Abstract
Background
Mucosal apoptosis is the initiating event in models of necrotizing enterocolitis (NEC) within rodents. It is possible there are species-specific differences that make apoptosis a more prominent feature of NEC in rodents than in humans.
Hypothesis
A lower threshold for mucosal apoptosis in the rodent distal intestine might have evolutionary advantages (via enhanced opsonization with the neonatal Fc receptor [FcRn]), since many short-gestation mammals are comparatively premature (histomorphologically) but are protected from NEC by breast milk.
Methods
We utilized a rat intestinal epithelial cell (IEC-18) model to determine if cell death alters FcRn – IgG binding, and rodent models of NEC to determine if cell death results in increased opsonization of IgG. Cultured IEC-18 cells were treated with H2O2 and analyzed. Neonatal Sprague-Dawley rats were cold and hypoxia stressed and intestinal sections were frozen for analysis.
Results
IgG binding was increased in H2O2-treated cells. Co-incubation of treated cells with either insulin-like growth factor or tunicamycin decreased IgG binding. Sprague-Dawley rats formula fed with exogenous bacteria showed a significant decrease in intestinal FcRn mRNA but increased ileal IgG binding.
Conclusions
We speculate that FcRn plays a role in passive opsonization and subsequent bacterial pathogen clearance, making rodents resistant to NEC.
Keywords: necrotizing enterocolitis, neonatal Fc receptor, intestinal epithelial cell, glycosylation, immunoglobulin
INTRODUCTION
Passive immunity in the neonatal intestine is critical to the prevention of necrotizing enterocolitis (NEC). The principle source of passive immunity to the intestine in all mammals is immunoglobulins (IgGs) in breast milk. The neonatal Fc receptor (FcRn) is also a crucial component of this passive immunity. Rodents and other short gestation mammals take up most of the maternal IgG postnatally via FcRn in the proximal intestine.1 In contrast, humans take up the majority of their IgG across the placenta in the third trimester using the same receptor and thus are born with a form of intact passive immunity.2 FcRn is an approximately 60 kD protein that is also known to regulate the catabolism of IgG via a recycling mechanism that has been well described.3
FcRn follows a proximal to distal gradient in its distribution with decreasing abundance in the ileum and colon of both fetal and adult human tissue.4 Similar findings have also been reported for rodents.5 Due to its paucity in the ileum, we wondered if its presence in the distal intestine might be important for host defense in short gestation mammals, rather than for generation of passive immunity. For example, human FcRn has only one site for N-linked glycosylation, whereas rodents have four potential sites.6,7 Glycosylation is a well documented mechanism for regulating IgG – Fc receptor complex binding, thus providing a possible switch for altering the affinity for IgG in times of host defense (as might happen with an infected or dying cell that could still serve as a super-opsin by aggregating IgG-bound bacteria with FcRn).8–10
The aim of this study was to utilize a rat intestinal epithelial (IEC-18) cell model of FcRn – IgG binding to determine if cell death alters FcRn – IgG binding, and to survey for IgG binding within in vivo rodent models of NEC. These findings would be novel and possibly provide the first evidence for a previously unrecognized mechanism by which short gestation mammals avoid NEC in the perinatal period, as well as investigate an important potential cofounder of rodent NEC studies.
MATERIALS & METHODS
Cell culture
Rat intestinal epithelial cells (IEC-18; American Type Culture Collection, Rockville, MD) were grown to 80% confluence in Dulbecco’s Modified Eagles Medium (DMEM) (Invitrogen, Carlsbad, CA) 10% fetal bovine serum and 0.01% insulin in six-well plates with and without coverslips. Time period to reach 80% confluence was 20 to 24 hrs. Cells were treated with 200 μM hydrogen peroxide (H2O2) for three hours to induce cell death and were co-incubated with either 5×10−6 M insulin-like growth factor – I (IGF-I), 5×10−6 M insulin-like growth factor – II (IGF-II), orthovanadate (either 1 or 10 μM), or tunicamycin (either 0.1 μg/ml or 1 μg/ml). All reagents were obtained from Sigma, St. Louis, MO. After treatment, cells were harvested for immunocytochemistry, western blot analysis, DNA fragmentation or real time RTPCR analysis.
