Abstract
The validation of putative biomarker candidates has become the major bottle-neck in protein biomarker development. Conventional immunoaffinity methods are limited by the availability of antibodies and kits. Here we demonstrated the feasibility of using the selected reaction monitoring (SRM) without isotope labeling to achieve fast and reproducible quantification of serum proteins. The SRM/MRM assays for three standard serum proteins, including ceruloplasmin (CP), serum aymloid A (SAA) and sex hormone binding globulin (SHBG) have good linear ranges, generally 103 – 104. There are almost perfect correlations between SRM intensities and the loaded peptide amounts (R2 is usually ~0.99). Our data suggest that SRM/MRM is able to quantify proteins at 0.2 – 2 fmol level, which are comparable to the commercial ELISA/LUMINEX kits for these proteins. Excellent correlations between SRM/MRM and ELISA/LUMINEX assays were observed for SAA and SHBG (R2 = 0.928 and 0.851 respectively). The correlation between SRM/MRM and ELISA for CP is less desirable (R2 = 0.565). The reproducibility for SRM/MRM assays is generally very good but may depend on the proteins/peptides (R2 = 0.931 and 0.882 for SAA and SHBG, and 0.723 for CP). SRM/MRM assay without isotope labeling is a rapid and useful method for protein biomarker validation in a modest number of samples and is especially useful when other assays such as ELISA or Luminex beads are not available.
Keywords: SRM/MRM, ELISA, Biomarker validation
Introduction
Recent advances in comparative proteomic technologies, such as isobaric labeling [1], SILAC[2] and spectral counting-based non-labeling methods [3], have made possible the rapid discovery of many putative protein biomarkers from complex proteomes such as serum [4]. However, validation of the putative biomarker candidates has become the major bottle-neck in protein biomarker development. Immunoaffinity-based assays, such as ELISA and LUMINEX technologies, have been widely used for the validation purpose. These assays usually have excellent specificity and excellent sensitivity as well as high-throughput capacity. However, the application of immunoaffinity-based techniques is severely hindered by the limited availability of antibodies and kits because of the high cost and lengthy procedure required for the production of specific antibodies and the development of assay kits [5].
Selected reaction monitoring (SRM) and its extension, multiple reaction monitoring (MRM), have been widely used in the quantification of small molecules [6]. Recently, these techniques have been adopted for protein/peptide analysis [7–12]. The basic concept of the technique is to monitor the presence and intensity of specific transitions consisting of pairs of parent ion m/z and its daughter ion m/z. The selected monitoring and double selection criteria (parent/daughter ions) provide high specificity for peptide selection since only desired transitions are recorded and other signals are regarded as noise. Furthermore, dependent MS/MS scans can be triggered by SRM/MRM scans to provide sequence information for the selected peptides, further increasing the specificity of the technique. Multiple transitions (50–100) corresponding to multiple proteins of interest can be monitored and sequenced in a single MRM analysis, providing great potentials for quantitative analyses of a relatively large number of proteins in a single assay.
In previous SRM/MRM studies, protein/peptide quantification was achieved using isobaric labeling (iTRAQ) of the target proteins [11] or by spiking isotopic peptides into samples as internal controls [8–10,13]. While both approaches have been successfully used, there are significant hurdles to apply these approaches to large scale biomarker validation experiments because the high cost of the reagents and the incompleteness of isotopic labeling of target proteins. Therefore, here we evaluated the possibility of direct quantification of proteins/peptides from human serum using SRM/MRM method and compared it with immunoaffinity methods. Three important issues, including the sensitivity and linear range of SRM/MRM method, the correlation between SRM/MRM results and immunoaffinity results and the reproducibility of the SRM/MRM method, were addressed in this study.
