Abstract
The inference of gene regulatory network (GRN) from gene expression data is an unsolved problem of great importance. This inference has been stated, though not proven, to be underdetermined implying that there could be many equivalent (indistinguishable) solutions. Motivated by this fundamental limitation, we have developed new framework and algorithm, called TRaCE, for the ensemble inference of GRNs. The ensemble corresponds to the inherent uncertainty associated with discriminating direct and indirect gene regulations from steady-state data of gene knock-out (KO) experiments. We applied TRaCE to analyze the inferability of random GRNs and the GRNs of E. coli and yeast from single- and double-gene KO experiments. The results showed that, with the exception of networks with very few edges, GRNs are typically not inferable even when the data are ideal (unbiased and noise-free). Finally, we compared the performance of TRaCE with top performing methods of DREAM4 in silico network inference challenge.
Introduction
The discovery and analysis of biological networks have important applications, from finding treatment of diseases to engineering of microbes for the production of drugs and biofuels [1]–[4]. With continued advances in high throughput and omics technology, the inference of biological networks from omics data has received a great deal of interest. In particular, the inference of gene regulatory networks from gene expression data constitutes a major research topic in systems biology. In the last decade, the number of methodologies that are dedicated for the GRN inference has increased tremendously [5]–[7].
The Dialogue for Reverse Engineering Assessments and Methods (DREAM) project is a community-wide effort initiated to fulfill the need for a rigorous and fair comparison of the strengths and weaknesses of methods for the reverse engineering of biological networks from data. To this end, challenges involving the inference of cellular networks are organized on a yearly basis (http://www.the-dream-project.org/challenges). Specifically, the inference of GRN has become a major focus of several DREAM challenges. The outcomes of these challenges indicate that the state-of-the-art algorithms for GRN inference are unable to provide accurate and reliable network predictions, even when large expression datasets are available and the number of genes is small (10–100 genes) [8]–[10]. Nonetheless, a crowd-sourcing strategy that combines the predictions of different inference methods has been shown to be more reliable than any individual method [10].
Whether or not a direct regulation of one gene by another can be correctly inferred depends not only on the ability of an inference method to extract the relevant information from data, but also on the availability of such information in the data. In general, the information content of data is determined first and foremost by the conditions of the experiments. If the required information is unavailable or incompletely available, then the inference problem is underdetermined. In such a case, the network is not inferable from the data regardless of the method used.
The underdetermined nature is not exclusive to the inference of GRNs. Much of the difficulty in the inverse modeling of signaling and metabolic networks can also be attributed to the lack of inferability or identifiability of model structure and parameters [11]–[14]. As the inference problem is underdetermined, there exist multiple solutions which are indistinguishable. The lack of model identifiability has motivated a paradigm shift toward ensemble modeling [15]–[18]. While such a strategy has begun to gain traction in the modeling of signaling and metabolic networks, the ensemble paradigm has not been widely used in the inference of GRNs. Also, since the network representation and data for GRNs differ markedly from those for signaling and metabolic networks, existing algorithms for ensemble modeling cannot be directly applied for the inference of GRN.
In this work, we introduce new framework and algorithms, called Transitive Reduction and Closure Ensemble (TRaCE), for the ensemble inference of GRNs. Specifically, TRaCE produces the lower and upper bounds of the ensemble, i.e. the smallest network and the largest network that limit the complexity of networks in the ensemble. As the size of the ensemble reflects the uncertainty about the GRN inference, the bounds can also be used to analyze the inferability of GRNs. In this study, we have used TRaCE in two applications. First, we investigated the inferability of random GRNs and the GRNs of E. coli and S. cereviseae given steady-state gene expression data of single- and double-gene KO experiments. Then, we applied TRaCE to simulated gene expression data, generated in the same manner as the DREAM 4 in silico network inference challenge, and compared the performance of TRaCE with existing methods.
Methods
Theoretical Foundation
Definitions
Here, we provide a short synopsis of basic concepts in graph theory that are necessary for the development of our algorithm. A graph
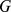 is an ordered pair
is an ordered pair 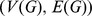 , where
, where 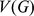 is the set of vertices (or nodes) and
is the set of vertices (or nodes) and  is the set of edges. The number of vertices
is the set of edges. The number of vertices  and the number of edges
and the number of edges  are called the order and the size of the graph, respectively. An edge
are called the order and the size of the graph, respectively. An edge  is defined by the pair
is defined by the pair 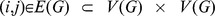 , describing the existence of a relationship between two vertices
, describing the existence of a relationship between two vertices  and
and 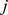 . In this case, the edge
. In this case, the edge 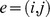 is said to be incident to the vertices
is said to be incident to the vertices  and
and 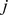 . The set of edges of a graph
. The set of edges of a graph  that are incident to a vertex
that are incident to a vertex  is denoted by
is denoted by 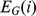 , while the cardinality of
, while the cardinality of 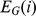 is called the degree of the vertex
is called the degree of the vertex  . Similarly, the set of edges that are incident to a set of vertices
. Similarly, the set of edges that are incident to a set of vertices  is denoted by
is denoted by 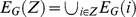 . A graph
. A graph 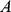 is a subgraph of
is a subgraph of 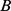 , denoted by
, denoted by 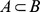 , if
, if 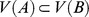 and
and  . In this case,
. In this case,  is called the supergraph of
is called the supergraph of 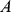 , and is also said to contain
, and is also said to contain 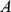 . Furthermore, the union of two graphs
. Furthermore, the union of two graphs 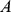 and
and 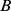 is denoted by
is denoted by 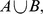 where
where 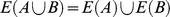 and
and 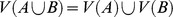 . The intersection of two graphs
. The intersection of two graphs  and
and 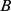 is denoted by
is denoted by  , where
, where  and
and 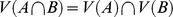 . Finally, the difference between two graphs
. Finally, the difference between two graphs  and
and 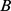 with
with 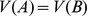 is denoted by
is denoted by  and defined as the set of edges in the graph
and defined as the set of edges in the graph 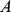 that do not belong to the graph
that do not belong to the graph  , i.e. edges in the set difference
, i.e. edges in the set difference 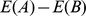 .
.
A directed edge is an ordered pair 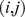 , representing an edge from the vertex
, representing an edge from the vertex  pointing to the vertex
pointing to the vertex  . A directed graph or digraph
. A directed graph or digraph
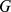 is a graph in which all of its edges are directed. A directed path is a sequence of vertices such that there exists a directed edge from one vertex to the next vertex in the graph. The first vertex in a directed path is called the start vertex, and the last is called the end vertex. A directed cycle is a directed path where the start and the end vertices are the same. A directed acyclic graph (DAG) is a digraph which does not contain any cycle. The adjacency matrix of a digraph
is a graph in which all of its edges are directed. A directed path is a sequence of vertices such that there exists a directed edge from one vertex to the next vertex in the graph. The first vertex in a directed path is called the start vertex, and the last is called the end vertex. A directed cycle is a directed path where the start and the end vertices are the same. A directed acyclic graph (DAG) is a digraph which does not contain any cycle. The adjacency matrix of a digraph 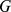 of order
of order  , denoted by
, denoted by 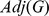 , is an
, is an 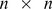 matrix with
matrix with 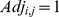 when
when  , and
, and 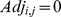 otherwise. In other words, the non-zero elements of the adjacency matrix represent all directed edges from any node
otherwise. In other words, the non-zero elements of the adjacency matrix represent all directed edges from any node  to another node
to another node 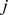 in the graph
in the graph 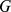 . Meanwhile, the accessibility matrix of
. Meanwhile, the accessibility matrix of 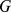 , denoted by
, denoted by 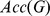 , is an
, is an 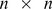 matrix with
matrix with 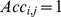 when there exists a directed path from node
when there exists a directed path from node  to node
to node 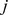 , and
, and 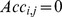 otherwise. When
otherwise. When 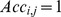 , vertex
, vertex 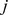 is said to be accessible from the vertex
is said to be accessible from the vertex  .
.
A strongly connected component or strong component of a digraph 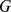 is a maximal subset of nodes in
is a maximal subset of nodes in 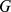 where any two nodes in the subset are mutually accessible. Every pair of nodes that are part of a directed cycle belong to the same strong component, while any node that is not part of a cycle is a strong component of its own. The condensation of a digraph
where any two nodes in the subset are mutually accessible. Every pair of nodes that are part of a directed cycle belong to the same strong component, while any node that is not part of a cycle is a strong component of its own. The condensation of a digraph  is the DAG of the strong components of
is the DAG of the strong components of  , which is generated by lumping the nodes belonging to a cycle into a single node and replicating the edges that are incident to any of these nodes onto the lumped node [19].
, which is generated by lumping the nodes belonging to a cycle into a single node and replicating the edges that are incident to any of these nodes onto the lumped node [19].
A digraph is transitive if for every pair of vertices  and
and 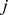 , there exists an edge
, there exists an edge 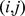 when there is a directed path from
when there is a directed path from  to
to 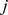 . The transitive closure of a digraph
. The transitive closure of a digraph 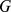 , denoted by
, denoted by 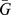 , is the smallest transitive supergraph of
, is the smallest transitive supergraph of 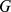 (with the fewest edges) [20]. When
(with the fewest edges) [20]. When 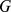 is a DAG, we denote the transitive closure of
is a DAG, we denote the transitive closure of 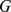 as
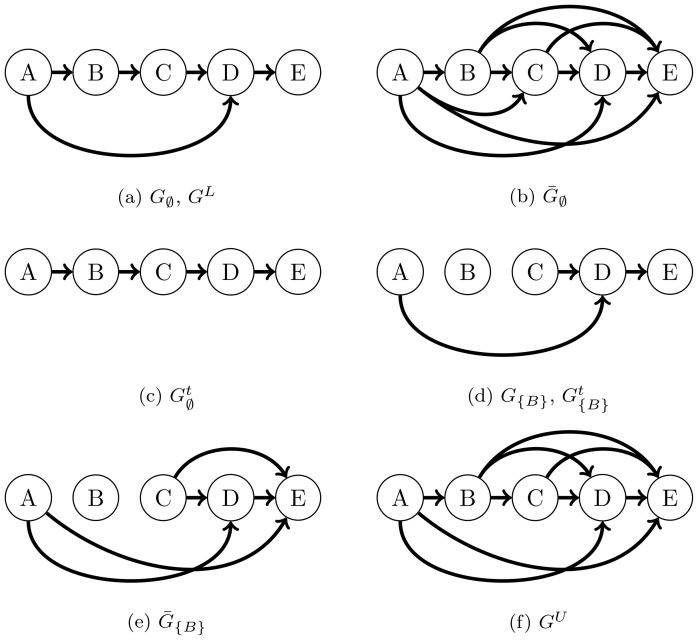
as 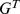 . As shown in Fig. 1(a)-(b), the transitive closure of a digraph can be generated by adding a directed edge
. As shown in Fig. 1(a)-(b), the transitive closure of a digraph can be generated by adding a directed edge 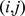 , whenever a directed path exists from vertex
, whenever a directed path exists from vertex  to vertex
to vertex 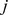 . Note that the accessibility matrix of a digraph is the adjacency matrix of its transitive closure, i.e.
. Note that the accessibility matrix of a digraph is the adjacency matrix of its transitive closure, i.e. 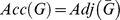 . For a digraph
. For a digraph  , the set of digraphs that have the same transitive closure
, the set of digraphs that have the same transitive closure 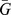 is denoted by
is denoted by  . The transitive reduction of
. The transitive reduction of 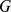 , denoted by
, denoted by 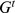 , is defined as the smallest member of
, is defined as the smallest member of 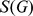 in size (i.e. the graph with the fewest edges). The transitive reduction of a DAG is unique, given by
in size (i.e. the graph with the fewest edges). The transitive reduction of a DAG is unique, given by 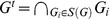 [20]. An algorithm for obtaining transitive reduction has been previously developed [19], in which any directed edge
[20]. An algorithm for obtaining transitive reduction has been previously developed [19], in which any directed edge  is pruned whenever there exists a directed path from
is pruned whenever there exists a directed path from  to
to 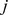 that does not include
that does not include 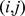 (for example, see Fig. 1(c)). Note that the transitive reduction of a digraph with cycles is not unique.
(for example, see Fig. 1(c)). Note that the transitive reduction of a digraph with cycles is not unique.
Figure 1. (a) An example of a directed graph 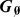 .
.
(b) The transitive closure 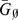 (in this case,
(in this case,  since
since 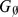 is a DAG). (c) The transitive reduction
is a DAG). (c) The transitive reduction 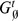 of
of 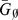 . (d) The directed graph
. (d) The directed graph 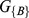 associated with
associated with 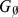 . (e) The transitive closure
. (e) The transitive closure 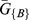 . In this case, the transitive reduction
. In this case, the transitive reduction 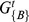 happens to be the graph
happens to be the graph 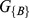 . (f) The ensemble upper bound
. (f) The ensemble upper bound 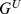 obtained from
obtained from 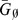 and
and  . The ensemble lower bound
. The ensemble lower bound  obtained from
obtained from  and
and  happens to be the graph
happens to be the graph 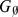 .
.
Inference of Network Ensemble Bounds
In this work, we consider the inference of GRNs as digraphs, where the nodes correspond to the genes and the directed edges represent the gene regulatory interactions. An edge 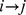 implies that the expression of gene
implies that the expression of gene  influences the expression of gene
influences the expression of gene 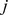 . In the following, the GRN of interest is denoted by the digraph
. In the following, the GRN of interest is denoted by the digraph 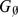 . For any set of genes
. For any set of genes 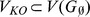 , we use
, we use 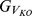 to denote a subgraph of
to denote a subgraph of 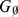 that results from removing all edges incident to the genes in the set
that results from removing all edges incident to the genes in the set 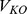 ,
, 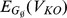 . In other words,
. In other words, 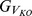 is the digraph with
is the digraph with 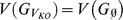 and
and 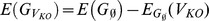 . For example,
. For example, 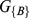 associated with
associated with 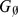 in Fig. 1(a) is the graph with all edges incident to gene
in Fig. 1(a) is the graph with all edges incident to gene  removed, as shown in Fig. 1(d). Here, we interchangeably use the notations for a graph
removed, as shown in Fig. 1(d). Here, we interchangeably use the notations for a graph 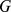 and its adjacency matrix
and its adjacency matrix 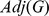 .
.