Immunocytochemistry
Coverslips with IEC-18 were removed from the six-well plates and fixed with 10% phosphate-buffered formalin overnight. Slides were then washed five times with DIG buffer (4% 1M Tris base, 6% 5M NaCl, 16% 1M Tris-HCl) and blocked (1% BSA) for one hour at room temperature. When used, slides were treated with a polyclonal rabbit anti-rat FcRn alpha-chain serum antibody (provided by Neil Simister, Brandeis University) for one hour and then washed with DIG buffer.7 Slides were then treated with biotin-conjugated goat anti-rabbit secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA) for one hour and then washed again as above. ABC (Elite Series, Vector Laboratories) was applied per manufacturer’s directions, washed with DIG buffer and slides were developed with diaminobenzadine (Sigma, St. Louis, MO), counterstained with hematoxylin and coverslipped. Pictures were taken with a PixCell II Laser Capture Microscope® at 20x magnification. At least 10 pictures of each treatment condition were taken randomly and cell counts were obtained by one author (JRS) who was blinded to the treatment conditions.
Double-labeling of slides was done in a similar fashion. Coverslips were fixed overnight, then washed, blocked, stained with anti-FcRn then visualized using a peroxidase-conjugated secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA) as described above. Coverslips were then stained with anti-caspase-3 rabbit polyclonal IgG conjugated to alkaline phosphatase (Upstate Biotechnologies, Lake Placid, NY) and detected with 5-bromo-4-chloro-3-indolyphosphate p-toluidine salt and nitroblue tetrazolium (BCIP/NBT System, R&D Systems Inc., Minneapolis, MN).
Western blot analysis
After media removal from six-well plates, cells were washed with phosphate-buffered saline (PBS) at 4°C. Cell samples were collected in lysis buffer (0.05 M Tris-HCl ph 7.4, 0.25 M NaCl, 1% Nonidet P 40 substitute, and one Complete, Mini EDTA-free protease inhibitor tablet [Roche, Indianapolis, IN]) and then centrifuged for 10 min at 4°C. Lysate samples were then diluted 4:1 with running buffer (62.5 mM Tris-HCl pH 6.8, 20% glycerol, 2% SDS, 5% β-mercaptoethanol) and run on 10% acrylamide gels. Gels were then transferred to nitrocellulose membranes and blocked in 5% milk for at least 1 hr. Primary antibodies (IGFBP3 goat polyclonal IgG, Jackson Immunoresearch Laboratories, West Grove, PA; FcRn rabbit polyclonal, Neil Simister, Brandeis University) were applied 1:1000 (10 μl antibody: 10 ml 5% milk) for 1 hr and then washed for three times in TBS-Tween for 5 min per wash. Biotin-conjugated rabbit anti-goat secondary antibody or biotin-conjugated donkey anti-rabbit secondary antibody (Jackson Immunoresearch Laboratories, West Grove, PA) was applied 1:2000 (5 μl antibody: 10 ml 5% milk) for 1 hr and then membranes were washed as above. ABC was applied per manufacturer’s guidelines. After washing as above, membranes were visualized using SuperSignal Chemiluminescent Subtrate (Pierce, Rockford, IL) on radiographic film. Densitometric analyses of protein bands were performed, in order to quantitatively determine IgG binding, using a Molecular Dynamics densitometer with Image Quant 5.0 (Amersham Pharmacia Biotech, Cambridge, MA). To determine equal protein loading, samples were analyzed for protein concentration with a bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL) per manufacturer’s recommendations prior to loading in gel.
