Abstract
A 38-kDa lipoprotein of Treponema pallidum (Tp38) was predicted to be a periplasmic sugar-binding protein based on its sequence similarity to the glucose/galactose-binding (MglB) protein of Escherichia coli (P. S. Becker, D. R. Akins, J. D. Radolf, and M. V. Norgard, Infect. Immun. 62:1381-1391, 1994). Inasmuch as glucose is believed to be the principal, if not sole, carbon and energy source for T. pallidum and is readily available to the spirochete during its obligate infection of humans, we hypothesized that Tp38 may serve as the organism's requisite glucose receptor. For the present study, a nonacylated recombinant form of Tp38 was coexpressed with GroES and GroEL in E. coli to facilitate the isolation of soluble, properly folded Tp38. The highly sensitive method of intrinsic fluorescence spectroscopy, predicated on the manner in which tryptophan residues reside and move within protein microenvironments, was then used to assess sugar binding to Tp38. The intrinsic fluorescence of Tp38 was essentially unaltered when it was exposed to d-mannose, d-fucose, d-ribose, l-glucose, or l-galactose, but it changed markedly in the presence of d-glucose, and to a lesser extent, d-galactose, indicating binding. The Kd values for d-glucose and d-galactose binding to Tp38 were 152.2 ± 20.73 nM and 251.2 ± 55.25 nM, respectively. Site-directed mutagenesis of Trp-145, a residue postulated to contribute to the sugar-binding pocket in a manner akin to the essential Trp-183 in E. coli MglB, abolished Tp38's conformational change in response to d-glucose. The combined data are consistent with Tp38 serving as a glucose receptor for T. pallidum. These findings potentially have important implications for syphilis pathogenesis, particularly as they may pertain to glucose-mediated chemotactic responses by T. pallidum.
Treponema pallidum, the spirochetal agent of syphilis, continues to be highly enigmatic. Despite the availability of T. pallidum genome sequence information (11), many fundamental aspects of the organism's basic physiology and metabolism remain obscure (30). In this regard, members of our laboratory previously reported that a 38-kDa membrane lipoprotein of T. pallidum (Tp38) was 58.9% similar to the MglB (glucose and galactose binding) proteins of Escherichia coli and Salmonella enterica serovar Typhimurium (3), suggesting that it is a homolog of E. coli MglB (38). Genomic sequence analysis actually has predicted two genes encoding MglB orthologs in T. pallidum (TpMglB-1 [Tp0545] and TpMglB-2 [Tp0684]) (11), with Tp38 corresponding to TpMglB-2. Notably, we and others have reported that the tp38 (tp-mglB) gene (Tp0684) is carried within a typical ATP-binding cassette (ABC) transport-like operon, inclusive of a downstream probable ATPase (tp-mglA) and a putative channel-forming polypeptide (tp-mglC) (11, 31, 39). No such ABC-like operonic arrangement has been noted for the gene encoding TpMglB-1 (Tp0545) (11), prompting efforts first to examine the biochemical properties of TpMglB-2, which is designated Tp38 herein for simplicity.
Whereas analogous receptors of ABC transport systems tend to be heterologous at the primary sequence level, they often possess similarities with respect to the manner in which their binding clefts are configured (6, 33, 40). In this regard, of the 19 amino acids that form the carbohydrate binding pocket of E. coli MglB (34), Tp38 has identity with 11, including Trp-145, which likely corresponds to the essential Trp-183 in E. coli MglB (3). Topologically, Tp38 is believed to be tethered via its lipid moieties to the outer leaflet of the cytoplasmic membrane in T. pallidum, giving rise to a periplasmic location consistent with it serving as a receptor (E. coli MglB is also periplasmic). By analogy with E. coli MglB, it is thus plausible that Tp38 functions as a carbohydrate receptor in T. pallidum, most likely for glucose. Notably, glucose is believed to be the principal, if not sole, carbon and energy source for T. pallidum (1, 2, 28, 30, 36). If so, Tp38 (and/or TpMglB-1, perhaps) may play a central role in T. pallidum virulence by comprising the uptake pathway(s) for the organism's requisite energy source. However, all predictions to date regarding the putative function of Tp38 or TpMglB-1 in T. pallidum have been based solely on theory. In the present study, we provide the first direct evidence that a recombinant version of Tp38 binds glucose in a manner consistent with the native molecule serving as a receptor for glucose transport in T. pallidum.
MATERIALS AND METHODS
Recombinant DNA techniques.
A nonacylated version of Tp38 was constructed as a His-tagged fusion protein encoded by pQE32 (Qiagen). To accomplish this, a DNA fragment encoding amino acid residues 2 to 378 (residue 1 is a Cys of the mature [processed] form) of Tp38 (3) was amplified from T. pallidum DNA by PCR using high-fidelity Pfu DNA polymerase (Stratagene). Oligonucleotide primers for PCR were synthesized by IDT Inc. (Coralville, Iowa). The sense primer (5′-ATGCATGCCCTCATCCAAGACGGATGTCAC-3′) consisted of an SphI site appended to the codons for residues 2 to 8. The antisense primer (5′-ATAAGCTTCAGTATTTGAGCTTGTCTGTATAGT-3′) contained a HindIII site attached to the complementary sequence for residue 371 through the termination codon. The resulting PCR fragment was cloned between the SphI and HindIII sites within pQE32. The vector containing the insert was transformed into E. coli XL1-Blue competent cells (Stratagene). Transformants were selected on Luria-Bertani (LB) agar medium containing 100 μg of ampicillin per ml. Plasmid DNA was isolated and the construct was verified by DNA sequencing. The resulting construct was referred to as pTp38.
