SUMMARY
Recombinant tissue plasminogen activator (r-tPA) is the drug of choice for thrombolysis, but it is associated with a significant risk of bleeding and is not always successful. By cleaving von Willebrand factor (VWF), the metalloprotease ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type I repeats 13) down-regulates thrombus formation in injured vessels. We investigated whether recombinant ADAMTS13 (r-ADAMTS13) induces thrombolysis in vivo in mice. Thrombosis was produced by ferric chloride-induced (FeCl3) injury in the venules of a dorsal skinfold chamber. Vehicle, r-tPA or r-ADAMTS13, supplemented with r-hirudin (to stop on-going thrombin generation), was directly applied onto the occluded vessel and thrombus dissolution was evaluated by intravital microscopy. The incidence of blood flow restoration significantly increased 30 minutes after r-ADAMTS13 versus PBS treatment (60% versus 0%, P<0.05) and 60 minutes after r-tPA treatment (75% versus 17%, P<0.05). Both r-tPA and r-ADAMTS13 significantly reduced thrombus size 60 minutes after their superfusion (53.2% and 62.3% of the initial thrombus size, P<0.05 and P<0.01, respectively). Bleeding occurred in all r-tPA-treated chambers, while it was absent in mice treated with r-ADAMTS13 or PBS.
We observed that, similar to r-tPA, r-ADAMTS13 can dissolve occlusive thrombi induced by FeCl3 injury in venules. The in vivo thrombolytic effect of the drug was not associated with any sign of haemorrhage. ADAMTS13 could represent a new therapeutic option for thrombolysis.
INTRODUCTION
Vascular injury and subsequent thrombus formation are key events of thrombotic disorders such as myocardial infarction, stroke or deep vein thrombosis and they are a major cause of mortality in western countries. Thrombolytic therapy is an important means of establishing reperfusion after a thrombotic event. Currently, only plasminogen activators have been approved by regulatory agencies as thrombolytics (1). These drugs convert the pro-enzyme, plasminogen, into plasmin, which digests the fibrin network stabilizing the thrombus thus causing its lysis (2). The most commonly used thrombolytic is recombinant tissue plasminogen activator (r-tPA). It has been shown to significantly improve the survival rate in patients with acute myocardial infarction (3) and to ameliorate neurologic function in acute stroke (4). However, haemorrhagic complications are the most dangerous adverse events associated with r-tPA treatment and alternative thrombolytic therapies are needed (5, 6). von Willebrand factor (VWF) is a multimeric protein present in platelet α-granules and in the Weibel-Palade bodies of endothelial cells that plays an essential role in thrombogenesis (7, 8). It is released as ultra large multimers (UL-VWF) which are highly biologically active in binding platelet GPIbα and promoting platelet adhesion to the subendothelium (9). VWF is also a critical mediator of platelet-platelet firm adhesion especially while the thrombus is growing and the shear rate increasing (10). If not rapidly consumed for platelet adhesion, UL-VWF is cleaved by the metalloprotease ‘a disintegrin-like and metalloprotease with thrombospondin type I repeats – 13′ (ADAMTS13) to smaller, less adhesive multimers (11). ADAMTS13 has a powerful antithrombotic activity in vivo in both low venous and high arterial shear stress conditions. It has been shown to regulate platelet interaction with the “activated” vessel wall of venules, to significantly prolong occlusion time in FeCl3-injured arterioles, but also to destabilize the growing thrombus when infused in mice (12). Moreover, ADAMTS13 reduces infarct size and improves functional outcome in experimental stroke (13). Using intravital microscopy, we evaluated the efficacy of recombinant ADAMTS13 (r-ADAMTS13) to re-open occlusive thrombi in a mouse model of thrombolysis using FeCl3-induced injury and dorsal skinfold chambers (14).
MATERIALS AND METHODS
Mice
C57BL/6J male mice (8- to 10-week-old) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Animals were bred at the Immune Disease Institute and experimental procedures were approved by its Animal Care and Use Committee.
