The purpose of this review is to examine the epidemiology, etiology, and not-yet-defined pathogenic characteristics of non-AIDS-defining cancers (NADCs) and discuss topics such as treatment strategies, comorbidity, and multidrug interactions. Four types of NADCs that deserve special attention are examined: anal cancer, Hodgkin lymphoma, hepatocellular carcinoma, and lung cancer.
Keywords: HIV, Non-AIDS-defining cancers, Chemotherapy, Immunodeficiency
Abstract
Background.
The impact of highly active antiretroviral therapies (HAART) on the risk of non-AIDS-defining cancers (NADCs) and the role of biological and clinical factors in their pathogenesis are debated issues. The purpose of this review is to examine the epidemiology, etiology, and not-yet-defined pathogenic characteristics of NADCs and discuss topics such as treatment strategies, comorbidity, and multidrug interactions. Four types of NADCs that deserve special attention are examined: anal cancer, Hodgkin lymphoma (HL), hepatocellular carcinoma, and lung cancer.
Methods.
The PubMed database and the Cochrane Library were searched by focusing on NADCs and on the association among NADCs, HAART, aging, and/or chronic inflammation. All articles were reviewed to identify those reporting variables of interest.
Results.
NADC incidence is twofold higher in patients with HIV/AIDS than in the corresponding general population, and this elevated risk persists despite the use of HAART. The mechanisms that HIV may use to promote the development of NADCs are presently unclear; immunological mechanisms, either immunodeficiency and/or immunoactivation, may play a role.
Conclusion.
Recent clinical studies have suggested that equivalent antineoplastic treatment is feasible and outcome can be similar in HIV-infected patients on HAART compared with uninfected patients for the treatment of HL and anal and lung cancers. However, patients with advanced HIV disease and/or aging-related comorbidities are likely to experience worse outcomes and have poorer tolerance of therapy compared with those with less advanced HIV disease.
Implications for Practice:
This review provides information on the epidemiology of non-AIDS-defining cancers (NADCs), important in view of the changing spectrum of AIDS-related malignancies resembling cancers present in HIV-negative population. HIV patients with NADCs experience advanced stages at diagnosis, aggressive behaviors of the disease, poor prognoses, are frequently undertreated and die prematurely. The data reported here have implications in the management of HIV-associated cancers, since prevention and screening programs for HIV-infected persons need to be enforced. Specialists must be aware that evidences suggest that equivalent treatment is feasible, and outcomes can be similar, in HIV-infected patients on effective HAART compared to uninfected patients. Further progress in the treatment of NADCs strongly depends on prospective clinical trials, including drug-drug interaction studies.
Introduction
Life expectancy of HIV-infected persons has constantly improved because of increasingly effective antiretroviral drugs and enhanced management of the global burden of infection [1]. However, the growing proportion of aged HIV-infected persons, the residual immune deficit not controlled by antiretroviral drugs, risky lifestyle factors, and coinfections still put HIV-infected persons at higher risk of cancer than their uninfected counterparts [2–4]. Although AIDS-defining cancers declined overall, the frequency of non-AIDS-defining cancers (NADCs) has been steadily increasing over the past two decades [2, 5–7]. Coinfections with oncogenic viruses are responsible for the largest number of NADCs in HIV-infected persons [8], but NADCs not associated with viral infections (e.g., lung cancer) also play an important role [2, 6, 9].
The impact of antiretroviral therapies on the risk of NADCs and the role of chronic immunoactivation and inflammation in the NADC pathogenesis are debated issues, given that in the era before highly active antiretroviral therapies (HAART), competing causes of mortality concealed a potential association of immune activation and cancer risk. HIV patients with NADCs experience advanced stages at diagnosis, aggressive behaviors of the disease, and poor prognoses. In addition, these patients are frequently undertreated and die prematurely [2, 6, 10, 11].
Immune reconstitution induced by HAART, with improved life expectancy, has modified patterns of morbidity and mortality for most NADCs. Consequently, the interaction between anticancer and antiretroviral drugs became exceedingly complex, and the aging of HIV-infected persons has produced an extra burden of comorbid conditions, polypharmacy, and functional decline. In this evolving scenario, NADC management represents a new challenge in the care of HIV-infected persons.
The purpose of this review is to examine epidemiology, etiology, and some not well-defined pathogenic characteristics of NADCs. Implications related to treatment strategies, comorbidity, and multidrug interactions are also discussed.
Search Strategy
We searched the PubMed database and the Cochrane Library by focusing on NADCs and on the association among NADCs, HAART, aging, and/or chronic inflammation. We then combined the searches with the “AND” boolean operator. We searched the PubMed database using the following combined heading search strategy: (non-aids-defining*[title abstract (tiab)] OR non-AIDS*[tiab] OR (HIV-associated*[ti] AND “not”[ti]) OR nonacquired immune deficiency syndrome[tiab] OR nonaids*[tiab]) AND (neoplasms[mesh] OR malignan*[tiab] OR cancer[tiab] OR tumor*[tiab] OR tumor*[tiab] OR NHL[tiab] OR non-Hodgkin lymphoma[tiab] OR lymphoma*[tiab] OR leukemia*[tiab] OR sarcoma*[tiab] OR lung[tiab] OR anal[tiab] OR hepatocellular[tiab] OR vulva*[tiab] OR vagina*[tiab] OR cervi*[tiab] OR genital*[tiab] OR skin[tiab] OR carcinoma*[tiab] OR commorbities[tiab] OR malignan*[tiab]) (* is the truncation symbol). No restrictions of language or time use included.
