Abstract
Background:
Its being long recognized about the highly debilitating and destructive nature of cerebrovascular accidents (CVAs). Around the world CVAs has posed as a major factor in medical morbidity and mortality. It has thrown up challenges with regards to their medical management and also towards posttreatment rehabilitation. It is well-known that neurologic disorder contributes variously towards varied electrocardiogram (ECG) changes and stroke is no exception.
Objective:
To study the ECG changes and its relation to mortality in cases of CVA.
Materials and Methods:
A total of 100 patients with acute stroke were enrolled in the study. All the 100 patients underwent ECG recording within first 24 h of admission. The patients were divided into ischemic and hemorrhagic group depending on the nature of lesion.
Results:
Out of 100 cases, 58 were ischemic and 42 were hemorrhagic. The ECG changes were noted in 78 patients. Among the ischemic group, the changes noted in the ECG were: T wave inversion (34.48%), ST segment depression (32.75%), QTc prolongation (29.31%), and presence of U waves (27.58%). In cases of hemorrhagic stroke, it was: T wave inversion (33.33%), arrhythmias (33.33%), U waves (30.95%), and ST segment depression (23.80%). Mortality was higher in patients with ST-T changes in ischemic group (66.66%) and in patients with positive U waves (60%) in hemorrhagic group.
Conclusion:
In acute stroke patients, changes in ECG were commonly seen. The changes varied from T-wave inversion to ST segment depression in ischemic stroke. In hemorrhagic stroke it consisted of T wave inversion and arrhythmias. Overall mortality was high in cases of hemorrhagic compared to ischemic group.
Keywords: Cerebrovascular accident, ECG, stroke
INTRODUCTION
Cerebrovascular accidents (CVAs) pose a serious threat to adult health. It causes physical and emotional debilitation in an individual.[1] Recently many studies have been done which accentuate the importance of investigations, early treatment and rehabilitation of a stroke patient; but there are only scarce studies which shows the correlation between electrocardiogram (ECG) changes and CVA. A variety of cardiovascular events like cardiac arrest, arrhythmias, and severe hypotension can be seen in stroke victims. These patients are often found not to have any preexisting cardiac disease.[2] The unexpected event of sudden death has its correlation to cerebral and coronary arteriosclerotic disease. These etiologies have common risk factors.[3] The need for ECG observation in stroke patients has to be increased to reduce the excess risk of mortality in CVA. The intent of this study is to focus on the gravity of ECG changes in CVA.
MATERIALS AND METHODS
The study was conducted in the Department of Neurology in collaboration with Department of Physiology, AJ Institute of Medical Sciences and Research Centre, Mangalore. The study period was from December 2009-May 2011. A total of 100 patients were enrolled in the study. The inclusion criteria were, age above 45 years who were admitted within 24 h of appearance of stroke. Stroke due to trauma, dissecting aortic aneurysm, and patients with previous documented cardiac disease were excluded from the study. Written and informed consent was obtained. The study was approved by the Institutes Ethics Committee. In all patients, detailed history was taken and was followed by medical and neurological examination. The clinical assessment was followed by computed tomography (CT) scan. The patients were then categorized into ischemic and hemorrhagic stroke depending on the radiologic findings in the CT scan. ECG recording was done within 24 h of admission. ECG findings and its relation to mortality were documented and tabulated. Descriptive statistics was used to analyze the data.
RESULTS
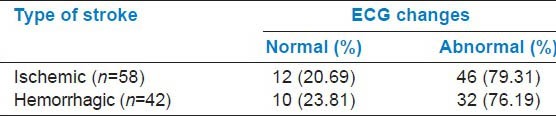
In the study group, 71 were males and 29 were females (2.4:1). Out of 100 CVA patients, 58 had ischemic stroke and 42 had hemorrhagic stroke. In 78 out of 100 patients (78%), the ECG changes were found to be significant. The abnormalities were more common in ischemic group (79.31%) compared to hemorrhagic group (76.19%) [Table 1]. The changes seen in ECG in patients with stroke were found to be independent of the type of stroke, that is, they were evenly distributed among the various types of stroke.
Table 1.