FeCl3-induced injury and thrombolysis model
Mice bearing dorsal skinfold chambers (15) were anaesthetized with an intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. Following surgery, mice were injected subcutaneously with 0.1 mg/kg buprenorphine and then again every 8–12 hours (h) for 3 days for analgesia. Vessels in dorsal skinfold chambers were exposed by removing the chamber cover glass. Occlusive thrombosis was generated by using a 0.5 × 1 mm filter paper soaked with a 15% FeCl3 solution and placed over exposed subcutaneous venules (100 to 160 μm in diameter) for 4 minutes (min), as previously described (14, 16). Thrombus formation at the site of injury was detected by infusion in the blood circulation of Syto 62 (Invitrogen, Carlsbad, CA, USA) (17) and was monitored until vessel occlusion, defined as complete arrest of blood flow, for at least 5 min. Five minutes after vessel occlusion, 50 μl of PBS containing r-tPA (140 μM) (Genentech Inc., San Francisco, CA, USA) or r-ADAMTS13 (4 μM) (Baxter Innovations GmbH, Vienna, Austria), supplemented with r-hirudin (100 μM) (Pentapharm, Basel, Switzerland) was applied topically in the chamber. r-hirudin, a thrombin inhibitor, was used to block the on-going thrombin activity at the site of FeCl3-induced injury and to prevent rethrombosis after the thrombolytic treatment. Thrombolysis was analysed by measuring the occurrence of blood flow restoration in the occluded vessels and the decrease in thrombus size at 30 min and 1 h after r-tPA or r-ADAMTS13 treatment. During observation, DSCs were kept open and regularly superfused with saline to prevent drying of the tissue. Typically, one injury was performed per mouse.
Real-time intravital microscopy
Data were obtained using a Zeiss Axioplan upright fluorescence microscope with a LED 4-Color Light Engine (Lumencor Inc., Beaverton, OR, USA) and a cooled Hamamatsu CCD Camera coupled to an image intensifier (Video Scope International, Dulles, VA, USA). Data acquisition and analysis were performed with Slidebook 5.0 software (Intelligent Imaging Innovations Inc., Denver, CO, USA) as previously described (18).
Haemorrhage measurement
Haemorrhage in dorsal skinfold chambers was visualized 24 h post-treatment by light microscopy (Zeiss CCD camera) using software from the same manufacturer (Axiovision 4.6.3, Zeiss, Germany). Full-size skinfold photographs were taken with a Nikon digital camera.
Statistical analysis
For comparisons of the incidence of recanalization and of tissue haemorrhage, an overall contingency table was constructed, followed by a chi-square test to compare individual groups. Data relative to thrombus size are expressed as mean ± SEM and were compared by one-way ANOVA followed by the Tukey test. P values < 0.05 were regarded as statistically significant. GraphPad Prism 4.0 software (GraphPad Software, La Jolla, CA, USA) was used for all statistical analyses.
RESULTS
r-tPA and r-ADAMTS13 restore blood flow and reduce the size of occlusive thrombi
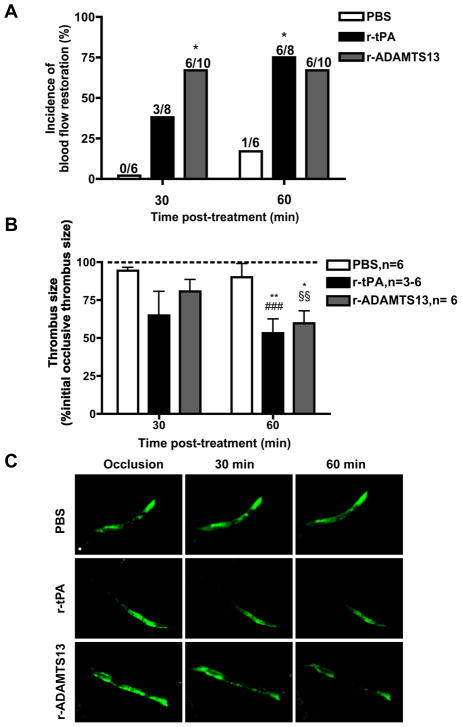
Occlusive thrombi were produced by FeCl3 application; time to vessel occlusion was 63.5 ± 4.1 minutes (mean ± SEM, n=24). Thirty minutes after vehicle (PBS), r-tPA and r-ADAMTS13 treatment, recanalization was observed in 0%, 38% and 60% of mice, respectively. The incidence of blood flow restoration was significantly higher in mice treated with r-ADAMTS13 than in mice treated with PBS. Sixty minutes after drug application, the incidence of blood flow restoration was 17%, 75% and 60% in mice treated with PBS, r-tPA and r-ADAMTS13, respectively, and was significantly higher in the r-tPA than in the PBS treatment group (Fig. 1A).