Gene KO experiments are commonly performed for the purpose of GRN inference. In these experiments, the resulting data typically consist of gene expression profiles taken after the effects of the gene perturbation have reached steady state. While temporal gene expressions are increasingly measured, here we focus on using more commonly available steady-state expression data. The treatments of time-series measurements and observational data are left to future publications. Many network inference algorithms have been developed for using data of gene KOs [7], [9], and most of these algorithms produce a single network prediction. In contrast, an ensemble inference strategy is adopted in this work.
In order to illustrate the limitation of using steady-state gene expression data for GRN inference, we consider a GRN 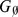 described by the graph in Fig. 1(a). Here, KO of gene
described by the graph in Fig. 1(a). Here, KO of gene 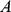 is expected to cause changes in the expression of genes
is expected to cause changes in the expression of genes 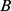 ,
,  ,
, 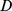 and
and  at steady state, even though
at steady state, even though 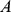 directly regulates only
directly regulates only 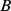 and
and 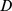 . This simple illustration demonstrates that we cannot in principle discriminate direct and indirect gene regulations from steady-state gene KO expression data [19]. In general, genes that are differentially expressed upon knocking out gene
. This simple illustration demonstrates that we cannot in principle discriminate direct and indirect gene regulations from steady-state gene KO expression data [19]. In general, genes that are differentially expressed upon knocking out gene  in the GRN correspond to those that are directly and indirectly regulated by gene
in the GRN correspond to those that are directly and indirectly regulated by gene  , i.e. vertices in
, i.e. vertices in 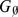 that are accessible from the vertex
that are accessible from the vertex  . Motivated by such a limitation, in TRaCE we first convert gene KO data into gene accessibility lists or matrices. As the minimum input, TRaCE requires the complete dataset of single-gene KO experiments, from which one can construct the accessibility matrix
. Motivated by such a limitation, in TRaCE we first convert gene KO data into gene accessibility lists or matrices. As the minimum input, TRaCE requires the complete dataset of single-gene KO experiments, from which one can construct the accessibility matrix 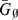 . More specifically, the
. More specifically, the 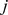 -th element in the
-th element in the  -th row of
-th row of 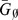 (i.e.
(i.e. 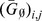 ) is set to 1 when gene
) is set to 1 when gene 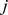 is differentially expressed in the KO experiment of gene
is differentially expressed in the KO experiment of gene  . The other elements of
. The other elements of 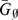 are set to
are set to  . The detailed procedure of differential expression analysis adopted in this work is described in the Numerical Implementation section.
. The detailed procedure of differential expression analysis adopted in this work is described in the Numerical Implementation section.
For data of multi-gene KO experiments, we consider the accessibility matrix of 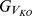 for an appropriately chosen set of genes
for an appropriately chosen set of genes 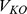 . In principle, we can determine
. In principle, we can determine 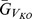 from the complete set of experiments involving KOs of the genes in the set
from the complete set of experiments involving KOs of the genes in the set 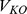 and an additional gene
and an additional gene  for all
for all 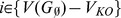 . These experiments are equivalent to performing single-gene KOs of the GRN
. These experiments are equivalent to performing single-gene KOs of the GRN 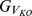 , and therefore
, and therefore  can be obtained by following the same procedure as that for
can be obtained by following the same procedure as that for 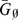 above. As an illustration, consider the GRN in Fig. 1(a) with
above. As an illustration, consider the GRN in Fig. 1(a) with  . The graph
. The graph  is given in Fig. 1(d). In this case, we can construct the accessibility matrix
is given in Fig. 1(d). In this case, we can construct the accessibility matrix 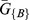 from the data of two-gene KO experiments, namely
from the data of two-gene KO experiments, namely 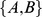 ,
, 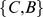 ,
, 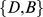 and
and 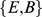 KOs. As these experiments differ from each other in only one gene while sharing the KO of gene
KOs. As these experiments differ from each other in only one gene while sharing the KO of gene 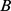 , the differential expression analysis of the data thus correspond to changes in the expression of the GRN
, the differential expression analysis of the data thus correspond to changes in the expression of the GRN 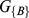 caused by a single-gene KO. Consequently, in this analysis, genes that are found to be differentially expressed in the KO of
caused by a single-gene KO. Consequently, in this analysis, genes that are found to be differentially expressed in the KO of 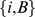 are those that are accessible from gene
are those that are accessible from gene  (
(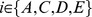 ) in the graph
) in the graph 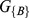 . For example, the KO of
. For example, the KO of 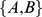 is expected to cause differential expression in genes
is expected to cause differential expression in genes 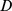 and
and 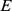 . The full accessibility matrix of
. The full accessibility matrix of 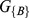 is illustrated by the digraph in Fig. 1(e).
is illustrated by the digraph in Fig. 1(e).
We can generalize the simple example above to any set of genes 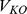 that could be derived from the available multi-gene KO experiments. More specifically, we set
that could be derived from the available multi-gene KO experiments. More specifically, we set 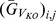 to 1 when knocking-out
to 1 when knocking-out 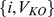 leads to a differential expression of gene
leads to a differential expression of gene 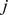 with respect to its expression level in
with respect to its expression level in 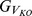 . The remaining elements of
. The remaining elements of 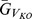 are set to 0. Unfortunately, the construction of
are set to 0. Unfortunately, the construction of 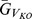 of a large GRN
of a large GRN  would proportionally require a high number of KO experiments (the number of KO experiments is
would proportionally require a high number of KO experiments (the number of KO experiments is  , where
, where  and
and  is the number of genes in
is the number of genes in 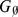 and
and  , respectively). However, when
, respectively). However, when 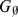 is sparse,
is sparse, 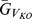 differs from
differs from  for only a few elements and importantly, these elements can be determined from
for only a few elements and importantly, these elements can be determined from 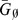 (see the next section).
(see the next section).
In the theoretical development below, we assume that the accessibility matrices 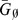 and
and 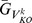 for
for 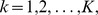 have already been obtained from the expression data. Here,
have already been obtained from the expression data. Here, 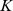 denotes the total number of accessibility matrices involving subgraphs of the GRN
denotes the total number of accessibility matrices involving subgraphs of the GRN 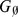 that can be constructed from data. For example, from the dataset of the complete double-gene KO experiments, we can obtain the accessibility matrix
that can be constructed from data. For example, from the dataset of the complete double-gene KO experiments, we can obtain the accessibility matrix 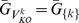 for
for 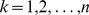 (here,
(here, 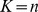 ). In TRaCE, we consider the ensemble containing all digraphs that are consistent with the accessibility matrices
). In TRaCE, we consider the ensemble containing all digraphs that are consistent with the accessibility matrices 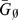 and
and 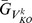 's, which is the set:
's, which is the set:
| (1) |
where 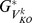 is the digraph with
is the digraph with  and
and 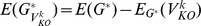 . Note that the GRN
. Note that the GRN 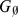 is a member of the ensemble
is a member of the ensemble 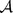 . The size of the ensemble is a direct measure of uncertainty in the network inference problem. A GRN is therefore deemed inferable when the ensemble only contains a single (unique) network.
. The size of the ensemble is a direct measure of uncertainty in the network inference problem. A GRN is therefore deemed inferable when the ensemble only contains a single (unique) network.
As the number of digraphs in the ensemble is often very large, in TRaCE we generate only the lower and upper bounds of the ensemble, denoted by 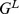 and
and 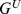 , respectively. The bounds are defined such that each digraph in the ensemble is a supergraph of
, respectively. The bounds are defined such that each digraph in the ensemble is a supergraph of  and a subgraph of
and a subgraph of 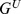 . For GRNs without any cycle (i.e. DAGs), the lower and upper bound GRNs can be obtained from the accessibility matrices of
. For GRNs without any cycle (i.e. DAGs), the lower and upper bound GRNs can be obtained from the accessibility matrices of  and
and 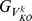 's (i.e.
's (i.e. 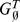 and
and  's) and their transitive reductions (i.e.
's) and their transitive reductions (i.e. 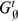 and
and  's), using the following equations (for details see the Numerical Implementation section):
's), using the following equations (for details see the Numerical Implementation section):
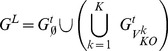 |
(2) |
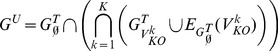 |
(3) |
where 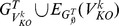 denotes the digraph with vertices
denotes the digraph with vertices 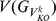 and edges
and edges 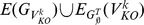 . Without any
. Without any 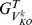 , the upper bound of the ensemble is simply given by the accessibility matrix
, the upper bound of the ensemble is simply given by the accessibility matrix 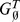 and the lower bound is the transitive reduction
and the lower bound is the transitive reduction  . As
. As 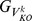 is a subgraph of
is a subgraph of 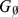 , the transitive closure
, the transitive closure 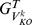 is also a subgraph of
is also a subgraph of 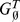 . In Eq. (3), the upper bound is constructed starting from
. In Eq. (3), the upper bound is constructed starting from  in which edges are removed based on
in which edges are removed based on 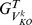 . Here, edges incident to
. Here, edges incident to 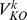 are not altered during the intersection of the accessibility matrix
are not altered during the intersection of the accessibility matrix 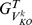 . For example, consider again the GRN in Fig. 1(a) with the accessibility matrices
. For example, consider again the GRN in Fig. 1(a) with the accessibility matrices  and
and 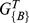 in Figs. 1(b) and 1(e). The resulting upper bound
in Figs. 1(b) and 1(e). The resulting upper bound 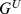 from the combination of these accessibility matrices will have one fewer edge than
from the combination of these accessibility matrices will have one fewer edge than 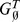 , which is the edge
, which is the edge 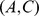 (see Fig. 1(f)). Thus, the size of the upper bound is generally reduced with the incorporation of
(see Fig. 1(f)). Thus, the size of the upper bound is generally reduced with the incorporation of 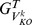 's. On the other hand, according to Eq. (2), the lower bound becomes larger with the inclusion of every available
's. On the other hand, according to Eq. (2), the lower bound becomes larger with the inclusion of every available  . In the same example above, the transitive reduction of
. In the same example above, the transitive reduction of 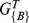 happens to be the graph
happens to be the graph 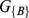 (i.e. in this case
(i.e. in this case 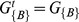 ). Here, the combination of
). Here, the combination of 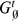 and
and 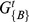 in Figs. 1(c) and 1(d), respectively, gives the lower bound
in Figs. 1(c) and 1(d), respectively, gives the lower bound 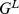 that is equal to
that is equal to 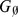 . However, in general,
. However, in general, 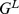 is a subgraph of
is a subgraph of 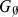 .
.
Theorem 1 establishes 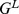 and
and 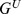 in Eqs. (2)-(3) as valid lower and upper bounds of the set
in Eqs. (2)-(3) as valid lower and upper bounds of the set 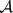 for DAGs.
for DAGs.
Theorem 1: For 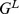 and
and  described in Eqs. (2)-(3), the following relationship applies:
described in Eqs. (2)-(3), the following relationship applies:
Proof of
 For any edge
For any edge 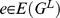 , Eq. (2) implies that either
, Eq. (2) implies that either 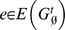 or
or 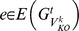 . Therefore, we have either:
. Therefore, we have either:
 , or
, or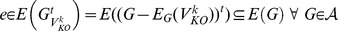 .
. 
Proof of
 If
If 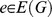 for some
for some  , then
, then 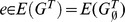 . In addition, this edge satisfies either:
. In addition, this edge satisfies either:
Therefore, 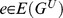 .
. 
Remark: Since 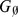 is a member of
is a member of 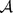 ,
, 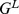 and
and 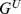 can also be thought as the lower and upper bounds of
can also be thought as the lower and upper bounds of  , i.e.
, i.e. 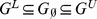 . For DAGs, the members of the set
. For DAGs, the members of the set  can be obtained by combinatorially adding edges in the set
can be obtained by combinatorially adding edges in the set 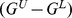 to
to 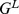 . Therefore, the dimension of
. Therefore, the dimension of  is equal to
is equal to 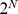 where
where 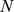 is the difference between the number of edges in
is the difference between the number of edges in 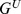 and
and 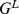 . Finally, Theorem 1 guarantees that when
. Finally, Theorem 1 guarantees that when 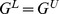 ,
, 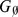 is fully identifiable, i.e.
is fully identifiable, i.e. 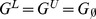 .
.
For digraphs with cycles, the upper bound can still be constructed using Eq. 3 with 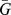 replacing
replacing 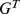 . In this more general case, the relationship
. In this more general case, the relationship 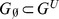 in Theorem 1 is still valid. However, as mentioned earlier, the transitive reduction of digraphs with cycles is not unique. In a previous publication [19], Wagner proposed a procedure in which digraphs are first condensed into DAGs before constructing the transitive reduction [19]. Similarly, in TRaCE, each input accessibility matrix is first condensed and the transitive reduction algorithm is subsequently applied to the DAG of the strong components. Here, edges incident to the condensations of cycles are also removed. Afterwards, the transitive reduction graph is expanded, reversing the condensation step. Except for cycles involving two nodes, edges of any directed cycle cannot be uniquely prescribed and are therefore pruned. The above procedure for reducing digraphs with cycles is referred to as Condensation, Transitive Reduction and Expansion (ConTREx). The ConTREx of an accessibility matrix
in Theorem 1 is still valid. However, as mentioned earlier, the transitive reduction of digraphs with cycles is not unique. In a previous publication [19], Wagner proposed a procedure in which digraphs are first condensed into DAGs before constructing the transitive reduction [19]. Similarly, in TRaCE, each input accessibility matrix is first condensed and the transitive reduction algorithm is subsequently applied to the DAG of the strong components. Here, edges incident to the condensations of cycles are also removed. Afterwards, the transitive reduction graph is expanded, reversing the condensation step. Except for cycles involving two nodes, edges of any directed cycle cannot be uniquely prescribed and are therefore pruned. The above procedure for reducing digraphs with cycles is referred to as Condensation, Transitive Reduction and Expansion (ConTREx). The ConTREx of an accessibility matrix  , denoted by
, denoted by 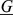 , may no longer be a valid transitive reduction (i.e.
, may no longer be a valid transitive reduction (i.e. 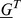 may not necessarily be equal to
may not necessarily be equal to 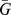 ). Nonetheless, the lower bound constructed using Eq. (2) with
). Nonetheless, the lower bound constructed using Eq. (2) with 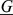 's replacing
's replacing  's, satisfies
's, satisfies 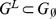 . The proof of this relationship is analogous to the one presented for Theorem 1. However,
. The proof of this relationship is analogous to the one presented for Theorem 1. However, 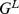 may not be a member of
may not be a member of 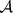 (see Fig. S1). Finally, the enumeration of digraphs with cycles from
(see Fig. S1). Finally, the enumeration of digraphs with cycles from 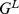 and
and 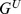 is more complicated than that for DAGs. The main difference is in the generation of all possible cycles among nodes belonging to a particular strong component, constrained by
is more complicated than that for DAGs. The main difference is in the generation of all possible cycles among nodes belonging to a particular strong component, constrained by 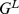 and
and 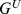 (see an example in Fig. S2).
