Abstract
Purpose
Tumor gene mutation status is becoming increasingly important in the treatment of patients with cancer. A comprehensive catalog of tumor gene–response outcomes from individual patients is needed, especially for actionable mutations and rare variants. We created a proof-of-principle database [DNA-mutation Inventory to Refine and Enhance Cancer Treatment (DIRECT)], starting with lung cancer-associated EGF receptor (EGFR) mutations, to provide a resource for clinicians to prioritize treatment decisions based on a patient’s tumor mutations at the point of care.
Methods
A systematic search of literature published between June 2005 and May 2011 was conducted through PubMed to identify patient-level, mutation–drug response in patients with non–small cell lung cancer (NSCLC) with EGFR mutant tumors. Minimum inclusion criteria included patient’s EGFR mutation, corresponding treatment, and an associated radiographic outcome.
Results
A total of 1,021 patients with 1,070 separate EGFR tyrosine kinase inhibitor therapy responses from 116 different publications were included. About 188 unique EGFR mutations occurring in 207 different combinations were identified: 149 different mutation combinations were associated with disease control and 42 were associated with disease progression. Four secondary mutations, in 16 different combinations, were associated with acquired resistance.
Conclusions
As tumor sequencing becomes more common in oncology, this comprehensive electronic catalog can enable genome-directed anticancer therapy. DIRECT will eventually encompass all tumor mutations associated with clinical outcomes on targeted therapies. Users can make specific queries at http:// www.mycancergenome.org/about/direct to obtain clinically relevant data associated with various mutations.
Introduction
The treatment of patients with cancer in the 21st century has evolved into a complicated algorithm, requiring knowledge of an individual patient’s tumor mutation status before initiating therapy. Making mastery of knowledge even more difficult, mutational profiling studies have revealed that within a single type of cancer, even a single gene can harbor multiple different mutations in different individuals. EGF receptor (EGFR) mutations in non–small cell lung cancer (NSCLC) represent a prime example of the complexity of disease at the molecular level. In North America, about 17% of patients with non–small cell lung cancer (NSCLC) harbor EGFR mutations (1), of which approximately 80% to 90% are composed of exon 19 deletions and L858R point mutations (2), which inherently confer sensitivity to therapy with the EGFR tyrosine kinase inhibitors (TKI), gefitinib or erlotinib (3). However, there are rare mutations that can be associated with primary drug sensitivity, primary drug resistance, or secondary resistance, while other rarer EGFR mutations are of less clear clinical significance (4). This rapid evolution of information promises to overwhelm practicing clinicians who will need a mechanism whereby they can quickly and conveniently reference the most accurate therapeutic options for patients with known tumor mutations.
Here, we report on the development of a catalog of clinically relevant somatic mutations, named the DNA-mutation Inventory to Refine and Enhance Cancer Treatment (DIRECT). This clinical database is designed to enable a genetically informed approach to cancer medicine by providing clinicians access to tumor gene therapy–response information based on individual patient data published in the literature. As proof-of-principle, we started the catalog on EGFR mutations in lung cancer, aiming to provide a comprehensive database on rare and common mutations associated with disease control and disease progression to EGFR TKI therapy.
Materials and Methods
Searches of the PubMed database from June 2005 to May 2011 were completed to identify relevant studies that reported on EGFR mutations in patients with NSCLCs. The search strategy used the terms Receptor, Epidermal Growth Factor [Mesh] OR (EGFR protein, human [Substance Name]) AND Lung Neoplasms [Mesh]. Additional articles were identified from searching the bibliographies of retrieved articles. An English language restriction was applied. Details of the search strategies are available on request from the authors. All articles were manually reviewed to determine whether the publication met minimum criteria for inclusion by 2 independent reviewers (P. Yeh and J. Andrews). Additional articles were identified from searching the bibliographies of retrieved articles. The review of articles was conducted manually as most of the individual patient-level data, if presented, was within a table or image form, undetectable by automated, computerized methods. After the cutoff date for the PubMed search query, a retrospective search on the database was conducted to identify duplicate patient data published in multiple studies. The database was further verified by having 2 independent investigators (L. Horn and W. Pao) recheck at least every tenth patient data entry with the primary source reference. Any discrepancies were resolved through collective agreement.
All data was entered into an open source, electronic data capture program, the Research Electronic Data Capture (REDCap) project, from Vanderbilt University (Nashville, TN; refs. 5, 6). Only articles with individual patient data that provided the minimum following criterion were included: EGFR mutation, type of systemic treatment, and associated outcome including response rate (RR), progression-free survival (PFS), and/or overall survival (OS). At this time, only patients treated with the EGFR TKIs, gefitinib and erlotinib, were included. Data from studies with second-generation EGFR TKIs, such as afatinib (BIBW2992), neratinib (HKI-272), dacomitinib (PF-00299804), and XL647, will be included in future studies.