EXPERIMENTAL
Materials
Ceruloplasmin (purity >95%), serum aymloid A (purity >98%) and sex hormone binding globulin (purity >90%) were purchased from Athens Research & Technology (Athens, GA, USA), Santa Cruz Biotechnology (Santa Cruz, CA, USA) and GenWay Biotech (San Diego, CA, USA), separately. Other reagents were purchased from Fisher Scientific (Pittsburgh, PA, USA), unless otherwise indicated.
Serum Preparation
Blood samples from healthy volunteers were obtained using serum separator tubes (BD Biosciences, Franklin Lakes, NY, USA) after consent. Samples were allowed to clot at room temperature for 30 minutes before centrifugation at 3000g at 4°C for 10 minutes. Serum samples were then aliquoted and stored at −80°C until use. No more than three cycles of freezing/thaw were allowed for any samples.
In-solution Protein Digestion
Lyophilized standard proteins were dissolved into 50 mM ammonia bicarbonate to achieve final concentrations of 0.5mg/ml. For pre-dissolved standard protein, the buffer of protein sample was first exchanged to 50mM ammonia bicarbonate by loading onto gel-filtration spin column (Micro-spin 6, BioRad Laboratories, Hercules, CA, USA) pre-equilibrated with 50 mM ammonia bicarbonate and centrifugating at 1000 g for 4 minutes. The sample was then diluted to 0.5mg/ml using 50mM ammonia bicarbonate. Each neat serum sample (1uL) was diluted with 100 uL 50mM ammonia bicarbonate to a final concentration of ~0.5mg/ml. All protein samples were next denatured and reduced by adding dithiotheritol (DTT) to a final concentration of 10mM and incubating at 95 °C for 5 minutes. Alkylation was performed by adding 1/10 volume of 200mM iodoacetamide (IAA) to the mixture and incubation at room temperature for 30 minutes in the dark. Sequencing-grade trypsin (Promega, Madison, WI, USA) was then added at the enzyme to protein ratio of 1:50 (w/w); the mixture was incubated at 37°C for 12 hours. Digestion was terminated by adding formic acid to a final concentration of 1%. Digested peptide mix was lyophilized on a Freezone 6 freeze dry system (Labconco, Kansas City, MO, USA). Digested standard proteins and neat serum were reconstituted with 10ul 0.1% formic acid each and stored at −80°C for further analysis.
Development of transitions for purified proteins
Transitions were developed using the MIDAS Workflow Designer software (Ver. 1.1 Applied Biosystems, Foster City, CA, USA). Briefly, protein sequence was imported into the software and preferred Q1 and Q3 m/z were generated to obtain the transition list using the following settings: enzyme=trypsin, missed cleavages=0, fixed modification=Carbamidomethylation (C), fragments=1y>precursor, 2y>precursor and y from sequence, and max. modifications in peptide=3. Filters used to narrow down the MRM transitions list included Q1 m/z>400, Q3 m/z<1200, number of AA between 6 and 30. MRM dwell time was fixed at 80 ms. To increase the specificity of the method, two or three fragments were selected to build two or three transitions for each peptides. MRM methods were therefore built based on these transitions for each protein. Transitions for each protein were further refined by examining the peak areas of the candidate transitions after analyzing 100 fmol digest of each purified protein on LC-SRM/MRM MS using the above method. The top 3 or 4 peptides for each protein with the highest SRM/MRM response (peak areas of transitions) were selected as signature peptides and included in the final transition list. For any signature peptide, if three fragments were selected in the transition list, only the top 2 fragments with largest peak areas were included in the final list. Therefore, a pair of transitions was created for each signature peptide resulting in a easy-to-distinguish overlapping double-peak with the same elution time in spectral (Supplementary Fig. 1).
LC-SRM/MRM MS analysis
LC-SRM/MRM MS analysis was performed using MRM Initiated Detection And Sequencing (MIDAS, Applied Biosystems, Foster City, CA, USA) workflow comprising a Agilent 1100 nano-HPLC (Santa Clara, CA, USA) coupled with 4000 QTRAP MS system (Applied Biosystems, Foster City, CA, USA).