(see an example in Fig. S2).
Error Correction and Filter
In practice, the accessibility matrices constructed from data contain errors. Some elements of the accessibility matrices maybe identified as 1 when they should be 0 (i.e. false positive, FP), and some maybe identified as 0 when they should be 1 (i.e. false negative, FN). These errors can affect the lower and upper bound constructed by Eqs. (2)-(3). We denote the erroneous lower and upper bound by 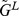 and
and 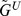 , respectively. In this case, neither
, respectively. In this case, neither 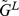 is guaranteed to be a subgraph of
is guaranteed to be a subgraph of  , nor
, nor 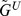 a supergraph of
a supergraph of  and
and 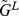 .
.
There are several types of errors affecting 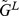 and
and 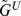 . In the first case (Type A error), an edge which is not present in
. In the first case (Type A error), an edge which is not present in 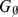 (
(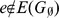 ) erroneously appears in
) erroneously appears in 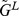 and
and  (
(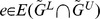 ). Or, an edge in
). Or, an edge in 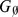 (
(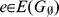 ) is missing from both
) is missing from both 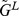 and
and 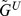 (
(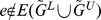 ). As such error affects both
). As such error affects both 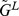 and
and 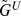 in the same manner, this error is not detectable from
in the same manner, this error is not detectable from  and
and 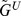 . The second case (Type B error) involves either a FP in the accessibility matrix or a FN in the ConTREx matrix. In this case, the resulting bounds are still consistent with each other and are still valid for
. The second case (Type B error) involves either a FP in the accessibility matrix or a FN in the ConTREx matrix. In this case, the resulting bounds are still consistent with each other and are still valid for  . However, the ensemble size and the network uncertainty increase due to this error. In the third case (Type C error), an edge erroneously appears only in
. However, the ensemble size and the network uncertainty increase due to this error. In the third case (Type C error), an edge erroneously appears only in 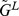 , or vice versa, an edge is errorneously missing only from
, or vice versa, an edge is errorneously missing only from 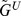 (
(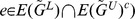 , where
, where  denote the complement of a set). Here, the bounds become inconsistent with each other (i.e.,
denote the complement of a set). Here, the bounds become inconsistent with each other (i.e., 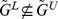 ). Thus, we refer such errors as inconsistent edges, which can be identified by searching for edges belonging to
). Thus, we refer such errors as inconsistent edges, which can be identified by searching for edges belonging to 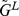 that are not in
that are not in  (i.e., from
(i.e., from 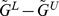 ). Table 1 illustrates the three types of errors mentioned above.
). Table 1 illustrates the three types of errors mentioned above.
Table 1. Types of Errors in 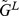 and
and 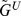 .
.
| Error | ||||
| Type A | Type B | Type C | ||
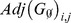
|
0 | 1 | 0 or 1 | 0 or 1 |
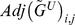
|
1 | 0 | 1 | 0 |
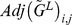
|
1 | 0 | 0 | 1 |
A closer scrutiny of Eqs. (2)-(3) reveals that errors from the input accessibility matrices are passed on and compounded in the bounds. For example, FN errors in 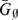 or any
or any 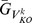 will end up in
will end up in  , while FPs in
, while FPs in  and any
and any  will also appear in
will also appear in  . In order to reduce the transmission of errors, we have developed a filter such that only a subset of edges of
. In order to reduce the transmission of errors, we have developed a filter such that only a subset of edges of 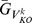 are used for the construction of
are used for the construction of 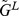 and
and  . The filter is based on the concept of testable edges. Specifically, the testable edges of
. The filter is based on the concept of testable edges. Specifically, the testable edges of 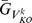 are any edge
are any edge 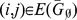 with
with  , such that there exists a directed path from
, such that there exists a directed path from  to
to 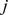 involving one or more genes in
involving one or more genes in 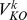 . As directed paths involving genes in
. As directed paths involving genes in 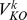 are disconnected in
are disconnected in 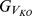 , we can potentially verify the existence of the testable edges from
, we can potentially verify the existence of the testable edges from 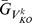 and
and 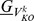 . For example, the existence of the edge
. For example, the existence of the edge 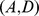 in Fig. 1(a) can be verified using the transitive reduction of
in Fig. 1(a) can be verified using the transitive reduction of 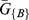 , which is the graph shown in Fig. 1(d). Meanwhile, we can establish the absence of the edge
, which is the graph shown in Fig. 1(d). Meanwhile, we can establish the absence of the edge 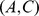 in
in 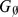 using the accessibility matrix
using the accessibility matrix  (see Fig. 1(e)). The number of testable edges can also be used to estimate the information content of a
(see Fig. 1(e)). The number of testable edges can also be used to estimate the information content of a 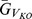 , where a higher number of testable edges indicates more informative
, where a higher number of testable edges indicates more informative 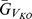 .
.
For a given  , it is straightforward to show that the testable edges are the non-zero entries of the testability matrix:
, it is straightforward to show that the testable edges are the non-zero entries of the testability matrix:
| (4) |
where 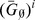 and
and 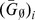 are the i-th column and i-th row of
are the i-th column and i-th row of 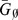 , respectively, and
, respectively, and  denotes the outer product. During the construction of the lower and upper bound GRNs, the incorporation of each
denotes the outer product. During the construction of the lower and upper bound GRNs, the incorporation of each 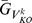 will only need to be performed for the associated testable edges, i.e. non-zero elements of
will only need to be performed for the associated testable edges, i.e. non-zero elements of 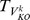 . Moreover, as the number of testable edges corresponding to
. Moreover, as the number of testable edges corresponding to 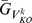 is typically small and as such edges can be determined from
is typically small and as such edges can be determined from 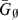 , only a few rows of
, only a few rows of 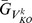 need to be determined from data, i.e. rows of
need to be determined from data, i.e. rows of 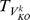 with non-zero entries. Thus, the number of KO experiments for constructing
with non-zero entries. Thus, the number of KO experiments for constructing 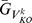 could be relaxed by considering only testable edges.
could be relaxed by considering only testable edges.
Numerical Implementation
The pseudo-codes and the MATLAB implementations of TRaCE are provided in the supporting material and the following website (http://www.cabsel.ethz.ch/tools/trace). Given steady-state gene expression data, we first group the data into datasets according to the KO experiments required for the construction of the accessibility matrices. We perform differential expression analysis for each dataset using Z-score transformation and obtain the corresponding accessibility matrix. We provide two implementations of TRaCE, one with and another without error correction. TRaCE without error correction should only be applied when the input accessibility matrices are error free (e.g. for inferability analysis). In any other scenario, TRaCE with error correction should be used. If desired, a ranked list of gene regulatory predictions can also be generated using the lower and upper bounds of the ensemble and the differential expression analysis.
Constructing Accessibility Matrices from Expression Data
In the case studies, we have employed the Z-score transformation for differential gene expression analysis [21]. Without loss of generality, we describe below the procedure for constructing  from the complete set of single-gene KOs of
from the complete set of single-gene KOs of 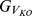 , i.e. all possible combinations of
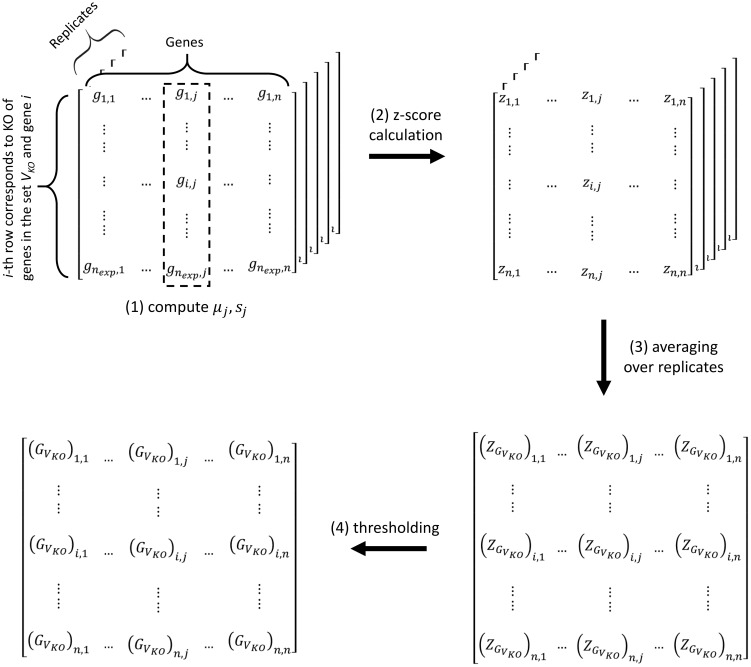
, i.e. all possible combinations of 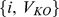 genes KOs. The gene expression dataset is organized into a matrix in which the rows correspond to the experiments and the columns correspond to the genes. Technical replicates are arranged into separate data matrices. For microarray data, the gene expression is typically represented by log-10 transformed fluorescence intensity data. The following procedure is also illustrated in Fig. 2.
genes KOs. The gene expression dataset is organized into a matrix in which the rows correspond to the experiments and the columns correspond to the genes. Technical replicates are arranged into separate data matrices. For microarray data, the gene expression is typically represented by log-10 transformed fluorescence intensity data. The following procedure is also illustrated in Fig. 2.
Figure 2. Construction of accessibility matrix  from expression data.
from expression data.
The data come from KOs of genes in the set 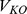 and an additional gene
and an additional gene  ,
, 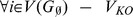 . For each replicate, the expression data are arranged into a matrix where the rows correspond to the experiments and the columns correspond to the genes. (1) The sample mean and standard deviation of the expression of gene
. For each replicate, the expression data are arranged into a matrix where the rows correspond to the experiments and the columns correspond to the genes. (1) The sample mean and standard deviation of the expression of gene 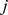 , denoted by
, denoted by 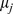 and
and 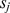 , respectively, are obtained using the expression data in the
, respectively, are obtained using the expression data in the 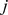 -th column of the data matrix. (2) For each replicate, a z-score matrix is computed according to Eq. (5). (3) Subsequently, the z-score matrices are averaged over the technical replicates to give
-th column of the data matrix. (2) For each replicate, a z-score matrix is computed according to Eq. (5). (3) Subsequently, the z-score matrices are averaged over the technical replicates to give 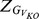 . (4) The accessibility matrix
. (4) The accessibility matrix  is determined from
is determined from 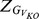 based on a threshold criterion in Eq. (6).
based on a threshold criterion in Eq. (6).
We first obtain the sample mean
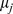 and standard deviation
and standard deviation 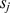 of the expression of each gene
of the expression of each gene 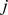 in the dataset. More specifically, for each technical replicate, we calculate the sample mean and standard deviation of the
in the dataset. More specifically, for each technical replicate, we calculate the sample mean and standard deviation of the 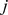 -th column in the data matrix. Then, we identify expressions that differ from the mean by more than a specified multiple
-th column in the data matrix. Then, we identify expressions that differ from the mean by more than a specified multiple 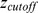 of the standard deviation. We subsequently recompute the sample mean and standard deviation
of the standard deviation. We subsequently recompute the sample mean and standard deviation  and
and 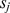 by excluding the data beyond
by excluding the data beyond  . When available, we also use the expression data from the KO experiment of genes
. When available, we also use the expression data from the KO experiment of genes 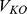 in calculating
in calculating 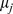 and
and 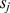 .
.-
For each replicate, we compute a z-score matrix
 for
for  according to [21]
according to [21]
where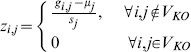
(5) 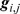 is the expression level of gene
is the expression level of gene 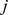 associated with knocking out gene
associated with knocking out gene  and genes in
and genes in  . These z-scores reflect the significance of changes in the gene expression with respect to the GRN
. These z-scores reflect the significance of changes in the gene expression with respect to the GRN 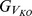 .
. Subsequently, we average the z-score matrices over the technical replicates, producing the overall z-score matrix
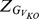 .
.- We determine the accessibility matrix
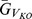 from
from 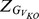 using a threshold, as follows:
using a threshold, as follows: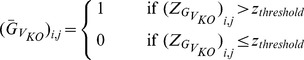
(6)
In our experience, 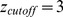 and
and 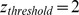 provide reliable network ensembles. In general, choosing higher
provide reliable network ensembles. In general, choosing higher 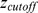 and
and 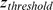 will lead to fewer FPs but more FNs in the accessibility matrix. For the GRN examples considered in this work, the performance of TRaCE does not vary considerably within the selected ranges of
will lead to fewer FPs but more FNs in the accessibility matrix. For the GRN examples considered in this work, the performance of TRaCE does not vary considerably within the selected ranges of  between 1.5 and 2.5 and
between 1.5 and 2.5 and 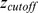 between 2 and 3 (see Results).
between 2 and 3 (see Results).