Response rate was classified into two categories, disease control and disease progression. Disease control was used to describe patients who achieved a complete response (CR), partial response (PR), or stable disease (SD), as defined by Response Evaluation Criteria in Solid Tumors (RECIST; ref. 7), following treatment with gefitinib or erlotinib. Disease progression was categorized as patients who had progressive disease (PD) as defined by the RECIST (7) following treatment with gefitinib or erlotinib. If a particular mutation had patients in both groups, that particular mutation was classified in the disease control or disease progression group based on whatever group had the higher percentage of a particular response. Additional data included if available were patient age, gender, ethnicity, smoking status, tumor histology, cancer stage at diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status, and number of prior therapies. The PubMed ID, primary author name, journal name, and year of publication were recorded for each individual patient. Patients with acquired resistance to EGFR TKI therapy were catalogued separately (n = 61 from 9 different studies) from those with de novo mutations (i.e., disease previously untreated with EGFR TKIs).
Descriptive statistics, including medians, and ranges of continuous variables, as well as percentages and frequencies for categorical variables, were tabulated. The demographic analysis of the final DIRECT patient pool for this article was conducted using R Statistical Computing Program Version 14·1 (8).
Results
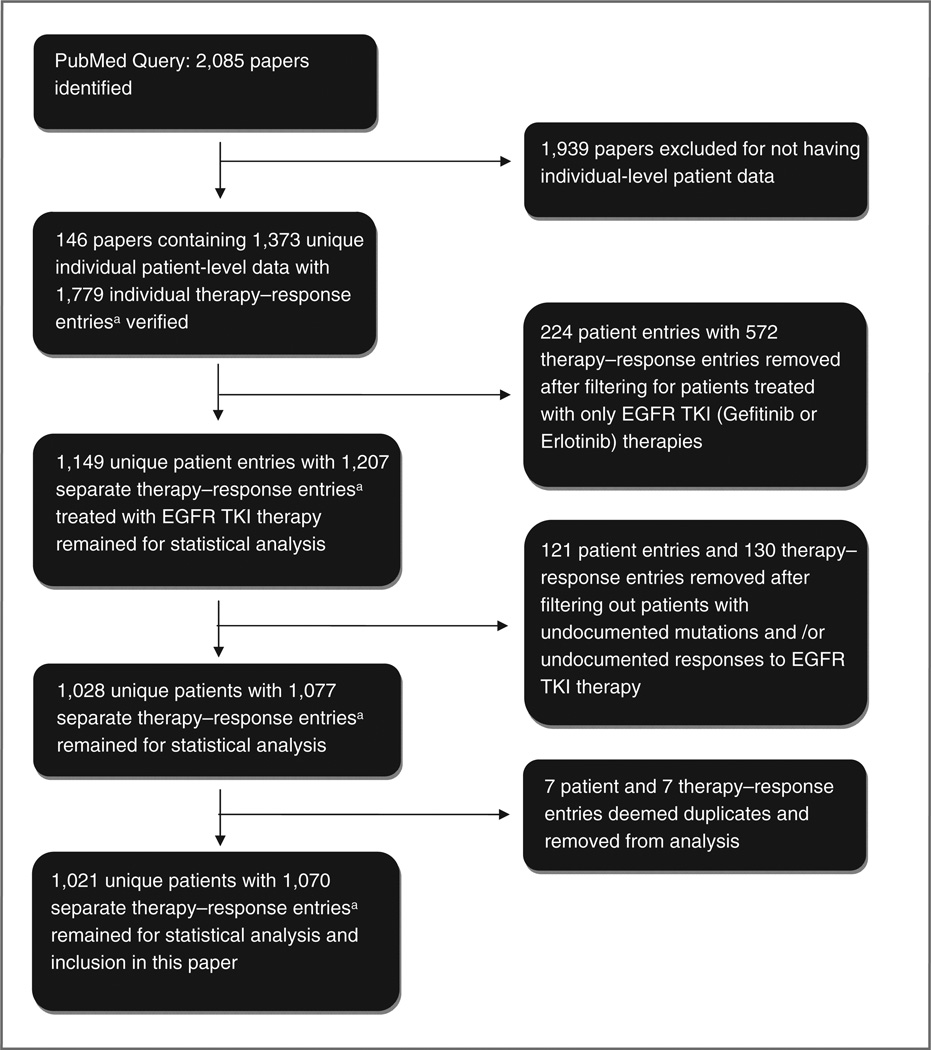
The initial search identified 2085 publications including prospective studies, retrospective studies, and case reports (Fig. 1). Of the 2085 publications, 1,939 articles were excluded for not meeting minimum inclusion criteria. The most common reason for exclusion was data presented in an aggregate population form such as a clinical trial. Of the 2085 publications, 146 papers met minimum criterion for inclusion into the DIRECT database. The number of therapy–response entries exceeds the number of individual patient entries, as some patient reports included more than one line of treatment.
