Significance
Cyanobacterial flavodiiron proteins (FDPs) comprise a protein family with unique modular structure and photoprotective functions in an oxygenic environment. It is conceivable that FDPs have made the development of oxygenic photosynthesis possible in cyanobacteria. Here, we report the ability of specific FDPs to reduce O2 directly to water in heterocyst-forming filamentous cyanobacteria, not only to support the photosynthetic machinery, but also to prevent oxidative damage of the N2-fixing enzyme nitrogenase. Whilst in the ancient environment, N2 fixation was secured from O2 inhibition, the later increase of atmospheric O2 may have initiated an important role for FDP-mediated protection of nitrogenase in maintaining the N2-fixing activity of cyanobacteria.
Keywords: nitrogen fixation, oxygen protection, photosynthesis
Abstract
Flavodiiron proteins are known to have crucial and specific roles in photoprotection of photosystems I and II in cyanobacteria. The filamentous, heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120 contains, besides the four flavodiiron proteins Flv1A, Flv2, Flv3A, and Flv4 present in vegetative cells, two heterocyst-specific flavodiiron proteins, Flv1B and Flv3B. Here, we demonstrate that Flv3B is responsible for light-induced O2 uptake in heterocysts, and that the absence of the Flv3B protein severely compromises the growth of filaments in oxic, but not in microoxic, conditions. It is further demonstrated that Flv3B-mediated photosynthetic O2 uptake has a distinct role in heterocysts which cannot be substituted by respiratory O2 uptake in the protection of nitrogenase from oxidative damage and, thus, in an efficient provision of nitrogen to filaments. In line with this conclusion, the Δflv3B strain has reduced amounts of nitrogenase NifHDK subunits and shows multiple symptoms of nitrogen deficiency in the filaments. The apparent imbalance of cytosolic redox state in Δflv3B heterocysts also has a pronounced influence on the amounts of different transcripts and proteins. Therefore, an O2-related mechanism for control of gene expression is suggested to take place in heterocysts.
Flavodiiron proteins (FDPs, also called A-type flavoproteins) belong to a large family of proteins originally discovered and investigated in strict or facultative anaerobic bacteria, archaea, and some eukaryotic parasites (1). They have been proposed to help anaerobic species in coping with oxidative and/or nitrosative stress and to play an important role in maintaining the anaerobic metabolism. Homologs of genes encoding FDPs were later found in the genomes of oxygenic photosynthetic organisms: cyanobacteria, green algae, mosses, and lycophytes. FDPs are widespread in cyanobacteria, but have gradually disappeared in the course of higher plant evolution (2, 3). Intriguingly, the structures of the FDPs in oxygenic photosynthetic organisms are unique, because they possess an extra C-terminal flavin-reductase domain. This domain is in addition to the common core that is comprised of two redox centers, a β-lactamase–like domain including the nonheme catalytic diiron center at the N terminus and the flavin mononucleotide-containing flavodoxin-like domain at the C terminus (1, 4). Therefore, cyanobacterial-type FDPs are likely capable of donating electrons to O2/NO directly from NAD(P)H. Cyanobacterial FDPs have mostly been studied in Synechocystis sp. strain PCC 6803 (hereafter Synechocystis), a unicellular non-N2–fixing cyanobacterium.
The Flv2 and Flv4 proteins are present only in β-cyanobacteria and their heterodimer functions in photoprotection of photosystem (PS) II (2, 5, 6). The Flv1 and Flv3 proteins can be found in α- and β-cyanobacteria, but also in green algae, mosses, and lycophytes (2). Initial in vitro studies with recombinant Flv3 protein from Synechocystis provided evidence that it may function as an NAD(P)H:O2 oxidoreductase reducing O2 directly to water (4). Afterward, Helman et al. (7) demonstrated that the Δflv1 and Δflv3 mutants lack the light-induced O2 uptake and proposed that Flv1 and Flv3 reduce molecular O2 to water with NADPH produced on the acceptor side of PS I without formation of reactive oxygen species (ROS). Under certain conditions, up to 60% of electrons originating from water-splitting PS II could be forwarded to O2 via Flv1 and Flv3 proteins (8). The importance of Flv1 and Flv3 for the survival of cyanobacteria was unambiguously proven only recently by application of fluctuating light to mimic the constantly changing natural illumination conditions in aquatic environments (9).
We have demonstrated the existence of two “extra” genes that represent copies of flv1 and flv3 in Anabaena sp. strain PCC 7120 (hereafter Anabaena), a model filamentous N2-fixing, heterocyst-forming cyanobacterium. We have detected in the genome of Anabaena the following flv genes: flv1A (all3891), flv1B (all0177), flv2 (all4444), flv3A (all3895), flv3B (all0178), and flv4 (all4446) (10). Anabaena forms long filaments comprised only of vegetative cells when grown in medium containing nitrate or ammonium. In the absence of combined nitrogen, some vegetative cells differentiate into heterocysts, cells with specific morphology and metabolism providing the conditions for fixation of atmospheric N2 (11). Oxygenic photosynthesis in N2-fixing filaments is restricted to vegetative cells. Heterocysts, in contrast, bear an extra, O2-impermeable envelope with glycolipid and polysaccharide layers outside of the outer membrane, so that diffusion of gases, including N2, occurs mainly through terminal pores connecting heterocysts and vegetative cells (12). Vegetative cells also supply heterocysts with energy for N2 fixation, mainly in the form of sucrose, whereas heterocysts, in turn, provide the whole filament with fixed nitrogen.
An obligatory condition for the activity of nitrogenase, the key enzyme in N2 fixation, is a low partial pressure of O2 inside the cells (13). O2 in heterocysts is thought to be eliminated immediately by respiratory complexes (12). Two clusters encoding respiratory terminal oxidases, cox2 and cox3, were found to be expressed specifically in heterocysts and shown to be essential for diazotrophic growth in Anabaena (14, 15). Additionally, several other systems of protection against O2 and ROS were recently found to be required to maintain the activity of nitrogenase or diazotrophic growth, emphasizing the ultimate importance of microoxic conditions for proper functioning of the N2-fixing machinery (16–19).