Figure 1. Evaluation of the thrombolytic effect of r-tPA and r-ADAMTS13.
A. Incidence of blood flow restoration. The number of mice studied in each group is the denominator above each bar. *P<0.05 vs. PBS group. B. Analysis of thrombus size. Data (mean ± SEM) represent percent of the initial occlusive thrombus size (dashed line). *P<0.05 and **P<0.01 versus initial occlusive thrombus size, §§P<0.01 and ###P<0.001 versus PBS group at 60 min post-treatment. C. Intravital microscopy photographs representative of thrombus size at occlusion time and at 30 and 60 min after drug superfusion. Bar = 100 μm.
In vessels where blood flow was restored, the size of the thrombus was calculated as an additional parameter to evaluate the thrombolytic activity of the tested drugs. r-tPA and r-ADAMTS13 both significantly reduced thrombus size 60 min after treatment (to 53.2% and 62.3% of initial thrombus size, respectively) and the residual thrombus was significantly smaller than after PBS treatment (Fig. 1B and C).
Evaluation of the haemorrhagic risk associated with r-tPA and r-ADAMTS13 treatment
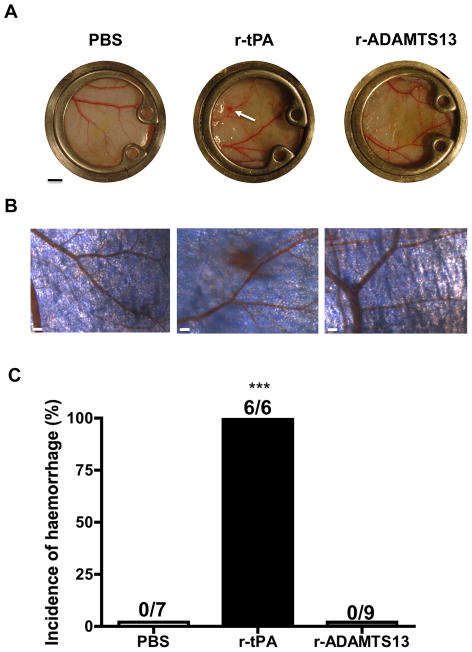
Skinfold chambers were examined 24 h after thrombolytic treatment to evaluate the presence of tissue haemorrhage. Petechial bleeding, originating from vessels surrounding the FeCl3-injured area, was observed in all the mice treated with r-tPA, while no signs of haemorrhage were found in mice treated with r-ADAMTS13 or PBS (Fig. 2A–C).
Figure 2. Tissue haemorrhage evaluation 24 hours after thrombolytic treatment.
A. Photographs of representative skinfold chambers. Bar = 2 mm. The arrow indicates a petechial spot. B. Microscopic views of vessels surrounding FeCl3 injury. Bar = 200 μm. C. Incidence of tissue haemorrhage. The number of mice studied in each group is reported as denominator above the bars. *** P<0.001 versus PBS group or versus r-ADAMTS13 group.
DISCUSSION
Here, by using real-time intravital microscopy, we demonstrate that r-ADAMTS13 acts as a thrombolytic drug in vivo. In fact, we found that, as with the gold standard thrombolytic agent r-tPA, application of r-ADAMTS-13 on occlusive thrombi in dorsal skinfold chamber venules induced a significant reduction in thrombus size and dramatically increased the rate of recanalization within the first hour following treatment. Our results are in line with previous publications demonstrating that thrombolysis by fibrinolytic agents is enhanced by blocking platelet interaction with VWF (19, 20). In this work, thrombolysis was studied by real-time intravital microscopy in dorsal skinfold chamber venules injured with FeCl3. We chose to apply the drugs topically onto the thrombus, instead of infusing them systemically because of the easy access to and the increased permeability of FeCl3-injured vessels in dorsal skinfold chambers (16). Moreover, delivery of the thrombolytic agent directly into the thrombus offers substantial advantages over systemic administration. During systemic administration, drug may fail to reach a therapeutic concentration in part because of the impaired blood flow in occluded vessels and in part because of the short circulation half-life of thrombolytics that necessitates their continuous infusion. Although topical drug application is unlikely to be utilized in patients, in situ drug administration can be achieved by catheter-directed drug delivery, a method that has proven a better efficacy and safety over systemic infusion in the treatment of stroke (21), limb arterial occlusion (22), acute deep vein thrombosis (23–25) and acute massive pulmonary embolism (26, 27).