TRaCE without error correction
TRaCE without error correction is implemented as matrix-operations of Eqs. (2) and (3). Briefly, the upper bound 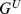 is constructed by performing Hadamard (element wise) multiplications of the accessibility matrices, excluding the rows and columns corresponding to genes in
is constructed by performing Hadamard (element wise) multiplications of the accessibility matrices, excluding the rows and columns corresponding to genes in 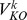 . On the other hand, the transitive reduction is based on the algorithm by Wagner [19], which has been re-implemented using matrix operations. When there is no cycle in
. On the other hand, the transitive reduction is based on the algorithm by Wagner [19], which has been re-implemented using matrix operations. When there is no cycle in 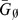 and
and 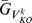 's, the transitive reduction algorithm is applied to each accessibility matrix and the construction of
's, the transitive reduction algorithm is applied to each accessibility matrix and the construction of 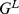 is done by binary additions of the transitive reductions, following Eq. (2). Cycles and genes involved in cycles can be detected from entries of
is done by binary additions of the transitive reductions, following Eq. (2). Cycles and genes involved in cycles can be detected from entries of  [22]. For GRNs with cycles, the ConTREx procedure is applied to each available accessibility matrix, and the resulting
[22]. For GRNs with cycles, the ConTREx procedure is applied to each available accessibility matrix, and the resulting 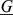 matrices are again combined using binary additions to produce
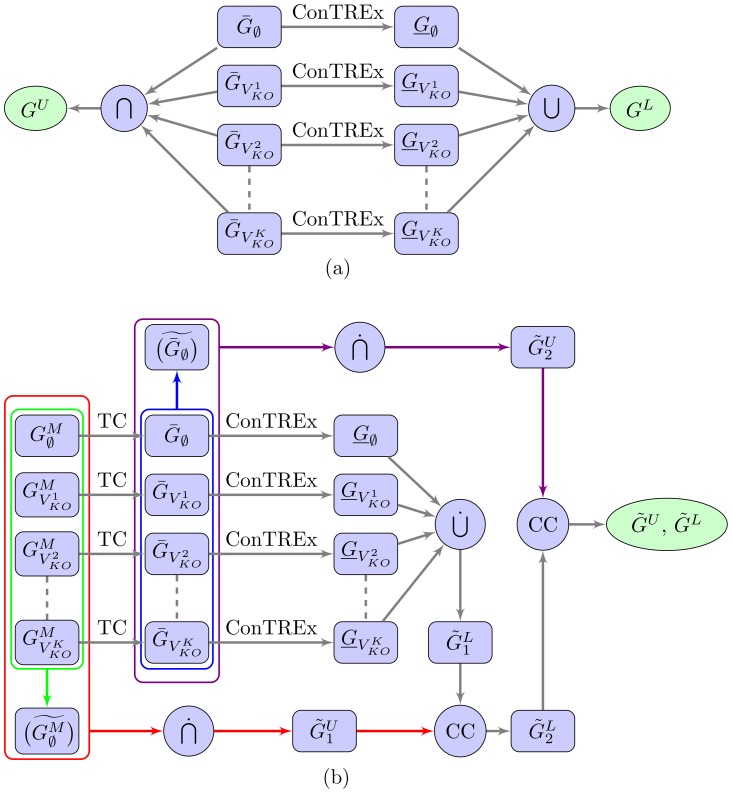
matrices are again combined using binary additions to produce 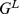 . The schematic diagram of the error-free implementation is shown in Fig. 3(a).
. The schematic diagram of the error-free implementation is shown in Fig. 3(a).
Figure 3. Schematic diagrams of TRaCE with and without error correction.
(a) Construction of the lower bound 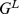 and upper bound
and upper bound 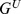 from
from 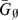 and
and  's using TRaCE without error correction. Expression data from gene KO experiments are first converted into accessibility matrices. ConTREx is then applied to each accessibility matrix, removing feed-forward edges and edges incident to vertices belonging cycles with more than 2 nodes. The upper bound is constructed by taking the intersection of the accessibility matrices, while the lower bound is constructed by taking the union of the ConTREx outputs. (b) Construction of the lower bound
's using TRaCE without error correction. Expression data from gene KO experiments are first converted into accessibility matrices. ConTREx is then applied to each accessibility matrix, removing feed-forward edges and edges incident to vertices belonging cycles with more than 2 nodes. The upper bound is constructed by taking the intersection of the accessibility matrices, while the lower bound is constructed by taking the union of the ConTREx outputs. (b) Construction of the lower bound  and upper bound
and upper bound 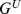 from
from  and
and 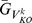 's using TRaCE with error correction. Expression data from gene KO experiments are converted into accessibility matrices
's using TRaCE with error correction. Expression data from gene KO experiments are converted into accessibility matrices 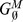 and
and 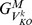 's, where the superscript
's, where the superscript 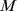 indicates that these matrices may not be transitive due to noise in the measured gene expression levels. Subsequently, the transitive closures of
indicates that these matrices may not be transitive due to noise in the measured gene expression levels. Subsequently, the transitive closures of 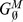 and
and 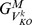 's are created, denoted respectively by
's are created, denoted respectively by  and
and 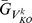 's, and the ConTREx of these closures are evaluated. TRaCE with error correction begins with the preprocessing of
's, and the ConTREx of these closures are evaluated. TRaCE with error correction begins with the preprocessing of  's and
's and 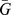 's to produce the corrected matrices
's to produce the corrected matrices 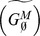 and
and 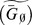 , which are required to determine testable edges. For the construction of the lower and upper bounds, the union and intersection of matrices are performed with filtering, denoted by
, which are required to determine testable edges. For the construction of the lower and upper bounds, the union and intersection of matrices are performed with filtering, denoted by  and
and 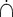 , respectively, where only the relevant testable edges are updated. Two candidate upper bounds are obtained, the first from the matrices
, respectively, where only the relevant testable edges are updated. Two candidate upper bounds are obtained, the first from the matrices 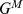 's, denoted by
's, denoted by  , and the second from the matrices
, and the second from the matrices 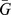 's, denoted by
's, denoted by  . Meanwhile, the initial lower bound estimate, denoted by
. Meanwhile, the initial lower bound estimate, denoted by 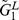 , is obtained from the ConTREx matrices. The consistency check (CC) is first applied to the pair
, is obtained from the ConTREx matrices. The consistency check (CC) is first applied to the pair 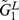 and
and 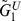 to produce the corrected lower bound
to produce the corrected lower bound 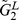 , and then to the pair
, and then to the pair 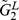 and
and 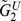 to produce the final estimates of the bounds
to produce the final estimates of the bounds 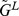 and
and 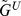 . More detailed descriptions of the filtering and consistency check can be found in supporting material (text S1 and text S2).
. More detailed descriptions of the filtering and consistency check can be found in supporting material (text S1 and text S2).
TRaCE with error correction
The procedure for TRaCE with error correction is illustrated in Fig. 3(b). There are two main steps in this procedure: (1) the construction of lower and upper bounds with filtering and (2) the correction of inconsistent edges. The first main step refers to an implementation of Eqs. (2) and (3) in which the intersection and union operations involving  are performed only for testable edges associated with non-zero entries of
are performed only for testable edges associated with non-zero entries of  . As testable edges are determined from
. As testable edges are determined from 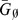 , a pre-processing step is performed to reduce errors in
, a pre-processing step is performed to reduce errors in 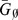 . The premise behind the pre-processing step is that an error unlikely affects the same edge, and that testable edges of any
. The premise behind the pre-processing step is that an error unlikely affects the same edge, and that testable edges of any 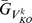 constitute only a small subset of edges in
constitute only a small subset of edges in 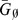 (i.e. the network is sparse). Following this premise, edges that appear in a majority of the accessibility matrices (above a certain threshold) are kept, but are otherwise removed. In our experience, a threshold of 65% gives a good and reliable performance, but any value between 50% to 80% works quite well in the case studies (see Results). A more detailed description of the pre-processing method can be found in text S1, while the filtering algorithm is provided in text S2.
(i.e. the network is sparse). Following this premise, edges that appear in a majority of the accessibility matrices (above a certain threshold) are kept, but are otherwise removed. In our experience, a threshold of 65% gives a good and reliable performance, but any value between 50% to 80% works quite well in the case studies (see Results). A more detailed description of the pre-processing method can be found in text S1, while the filtering algorithm is provided in text S2.
The schematic diagram of TRaCE with error correction is given in Fig. 3(b). We consider two sets of accessibility matrices; the first set comes from differential expression analysis (based on 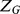 's) and the second set comes from the transitive closure of the first set. We create the second set of matrices since the accessibility matrices identified from differential expressions may not satisfy the transitivity condition due to errors. The pre-processing step above is applied to both sets of matrices. Subsequently, two candidate upper bounds are generated using TRaCE with filtering. The upper bound obtained from the first set of accessibility matrices, denoted by
's) and the second set comes from the transitive closure of the first set. We create the second set of matrices since the accessibility matrices identified from differential expressions may not satisfy the transitivity condition due to errors. The pre-processing step above is applied to both sets of matrices. Subsequently, two candidate upper bounds are generated using TRaCE with filtering. The upper bound obtained from the first set of accessibility matrices, denoted by  , is expectedly smaller (in size) than the bound from the second set, denoted by
, is expectedly smaller (in size) than the bound from the second set, denoted by 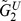 . Note that ConTREx is only applicable to transitive digraphs, and therefore is applied only to the transitive closures (i.e. the second set). Using TRaCE with filtering, a candidate lower bound, denoted by
. Note that ConTREx is only applicable to transitive digraphs, and therefore is applied only to the transitive closures (i.e. the second set). Using TRaCE with filtering, a candidate lower bound, denoted by  , is generated from the results of ConTREx.
, is generated from the results of ConTREx.
The last step in the procedure is to correct inconsistent edges, which is done by voting. For each inconsistent edge, we compared the number of times that the edge is present in the accessibility matrices and the ConTREx results (supporting the presence of the edge), with the number of times that the edge is absent from the accessibility and ConTREx matrices (supporting the absence of the edge). The upper bound is corrected (by addition of this edge) when the presence of the edge receives a (simple) majority vote. Vice versa, the lower bound is corrected (by removal of this edge) when the absence of the edge receives a majority vote. In the case of no majority vote, the edge is added to the upper bound and removed from the lower bound. The detail of the consistency check is described in text S3. As shown in Fig. 3(b), the consistency check and correction are first performed for the pair 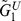 and
and 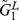 , and subsequently the corrected lower bound, denoted by
, and subsequently the corrected lower bound, denoted by 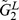 , is compared with
, is compared with 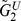 to obtain the final corrected
to obtain the final corrected 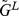 and
and 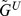 .
.
Ranking of Edges from Ensemble Bounds
If desired, a ranked list of edges can be generated using the lower and upper bounds of TRaCE in conjunction with the average z-scores for 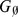 , i.e.
, i.e. 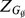 . Here, we carry out the ranking of regulatory edges in two phases. In the first phase, we rank subsets of edges according to the lower and upper bounds in the following order: edges in
. Here, we carry out the ranking of regulatory edges in two phases. In the first phase, we rank subsets of edges according to the lower and upper bounds in the following order: edges in 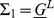 , edges in
, edges in 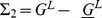 , edges in
, edges in 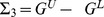 , edges in
, edges in 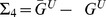 , and finally edges in
, and finally edges in 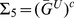 . In the second phase, we rank the edges within individual subsets according to the average z-scores. We implement the second phase by first computing the overall scores
. In the second phase, we rank the edges within individual subsets according to the average z-scores. We implement the second phase by first computing the overall scores 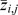 according to
according to
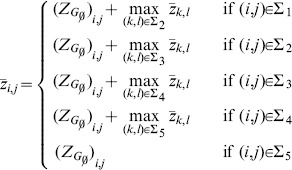 |
(7) |
Following the submission requirement of DREAM 4 network inference challenge, we then assign a confidence score 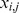 to the edge
to the edge 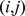 according to:
according to:
| (8) |
A score 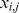 of 1 reflects the highest confidence of the existence of an edge
of 1 reflects the highest confidence of the existence of an edge 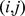 , and vice versa a zero confidence score indicates certainty in the inexistence of
, and vice versa a zero confidence score indicates certainty in the inexistence of 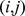 . Finally, the ranked list of edges is generated by sorting the edges in decreasing order of confidence scores. A similar procedure, called down-ranking, has been presented in Pinna et al. [23], where feed-forward edges are ranked lower than edges in the transitive reduction of the accessibility matrix. However, the down-ranking algorithm is described only for data of single-gene KO experiments.
. Finally, the ranked list of edges is generated by sorting the edges in decreasing order of confidence scores. A similar procedure, called down-ranking, has been presented in Pinna et al. [23], where feed-forward edges are ranked lower than edges in the transitive reduction of the accessibility matrix. However, the down-ranking algorithm is described only for data of single-gene KO experiments.
Results
Inferability Analysis
We first applied TRaCE to error-free accessibility matrices of 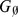 and
and 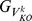 by assuming ideal data (unbiased and error free) for the purpose of inferability analysis. Such an analysis is analogous to a priori identifiability analysis in the kinetic modeling of biological networks [12]. Here, we evaluated the network distances between the lower and upper bounds and the GRN, i.e. the numbers of edges in the set
by assuming ideal data (unbiased and error free) for the purpose of inferability analysis. Such an analysis is analogous to a priori identifiability analysis in the kinetic modeling of biological networks [12]. Here, we evaluated the network distances between the lower and upper bounds and the GRN, i.e. the numbers of edges in the set 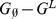 and
and 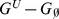 , respectively.
, respectively.
Random GRNs
We investigated the inferability of random GRNs of orders 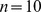 and
and 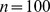 genes. We set the network size (i.e. number of edges) between
genes. We set the network size (i.e. number of edges) between  and
and  randomly with equal probability, and assigned the edges without any preference. The upper size limit of
randomly with equal probability, and assigned the edges without any preference. The upper size limit of 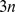 was chosen based on the ratio between the number of edges and the number of nodes in E. coli and yeast GRNs [24]. For each random network, we generated
was chosen based on the ratio between the number of edges and the number of nodes in E. coli and yeast GRNs [24]. For each random network, we generated 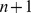 accessibility matrices associated with
accessibility matrices associated with 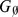 and
and 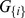 for every
for every 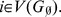 These accessibility matrices correspond to performing the full set of single- and double-gene KO experiments.
These accessibility matrices correspond to performing the full set of single- and double-gene KO experiments.