Fluorescent protein tagging revealed that the duplicates of Flv1 and Flv3 are spatially segregated in the filaments of Anabaena (10). Flv1A and Flv3A were detected only in vegetative cells, whereas Flv1B and Flv3B were expressed only after combined nitrogen stepdown and were found exclusively in heterocysts. Flv1B and Flv3B could possibly form an operon, and the expression of Flv3B may be activated by NtcA, a global regulator of heterocyst differentiation (20, 21). Here, we investigate Anabaena strains lacking the heterocyst-specific flavodiiron proteins and demonstrate the existence of Flv-mediated light-induced O2 uptake inside heterocysts, which is important for N2 fixation and diazotrophic growth of this filamentous cyanobacterium.
Results
Growth Phenotype of the Mutants Lacking Heterocyst-Specific FDPs.
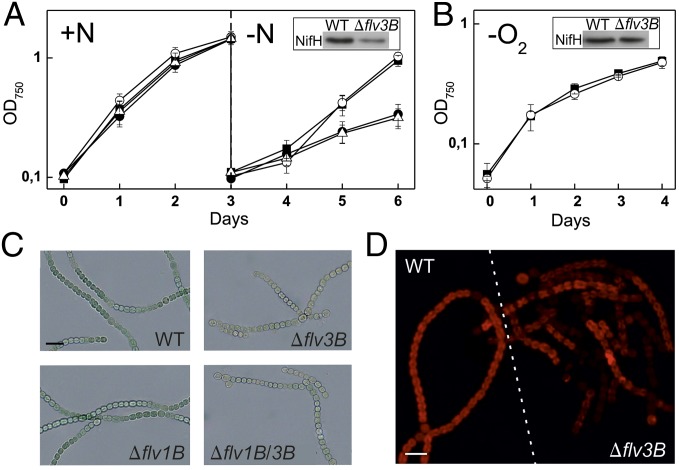
To clarify the roles of Flv1B and Flv3B in heterocysts, we constructed mutant strains Δflv1B and Δflv3B, as well as the double mutant Δflv1B/3B (Fig. S1 and Table S1). In the presence of combined nitrogen, wild-type (WT) and mutant strains grew similarly (Fig. 1A, +N). Next, N2 prototrophy of the mutants was addressed by shifting the nitrate-grown filaments to nitrate-free medium. Whereas the Δflv1B mutant grew similarly to WT cells, the strains lacking Flv3B protein demonstrated approximately 50% reduced growth rate (0.40 ± 0.06, P < 0.001) compared with WT (0.79 ± 0.08) based on OD750 measurements (Fig. 1A, -N). Additionally, Flv3B-depleted strains had approximately 50% lower chlorophyll (Chl) a and protein ratio compared with OD750 after 4 d in N2-fixing conditions (Fig. S2). To test the role of O2 in slowing growth in the absence of Flv3B, the growth of the Δflv3B mutant was next examined in the absence of O2, i.e., while flushing with a gas mixture of 99.96% N2 and 0.04% CO2. The O2-depleted atmosphere rescued the growth of the Δflv3B mutant in N2-fixing conditions similar to that of the WT (Fig. 1B). Furthermore, the protein amounts of the nitrogenase subunit NifH were similar in the mutant and the WT in microoxic conditions (Fig. 1B, Inset), which is in contrast to heavily reduced amount of NifH in the Δflv3B mutant under oxic conditions (Fig. 1A, Inset).
Fig. 1.
Growth phenotype of Anabaena WT and the flv mutants. (A) Filaments were grown with combined N (+N) for 3 d followed by adjustment of OD750 to 0.1 and shifting to N2-fixing conditions (-N). ■, WT; mutant strains: ○, Δflv1B; ●, Δflv3B; ∆, Δflv1B/3B. (B) Growth of the WT and Δflv3B in O2-depleted atmosphere after combined N stepdown: ■, WT; ○, Δflv3B. (C) Filaments of the WT and mutants after 4 d in N2-fixing conditions visualized with bright field microscopy. (D) Chl a autofluorescence of the WT and Δflv3B in N2-fixing conditions. (A and B) Values are mean ± SD, n = 3; Insets demonstrate amount of the NifH protein in the growth conditions. (Scale bars: 10 μm.)
To test for possible modifications in the morphology of the mutant strains, the phenotype of the filaments under N2-fixing conditions in oxic environment was investigated in more detail. Bright field microscopy revealed that single and double mutant strains lacking Flv3B consisted of only short pale-green chains of vegetative cells, typically with one or two terminal heterocysts, whereas the Δflv1B strain had long filaments similar to the WT in color and heterocyst content (Fig. 1C and Table S2). Simultaneous monitoring of Chl a autofluorescence from WT and Δflv3B mutant filaments confirmed that vegetative cells of the mutant contained lower amounts and uneven distribution of Chl a (Fig. 1D).
Gas Exchange in Filaments and Heterocysts of WT and the flv Mutants.
We previously demonstrated that Flv1A and Flv3A proteins, located exclusively in vegetative cells, are essential for the growth of Anabaena under fluctuating light (9). Importantly, after nitrogen stepdown, under both the steady-state (Fig. 1A) and fluctuating light conditions (Fig. S3), the growth of Δflv1B did not differ from that of the WT. However, the Δflv3B mutant had approximately 50% lower growth rates under both light conditions compared with WT cells. This observation suggested a different role for the Flv1B and Flv3B proteins in heterocysts from that of the Flv1A and Flv3A in vegetative cells under fluctuating light conditions. The next question to address was whether Flv1B and Flv3B, like their homologs Flv1A and Flv3A, are involved in O2 photoreduction. To this end, membrane inlet mass spectrometry (MIMS) was applied to monitor online gas exchange from the whole filaments incubated in N2-fixing conditions. The method allows simultaneous measurement of the amount of naturally abundant 16O2 evolved by PS II and the heavy isotope 18O2 injected into the experimental medium before the analysis, thus making it possible to specifically distinguish the O2 uptake.