The occurrence of bleeding episodes, especially intracranial haemorrhage, represents the major complication of thrombolytic therapy with r-tPA (4, 5, 28–30). Haemorrhage may occur either when r-tPA is administered systemically or when it is directly infused into the thrombus by using catheter-based techniques (6). In our experimental model, where drugs are delivered topically to the thrombus, no evident signs of haemorrhage could be detected 24 h after treatment with r- ADAMTS13, while bleeding was found in all r-tPA treated mice. These results reinforce previous data from our lab demonstrating that r-ADAMTS13 exerts a protective role in stroke without producing cerebral haemorrhage (13). In our experimental settings, the difference between r-tPA and r-ADAMTS13 in inducing bleeding is clear-cut and may be due to their diverse target specificity. In fact, while ADAMTS13 has rather restricted proteolytic action and specifically cleaves VWF (31), tPA not only activates zymogen plasminogen but also degrades the major protein components of the extracellular matrix and vessel wall either directly or by inducing matrix metalloproteinase activation (32).
The bleeding rate we observed in mice after local r-tPA treatment was higher than in humans following i.v. drug infusion. This could be in part due to the dose of r-tPA used (140 μM which corresponds to 10 mg/kg) which is 10 times higher than what is currently used in patients. However, since human r-tPA has a modest efficacy in activating mouse plasminogen (33–35), higher doses of r-tPA are required in mice for effective thrombolysis (36–38). Furthermore, in our experimental model, the topical route of administration made it easy for r-tPA to exert cytotoxic effects in the extravascular space. By contrast, in patients r-tPA is mainly infused i.v. and it has to leak into tissues to cause cytotoxicity.
In our study, both r-tPA and r-ADAMTS13 were supplemented with r-hirudin to prevent thrombin activity in the area of the injury and the formation of a new thrombus that could interfere with the evaluation of their thrombolytic efficacy. We can exclude that r-hirudin exerted a thrombolytic effect by itself because PBS-treated mice showed neither significant vessel recanalization nor a reduction of thrombus size. Moreover, in a previous work using this experimental setting, it has been shown that r-hirudin does not increase the ability of r-tPA to cause haemorrhage because the proportion of mice with skin bleeding after r-tPA treatment in dorsal skinfold chambers was not modified by r-hirudin (16).
Many lines of research are on-going to improve the efficacy of thrombolysis in patients, such as looking for a more effective dose of r-tPA, reducing bleeding complications, testing new thrombolytic drugs, or increasing the extent of lysis with ultrasounds, micro-vesicle tools, anti-coagulant and antiplatelet agents (4, 6). Although our results remain to be confirmed in models that better mimic macrovascular thrombosis, we propose that r-ADAMTS13, inducing the lysis of occlusive thrombi without causing haemorrhage, could represent a new safer therapeutic option for thrombolysis. It could be used alone or in combination with lower doses of fibrinolytics to obtain an improved thrombolytic effect with lower risk of bleeding.
1. What is known about this topic?
Currently, only plasminogen activators have been approved as thrombolytics. Among them t-PA is effective in different thrombotic disorders, but its use is often associated with haemorrhagic complications and alternative therapies are needed.
ADAMTS13, a metalloprotease that cleaves VWF, exerts an antithrombotic effect in vivo in both low venous and high arterial shear stress conditions
2. What does this paper add?
Recombinant ADAMTS13 (r-ADAMTS13), similarly to the gold standard thrombolytic agent recombinant tPA (r-tPA), dissolves occlusive thrombi in the venous microcirculation.
In contrast to r-tPA, r-ADAMTS13 does not produce haemorrhage near the treatment site
r-ADAMTS13 could represent a new safer therapeutic approach for thrombolysis.
Acknowledgments
We thank L. Cowan for help in preparing the manuscript, F. Scheiflinger and H. Rottensteiner for kindly providing us with r-ADAMTS13 and helpful discussion.