The authors reviewed all the articles to identify those reporting quantitative estimates or demographic, epidemiological, clinical, and/or laboratory variables of interest. Studies regarding the pathogenetic mechanisms involved in NADC development were also included.
Epidemiological Considerations
In HIV-infected persons, the spectrum of cancer sites with significantly elevated incidence rates is large. The overall relative risk for all NADCs is about twofold higher than in the general population of the same age and sex, with substantial variations in risk estimates according to cancer types and sites, study period, and geographic area [8]. Although for some cancers, mostly related to viral infections, the relative risk may be elevated more than 50-fold, for the most common epithelial cancers (e.g., colon, breast, and prostate), little evidence of increased risk in patients with HIV/AIDS (PWHA) has accumulated [12–14]. Four types of NADCs deserving special attention are examined in this review: anal cancer (AC), Hodgkin lymphoma (HL), hepatocellular carcinoma (HCC), and lung cancer. Attention was restricted to these malignancies because they were documented to arise at particularly elevated frequencies in nearly all investigations, and they represent a major cause of morbidity and mortality in HIV-infected patients in the HAART era. Moreover, these NADCs still present intriguing etiopathogenetic and clinical issues that are of potential interest for both researchers and clinicians in the field of HIV-associated cancers.
The overall relative risk for all NADCs is about twofold higher than in the general population of the same age and sex, with substantial variations in risk estimates according to cancer types and sites, study period, and geographic area. Although for some cancers, mostly related to viral infections, the relative risk may be elevated more than 50-fold, for the most common epithelial cancers, little evidence of increased risk in patients with HIV/AIDS has accumulated.
Anal Cancer
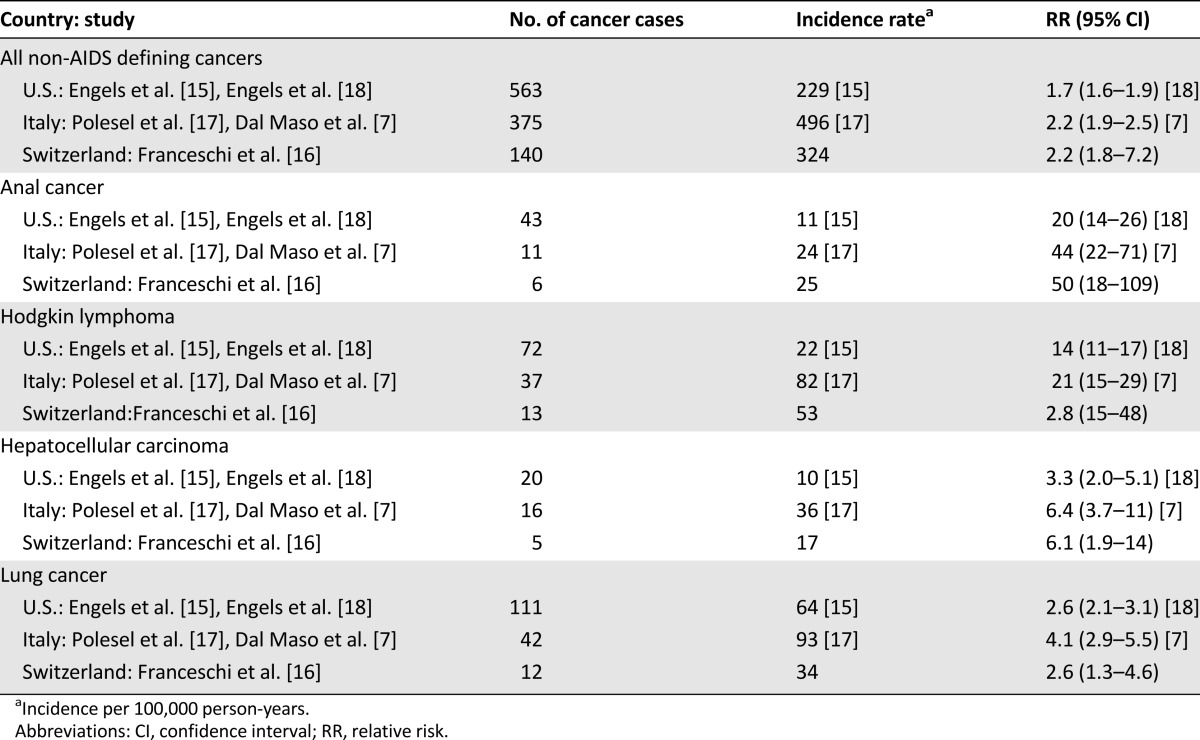
Incidence rates of invasive AC among HIV-infected persons were between 11 and 24 cases per 100,000 HIV-infected people per year in the U.S. and Europe [15–18], with up to 50-fold excess risk (Table 1). Groups at high risk include men who have sex with men, current smokers, and HIV-infected persons with a low number of CD4+ cell counts up to 7 years prior to cancer diagnosis [19]. AC occurs as a consequence of human papillomavirus (HPV) infection, mainly with HPV-16, but the oncogenic mechanisms have yet to be defined; HPV has been postulated to have similar oncogenic mechanisms in the cervix and in the anus, but preclinical models of anal carcinogenesis are not currently available [20, 21]. The role of immune deficiency is uncertain, with AC risk associated with the cumulative duration of low CD4 count and/or high HIV RNA [22], particularly 6–7 years prior to cancer diagnosis [19]. This observation suggests that cancer progression is likely to take place even with immune reconstitution [23].