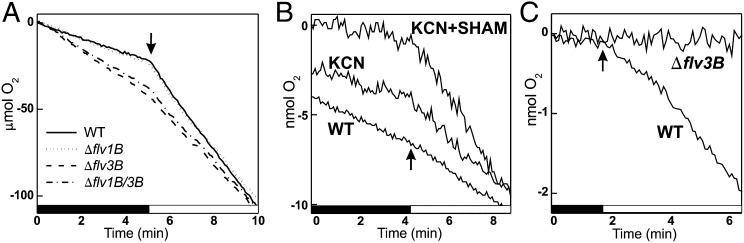
As demonstrated by the MIMS gas exchange measurements (Fig. 2A and Table 1), illumination of the dark-adapted WT filaments with white light strongly stimulated the rate of O2 uptake (69.5 μmol⋅[mg of Chl a]−1⋅h−1, hereafter rates are average; details in Table 1) compared with that in darkness (respiration, 20.3 μmol⋅[mg of Chl a]−1⋅h−1). The fraction of O2 consumption during illumination, after subtraction of the dark O2 uptake rate, is referred to as light-induced O2 uptake (49.1 μmol⋅[mg of Chl a]−1⋅h−1). In contrast to the WT, both Δflv3B and Δflv1B/3B mutants in darkness consumed more O2 (32.4 and 30.4 μmol⋅[mg of Chl a]−1⋅h−1), but upon transfer to the light showed less light-induced O2 uptake (21.8 and 25.4 μmol⋅[mg of Chl a]−1⋅h−1). The Δflv1B mutant showed dark respiration and light-induced O2 uptake rates similar to those of WT.
Fig. 2.
O2 uptake by N2-fixing filaments and heterocysts of the WT and mutant strains. (A) MIMS measurements of O2 consumption during the dark-to-light transition by whole filaments of the WT (solid line) and mutants: ∆flv1B (dotted line), ∆flv3B (dashed line), ∆flv1B/3B (dash-dot line). (B) O2 uptake by WT heterocysts with or without inhibitors. (C) Light-induced O2 uptake by WT and ∆flv3B heterocysts; dark O2 uptake rates were subtracted for better legibility. Arrows indicate the beginning of illumination. Chl a content of samples was adjusted to 15 μg⋅ml−1.
Table 1.
The rates of CO2 uptake in whole filaments and O2 exchange in whole filaments and isolated heterocysts of the WT and the flv mutant strains
| Strain and inhibitors | Filaments | Heterocysts | |||||||
| CO2 uptake | O2 uptake | Gross O2 evolution | Net O2 evolution | O2 uptake | |||||
| Dark | Light | Light-induced | Dark | Light | Light-induced | ||||
| WT | 7.0 ± 0.3 | 20.3 ± 1.1 | 69.5 ± 0.8 | 49.1 ± 0.8 | 187.5 ± 5.5 | 118.0 ± 5.9 | 3.4 ± 0.5 | 5.6 ± 0.5 | 2.2 ± 0.7 |
| + KCN | — | — | — | — | — | — | 1.3 ± 0.4** | 5.5 ± 0.8 | 4.2 ± 0.4** |
| + KCN, SHAM | — | — | — | — | — | — | 0.7 ± 0.3** | 7.5 ± 0.5** | 6.8 ± 0.2** |
| + Antimycin A | — | — | — | — | — | — | 1.4 ± 0.6** | 5.7 ± 0.7 | 4.4 ± 0.1** |
| ∆flv1B | 7.5 ± 2.1 | 21.1 ± 1.6 | 58.5 ± 0.5** | 37.4 ± 2.1** | 181.5 ± 5.8 | 123.0 ± 5.0 | 4.9 ± 0.8* | 6.7 ± 0.5* | 1.8 ± 0.3 |
| ∆flv3B | 5.2 ± 0.3** | 32.4 ± 3.15* | 54.3 ± 2.1** | 21.8 ± 5.3** | 142.8 ± 6.8** | 88.5 ± 4.6* | 7.4 ± 0.7** | 6.5 ± 0.1* | Nd |
| ∆flv1B/3B | 5.1 ± 0.4** | 30.4 ± 1.4** | 55.8 ± 1.7** | 25.4 ± 3.1** | 145.5 ± 5.6** | 89.7 ± 7.2* | 6.5 ± 0.6** | 6.4 ± 0.1* | Nd |
Gas exchange rates are presented as micromoles per milligram of Chl a per hour. Filaments were grown in N2-fixing conditions. Light-induced O2 uptake was calculated by subtracting the O2 uptake rate in darkness from the O2 uptake rate in the light. Net O2 evolution was deducted as a residual rate of gross O2 evolution after subtraction of the light O2 uptake rate. Mean ± SD, n = 3, asterisks indicate statistically significant differences with the WT (*P < 0.05; **P < 0.01). Nd, not detected.
To specifically address the O2 exchange reactions in heterocysts, we isolated heterocysts from WT and mutants and checked their ability to take up O2 by using a Clark-type O2 electrode. WT heterocysts demonstrated a dark-respiration rate of 3.4 μmol⋅[mg of Chl a]−1⋅h−1 and also a substantial light-induced O2 uptake (2.2 μmol⋅[mg of Chl a]−1⋅h−1; Fig. 2B). The origin of the light-induced O2 uptake was tested by supplementation with well-known inhibitors of respiratory chain components. Incubation of WT heterocysts with potassium cyanide (KCN), salicylhydroxamic acid (SHAM), or antimycin A resulted in significant inhibition of dark O2 uptake, whereas the light-induced O2 uptake was stimulated by the presence of these inhibitors (Fig. 2B and Table 1). The Δflv1B heterocysts exhibited a 44% higher dark O2 uptake rate than the WT, whereas the light-induced O2 uptake rate was not significantly different from that in the WT. In contrast to Δflv1B, the heterocysts of the Δflv3B and Δflv1B/3B mutants showed notably higher rates (approximately twofold) of dark respiration than the WT, whereas light-induced O2 uptake was completely absent (Fig. 2C and Table 1).