Figure 1. Schematic flowchart of DIRECT database and patient cohort formation. Number of unique patients does not match the number of therapy-response entries as one patient may have been treated with EGFR TKIs in different lines of treatment and thus have multiple associated therapy-response entries.
After filtering out patients treated with only with other therapies (e.g., chemotherapy regimens), 1,149 patients with a total of 1,207 unique therapy–response entries still remained. Forty-two patients (with 47 therapy–response entries) were excluded because the exact EGFR mutation was unreported, and 79 patients (with 83 therapy–response entries) were excluded because the responses to EGFR TKI therapies were not reported. An additional 7 patients (with 7 therapy–response entries) were deemed duplicate data and deleted. About 1,070 separate therapy–response entries to gefitinib or erlotinib therapy from 1,021 patients and 116 different studies were used in our analysis. The number of unique patients does not match the number of therapy– response entries as one patient may have been treated with EGFR TKIs in different lines of treatment and thus have multiple associated therapy–response entries.
The median age of patients was 62 years (Table 1). The majority of the DIRECT patients with reported clinical features was female (42.8% vs. 28.0% male), Asian (66.6% vs. 18.5% Caucasian), never smokers (42.4% vs. 26.5% current/former smokers) with adenocarcinoma histology (65.8%vs. 24.8% other histology). About 63.6% had stage IIIB/IV or IV lung cancer (32.5% had unrecorded cancer staging). A significant portion of the patient pool had unknown demographic features (Table 1).
Table 1.
Demographic data for DIRECT patients treated with EGFR TKIs
| Category | n (% of total cohort) |
|---|---|
| Median age (range), y | 62 (27–92) |
| Gender | |
| Female | 437 (42.8) |
| Male | 286 (28) |
| Unknown | 298 (29.2) |
| Ethnicity | |
| Caucasian | 189 (18.5) |
| Asian | 680 (66.6) |
| African-American | 2 (0.2) |
| Unknown | 150 (14.7) |
| Histologya | |
| Adenocarcinoma | 672 (65.8) |
| Adenosquamous | 5 (0.5) |
| Bronchioloalveolar | 23 (2.3) |
| Large cell | 15 (1.5) |
| Squamous | 34 (3.3) |
| Spindle | 1 (0.1) |
| Unknown | 96 (9.4) |
| Smoking status | |
| Current | 55 (5.4) |
| Former smokers | 215 (21.1) |
| Never smokers | 433 (42.4) |
| NSCLC nosb | 175 (17.5) |
| Unknown | 318 (31.2) |
| Stage | |
| I | 4 (0.4) |
| IB | 1 (0.1) |
| IIA | 3 (0.3) |
| IIB | 5 (0.5) |
| III | 2 (0.2) |
| IIIA | 25 (0.25) |
| IIIB | 39 (3.8) |
| IIIB/IV | 401 (39.3) |
| IV | 209 (20.5) |
| Unknown | 332 (32.5) |
| ECOG performance status | |
| 0 | 11 (1.1) |
| 1 | 35 (3.4) |
| 2 | 21 (2.1) |
| 3 | 8 (0.8) |
| 4 | 5 (0.5) |
| Unknown | 941 (92.1) |
| Number of prior therapies before starting EGFR TKI therapy | |
| 0 | 179 (17.5) |
| 1 | 49 (4.8) |
| 2 | 86 (8.4) |
| 3 | 18 (1.8) |
| 4 | 9 (0.9) |
| 6 | 2 (0.2) |
| Unknown | 678 (66.4) |
| Total number of mutation combinations by response category | |
| De novo disease control | 149 (72.0) |
| De novo disease progression | 42 (20.3) |
| Acquired resistance | 16 (7.7) |
| TOTAL unique mutation combinations | 207 (N/A) |
Abbreviation: N/A, not applicable.
2004 World Health Organization (WHO) Classification used.
NSCLC not otherwise specified.
The mutations from each of the 1,021 patients were then classified into mutations associated with disease control, disease progression, or acquired resistance (Supplementary Table S1, Tables 2 and 3). Several mutations had patients that fit more than one category. For example, 2 of 3 patients with L861R mutation had disease control with EGFR TKI treatment; L861R was classified as a mutation with EGFR TKI disease control. Five mutation combinations that had a perfectly even split between 2 groups were classified as mutations associated with disease control because half of the patient sample achieved a SD, PR, or CR response, indicating the mutation seems at least somewhat sensitive to EGFR TKI therapy.