Cell Death Assays
To determine the amount of cell death, DNA fragmentation was performed as previously described.11 Briefly, media and cells were centrifuged at 1800 rpm at 4°C for 5 min. Supernatant was discarded, and the pellet was resuspended in 1 ml of 4°C PBS and spun down again at 6000 rpm for 1 min. Lysis buffer (0.01 M Tris-HCl pH 7.4, 0.01 M EDTA, and 0.5% Triton X-100) was added and samples were placed on ice for 30 min and subsequently spun down at 13200 rpm for 10 min at 4°C. Supernatant was separated from the pellet and both incubated on ice for 30 min in 70% perchloric and cell lysis buffer. Samples were then centrifuged for 20 min at 13200 rpm at 4°C. Supernatant was removed and 70% perchloric acid was added. Samples were incubated in a 70°C water bath for 20 min and then transferred to 4°C for 5 min. Diphenylamine solution was added to all samples and kept at room temperature overnight in a darkroom. Samples were then measured on an ELISA reader at 570 nm.
Caspase-3 production was also measured via immunocytochemistry and western blot analysis as described above. Primary antibody for caspase-3 was a rabbit polyclonal IgG anti-caspase-3 (Upstate Biotechnologies, Lake Placid, NY).
Real time RT-PCR
IEC-18 in 150 mm plates were treated with H2O2 and IGF-I or IGF-II as described in the cell culture methods. RNA was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA). Real time RT-PCR was run at the University of Virginia Digestive Health Center of Excellence Core Lab using pre-labeled primers and TaqMan probes (Applied Biosystems, Foster City, CA) for three Fc receptors (FcRn, FcGr2b, and FcGr3).
Animal Experiments
To look at in vivo relevance to our model, we used neonatal rats delivered via cesarean section at 21 days from time-dated pregnant Sprague-Dawley dams at Evanston Northwestern Healthcare Research Institute (JL and TJ). After stabilization and isolation from their mothers, litters were randomized into three groups; pups were either fed dam milk, Esbilac puppy formula every 3 hr via an orogastric tube or Esbilac puppy formula every 3 hr with a mixture of Klebsiella pneumoniae, Serratia marcesens, and viridins Streptococcus. All groups were fed ~200 kcal/kg/day. Experimental NEC was generated using a previously described method.12 Briefly, pups were exposed to 100% nitrogen for 1 minute followed by cold (4°C) exposure for 10 min twice daily. Pups were euthanized at 48 – 72 hrs of life; those that died prior to 24 hrs were excluded as NEC histology does not occur before 24 hrs of life. Intestinal sections of the duodenum and ileum were obtained and frozen in OCT or snap frozen. Five micron sections frozen in OCT were cut and placed on a slide. Slides were treated and pictures taken as per our immunocytochemistry protocol outlined above. A blinded observer scored each slide from zero (no binding) to four (saturated binding) based on the amount of IgG binding present. Sections that were snap frozen were analyzed for FcRn RNA using real-time RT-PCR as outlined above.
Statistics
Appropriate statistical tests including paired two-tailed student’s t-test, ANOVA, and Mann-Whitney were run on GraphPad Prism 4 for Windows (GraphPad Software, Inc, San Diego, CA). In all statistical tests, a p value ≤ 0.05 was considered significant.
RESULTS
IEC-18 express FcRn and bind IgG when undergoing cell death
IEC-18 express FcRn abundantly. (Figure 1A) Western blot analysis of IEC-18 that were treated with H2O2 and subsequently stained with a non-specific antibody demonstrated a 65 kD band that was not seen in untreated controls. (Figure 1B) Using a primary antibody to IGFBP3, found abundantly in IEC-18, confirmed similar protein loading in both lanes. When using only a secondary antibody on western blot analysis this 65 kD doublet is still visible (Figure 1C), indicating this protein is not a specific antigen and likely represents an Fc receptor.
Figure 1.