Light-saturated photosynthesis was measured by MIMS as O2 evolution from whole filaments incubated in the absence of combined nitrogen. The WT and the Δflv1B mutant showed comparable rates of net O2 evolution (approximately 118 μmol⋅[mg of Chl a]−1⋅h−1), whereas in the Δflv3B strain, the rate was reduced by 25% (Table 1). Simultaneous analysis of CO2 uptake in the presence of 10 mM NaHCO3 revealed significantly lower light-induced CO2 uptake in mutants lacking Flv3B compared with the WT and Δflv1B (Table 1). As to the yields of PS I and PS II, the values in the Δflv3B mutant under normal growth light conditions were similar to those of the WT, but upon increasing light intensity, the yields of PS I and PS II dropped more rapidly in Δflv3B than in the WT (Fig. S4).
Lack of Heterocyst-Specific Protein Flv3B Modulates the Transcriptome of Whole Filaments.
To get insights into the role of the Flv1B and Flv3B proteins in cellular metabolism, we next compared the gene expression profiles of the WT and the Δflv1B, Δflv3B, and Δflv1B/3B mutant strains. To identify the genes with differential expression, we first isolated total RNA from whole filaments incubated under N2-fixing conditions and subjected it to RNA sequencing. The genes with prominent differences in expression between the strains were then subjected to more precise transcript analysis by quantitative real-time PCR (RT-q-PCR). In Fig. 3, the RT-q-PCR results are presented as a ratio of the gene transcript abundance in each mutant over the transcript abundance in the WT.
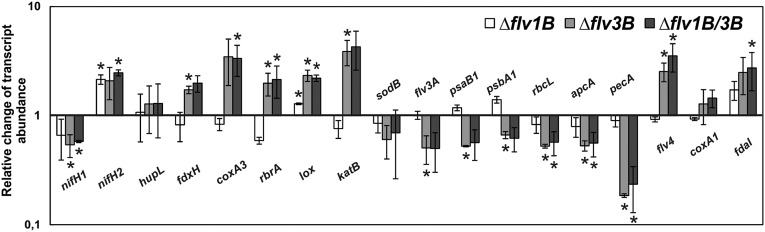
Fig. 3.
RT-q-PCR analysis of gene expression in whole filaments of the WT and mutants incubated in the absence of combined nitrogen. The fold change of relative transcript abundance of the selected genes in mutant cells compared with the WT is shown: white bars, ∆flv1B; light gray bars, ∆flv3B; dark gray bars, ∆flv1B/3B. Mean ± SD, n = 3. Asterisks indicate statistically significant differences with the WT (*P < 0.05).
The filaments of both mutants lacking Flv3B (the Δflv3B and Δflv1B/3B mutants) demonstrated similar changes in the transcript profile in comparison with the WT. Notably, we observed decreased amounts of nifH1 mRNA (0.54 ± 0.13, hereafter data indicated is only for the Δflv3B mutant), but elevated transcript abundance of nifH2 (2.09 ± 0.68). The latter gene represents a copy of nifH1 with nearly identical sequence but is not accompanied by copies of nifD and nifK genes. The physiological role of nifH2 is not clear; however, it might be involved in biosynthesis of the Fe-Mo cofactor of nitrogenase (22). The transcript abundance of heterocyst-specific ferredoxin, considered to be a donor of electrons for nitrogenase (23), was also up-regulated in Flv3B-deficient mutants (fdxH, 1.73 ± 0.13). Importantly, genes encoding proteins involved in O2 and H2O2 reduction were significantly up-regulated in the absence of Flv3B under N2-fixing conditions (coxA3, 3.47 ± 1.58; rbrA, 1.98 ± 0.47; lox, 2.34 ± 0.28; katB, 3.88 ± 1.01). On the contrary, a specific group of photosynthesis-related genes was down-regulated (psaB1, 0.53 ± 0.01; psbA1, 0.67 ± 0.05; rbcL, 0.53 ± 0.02; flv3A, 0.51 ± 0.15), and the most prominent drop in transcript level was observed for genes encoding components of phycobilisomes (PBS): both the core (apcA, 0.53 ± 0.06) and the rods (pecA, 0.19 ± 0.01). However, the transcript amount of flv4, a homolog of the gene encoding the flavodiiron protein that is specifically connected to PS II and PBS in photoprotection of Synechocystis (6), was higher in the mutants lacking Flv3B than in WT (2.54 ± 0.49).
In the Δflv1B mutant, the transcript data obtained on the level of whole N2-fixing filaments demonstrated no prominent differences from that of the WT with regard to light harvesting, photosynthesis, and N2 fixation-related genes. However, nifH1 gene was down-regulated compared with the WT (0.66 ± 0.26), whereas expression of the nifH2 gene was higher (2.15 ± 0.21).
Protein Composition of Heterocyst-Specific flv Mutants.
To track changes on the protein level, we compared Δflv1B, Δflv3B, and WT, using both the whole filaments incubated in the absence of combined nitrogen and heterocyst-enriched cell fractions. For protein comparison, the differential gel electrophoresis (DIGE) approach was taken to identify differentially abundant proteins (Fig. S5). The difference in expression of proteins between Δflv3B and WT is hereafter shown as a ratio of the relative protein amount in the mutant compared with the WT; a full list of proteins and ratio values are presented in Table S3. Whole filaments of the Δflv3B mutant completely lacked the PecA and PecC proteins, which are the phycoerythrocyanin component of PBS rods and its linker protein, respectively. The down-regulated proteins included the large subunit of ribulose bisphosphate carboxylase (RbcL, 0.6 ± 0.1), fructose bisphosphate aldolase (Fda II, 0.7 ± 0.1), glyceraldehyde-3-phosphate dehydrogenase (Gap2, 0.6 ± 0.0), d-fructose-1,6-bisphosphatase class 2/sedoheptulose-1,7-bisphosphatase (GlpX, 0.7 ± 0.0), thioredoxin reductase (TrxB, 0.6 ± 0.1), and several proteins involved in various biosynthesis pathways: sulfolipid biosynthesis protein (Alr1744), ketol-acid reductoisomerase (IlvC), protochlorophyllide oxidoreductase (Por), and glucose-1-phosphate adenylyltransferase (GlgC).
