Abstract
Background and Aims
Liver injury serves as an excellent model of wound healing, characterized by increased synthesis of various cytokines and peptides, including the vasoactive peptide endothelin-1. In the liver, wound healing is mediated by effector cells such as hepatic stellate cells, which cause tissue contraction. Endothelin-1 has autocrine effects on stellate cells and induces their contractile activities. We explored the role of various extracellular matrix components, particularly of fibronectin, in regulating endothelin-1 production during liver injury.
Methods
Hepatic stellate cells were isolated from normal and injured rats. Real-time PCR, immunoblot and ELISA analyses were used to measure specific variables, including endothelin-1 production. Preproendothelin-1 promoter activity was determined by a luciferase assay. Stellate cell contraction was measured by a gel contraction assay.
Results
Fibronectin stimulated transcription of preproendothelin-1 mRNA and expression of endothelin through an integrin-dependent pathway in activated hepatic stellate cells. In these cells, fibronectin induced phosphorylation/activation of extracellular signal-regulated kinase (ERK) through a Shc- and Src-dependent mechanism; ERK activation was required for fibronectin-induced endothelin-1 expression. Fibronectin stimulation by stellate cells induced expression of smooth muscle α-actin and endothelin-1–mediated autocrine stellate cell contraction. Stellate cells isolated from injured livers of rats exhibited increased basal phosphorylation levels of Src, Shc and ERK, as well as increased endothelin-1 synthesis.
Conclusions
Fibronectin stimulates activated stellate cells to produce endothelin-1 and contract, via an ERK-dependent signaling pathway. The resulting autocrine functional effects of endothelin-1 are likely to be important in the wound-healing process in injured liver.
Keywords: fibrosis, vascular biology, cirrhosis, wound healing, cell biology, portal hypertension
Introduction
Wound healing is a typical response of multiple organs to injury. Many different forms of liver injury induce a wounding process, which is characterized by activation of hepatic stellate cells, also liver specific myofibroblasts. It is commonly appreciated that soluble factors (e.g. transforming growth factor-β, etc) found in the injury milieu may trigger stellate cell activation. Additionally, the extracellular matrix (ECM) itself may stimulate stellate cell activation 1. The ECM signals through integrin receptors, heterodimeric transmembrane proteins consisting of α and β subunits. Multiple combinations of α and β subunits form a wide variety of integrin receptors and many integrin receptors, including α1β1, α2β1, α5β1, αvβ3, among others, are expressed on stellate cells 2, 3.
Endothelin-1, a 21 amino acid peptide, is a potent vasoconstrictor that appears to be important in the pathogenesis of hepatic fibrogenesis and portal hypertension 4, 5. Endothelin-1 production is regulated, at least in part, at the level of preproendothelin-1 mRNA transcription, by a number of soluble factors6. The endothelin-1 peptide arises by proteolytic processing of large precursors (derived from preproendothelin-1 mRNA) to an intermediate known as big endothelin-1, which is cleaved at Trp-21-Val/Ile-22 to produce the mature 21-residue, biologically active, peptide. The enzyme responsible for the specific cleavage at Trp-21, known as endothelin converting enzyme (ECE) has several isoforms, of which ECE-1 is the most prominent 7. ECE-1 may also be regulated, and is important in determining endothelin-1 levels 8.
Given data supporting prominent cell-matrix interactions involving stellate cells, we hypothesized that ECM molecules could stimulate endothelin-1. We further postulated that ECM-stimulated endothelin-1 synthesis might have functional autocrine effects on stellate cells. Here, we have elucidated a unique signaling pathway from fibronectin to endothelin-1 synthesis, and in addition, have demonstrated important functional effects of ECM-generated endothelin-1 on stellate cells.
Methods
Materials
Human plasma and cellular fibronectin, laminin, collagen I, and collagen IV were from Sigma. GRGDSP, PD98059, U0126, Src inhibitor-1, and phosphoramidon were from Calbiochem. Blocking antibodies to fibronectin receptors (integrin α5β1 and integrin α5) were from Chemicon and integrin β1 (9EG7) was from BD Bioscience. Polyclonal antibodies directed against phospho-Erk, total Erk, phospho-Src, total Src, and Shc were from Cell Signaling. Monoclonal antibodies to detect actin species were from Sigma. Monoclonal antibody to detect GAPDH was from Santa Cruz. Src adenoviruses were gifts from Dr. Roland Baron (Yale). The Fyn adenovirus was a gift from Dr. Cynthia Corley Mastick (University of Nevada). The FAK and its mutant FRNK adenoviruses were gifts from Dr. David D. Schlaepfer (University of California). The pGL3-preproendothelin-1 (luciferase) plasmid was a gift from Dr. Bin Ren (University of California). Shc and control siRNA was from Santa Cruz.
Liver injury, cell isolation and cell culture
Liver injury and rat stellate cell isolation in male Sprague Dawley rats (450–600 grams) as described 5 9. All animal procedures were approved by the UTSW animal care committee.
Cell transfection
A reporter cDNA plasmid containing the human endothelin-1 promoter linked to luciferase (pGL3-preproendothelin-1) was transiently transfected into activated stellate cells using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen). pGL3-preproendothelin-1 was cotransfected with pCMV-βgal, which was used to adjust for the differences in transfection efficiencies between samples. Luciferase activity was measured by luminometry (Centro XS LB960).
Immunoblotting and immunoprecipitation
Immunoblotting was performed as described 5 and immunoreactive bands were visualized by ECL as per the manufacturer’s instructions (Pierce Biotechnology); intensities of specific bands were quantified using standard software (Gene Tool). Immunoprecipitation was also as per the manufacturer’s instructions (Cell Signaling).
RNA extraction, RT, and real-time PCR
Total RNA was extracted using TRIzol reagent according to the manufacturer’s instructions (Invitrogen). The RT reaction was performed using 1 μg total RNA that was reverse-transcribed into the first-strand cDNA by Superscript II reverse transcriptase with random primers (Invitrogen). Primers are shown in Supplemental Table 1. RT-PCR was performed as per the manufacturer’s instructions (Applied Biosystems).
Measurement of endothelin-1 in conditioned supernatants
Immunoreactive endothelin-1 was measured in conditioned medium using an ELISA kit according to the manufacturer’s instruction (Assay Design Inc.).
Cell contraction assay
Stellate cell contraction was evaluated using a collagen gel lattice assay 10, with minor modifications. Primary stellate cells were allowed to become activated, trypsinized and added to collagen so as to yield 3 × 105 cells/mL. After polymerization of gels, serum free conditions were introduced by addition of 500 μL of serum-free medium; specific compounds were added and lattices were gently dislodged with a pipet tip.
Adenovirus Production and Infection
Src and Src dominant negative expressing adenoviruses were as described 11. Src adenoviruses were propagated in 293 cells and tittered using standard plaque assays. Activated stellate cells were infected at equivalent MOI (5 plaque-forming units/cell) and were routinely harvested 2 days after infection.
Statistics
Data are expressed as means ± SEM. Statistical analysis was performed by using an independent Student t test or one-way analysis of variance with the Tukey post hoc test when appropriate. A p value less than 0.05 was considered statistically significant.
Results
Fibronectin stimulates endothelin-1 expression through integrin pathways in activated hepatic stellate cells
We initially tested the ability of different ECM proteins to induce endothelin-1 production in stellate cells. We found that while several ECM proteins stimulated endothelin-1, the most prominent effect was observed with fibronectin (Supplemental Figure 1). Expression of preproendothelin-1 mRNA after exposure to ECM proteins paralleled that of endothelin-1 itself (Supplemental Figure 1).