Financial support: This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grant HL041002 (to D.D.W.)
Footnotes
CONFLICT OF INTEREST DISCLOSURE
F. Scheiflinger and H. Rottensteiner (mentioned in Acknowledgements) are employees of Baxter Bioscience. The authors declare that they have no other conflict of interest.
References
- 1.Marder VJ. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 5. Lippincott, Williams & Wilkins; 2006. Foundations of thrombolytic therapy; pp. 1739–52. [Google Scholar]
- 2.Collen D, Lijnen HR. Thrombolytic agents. Thromb Haemost. 2005;93:627–30. doi: 10.1160/TH04-11-0724. [DOI] [PubMed] [Google Scholar]
- 3.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343:311–22. [PubMed] [Google Scholar]
- 4.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 5.Wechsler LR. Intravenous thrombolytic therapy for acute ischemic stroke. N Engl J Med. 2011;364:2138–46. doi: 10.1056/NEJMct1007370. [DOI] [PubMed] [Google Scholar]
- 6.Marder VJ. Historical perspective and future direction of thrombolysis research: the rediscovery of plasmin. J Thromb Haemost. 2011;9 (Suppl 1):364–73. doi: 10.1111/j.1538-7836.2011.04370.x. [DOI] [PubMed] [Google Scholar]
- 7.Brill A, Fuchs TA, Chauhan AK, et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–7. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Meyer SF, Schwarz T, Deckmyn H, et al. Binding of von Willebrand factor to collagen and glycoprotein Ibalpha, but not to glycoprotein IIb/IIIa, contributes to ischemic stroke in mice--brief report. Arterioscler Thromb Vasc Biol. 2010;30:1949–51. doi: 10.1161/ATVBAHA.110.208918. [DOI] [PubMed] [Google Scholar]
- 9.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thromb Res. 2007;120 (Suppl 1):S5–9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni H, Denis CV, Subbarao S, et al. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–92. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plaimauer B, Zimmermann K, Volkel D, et al. Cloning, expression, and functional characterization of the von Willebrand factor-cleaving protease (ADAMTS13) Blood. 2002;100:3626–32. doi: 10.1182/blood-2002-05-1397. [DOI] [PubMed] [Google Scholar]
- 12.Chauhan AK, Motto DG, Lamb CB, et al. Systemic antithrombotic effects of ADAMTS13. J Exp Med. 2006;203:767–76. doi: 10.1084/jem.20051732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao BQ, Chauhan AK, Canault M, et al. von Willebrand factor-cleaving protease ADAMTS13 reduces ischemic brain injury in experimental stroke. Blood. 2009;114:3329–34. doi: 10.1182/blood-2009-03-213264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulaftali Y, Ho-Tin-Noe B, Pena A, et al. Platelet protease nexin-1, a serpin that strongly influences fibrinolysis and thrombolysis. Circulation. 2011;123:1326–34. doi: 10.1161/CIRCULATIONAHA.110.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehr HA, Leunig M, Menger MD, et al. Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol. 1993;143:1055–62. [PMC free article] [PubMed] [Google Scholar]
- 16.Boulaftali Y, Lamrani L, Rouzaud MC, et al. The mouse dorsal skinfold chamber as a model for the study of thrombolysis by intravital microscopy. Thromb Haemost. doi: 10.1160/TH11-10-0705. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas GM, Panicot-Dubois L, Lacroix R, et al. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206:1913–27. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubois C, Atkinson B, Furie BC, et al. Platelets. Elsevier/Academic Press; 2006. Real-time in vivo imaging of platelets during thrombus formation; pp. 611–28. [Google Scholar]
- 19.Yamamoto J, Kawano M, Hashimoto M, et al. Adjuvant effect of antibodies against von Willebrand Factor, fibrinogen, and fibronectin on staphylokinase-induced thrombolysis as measured using mural thrombi formed in rat mesenteric venules. Thromb Res. 2000;97:327–33. doi: 10.1016/s0049-3848(99)00184-x. [DOI] [PubMed] [Google Scholar]
- 20.Gurevitz O, Goldfarb A, Hod H, et al. Recombinant von Willebrand factor fragment AR545C inhibits platelet aggregation and enhances thrombolysis with rtPA in a rabbit thrombosis model. Arterioscler Thromb Vasc Biol. 1998;18:200–7. doi: 10.1161/01.atv.18.2.200. [DOI] [PubMed] [Google Scholar]