In the heterocyst fraction of Δflv3B, the most prominent decrease was observed for subunits of nitrogenase (NifH, 0.5 ± 0.1; NifD, 0.5 ± 0.1; NifK, 0.6 ± 0.0) and uptake hydrogenase (HupL, 0.3 ± 0.0). Further, down-regulation was recorded for GlpX (0.7 ± 0.1), 6-phosphogluconate dehydrogenase (6-PGD, 0.5 ± 0.1), aconitate hydratase (AcnB), TrxB, enzymes of Chl biosynthesis geranylgeranyl hydrogenase (ChlP) and Por, and several other proteins. As expected, heterocysts lacked Flv3B protein, but Flv1B was detected in a higher amount than in WT (3.3 ± 0.5). Among other up-regulated proteins, we identified Fda I (1.9 ± 0.1), transaldolase (Tal, 1.5 ± 0.0), succinate-semialdehyde dehydrogenase (1.8 ± 0.3), and alanine dehydrogenase (Ald, 3.5 ± 0.4). Likewise, two porins, Alr0834 and All4499, possibly involved in cell wall remodeling of heterocysts (11), were found in higher amounts in the Δflv3B mutant.
In contrast to the Δflv3B mutant, the Δflv1B strain, which did not differ much in growth characteristics from the WT (Fig. 1A), demonstrated only subtle changes in protein amounts in both heterocysts and whole N2-fixing filaments (Fig. S5 and Table S4). However, several proteins up-regulated in whole filaments of the Δflv1B mutant were Fda I, Fda II, and Gap2. PecA and 6-PGD showed decreased amounts in Δflv1B filaments. In heterocysts of the Δflv1B mutant, Flv1B was absent, as expected, but the amount of Flv3B was similar to that in the WT. Decreased amounts of HupL, TrxB, and 6-PGD were also observed in Δflv1B heterocysts, but the mutation did not have an effect on protein amounts of the nitrogenase subunits.
Nitrogenase Activity of the Mutant Strains.
In oxic acetylene reduction assays, the WT showed a nitrogenase activity rate of 19.6 ± 0.2 μmol⋅[mg of Chl]−1⋅h−1. All mutants studied had a significantly lower nitrogenase activity compared with the WT: 10.3 ± 4.1, 11.1 ± 1.6, and 8.9 ± 1.5 μmol⋅[mg of Chl]−1⋅h−1 by Δflv1B, Δflv3B, and the double mutant, respectively (mean ± SD, n = 3, P < 0.05).
Discussion
Flavodiiron Protein Flv3B Is Responsible for Light-Induced O2 Uptake in Heterocysts.
N2 fixation in heterocysts is an energetically expensive process that requires large amounts of reducing equivalents and ATP, which are ultimately provided by photosynthesis occurring in vegetative cells during illumination. It is conceivable that the efficient diffusion of PS II-generated O2 into the heterocyst occurs mainly through terminal pores according to the concentration gradient (12).
Besides respiratory O2 uptake pathways, this extra O2 might be taken in charge by the PS I-driven light-induced O2 quenching system in heterocysts (13). Such light-induced O2 uptake of unknown nature has been reported and discussed in respect to protection of the nitrogenase activity against oxidative damage (24, 25). Here, we demonstrate O2 uptake by isolated heterocysts in darkness (Fig. 2B), i.e., respiratory activity that has been postulated as a means to decrease partial O2 pressure in heterocysts (12). We also show that O2 uptake increases 1.6-fold in WT heterocysts upon a dark-to-light transition. Whereas dark O2 uptake is inhibited by respiratory electron transfer inhibitors, light-induced O2 uptake remains unaffected (Fig. 2B). However, light-induced O2 uptake is completely eliminated by disruption of the flv3B gene (Fig. 2C). This unambiguously indicates that the flavodiiron protein Flv3B is responsible for O2 photoreduction in heterocysts.
Flv3B-dependent O2 reduction in heterocysts appears to be crucial for growth and photosynthesis of Anabaena filaments upon removal of combined nitrogen (Fig. 1). Only the Δflv3B mutant, but not Δflv1B, has lost the capability for light-induced O2 uptake in heterocysts. In line with this observation, the presence of the Flv1B protein in Δflv3B heterocysts did not rescue the severe Δflv3B phenotype. We suggested earlier that flavodiiron proteins in cyanobacteria function as heterodimers, but in the case of heterocyst-specific FDPs, Flv3B is clearly capable of functioning independently. Therefore, the function of Flv1B remains unclear.
Extra FDP-coding genes are present in genomes of all heterocyst-forming cyanobacteria sequenced to date (10). Thus, Flv3B seems to have a unique role in O2 quenching in heterocysts. It is dispensable for N2 fixation-dependent growth when Anabaena is incubated under microoxic conditions (Fig. 1B). However, its absence leads to strong growth impairment in oxic conditions, even if dark O2 uptake appears stimulated in mutants. Therefore, Flv3B function cannot be replaced by up-regulation of common respiratory pathways, which likewise consume O2. Although terminal oxidases are concentrated in the membranes near to cell junctions (26), Flv3B protein is equally distributed across the heterocysts (10), likely following the localization of the nitrogenase enzyme. By eliminating molecular O2, Flv3B may participate in the control of the redox status of the cytosol and provide appropriate conditions for the function of nitrogenase, and probably many other enzymes, under illumination.
Possible Donors of Electrons for Flv3B.
Identification of Flv3B as a responsible protein for light-induced O2 uptake in heterocysts raises a question about its electron donor. NADPH and NADH were both proven to donate electrons to Synechocystis Flv3 in vitro (4). However, the electron donor might also be ferredoxin, because FdxI, one of ferredoxins of Chlamydomonas reinhardtii, was recently found to interact with Flv3, a homolog of cyanobacterial-like flavodiiron proteins (27). Current knowledge of heterocyst-specific metabolism does not allow us to make a direct distinction between possible electron donors to Flv3B. Nevertheless, it is known that heterocysts harbor only the “short” form of ferredoxin:NADPH oxidoreductase (FNR), i.e., the form that lacks the PBS-binding domain and is active in oxidation of NADPH, making it unlikely that NADP+ is reduced on the reducing side of PS I in heterocysts (28). Therefore, it is conceivable that both NADPH and NADH are produced by glycolysis and oxidative pentose phosphate pathway (OPPP) equally in darkness and under the light. As to ferredoxin serving as an electron donor to Flv3B, two heterocyst-specific ferredoxins are known in Anabaena. The fdxN gene within the main nif gene cluster encodes a “bacterial-type” ferredoxin (29). Although its role remains unclear, the disruption of fdxN did not affect the diazotrophic growth of Anabaena variabilis (30). Another ferredoxin, encoded by fdxH, has been shown to function as a physical donor of electrons to nitrogenase in vitro and was suggested to play a role as a common pool where reducing equivalents from different reactions are collected to be tunneled further to N2 fixation (23). FdxH takes electrons from PS I in the light but can also be reduced by FNR in darkness with the use of NADPH produced in OPPP.