Relationship between stroke type and electrocardiogram (ECG) changes
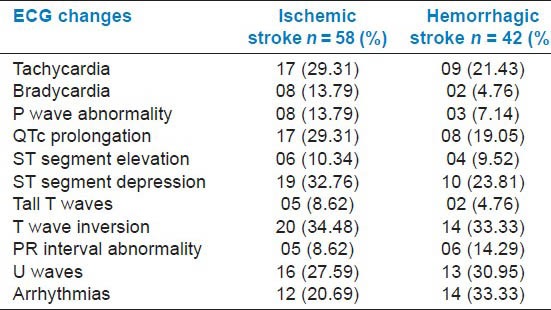
From the Table 2, it is evident that among the ischemic group, T wave inversion (34.48%) and ST segment depression (32.76%) were the most common abnormalities. This was followed by QTc prolongation (29.31%) and presence of U waves (27.59%). In case of hemorrhagic group, T wave inversion (33.33%) and arrhythmias (33.33%) occurred in equal proportions, followed by U waves (30.95%) and ST segment depression (23.81%).
Table 2.
Electrocardiogram (ECG) changes in stroke patients
The mortality in the study group was 16 (16%). The majority of deaths occurred in the hemorrhagic group (14/42, 23.80%) when compared to ischemic group (6/58, 10.34%). ECG changes were seen in 12 out of 16 (75%) who died of stroke. The mortality in the ischemic group was higher in patients showing ST-T changes in ECG (66.66%). In the hemorrhagic group, it was in patients showing positive U waves in ECG (60%) [Table 3]. The patients with abnormal ECG (17.95%) had high mortality in contrast to those who had normal ECG (9.09%) [Table 4].
Table 3.
Mortality in stroke types and its relation with electrocardiogram (ECG) changes
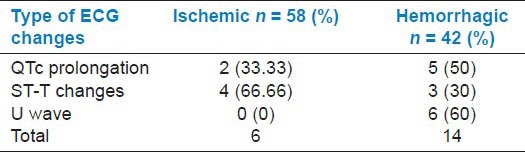
Table 4.
Relationship of mortality with abnormal electrocardiogram (ECG) in stroke patients

DISCUSSION
The average incidence of CVA cases in AJ Institute of Medical Sciences during the study period from December 2009 to May 2011 was 6.02%. It is comparable to Kuruvilla and Bharucha's study[4] in which the percentage of stroke cases in India was 4.5% of total medical admission and Llibre et al.,'s, study[5] where the percentage was 7.8%. The incidence of stroke is higher in India due to increased prevalence of risk factors like hypertension and diabetes.[4] Out of 100 patients, 71 were males and 29 were females. The male: female ratio was 2.4:1 which is comparable to other studies done by Anand et al.,[6] (1.7:1) and Nagaraja et al.,[7] (2:1). The lower incidence of stroke seen in women is attributable to a variety of factors which include genetic susceptibility, estrogenic effects on the cerebral circulation, and to reduced blood pressure values compared to men. Moreover, men showed an increased prevalence of ischemic heart disease, peripheral vascular disease, and smoking.[8] In this study, 58% of the patients had ischemic stroke, which is comparable with that found in the studies of Kuruvilla and Bharucha[4] and Kumar et al.,[9] that is, 57.3 and 56%, respectively. The most common cause for ischemic stroke is atherosclerosis of the small and medium cerebral arteries.[10] Forty-two percent had hemorrhagic stroke in the present study that is comparable with 37.9 and 44% in the Kuruvilla and Bharucha[4] and Kumar et al.,[9] study group, respectively. Hemorrhagic stroke are less common than ischemic stroke, but cause a significant number of deaths worldwide. Hemorrhagic stroke causes severe, morbid damage to cerebral tissue that can leave individuals paralyzed or weak, with difficulty in motor activities and cognitive abilities.[11] A vast majority of stroke patients demonstrated ECG changes in the current study (78%). This conforms to the previous studies of Goldstein[12] and Bozluolcay et al.,[13] where ECG changes were demonstrated in 92 and 62.1% of patients, respectively.
Increased QTc in our study was seen in 25% of cases. This is similar to observation in a large scale study done by Goldstein where it was seen in 32% of cases. T-wave inversion (15%), ST-segment depression (13%), and U-wave (28%). A similar study was done by Familoni et al.,[14] in 2006 where QTc prolongation was seen in 28% of the cases, T wave inversion in 21.8%, ST segment depression in 29.7%, U wave in 9.3%, and arrhythmia in 34.4% of the cases in study group. Various other studies showed highly variable values and this may be due to the fact that ECG changes occurring in stroke are highly variable over time and cannot be standardized unless continuous ECG monitoring is done.