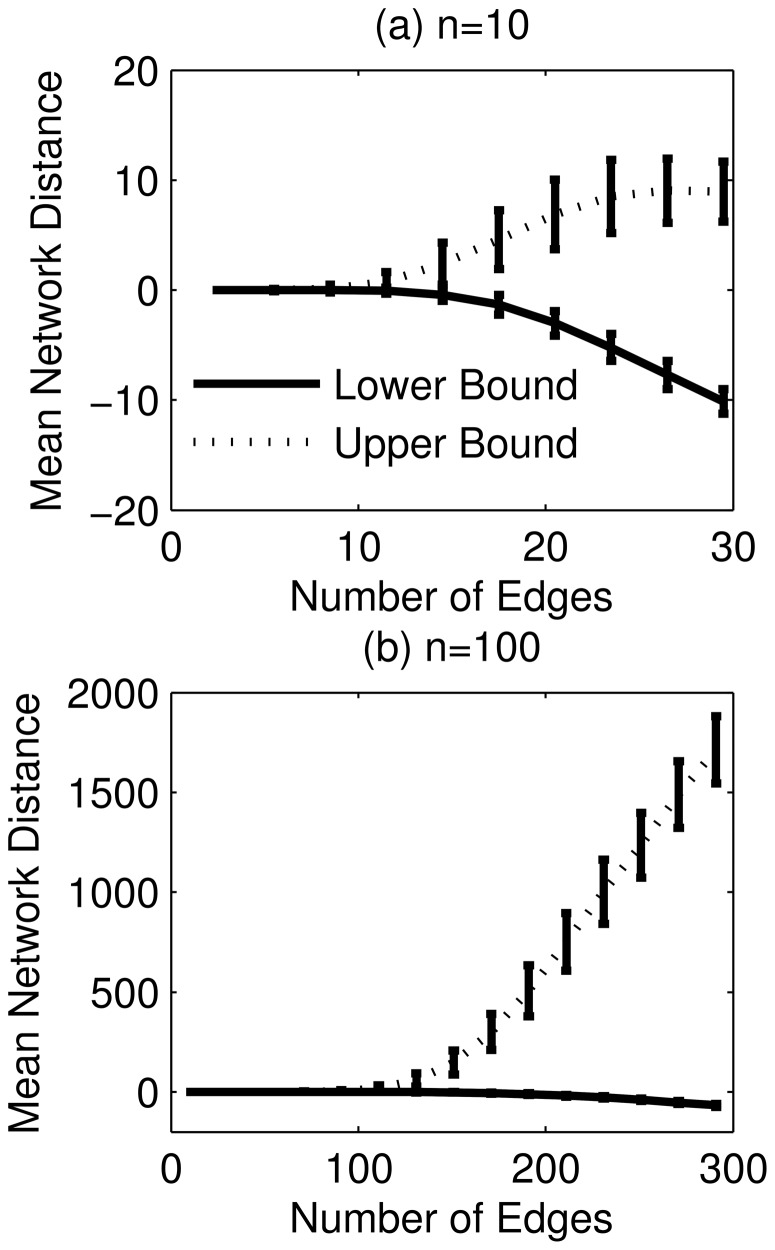
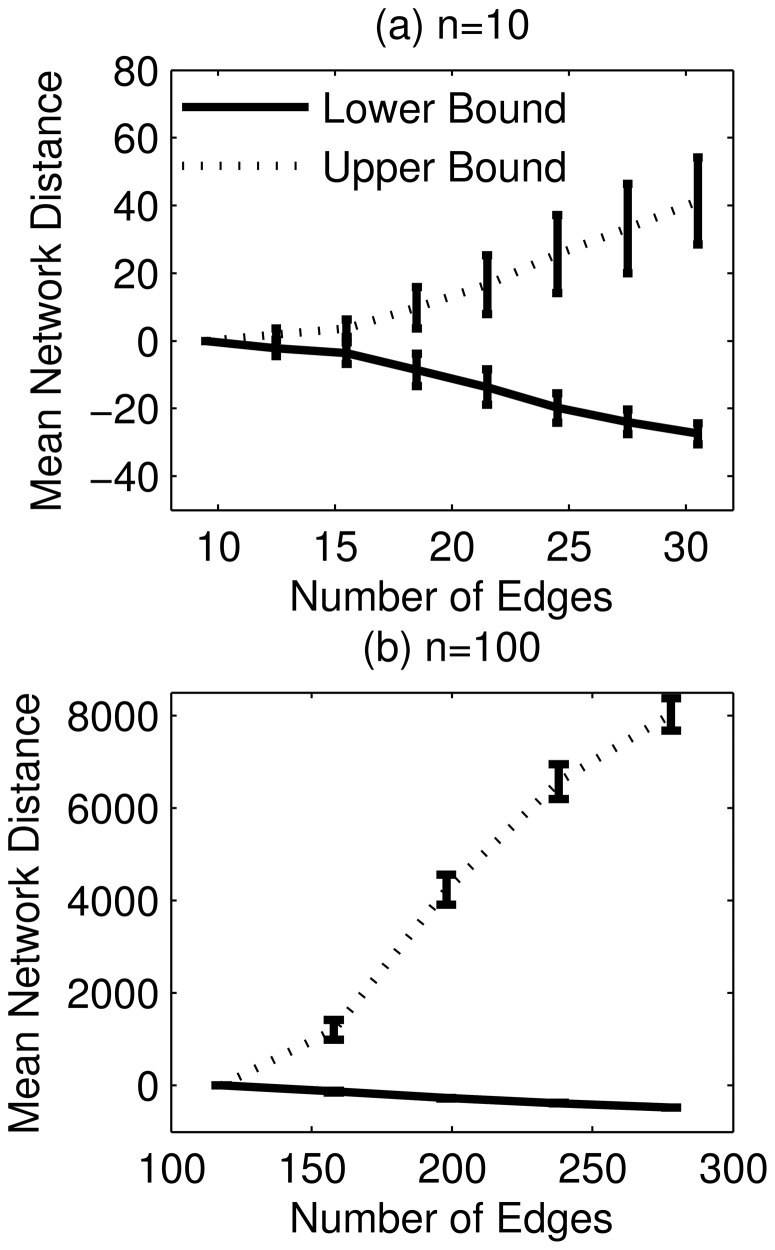
We applied TRaCE without error correction to construct the ensemble lower and upper bounds for each random network using the aforementioned accessibility matrices. The mean network distances of the bounds from 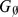 are shown in Figs. 4(a) and (b) as a function of network size. Here, we plotted the network distances of the lower bound using negative numbers and those of the upper bound using positive numbers. By doing so, we could illustrate the distance between the lower and upper bounds in the same plot. In particular, the number of edges in the set
are shown in Figs. 4(a) and (b) as a function of network size. Here, we plotted the network distances of the lower bound using negative numbers and those of the upper bound using positive numbers. By doing so, we could illustrate the distance between the lower and upper bounds in the same plot. In particular, the number of edges in the set 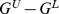 is equal to the distance between the two network distance curves in Fig. 4. Not surprisingly, the network distance increased with the size of the networks, i.e. larger networks are more difficult to infer than smaller networks. The difference between the lower and upper bounds also broadened with network size, indicating higher network uncertainty in the inference of larger GRNs. For networks containing fewer edges than nodes, the GRN
is equal to the distance between the two network distance curves in Fig. 4. Not surprisingly, the network distance increased with the size of the networks, i.e. larger networks are more difficult to infer than smaller networks. The difference between the lower and upper bounds also broadened with network size, indicating higher network uncertainty in the inference of larger GRNs. For networks containing fewer edges than nodes, the GRN 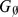 could generally be recovered from
could generally be recovered from 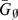 and
and 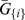 's. Nevertheless, Fig. 4 demonstrated that the GRNs were typically (64% for 10 gene networks and 76% for 100 gene networks) not inferable, since the lower and upper bounds did not converge.
's. Nevertheless, Fig. 4 demonstrated that the GRNs were typically (64% for 10 gene networks and 76% for 100 gene networks) not inferable, since the lower and upper bounds did not converge.
Figure 4. Ensemble inference and inferability of 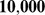 random networks of order (a)
random networks of order (a) 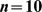 and (b)
and (b)  genes.
genes.
The mean network distances of the lower and upper bounds from 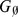 are shown as a function of network size (i.e. number of edges). The error bars indicate the standard deviations.
are shown as a function of network size (i.e. number of edges). The error bars indicate the standard deviations.
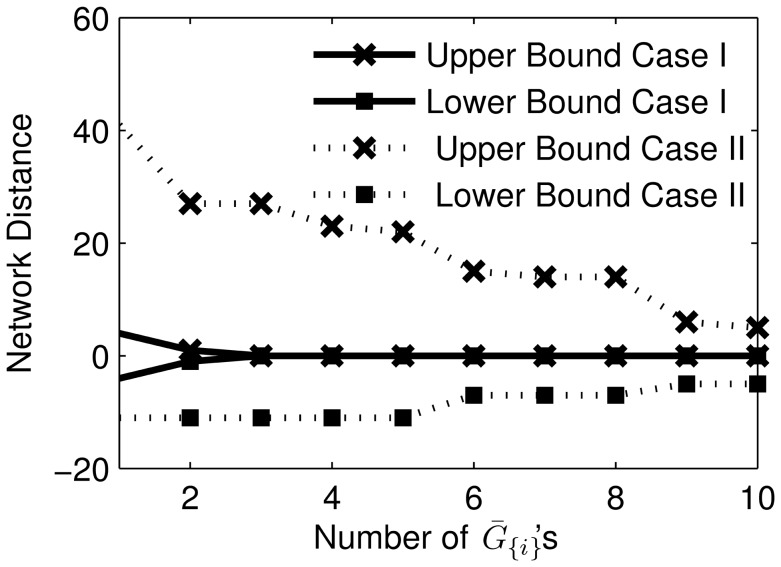
Fig. 5 shows two examples of GRN inference of order 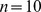 genes. In the first case (case I,
genes. In the first case (case I, 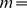 8 edges),
8 edges), 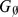 could be recovered from
could be recovered from 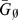 and as few as 3
and as few as 3 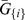 's, while in the second case (case II,
's, while in the second case (case II, 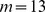 edges), the inference problem was underdetermined. Moreover, the results suggested that
edges), the inference problem was underdetermined. Moreover, the results suggested that 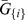 's were not equally informative, as the reduction in the distance between the lower and upper bounds by incorporating an additional
's were not equally informative, as the reduction in the distance between the lower and upper bounds by incorporating an additional 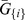 was not uniform.
was not uniform.
Figure 5. Examples of the ensemble inference of random networks with 10 genes.
In case I, the GRN has 8 edges and is inferable from the accessibility matrices  and as few as three
and as few as three 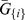 's. In case II, the GRN has 13 edges and is not inferable.
's. In case II, the GRN has 13 edges and is not inferable.
Random scale-free GRNs
Many cellular networks have been shown to be scale-free with a power-law degree distribution [25], where the majority of the nodes have low degrees (1 to 2) and a few nodes (called hubs) are of high degrees. We also tested the performance of TRaCE using random scale-free networks. Here, we constructed two sets of 5000 scale-free GRNs with order 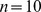 and
and 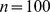 genes using the Barabási–Albert model [26]. Briefly, the GRNs were grown from a random seed network of small size (with 3 vertices) by sequentially adding nodes to the network. For each node addition, between 1 and 5 new edges were inserted to the network connecting the new node with existing ones, in a manner such that the degree distribution decayed exponentially. Again, for the purpose of inferabiliity analysis, we generated
genes using the Barabási–Albert model [26]. Briefly, the GRNs were grown from a random seed network of small size (with 3 vertices) by sequentially adding nodes to the network. For each node addition, between 1 and 5 new edges were inserted to the network connecting the new node with existing ones, in a manner such that the degree distribution decayed exponentially. Again, for the purpose of inferabiliity analysis, we generated 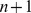 error-free accessibility matrices
error-free accessibility matrices 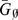 and all
and all 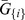 's, equivalent to having ideal data from single- and double-gene KO experiments.
's, equivalent to having ideal data from single- and double-gene KO experiments.
We used the error-free implementation of TRaCE to construct the ensemble lower and upper bounds for each of the random scale-free GRNs. Fig. 6 shows the mean network distances of the bounds as a function of network size. Similar to the random GRNs, most (79% for 10 gene networks and 75% for 100 gene networks) scale-free GRNs were not inferable from single and double-gene KO experiments, as the ensemble lower and upper bound did not meet for the majority of the networks. The mean network distance of the lower and upper bounds again increased with network size. However, the inference of scale-free GRNs from the accessibility matrices 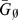 and
and  's appeared to be more difficult than that of random GRNs, as suggested by the larger distances between the lower and upper bounds for scale-free GRNs than for random GRNs of the same size.
's appeared to be more difficult than that of random GRNs, as suggested by the larger distances between the lower and upper bounds for scale-free GRNs than for random GRNs of the same size.
Figure 6. Ensemble inference and inferability of  random scale-free networks of order (a)
random scale-free networks of order (a) 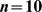 and (b)
and (b) 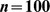 genes.
genes.
The mean network distances of the lower and upper bounds from  are shown as a function of network size (i.e. number of edges). The error bars indicate the standard deviations.
are shown as a function of network size (i.e. number of edges). The error bars indicate the standard deviations.
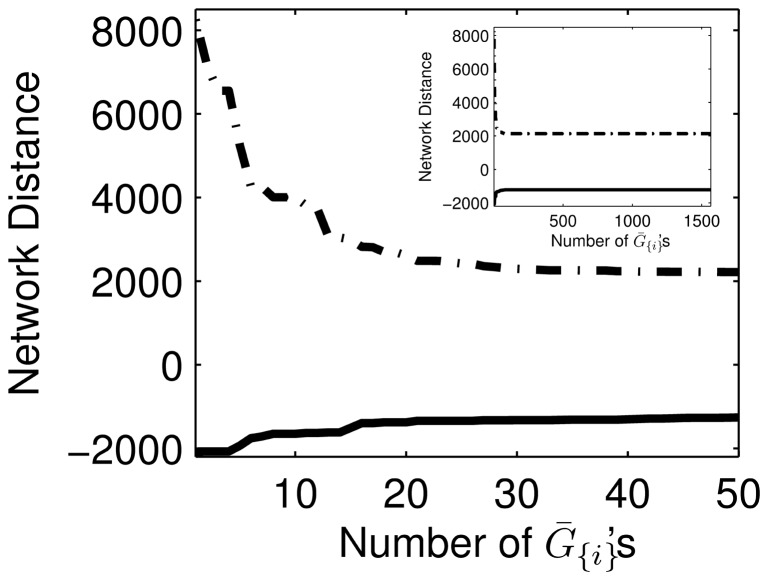
E. coli and S. cerevisiae GRNs
Finally, we investigated the inferability of large, realistic GRNs of E. coli and S. cerevisiae available in GeneNetWeaver [24]. The E. coli GRN consists of 1565 genes and 3758 edges, while the yeast GRN comprise 4441 genes and 12873 edges. For E. coli, we generated the accessibility matrices of 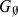 and all
and all 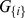 's. To reduce computational complexity, in the case of yeast, we used only the 100 most informative
's. To reduce computational complexity, in the case of yeast, we used only the 100 most informative  's based on the number of testable edges (i.e. the number of non-zero elements in the testability matrix
's based on the number of testable edges (i.e. the number of non-zero elements in the testability matrix 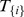 in Eq. (4)). The results are shown in Figs. 7 and 8. Not surprisingly, both E. coli and yeast GRNs could not be completely inferred from the above accessibility matrices. There was a diminishing return of information after about 25 and 50
in Eq. (4)). The results are shown in Figs. 7 and 8. Not surprisingly, both E. coli and yeast GRNs could not be completely inferred from the above accessibility matrices. There was a diminishing return of information after about 25 and 50 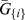 's for the inference of E. coli and yeast GRNs, respectively.