Representative western blots of IEC-18 lysates using (A) anti-FcRn primary antibody (SP1°), (B) a non-specific anti-IGFBP-3 primary antibody (NS1°), or (C) a secondary antibody. FcRn is abundant in the IEC-18 cell line (A), and when stained with a non-specific antibody (B) we still show a 65 kD doublet in the H2O2 treated cells (which is also seen in the C– indicating the 65 kD doublet is not a specific antigen in these two conditions). The use of the non-specific primary antibody also serves to demonstrate equitable loading of the two treatment conditions. We conclude that the 65 kD doublet seen with secondary antibody alone is likely FcRn.
Untreated IEC-18 were screened for three different Fc receptors that have been reported to be found in rat intestinal tissue using real time RT-PCR. The three receptors were the neonatal Fc receptor (FcRn) and two gamma receptors, FcγRIII and FcγRb. FcRn was the most abundant member of the Fc receptor family found in untreated IEC-18, confirming that the antigen found on our western blots with non-specific antibody was in fact FcRn. (Figure 2)
Figure 2.
Real time RTPCR demonstrating that FcRn is the only Fc receptor mRNA in appreciable quantity within the IEC-18 cell line.
IEC-18 bind IgG in a pattern suggestive of programmed cell death
During immunocytochemistry analysis (using only secondary antibody for detection), it was noted that IEC-18 with IgG binding demonstrated stereotypic stages of cell death when treated with H2O2. Early onset of increased IgG binding is highly compartmentalized (Figure 3A, note the polarized staining around vacuoles) and staining intensity seems to progress coincident with cellular retraction from surrounding cells (Figure 3B). Cells undergo anoikis in parallel with the most intense staining (Figure 3C), detaching themselves from the surrounding cells and eventually jettisoning their nuclei to become cellular ghosts. (Figure 3D)
Figure 3.
Photomicrographs demonstrating the relationship between IgG binding seen in immunocytochemistry (40x MAG) and stereotypic examples of cell morphology: A) early IgG binding, characterized by polar staining in association with intracellular vacuoles; B) increased intensity of IgG binding associated with cellular retraction; C) maximum intensity of IgG binding associated with anoikis; D) maximum intensity of IgG binding associated with nuclear ejection.
H2O2-induced IgG binding is a post-transcriptional event and IGFs decrease IgG binding, but not FcRn expression
IEC-18 were stained with anti-FcRn rabbit polyclonal antibody. All cells, whether treated or untreated, showed some baseline amount of FcRn detection in a global pattern reminiscent of ostrich skin (i.e. all cells show a small amount of perinuclear staining – Figure 4A). In cells that were treated with H2O2, there is a general increase in the amount of FcRn staining within all cells and a marked increase within cells that appear to be that are undergoing cell death (evident by their retraction – Figure 4B). When H2O2 treated cells were co-incubated with either IGF-I (Figure 4C) or IGF-II (Figure 4D), IEC-18 returned to baseline staining similar to that of control cells.
Figure 4.
Photomicrograph of IEC-18 stained with an anti-FcRn rabbit polyclonal antibody (20x MAG). Untreated control cells (A) have a baseline expression of FcRn. H2O2-treated cells (B) show increased amounts of FcRn staining visualized with horseradish peroxidase. It is especially dark in cells that are undergoing cell death (evident by detachment). IEC-18 co-incubated with IGF-I (C) or IGF-II (D) show similar amounts of FcRn staining seen in control cells.
