Abstract
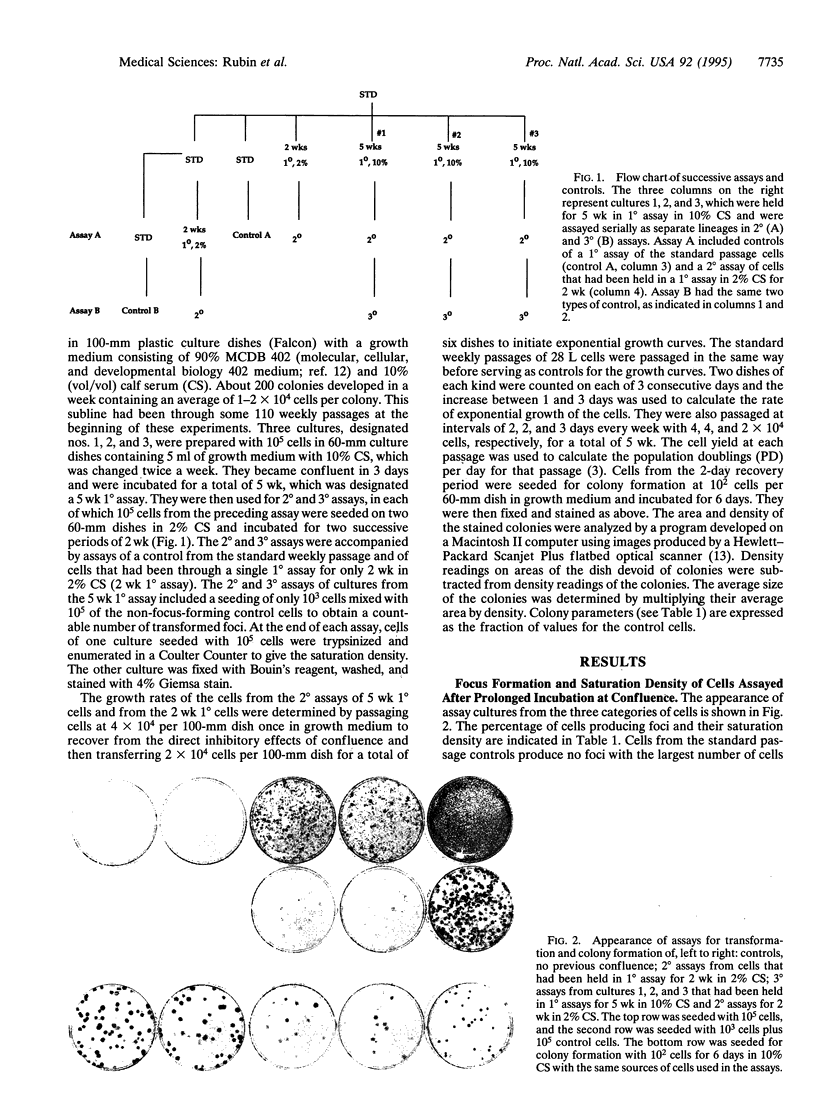
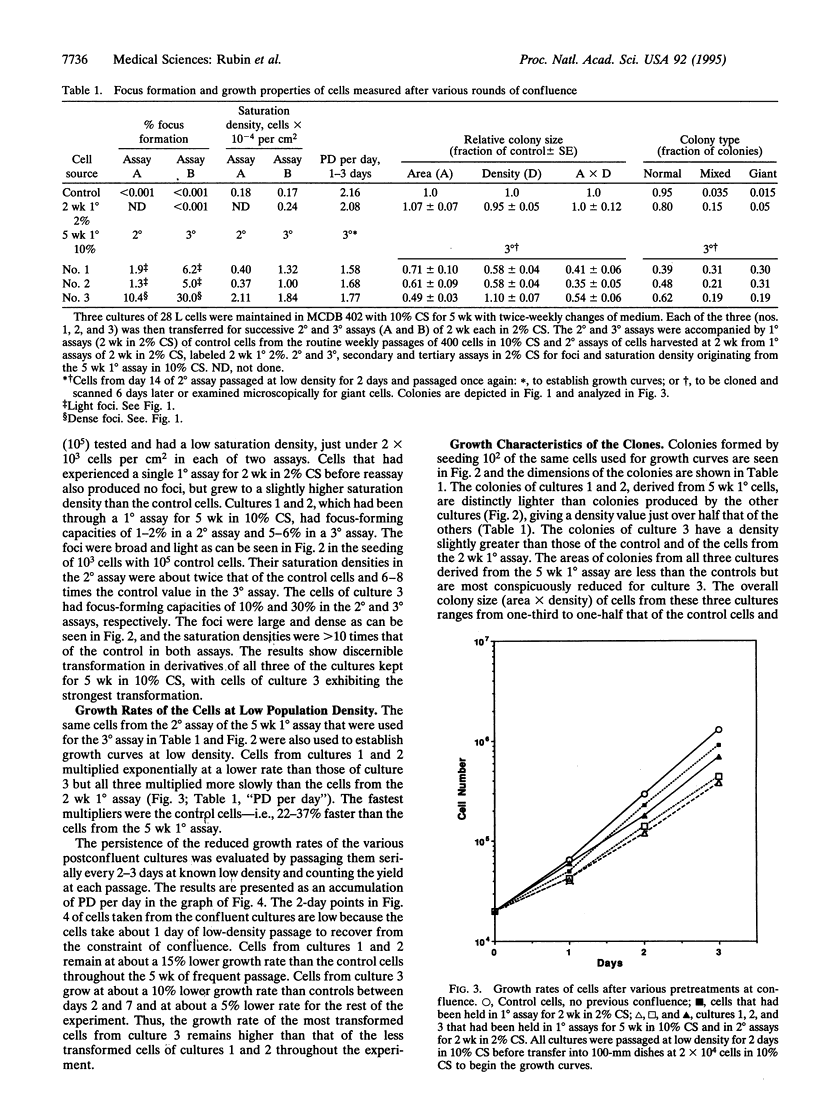
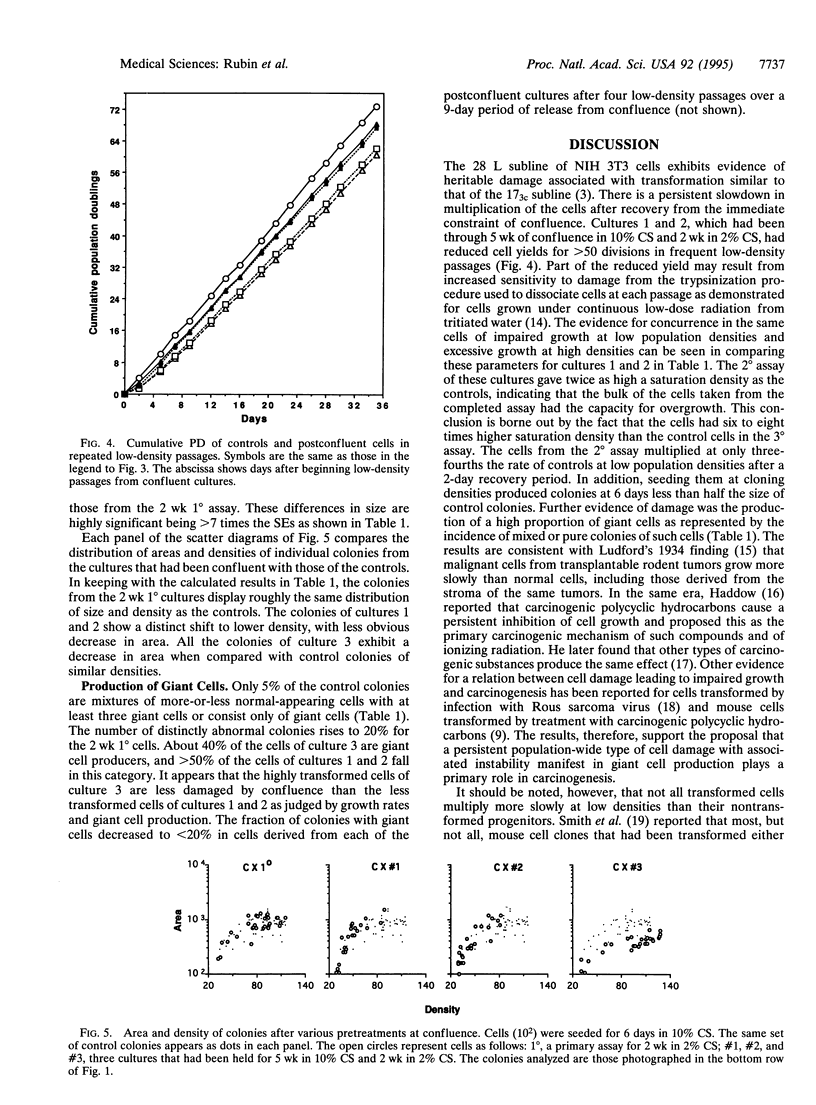
The role of heritable, population-wide cell damage in neoplastic development was studied in the 28 L subline of NIH 3T3 cells. These cells differ from the 17(3c) subline used previously for such studies in their lower frequency of "spontaneous" transformation at high population density and their greater capacity to produce large, dense transformed foci. Three cultures of the 28 L subline of NIH 3T3 cells were held under the constraint of confluence for 5 wk (5 wk 1 degree assay) and then assayed twice in succession (2 degrees and 3 degrees assays) for transformed foci and saturation density. After the 2 degrees assay, the cells were also passaged at low density to determine their exponential growth rates and cloned to determine the size and morphological features of the colonies. Concurrent measurements were made in each case with control cells that had been kept only in frequent low-density passages and cells that had been kept at confluence for only 2 wk (2 wk 1 degree). Two of the three cultures transferred from the 2 degrees assay of the 5 wk 1 degree cultures produced light transformed foci, and the third produced dense foci. The light focus-forming cultures grew to twice the control saturation density in their 2 degrees assay and 6-8 times the control density in the 3 degrees assay; saturation densities for the dense focus formers were about 10 times the control values in both assays. All three of the cultures transferred from the 2 degrees assay of the 5 wk 1 degree cultures multiplied at lower rates than controls at low densities, but the dense focus formers multiplied faster than the light focus formers. The reduced rates of multiplication of the light focus formers persisted for > 50 generations of exponential multiplication at low densities. Isolated colonies formed from single cells of the light focus formers were of a lower population density than controls; colonies formed by the dense focus formers were slightly denser than the controls but occupied only half the area. A much higher proportion of the colonies from the 5 wk 1 degree cultures than the controls consisted of giant cells or mixtures of giant and normal-appearing cells. The results reinforce the previous conclusion that the early increases in saturation density and