The expression of Tp38 from pTp38 resulted in the production of insoluble protein in E. coli. To obtain soluble Tp38, we used a strategy whereby Tp38 was coexpressed in the presence of high amounts of the E. coli chaperonins GroES and GroEL. Plasmid pGroESL, which carries the genes encoding GroES and GroEL under the transcriptional control of the lac promoter (13), was kindly provided by Philip J. Thomas (Department of Physiology, UT Southwestern Medical Center). This plasmid was electroporated into the XL1-Blue strain of E. coli containing the pTp38 construct. Double transformants were selected on LB agar plates containing 100 μg of ampicillin per ml and 100 μg of chloramphenicol per ml. Test expression of these coresident plasmids was performed by incubating cultures at 30°C for 5 h after the addition of 0.6 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and then subjecting induced E. coli cells to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 1).
FIG. 1.

Expression of Tp38 in E. coli and purification of recombinant protein. Cell lysates were prepared from E. coli harboring pTp38 and pGroESL (encoding GroEL and GroES) either prior to (lane 1) or after (lane 2) induction with IPTG. Recombinant Tp38 was then purified from induced cultures by Ni-affinity column chromatography followed by gel filtration chromatography via FPLC (lane 3). Proteins were subjected to SDS-PAGE (12.5% resolving gel) and visualized by Coomassie blue staining. The positions of GroEL and Tp38 are noted, whereas low-molecular-weight GroES migrated out of this gel. Molecular mass markers (in kilodaltons) are indicated at the left.
Site-directed mutations within tp38 on the pTp38 plasmid were created by using the Stratagene QuikChange mutagenesis system. For creation of the W37F mutant, the sequence of the forward oligonucleotide primer was 5′-CATGGCTGCCATGAACTTTAACGACAAAAC-3′. The reverse primer sequence was 5′-GTTTTGTCGTTAAAGTTCATGGCAGCCATG-3′. The bold letters indicate the site of mutagenesis, resulting in a change from Trp to Phe. For the W145F mutant, the forward primer sequence was 5′-GACGGTTCCACTTTTAATACGAATTCTGCA-3′ and the reverse primer sequence was 5′-TGCAGAATTCGTATTAAAAGTGGAACCGTC-3′. All mutations were verified by DNA sequencing.
Culture conditions, protein purification, and mass spectrometry.
An overnight culture of E. coli containing the coresident plasmids was diluted 1:50 in 1 liter of LB broth containing ampicillin and chloramphenicol (100 μg/ml [each]). The culture was incubated at 37°C on a rotary shaker at 250 rpm. When the culture reached the mid-logarithmic phase of growth (optical density at 600 nm of about 0.6), the culture was shifted to 30°C, IPTG was added to a final concentration of 0.6 mM, and the culture was incubated with shaking for an additional 5 h.
Unless otherwise stated, all protein purification steps were performed at 4°C. Cells were first harvested by centrifugation at 4,000 × g for 15 min and were suspended in 50 ml of 50 mM Tris-HCl buffer (pH 8.5) containing 500 U of DNase I and 20 mg of lysozyme (Sigma). After 1 h on ice, cells were further disrupted by sonication (Branson sonifier; Branson Ultrasonic Corp.) (output control = 7, duty cycle = 50%, and total time = 3 min), and the lysate was cleared by centrifugation at 15,000 × g for 20 min.
Soluble protein was purified from the lysate by standard Ni affinity column chromatography (Qiagen). The eluate was buffer exchanged by using a PD-10 column (Amersham Biosciences) and 50 mM sodium phosphate buffer (pH 7.4) plus 20 mM NaCl. The protein was further concentrated by using a centrifuge membrane filter device with a molecular exclusion of 30,000 Da (Millipore). The concentrate was then loaded onto a Superdex S-200 column that was preequilibrated with 50 mM sodium phosphate buffer (pH 7.4) plus 20 mM NaCl, using an Äkta fast-performance liquid chromatography (FPLC) system (Amersham Biosciences). Subsequent to elution of the protein from the column in the same buffer, the protein-containing fractions were analyzed by SDS-PAGE. At this stage, the protein was pure to apparent homogeneity (i.e., >95%). Fractions containing purified Tp38 were pooled and stored at 4°C for no more than 2 weeks prior to being used for biochemical studies. The molecular weight of the purified protein was estimated by size exclusion chromatography, using a calibration curve made with known protein standards (Bio-Rad). Mutant versions of Tp38 were purified in the same manner as wild-type Tp38.