We identified more than 188 unique EGFR mutations, occurring in 207 different combinations. For example, some patients had either L858R alone (n = 225 from 62 studies), T790M alone (n = 6 from 3 studies), or both L858R and T790M in combination (n = 27 from 11 studies), resulting in an analysis of just 2 unique mutations (L858R and T790M) but 3 different noted mutation combinations. Of the 207 EGFR mutation combinations, 149 (72.0%) met criteria for disease control, 42 (20.3%) were associated with disease progression, and 16 (7.7%) were linked to acquired resistance (Table 1).
L858R
About 210 of the 235 therapy–response instances (89%) from 225 patients with a single L858R mutation achieved disease control with either gefitinib or erlotinib. L858R mutations were also frequently found with a second de novo mutation (n = 31). Including all combinations of L858R mutation with concurrent de novo mutations, 237 of the 266 therapy–response instances (89%) from 256 unique patients achieved disease control. L858R mutations occurring in combination with either A871E, L747S, de novo G719S, de novo T790M, L861P, or R776G were associated with disease progression.
Del19
A total of 191 of the 215 therapy–response instances (89%) from 204 separate patients with a single del19 E746-A750 mutation achieved disease control. Including patients with del19 E746-A750 mutation and a concurrent de novo mutation, 201 of the >225 therapy–response instances (89%) from 213 separate patients achieved disease control. Factoring in all patients from the 53 mutation combinations involving a del19 mutation, including those with an unspecified amino acid deletion in exon 19 (del19 unspecified) and those with concurrent de novo mutation(s), 424 of the 466 therapy–response instances (91%) from 439 patients were classified as having disease control. Del19 unspecified in combination with either ins20 unspecified or V769M were noted to be associated with disease progression. Only one patient with each combination was in DIRECT, making any determination of their therapeutic significance difficult.
G719X
Fourteen of 18 therapy–responses and 18 patients (78%) with a single G719X mutation—G719A, G719C, G719D, and G719S—achieved disease control. Expanding the pool to all patients with a G719X and concurrent de novo mutations, 20 of 24 therapy–response instances and 24 patients (83%) fit disease control criteria.
L861Q
Twelve of 14 therapy–response instances (86%) from 13 patients with a single L861Q mutation achieved disease control. Including all patients with L861Q and concurrent de novo mutations, 17 of 19 therapy–response instances (89%) from 18 patients had disease control. However, only 7 of these 17 (41%) disease control therapy–responses were partial responses; the other 10 met stable disease criteria. In contrast, 177 of the 237 disease control therapy–responses (75%) for L858R, 332 of the 424 disease control therapy– responses (78%) for del19, and 16 of the 20 disease control therapy–responses (80%) for G719X were partial responses, suggesting that L858R, exon 19 deletion, and G719X mutations respond more favorably to gefitinib or erlotinib than L861Q.
T790M
Both therapy–responses from 2 patients in DIRECT with a de novo T790M mutation had progressive disease. Five of 6 therapy–response instances (83%) and 6 patients with de novo L858R + T790M had disease progression.
Acquired resistance
Fifty-seven of 60 patients (95%) with acquired resistance to EGFR TKI therapy had T790M. The other 3 patients developed a D761Y, L747S, or T854A resistance mutation, in line with known acquired resistance mutations to EGFR TKI therapy (9). Other resistance mechanisms (e.g., MET amplification, PIK3CA mutation, etc.) were outside the focus of this current report but will be included in future studies. Del19 E746-A750 was found with T790M acquired resistance mutation in 14 patients from 5 studies, whereas the L858R mutation was found with acquired resistance mutation T790M in 21 patients from 6 studies.
Discussion
The DIRECT database represents the most comprehensive catalog of EGFR mutations and response to gefitinib or erlotinib therapy to date. The majority of the mutations in the DIRECT database are rare mutations with limited clinical data that may not be readily accessible to the busy clinician. As expected for more common mutations, L858R and exon 19 deletions were associated with disease control, whereas the T790M mutation was associated with disease progression on an EGFR TKI. We further confirm that G719X and L861Q mutations confer sensitivity to EGFR TKIs, although L861Q mutations were associated with a lower response rate. As an increasing number of patients have their tumors genotyped, there will be (and is already) a dire need for an interactive easily accessible educational tool that provides up-to-date information to physicians on the clinical relevance of mutations in cancers. The database is currently available for query at http://www.mycancergenome.org/about/direct, where users can complete a brief questionnaire on their mutation of interest, and a detailed report on the specific mutation is emailed back (10). Since its inception, we have averaged 5 to 6 queries per month from around the world, the majority (>80%) for rare mutations. The number of queries continues to increase monthly. With time and with proper data sharing agreements for data from large clinical trials, we intend to change this into an interactive platform that will provide immediate data at the point of care to aid in therapeutic decisions.