light focus formation are associated with, and perhaps caused by, heritable, population-wide damage to cells that is essentially epigenetic in nature. The more advanced transformation characterized by large increases in saturation density and dense focus formation could have originated from rare genetic changes, such as chromosome rearrangements, known to occur at an elevated frequency in cells destabilized by antecedent cellular damage.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban G. B., Herlyn M., Clark W. H., Jr, Nowell P. C. Karyotypic evolution in human malignant melanoma. Cancer Genet Cytogenet. 1986 Jan 1;19(1-2):113–122. doi: 10.1016/0165-4608(86)90378-x. [DOI] [PubMed] [Google Scholar]
- Bignami M., Ficorella C., Dogliotti E., Norman R. L., Kaighn M. E., Saffiotti U. Temporal dissociation in the exposure times required for maximal induction of cytotoxicity, mutation, and transformation by N-methyl-N'-nitro-N-nitrosoguanidine in the BALB/3T3 ClA31-1-1 cell line. Cancer Res. 1984 Jun;44(6):2452–2457. [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Delayed reproductive death in X-irradiated Chinese hamster ovary cells. Int J Radiat Biol. 1991 Sep;60(3):483–496. doi: 10.1080/09553009114552331. [DOI] [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Persistently elevated frequency of spontaneous mutations in progeny of CHO clones surviving X-irradiation: association with delayed reproductive death phenotype. Mutat Res. 1992 Nov 16;270(2):191–199. doi: 10.1016/0027-5107(92)90130-t. [DOI] [PubMed] [Google Scholar]
- Cram L. S., Bartholdi M. F., Ray F. A., Travis G. L., Kraemer P. M. Spontaneous neoplastic evolution of Chinese hamster cells in culture: multistep progression of karyotype. Cancer Res. 1983 Oct;43(10):4828–4837. [PubMed] [Google Scholar]
- Croce C. M., Bakay B., Nyhan W. L., Koprowski H. Reexpression of the rat hypoxanthine phosphoribosyltransferase gene in rat-human hybrids. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2590–2594. doi: 10.1073/pnas.70.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domann F. E., Freitas M. A., Gould M. N., Clifton K. H. Quantifying the frequency of radiogenic thyroid cancer per clonogenic cell in vivo. Radiat Res. 1994 Mar;137(3):330–337. [PubMed] [Google Scholar]