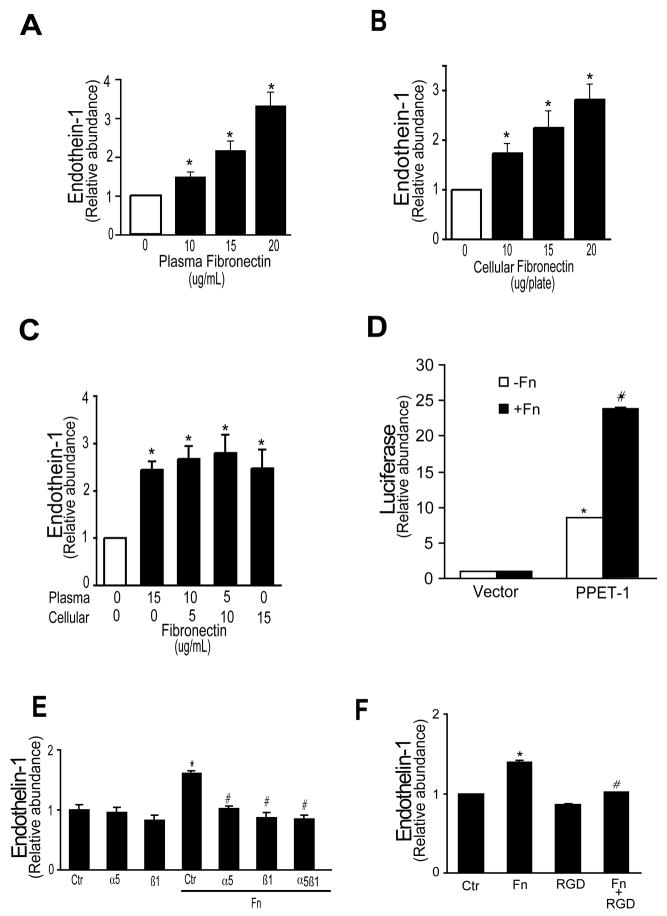
Because of the prominent effect of fibronectin on endothelin-1 synthesis, this ECM component was chosen for further study. We first examined the effect of fibronectin coated on the culture (plastic) substrata compared to fibronectin added directly to monolayers of cells adherent to plastic substrata. We found that there was no difference in production of preproendothelin-1 mRNA or endothelin-1 peptide after exposure to cellular or plasma fibronectin precoated on plastic culture dishes compared to either fibronectin species when added directly to stellate cells already growing on plastic substrata. Thus, we developed a model system in which we simply added plasma fibronectin to cultures to assess its affects. We also examined the effect of the two major fibronectin isoforms, plasma and cellular fibronectin (Figure 1A/B); we found that each stimulated endothelin-1 synthesis, and that the effect of both isoforms was proportional to fibronectin concentration (Figure 1A/B). Combinations of the two fibronectin species were not different than for either species alone (Figure 1C). Preproendothelin-1 mRNA, detected by qRT-PCR, was identified within 4 hours of exposure to fibronectin (not shown). Fibronectin also led to robust preproendothelin-1 mRNA transcription (Figure 1D).
Figure 1. Fibronectin induced endothlein-1 synthesis.
In (A), stellate cells were isolated as in Methods and grown for 5 days in 20% serum medium to induce activation. Cells were serum starved overnight, then stimulated with plasma fibronectin in serum-free medium for 24 hours. Medium was collected and immunoreactive endothelin-1 (ET-1) was measured (n = 4; *p < 0.05 compared to control or “0”) as in Methods. In (B) and (C), activated stellate cells were grown as in (A), then stimulated with cellular fibronectin (B) or with plasma fibronectin and (or) cellular fibronectin (C) in for 24 hours. Immunoreactive ET-1 was detected as in (A) (n = 3; *p < 0.05 compared to control or “0”). In (D), activated stellate cells were grown as in (A) for 4 days, and luciferase assays using pGL3 (Vector) and pGL3-preproendothelin-1 (PPET-1) were performed as in Methods (n = 4; *p < 0.05 for the pGL3-preproendothelin-1 compared to the pGL3 without fibronectin, and #p < 0.05 for the pGL3-preproendothelin-1 compared to the pGL3 with fibronectin). In (E), activated stellate cells as in (A), were stimulated with plasma fibronectin (10 μg/mL) for 24 hours, with or without α5 antibody, β1 antibody, α5β1 antibody (1:500) or in (F), RGD (150 μg/mL), each of which was added 1 hour prior to plasma fibronectin. Immunoreactive ET-1 was detected as described in Methods (n = 5, *p< 0.05 compared to control, #p < 0.05 compared to fibronectin alone). Abbreviations: PPET-1 = pGL3-preproendothelin-1, Ctr = control, Fn = fibronectin
Although we found no significant changes in endothelin converting enzyme-1 (ECE-1) mRNA (Supplemental Figure 2A) or protein expression (Supplemental Figure 2B) as a result of exposure of stellate cells to fibronectin, phosphoramidon, a well-known inhibitor of ECE activity, significantly inhibited fibronectin-induced endothelin-1 production (Supplemental Figure 2C), consistent with fibronectin’s induction of endothelin-1 synthesis through the canonical endothelin synthesis pathway.
Pre-incubation of stellate cells with neutralizing antibodies directed against the integrin subunits, α5 and β1 as well as α5β1, inhibited preproendothelin-1 mRNA (Supplemental Figure 3A) and endothelin-1 peptide synthesis induced by fibronectin (Figure 1E). The RGD peptide preferentially binds to the fibronectin III10 region and inhibits the ability of fibronectin to interact with its integrin(s) 12. Thus, as predicted, pre-incubation of stellate cells with RGD also prevented preproendothelin-1 mRNA (Supplemental Figure 3B) and endothelin-1 synthesis induced by fibronectin (Figure 1F). As a further control, we tested the effect of the RGD peptide (which is specific for fibronectin) on type collagen I mediated preproendothelin-1 mRNA and endothelin-1 production; as predicted, RGD had no effect (Supplemental Figure 4A/B).
To further document the specificity of the effect of fibronectin in this system, we also examined the effect of α5 and β1 neutralizing antibodies on type collagen I mediated preproendothelin-1 mRNA and endothelin-1 production. As predicted, we found that anti-β1 integrin antibody blocked preproendothelin-1 mRNA expression and endothelin-1 production induced by type I collagen, but anti- α5β1 and α5 antibodies had no effect (Supplemental Figure 4A/B). Also, as predicted, antibodies directed against. In aggregate, these data indicate that fibronectin’s effect on endothelin-1 is specific and proceeds through a typical cell-integrin interaction.
TGFβ has been shown to stimulate ET-1 production in fibroblasts and myofibroblasts 8, 13, and it is possible that fibronectin’s effect on ET-1 expression in our system could be TGFβ dependent. Therefore, we measured TGFβ-1 levels after exposure of stellate cells to fibronectin; we found no change (Supplemental Figure 5).