Protein concentrations were determined from molar extinction coefficients at 280 nm (12); these values were 49,500 M−1 cm−1 (Tp38) and 38,120 M−1 cm−1 (W145F and W37F). The masses of recombinant or mutant Tp38 proteins were determined by electrospray ionization mass spectrometry (ESI-MS) (9).
Recombinant TroA was purified from E. coli as previously described (8). Recombinant Tp47 was isolated according to previously published methods (9).
Preparation of ligand-free Tp38.
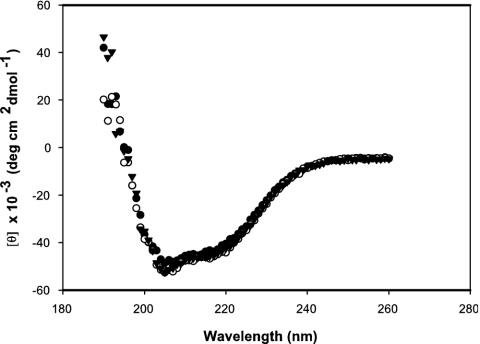
For release of any ligand that was potentially bound to recombinant Tp38 purified from E. coli, the protein was mildly denatured in 3 M guanidine-HCl (pH 7.4). Tp38 was then exhaustively dialyzed against 50 mM sodium phosphate buffer (pH 7.4) plus 20 mM NaCl (26). After dialysis and renaturation, residual insoluble protein was removed from the soluble refolded protein by centrifugation at 10,000 × g for 20 min. The resultant soluble protein was considered to be ligand-free Tp38. Note that the Kd for d-glucose binding to ligand-free Tp38 was 10-fold lower than that for binding to untreated Tp38 (not shown), suggesting that Tp38 purified from E. coli contained some endogenously bound ligand. Proper folding of the renatured protein was verified by circular dichroism (CD) (see below). Unless otherwise noted, all experiments were carried out by using this renatured protein.
CD measurements.
All CD spectra were collected on an Aviv 62DS spectropolarimeter (Aviv Inc.), using a 1-mm path-length quartz cuvette and averaging three repetitive scans between 260 and 190 nm. Typically, measurements were performed at 25°C for 1 μM protein in 10 mM sodium phosphate buffer plus 20 mM NaCl (pH 7.4). The mean residue ellipticity (θ) was calculated from the following equation: [θ] = θ × M/10 × d × c, in which 107.7 was used for M (the mean amino acid residue weight), d was the cell path (in centimeters), and c was the concentration of the protein in milligrams per milliliter.
Intrinsic fluorescence spectroscopy.
Intrinsic fluorescence measurements, based on tryptophan microenvironments within proteins, were performed with Tp38 or mutant proteins in an LS50B spectrofluorometer (Perkin-Elmer). The tryptophan residues in Tp38 were excited at 295 nm (to avoid excitation of tyrosine or phenylalanine residues), and the emission spectra and fluorescence intensities, which were measured in relative fluorescence units, were obtained prior to and after the addition of ligands. Spectra were recorded by using the following parameters: excitation wavelength, 295 nm (slit width of 5 nm); scanning speed, 100 nm/min; emission slit width, 2.5 nm; and recording (emission) range, 310 to 400 nm. All measurements (averaging three repetitive scans) were typically performed at 25°C in a 1.0-ml volume quartz cuvette containing 1 μM protein, 10 mM sodium phosphate buffer (pH 7.4), and 20 mM NaCl. The effect of the ligands on the intrinsic fluorescence of Tp38 was measured by adding ligands at various concentrations. Care was taken to ensure that the volume of ligand added was not more than 1% of the total volume. The relative fluorescence change of Tp38, ΔF at 347.5 nm, upon ligand binding was defined as ΔF = F0 − FL, where F0 is the initial fluorescence of the sample and FL is the fluorescence of the sample at a given concentration of ligand. The resultant fluorescence changes (ΔF at 347.5 nm) were subsequently subjected to kinetic analyses according to the equilibrium equation described below.
Analysis of kinetic data.
Additions of either d-glucose or d-galactose specifically decreased the intensity of the fluorescence emission spectra when purified Tp38 was excited at 295 nm. For nonlinear regression analysis, the dependence of the change in fluorescence intensity (ΔF) at a ligand concentration ([L]) was fitted to the following equation (equation 1): ΔF = ΔFmax × [L]/([L] + Kd), where [L] is the ligand (sugar) concentration, ΔFmax is the maximal change in fluorescence intensity for a saturating [L], and Kd is the dissociation constant of the protein-ligand complex (derived from the concentration of ligand at which ΔF = ΔFmax/2). Fits of data to the equation were performed with Prism 3.03 software (GraphPad Software). Whereas data are shown for representative experiments, all experiments were performed at least three times, using independently purified batches of protein.
RESULTS AND DISCUSSION
Expression, purification, and characterization of recombinant Tp38.
The expression of nonacylated, His-tagged Tp38 in E. coli cultivated at either 30 or 37°C was inefficient and gave rise mostly to insoluble protein. This problem appeared to be largely due to protein misfolding, because it was circumvented by coexpression of the same fusion construct in E. coli with the chaperonins GroEL (Fig. 1, lane 2) and GroES (not shown; low-molecular-mass [10-kDa] GroES expression was verified on a separate 15% polyacrylamide gel) (13). The purification of Tp38 from 1 liter of cell culture, based on Ni-affinity chromatography followed by gel filtration chromatography using FPLC, yielded about 20 mg of protein that was >95% pure (Fig. 1).