Digested sample (1 uL) was loaded onto a ZORBAX 300SB-C18 column (3.5μm, 150mm×0.075mm, Agilent Technologies, Santa Clara, CA, USA) and separated by a 5%~40% gradient of acetonitrile (0.1% formic acid) over 40 minutes. Eluted peptides were introduced into 4000 QTRAP MS system via electrospray ionization using MicroIonSpray II head (Applied Biosystems, Foster City, CA, USA), with temperature of 125°C, spray voltage of 2500V and ion source gas1 of 12. The MRM transitions were monitored with Q1 and Q3 setting of low and unit respectively and three enhanced product ion scan (MS/MS scan) were triggered corresponding to the most intense three peaks when the intensities of transitions were above 200 cps (Information dependent acquisition, IDA). To monitor as much as designated transitions as possible, dynamic exclusion feature of IDA was disabled. The peak areas of transitions were calculated using the automatic integration feature of the Analyst software (Ver 1.4, Applied Biosystems, Foster City, CA, USA).
LUMINEX assay
Serum amyloid A (SAA) was measured using Human Cardiovascular Disease Panel 2 Lincoplex Kit (Millipore, Billerica, MA, USA) on a LUMINEX 100 system (LUMINEX Corporation, Austin, TX, USA). All analyses are performed according to manufacturer’s protocol. Briefly, the kit is a sandwich immuno-assay, which consists of dyed microspheres conjugated with a monoclonal antibody specific for SAA as a capture antibody. Serum samples (1:1000 diluted) were incubated with the antibody-coupled microspheres, and then with biotinylated detection antibody, and then streptavidin-phycoerythrin. The captured bead-complexes were read by the Bioplex array reader (Biorad Laboratories, Hercules, CA, USA). Instrument settings were: events/bead: 50, minimum events: 0, Flow rate: 60ul/min, Sample size: 50ul, doublet discriminator gates at 6800 and 12800. For all the assays Median fluorescence Intensity (MFI) were collected.
ELISA assays
Sex hormone binding protein (SHBG) in serum was measured using an ELISA kit from Diagnostic Systems Laboratories (Webster, Texas, USA). The kit is a two-step “sandwich” assay. Briefly, standards, controls and samples were incubated in microtitration wells coated with anti-SHBG antibody. After incubation and washing, anti-SHBG detection antibody labeled with enzyme-horseradish peroxidase (HRP) was added to each well. After a second incubation and washing step, the substrate tetramethylbenzidine (TMB) was added to the wells. Lastly, an acidic stopping solution was added. The degree of enzymatic turnover of the substrate was determined by dual wavelength absorbance measurement at 450 nm and between 600 and 630 nm.
Ceruloplasmin (CP) in serum was measured using an ELISA kit from Assaypro (St. Charles, MO, USA). This assay employs a quantitative competitive enzyme immunoassay technique. Briefly, a polyclonal antibody specific for CP was pre-coated onto a 96-well microplate. CP in standards and samples was competed by a biotinylated CP sandwiched by the immobilized antibody and streptavidin-peroxidase conjugate. All unbound material was then washed away and a peroxidase enzyme substrate was added. The color development was stopped and the intensity of the color was measured.
Results and Discussion
Three purified human serum proteins, including ceruloplasmin (CP), serum aymloid A (SAA) and sex hormone binding globulin (SHBG) were first tested for SRM/MRM responses and linear ranges. Transitions for three or four peptides were developed for each of the three proteins (Supplementary Table 1), as described in Materials and methods section.