We suggest that illumination modifies the redox status of heterocysts and activates the Flv3B-mediated electron transfer from the reducing side of PS I, likely from FdxH, to O2. In this case, Flv3B uses light-driven electrons originating from reductants provided by vegetative cells. Based on the model structure of the Flv2/Flv4 heterodimer (5), arrangement of an Flv3B homodimer positions two cysteine residues in close proximity on the surface of the protein, where they may be available for redox regulation. Nevertheless, a possibility of a so far unknown electron carrier for reducing Flv3B cannot be excluded.
Flv3B-Mediated Protection of Nitrogenase Ensures Sufficient Supply of Filaments with Nitrogen.
Although flv mutants demonstrated approximately 50% decreased nitrogenase activity compared with WT in acetylene reduction assay, it is conceivable that the maximum capacity of enzyme activity is not reached in native conditions. In the Δflv1B mutant, a lower nitrogenase activity does not reduce the growth of filaments. Mutants lacking Flv3B have a similar potential for N2 fixation; however, in practice, the absence of light-induced O2 uptake in heterocysts results in an insufficient supply of amino acids to filaments. This notion is indicated by the multiple symptoms of nitrogen starvation in filaments of the Δflv3B mutant. Most prominent is the impaired growth and modified shape of the mutant filaments (Fig. 1 A and C), as well as lower CO2 uptake and gross O2 evolution rates at saturating light intensity (Table 1). The decreased levels of gas exchange indicate programmed down-regulation of photosynthesis and carbon assimilation activities in response to nitrogen starvation. At the gene expression level, these symptoms are seen as a scarcity of transcripts and, consequently, of proteins from PBS-coding genes, down-regulation of photosynthesis-related genes, and reduced amounts of proteins involved in Chl synthesis (ChlP and Por), the Calvin cycle (RbcL, Fda II, and Gap2), and sugar anabolism (GlpX and GlgC). These defects are typically connected to nitrogen starvation (31). The elevated dark O2 uptake rate by filaments of the Δflv3B mutant might also indicate a decreased N:C ratio, because nitrogen starvation causes an accumulation of succinate, fumarate, and malate in the citric acid cycle (TCA), and respiratory activity helps to dissipate the excess carbon (32).
Role of Flv3B in Regulation of Expression of Genes and Proteins.
The flv3B gene might be classified as a “late heterocyst differentiation gene” because it is actively transcribed in fully formed heterocysts, similar to the nif genes (33). Therefore, the lack of Flv3B does not have an effect on the formation of heterocysts, but instead modifies the fitness of the cells. The decreased amounts of NifHDK protein subunits of nitrogenase and the HupL subunit of uptake hydrogenase in the Δflv3B mutant is in line with elevated O2 levels in Δflv3B heterocysts upon illumination. Both enzymes are known to be extremely sensitive to O2 because oxidation of iron centers is expected to trigger degradation of the proteins. However, in the case of nitrogenase subunits, the lower protein amount might simply result from the transcript scarcity.
Control of gene expression in heterocysts under steady-state conditions has not yet been thoroughly investigated. However, it is conceivable that once nitrogenase is active, nitrogen deprivation is no more an ultimate driving factor for modulating nitrogenase gene expression, and other mechanisms might take over. Unlike the direct transcriptional regulation by O2 of the nitrogenase genes in nonphotosynthetic diazotrophs (34), the expression of nifHDK in Anabaena has been considered to be mainly subject to developmental control (35). In line with this idea, the WT level of nif gene expression was observed in developing heterocysts of a double cox2 cox3 mutant of Anabaena (15) despite apparently elevated O2 partial pressure. However, we cannot exclude that regulation of gene expression in heterocysts is carried out by O2 indirectly, through subsequent O2 interactions in a cell. Indeed, O2-induced modification of cytosolic redox status or degradation products of the nitrogenase protein might trigger a signaling cascade resulting in strong transcriptional changes of genes encoding nitrogenase, O2 scavenging enzymes, and other proteins. In accordance with this notion, up-regulation of coxA3, part of an operon encoding a heterocyst-specific terminal oxidase; lox, which encodes lactate oxidase that is thought to reduce O2; and genes encoding rubrerythrin and Mn catalase, both reducing H2O2, were found in the mutants lacking Flv3B (Fig. 3). Lactate oxidase and rubrerythrin have been shown to be important for diazotrophic growth and nitrogenase activity, respectively (17, 18).
Our data also demonstrate that Flv3B is crucial for protein composition in Anabaena. Many proteins were present in different amounts in the Δflv3B mutant compared with WT, especially in the heterocyst-enriched fraction. The profound adjustment of protein composition likely allows the mutant to partially overcome the severe effects of the mutation and maintain a level of nitrogenase activity. Our findings reiterate the importance of mechanisms for gene and protein regulation in mature heterocysts that are yet to be investigated.
Concluding Remarks.
Understanding the ultimate importance of flavodiiron proteins for the well-being of cyanobacteria has emerged only during the past few years. It is highly likely that evolution of FDPs made the appearance of oxygenic photosynthesis possible by providing protection at multiple sites: the Flv2/Flv4 heterodimer protects against photoinhibition of PS II, whereas Flv1(A) and Flv3(A) prevent the photodamage of PS I. Here, we demonstrate that a heterocyst-specific flavodiiron protein Flv3B is crucial for elimination of O2 in illuminated heterocysts, thereby contributing to solve the long-lasting problem of O2 protection of nitrogenase in the light. The ability of FDPs to transfer light-driven electrons from downstream of PS I directly to O2 can be used in heterocysts to prevent oxidative damage of the N2-fixing machinery and to create appropriate redox conditions for heterocyst metabolism.