ERK activation is required for fibronectin-induced endothelin-1 expression
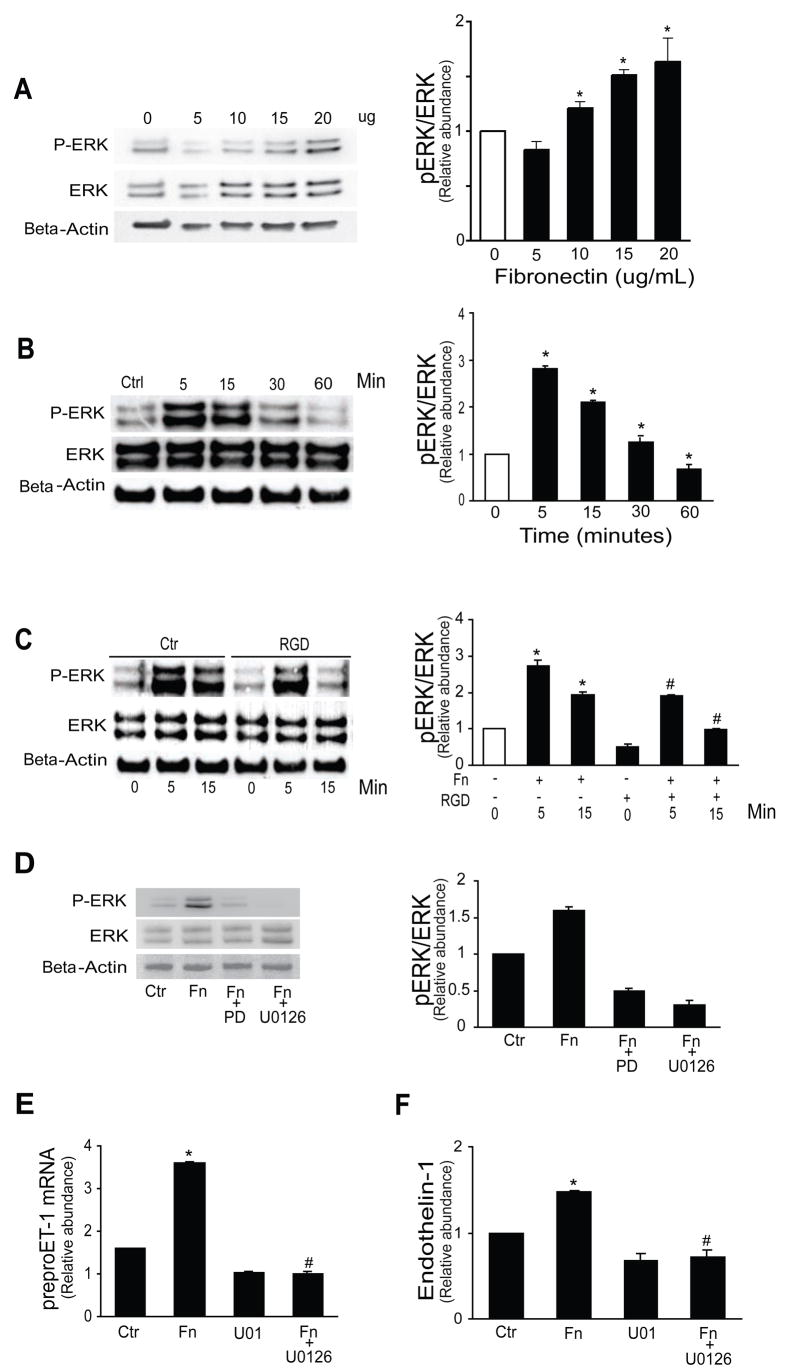
Fibronectin caused a dose dependent increase in phosphorylation of ERK expression, reaching a peak no later than 5 minutes after fibronectin exposure (Figure 2A/B). An RGD peptide significantly abrogated ERK phosphorylation (Figure 2C). Additionally, exposure of stellate cells stimulated with fibronectin to anti-α5β1 antibody or echistatin led to significant reductions in phospho-ERK, but not total ERK (not shown). We also exposed stellate cells to a MEK inhibitor, U0126, and a MAPK inhibitor, PD98059 (PD); each completely blocked ERK phosphorylation (Figure 2D). The effect of PD98059 was the same as U0126 on preproendothelin-1 mRNA and endothelin-1 synthesis (Figure 2E–F). These data indicate that ERK activation induced by fibronectin is critical in the signal transduction pathway activated by fibronectin.
Figure 2. The ERK signaling pathway mediates fibronectin induced ET-1 synthesis.
In (A), activated stellate cells as in Figure 1 were exposed to different concentrations of plasma fibronectin for 1 hour and ERK phosphorylation was measured as in Methods. In (B), stellate cells were exposed to 10 μg/mL fibronectin for the indicted times and cell lysates were subjected to immunoblotting as in (A). In (C), stellate cells were exposed to fibronectin for the indicted times with or without 150 μg/mL RGD prior to fibronectin for 1 hour. In (A–C), representative immunoblot images are shown on the left and graphs in which specific signals were quantitated, normalized to appropriate control values, and expressed graphically are shown on the right (n = 5, *p < 0.05 compared to control as indicated in empty columns; #p < 0.05 compared to corresponding times points with fibronectin alone). In (D), stellate cells were exposed to MAPK inhibitors PD 98059 (25 μM) and U0126 (5 μM). In the image to the left, representative blots are shown and in the graph to the right, specific bands were scanned, quantified, normalized, and the data expressed graphically (n = 5, * p < 0.05 compared to control, #p < 0.05 compared to fibronectin alone). In (E), stellate cells as in (A) were exposed to 10 μg/mL fibronectin for 24 hours with or without the ERK inhibitor U0126 (5 μM) which was added 1 hour prior to fibronectin. Preproendothelin-1 mRNA was detected by RT-qPCR as in Methods and in (F) immunoreactive ET-1 peptide was measured (n = 5, *p < 0.05 compared to control; #p < 0.05 compared to fibronectin alone). Abbreviations: Ctr = control, PD = PD 98059, Fn = fibronectin
We also considered the possibility that endothelin-1 itself, after being induced by fibronectin, could be responsible for stimulation of endothelin-1 production 14 and thus asked whether endothelin-1 could stimulate ERK. We found that endothelin-1 stimulated ERK, but over a rapid time course (Supplemental Figure 6) that would not be expected to be a factor in fibronectin stimulation of endothelin-1.