Inasmuch as the receptors of ABC transporters tend to bind their cognate ligands with high affinities, it was possible that recombinant Tp38 from E. coli might have contained endogenously bound ligand. The presence of such endogenously bound ligand is not unusual for ligand-binding proteins (21, 26, 27) and can give rise to aberrant behaviors in in vitro binding studies (26). Thus, to obtain Tp38 devoid of endogenous ligand, we first partially denatured Tp38 (verified by CD and fluorescence spectroscopy; not shown), followed by renaturation by dialysis. The conformational homogeneity of refolded, ligand-free Tp38 was initially assessed by size exclusion chromatography; like untreated Tp38, the refolded protein resolved as a single elution peak (consistent with conformational homogeneity) (not shown) that had a slightly lower molecular mass than the ovalbumin standard (44,000 Da). This observed mass and the mass determined by ESI-MS (42,205 Da) were both near the theoretical mass of Tp38 (42,208 Da), indicating that Tp38 behaved as a monomer in solution.
CD analyses.
CD was used to define the structural features of Tp38 in either its untreated or ligand-free form. The far UV CD spectra of untreated and ligand-free Tp38 were very similar, indicating that the proteins were correctly folded and that the partial denaturation process was reversible (Fig. 2). The two minima of the spectra, at about 205 and 216 nm, are typical of a fold with a majority of α-helical secondary structure. These observed α-helical determinations were also in agreement with PredictProtein secondary structure predictions indicating a 35% helical content for Tp38 (http://cubic.bioc.columbia.edu/predictprotein/).
FIG. 2.
CD spectra of various Tp38 preparations. Samples (1 μM) were scanned from 260 to 190 nm. Mean residue ellipticities are shown as a function of the wavelength. Inverted triangles, untreated Tp38; solid circles, ligand-free Tp38; open circles, W145F mutant Tp38.
Intrinsic fluorescence assessments of sugar binding by Tp38.
Intrinsic fluorescence is widely used as a sensitive measure of ligand-induced conformational changes within ligand-binding proteins (20). Several sugars were thus evaluated by intrinsic fluorescence for the ability to bind to Tp38. Among these were d-glucose and d-galactose, inasmuch as MglB orthologs typically bind both d-glucose and d-galactose (C4 epimer of d-glucose) (26). l-Glucose and l-galactose were selected as negative controls because l-isomers typically cannot substitute for d-isomers in binding to MglB (44). d-Mannose (C2 epimer of d-glucose) and d-fucose (6-deoxy derivative of d-galactose) are two additional hexoses that are closely related to d-glucose. d-Ribose was selected as a nonhexose monosaccharide which can sometimes interact with MglB orthologs (24).
The intrinsic fluorescence emission of Tp38 exposed to l-glucose or l-galactose (negative controls) was changed only about 5% (Fig. 3). Changes in the intrinsic fluorescence of recombinant TroA, a periplasmic ABC metal-binding protein of T. pallidum (8, 19), or Tp47, a T. pallidum periplasmic penicillin-binding protein (9, 46), in the presence of d-glucose were below 2% (not shown). In comparison with all of these baseline (negative control) values, the intrinsic fluorescence of Tp38 changed markedly only in the presence of d-glucose or d-galactose (Fig. 3), denoting a specificity for one or both of these hexoses among the sugars tested. However, the relative reduction in fluorescence was significantly higher for d-glucose (37.6%) than for d-galactose (14.2%), suggesting that d-glucose is the preferred ligand for Tp38.
FIG. 3.
Percent change in intrinsic fluorescence of ligand-free Tp38 exposed to various sugars. Experiments were performed using 1 μM protein either in the absence or presence of each sugar (1 μM). Changes in intrinsic fluorescence in the range of 5% (dotted line) were considered negligible. d-Glu, d-glucose; d-Gal, d-galactose; d-Man, d-mannose; d-Fuc, d-fucose; d-Rib, d-ribose; l-Glu, l-glucose; l-Gal, l-galactose.
Sugar-induced conformational changes in Tp38.
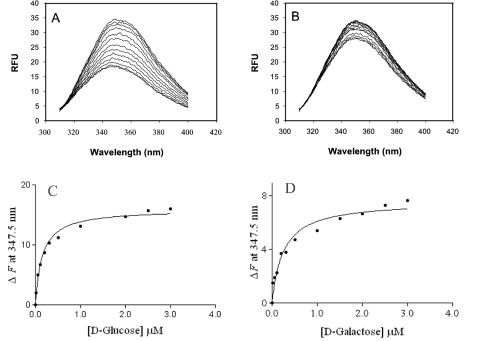
The intrinsic fluorescence emission of Tp38 decreased in response to increasing concentrations of d-glucose or d-galactose (Fig. 4A and B), indicating the presence of ligand-induced, concentration-dependent conformational changes. Such conformational changes, however, were not accompanied by apparent changes in Tp38's secondary structure, in that the CD spectra of Tp38 in the presence or absence of d-glucose were identical (not shown). These combined findings imply that upon binding of d-glucose, there is a reshaping of the protein's tertiary structure without a concomitant change in the overall secondary structural elements.