Materials and Methods
Anabaena sp. (also known as Nostoc sp.) strain PCC 7120 and mutant strains were grown in BG-11o medium (lacking combined nitrogen) buffered with 10 mM TES–KOH (pH 8.2) in the presence of 3% (vol/vol) CO2 under gentle agitation at 30 °C and constant illumination of 50 μmol photons⋅m−2⋅s−1. Where indicated, the cells were cultivated in BG-11 medium containing 17.6 mM NaNO3. The cells at the exponential growth phase (about OD750 = 1) were harvested, resuspended in a fresh medium, and subjected to the activity measurements or to RNA and protein analyses. The growth rate constants were determined as tangents of growth curves plotted in logarithmic scale. To create microoxic conditions, the cultures were grown under continuous bubbling with gas mixture containing 99.96% N2 and 0.04% CO2. Filaments were visualized with a Zeiss Axiovert 200M microscope. Chl a was excited with a 488-nm argon ion laser, and fluorescence was collected across 680–720 nm.
The construction of the Δflv1B, Δflv3B, and Δflv1B/3B mutant strains of Anabaena by triparental mating, isolation of heterocysts, nitrogenase activity assay, immunodetection, and DIGE are described in detail in SI Materials and Methods. Isolation of total RNA from whole filaments of WT and mutant strains and RT-q-PCR analysis were performed as described (10); the list of primers is provided in Table S1. Strand-specific transcriptome sequencing was performed by Illumina HiSeq2000 (BGI Tech Solutions, Co., Ltd.).
O2 (mass 32), 18O2 (mass 36), and CO2 exchange were monitored by MIMS as described earlier (8). Actinic light at the intensity of 600 μmol photons⋅m−2⋅s−1 was applied by a LED-powered fiber optic illuminator (PerkinElmer Life Sciences) when needed. O2 consumption by isolated heterocysts was measured with a Clark type oxygen electrode (DW1, Hansatech) in darkness and at a saturating light intensity of 400 μmol photons⋅m−2⋅s−1. The final concentration of inhibitors used was 1 mM KCN, 10 mM SHAM, and 10 μM Antimycin A. Chl a fluorescence and P700 oxidoreduction were recorded by DUAL-PAM-100 (Walz) upon application of saturating pulses (4,000 μmol photons⋅m−2⋅s−1, 300 ms) at stepwise increments of actinic light intensities duration of 30 s.
Supplementary Material
Acknowledgments
We thank Cell Imaging Core and Proteomics Facility of Turku Centre for Biotechnology. Research was financially supported by the Academy of Finland Projects 118637, 271832, and 273870 (to E.-M.A.), Kone Foundation (Y.A.), and Varsinais-Suomi Cultural Foundation regional fund (M.E.). Support was provided by the HélioBiotec platform, funded by the European Union (European Regional Development Fund), the Région Provence Alpes Côte d’Azur, the French Ministry of Research, and the “Commissariat à l’Energie Atomique et aux Energies Alternatives.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407327111/-/DCSupplemental.
References
- 1.Vicente JB, Justino MC, Gonçalves VL, Saraiva LM, Teixeira M. Biochemical, spectroscopic, and thermodynamic properties of flavodiiron proteins. Methods Enzymol. 2008;437:21–45. doi: 10.1016/S0076-6879(07)37002-X. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Allahverdiyeva Y, Eisenhut M, Aro EM. Flavodiiron proteins in oxygenic photosynthetic organisms: Photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE. 2009;4(4):e5331. doi: 10.1371/journal.pone.0005331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peltier G, Tolleter D, Billon E, Cournac L. Auxiliary electron transport pathways in chloroplasts of microalgae. Photosynth Res. 2010;106(1-2):19–31. doi: 10.1007/s11120-010-9575-3. [DOI] [PubMed] [Google Scholar]
- 4.Vicente JB, Gomes CM, Wasserfallen A, Teixeira M. Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem Biophys Res Commun. 2002;294(1):82–87. doi: 10.1016/S0006-291X(02)00434-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P, et al. Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell. 2012;24(5):1952–1971. doi: 10.1105/tpc.111.094417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bersanini L, et al. Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in Cyanobacteria. Plant Physiol. 2014;164(2):805–818. doi: 10.1104/pp.113.231969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helman Y, et al. Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol. 2003;13(3):230–235. doi: 10.1016/s0960-9822(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 8.Allahverdiyeva Y, et al. Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J Biol Chem. 2011;286(27):24007–24014. doi: 10.1074/jbc.M111.223289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allahverdiyeva Y, et al. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA. 2013;110(10):4111–4116. doi: 10.1073/pnas.1221194110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ermakova M, Battchikova N, Allahverdiyeva Y, Aro EM. Novel heterocyst-specific flavodiiron proteins in Anabaena sp. PCC 7120. FEBS Lett. 2013;587(1):82–87. doi: 10.1016/j.febslet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Kumar K, Mella-Herrera RA, Golden JW. Cyanobacterial heterocysts. Cold Spring Harb Perspect Biol. 2010;2(4):a000315. doi: 10.1101/cshperspect.a000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsby AE. Cyanobacterial heterocysts: Terminal pores proposed as sites of gas exchange. Trends Microbiol. 2007;15(8):340–349. doi: 10.1016/j.tim.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Böthe H, Schmitz O, Yates MG, Newton WE. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol Mol Biol Rev. 2010;74(4):529–551. doi: 10.1128/MMBR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones KM, Haselkorn R. Newly identified cytochrome c oxidase operon in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120 specifically induced in heterocysts. J Bacteriol. 