Fibronectin induces ERK phosphorylation in stellate cells through a Src-dependent mechanism
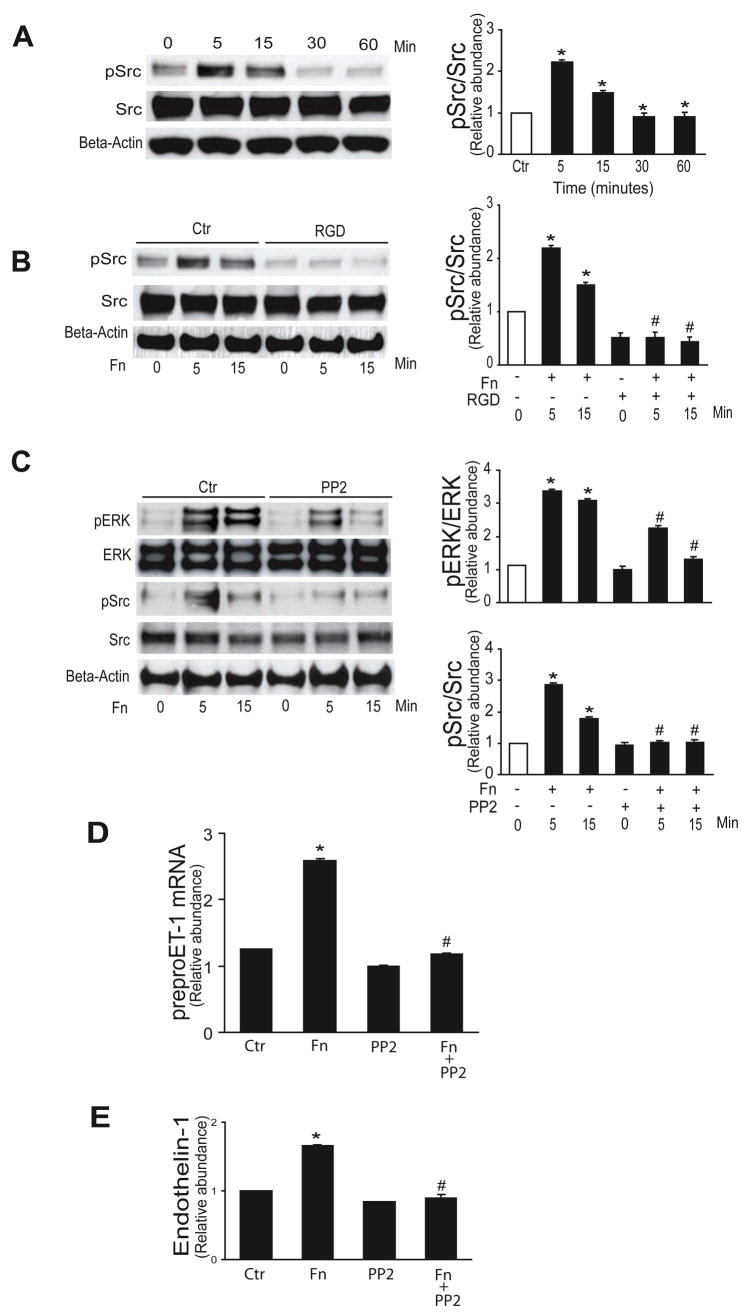
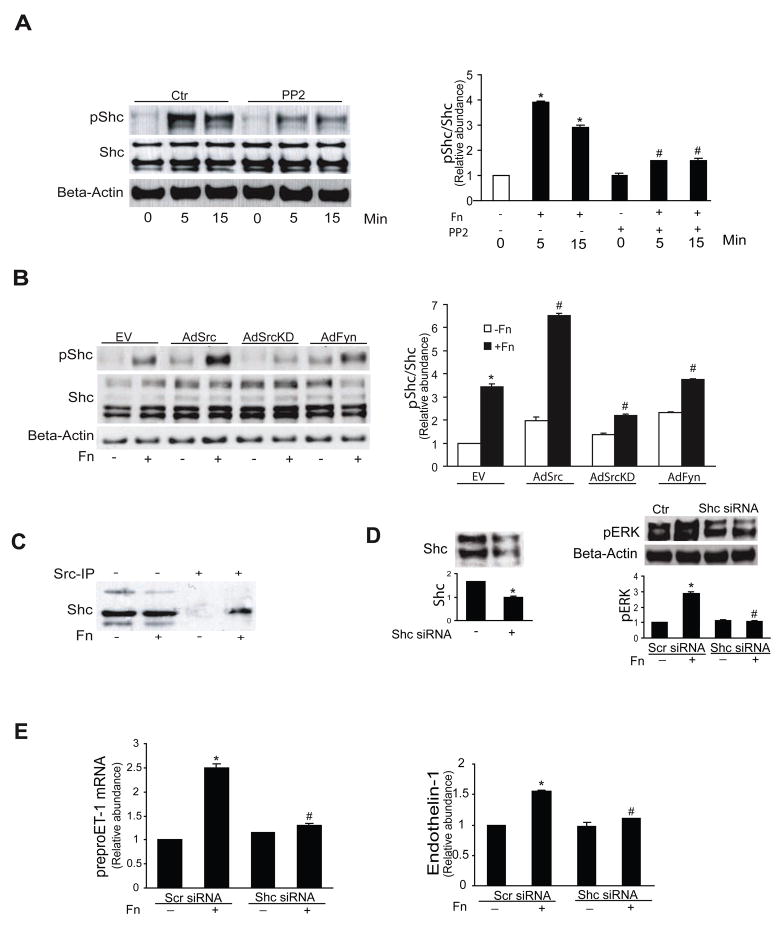
Src and Src family members are important in mediating various cellular functions including migration, adhesion, and cell spreading, many of which may be fibronectin-mediated 12. Thus, we examined whether Src was involved in the current fibronectin signaling pathway; Src family kinases were rapidly phopshorylated (Figure 3A), and this increase was blocked by interrupting the fibronectin-stellate cell integrin interaction with RGD (Figure 3B). To determine whether Src family kinases play a role in fibronectin mediated MAPK activation, we utilized the Src family kinase inhibitor, PP2 - which significantly reduced fibronectin-induced ERK1/2 and Src family kinase phosphorylation at all time points compared with controls (Figure 3C). As expected, Src family inhibitor 1 also prevented fibronectin mediated Src phosphorylation (Supplemental Figure 7). Additionally, PP2 inhibited fibronectin-induced preproendothelin-1 mRNA synthesis and endothelin-1 production (Figure 3D/E).
Figure 3. Src mediates fibronectin induced ET-1 synthesis.