Quantification of peptides/proteins is based on the intensities (integrated peak area) of the transitions using MRM scan at the triple quadruple followed by three enhanced product ion scans in the ion-trap. To evaluate the feasibility of direct quantification by MRM, serial dilutions of the digested peptide mixture from each protein were analyzed and the intensities of the transitions were correlated with loaded amount of peptides. To minimize absorption of target peptides by glass vial, especially at very low concentration, the digested peptide mixture was diluted using 100ng/μl human transferrin digest, which saturates the vial surface and also increased the complexity of the sample. Due to the relative high concentration of these three proteins in serum, it is not feasible to use serum digest as matrix to evaluate the performance of the method. However, based on the well-known high specificity/selectivity of the SRM/MRM method, increased sample complexity would have minimal impact on the performance of the SRM/MRM method. Co-eluted other peptides from the increased complexity will be ignored since most of them do not have the same Q1/Q3 values. Meanwhile, the accompany MS/MS sequencing feature triggered by SRM/MRM scan would exclude the false positive results caused by transitions from other peptides that have similar Q1/Q3 values to target transitions.
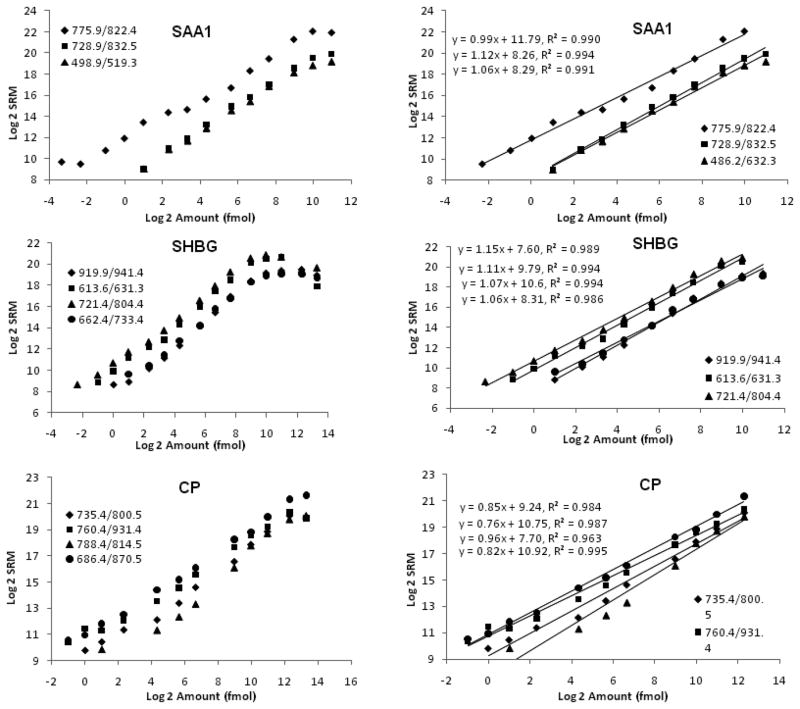
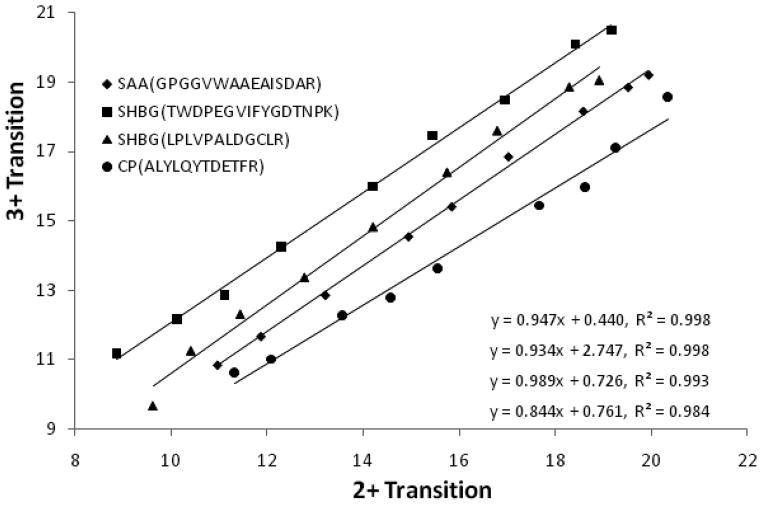
As shown in Fig. 1, the MRM intensity-peptide amount plots for all three proteins have typical S-shapes. However, the assays have excellent linear range, generally in the range of 103 – 104 and there are almost perfect correlations between MRM intensities and the loaded peptide amounts (R2 are usually ~0.99) (Fig. 1). Furthermore, the linear range is transition-specific and transitions with higher intensity for the same protein tend to have better linear ranges (Fig. 1). Compared to the linear ranges for the ELISA/LUMINEX kits for these three proteins (<103) (Supplementary Fig. 2), the ones for MRM-based assays are much wider. An interesting finding of our study is that the transitions with different charges (2+ and 3+) for the same peptide have differently intensities but the ratios remain constant at different peptide concentration (Fig. 2). These results suggest that the electro-spray ionization of peptides produces fairly constant ratios of 2+ and 3+ charged ions but may have preference of producing ions with either of the charge states. Our results are consistent with the view that SRM/MRM transitions should be designed assuming both 2+ and 3+ charged precursors in Q1, because the predominant charge state in positive-ion mode cannot be safely assumed to be the same as in negative-ion mode [14].