- Kamiya K., Yasukawa-Barnes J., Mitchen J. M., Gould M. N., Clifton K. H. Evidence that carcinogenesis involves an imbalance between epigenetic high-frequency initiation and suppression of promotion. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1332–1336. doi: 10.1073/pnas.92.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy A. R., Cairns J., Little J. B. Timing of the steps in transformation of C3H 10T 1/2 cells by X-irradiation. Nature. 1984 Jan 5;307(5946):85–86. doi: 10.1038/307085a0. [DOI] [PubMed] [Google Scholar]
- Kennedy A. R., Fox M., Murphy G., Little J. B. Relationship between x-ray exposure and malignant transformation in C3H 10T1/2 cells. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7262–7266. doi: 10.1073/pnas.77.12.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVAN A., BIESELE J. J. Role of chromosomes in cancerogenesis, as studied in serial tissue culture of mammalian cells. Ann N Y Acad Sci. 1958 Sep 30;71(6):1022–1053. doi: 10.1111/j.1749-6632.1958.tb46820.x. [DOI] [PubMed] [Google Scholar]
- Nias A. H., Ockey C. H. Change in chromosome number during continuous irradiation. Nature. 1965 May 22;206(4986):840–841. doi: 10.1038/206840a0. [DOI] [PubMed] [Google Scholar]
- Paquette B., Little J. B. Genomic rearrangements in mouse C3H/10T1/2 cells transformed by X-rays, UV-C, and 3-methylcholanthrene, detected by a DNA fingerprint assay. Cancer Res. 1992 Oct 15;52(20):5788–5793. [PubMed] [Google Scholar]
- Rabinovitch P. S., Reid B. J., Haggitt R. C., Norwood T. H., Rubin C. E. Progression to cancer in Barrett's esophagus is associated with genomic instability. Lab Invest. 1989 Jan;60(1):65–71. [PubMed] [Google Scholar]
- Rao J. Y., Hemstreet G. P., 3rd, Hurst R. E., Bonner R. B., Jones P. L., Min K. W., Fradet Y. Alterations in phenotypic biochemical markers in bladder epithelium during tumorigenesis. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8287–8291. doi: 10.1073/pnas.90.17.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Rowley J. D. Biological implications of consistent chromosome rearrangements in leukemia and lymphoma. Cancer Res. 1984 Aug;44(8):3159–3168. [PubMed] [Google Scholar]
- Rubin H. Cellular epigenetics: effects of passage history on competence of cells for "spontaneous" transformation. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10715–10719. doi: 10.1073/pnas.90.22.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Experimental control of neoplastic progression in cell populations: Foulds' rules revisited. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6619–6623. doi: 10.1073/pnas.91.14.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Incipient and overt stages of neoplastic transformation. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12076–12080. doi: 10.1073/pnas.91.25.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. The inhibition of chick embryo cell growth by medium obtained from cultures of Rous sarcoma cells. Exp Cell Res. 1966 Jan;41(1):149–161. doi: 10.1016/0014-4827(66)90555-6. [DOI] [PubMed] [Google Scholar]
- Rubin H., Xu K. Evidence for the progressive and adaptive nature of spontaneous transformation in the NIH 3T3 cell line. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Yao A., Chow M. Heritable, population-wide damage to cells as the driving force of neoplastic transformation. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4843–4847. doi: 10.1073/pnas.92.11.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINCLAIR W. K. X-RAY-INDUCED HERITABLE DAMAGE (SMALL-COLONY FORMATION) IN CULTURED MAMMALIAN CELLS. Radiat Res. 1964 Apr;21:584–611. [PubMed] [Google Scholar]
- Shipley G. D., Ham R. G. Improved medium and culture conditions for clonal growth with minimal serum protein and for enhanced serum-free survival of Swiss 3T3 cells. In Vitro. 1981 Aug;17(8):656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- Smith G. J., Bell W. N., Grisham J. W. Clonal analysis of the expression of multiple transformation phenotypes and tumorigenicity by morphologically transformed 10T1/2 cells. Cancer Res. 1993 Feb 1;53(3):500–508. [PubMed] [Google Scholar]
- Terzi M., Hawkins T. S. Chromosomal variation and the establishment of somatic cell lines in vitro. Nature. 1975 Jan 31;253(5490):361–362. doi: 10.1038/253361a0. [DOI] [PubMed] [Google Scholar]
- Yao A., Rubin H. Automatic enumeration and characterization of heterogeneous clonal progression in cell transformation. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10524–10528. doi: 10.1073/pnas.90.22.10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Nelkin B. D., Celano P., Yen R. W., Falco J. P., Hamilton S. R., Baylin S. B. High expression of the DNA methyltransferase gene characterizes human neoplastic cells and progression stages of colon cancer. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3470–3474. doi: 10.1073/pnas.88.8.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]