Currently, DIRECT catalogs only patients with NSCLCs and published individual patient data on EGFR mutations. We are working with collaborators to expand the database to include individual patient data from large randomized phase II and III trials on patients with EGFR mutations and response to EGFR TKIs and chemotherapy. We also plan to incorporate other known mutations in solid tumors (e.g. melanoma, gastrointestinal stromal tumors, etc.) for which mutation–response data exist. To truly realize the promise of personalized medicine, all publications involving genotype-driven trials should at a minimum publish Supplementary Tables listing individual patients by tumor mutation status and treatment outcome.
This study has several limitations. First, not all demographic data including gender, smoking status, tumor histology, and stage were available for inclusion. These data do not affect the reported mutation–response data but do not allow classification of specific mutations within demographic categories. Second, of our 207 mutation combinations, 67% or 138 mutation combinations (97 disease control, 33 disease progressing, and 8 acquired resistance) are rare mutations for which there are only data from one patient, making any determination of their therapeutic significance difficult. Finally, we did not record the specific method of EGFR mutation testing from each study and thus this article does not take into account the variance in EGFR mutation detection between differing studies. Nevertheless, these data may serve as an initial guide in selecting therapy. As more and more patients have their tumors genotyped, the number of patients with such "rare mutations" will grow and their clinical significance will emerge.
Supplementary Material
Table 2.
EGFR mutations associated with disease progression
|
N |
Number of unique |
||||
|---|---|---|---|---|---|
| Mutation | Therapy–response instances |
PD responses | Patientsa | Studiesb | References |
| A763V | 1 | 1 | 1 | 1 | 11 |
| A859T | 2 | 2 | 2 | 2 | 12, 13 |
| Del19 A767-V769 | 1 | 1 | 1 | 1 | 11 |
| Del19 unspecified + ins20 unspecified | 1 | 1 | 1 | 1 | 14 |
| Del19 unspecified + V769M | 1 | 1 | 1 | 1 | 14 |
| E709G + G719C | 1 | 1 | 1 | 1 | 14 |
| E711K | 1 | 1 | 1 | 1 | 15 |
| G719A + S768I | 2 | 2 | 2 | 2 | 16, 17 |
| G721D | 1 | 1 | 1 | 1 | 18 |
| G729R | 1 | 1 | 1 | 1 | 15 |
| I744M | 1 | 1 | 1 | 1 | 19 |
| I759T | 1 | 1 | 1 | 1 | 20 |
| Ins20 769–770insGVV | 1 | 1 | 1 | 1 | 18 |
| Ins20 A767-V769dupASV | 1 | 1 | 1 | 1 | 17 |
| Ins20 D770-N771insD | 1 | 1 | 1 | 1 | 17 |
| Ins20 P772-H773insYNP + H773Y | 1 | 1 | 1 | 1 | 17 |
| Ins20 S768-D770dupSVD | 4 | 3 | 4 | 1 | 17 |
| Ins20 SVD768–770 | 3 | 2 | 3 | 1 | 21 |
| Ins20 unspecified | 3 | 3 | 3 | 1 | 22 |
| K806E | 1 | 1 | 1 | 1 | 17 |
| K860E | 1 | 1 | 1 | 1 | 15 |
| L703F | 1 | 1 | 1 | 1 | 15 |
| L747P | 2 | 2 | 2 | 1 | 23 |
| L777G | 1 | 1 | 1 | 1 | 18 |
| L838P + E868G | 1 | 1 | 1 | 1 | 19 |
| L858R + A871E | 1 | 1 | 1 | 1 | 14 |
| L858R + G719S | 3 | 2 | 3 | 1 | 24 |
| L858R + L747S (de novo) | 1 | 1 | 1 | 1 | 14 |
| L858R + L861P | 1 | 1 | 1 | 1 | 15 |
| L858R + T790M (de novo) | 6 | 5 | 6 | 5 | 17, 18, 21, 25, 26 |
| L858R + R776G | 1 | 1 | 1 | 1 | 17 |
| L861Q + G719S | 1 | 1 | 1 | 1 | 11 |
| N826Y | 1 | 1 | 1 | 1 | 14 |
| N842S | 1 | 1 | 1 | 1 | 14 |
| S768I + V769L | 1 | 1 | 1 | 1 | 27 |
| S784F | 1 | 1 | 1 | 1 | 14 |
| T847A + G863S | 1 | 1 | 1 | 1 | 15 |
| T847I | 1 | 1 | 1 | 1 | 14 |
| T790M (de novo) | 2 | 2 | 2 | 2 | 25, 28 |
| V774M | 1 | 1 | 1 | 1 | 14 |
| V802I | 1 | 1 | 1 | 1 | 25 |
| V852I | 1 | 1 | 1 | 1 | 29 |
NOTE: Disease progression noted as a PD response as defined by the RECIST criteria (7), following treatment with EGFRTKI(gefitinib or erlotinib therapy).