In (A – C), activated stellate cells as in Figure 1 were exposed to 10 μg/mL fibronectin as indicted and whole-cell lysates were subjected to immunoblotting to detect total protein or phosphorylated proteins as indicated, as in Methods. In the panels on the left are shown representative immunoblots, and in the graphs on the right, specific signals were quantitated, normalized to appropriate control values (n = 5, *p < 0.05 compared to control, #p < 0.05 compared to fibronectin alone). In (B) and (C), RGD (150 μg/mL) and the Src kinase inhibitor, PP2 (5 μM) were each added 1 hour prior to fibronectin. In (D) and (E), stellate cells were exposed to 10 μg/mL fibronectin for 24 hours with or without the Src inhibitor PP2 (5 μM). Preproendothelin-1 mRNA was detected by RT-qPCR and immunoreactive ET-1 peptide was detected as in Methods (n = 3, *p < 0.05 compared to control; #p < 0.05 compared to fibronectin alone). Abbreviations: Ctr = control, Fn = fibronectin
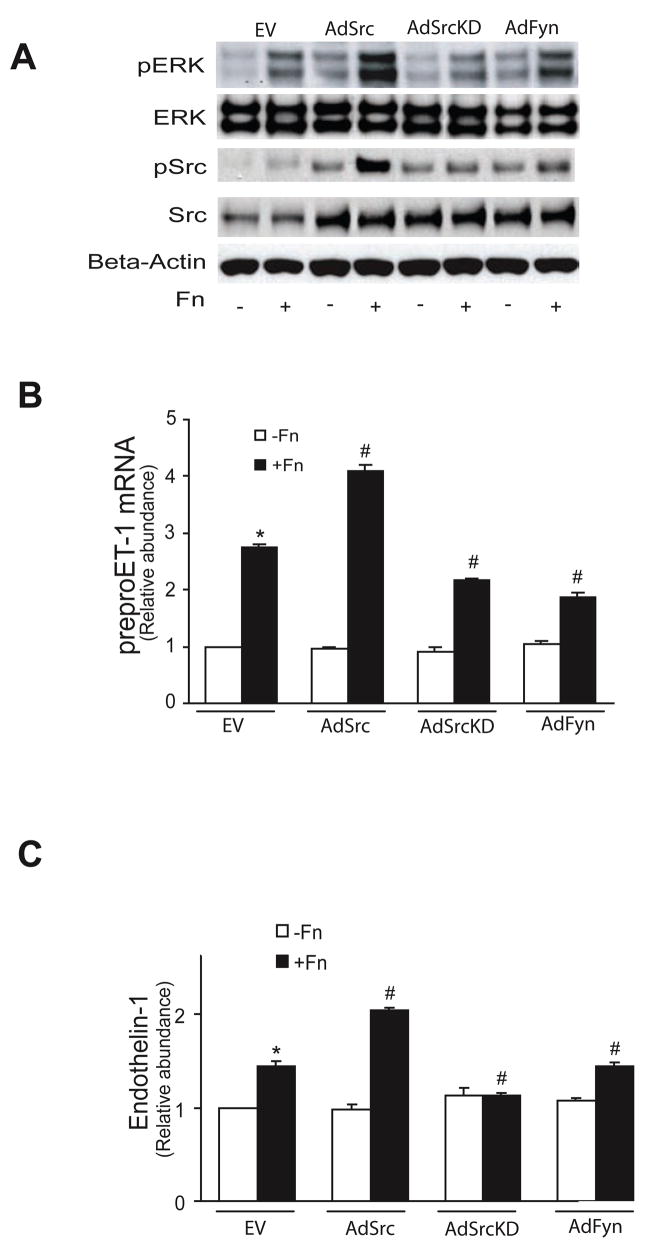
To better define the role of specific Src family kinases, we infected stellate cells with adenoviruses encoding the following specific constructs: wild-type Fyn (AdFyn), wild-type c-Src (AdSrc), and inactive (dominant negative) c-Src (SrcK295M, AdSrcKD). Over-expression of wild type Src and Fyn led to ERK1/2 phosphorylation (though the effect of Src was greater than that of Fyn), and the dominant negative Src construct inhibited this response (Figure 4A, top row). Fibronectin stimulated Src family kinase phosphorylation in Src and Fyn over-expressing cells (Figure 4A, third row), but the dominant negative Src construct inhibited Src family kinase phosphorylation (Figure 4A, third row). Over-expression of wild type Src led to an increase in preproendothelin-1 mRNA (Figure 4B) as well as endothelin-1 (Figure 4C) production, while overexpression of wild-type Fyn had no effect (Figure 4B/C). Together, these data indicate that fibronectin-mediated endothelin -1 synthesis is dependant on ERK 1/2 activity which is stimulated by Src.
Figure 4. Src, but not Fyn, mediates ET-1 synthesis.
In (A), activated stellate cells as in Figure 1 were infected with the specific constructs overnight (MOI = 5). Cells were then stimulated with 10 μg/mL fibronectin for 15 minutes, and cell lysates were subjected to immunoblotting to detect total ERK and Src, phospho-ERK and phospho-Src, and beta-actin as in Methods. A representative image is shown (n = 4). In (B), preproendothelin-1 mRNA was detected by RT-qPCR as in Methods and in (C), immunoreactive ET-1 was detected as in Methods (n = 3, *p < 0.05 compared to control; #p < 0.05 compared to fibronectin alone). Abbreviations: EV = empty virus, AdSrc = adenovirus wild type Src, AdSrcKD = adenovirus Src kinase domain negative, AdFyn = adenovirus Fyn control,
Focal adhesion kinase (FAK) plays an important role in cell migration and cell attachment, including in stellate cells 15. Additionally, activated FAK (i.e. phosphorylated at Y397) binds to Src, and not only autophosphorylates itself, but also several down-stream kinases, including Ras and ERK. Therefore, we queried whether fibronectin may activate FAK and subsequently ERK. FAK phosphorylation at Y397 and Y576/577 appeared to be greatest (Supplemental Figure 8A). However, either over-expression of wild-type FAK (AdFAK), or a dominant negative FAK (AdFRNK), did not lead to changes in ERK1/2 phosphorylation (Supplemental Figure 8B). Additionally, modulation of FAK had no effect on preproendothelin-1 mRNA or endothelin-1 levels (Supplemental Figure 8C/D).
Fibronectin induced Shc phosphorylation is dependent on Src activation
We further postulated that Shc may be involved in fibronectin-integrin down-stream signaling. Fibronectin led to rapid Shc phosphorylation (Tyr 239/240); PP2 inhibited this response (Figure 5A). Additionally, Shc phosphorylation was exaggerated in Src expressing cells, but not in those expressing a dominant negative Src construct or in those expressing the Fyn construct (Figure 5B). We also found that fibronectin dependent Shc phosphorylation required interaction with Src (Figure 5C). To further evaluate the effect of Shc, we designed experiments in which Shc expression was inhibited. Shc siRNA knocked down Shc expression (Figure 5D, left panel); additionally, Shc siRNA reduced fibronectin mediated ERK phosphorylation (Figure 5D, right panel). Finally, Shc knock down reduced both fibronectin induced preproendothelin-1 mRNA expression as well as endothelin-1 synthesis (Figure 5E, left and right panels, respectively).
Figure 5. Src signals through Shc in activated stellate cells.
In (A), activated stellate cells as in Figure 1 were exposed to 10 μg/mL fibronectin for the indicted times with or without PP2 (cells were exposed to 5 μM PP2 for 1 hour prior to addition of fibronectin). Specific signals were detected by immunoblotting, quantitated, normalized to control values, and expressed graphically as the ratio of pShc to total Shc (n = 4, *p < 0.05 compared to control; #p < 0.05 compared to fibronectin alone - 5 minutes). In (B), activated stellate cells as above were infected with the same adenoviral constructs as in Figure 4, and certain cells were exposed to fibronectin (10 μg/mL) for 15 minutes. Specific signals were detected by immunoblotting, quantitated, normalized to control values, and expressed graphically as the ratio of pShc to total Shc in the graph shown below a representative image (n = 4, *p < 0.05 compared to control; #p < 0.05 compared to fibronectin alone - 15 minutes). In (C), cells as in (A), were infected with wild type Src adenovirus, and cell lysates were subjected to immunoprecipitatation with anti-Src antibody, with or without fibronectin (10 μg/mL) for 15 minutes and were immunoblotted to detect Shc. The image is representative of 3 others. In (D), stellate cells as in (A) were transfected with 50 μM control (scrambled) siRNA or specific Shc siRNA for 24 hours. Cells were harvested and imunoblotting was performed using anti-Shc antibody and β-actin antibody as control (left panel). Cells were exposed to 10 μg/mL fibronectin for 15 min after siRNA transfection for 24 hours, cell lysates were harvested and immunoblotted with anti-phospho-ERK antibody and β-actin antibody. For each of these experiments, specific signals were quantitated, normalized to control values, and expressed graphically (graphs are shown below a representative image, n = 3, *p < 0.05 compared to scrambled siRNA on the left, or no fibronectin on the right; #p < 0.05 compared to scrambled siRNA with fibronectin). In (E), the same cells and their medium as in (D) were harvested and preproET-1 mRNA and immunoreactive ET-1 were detected as described in Methods (n = 3, *p < 0.05 compared to no fibronectin; #p < 0.05 compared to scrambled siRNA with fibronectin). The abbreviations in this Figure are as the same as shown in Figure 4.