FIG. 4.
Changes in intrinsic fluorescence emission spectra of Tp38 exposed to various concentrations of d-glucose or d-galactose. Ligand-free Tp38 was incubated with 0.00, 0.01, 0.05, 0.1, 0.2, 0.3, 0.5, 1.0, 2.0, 2.5, or 3.0 μM (top line to bottom line, respectively) d-glucose (A) or d-galactose (B). Emission spectra were recorded from 310 to 400 nm. RFU, relative fluorescence units. The observed changes in intrinsic fluorescence (ΔF) were then plotted as a function of either d-glucose (C) or d-galactose (D) concentration. The solid lines in panels C and D reflect the best-fit curves, calculated by use of equation 1 (see Materials and Methods), that were used to derive the respective Kd values.
In general, the fluorescence characteristics of a tryptophan residue, the underlying principle of intrinsic fluorescence as an analytical approach, are highly dependent on its microenvironment; fluorescence emission maxima can vary from 320 nm (in a nonpolar environment) to 355 nm (in a polar environment) (10). As shown in Fig. 4A and B, the emission maximum for Tp38 with either d-glucose or d-galactose was 347.5 nm, just below the emission maximum for a free tryptophan in an aqueous environment (10, 20). These results imply that of the six tryptophan residues within Tp38, at least one is partially exposed in a hydrophilic microenvironment, potentially very near or perhaps comprising the binding pocket.
Fluorescence titrations (Fig. 4A and 4B) were then used to determine the binding affinities (Kd values) for d-glucose and d-galactose binding to Tp38. ΔF versus the concentration of d-glucose or d-galactose was determined by use of equation 1, and the apparent Kd for each case was estimated from the respective curves (Fig. 4C and D). By this approach, the calculated Kd of d-glucose was 152.2 ± 20.73 nM (Fig. 4C), which compares favorably to the Kd value (117 nM) for E. coli MglB (26). These data are also consistent with the simple binding character of other bacterial ligand-binding proteins, and in addition, Scatchard analysis yielded a linear plot (not shown). In analogous experiments, the derived Kd of d-galactose was 251.2 ± 55.25 nM (Fig. 4D), which is almost twofold higher than the Kd of d-glucose. The lower Kd for d-glucose again was consistent with a possible predilection by Tp38 for d-glucose.
Trp-145 is implicated in the sugar-binding pocket of Tp38.
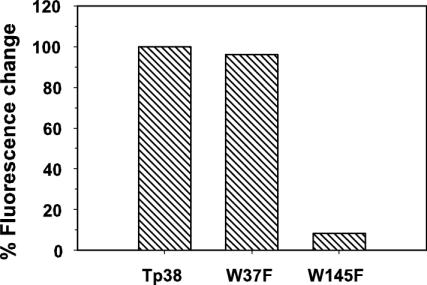
Tp38 contains six Trp residues, at positions 37, 106, 145, 157, 262, and 280 (3). Members of our laboratory previously postulated (3) that Trp-145 is equivalent in importance to Trp-183, which contributes to the binding pocket of E. coli MglB and mediates the hydrophobic contact between the protein and d-glucose or d-galactose (6). To test this hypothesis, we performed site-directed mutagenesis to create a Tp38 mutant protein (W145F) wherein Trp-145 was replaced with Phe. For comparative purposes, one other mutant Tp38 protein was created wherein Phe was substituted for Trp-37 (W37F). In the presence of 1.0 μM d-glucose, the mutant protein W37F displayed a fluorescence change comparable to that of wild-type Tp38 (Fig. 5), indicating normal binding of d-glucose by the W37F protein. In contrast, the fluorescence emission spectrum of the W145F mutant protein was only 8.4% of that observed for wild-type Tp38 in the presence of d-glucose, denoting the failure of the W145F protein to bind d-glucose (Fig. 5). The failure of the W145F mutant protein to bind d-glucose was not attributable to changes in the protein's secondary structure, in that the CD spectra of the W145F mutant protein and wild-type Tp38 were identical (Fig. 2). The simplest explanations for these combined observations are that Trp-145 in wild-type Tp38 is an important residue for the binding of d-glucose and that Trp-145 likely resides in or very near the glucose-binding pocket.
FIG. 5.
Percent change in intrinsic fluorescence of wild-type Tp38 and mutant proteins W37F and W145F exposed to d-glucose (1 μM). The percent change in intrinsic fluorescence of wild-type Tp38 exposed to d-glucose was considered to be 100%; all other values were expressed as a proportion of this maximal value.
Conclusions and implications.