Figure 1.
Standard curves for three purified human proteins for SRM/MRM analysis. Serial dilutions of the digested peptides were loaded onto a nano-RP column and separated by an acetonitrile gradient of 5%~30% in 30 minutes. MRM intensities for the peptides were derived from the integrated peak area for the daughter ion with higher peak-height. MRM intensities and loaded peptide amounts were Log2 transformed before plotting. The entire series of dilutions were presented on the left panel, while the linear ranges of the standard curves were presented on the right panel. The linear regression results were listed for each transition.
Figure 2.
Correlation between MRM intensities of doubly and triply charged ions for the same peptides.
Analyses of the purified proteins suggest that SRM/MRM is able to quantify proteins at levels as low as 0.2 fmol based on our results on the three proteins (Fig. 1). This detection level is comparable to the ones of the commercial ELISA/LUMINEX kits for these proteins (Supplementary Fig. 2), suggesting that SRM/MRM has good sensitivity in detecting purified proteins/peptides. In reality, we were able to detect, in human serum, all three proteins including SHBG, CP and SAA (Fig. 3) with serum concentration at μg/ml to mg/ml level. However, SRM/MRM is not able to reliably detect and quantify low-abundance serum proteins, such as myeloperoxidase (MPO), directly in human neat serum, the mean concentration of which is ~3.6pmol/ml based on our LUMINEX results (data not shown). Approximately, only 0.3fmol of MPO protein is subjected to SRM/MRM analysis using our protocol (equivalent of ~0.1ul of neat serum) due to the limited sample loading capacity of nano-C18 column. This is near the lower end of the detection limit of the SRM/MRM method. The sample loading capacity on the nano-C18 column (0.1 ul) is much lower than the possible processing volume of ELISA/LUMINEX assays (up to several ul), which therefore leads to the much lowered sensitivity (expressed in concentration, for example, ng/ul) of the SRM/MRM method. This technical hurdle could be overcome by various protein enrichment techniques [7,8]. For example, MPO as well as other low abundance serum proteins have been readily detected and quantified from the serum samples normalized with a combinatorial hexpeptide ligand libraries beads (ProteoMiner kit from Bio-Rad) in our laboratory (data not shown). However, adding further prefractionation step prior to SRM/MRM assays could lead to increased variation of the results and also more analysis time and efforts, the extent of both are now being evaluated by our group.