's for the inference of E. coli and yeast GRNs, respectively.
Figure 7. Ensemble inference of E. coli GRN from error-free 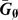 and the complete set of
and the complete set of 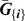 's.
's.
The plot shows the network distances of the lower and upper bounds from  as a function of the number of
as a function of the number of 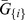 's for the 50 most informative
's for the 50 most informative 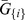 's, i.e. the top 50 highest number of testable edges. The incorporation of
's, i.e. the top 50 highest number of testable edges. The incorporation of  's and
's and 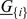 's was performed sequentially in decreasing number of testable edges. The inset shows the result for the complete set of
's was performed sequentially in decreasing number of testable edges. The inset shows the result for the complete set of 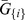 's.
's.
Figure 8. Ensemble inference of S. cerevisieae GRN from error-free 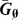 and the 100 most informative
and the 100 most informative 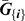 's based on the number of testable edges.
's based on the number of testable edges.
The plot shows the network distances of the lower and upper bounds from 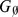 as a function of the number of
as a function of the number of 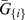 's. The incorporation of
's. The incorporation of 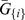 's and
's and 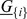 's was performed sequentially in decreasing number of testable edges.
's was performed sequentially in decreasing number of testable edges.
Ensemble inference from errorneous accessibility matrices
We evaluated the performance of TRaCE with error correction using E. coli GRN and subnetworks, as well as yeast GRN. False positive errors were simulated by randomly adding edges to the accessibility matrices, while false negatives were simulated by randomly removing edges from the accessibility matrices. The performance of error correction in TRaCE was judged by the number of erroneous edges that remained in the bounds after correction for different FP and FN rates (abbreviated as FPR and FNR, respectively), defined with respect to the size of 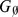 .
.
E. coli GRNs
We first used TRaCE with error correction for the ensemble inference of 50 random subnetworks of E. coli GRN with 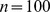 genes, generated using GeneNetWeaver [24]. The average number of edges was
genes, generated using GeneNetWeaver [24]. The average number of edges was  . As in the above case study, we created the accessibility matrices of
. As in the above case study, we created the accessibility matrices of 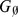 and every
and every 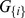 . We subsequently contaminated these matrices with FP and FN errors at the specified rates without any preference. The accuracy of the lower and upper bounds constructed using TRaCE with and without error correction is summarized in Table 2.
. We subsequently contaminated these matrices with FP and FN errors at the specified rates without any preference. The accuracy of the lower and upper bounds constructed using TRaCE with and without error correction is summarized in Table 2.
Table 2. Ensemble inference of E. coli subnetworks (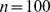 genes).
genes).
| FPR | FNR | Before Correction | After Correction | |||
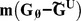
|
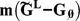
|
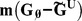
|
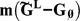
|

|
||
| 0.00 | 0.00 | 0 | 0 | 0 | 0 | 122.62 |
| 0.00 | 0.10 | 183.4 | 35.86 | 3.7 | 1.88 | 118.2 |
| 0.00 | 0.20 | 191.1 | 67.36 | 16.56 | 8.7 | 113.52 |
| 0.10 | 0.00 | 0 | 1412.42 | 0 | 0.24 | 150.4 |
| 0.10 | 0.10 | 183.02 | 1441.68 | 2.72 | 1.42 | 146.3 |
| 0.10 | 0.20 | 191 | 1460.82 | 12.06 | 2.1 | 146.74 |
| 0.20 | 0.00 | 0 | 2324.46 | 0 | 0.18 | 213.42 |
| 0.20 | 0.10 | 184.02 | 2354.16 | 2.72 | 0.46 | 197.28 |
| 0.20 | 0.20 | 191 | 2386.88 | 9.94 | 1.62 | 210.12 |
The reported values represent the averages over 50 subnetworks. FPR (FNR) is the ratio between the number of FP (FN) in the accessibility matrices and the number of edges in  . Let
. Let 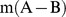 of any two digraphs
of any two digraphs 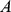 and
and  denote the number of edges in the set
denote the number of edges in the set  .
.
As in many scenarios above, none of the subnetworks was inferable. FP errors could be very effectively eliminated by error correction. FNs errors expectedly led to missing edges from the upper bound, as indicated by the number of edges of 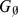 that did not appear in
that did not appear in 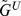 (see
(see 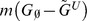 in Table 2). The error correction could not completely eliminate Type A errors, leading to erroneous edges that appeared in the lower bound
in Table 2). The error correction could not completely eliminate Type A errors, leading to erroneous edges that appeared in the lower bound 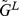 but did not belong to
but did not belong to 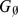 (see
(see  in Table 2). A combination of FP and FN errors were more easily corrected than FN errors alone. While FNs were more difficult to eliminate than FPs, correcting FP errors tended to produce larger network ensembles than FNs, indicating higher network uncertainty (see
in Table 2). A combination of FP and FN errors were more easily corrected than FN errors alone. While FNs were more difficult to eliminate than FPs, correcting FP errors tended to produce larger network ensembles than FNs, indicating higher network uncertainty (see 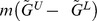 in Table 2). Nevertheless, even in the worst case (0% FP, 20% FN), roughly 90% of the errors in the lower and upper bounds could be removed by the error correction (compare
in Table 2). Nevertheless, even in the worst case (0% FP, 20% FN), roughly 90% of the errors in the lower and upper bounds could be removed by the error correction (compare 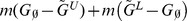 before and after correction).
before and after correction).
For the inference of E. coli GRN, we generated erroneous accessibility matrices 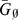 and the 100 most informative
and the 100 most informative  's corresponding to the top 100 highest numbers of testable edges. The performance of TRaCE with error correction for different FP and FN rates is summarized in Table 3. In addition, the structural Hamming distances of the lower and upper bounds before and after correction are reported in tables S1 and S2. As before, TRaCE with error correction could handle FPs more effectively than FNs, and a mixture of FP and FN errors in the accessibility matrices were more easily eliminated than FN alone. In the worst case (0% FP, 20% FN), more than 95% of the errors were corrected. The size of the ensemble also depended strongly on the FP errors, and at 20% FP, the number of edges between the lower and upper bound reached three times the size of the full GRN.
's corresponding to the top 100 highest numbers of testable edges. The performance of TRaCE with error correction for different FP and FN rates is summarized in Table 3. In addition, the structural Hamming distances of the lower and upper bounds before and after correction are reported in tables S1 and S2. As before, TRaCE with error correction could handle FPs more effectively than FNs, and a mixture of FP and FN errors in the accessibility matrices were more easily eliminated than FN alone. In the worst case (0% FP, 20% FN), more than 95% of the errors were corrected. The size of the ensemble also depended strongly on the FP errors, and at 20% FP, the number of edges between the lower and upper bound reached three times the size of the full GRN.
Table 3. Ensemble inference of E. coli GRN.
| FPR | FNR | Before Correction | After Correction | |||
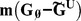
|

|
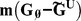
|

|
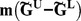
|
||
| 0.00 | 0.00 | 0 | 0 | 0 | 0 | 3351 |
| 0.00 | 0.10 | 3550 | 1029 | 25 | 46 | 3281 |
| 0.00 | 0.20 | 3746 | 1685 | 56 | 173 | 3092 |
| 0.10 | 0.00 | 0 | 34796 | 0 | 1 | 4636 |
| 0.10 | 0.10 | 3573 | 35566 | 14 | 1 | 4624 |
| 0.10 | 0.20 | 3746 | 35975 | 49 | 4 | 4556 |
| 0.20 | 0.00 | 0 | 62035 | 0 | 1 | 14933 |
| 0.20 | 0.10 | 3554 | 62632 | 12 | 7 | 14773 |
| 0.20 | 0.20 | 3741 | 63053 | 40 | 5 | 14392 |
FPR (FNR) is the ratio between the number of FP (FN) in the accessibility matrices and the number of edges in 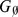 . Let
. Let 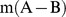 of any two digraphs
of any two digraphs 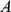 and
and 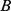 denote the number of edges in the set
denote the number of edges in the set  .
.
S. cerevisiae GRN
For yeast GRN, we generated erroneous 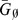 and the 100 most informative
and the 100 most informative 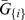 's. The results of TRaCE with error correction using these accessibility matrices are summarized in Table 4. The performance of TRaCE here was notably better than the inference of E. coli GRN. In all cases, TRaCE could rectify almost all erroneous edges. However, the correction came at a price of high uncertainty, where the difference between the lower and upper bounds exceeded 20 times the number of edges in
's. The results of TRaCE with error correction using these accessibility matrices are summarized in Table 4. The performance of TRaCE here was notably better than the inference of E. coli GRN. In all cases, TRaCE could rectify almost all erroneous edges. However, the correction came at a price of high uncertainty, where the difference between the lower and upper bounds exceeded 20 times the number of edges in 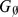 . Despite such high uncertainty, the gap between the bounds represented only 1.3% of the total possible edges.
. Despite such high uncertainty, the gap between the bounds represented only 1.3% of the total possible edges.
Table 4. Ensemble inference of S. cerevisiae GRN.
| FPR | FNR | Before Correction | After Correction | |||
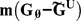
|
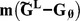
|
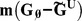
|
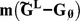
|
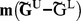
|
||
| 0.00 | 0.00 | 0 | 0 | 0 | 0 | 131595 |
| 0.00 | 0.10 | 4048 | 604 | 9 | 4 | 131879 |
| 0.00 | 0.20 | 6934 | 788 | 19 | 8 | 132155 |
| 0.10 | 0.00 | 0 | 121370 | 0 | 4 | 198624 |
| 0.10 | 0.10 | 4096 | 121563 | 8 | 4 | 198762 |
| 0.10 | 0.20 | 6879 | 121747 | 16 | 6 | 198883 |
| 0.20 | 0.00 | 0 | 227013 | 0 | 2 | 260484 |
| 0.20 | 0.10 | 4113 | 227087 | 4 | 3 | 260443 |
| 0.20 | 0.20 | 6909 | 227150 | 8 | 2 | 260313 |
Let 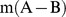 of any two digraphs
of any two digraphs 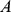 and
and 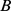 denote the number of edges in the set
denote the number of edges in the set  .
.
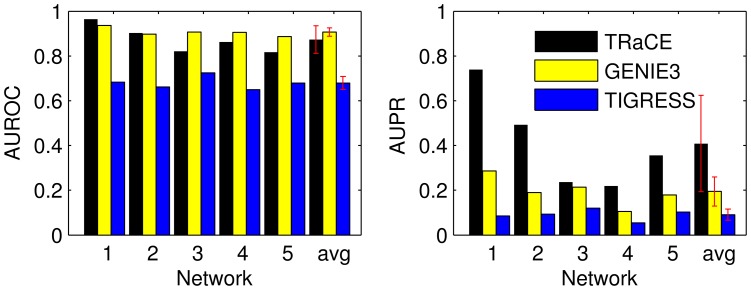
Ensemble inference from expression data
We further evaluated the performance of TRaCE using in silico noisy gene expression data generated using GeneNetWeaver [24]. We simulated steady-state gene expression data using the same settings as those in DREAM4 100-gene in silico network inference subchallenge. The simulated data are available at http://www.cabsel.ethz.ch/tools/trace or upon request. In the following case studies, we analyzed and converted the data into accessibility matrices following the procedure described in the Numerical Implementation section. Subsequently, we used the resulting accessibility matrices with TRaCE to produce the ensemble lower and upper bounds. For the purpose of comparison with existing network inference methods, we also ranked the gene regulatory interactions according to their confidence scores. In particular, we compared the rankings with those from top performing inference methods in DREAM4, namely the downranking method by Pinna et al. [27], GENIE3 [28] and TIGRESS [29]. As mentioned earlier, the downranking method follows a two-phase procedure in ranking edges similar to our implementation, but the method only down-ranks feed-forward edges. On the other hand, GENIE3 uses a machine learning strategy called random forest, and TIGRESS is a regression-based method. For each method, we calculated the area under receiver operating characteristics (AUROC) and precision-recall (AUPR) using a redefined confusion matrix, in which methods were not penalized for any error within the set of non-inferable edges. We define non-inferable edges as edges belonging to the upper bound that are missing from the lower bound (i.e. all edges in the set  ), which are determined from error-free accessibility matrices of the gold standard GRNs. More details of the calculation of the AUROC and AUPR can be found in a recent publication [30].
), which are determined from error-free accessibility matrices of the gold standard GRNs. More details of the calculation of the AUROC and AUPR can be found in a recent publication [30].
DREAM4 in silico network inference 100-gene subchallenge
We first simulated 5 replicates of steady state gene expression data associated with the complete single-gene KO experiments. We then used the data to construct the accessibility matrices of the gold standard GRNs. In this case, the upper bound of the ensemble was simply given by the accessibility matrix, and the lower bound was the ConTREx of the upper bound. Table 5 shows the FPR and FNR in the accessibility matrices, the errors in the lower and upper bounds (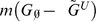 and
and 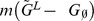 ), and the size of the ensemble (
), and the size of the ensemble (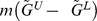 ) constructed using TRaCE. We noted that the majority (90%) of FN errors in the accessibility matrices were associated with fan-in motifs, in which a gene was regulated by several genes. In such a case, the effect of knocking-out one of the regulator genes could be compensated by the others and thus, the KO experiment did not show any significant differential expression of the downstream genes. As FNs affected the accessibility matrices, the errors in the upper bound
) constructed using TRaCE. We noted that the majority (90%) of FN errors in the accessibility matrices were associated with fan-in motifs, in which a gene was regulated by several genes. In such a case, the effect of knocking-out one of the regulator genes could be compensated by the others and thus, the KO experiment did not show any significant differential expression of the downstream genes. As FNs affected the accessibility matrices, the errors in the upper bound 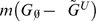 were higher than those in the lower bound
were higher than those in the lower bound 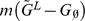 .
.