2002;184(9):2491–2499. doi: 10.1128/JB.184.9.2491-2499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valladares A, Maldener I, Muro-Pastor AM, Flores E, Herrero A. Heterocyst development and diazotrophic metabolism in terminal respiratory oxidase mutants of the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol. 2007;189(12):4425–4430. doi: 10.1128/JB.00220-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao W, Guo Q, Zhao J. A membrane-associated Mn-superoxide dismutase protects the photosynthetic apparatus and nitrogenase from oxidative damage in the Cyanobacterium Anabaena sp. PCC 7120. Plant Cell Physiol. 2007;48(4):563–572. doi: 10.1093/pcp/pcm025. [DOI] [PubMed] [Google Scholar]
- 17.Zhao W, Ye Z, Zhao J. RbrA, a cyanobacterial rubrerythrin, functions as a FNR-dependent peroxidase in heterocysts in protection of nitrogenase from damage by hydrogen peroxide in Anabaena sp. PCC 7120. Mol Microbiol. 2007;66(5):1219–1230. doi: 10.1111/j.1365-2958.2007.05994.x. [DOI] [PubMed] [Google Scholar]
- 18.Hackenberg C, et al. Cyanobacterial lactate oxidases serve as essential partners in N2 fixation and evolved into photorespiratory glycolate oxidases in plants. Plant Cell. 2011;23(8):2978–2990. doi: 10.1105/tpc.111.088070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekman M, Sandh G, Nenninger A, Oliveira P, Stensjö K. Cellular and functional specificity among ferritin-like proteins in the multicellular cyanobacterium Nostoc punctiforme. Environ Microbiol. 2014;16(3):829–844. doi: 10.1111/1462-2920.12233. [DOI] [PubMed] [Google Scholar]
- 20.Mitschke J, Vioque A, Haas F, Hess WR, Muro-Pastor AM. Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc Natl Acad Sci USA. 2011;108(50):20130–20135. doi: 10.1073/pnas.1112724108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picossi S, Flores E, Herrero A. ChIP analysis unravels an exceptionally wide distribution of DNA binding sites for the NtcA transcription factor in a heterocyst-forming cyanobacterium. BMC Genomics. 2014;15:22. doi: 10.1186/1471-2164-15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangaraj P, Ludden PW. Accumulation of 99Mo-containing iron-molybdenum cofactor precursors of nitrogenase on NifNE, NifH, and NifX of Azotobacter vinelandii. J Biol Chem. 2002;277(42):40106–40111. doi: 10.1074/jbc.M204581200. [DOI] [PubMed] [Google Scholar]
- 23.Böhme H, Haselkorn R. Molecular cloning and nucleotide sequence analysis of the gene coding for heterocyst ferredoxin from the cyanobacterium Anabaena sp. strain PCC 7120. Mol Gen Genet. 1988;214(2):278–285. doi: 10.1007/BF00337722. [DOI] [PubMed] [Google Scholar]
- 24.Peterson RB, Burris RH. Properties of heterocysts isolated with colloidal silica. Arch Microbiol. 1976;108(1):35–40. doi: 10.1007/BF00425090. [DOI] [PubMed] [Google Scholar]
- 25.Milligan AJ, et al. Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection. J Phycol. 2007;43(5):845–852. [Google Scholar]
- 26.Murry MA, Olafsen AG, Benemann JR. Oxidation of diaminobenzidine in the heterocysts of Anabaena cylindrica. Curr Microbiol. 1981;6(4):201–206. [Google Scholar]
- 27.Peden EA, et al. Identification of global ferredoxin interaction networks in Chlamydomonas reinhardtii. J Biol Chem. 2013;288(49):35192–35209. doi: 10.1074/jbc.M113.483727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omairi-Nasser A, Galmozzi CV, Latifi A, Muro-Pastor MI, Ajlani G. NtcA is responsible for accumulation of the small isoform of ferredoxin:NADP oxidoreductase. Microbiology. 2014;160(Pt 4):789–794. doi: 10.1099/mic.0.076042-0. [DOI] [PubMed] [Google Scholar]
- 29.Mulligan ME, Haselkorn R. Nitrogen fixation (nif) genes of the cyanobacterium Anabaena species strain PCC 7120. The nifB-fdxN-nifS-nifU operon. J Biol Chem. 1989;264(32):19200–19207. [PubMed] [Google Scholar]
- 30.Masepohl B, Görlitz K, Monnerjahn U, Moslerand B, Böhme H. The ferredoxin-encoding fdxN gene of the filamentous cyanobacterium Anabaena variabilis ATCC 29413 is not essential for nitrogen fixation. New Phytol. 1997;136(3):419–423. doi: 10.1046/j.1469-8137.1997.00771.x. [DOI] [PubMed] [Google Scholar]
- 31.Krasikov V, Aguirre von Wobeser E, Dekker HL, Huisman J, Matthijs HC. Time-series resolution of gradual nitrogen starvation and its impact on photosynthesis in the cyanobacterium Synechocystis PCC 6803. Physiol Plant. 2012;145(3):426–439. doi: 10.1111/j.1399-3054.2012.01585.x. [DOI] [PubMed] [Google Scholar]
- 32.Osanai T, et al. Capillary electrophoresis-mass spectrometry reveals the distribution of carbon metabolites during nitrogen starvation in Synechocystis sp. PCC 6803. Environ Microbiol. 2014;16(2):512–524. doi: 10.1111/1462-2920.12170. [DOI] [PubMed] [Google Scholar]
- 33.Flaherty BL, Van Nieuwerburgh F, Head SR, Golden JW. Directional RNA deep sequencing sheds new light on the transcriptional response of Anabaena sp. strain PCC 7120 to combined-nitrogen deprivation. BMC Genomics. 2011;12:332. doi: 10.1186/1471-2164-12-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Argudo I, Little R, Shearer N, Johnson P, Dixon R. Nitrogen fixation: Key genetic regulatory mechanisms. Biochem Soc Trans. 2005;33(Pt 1):152–156. doi: 10.1042/BST0330152. [DOI] [PubMed] [Google Scholar]
- 35.Elhai J, Wolk CP. Developmental regulation and spatial pattern of expression of the structural genes for nitrogenase in the cyanobacterium Anabaena. EMBO J. 1990;9(10):3379–3388. doi: 10.1002/j.1460-2075.1990.tb07539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.