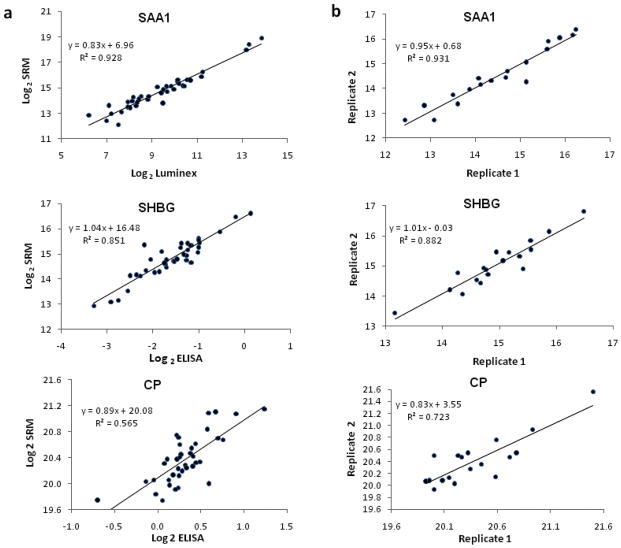
Figure 3.
Performance of MRM for quantitative analysis of serum proteins. (a) Correlation between protein concentrations measured by MRM and by LUMINEX or ELISA in 40 different serum samples. All data were Log2 transformed. (b) Reproducibility of two replicate MRM analyses for 20 different serum samples. Data were Log2 transformed.
To compare the quantification of proteins in serum by SRM/MRM and immunoaffinity methods, we measured the concentration of SAA, SHBG and CP in 40 neat serum samples using both SRM/MRM and ELISA/LUMINEX assays. For SRM/MRM analysis, equal volume (1ul) of digested and diluted neat serum from each sample was analyzed sequentially for the transitions developed with the purified proteins. Using the multiplexing capability of the 4000 QTRAP instrument, all transitions for the three proteins were analyzed simultaneously for all samples. Due to the good correlation between different transitions within the same protein, as indicated in Supplementary Fig. 3 for CP and our other unpublished data, only the MRM intensity for the strongest transition of each protein were plotted against the ELISA/LUMINEX results for each protein (Fig. 3a). Excellent correlations between SRM/MRM and ELISA/LUMINEX assays were observed for SAA and SHBG (R2 = 0.928 and 0.851 respectively), suggesting that SRM/MRM can be used for serum protein quantification without isotope labeling or internal standard. The correlation between SRM/MRM and ELISA for CP for the selected transition (R2 = 0.565) is lower than for the other two proteins. Although SRM/MRM analysis with other transitions for CP may improve the results, the lower correlation between MRM and ELISA for CP may be largely caused by the poor performance of the ELISA kit used in this study as shown in Supplementary Fig. 2c. The CP ELISA kit was based on competitive principle and the standard curve is not linear even after double log transform, as shown in Supplementary Fig 2c, which could lead to the decreased accuracy of the calculation results of the CP concentration in serum samples and therefore results in the lower correlation between ELISA and SRM/MRM results. On the other hand, there seems to be a positive connection between the reproducibility of transitions and the correlation between SRM/MRM and ELISA/LUMINEX result as shown in Fig. 3a and b, which further addressed the importance of selection good transitions for reproducible SRM/MRM assays.