Table 5. Ensemble inference of DREAM4 100-gene gold standard networks: single-gene KO dataset.
| Network | FPR | FNR |
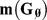
|
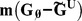
|
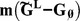
|
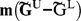
|
| 1 | 0 | 2.28 | 176 | 44 | 9 | 136 |
| 2 | 0 | 1.96 | 249 | 122 | 6 | 123 |
| 3 | 0 | 11.04 | 195 | 86 | 21 | 144 |
| 4 | 0 | 9.69 | 211 | 91 | 7 | 208 |
| 5 | 0 | 3.47 | 193 | 108 | 12 | 237 |
FPR (FNR) is the ratio between the number of FP (FN) in the accessibility matrices and the number of edges in 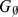 . Let
. Let 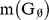 denote the number of edges in
denote the number of edges in  , and
, and 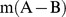 of any two digraphs
of any two digraphs 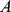 and
and 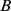 denote the number of edges in the set
denote the number of edges in the set  .
.
Subsequently, we created a ranked list of gene regulatory predictions and compared the list against those produced by the downranking method, GENIE3 and TIGRESS. Fig. 9 provides the comparison of AUROC and AUPR of the four methods. The comparison showed that TRaCE and the downranking method outperformed GENIE3 and TIGRESS, especially considering the AUPR values. Here, TRaCE performed as well as the downranking method, which was the best overall performer in DREAM 4 100-gene network inference subchallenge [27].
Figure 9. Comparison of TRaCE and top performing methods in DREAM4 100-gene network inference subchallenge: single-gene KO dataset.
The error bars represent the standard deviations. Based on the AUROC values, TRaCE performed as well as the downranking method (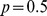 ) and GENIE3 (
) and GENIE3 (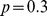 ), but better than TIGRESS (
), but better than TIGRESS (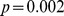 ). Based on the AUPR values, TRaCE performed as well as the downranking method (
). Based on the AUPR values, TRaCE performed as well as the downranking method (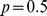 ), but better than GENIE3 (
), but better than GENIE3 (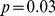 ) and TIGRESS (
) and TIGRESS (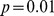 ). The statistical significance was evaluated using two sample t-test.
). The statistical significance was evaluated using two sample t-test.
Ensemble inference from single- and double-gene Kos
We further simulated 5 replicates of steady state gene expression data for the complete set of single- and double-gene KO experiments using the gold standard GRNs of DREAM4 100-gene subchallenge. We processed the data to obtain the accessibility matrices of 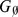 and all
and all 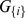 's. We subsequently applied TRaCE with error correction to the accessibility matrices to obtain the ensemble lower and upper bounds, which are summarized in Table 6. The average FPR and FNR were similar to the single-gene KO data since both datasets had the same number of replicates. Again, the majority (80%) of errors in the accessibility matrices were associated with fan-in motifs. By comparing Tables 5 and 6, the errors in the lower bounds improved slightly in comparison with those from only single-gene KO dataset (compare
's. We subsequently applied TRaCE with error correction to the accessibility matrices to obtain the ensemble lower and upper bounds, which are summarized in Table 6. The average FPR and FNR were similar to the single-gene KO data since both datasets had the same number of replicates. Again, the majority (80%) of errors in the accessibility matrices were associated with fan-in motifs. By comparing Tables 5 and 6, the errors in the lower bounds improved slightly in comparison with those from only single-gene KO dataset (compare 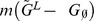 values). However, the errors in the upper bounds increased due to the accumulation of FN errors from fan-in motifs (compare
values). However, the errors in the upper bounds increased due to the accumulation of FN errors from fan-in motifs (compare 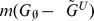 values). Nevertheless, the additional data from double-gene KO experiments led to lower network uncertainties (compare
values). Nevertheless, the additional data from double-gene KO experiments led to lower network uncertainties (compare 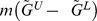 values).
values).
Table 6. Ensemble inference of DREAM4 100-gene gold standard networks: single- and double-gene KO dataset.
| Network | FPR | FNR |
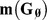
|
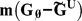
|
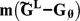
|

|
| 1 | 0.02 | 2.21 | 176 | 50 | 5 | 27 |
| 2 | 0.01 | 1.91 | 249 | 137 | 5 | 37 |
| 3 | 0 | 10.43 | 195 | 103 | 15 | 30 |
| 4 | 0 | 9.31 | 211 | 118 | 8 | 35 |
| 5 | 0.01 | 3.40 | 193 | 131 | 12 | 21 |
FPR (FNR) is the average ratio between the number of FP (FN) in the accessibility matrices and the number of edges in  . Let
. Let 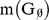 denote the number of edges in
denote the number of edges in 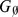 , and
, and  of any two digraphs
of any two digraphs  and
and  denote the number of edges in the set
denote the number of edges in the set 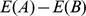 .
.
For each gold standard GRN, we also generated a ranked list of edges based on the confidence scores and compared the list with those using GENIE3 and TIGRESS. The downranking method could not be applied to double-gene KO data and was left out from the comparison. The AUROC and AUPR of the three methods are compared in Fig. 10. The AUPR of TRaCE was higher than GENIE3 and TIGRESS. Meanwhile, the AUROC values were generally high for all three methods, with TIGRESS having the lowest value. In comparison to single-gene KO data, the inclusion of double-gene KO data led to no change in the average AUROC (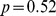 , two sample t-test) and an increase in AUPR (
, two sample t-test) and an increase in AUPR (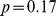 , two sample t-test). Finally, as shown in Tables 7 and 8, the AUROC and AUPR values of TRaCE were insensitive to
, two sample t-test). Finally, as shown in Tables 7 and 8, the AUROC and AUPR values of TRaCE were insensitive to 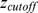 and
and 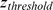 used in the construction of the accessibility matrices, and the threshold value in the preprocessing of
used in the construction of the accessibility matrices, and the threshold value in the preprocessing of 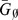 . Because of the trade-off between FPs and FNs when using different
. Because of the trade-off between FPs and FNs when using different 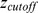 and
and  , the maximum of AUROC and AUPR corresponded to intermediate values within the selected ranges of
, the maximum of AUROC and AUPR corresponded to intermediate values within the selected ranges of 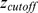 and
and 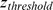 .
.
Figure 10. Comparison of TRaCE and top performing methods in DREAM4 100-gene network inference subchallenge: single- and double-gene KO dataset.
The error bars represent the standard deviations. Based on the AUROC values, TRaCE performed as well as GENIE3 (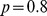 ) and better than TIGRESS (
) and better than TIGRESS (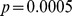 ). Similarly, based on the AUPR values, TRaCE performed better than GENIE3 (
). Similarly, based on the AUPR values, TRaCE performed better than GENIE3 (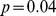 ) and TIGRESS (
) and TIGRESS (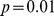 ). The statistical significance was evaluated using two sample t-test.
). The statistical significance was evaluated using two sample t-test.
Table 7. Effects of  and
and  on AUROC and AUPR of TRaCE in DREAM4 100-gene subchallenge: single gene KO data.
on AUROC and AUPR of TRaCE in DREAM4 100-gene subchallenge: single gene KO data.
|

|
AUROC | AUPR |
| 1.5 | 2 | 0.871 0.0616 0.0616 |
0.3085 0.1416 0.1416 |
| 1.5 | 3 | 0.8723 0.0673 0.0673 |
0.3209 0.1534 0.1534 |
| 2 | 2 | 0.8756 0.0595 0.0595 |
0.2922 0.154 0.154 |
| 2 | 3 |
0.8758
 0.0642
0.0642
|
0.2889
 0.1596
0.1596
|
| 2.5 | 3 | 0.8758 0.0642 0.0642 |
0.2438 0.1545 0.1545 |
The AUROC and AUPR values are the average  standard devation over 5 gold standard networks. The values used in the comparison with existing methods are highlighted in bold.
standard devation over 5 gold standard networks. The values used in the comparison with existing methods are highlighted in bold.
Table 8. Effects of  ,
, 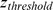 and the preprocessing threshold of
and the preprocessing threshold of 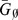 on AUROC and AUPR of TRaCE in DREAM4 100-gene subchallenge: single- and double-gene KO data.
on AUROC and AUPR of TRaCE in DREAM4 100-gene subchallenge: single- and double-gene KO data.
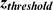
|
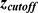
|

|
AUROC | AUPR |
| 1.5 | 2 | 0.5 | 0.8723 0.0595 0.0595 |
0.4327 0.1931 0.1931 |
| 0.65 | 0.8718 0.0588 0.0588 |
0.4197 0.1959 0.1959 |
||
| 0.8 | 0.8724 0.0600 0.0600 |
0.4184 0.2022 0.2022 |
||
| 1.5 | 3 | 0.5 | 0.8728 0.0596 0.0596 |
0.4204 0.1986 0.1986 |
| 0.65 | 0.8733 0.0610 0.0610 |
0.4212 0.2059 0.2059 |
||
| 0.8 | 0.8735 0.0617 0.0617 |
0.4201 0.2100 0.2100 |
||
| 2 | 2 | 0.5 | 0.8726 0.0603 0.0603 |
0.4177 0.2158 0.2158 |
| 0.65 | 0.8727 0.0607 0.0607 |
0.4167 0.2170 0.2170 |
||
| 0.8 | 0.8728 0.0608 0.0608 |
0.4091 0.2139 0.2139 |
||
| 2 | 3 | 0.5 | 0.8735 0.0618 0.0618 |
0.4170 0.2160 0.2160 |
| 0.65 |
0.8734
 0.0620
0.0620
|
0.4086
 0.2149
0.2149
|
||
| 0.8 | 0.8698 0.0604 0.0604 |
0.4045 0.2108 0.2108 |
||
| 2.5 | 3 | 0.5 | 0.8695 0.0599 0.0599 |
0.3893 0.2054 0.2054 |
| 0.65 | 0.8696 0.0598 0.0598 |
0.3837 0.1975 0.1975 |
||
| 0.8 | 0.8695 0.0600 0.0600 |
0.3764 0.2023 0.2023 |
The AUROC and AUPR values are the average  standard devation over 5 gold standard GRNs. The values used in the comparison with existing methods are highlighted in bold.
standard devation over 5 gold standard GRNs. The values used in the comparison with existing methods are highlighted in bold.
E. coli and Yeast GRNs
Finally, we simulated the complete set of single-gene KO experiments for E. coli and yeast. For each organism, we generated 10 replicates of steady state gene expression data. We performed differential expression analysis using the Z-score transformation and obtained the accessibility matrices using either 5 or 10 replicates. We then applied TRaCE with error correction to construct the ensemble lower and upper bounds, and created ranked lists of edges as done earlier. The errors in the accessibility matrices and in the bounds are reported in Table 9, along with the AUROC and AUPR values. Here, FPR in the accessibility matrices decreased with increasing number of replicates, but FNR did not change with the number of replicates. The errors in the upper bounds changed little with increasing technical replicates from 5 to 10, but those in the lower bounds dropped considerably. The sizes of the ensemble also decreased with increasing replicates. Finally, the AUPR values improved slightly with higher replicates, while the AUROC values were insensitive with respect to the number of replicates.
Table 9. Ensemble inference of E. coli and Yeast GRNs from single-gene KO data.
| Network | Replicates | FPR | FNR |
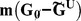
|

|
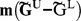
|
AUROC | AUPR |
| E. coli | 5 | 0.117 | 2.61 | 1611 | 687 | 1724 | 0.8601 | 0.4836 |
| E. coli | 10 | 0.005 | 2.61 | 1612 | 297 | 1673 | 0.8639 | 0.5192 |
| Yeast | 5 | 0.582 | 24.97 | 8101 | 9159 | 7907 | 0.7699 | 0.2464 |
| Yeast | 10 | 0.141 | 24.98 | 8098 | 3753 | 7200 | 0.7569 | 0.2768 |
FPR (FNR) is the ratio between the number of FP (FN) in the accessibility matrices and the number of edges in 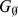 . Let
. Let 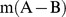 of any two digraphs
of any two digraphs 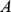 and
and 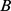 denote the number of edges in the set
denote the number of edges in the set  .
.
Discussion
The inference of GRNs from data of gene perturbation experiments is an important but unsolved problem. The difficulty stems from the underdetermined nature of such an inference [7], [9], as the data do not contain the necessary information to establish the complete causal interactions among the genes. Consequently, there exist many indistinguishable solutions. We have developed TRaCE with this consequence in mind by employing an ensemble inference strategy. Specifically, we have taken into consideration the fundamental limitation in using steady-state expression data of gene KO experiments for establishing direct causal relationships among genes. In TRaCE, we first transform the expression data into accessibility relationships (matrices) among genes. The novel contribution of TRaCE is an algorithm for the construction of lower and upper bounds of network ensemble, where each member of the ensemble satisfies the accessibility matrices. Edges of the upper bound that do not appear in the lower bound are considered non-inferable, as the existence of such edges can not be verified. Here, the size of the ensemble provides a metric of uncertainty in the network inference problem, with which the GRN inferability can be rigorously assessed. The GRN is inferable when the lower and upper bounds coincide (i.e. the ensemble only contains one network). Thus, in TRaCE, the inference and inferability analysis are accomplished simultaneously.
In the case studies, we have demonstrated the use of TRaCE for analyzing the inferability of GRNs. With the exception of networks of low order and small size, the majority of the GRNs were not inferable even when using error-free accessibility matrices. As we have used sparse networks in the case studies, the lower bound of the ensemble was a better estimate of the GRN than the upper bound. Finally, the majority of double-gene KO experiments were non-informative as the reduction in the size of the ensemble diminished after only a small number of  's. The observation above suggests that experimental design contributes significantly to the underdetermined nature of the typical GRN inference. In this regard, the lower and upper bounds of the ensemble could be used for optimizing the gene perturbation experiments, for example by finding the KO experiment that provides the maximum reduction in the difference between the lower and upper bounds. A strategy for optimal design of experiments using ensemble inference will be presented in a future publication.
's. The observation above suggests that experimental design contributes significantly to the underdetermined nature of the typical GRN inference. In this regard, the lower and upper bounds of the ensemble could be used for optimizing the gene perturbation experiments, for example by finding the KO experiment that provides the maximum reduction in the difference between the lower and upper bounds. A strategy for optimal design of experiments using ensemble inference will be presented in a future publication.
We have also used the ensemble lower and upper bounds in conjunction with the z-scores to produce a ranked list of gene regulatory predictions. In comparison with the top methods of DREAM4 network inference challenge, TRaCE could match the performance of the downranking method, the best overall performer in the 100-gene subchallenge. For single-gene KO dataset, TRaCE and the downranking method differed only for edges that were involved in cycles of more than 2 nodes. However, the two methods were fundamentally different, as TRaCE was developed for ensemble inference. Furthermore, the downranking method was created for single-gene KO experiments. Meanwhile, TRaCE significantly outperformed GENIE3 and TIGRESS when using single- and double-gene KO data. We note that GENIE3 and TIGRESS were also among the best performers in DREAM5 network inference challenge [10].