- 21.Mazighi M, Serfaty JM, Labreuche J, et al. Comparison of intravenous alteplase with a combined intravenous-endovascular approach in patients with stroke and confirmed arterial occlusion (RECANALISE study): a prospective cohort study. Lancet Neurol. 2009;8:802–9. doi: 10.1016/S1474-4422(09)70182-6. [DOI] [PubMed] [Google Scholar]
- 22.Ouriel K. Current status of thrombolysis for peripheral arterial occlusive disease. Ann Vasc Surg. 2002;16:797–804. doi: 10.1007/s10016-001-0318-y. [DOI] [PubMed] [Google Scholar]
- 23.Alesh I, Kayali F, Stein PD. Catheter-directed thrombolysis (intrathrombus injection) in treatment of deep venous thrombosis: a systematic review. Catheter Cardiovasc Interv. 2007;70:143–8. doi: 10.1002/ccd.21079. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Preece SR, Black JH, et al. Safety of catheter-directed thrombolysis for deep venous thrombosis in cancer patients. J Vasc Surg. 2008;47:388–94. doi: 10.1016/j.jvs.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Mewissen MW, Seabrook GR, Meissner MH, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: report of a national multicenter registry. Radiology. 1999;211:39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- 26.Kuo WT, van den Bosch MA, Hofmann LV, et al. Catheter-directed embolectomy, fragmentation, and thrombolysis for the treatment of massive pulmonary embolism after failure of systemic thrombolysis. Chest. 2008;134:250–4. doi: 10.1378/chest.07-2846. [DOI] [PubMed] [Google Scholar]
- 27.Kuo WT, Gould MK, Louie JD, et al. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20:1431–40. doi: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Grines CL, Serruys P, O’Neill WW. Fibrinolytic therapy: is it a treatment of the past? Circulation. 2003;107:2538–42. doi: 10.1161/01.CIR.0000075292.29458.BB. [DOI] [PubMed] [Google Scholar]
- 29.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3–4. 5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372:1303–9. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- 30.Westerhout CM, Bonnefoy E, Welsh RC, et al. The influence of time from symptom onset and reperfusion strategy on 1-year survival in ST-elevation myocardial infarction: a pooled analysis of an early fibrinolytic strategy versus primary percutaneous coronary intervention from CAPTIM and WEST. Am Heart J. 2011;161:283–90. doi: 10.1016/j.ahj.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Crawley JT, de Groot R, Xiang Y, et al. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 2011;118:3212–21. doi: 10.1182/blood-2011-02-306597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin R, Yang G, Li G. Molecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activator. Neurobiol Dis. 2010;38:376–85. doi: 10.1016/j.nbd.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korninger C, Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981;46:561–5. [PubMed] [Google Scholar]
- 34.Lijnen HR, van Hoef B, Beelen V, et al. Characterization of the murine plasma fibrinolytic system. Eur J Biochem. 1994;224:863–71. doi: 10.1111/j.1432-1033.1994.00863.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Carmeliet P, Fay WP. Plasminogen activator inhibitor-1 is a major determinant of arterial thrombolysis resistance. Circulation. 1999;99:3050–5. doi: 10.1161/01.cir.99.23.3050. [DOI] [PubMed] [Google Scholar]
- 36.Kilic E, Hermann DM, Hossmann KA. Recombinant tissue-plasminogen activator-induced thrombolysis after cerebral thromboembolism in mice. Acta Neuropathol. 2000;99:219–22. doi: 10.1007/pl00007430. [DOI] [PubMed] [Google Scholar]
- 37.Orset C, Macrez R, Young AR, et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007;38:2771–8. doi: 10.1161/STROKEAHA.107.487520. [DOI] [PubMed] [Google Scholar]
- 38.Lapergue B, Moreno JA, Dang BQ, et al. Protective effect of high-density lipoprotein-based therapy in a model of embolic stroke. Stroke. 2010;41:1536–42. doi: 10.1161/STROKEAHA.110.581512. [DOI] [PubMed] [Google Scholar]