Functional effect of fibronectin-mediated endothelin-1 synthesis
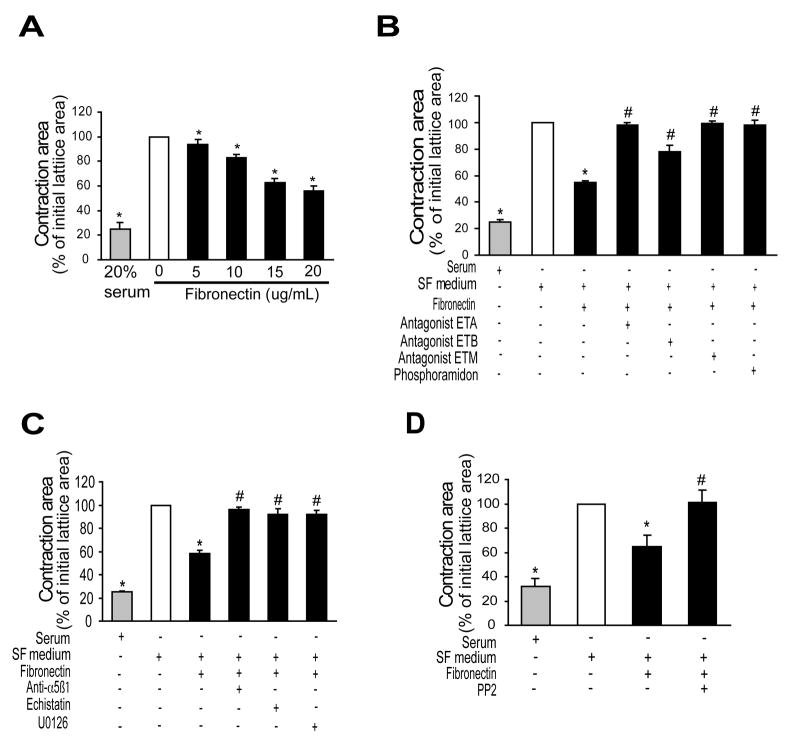
We next wished to determine whether endothelin-1 synthesis stimulated by fibronectin was functionally important. Therefore, we developed a novel assay system in which we added fibronectin to stellate cell/collagen lattices and measured the subsequent effect on lattice contraction. Fibronectin led to dose-dependent stimulation of stellate cell contraction (Figure 6A). Further, we demonstrated that the endothelin-1 produced by stellate cells was responsible for stimulating cellular contraction by showing that inhibition of de novo endothelin-1 synthesis with phosphoramidon inhibited cell contraction (Figure 6B). Similarly, blocking endothelin-1 binding to endothelin receptors with a mixed endothelin antagonist (A82086) reduced cell contraction (Figure 6B). Neither phosphoramidon nor an endothelin antagonist affected serum induced contraction (not shown), consistent with the effect of endothelin-1 after stimulation with fibronectin. Exposure of cells to either echistatin or fibronectin blocking antibody inhibited cell contraction (Figure 6C), emphasizing the importance of the fibronectin-stellate cell interaction. The MAPK inhibitor, U0126 also inhibited cell contraction stimulated by fibronectin (Figure 6C). Finally, PP2 inhibited fibronectin mediated stellate cell contraction (Figure 6D), consistent with a Src dependent endothelin-1 synthetic pathway.
Figure 6. Fibronectin induces stellate cell contraction by induction of endothelin-1 synthesis.
Stellate cells as in Figure 1 were seeded onto preformed collagen lattices as in Methods. In (A), cells were exposed to the indicated concentrations of fibronectin for 24 hours, gels were released from their plastic substrata, and gel contraction was measured 24 later (n = 5, *p < 0.05 compared to serum free medium alone). In (B), stellate cells as in (A) were exposed to the mixed endothelin receptor antagonist, ET-M (A-182086, 10−8 M), an ET-A receptor antagonist (A-147627, 10−8 M), or an ET-B receptor antagonist (A-192621 10−8 M), or the ECE inhibitor, phosphoramidon (100 μM) for 1 hour prior to addition of fibronectin (10 μg/mL) - and contraction was measured 24 hours later (n = 5, *p < 0.05 compared to control - serum free medium alone; #p < 0.05 compared to fibronectin alone). In (C) and (D), contraction assays were as in (A) and (B) above; specific agents (α5β1 antibody (1:500 dilution), echistatin (60 nM), PD98059 (25 μM), U0126 (5 μM), or PP2 (5 μM)) were added to cells for 1 hour prior to addition of fibronectin (10 μg/mL) (n = 5, *p < 0.05 compared to control - serum free medium alone; #p < 0.05 compared to fibronectin alone). Abbreviations: SF = serum free
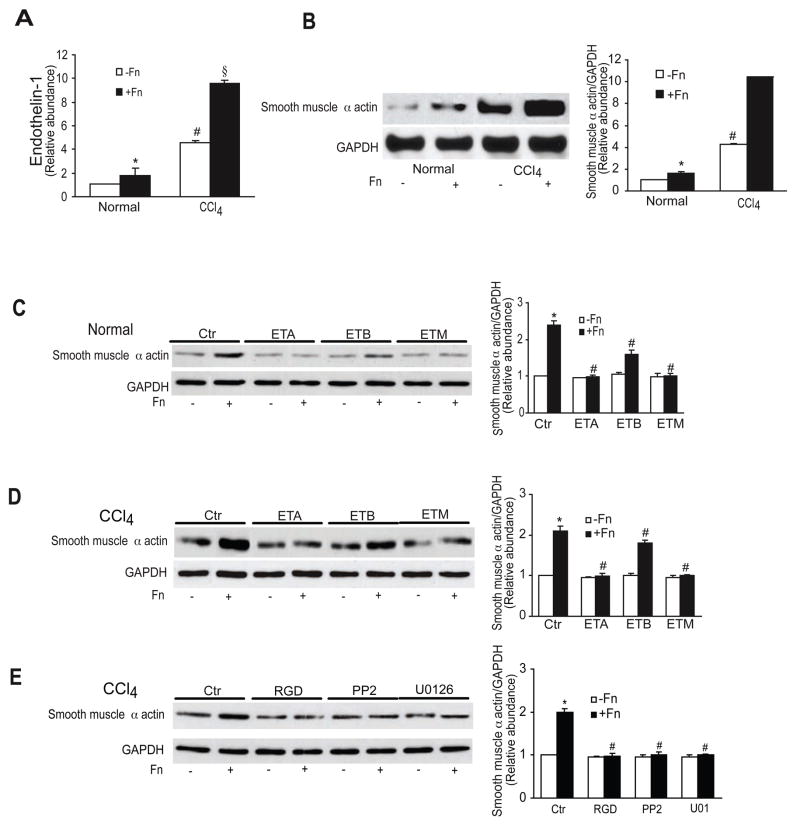
Another important functional effect of endothelin-1 is induction of smooth muscle α actin 4. Thus, we induced liver injury, isolated stellate cells, and then examined their response to fibronectin. The methodology used allowed recapitulation of the in vivo phenotype. First, we found that endothelin-1 was produced by stellate cells from injured livers to a greater extent than those from normal livers by fibronectin (Figure 7A). The same was true for and smooth muscle α actin synthesis. We also found that ET-A, ET-B, and a mixed endothelin receptor (ET-M) antagonist significantly reduced smooth muscle α actin expression in stellate cells isolated from injured livers (Figure 7C/D). When freshly isolated cells were exposed to RGD, PP2, or U0126, smooth muscle α actin was significantly reduced (Figure 7E). These data demonstrate that fibronectin stimulates endothelin-1 expression in activated stellate cells (i.e. those from injured livers) and the autocrine effect of endothelin-1 in turn leads to enhanced smooth muscle α actin expression through the signaling cascade highlighted above.