Number of unique patients does not match the number of therapy-response entries as one patient may have been treated with EGFR TKIs in different lines of treatment and thus may have multiple associated therapy-response entries.
Number of unique studies refers to the number of different studies that encompass all of the patients with a particular mutation in DIRECT.
Table 3.
EGFR mutations found in patients with acquired resistance to EGFR TKIs
| Number of unique |
||||
|---|---|---|---|---|
| Initial mutation | Acquired resistance mutationa | Patientsb | Studiesc | References |
| Del19 E746-A750 | T790M | 14 | 5 | 30–34 |
| Del19 E746-T751insA | T790M | 1 | 1 | 32 |
| Del19 E746-T751insV | T790M | 1 | 1 | 35 |
| Del19 L747-A750insP | T790M | 1 | 1 | 31 |
| Del19 L747-E749;A750P | T790M | 3 | 2 | 34, 35 |
| Del19 L747-P753insQ | T790M | 1 | 1 | 369 |
| Del19 L747-P753insS | T790M | 2 | 2 | 32, 34 |
| Del19 L747-S752 | T790M | 2 | 2 | 31, 32 |
| Del19 L747-T751 | T790M | 1 | 1 | 35 |
| Del19 L747-T751;K754E | T790M | 3 | 2 | 34, 35 |
| Del19 unspecified | T790M | 3 | 1 | 37 |
| L858R | D761Y | 1 | 1 | 32 |
| L858R | L747S | 1 | 1 | 38 |
| L858R | T854A | 1 | 1 | 35 |
| L858R | T790M | 21 | 6 | 31, 32, 34, 35, 37, 39 |
| Wild-type | T790M | 4 | 1 | 31 |
These resistance mutations were acquired after treatment with EGFR TKI.
Number of unique patients does not match the number of therapy-response entries as one patient may have been treated with EGFR TKIs in different lines of treatment and thus may have multiple associated therapy-response entries.
Number of unique studies refers to the number of different studies that encompass all of the patients with a particular mutation in DIRECT.
Translational Relevance.
Tumor gene mutation status is becoming increasingly important in the treatment of patients with cancer. A comprehensive catalog of tumor gene-response outcomes from individual patients is needed, especially for actionable mutations and rare variants. Currently, no such catalog exists. Therefore, we created a proof-of principle database [DNA-mutation Inventory to Refine and Enhance Cancer Treatment (DIRECT)], starting with lung cancer-associated EGF receptor mutations, to provide a resource for clinicians to prioritize treatment decisions based on a patient’s tumor mutations. As tumor sequencing becomes more common in oncology, this comprehensive electronic catalog can enable genome-directed anticancer therapy. DIRECT will eventually encompass all tumor mutations associated with clinical outcomes on targeted therapies.
Acknowledgments
The authors thank Christine Lovly, Christine Micheel, and Mia Levy for their help with the DIRECT database.
Grant Support
This work was supported by NIH NCI grants R01-CA121210 and P01-CA129243. Funding was also received from the Kleberg Foundation, the Martell Foundation, and an Anonymous Foundation. W. Pao received additional support from the VICC Cancer Center Core Grant (P30-CA68485).
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
W. Pao has received consulting fees from MolecularMD, AstraZeneca, Bristol-Myers Squibb, Symphony Evolution, and Clovis Oncology and research funding for other projects from Enzon, Xcovery, AstraZeneca, and Symphogen. W. Pao is part of a patent regarding EGFRT790M mutation testing that was licensed by Memorial Sloan-Kettering Cancer Center to Molecular MD. L. Horn is a consultant/advisory board member of Astellas, BI, Genentech. No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: W. Pao, L.