An important undercurrent in the postgenomics era is the necessity to verify theoretical predictions of protein function through direct experimentation (41). In the case of T. pallidum, this tenet poses particular challenges due to our inability either to cultivate the spirochete in vitro or, consequently, to genetically manipulate it in any manner (30). Contemporary investigations of a predicted T. pallidum gene and/or protein function(s) therefore must rely completely on surrogate systems or in vitro approaches (30). Perhaps among the best examples to date of such pursuits are studies with TroA, an ABC-like multiple metal transporter in T. pallidum (8, 19, 32). Along similar lines, but using different methods, we pursued a biochemical approach with a recombinant protein to establish that a nonacylated version of the Tp38 lipoprotein binds glucose in a manner consistent with it serving as a glucose receptor in T. pallidum. Tp38, therefore, is likely a functional equivalent of E. coli MglB, as previously predicted (3). Site-directed mutagenesis of Tp38's Trp-145, believed to be the functional equivalent of Trp-183 which is essential in the binding pocket of E. coli MglB (45), abolished Tp38's conformational change in response to d-glucose, further suggesting that even the binding pocket of Tp38 is functionally akin to that of E. coli MglB.
Ligand-binding proteins of ABC import systems tend to be divergent at the primary sequence level (42). However, based on selected sequence homologies, they can been classified into nine clusters (35). The sugar-binding proteins belong to cluster 2 and bind their ligands with relatively high affinities (Kd values are in the nanomolar range) (4, 26), consistent with the observed binding affinity for d-glucose to Tp38 described herein. However, even the high-affinity binding proteins can display dual specificities with comparable binding affinities, such as has been noted for d-glucose and d-galactose binding to E. coli MglB (26). Whereas the dual binding specificity of enteric MglB for d-glucose and d-galactose may have metabolic importance for E. coli, we challenge the biological relevance of the same observation herein for Tp38. First, it is questionable whether T. pallidum would even encounter meaningful levels of d-galactose in human tissues or body fluids, inasmuch as d-galactose levels in human plasma are >1,000-fold lower than those of d-glucose (29). Second, in extensive in vitro studies of carbohydrate utilization by T. pallidum, which can metabolize sugars in vitro or undergo limited replication in a tissue culture model system (30), d-galactose was not utilized by treponemes in any discernible fashion (7, 28, 30, 36). In fact, all studies thus far have suggested that T. pallidum utilizes d-glucose as its principal, if not sole, carbon and energy source (1, 2, 28, 30, 36). It is thus plausible that the observed d-galactose binding to Tp38 is a reflection merely of the monosaccharide's close structural relatedness (as a C4 epimer) to d-glucose and is therefore not likely to be biologically relevant to T. pallidum's sustenance within its human host.
MglB of enteric bacteria also serves an important role in the signal transduction events of chemotaxis. Upon binding carbohydrate, MglB interacts specifically with the sensory transducer Trg, a methyl-accepting chemotaxis protein (MCP), to induce a chemotactic response (18, 24). By extrapolation, Tp38 (or TpMglB-1) may also interact with a T. pallidum homolog of Trg or another T. pallidum MCP, at least four of which have been predicted for T. pallidum (Tp0040, Tp0488, Tp0639, and Tp0640) (11, 14, 16). Indeed, the clinical manifestations of syphilis (23) as well as the invasive behavior of treponemes in tissue culture model systems (43) reflect the propensity of T. pallidum to migrate through the skin, hematogenously disseminate, and ultimately invade targeted tissues. Treponemal motility is believed to be essential to all of these events, and thus it is likely that chemotaxis is an important facet of syphilis pathogenesis (5). It is therefore not implausible that via glucose binding to Tp38 (or to TpMglB-1) and an interaction with a cognate MCP, treponemal chemotaxis may be driven by local glucose concentration gradients in tissues and body fluids (25, 37). Finally, T. pallidum appears to contain all elements of the functional apparatuses that are requisite for glucose-mediated chemotaxis, such as the environmentally responsive sensory transducer (cognate MCP for Tp38 or TpMglB-1), response regulators and accessory molecules for chemotaxis (e.g., Che proteins), and the flagellar motor and switch proteins (11, 14-17, 22; S. R. Greene, N. R. Young, J. G. Frye, and L. V. Stamm, Abstr. 96th Gen. Meet. Am. Soc. Microbiol. 1996, abstr. D-49, p. 250, 1996). The discernment of the glucose-binding function for Tp38 herein thus is a potentially important first step towards evaluating Tp38's putative interaction with a cognate MCP and elucidating sensory transduction modules that are relevant to T. pallidum virulence and syphilis pathogenesis.
Acknowledgments
We thank Philip J. Thomas for supplying pGroESL and for guidance, Mischa Machius and W. Christian Wigley for helpful discussions, and Bikash Pramanik for performing mass spectrometry.
This study was supported by NIH grant AI-16692 from the National Institute of Allergy and Infectious Diseases and by grant I-0940 from the Welch Foundation.