Figure 7. Fibronectin regulates smooth muscle α actin expression through an endothelin-1, ERK, and Src dependent signaling pathway in liver injury.
In (A), stellate cells were isolated from injured (CCl4) livers and grown for 2 days, and then stimulated with fibronectin (10 μg/mL) for 24 hours under serum free conditions. Immunoreactive ET-1 was detected as in Methods (n = 5, *p < 0.05 compared to control - cells in serum free medium alone; #p < 0.05 compared to normal cells - either with or without fibronectin; §p <0.05 compared to CCl4 without fibronectin). In (B), cells were as in (A); specific signals were detected by immunoblotting (left panel), quantitated, normalized to GAPDH signals and control smooth muscle α actin expression after CCl4, and expressed graphically (right panel) (n = 5, * # § as in A). Cells from normal (C) or injured (D) livers were as in (A), with the exception that they were exposed to ET-A, ET-B or ET-M receptor antagonists (as in Figure 7, all 10−8 M) for 1 hour prior to fibronectin. Immunoblotting was as in (B); representative immunoblots are shown on the left, and quantitative data on the right (n = 5, *p < 0.05 compared to control; #p < 0.05 compared to cells exposed to fibronectin). In (E), cells were as in (C and D), but exposed to RGD (150 μg/mL), PP2 (5 μM) or U0126 (5 μM) for 1 hour prior to fibronectin. Immunoblotting was as in (C/D) (n = 4, *p < 0.05 compared to control – cells in serum free medium alone; #p < 0.05 compared to carbon tetrachloride with fibronectin). Abbreviations: Ctr = control; Fn = fibronectin; CCl4 = carbon tetrachloride
Additionally, we examined the effect of injury on signaling pathways in stellate cells (Supplemental Figure 9). Stellate cells isolated from injured livers exhibited increased basal phosphorylation of Src, Shc, and ERK. Activation induced injury was also associated with an exaggerated response to fibronectin.
Discussion
We have shown that endothelin-1 synthesis was stimulated by the prominent extracellular matrix component, fibronectin. While fibronectin is known to be important in a variety of important cell biologic processes, cellular differentiation and motility, our finding that fibronectin stimulates endothelin-1 is novel. A particularly novel aspect of the work is the demonstration of a specific signaling pathway from fibronectin to endothelin-1 synthesis. Another critical finding of this study was that fibronectin’s stimulation of endothelin-1 had functional effects, including stimulation of smooth muscle α actin (in an autocrine loop - Figure 7), and stimulation of cellular contraction (Figure 6), effects highly likely to important in vivo.
Fibronectin contains the canonical amino acid sequence, RGD, which binds α5β1 and αvβ3 16. As expected, a peptide blocking this interaction inhibited signaling in our system, while it had no effect on type I collagen induced endothelin-1 synthesis (Supplemental Figure 4). Additionally, echistatin, which also blocks the RGD binding site of integrins and anti-α5β1, anti-α5, and anti-β1 antibodies also inhibited fibronectin’s effect on stellate cell synthesis of endothelin-1. Stellate cells are known to express several integrin receptors, including α5β1 2, 3, 17. These data are thus all highly consistent with our observed potent effect of fibronectin on stellate cells. Interestingly, the fibronectin/integrin interaction more prominently stimulated endothelin than the collagen/integrin interaction, raising the possibility of the presence of a unique signaling pathway downstream of fibronectin-α5-β1.
The high molecular weight glycoprotein, fibronectin, exists in two major forms. The soluble form (plasma fibronectin) is produced primarily by hepatocytes while the cellular form (which itself may have a variety of splice variants that may induce components such as “EDA” or “EDB”) can be made by many cell types, in particular the endothelium 18. We found that plasma and cellular fibronectin seemed to be equally potent in stimulation of endothelin-1. That cellular fibronectin had potent effects was not surprising given previous data indicating its prominent effects on stellate cells 1. Further, recent work in a fibroblast cell line was consistent with our fibronectin stimulated endothelin-1 19. However, the finding that plasma fibronectin was potent, was noteworthy. Available data suggest that this finding may be highly relevant in wound healing. For example, circulating fibronectin is incorporated into the extracellular matrix 20 and can be stimulated after a wide range of injury, including in the liver 21. Notably, the concentrations of fibronectin found in blood far surpass those that were stimulatory in stellate cells. Thus, based on the data shown in Figure 7 and Supplemental Figure 9), we speculate that fibronectin stimulates endothelin-1 in normal cells minimally, while after stellate cells are stimulated, the effect of fibronectin is more exaggerated, and actively induces stellate cell production of endothelin-1, which in turn has a variety of effects on stellate cells, including further stimulation of their activation, as well as contraction and other effects 4.
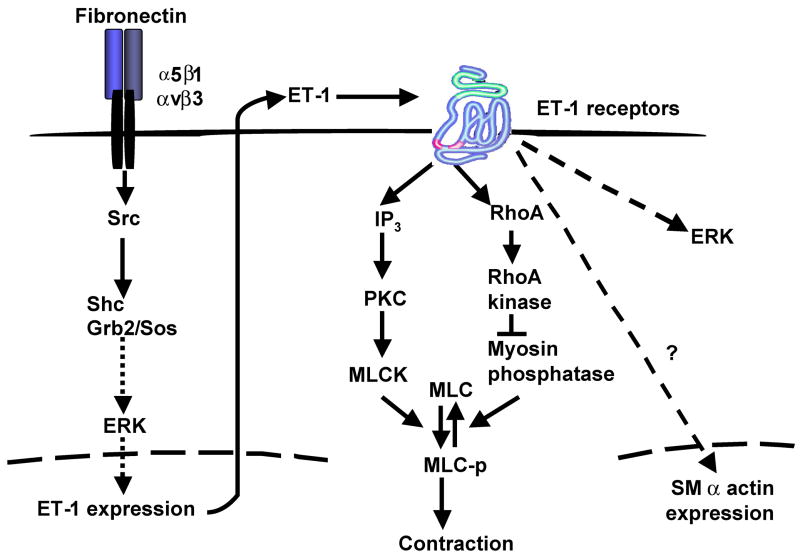
An important advance of this work is that we have investigated a specific signaling pathway from fibronectin to endothelin-1. An overview of the potential signaling pathway is shown in Figure 8. We have provided extensive evidence that a Src-Shc-ERK signaling pathway is involved. Other studies have demonstrated that fibronectin can activate Src 22, and it has been shown that fibronectin is able to stimulate accumulation of Src in stellate cell focal adhesions 17. Further, it has been established in other cell systems that Src signals to Shc 22. While we are not aware of systems in which fibronectin has been shown to stimulate signaling via Shc, the fact that we were able to link these two partners is not surprising. Finally, we have considered the possibility that endothelin-1 itself (after being induced by fibronectin), could stimulate further endothelin-1 production 14 and showed that endothelin-1 potently stimulates ERK (Supplemental Figure 6). However, given the time course over which mature peptide is produced (hours to days) and the fact that fibronectin stimulates Src and ERK within minutes and robust preproendothelin-1 transcription within hours, we believe that the effects of fibronectin on endothelin-1 synthesis reported here are convincing.