Ischemic-like and repolarization ECG changes that occur in patients with acute stroke have been thought to be due to neural myocardial stunning, changes in autonomic nervous system, and catecholamine-mediated injuries.[15] Some have attributed these to lesions in the insular cortex, which can lead to cardiac abnormalities such as ischemic-like changes, arrhythmias, and even myocytolysis. This sometimes makes it difficult to make a diagnosis of heart disease in the presence of acute stroke.[14]
CONCLUSION
ECG abnormalities are commonly seen in CVA patients. It varied from T-wave inversion and ST segment depression in ischemic stroke, to T wave inversion and arrhythmias in hemorrhagic stroke. The mortality was higher in stroke group with abnormal ECG.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rajinder KD, Mittal S, Bansal BC. Trends in Clinico-Epidemiological correlates of stroke in the community. J Indian Acad Clin Med. 2000;5:27–31. [Google Scholar]
- 2.Lavy S, Yaar I, Melamed E, Stern S. The effect of acute stroke on cardiac functions as observed in an intensive stroke care unit. Stroke. 1974;5:775–80. doi: 10.1161/01.str.5.6.775. [DOI] [PubMed] [Google Scholar]
- 3.Dimant J, Grob D. Electrocardiographic changes and myocardial damage in patients with acute cerebrovascular accidents. Stroke. 1977;8:448–55. doi: 10.1161/01.str.8.4.448. [DOI] [PubMed] [Google Scholar]
- 4.Kuruvilla T, Bharucha NE. Epidemiology of stroke in India. Neurol J Southeast Asia. 1998;3:5–8. [Google Scholar]
- 5.de Jesús Llibre J, Valhuerdi A, Fernández O, Llibre JC, Porto R, López AM, et al. Prevalence of stroke and associated risk factors in older adults in Havana City and Matanzas Provinces, Cuba (10/66 population-based study) MEDICC Rev. 2010;12:20–6. doi: 10.37757/MR2010.V12.N3.6. [DOI] [PubMed] [Google Scholar]
- 6.Anand K, Chowdhury D, Singh KB, Pandav CS, Kapoor SK. Estimation of mortality and morbidity due to strokes in India. Neuroepidemiology. 2001;20:208–11. doi: 10.1159/000054789. [DOI] [PubMed] [Google Scholar]
- 7.Nagaraja D, Gururaj G, Girish N, Panda S, Roy AK, Sarma GR, et al. Feasibility study of stroke surveillance: Data from Bangalore, India. Indian J Med Res. 2009;130:396–403. [PubMed] [Google Scholar]
- 8.Jorgensen HS, Weber U, Nakayama H, Kammersgaard LP, Olsen TS. Differences in risk factor distribution, initial stroke severity and outcome in men and women. The Copenhagen Stroke Study. Cerebrovasc Dis. 1999;9:19. [Google Scholar]
- 9.Kumar HH, Kalra B, Goyal N. A Study on stroke and its outcome in young adults (15-45 Years) from coastal South India. Indian J Community Med. 2011;36:62–5. doi: 10.4103/0970-0218.80798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandiyan U, Arjundas G, Deepak A. Risk factors and stroke outcome–An Indian Study. Ind J Physical Med and Rehabilitation. 2005;16:29–33. [Google Scholar]
- 11.Torpy JM, Burke AE, Glass RM. Hemorrhagic stroke. JAMA. 2010;303:2312. doi: 10.1001/jama.303.22.2312. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS. The electrocardiogram in stroke: Relationship to pathophysiological type and comparison with prior tracings. Stroke. 1979;10:253–9. doi: 10.1161/01.str.10.3.253. [DOI] [PubMed] [Google Scholar]
- 13.Bozluolcay M, Ince B, Celik Y, Harmanci H, Ilerigelen B, Pelin Z. Electrocardiographic findings and prognosis in ischemic stroke. Neurol India. 2003;51:500–2. [PubMed] [Google Scholar]
- 14.Familoni OB, Odusan O, Ogun SA. The pattern and prognostic features of QT intervals and dispersion in patients with acute ischemic stroke. J Natl Med Assoc. 2006;98:1758–62. [PMC free article] [PubMed] [Google Scholar]
- 15.Oppenheimer S. Cerebrogenic cardiac arrhythmias: Cortical lateralization and clinical significance. Clin Auton Res. 2006;16:6–11. doi: 10.1007/s10286-006-0276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