REFERENCES
- 1.Barbieri, J. T., F. E. Austin, and C. D. Cox. 1981. Distribution of glucose incorporated into macromolecular material by Treponema pallidum. Infect. Immun. 31:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri, J. T., and C. D. Cox. 1979. Glucose incorporation by Treponema pallidum. Infect. Immun. 24:291-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, P. S., D. R. Akins, J. D. Radolf, and M. V. Norgard. 1994. Similarity between the 38-kilodalton lipoprotein of Treponema pallidum and the glucose/galactose-binding (MglB) protein of Escherichia coli. Infect. Immun. 62:1381-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boos, W., and J. M. Lucht. 1996. Periplasmic binding protein-dependent ABC transporters, p. 1175-1209. In F. C. Neidhardt, J. L. Ingraham, B. Low, B. Magasanik, M. Schaechter, and H. E. Unbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 5.Charon, N. W., and S. F. Goldstein. 2002. Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annu. Rev. Genet. 36:47-73. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhuri, B. N., J. Ko, C. Park, T. A. Jones, and S. L. Mowbray. 1999. Structure of d-allose binding protein from Escherichia coli bound to d-allose at 1.8 Å resolution. J. Mol. Biol. 286:1519-1531. [DOI] [PubMed] [Google Scholar]
- 7.Cox, C. D. 1983. Metabolic activities, p. 57-70. In R. F. Schell and D. M. Musher (ed.), Pathogenesis and immunology of treponemal infection. Marcel Dekker, New York, N.Y.
- 8.Deka, R. K., Y.-H. Lee, K. E. Hagman, D. Shevchenko, C. A. Lingwood, C. A. Hasemann, M. V. Norgard, and J. D. Radolf. 1999. Physicochemical evidence that Treponema pallidum TroA is a zinc-containing metalloprotein that lacks porin-like structure. J. Bacteriol. 181:4420-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deka, R. K., M. Machius, M. V. Norgard, and D. R. Tomchick. 2002. Crystal structure of the 47-kilodalton lipoprotein of Treponema pallidum reveals a novel penicillin-binding protein. J. Biol. Chem. 277:41857-41864. [DOI] [PubMed] [Google Scholar]
- 10.Eftink, M. R. 1997. Fluorescence methods for studying equilibrium macromolecular-ligand interactions. Methods Enzymol. 278:221-257. [DOI] [PubMed] [Google Scholar]
- 11.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, C. Fujii, S. Garland, B. Hatch, K. Horst, K. Roberts, M. Sandusky, J. Weidman, H. O. Smith, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 12.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. [DOI] [PubMed] [Google Scholar]
- 13.Goloubinoff, P., A. A. Gatenby, and G. H. Lorimer. 1989. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature 337:44-47. [DOI] [PubMed] [Google Scholar]
- 14.Greene, S. R., and L. V. Stamm. 1998. Molecular characterization of Treponema pallidum mcp2, a putative chemotaxis protein gene. Infect. Immun. 66:2999-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene, S. R., L. V. Stamm, J. M. Hardham, N. R. Young, and J. G. Frye. 1997. Identification, sequences, and expression of Treponema pallidum chemotaxis genes. DNA Sequence 7:267-284. [DOI] [PubMed] [Google Scholar]
- 16.Hagman, K. E., S. F. Porcella, T. G. Popova, and M. V. Norgard. 1997. Evidence for a methyl-accepting chemotaxis protein gene (mcp1) that encodes a putative sensory transducer in virulent Treponema pallidum. Infect. Immun. 65:1701-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardham, J. M., J. G. Frye, and L. V. Stamm. 1995. Identification and sequences of the Treponema pallidum fliM′, fliY, fliP, fliQ, fliR, and flhB′ genes. Gene 166:57-64. [DOI] [PubMed] [Google Scholar]
- 18.Hazelbauer, G. L., H. C. Berg, and P. Matsumura. 1993. Bacterial motility and signal transduction. Cell 73:15-22. [DOI] [PubMed] [Google Scholar]
- 19.Hazlett, K. R. O., F. Rusnak, D. G. Kehres, S. W. Bearden, C. J. La Vake, M. E. La Vake, M. E. Maguire, R. D. Perry, and J. D. Radolf. 2003. The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc-dependent transcriptional repressor, and a semi-autonomously expressed phosphoglycerate mutase. J. Biol. Chem. 278:20687-20694. [DOI] [PubMed] [Google Scholar]
- 20.Lakowicz, J. R. 1999. Principles of fluorescence spectroscopy. Kluwer Academic/Plenum Press, New York, N.Y.
- 21.Lee, Y.-H., R. K. Deka, M. V. Norgard, J. D. Radolf, and C. A. Hasemann. 1999. Treponema pallidum TroA, a periplasmic zinc-binding protein with a helical backbone. Nat. Struct. Biol. 6:628-633. [DOI] [PubMed] [Google Scholar]
- 22.Limberger, R. J., L. L. Slivienski, M. C. T. El-Afandi, and L. A. Dantuono. 1996. Organization, transcription, and expression of the 5′ region of the fla operon of Treponema phagedenis and Treponema pallidum. J. Bacteriol. 178:4628-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukehart, S. A., and K. K. Holmes. 1994. Syphilis, p. 726-737. In K. J. Isselbacher, E. Braunwald, J. Wilson, J. B. Martin, A. S. Fauci, and D. L. Kasper (ed.), Harrison's principles of internal medicine. McGraw Hill, Inc., New York, N.Y.
- 24.Macnab, R. M. 1987. Motility and chemotaxis, p. 732-759. In F. C. Neidhardt, J. L. Ingraham, B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. ASM Press, Washington, D.C.