We next examined the reproducibility of the SRM/MRM analysis of serum proteins. For this purpose, 20 serum samples were firstly analyzed by SRM/MRM at two separate time points (Fig. 3b). Reproducibility was assessed using two different methods: the coefficient of variation (CV) and the square of correlation (R2). The mean CVs for all three transitions are surprisingly small (1.5 – 2.1% for the three proteins). Consistently, the R2 values for all three transitions were excellent (R2 = 0.882, 0.931 and 0.723 for SHBG, SAA and CP, respectively). Another 30 aliquots of the same neat serum sample were also analyzed using SRM/MRM assay and the reproducibility was assessed by examining the CVs of multiple transitions. As shown in Table 1, 8 of the 9 transitions had CVs of less than 20%. Furthermore, the peak areas measured by SRM/MRM for two different peptides/transitions were very consistent (Supplementary Fig. 3) among multiple samples. All these reproducibility tests were done without the use of standard curve of any kind, suggesting that direct SRM/MRM analyses of serum samples can be used to relatively quantify proteins/peptides in complex proteomes such as the serum. Our lab also performs a large number of ELISA/LUMINEX assays on a daily basis and the reproducibilities of these assays were checked routinely from time to time for a variety of molecules. Normally the intra-plate CVs are less than 20% (data not shown) and are generally comparable to the ones for SRM/MRM assays. A recently published paper also evaluated the robustness of the SRM/MRM methods across eight laboratories by progressively introducing sample preparation and instrumental analyses variables into the SRM/MRM analysis of seven spiked protein into human plasma, and reached satisfactory results as to inter- and intralaboratory reproducibility, transferability, precision and sensitivity, which could provide a fairly good basis to compare the robustness of the SRM/MRM method to ELISA/LUMINEX method [15].
Table 1.
SRM/MRM reproducibility test for 30 aliquots of neat serum sample
| Protein | Transitions | Mean | St. dev | CV |
|---|---|---|---|---|
| SAA | 775.9/822.4 | 1.85E+05 | 2.81E+04 | 15.2% |
| 728.9/832.5 | 3.91E+04 | 7.57E+03 | 19.4% | |
|
| ||||
| SHBG | 613.6/631.3 | 8.44E+05 | 1.17E+05 | 13.9% |
| 919.9/941.4 | 1.98E+05 | 3.51E+04 | 17.7% | |
| 721.4/804.4 | 8.16E+04 | 2.28E+04 | 27.9% | |
|
| ||||
| CP | 760.4/931.4 | 3.97E+05 | 5.44E+04 | 13.7% |
| 735.4/800.5 | 1.15E+06 | 1.55E+05 | 13.5% | |
| 788.4/814.5 | 1.67E+06 | 3.13E+05 | 18.7% | |
| 686.4/870.5 | 1.12E+06 | 6.43E+04 | 5.7% | |
Our non-labeling SRM/MRM assay is convenient and cost-effective method. Compared to the SRM/MRM approaches that use spiked isotopic labeling of peptides as internal standards or iTRAQ labeled target proteins, our approach is less expensive and saves time and money required for target protein labeling or spiked isotopic peptide controls. We believe that this is a very useful technique for the initial biomarker validation when other assays are not available and only a modest number of samples need to be analyzed. However, isotopic labeling MRM should offer better reproducibility over long periods of time and is recommended if thousands of samples must be analyzed. Furthermore, ELISA or Luminex assays are still preferred if they are available for the proteins of interest. The key advantage of MRM over ELISA and Luminex is that an assay can be developed in hours to days while months is required to develop a new ELISA/Luminex assay. Therefore, non-labeling MRM is a quick and dirty protein biomarker validation methods across a relatively modest number (hundreds) of samples.
Conclusions
Our data indicate that non-labeling SRM/MRM is a very useful addition to the proteomic toolbox for the rapid confirmation of protein differences across a relatively modest number (hundreds) of samples.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of health (4R33HD050196 and 2RO1HD37800) to Dr. Jin-Xiong She. Wenbo Zhi has been supported by a postdoctoral fellowship from the juvenile diabetes research foundation (JDRF3-2009-275).
