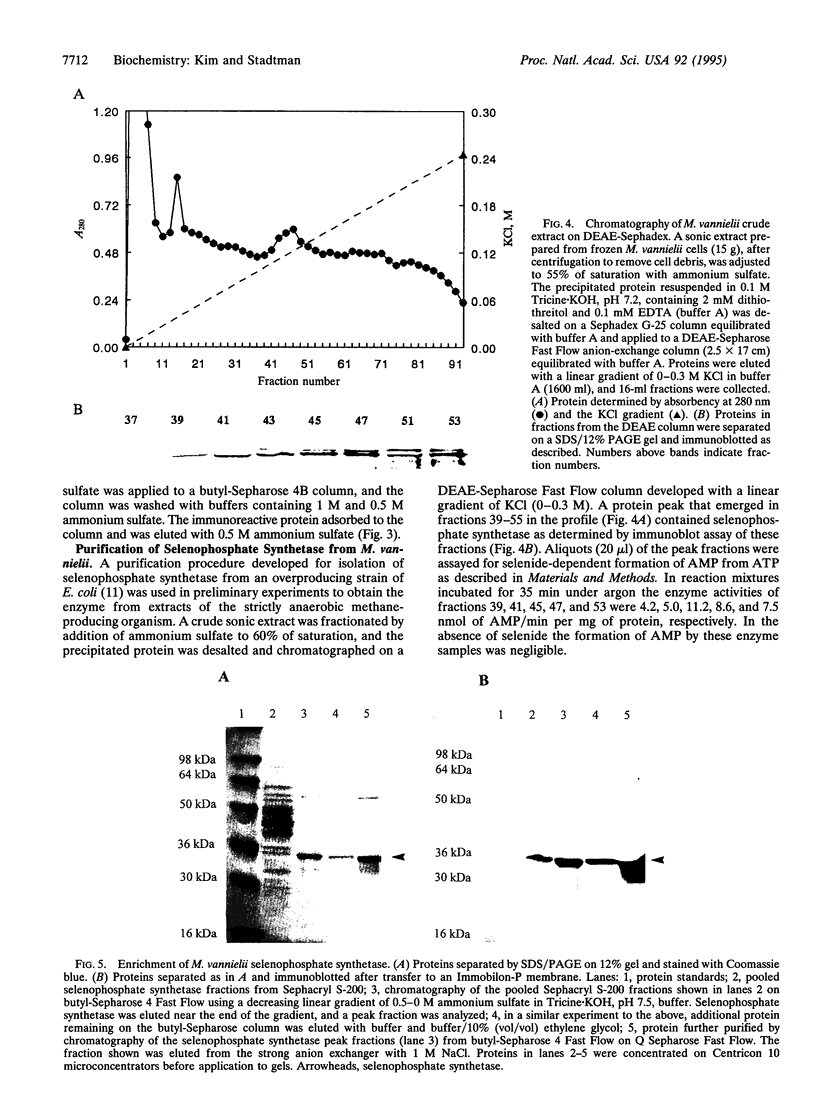

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ching W. M., Wittwer A. J., Tsai L., Stadtman T. C. Distribution of two selenonucleosides among the selenium-containing tRNAs from Methanococcus vannielii. Proc Natl Acad Sci U S A. 1984 Jan;81(1):57–60. doi: 10.1073/pnas.81.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich A., Forchhammer K., Tormay P., Veprek B., Böck A. Selenoprotein synthesis in E. coli. Purification and characterisation of the enzyme catalysing selenium activation. Eur J Biochem. 1992 Jun 15;206(3):767–773. doi: 10.1111/j.1432-1033.1992.tb16983.x. [DOI] [PubMed] [Google Scholar]
- Forchhammer K., Böck A. Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence. J Biol Chem. 1991 Apr 5;266(10):6324–6328. [PubMed] [Google Scholar]
- Forchhammer K., Leinfelder W., Boesmiller K., Veprek B., Böck A. Selenocysteine synthase from Escherichia coli. Nucleotide sequence of the gene (selA) and purification of the protein. J Biol Chem. 1991 Apr 5;266(10):6318–6323. [PubMed] [Google Scholar]
- Glass R. S., Singh W. P., Jung W., Veres Z., Scholz T. D., Stadtman T. C. Monoselenophosphate: synthesis, characterization, and identity with the prokaryotic biological selenium donor, compound SePX. Biochemistry. 1993 Nov 30;32(47):12555–12559. doi: 10.1021/bi00210a001. [DOI] [PubMed] [Google Scholar]
- Kim I. Y., Veres Z., Stadtman T. C. Biochemical analysis of Escherichia coli selenophosphate synthetase mutants. Lysine 20 is essential for catalytic activity and cysteine 17/19 for 8-azido-ATP derivatization. J Biol Chem. 1993 Dec 25;268(36):27020–27025. [PubMed] [Google Scholar]
- Kramer G. F., Ames B. N. Isolation and characterization of a selenium metabolism mutant of Salmonella typhimurium. J Bacteriol. 1988 Feb;170(2):736–743. doi: 10.1128/jb.170.2.736-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Leinfelder W., Forchhammer K., Veprek B., Zehelein E., Böck A. In vitro synthesis of selenocysteinyl-tRNA(UCA) from seryl-tRNA(UCA): involvement and characterization of the selD gene product. Proc Natl Acad Sci U S A. 1990 Jan;87(2):543–547. doi: 10.1073/pnas.87.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C., Davis J. N., Zehelein E., Böck A. Biochemical and genetic analysis of Salmonella typhimurium and Escherichia coli mutants defective in specific incorporation of selenium into formate dehydrogenase and tRNAs. Biofactors. 1989 Mar;2(1):35–44. [PubMed] [Google Scholar]
- Veres Z., Kim I. Y., Scholz T. D., Stadtman T. C. Selenophosphate synthetase. Enzyme properties and catalytic reaction. J Biol Chem. 1994 Apr 8;269(14):10597–10603. [PubMed] [Google Scholar]
- Veres Z., Stadtman T. C. A purified selenophosphate-dependent enzyme from Salmonella typhimurium catalyzes the replacement of sulfur in 2-thiouridine residues in tRNAs with selenium. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8092–8096. doi: 10.1073/pnas.91.17.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres Z., Tsai L., Politino M., Stadtman T. C. In vitro incorporation of selenium into tRNAs of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6341–6344. doi: 10.1073/pnas.87.16.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres Z., Tsai L., Scholz T. D., Politino M., Balaban R. S., Stadtman T. C. Synthesis of 5-methylaminomethyl-2-selenouridine in tRNAs: 31P NMR studies show the labile selenium donor synthesized by the selD gene product contains selenium bonded to phosphorus. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2975–2979. doi: 10.1073/pnas.89.7.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer A. J., Stadtman T. C. Biosynthesis of 5-methylaminomethyl-2-selenouridine, a naturally occurring nucleoside in Escherichia coli tRNA. Arch Biochem Biophys. 1986 Aug 1;248(2):540–550. doi: 10.1016/0003-9861(86)90507-2. [DOI] [PubMed] [Google Scholar]