As expected, data noise negatively influenced the GRN inference and increased the uncertainty in the GRN inference. In the case studies, random errors in the accessibility matrices expectedly led to a larger ensemble. While the error correction in TRaCE was able to eliminate the majority of errors, some of the errors remained in the bounds. In particular, FN errors were harder to correct than FPs. The reason was that more Type A errors originating from FNs passed through the correction than those originating from FPs (see Fig. S3 and text S4). Meanwhile, FP errors could cancel out some Type A errors associated with FNs at the cost of increased uncertainty (see Fig. S4 and text S4).
The application of TRaCE to simulated noisy gene expression data indicated that the majority of errors in the accessibility matrices were due to FNs associated with fan-in motifs in the GRN. In such motifs, the effects of knocking-out one regulator gene could be compensated by other regulator(s), and differential expression analysis could only reveal the dominant regulator(s) of a gene. Note that such a problem could not be improved by increasing technical replicates (see Table 9). Meanwhile, errors associated with fan-in motifs usually lead to type A errors where the affected edges are absent from the lower and upper bounds. However, if the available experiments permit the construction of 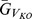 in which
in which  includes the dominant regulator(s) of a fan-in motif, then the related missing edge(s) may appear in
includes the dominant regulator(s) of a fan-in motif, then the related missing edge(s) may appear in 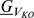 , leading to a detectable and correctable type C error. We expect the issue above would improve when using more sensitive measurements of gene expression, for example RNAseq.
, leading to a detectable and correctable type C error. We expect the issue above would improve when using more sensitive measurements of gene expression, for example RNAseq.
Conclusion
Inferring gene regulatory networks from DNA microarray data is an unsolved problem. Community-wide assessments of inference methods have shown that distinguishing direct and indirect regulatory interactions among genes is a common Achilles' heel of existing algorithms. In this study, we have adopted an ensemble inference strategy and develop new framework and algorithms for the creation of an ensemble of networks. Here, the ensemble represents the uncertainty associated with differentiating direct and indirect regulations using steady-state gene expression data of gene KO experiments. In particular, TRaCE produces the lower and upper bound of the ensemble. Using the bounds of the ensemble, a ranked list of gene regulatory predictions can also be generated. The case studies demonstrate that except for networks with few edges, most GRNs can not be fully inferred even when error-free data from complete single- and double-gene knock out experiments are available. In comparison with top performing methods of DREAM4 in silico network inference challenge, TRaCE performed equally well with the downranking method, the best overall performer in the challenge. However, the downranking method is not designed to handle data from multi-gene KO experiments. Meanwhile, TRaCE outperformed GENIE3 and TIGRESS when using single- and double-gene KO data. Nevertheless, the uncertainty in GRN inference is still significant, and systematic KOs of genes are often suboptimal as only a small fraction of the experiments are informative. We therefore hope that the shift of paradigm to ensemble inference will trigger further developments of methodologies for inferring and analyzing GRNs, in which network inference uncertainty is explicitly taken into consideration.
Supporting Information
An example of ConTREx of a simple GRN. The directed edges indicated by blue arrows in 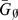 are in the set of indirect regulations. The edges between A and B are retained, because the cycle contains only two nodes. If the cycle had contained more than two nodes, all edges among the nodes would have been removed.
are in the set of indirect regulations. The edges between A and B are retained, because the cycle contains only two nodes. If the cycle had contained more than two nodes, all edges among the nodes would have been removed.
(TIFF)
Example of an ensemble involving GRN with cycle. Consider a GRN consisting of genes A, B and C, all of which are involved in a directed cycle. Further, let us assume that the edge 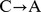 belongs to the lower bound and
belongs to the lower bound and 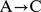 is not in the the upper bound. In this case, the ensemble comprises the graphs shown in (a)-(d).
is not in the the upper bound. In this case, the ensemble comprises the graphs shown in (a)-(d).
(TIFF)
Type A errors due to an FN. (a)  ; (b)
; (b)  ; (c)
; (c) 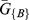 ; (d)
; (d) 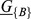 ; (e)
; (e) 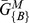 with an FN at
with an FN at  . In this case,
. In this case,  . (f)
. (f) 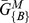 with an FN at
with an FN at 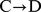 and an FP at
and an FP at 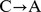 (g) ConTREx reduction of (f). (h)
(g) ConTREx reduction of (f). (h) 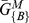 with an FN at
with an FN at  and an FP at
and an FP at  (i) ConTREx reduction of (h). See text S4 for details.
(i) ConTREx reduction of (h). See text S4 for details.
(TIFF)
Type A error due to an FP.
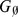 and
and 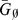 are shown in Fig. S3 (a) and (b), respectively. (a)
are shown in Fig. S3 (a) and (b), respectively. (a) 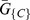 . In this case,
. In this case, 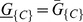 . (b)
. (b) 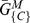 with an FP at
with an FP at 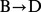 . Here,
. Here, 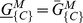 .
.
(TIFF)
Performance of TRaCE on inference of
E. coli
subnetworks (
 genes). The reported values represent the average over 50 subnetworks. Let
genes). The reported values represent the average over 50 subnetworks. Let  of any two digraphs
of any two digraphs  and
and  denote the structural Hamming distance (SHD) between them. The SHD is defined as the number of edges which differ or have opposite orientation between two networks [31].
denote the structural Hamming distance (SHD) between them. The SHD is defined as the number of edges which differ or have opposite orientation between two networks [31].
(PDF)
Performance of TRaCE on inference of
E. coli
GRN. Let 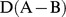 of any two digraphs
of any two digraphs 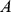 and
and 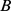 denote the structural Hamming distance between them.
denote the structural Hamming distance between them.
(PDF)
Preprocessing of
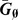 .
.
(PDF)
Filter Algorithms.
(PDF)
Consistency check.
(PDF)
Type A errors due to FN.
(PDF)
Acknowledgments
The authors would like to thank Caroline Siegenthaler, Erica Manesso and Heeju Noh for useful comments.
Funding Statement
The work was supported by funding from the Swiss National Science Foundation (grant 137614, http://www.snf.ch). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Apic G, Ignjatovic T, Boyer S, Russell RB (2005) Illuminating drug discovery with biological pathways. FEBS Lett 579: 1872–1877. [DOI] [PubMed] [Google Scholar]
- 2. Alper H, Stephanopoulos G (2009) Engineering for biofuels: exploiting innate microbial capacity or importing biosynthetic potential? Nat Rev Microbiol 7: 715–723. [DOI] [PubMed] [Google Scholar]
- 3. Oberhardt MA, Palsson B, Papin JA (2009) Applications of genome-scale metabolic reconstructions. Mol Syst Biol 5: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao XM, Iskar M, Zeller G, Kuhn M, van Noort V, et al. (2011) Prediction of drug combinations by integrating molecular and pharmacological data. PLoS Comput Biol 7: e1002323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hecker M (2009) Gene regulatory network inference: data integration in dynamic models–a review. Biosystems 96: 86–103. [DOI] [PubMed] [Google Scholar]
- 6. Margolin AA, Califano A (2007) Theory and limitations of genetic network inference from microarray data. Ann N Y Acad Sci 1115: 51–72. [DOI] [PubMed] [Google Scholar]
- 7. Smet RD, Marchal K (2010) Advantages and limitations of current network inference methods. Nature Reviews Microbiology 8: 717–729. [DOI] [PubMed] [Google Scholar]
- 8. Stolovitzky G, Prill RJ, Califano A (2009) Lessons from the dream2 challenges. Ann N Y Acad Sci 1158: 159–195. [DOI] [PubMed] [Google Scholar]
- 9. Marbach D, Prill RJ, Schaffter T, Mattiussi C, Floreano D, et al. (2010) Revealing strengths and weaknesses of methods for gene network inference. Proc Natl Acad Sci USA 107: 6286–6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marbach D, Costello JC, Küffner R, Vega NM, Prill RJ, et al. (2012) Wisdom of crowds for robust gene network inference. Nat Methods 9: 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gadkar KG, Gunawan R, Doyle FJ 3rd (2005) Iterative approach to model identification of biological networks. BMC Bioinformatics 6: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Srinath S, Gunawan R (2010) Parameter identifiability of power-law biochemical system models. J Biotechnol 149: 132–140. [DOI] [PubMed] [Google Scholar]
- 13. Chis OT, Banga JR, Balsa-Canto E (2011) Structural identifiability of systems biology models: A critical comparison of methods. PLoS ONE 6: e27755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szederkényi G, Banga J, Alonso A (2011) Inference of complex biological networks: distinguishability issues and optimization-based solutions. BMC systems biology 5: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuepfer L, Peter M, Sauer U, Stelling J (2007) Ensemble modeling for analysis of cell signaling dynamics. Nat Biotechnol 25: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 16. Miskovic L, Hatzimanikatis V (2011) Modeling of uncertainties in biochemical reactions. Biotechnol Bioeng 108: 413–423. [DOI] [PubMed] [Google Scholar]
- 17. Tan Y, Liao JC (2012) Metabolic ensemble modeling for strain engineers. Biotechnol J 7: 343–353. [DOI] [PubMed] [Google Scholar]
- 18. Jia G, Stephanopoulos G, Gunawan R (2012) Ensemble kinetic modeling of metabolic networks from dynamic metabolic profiles. Metabolites 2: 891–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wagner A (2001) How to reconstruct a large genetic network from n gene perturbations in fewer than n(2) easy steps. Bioinformatics 17: 1183–1197. [DOI] [PubMed] [Google Scholar]
- 20. Aho AV, Garey MR, Ullman JD (1972) The transitive reduction of a directed graph. SIAM J Comput 1: 131–137. [Google Scholar]
- 21.Jackson S (2011) Research Methods and Statistics: A Critical Thinking Approach. Cengage Learning, 138 pp.
- 22.Harray F (1969) Graph Theory. Addison-Wesley, Reading, Massachusetts.
- 23. Pinna A, Soranzo N, de la Fuente A (2010) From knockouts to networks: Establishing direct cause-effect relationships through graph analysis. PLoS ONE 5: e12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schaffter T, Marbach D, Floreano D (2011) Genenetweaver: in silico benchmark generation and performance profiling of network inference methods. Bioinformatics 27: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 25. Albert R (2005) Scale-free networks in cell biology. Journal of cell science 118: 4947–4957. [DOI] [PubMed] [Google Scholar]
- 26. Albert R, Barabási AL (2002) Statistical mechanics of complex networks. Rev Mod Phys 74: 47–97. [Google Scholar]
- 27. Pinna A, Soranzo N, de la Fuente A (2010) From knockouts to networks: Establishing direct cause-effect relationships through graph analysis. PLoS ONE 5: e12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huynh-Thu VA, Irrthum A, Wehenkel L, Geurts P (2010) Inferring regulatory networks from expression data using tree-based methods. PLoS ONE 5: e12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haury AC, Mordelet F, Vera-Licona P, Vert JP (2012) Tigress: Trustful inference of gene regulation using stability selection. BMC Systems Biology 6: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Siegenthaler C, Gunawan R (2014) Assessing network inference methods: How to cope with an underdetermined problem. PLoS One 9: e90481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsamardinos I, Brown LE, Aliferis CF (2006) The max-min hill-climbing bayesian network structure learning algorithm. Machine Learning 65: 31–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An example of ConTREx of a simple GRN. The directed edges indicated by blue arrows in 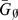 are in the set of indirect regulations. The edges between A and B are retained, because the cycle contains only two nodes. If the cycle had contained more than two nodes, all edges among the nodes would have been removed.
are in the set of indirect regulations. The edges between A and B are retained, because the cycle contains only two nodes. If the cycle had contained more than two nodes, all edges among the nodes would have been removed.
(TIFF)
Example of an ensemble involving GRN with cycle. Consider a GRN consisting of genes A, B and C, all of which are involved in a directed cycle. Further, let us assume that the edge 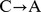 belongs to the lower bound and
belongs to the lower bound and 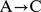 is not in the the upper bound. In this case, the ensemble comprises the graphs shown in (a)-(d).
is not in the the upper bound. In this case, the ensemble comprises the graphs shown in (a)-(d).
(TIFF)
Type A errors due to an FN. (a)  ; (b)
; (b)  ; (c)
; (c)  ; (d)
; (d)  ; (e)
; (e) 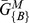 with an FN at
with an FN at 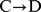 . In this case,
. In this case,  . (f)
. (f) 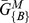 with an FN at
with an FN at 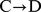 and an FP at
and an FP at 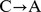 (g) ConTREx reduction of (f). (h)
(g) ConTREx reduction of (f). (h) 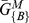 with an FN at
with an FN at  and an FP at
and an FP at  (i) ConTREx reduction of (h). See text S4 for details.
(i) ConTREx reduction of (h). See text S4 for details.
(TIFF)
Type A error due to an FP.
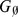 and
and 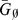 are shown in Fig. S3 (a) and (b), respectively. (a)
are shown in Fig. S3 (a) and (b), respectively. (a) 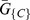 . In this case,
. In this case, 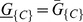 . (b)
. (b) 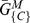 with an FP at
with an FP at 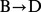 . Here,
. Here, 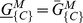 .
.
(TIFF)
Performance of TRaCE on inference of
E. coli
subnetworks (
 genes). The reported values represent the average over 50 subnetworks. Let
genes). The reported values represent the average over 50 subnetworks. Let  of any two digraphs
of any two digraphs 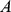 and
and  denote the structural Hamming distance (SHD) between them. The SHD is defined as the number of edges which differ or have opposite orientation between two networks [31].
denote the structural Hamming distance (SHD) between them. The SHD is defined as the number of edges which differ or have opposite orientation between two networks [31].
(PDF)
Performance of TRaCE on inference of
E. coli
GRN. Let 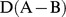 of any two digraphs
of any two digraphs 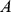 and
and 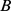 denote the structural Hamming distance between them.
denote the structural Hamming distance between them.
(PDF)
Preprocessing of
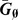 .
.
(PDF)
Filter Algorithms.
(PDF)
Consistency check.
(PDF)
Type A errors due to FN.
(PDF)