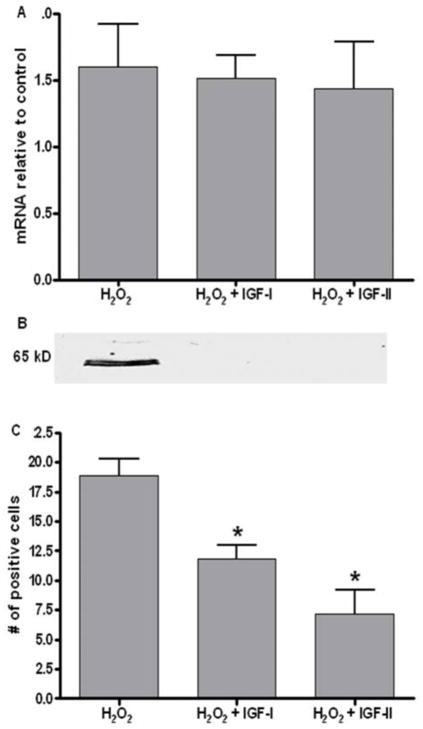
Real time RT-PCR of H2O2 treated cells demonstrated no significant change in FcRn mRNA (Figure 5A) when compared to untreated cells, suggesting that regulation of FcRn expression is a post-transcriptional event. When compared to cells co-treated with insulin-like growth factor–I (IGF-I) and –II (IGF-II), known inhibitors of apoptosis, there was no difference in FcRn mRNA, though on western blot analysis (Figure 5B) there was a decrease in IgG binding.13,14 Immunocytochemistry analysis of cells co-treated with H2O2 and IGF-I or IGF-II showed a significant decrease in the number cells showing IgG binding. (Figure 5C)
Figure 5.
(A) Real time RT-PCR of treated and IGF rescued cells showing no difference in the quantity of FcRn mRNA compared to untreated controls, but with a representative western blot (B) demonstrating minimal IgG binding with IGF rescue. In immunocytochemistry(C), there was a significant decrease in the number of cells with IgG binding (*p<0.01).
IEC-18 treated with H2O2 undergo apoptosis
Slides of IEC-18 treated with H2O2 and double-labeled with anti-FcRn and anti-caspase-3 antibodies show cells that are undergoing apoptosis (cleaved caspase-3, black arrows) have increased amounts of FcRn staining compared to cells not undergoing cell death. On western blot analysis (inset), cleaved caspase-3 was also found in cells treated with H2O2 and increased FcRn staining, but not in control cells or in adjacent cells on the same slide that did not demonstrate increased FcRn staining (Figure 6). Cells that were co-incubated with either IGF-I or IGF-II also did not demonstrate co-labeling for caspase-3 (data not shown).
Figure 6.

Photomicrograph of H2O2 treated cells double labeled with anti-FcRn (a brown precipitate) and anti-caspase-3 (a diffuese light purple/ blue precipitate) (20x MAG). Note the predominantly perinuclear staining indicative of caspase-3 activation. Inset demonstrates representative western blot demonstrating an increase in cleaved caspase-3 in H2O2 treated cells relative to untreated controls.
IEC-18 treated with orthovanadate have less cell death but there is no change in IgG binding
Orthovanadate is a known inhibitor of intestinal epithelial cell anoikis.15,16 IEC-18 co-treated with H2O2 and orthovanadate at concentrations of 1 or 10 μM showed no difference in the number of cells positively staining for IgG binding compared to H2O2-only treated cells (Figure 7A). Immunocytochemistry analysis showed a qualitative decrease in the number of cells undergoing cell death (cells with condensed nuclei or showing signs of retraction) at higher orthovanadate concentrations (black arrows) but no difference in the number of cells staining for IgG binding (white arrows – Figure 7B).
Figure 7.

(A) H2O2 treated cells with the addition of either 1 μM or 10 μM orthovanadate (OV) showing no change in the number of cells with IgG binding. (B) Photomicrograph showing a gualitative demonstration that a 1 μM concentration of OV has more evidence of cell death (black arrowheads) than does the 10 μM concentration, but that the amount of IgG binding appears comparable (white arrowheads) (20x MAG).
A pharmacologic inhibitor of glycosylation decreases IgG binding
When co-incubated with H2O2 and either 0.1 or 1 μg/ml of tunicamycin (a highly specific inhibitor of post-translational glycosylation) there was a significant decrease in IgG binding as shown by densitometry analysis of western blot samples (Figure 8).17 To determine if there was a change in cell death, cells were also assayed for DNA fragmentation. Analysis showed no significant change in the number of cells undergoing cell death between the controls and treated conditions.
Figure 8.