- 25.Maggs, D. G., R. Jacob, F. Rife, R. Lange, P. Leone, M. J. During, W. V. Tamborlane, and R. S. Sherwin. 1995. Interstitial fluid concentrations of glycerol, glucose, and amino acids in human quadriceps muscle and adipose tissue. J. Clin. Investig. 96:370-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, D. M., J. S. Olson, and F. A. Quiocho. 1980. The mechanism of sugar binding to the periplasmic receptor for galactose chemotaxis and transport in Escherichia coli. J. Biol. Chem. 255:2465-2471. [PubMed] [Google Scholar]
- 27.Newcomer, M. E., D. M. Miller, and F. A. Quiocho. 1979. Location of the sugar-binding site of l-arabinose-binding protein. J. Biol. Chem. 254:7529-7533. [PubMed] [Google Scholar]
- 28.Nichols, J. C., and J. B. Baseman. 1975. Carbon sources utilized by virulent Treponema pallidum. Infect. Immun. 12:1044-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning, C., and S. Segal. 2000. Plasma galactose and galactitol concentration in patients with galactose-1-phosphate uridyltransferase deficiency galactosemia: determination by gas chromatography/mass spectrometry. Metabolism 49:1460-1466. [DOI] [PubMed] [Google Scholar]
- 30.Norris, S. J., D. L. Cox, and G. M. Weinstock. 2001. Biology of Treponema pallidum: correlation of functional activities with genome sequence data. J. Mol. Microbiol. Biotechnol. 3:37-62. [PubMed] [Google Scholar]
- 31.Porcella, S. F., T. G. Popova, K. E. Hagman, C. W. Penn, J. D. Radolf, and M. V. Norgard. 1996. A mgl-like operon in Treponema pallidum, the syphilis spirochete. Gene 177:115-121. [DOI] [PubMed] [Google Scholar]
- 32.Posey, J. E., J. M. Hardham, S. J. Norris, and F. C. Gherardini. 1999. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc. Natl. Acad. Sci. USA 96:10887-10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quiocho, F. A., and P. S. Ledvina. 1996. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol. Microbiol. 20:17-25. [DOI] [PubMed] [Google Scholar]
- 34.Quiocho, F. A., N. K. Vyas, and J. C. Spurlino. 1991. Atomic interactions between proteins and carbohydrates, p. 23-35. In H. M. Einspahr (ed.), Transactions of the American Crystallographic Association. Protein-carbohydrate interactions. American Crystallographic Association, Buffalo, N.Y.
- 35.Saier, M. H. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64:354-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiller, N. L., and C. D. Cox. 1977. Catabolism of glucose and fatty acids by virulent Treponema pallidum. Infect. Immun. 16:60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt, F. J., W. J. Sluiter, and A. J. M. Schoonen. 1993. Glucose concentration in subcutaneous extracellular space. Diabetes Care 16:695-700. [DOI] [PubMed] [Google Scholar]
- 38.Scholle, A., J. Vreemann, V. Blank, A. Nold, W. Boos, and M. D. Manson. 1987. Sequence of the mglB gene from Escherichia coli K12: comparison of wild-type and mutant galactose chemoreceptors. Mol. Gen. Genet. 208:247-253. [DOI] [PubMed] [Google Scholar]
- 39.Stamm, L. V., N. R. Young, J. G. Frye, and J. M. Hardham. 1996. Identification and sequences of the Treponema pallidum mglA and mglC genes. DNA Sequence 6:293-298. [DOI] [PubMed] [Google Scholar]
- 40.Stock, A. M., and S. L. Mowbray. 1995. Bacterial chemotaxis: a field in motion. Curr. Opin. Struct. Biol. 5:744-751. [DOI] [PubMed] [Google Scholar]
- 41.Strauss, E. J., and S. Falkow. 1997. Microbial pathogenesis: genomics and beyond. Science 276:707-712. [DOI] [PubMed] [Google Scholar]
- 42.Tam, R., and M. H. Saier. 1993. Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol. Rev. 57:320-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas, D. D., M. Navab, D. A. Haake, A. M. Fogelman, J. N. Miller, and M. A. Lovett. 1988. Treponema pallidum invades intracellular junctions of endothelial cell monolayers. Proc. Natl. Acad. Sci. USA 85:3608-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vyas, N. K., M. N. Vyas, and F. A. Quiocho. 1988. Sugar and signal-transducer binding sites of the Escherichia coli galactose chemoreceptor protein. Science 242:1290-1295. [DOI] [PubMed] [Google Scholar]
- 45.Vyas, N. K., M. N. Vyas, and F. A. Quiocho. 1991. Comparison of the periplasmic receptors for l-arabinose, d-glucose/d-galactose, and d-ribose. J. Biol. Chem. 266:5226-5237. [PubMed] [Google Scholar]
- 46.Weigel, L. M., J. D. Radolf, and M. V. Norgard. 1994. The 47-kDa major lipoprotein immunogen of Treponema pallidum is a penicillin-binding protein with carboxypeptidase activity. Proc. Natl. Acad. Sci. USA 91:11611-11615. [DOI] [PMC free article] [PubMed] [Google Scholar]