Horn Development of methodology: J. Andrews, W. Pao, L. Horn
Acquisition of data (provided animals,acquired and managed patients, provided facilities, etc.): P. Yeh, J. Andrews, W. Pao, L. Horn
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): H. Chen, W. Pao, L. Horn
Writing, review, and/or revision of the manuscript: P. Yeh, J. Andrews, W. Pao, L. Horn
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): P. Yeh, R. Naser, W. Pao, L. Horn Study supervision: W. Pao, L. Horn
References
- 1.Kris MG, Johnson BE, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Aronson SL, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI's Lung Cancer Mutation Consortium (LCMC) J Clin Oncol. 2011;29(18 Suppl):CRA7506. [Google Scholar]
- 2.Ladanyi M, Pao W. Lung adenocarcinoma: guiding EGFR-targeted therapy and beyond. Mod Pathol. 2008;21(Suppl 2):S16–S22. doi: 10.1038/modpathol.3801018. [DOI] [PubMed] [Google Scholar]
- 3.Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- 4.Dahabreh IJ, Linardou H, Siannis F, Kosmidis P, Bafaloukos D, Murray S. Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2010;16:291–303. doi: 10.1158/1078-0432.CCR-09-1660. [DOI] [PubMed] [Google Scholar]
- 5.REDCAP. [cited 2012 May 1]; Available from: http;//project-redcap.org/
- 6.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Team RDC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for statistical computing; 2008. ISBN 3-90051-07-0. Available from: http://www.r-project.org/ [Google Scholar]
- 9.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10:281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yatabe Y, Pao W, Jett JR. Encouragement to submit data of clinical response to EGFR-TKIs in patients with uncommon EGFRmutations. J Thorac Oncol. 2012;7:775–776. doi: 10.1097/JTO.0b013e318251980b. [DOI] [PubMed] [Google Scholar]
- 11.Chou TY, Chiu CH, Li LH, Hsiao CY, Tzen CY, Chang KT, et al. Mutation in the tyrosine kinase domain of epidermal growth factor receptor is a predictive and prognostic factor for gefitinib treatment in patients with non-small cell lung cancer. Clin Cancer Res. 2005;11:3750–3757. doi: 10.1158/1078-0432.CCR-04-1981. [DOI] [PubMed] [Google Scholar]
- 12.Cappuzzo F, Bemis L, Varella-Garcia M. HER2 mutation and response to trastuzumab therapy in non-small-cell lung cancer. N Engl J Med. 2006;354:2619–2621. doi: 10.1056/NEJMc060020. [DOI] [PubMed] [Google Scholar]
- 13.Taron M, Ichinose Y, Rosell R, Mok T, Massuti B, Zamora L, et al. Activating mutations in the tyrosine kinase domain of the epidermal growth factor receptor are associated with improved survival in gefitinib-treated chemorefractory lung adenocarcinomas. Clin Cancer Res. 2005;11:5878–5885. doi: 10.1158/1078-0432.CCR-04-2618. [DOI] [PubMed] [Google Scholar]
- 14.Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17:3812–3821. doi: 10.1158/1078-0432.CCR-10-3408. [DOI] [PubMed] [Google Scholar]
- 15.Pallis AG, Voutsina A, Kalikaki A, Souglakos J, Briasoulis E, Murray S, et al. 'Classical' but not 'other' mutations of EGFR kinase domain are associated with clinical outcome in gefitinib-treated patients with non-small cell lung cancer. Br J Cancer. 2007;97:1560–1566. doi: 10.1038/sj.bjc.6604068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu SG, Chang YL, Hsu YC, Wu JY, Yang CH, Yu CJ, et al. Good response to gefitinib in lung adenocarcinoma of complex epidermal growth factor receptor (EGFR) mutations with the classical mutation pattern. Oncologist. 2008;13:1276–1284. doi: 10.1634/theoncologist.2008-0093. [DOI] [PubMed] [Google Scholar]
- 17.Wu JY, Wu SG, Yang CH, Gow CH, Chang YL, Yu CJ, et al. Lung cancer with epidermal growth factor receptor exon 20 mutations is associated with poor gefitinib treatment response. Clin Cancer Res. 2008;14:4877–4882. doi: 10.1158/1078-0432.CCR-07-5123. [DOI] [PubMed] [Google Scholar]
- 18.Ichihara S, Toyooka S, Fujiwara Y, Hotta K, Shigematsu H, Tokumo M, et al. The impact of epidermal growth factor receptor gene status on gefitinib-treated Japanese patients with non-small-cell lung cancer. Int J Cancer. 2007;120:1239–1247. doi: 10.1002/ijc.22513. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh MH, Fang YF, Chang WC, Kuo HP, Lin SY, Liu HP, et al. Complex mutation patterns of epidermal growth factor receptor gene associated with variable responses to gefitinib treatment in patients with nonsmall cell lung cancer. Lung Cancer. 2006;53:311–322. doi: 10.1016/j.lungcan.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Yamamoto N, Nukiwa T, Mori K, Tsuboi M, Horai T, et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer Res. 2010;30:557–63. [PubMed] [Google Scholar]