DNA fragmentation assay showed that there was no significant change in apoptosis between cells treated with H2O2 or cells treated with H2O2 and either 1 or 0.1 μg/ml tunicamycin. Also shown is a representative western blot and densitometry means (+ SD). Compared to IECs treated only with H2O2, IECs that were co-incubated with either 1 or 0.1 μg/ml of tunicamycin showed a significant decrease in IgG affinity (*p< 0.05)
FcRn expression is decreased in vivo in a neonatal rat model of NEC
FcRn mRNA was present in considerable quantity in duodenal tissue for both dam-fed and formula-fed rats. However, in those that were also fed the mixture of exogenous bacteria, there was a significant decrease (p<0.01) in the amount of FcRn mRNA present compared to dam-fed controls and those fed formula only (Figure 9). Dam-fed controls and formula-fed only rats had significantly less (p<0.01) FcRn mRNA present in ileal tissue. Rats fed the exogenous bacteria mixture also had significantly less (p<0.05) FcRn mRNA then the dam-fed treatment group in the ileal tissue as well.
Figure 9.

Real time RT-PCR for FcRn mRNA of neonatal Sprague-Dawley rats in either dam-fed, formula-fed, or formula-fed with a mixture of exogenous bacteria. There is a significant decrease (p<0.01) in FcRn mRNA in both duodenal and ileal tissue in those fed with the exogenous bacteria compared to those dam-fed or fed formula only (duodenal only).
IgG binding is increased in the ileum in a neonatal rat model of NEC
Using a blinded scoring system from zero (no binding) to four (saturated binding) we scored intestinal samples for the amount of IgG binding. In the duodenum, there was no difference between rats fed formula (F) and those fed formula with exogenous bacteria (F+B). In the ileum, there was a significant increase (p<0.05) in the amount of IgG binding in those fed F+B (Figure 10).
Figure 10.

Blinded IgG binding score of rats fed formula (F) versus formula fed with exogenous bacteria (F+B). There is a significant increase in the amount of IgG binding in the ileum of the rats fed the bacteria (*p<0.05).
DISCUSSION
We have demonstrated that early events associated with cell death alter IgG binding in IEC-18 cells. These experiments have generated a novel hypothesis and may represent a previously unrecognized mechanism by which short gestation mammals avoid NEC in the perinatal period. FcRn mRNA has been detected by others in the ileum, albeit at lower levels than the proximal intestine, potentially validating the in vivo relevance of our model. 5,18
These results also generate a possible confounder of rodent NEC studies. We looked at newborn rat duodenums and ileums to see if IgG binding was evident. We found minimal binding evident in the ileums of newborn rats that were dam-fed. In the rat pups that were stressed to generate experimental NEC, we were able to find intense IgG staining in the ileum, particularly in areas where NEC was evident, consistent with our hypothesis.19 This is especially true in the treatment group that received exogenous bacteria which has been shown to develop NEC over 50% of the time, compared to those fed formula only who develop NEC ~20% of the time.20 These descriptive findings also support our supposition that FcRn – IgG binding is associated with cell death. We were able to disrupt IgG binding with IGF, a strongly anti-apoptotic growth factor, but not orthovanadate, an agent that perturbs anoikis.13–16 This pair of findings point to the early events in cell death as being the window in which N-glycosylation is vulnerable to change, since anoikis is a late event and IGF only rescues cells if given concurrently.
Humans, as with all long-gestation mammals, receive the bulk of maternal immunoglobulins via placental transfer during the third trimester. However, preterm infants who are 28 weeks gestation or less have little or no systemic passive immunity and benefit from breast milk IgG occurs primarily as an opsin within the lumen of the intestine. While there are other antibacterial properties in breast milk that still make it preferable to formula, in comparison, premature infants are much more susceptible to NEC than are short gestation mammals – even though their intestinal tissues are histomorphologically similar.21–23
In rodents who are dam-fed (compared to formula-fed), we speculate that dying intestinal epithelial cells have altered N-glycosylation, resulting in improved FcRn – IgG binding and bacterial opsonization. As anoikis proceeds, these dying cells slough into the intestinal lumen, and evacuate bound pathogens as part of the feces (Figure 11). Additionally, our observation of diminished FcRn mRNA in the duodenum of rat pups fed bacteria (Figure 9) suggests that there may be a mechanism for reducing proximal IgG uptake through transcriptional down regulation, thereby allowing increased passage of IgG to the distal intestine in pathogen stressed animals. These postulated mechanisms, if accurate, could contribute to the paucity of NEC in dam-fed rodents compared to their formula-fed counterparts in experimental models of NEC.20
Figure 11.