Contributor Information
Wenbo Zhi, Email: wzhi@gerogiahealth.edu, Center for Biotechnology and Genomic Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA, 30912, USA
Meiyao Wang, Email: mewang@gerogiahealth.edu, Center for Biotechnology and Genomic Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA, 30912, USA
Jin-Xiong She, Email: jshe@gerogiahealth.edu, Center for Biotechnology and Genomic Medicine, Georgia Health Sciences University, 1120 15th Street, Augusta, GA, 30912, USA, Phone: 706-721-3410, Fax: 706-721-3688
Reference List
- 1.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, et al. Multiplexed Protein Quantitation in Saccharomyces cerevisiae Using Amine-reactive Isobaric Tagging Reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 2.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, et al. Stable Isotope Labeling by Amino Acids in Cell Culture, SILAC, as a Simple and Accurate Approach to Expression Proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 3.Asara JM, Christofk HR, Freimark LM, Cantley LC. A label-free quantification method by MS/MS TIC compared to SILAC and spectral counting in a proteomics screen. Proteomics. 2008;8:994–999. doi: 10.1002/pmic.200700426. [DOI] [PubMed] [Google Scholar]
- 4.Whiteaker JR, Zhao L, Zhang HY, Feng LC, Piening BD, Anderson L, et al. Antibody-based enrichment of peptides on magnetic beads for mass-spectrometry-based quantification of serum biomarkers. Anal Biochem. 2007;362:44–54. doi: 10.1016/j.ab.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteaker JR, Zhang H, Zhao L, Wang P, Kelly-Spratt KS, Ivey RG, et al. Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J Proteome Res. 2007;6:3962–3975. doi: 10.1021/pr070202v. [DOI] [PubMed] [Google Scholar]
- 6.Lee MS, Kerns EH. LC/MS applications in drug development. Mass Spectrom Rev. 1999;18:187–279. doi: 10.1002/(SICI)1098-2787(1999)18:3/4<187::AID-MAS2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Anderson L, Hunter CL. Quantitative Mass Spectrometric Multiple Reaction Monitoring Assays for Major Plasma Proteins. Mol Cell Proteomics. 2006;5:573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, Multiplexed Assays for Low Abundance Proteins in Plasma by Targeted Mass Spectrometry and Stable Isotope Dilution. Mol Cell Proteomics. 2007;6:2212–2229. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeSouza LV, Taylor AM, Li W, Minkoff MS, Romaschin AD, Colgan TJ, et al. Multiple Reaction Monitoring of mTRAQ-Labeled Peptides Enables Absolute Quantification of Endogenous Levels of a Potential Cancer Marker in Cancerous and Normal Endometrial Tissues. J Proteome Res. 2008 doi: 10.1021/pr800312m. [DOI] [PubMed] [Google Scholar]
- 10.Gerber SA, Rush J, Stemman O, Kirschner MW, Gygi SP. Absolute quantification of proteins and phosphoproteins from cell lysates by tandem MS. Proc Natl Acad Sci U S A. 2003;100:6940–6945. doi: 10.1073/pnas.0832254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf-Yadlin A, Hautaniemi S, Lauffenburger DA, White FM. Multiple reaction monitoring for robust quantitative proteomic analysis of cellular signaling networks. Proceedings of the National Academy of Sciences. 2007;104:5860–5865. doi: 10.1073/pnas.0608638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange V, Malmstrom JA, Didion J, King NL, Johansson BP, Schafer J, et al. Targeted quantitative analysis of Streptococcus pyogenes virulence factors by multiple reaction monitoring. Mol Cell Proteomics. 2008;7:1489–1500. doi: 10.1074/mcp.M800032-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitteringham NR, Jenkins RE, Lane CS, Elliott VL, Park BK. Multiple reaction monitoring for quantitative biomarker analysis in proteomics and metabolomics. Journal of Chromatography B. doi: 10.1016/j.jchromb.2008.11.013. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 14.Lenz C, Kühn-Hölsken E, Urlaub H. Detection of Protein-RNA Crosslinks by NanoLC-ESI-MS/MS Using Precursor Ion Scanning and Multiple Reaction Monitoring (MRM) Experiments. J Am Soc Mass Spectrom. 2007;18:869–881. doi: 10.1016/j.jasms.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Addona TA, Abbatiello SE, Schilling B, Skates SJ, Mani DR, Bunk DM, et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat Biotech. 2009;27:633–641. doi: 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.