- 21.Yang CH, Yu CJ, Shih JY, Chang YC, Hu FC, Tsai MC, et al. Specific EGFR mutations predict treatment outcome of stage IIIB/ IV patients with chemotherapy-naive non-small-cell lung cancer receiving first-line gefitinib monotherapy. J Clin Oncol. 2008;26:2745–2753. doi: 10.1200/JCO.2007.15.6695. [DOI] [PubMed] [Google Scholar]
- 22.Cappuzzo F, Ligorio C, Janne PA, Toschi L, Rossi E, Trisolini R, et al. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization-positive/phospho-Akt-positive or never smoker patients with advanced non-small-cell lung cancer: the ONCOBELL trial. J Clin Oncol. 2007;25:2248–2255. doi: 10.1200/JCO.2006.09.4300. [DOI] [PubMed] [Google Scholar]
- 23.Kimura H, Suminoe M, Kasahara K, Sone T, Araya T, Tamori S, et al. Evaluation of epidermal growth factor receptor mutation status in serum DNA as a predictor of response to gefitinib (IRESSA) Br J Cancer. 2007;97:778–784. doi: 10.1038/sj.bjc.6603949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hata A, Yoshioka H, Fujita S, Kunimasa K, Kaji R, Imai Y, et al. Complex mutations in the epidermal growth factor receptor gene in non-small cell lung cancer. J Thorac Oncol. 2010;5:1524–1528. doi: 10.1097/JTO.0b013e3181e8b3c5. [DOI] [PubMed] [Google Scholar]
- 25.Jackman DM, Miller VA, Cioffredi LA, Yeap BY, Janne PA, Riely GJ, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokumo M, Toyooka S, Ichihara S, Ohashi K, Tsukuda K, Ichimura K, et al. Double mutation and gene copy number of EGFR in gefitinib refractory non-small-cell lung cancer. Lung Cancer. 2006;53:117–121. doi: 10.1016/j.lungcan.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Asahina H, Yamazaki K, Kinoshita I, Yokouchi H, Dosaka-Akita H, Nishimura M. Non-responsiveness to gefitinib in a patient with lung adenocarcinoma having rare EGFR mutations S768I and V769L. Lung Cancer. 2006;54:419–422. doi: 10.1016/j.lungcan.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Donovan MJ, Kotsianti A, Bayer-Zubek V, Verbel D, Teverovskiy M, Cordon-Cardo C, et al. A systems pathology model for predicting overall survival in patients with refractory, advanced non-small-cell lung cancer treated with gefitinib. Eur J Cancer. 2009;45:1518–1526. doi: 10.1016/j.ejca.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 30.Costa DB, Nguyen KS, Cho BC, Sequist LV, Jackman DM, Riely GJ, et al. Effects of erlotinib in EGFR mutated non-small cell lung cancers with resistance to gefitinib. Clin Cancer Res. 2008;14:7060–7067. doi: 10.1158/1078-0432.CCR-08-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen HJ, Mok TS, Chen ZH, Guo AL, Zhang XC, Su J, et al. Clinicopathologic and molecular features of epidermal growth factor receptor T790M mutation and c-MET amplification in tyrosine kinase inhibitorresistant Chinese non-small cell lung cancer. Pathol Oncol Res. 2009;15:651–658. doi: 10.1007/s12253-009-9167-8. [DOI] [PubMed] [Google Scholar]
- 32.Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 33.Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 35.Bean J, Riely GJ, Balak M, Marks JL, Ladanyi M, Miller VA, et al. Acquired resistance to epidermal growth factor receptor kinase inhibitors associated with a novel T854A mutation in a patient with EGFR-mutant lung adenocarcinoma. Clin Cancer Res. 2008;14:7519–7525. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruppert AM, Beau-Faller M, Neuville A, Guerin E, Voegeli AC, Menne-cier B, et al. EGFR-TKI and lung adenocarcinoma with CNS relapse: interest of molecular follow-up. Eur Respir J. 2009;33:436–440. doi: 10.1183/09031936.00162307. [DOI] [PubMed] [Google Scholar]
- 37.Onitsuka T, Uramoto H, Nose N, Takenoyama M, Hanagiri T, Sugio K, et al. Acquired resistance to gefitinib: the contribution of mechanisms other than the T790M, MET, and HGF status. Lung Cancer. 2010;68:198–203. doi: 10.1016/j.lungcan.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Costa DB, Schumer ST, Tenen DG, Kobayashi S. Differential responses to erlotinib in epidermal growth factor receptor (EGFR)-mutated lung cancers with acquired resistance to gefitinib carrying the L747S or T790M secondary mutations. J Clin Oncol. 2008;26:1182–1184. doi: 10.1200/JCO.2007.14.9039. [DOI] [PubMed] [Google Scholar]
- 39.Inukai M, Toyooka S, Ito S, Asano H, Ichihara S, Soh J, et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. 2006;66:7854–7858. doi: 10.1158/0008-5472.CAN-06-1951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.