Schematic illustrating the role FcRn may play in the passive-opsonization of bacterial pathogens in the intestinal lumen. IECs, after undergoing some event leading towards cell death (A) begin to alter FcRn-IgG binding as they encounter early cell death events (such as activation of caspase-3). As the cell undergoes cell death (B). FcRn-IgG binding is enhanced and the bacterial pahtosen is opsonized and eventually flushed out of the intestinal lumen.
In support of our hypothesis is the existence of four glycosylation sites on mouse FcRn versus only one in human FcRn, potentially allowing a greater degree of IgG binding regulation.6,7 Studies in other Fc receptors have confirmed that N-glycosylation is a widespread mechanism for regulating IgG affinity in this protein family.8–10 It has also been noted that N-linked glycosylation is affected by infection, inflammation and a number of inflammatory agents including interleukins, tumor necrosis factor–α, transforming growth factor–β and interferon–γ.24,25 All of these agents can participate and lead to programmed cell death, further supporting our postulate that this is the event that triggers the alteration in IgG – FcRn binding.
In summary, we have data that supports the plausibility of the FcRn receptor as a component of a novel defense mechanism in the ileum of newborn rodents. If valid, this concept could have important implications for rodent models of NEC. For example, mutations in the N-glycosylation sites might result in formula-induced NEC models without the need for cold stress or hypoxia.14,26,27 Further research is required to determine the importance of our findings (if any) with regard to human NEC.
Acknowledgments
The authors would like to thank Nena Fox, PhD for assistance with cell cultures and Neil Simister, PhD for the generous donation of the anti-FcRn rabbit polyclonal antibody.
Footnotes
FINANCIAL DISCLOSURE: This study was funded in part by NIH grant T32 HL 7956 (JRS) and NIH grant 1KO8 DK/HD61553-01 (PVG)
References
- 1.Israel EJ, Taylor S, Wu Z, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Firan M, Bawdon R, Radu C, et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of γ-globulin in humans. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 3.Ghetie V, Ward ES. Transcytosis and catabolism of antibody. Immunol Res. 2002;25:97–114. doi: 10.1385/IR:25:2:097. [DOI] [PubMed] [Google Scholar]
- 4.Shah U, Dickinson BL, Blumberg RS, et al. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr Res. 2003;53:295–301. doi: 10.1203/01.PDR.0000047663.81816.E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin MG, Wu SV, Walsh JH. Ontogenetic development and distribution of antibody transport and Fc receptor mRNA expression in rat intestine. Dig Dis Sci. 1997;42:1062–1069. doi: 10.1023/a:1018853506830. [DOI] [PubMed] [Google Scholar]
- 6.Story CM, Mikulska JE, Simister NE. A major histocompatibility complex class I-like Fc receptor cloned from human placenta: possible role in transfer of immunoglobulin G from mother to fetus. J Exp Med. 1997;180:2377–2381. doi: 10.1084/jem.180.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 8.Drescher B, Witte T, Schmidt RE. Glycosylation of FcγRIII in N163 as mechanism of regulating receptor affinity. Immunology. 2003;110:335–340. doi: 10.1046/j.1365-2567.2003.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peppard JV, Hobbs SM, Jackson LE. Role of carbohydrate in binding of IgG to the Fc receptor of neonatal rat enterocytes. Mol Immunol. 1989;26:495–500. doi: 10.1016/0161-5890(89)90109-0. [DOI] [PubMed] [Google Scholar]