Figure 8. Schematic diagram of endothelin-1 synthesis signal transduction pathways and autocrine effects.
On the left, fibronectin promotes ET-1 production by activating Src, which, in turn, activates a Shc/Grb2/Sos complex by direct phosphorylation of Shc; phosphorylated Shc then activates the ERK signaling pathway, which leads to increased preproendothelin-1 expression. Increased endogenous ET-1 activity contributes to enhanced contraction and smooth muscle α actin expression in activated stellate cells. The proposed pathway to cellular contraction is based on previous literature in smooth muscle cells 27. Abbreviations: PKC = protein kinase C, MLC = myosin light chain, MLCK = myosin light chain kinase.
We were surprised to find that FAK signaling was not prominent upstream of Src in our system, since FAK can be activated by fibronectin 23. However, we provide extensive evidence indicating that FAK, though activated by fibronectin, did not play a role in endothelin-1 synthesis (Supplemental Figure 8).
The findings of this study have important implications not only for wound healing in general, but also for the pathogenesis of hepatic fibrosis and intrahepatic portal hypertension. Stellate cells are known to have extensive cytoplasmic cell extensions, and are believed to regulate microvascular blood flow in a manner analogous to tissue pericytes 24. Stellate cells possess abundant endothelin receptors, and thus appear to be major targets of endothelin-1 in the liver 25. Further, given that endogenous endothelin-1 has been found to exert potent effects on stellate cell contraction, this vasoactive peptide presumably contributes to the increased resistance typical of intrahepatic liver disease and portal hypertension 26. Indeed, consistent with this idea was our finding that fibronectin-mediated endothelin-1 synthesis led to autocrine induced smooth muscle α actin expression and cellular contraction. Thus, the data highlight in an in vivo system that an extracellular matrix - stellate cell interaction that likely contributes to wound healing and to intrahepatic portal hypertension.
Supplemental Materials
Acknowledgments
This work was supported by the NIH (Grant R01 DK 50574 to DCR).
Abbreviations
- ECM
extracellular matrix
- ET-1
endothelin-1
- ECE-1
endothelin converting enzyme-1
- FN
fibronectin
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
Footnotes
Disclosure: The authors have no conflict of interest to disclose regarding this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Racine-Samson L, Rockey DC, Bissell DM. The role of alpha1beta1 integrin in wound contraction. A quantitative analysis of liver myofibroblasts in vivo and in primary culture. J Biol Chem. 1997;272:30911–7. doi: 10.1074/jbc.272.49.30911. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Murphy FR, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem. 2004;279:23996–4006. doi: 10.1074/jbc.M311668200. [DOI] [PubMed] [Google Scholar]
- 4.Rockey DC, Fouassier L, Chung JJ, Carayon A, Vallee P, Rey C, Housset C. Cellular localization of endothelin-1 and increased production in liver injury in the rat: potential for autocrine and paracrine effects on stellate cells. Hepatology. 1998;27:472–80. doi: 10.1002/hep.510270222. [DOI] [PubMed] [Google Scholar]
- 5.Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. Implications for endothelin in the pathogenesis of wound healing. J Clin Invest. 1996;98:1381–8. doi: 10.1172/JCI118925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsen TA, Weber F, Egink G, Suckau G, Baldamus CA. Cyclosporin A induces prepro endothelin-1 gene transcription in human endothelial cells. Eur J Pharmacol. 1999;379:97–106. doi: 10.1016/s0014-2999(99)00447-1. [DOI] [PubMed] [Google Scholar]
- 7.Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 8.Shao R, Shi Z, Gotwals PJ, Koteliansky VE, George J, Rockey DC. Cell and molecular regulation of endothelin-1 production during hepatic wound healing. Mol Biol Cell. 2003;14:2327–41. doi: 10.1091/mbc.02-06-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Q, Shao R, Qian HS, George SE, Rockey DC. Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest. 2000;105:741–8. doi: 10.1172/JCI7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazaki T, Sanjay A, Neff L, Tanaka S, Horne WC, Baron R. Src kinase activity is essential for osteoclast function. J Biol Chem. 2004;279:17660–6. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- 12.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985;40:191–8. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 13.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, Du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 Promotes Myofibroblast Induction through the ETA Receptor via a rac/Phosphoinositide 3-Kinase/Akt-dependent Pathway and Is Essential for the Enhanced Contractile Phenotype of Fibrotic Fibroblasts. Mol Biol Cell. 2004;15:2707–19. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao R, Rockey DC. Effects of endothelins on hepatic stellate cell synthesis of endothelin- 1 during hepatic wound healing. J Cell Physiol. 2002;191:342–50. doi: 10.1002/jcp.10110. [DOI] [PubMed] [Google Scholar]
- 15.Reif S, Lang A, Lindquist JN, Yata Y, Gabele E, Scanga A, Brenner DA, Rippe RA. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J Biol Chem. 2003;278:8083–90. doi: 10.1074/jbc.M212927200. [DOI] [PubMed] [Google Scholar]
- 16.Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milliano MT, Luxon BA. Initial signaling of the fibronectin receptor (alpha5beta1 integrin) in hepatic stellate cells is independent of tyrosine phosphorylation. J Hepatol. 2003;39:32–7. doi: 10.1016/s0168-8278(03)00161-2. [DOI] [PubMed] [Google Scholar]
- 18.Nimmer D, Bergtrom G, Hirano H, Amrani DL. Regulation of plasma fibronectin biosynthesis by glucocorticoids in chick hepatocyte cultures. J Biol Chem. 1987;262:10369–75. [PubMed] [Google Scholar]
- 19.Kennedy L, Shi-Wen X, Carter DE, Abraham DJ, Leask A. Fibroblast adhesion results in the induction of a matrix remodeling gene expression program. Matrix Biol. 2008;27:274–81. doi: 10.1016/j.matbio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Oh E, Pierschbacher M, Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci U S A. 1981;78:3218–21. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson PN, Cho E, Blumenstock FA, Shah DM, Saba TM. Rebound elevation of fibronectin after tissue injury and ischemia: role of fibronectin synthesis. Am J Physiol. 1992;263:G437–45. doi: 10.1152/ajpgi.1992.263.4.G437. [DOI] [PubMed] [Google Scholar]
- 22.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. Embo J. 1998;17:5933–47. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–91. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 24.Pinzani M, Failli P, Ruocco C, Casini A, Milani S, Baldi E, Giotti A, Gentilini P. Fat-storing cells as liver-specific pericytes. Spatial dynamics of agonist-stimulated intracellular calcium transients. J Clin Invest. 1992;90:642–646. doi: 10.1172/JCI115905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Housset C, Rockey DC, Bissell DM. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci USA. 1993;90:9266–9270. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rockey DC. Vascular mediators in the injured liver. Hepatology. 2003;37:4–12. doi: 10.1053/jhep.2003.50044. [DOI] [PubMed] [Google Scholar]
- 27.Woodsome TP, Polzin A, Kitazawa K, Eto M, Kitazawa T. Agonist- and depolarization-induced signals for myosin light chain phosphorylation and force generation of cultured vascular smooth muscle cells. J Cell Sci. 2006;119:1769–80. doi: 10.1242/jcs.02805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.