- 10.Hobbs SM, Jackson LE, Hoadley J. Interaction of aglycosyl immunoglobulins with the IgG Fc transport receptor from neonatal rat gut: comparison of deglycosylation by tunicamycin treatment and genetic engineering. Mol Immunol. 1992;29:949–956. doi: 10.1016/0161-5890(92)90133-i. [DOI] [PubMed] [Google Scholar]
- 11.Fujii J, Matsui T, Heatherly DP, et al. Rapid apoptosis induced by shiga toxin in HeLa cells. Infect Immun. 2003;71:2724–2735. doi: 10.1128/IAI.71.5.2724-2735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan MS, Hedlund E, Adler L, et al. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Ped Pathology. 1994;14:1017–1028. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 13.Baregamian N, Song J, Jeschke NG, et al. IGF-1 protects intestinal epithelial cells from oxidative stress-induced apoptosis. J Surg Res. 2006;136:31–37. doi: 10.1016/j.jss.2006.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozen S, Akisu M, Baka M, et al. Insulin-like growth factor attenuates apoptosis and mucosal damage in hypoxia/reoxygenation-induced intestinal injury. Biol Neonate. 2005;87:91–96. doi: 10.1159/000081897. [DOI] [PubMed] [Google Scholar]
- 15.Kaeffer B, Bénard C, Blottière HM, et al. Treatment of rat proximal and distal colonic cells with sodium orthovanadate enhances their adhesion and survival in primary culture. Cell Biol Int. 1997;21:303–314. doi: 10.1006/cbir.1997.0141. [DOI] [PubMed] [Google Scholar]
- 16.Dzierzewicz Z, Orchel A, Parfiniewicz B, et al. The delay of anoikis due to the inhibition of protein tyrosine dephosphorylation enables the maintenance of normal rat colonocyte primary culture. Folia Histochem Cytobiol. 2003;41:223–228. [PubMed] [Google Scholar]
- 17.Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins SL, Wang J, Vazir M, et al. Role of passive and adaptive immunity in influencing enterocyte-specific gene expression. Am J Physiol Gastrointest Liver Physiol. 2003;285:G714–G725. doi: 10.1152/ajpgi.00130.2003. [DOI] [PubMed] [Google Scholar]
- 19.Jilling T, Lu J, Jackson M, et al. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622–629. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 20.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336:1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 22.Schanler RJ, Lau C, Hurst NM, et al. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116:400–406. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 23.McGuire W, Anthony MY. Donor human milk versus formula for preventing necrotizing enterocolitis in preterm infants: systematic review. Arch Dis Child Fetal Neonatal Ed. 2003;88:F11–F14. doi: 10.1136/fn.88.1.F11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackiewicz A, Laciak M, Gorny A, et al. Leukemia inhibitory factor, interferon gamma and dexamethasone regulate N-glycosylation of alpha 1-protease inhibitor in human hepatoma cells. Eur J Cell Biol. 1993;60:331–336. [PubMed] [Google Scholar]
- 25.Van den Steen P, Rudd PM, Dwek RA, et al. Cytokine and protease glycosylation as a regulatory mechanism in inflammation and autoimmunity. Adv Exp Med Biol. 1998;435:133–143. doi: 10.1007/978-1-4615-5383-0_13. [DOI] [PubMed] [Google Scholar]
- 26.Hsueh W, Caplan MS, Sun X, et al. Platelet-activating factor, tumor necrosis factor, hypoxia and necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:11–17. doi: 10.1111/j.1651-2227.1994.tb13234.x. [DOI] [PubMed] [Google Scholar]
- 27.Langer JC, Sohal SS, Mumford DA. Mucosal permeability in the immature rat intestine: effects of ischemia-reperfusion, cold stress, hypoxia, and drugs. J Pediatr Surg. 1993;28:1380–1383. doi: 10.1016/s0022-3468(05)80331-8. [DOI] [PubMed] [Google Scholar]