Table 1.
Crude incidence rates of selected cancer sites in people with HIV/AIDS from registry-linkage studies in the U.S., Italy, and Switzerland in the HAART era
In the HAART era, the prognosis in HIV-infected patients with invasive AC improved steadily, becoming similar to that in the uninfected population [24–26], except in one study [27] (Table 2) with 5-year survival rates ranging from 61% to 77%. Additionally, in the U.S., 2-year overall survival (OS) was 77% in HIV-positive patients and 75% in HIV-negative patients [28]. HAART has improved tolerability to concurrent chemoradiation (CRT), including mitomycin or cisplatin plus 5-fluorouracil. Significant predictors of survival were age, sex, metastasis at diagnosis, and comorbidity score >1 (i.e., 90% increased risk of death) but not HIV status [28].
Table 2.
Outcomes of patients with anal cancer treated with concurrent chemoradiotherapy, by HIV status
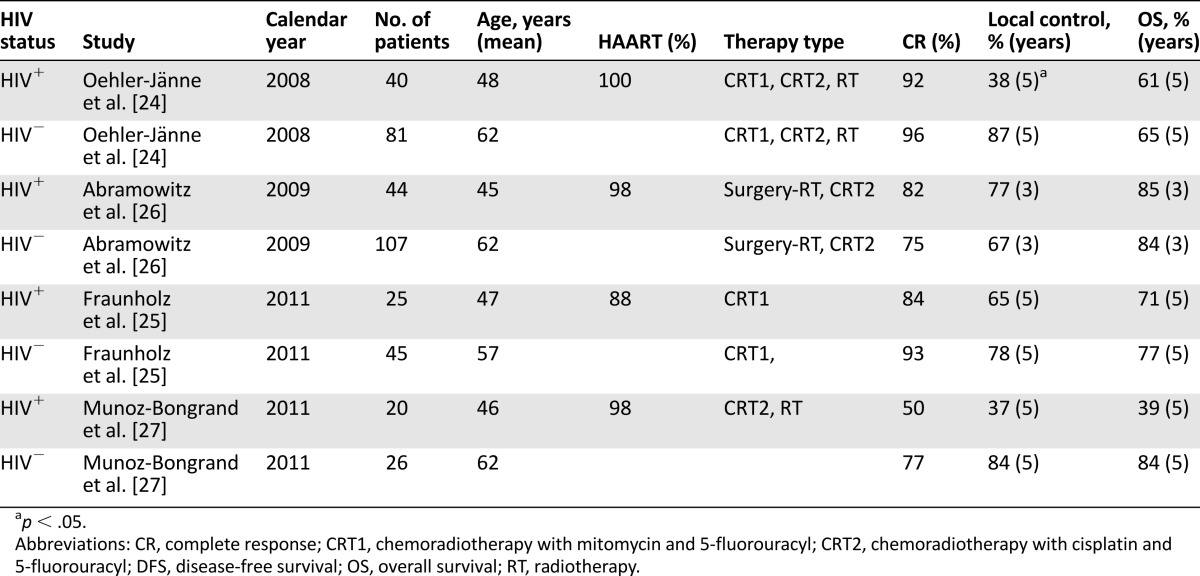
Long-term local tumor control and sphincter preservation remained a major problem in patients with low adherence to radiotherapy (RT) or CRT, longer duration of RT, and reduced use of mitomycin due to high toxicity rates [24]. When appropriate treatments and HIV multidisciplinary care are adopted, HIV-infected patients present treatment compliance and functional outcomes comparable to those observed in HIV-negative patients [25]. The evaluation of comorbidity and toxicity of treatment, including CRT plus HAART, and infection prophylaxis needs to become a key component of the multidisciplinary management of NADCs, including AC. The challenge facing clinicians is to reduce acute toxicity and to improve long-term quality of life of HIV-infected patients with AC. Preliminary results suggest that combination of chemotherapy and intensity-modulated radiation therapy may represent a successful therapeutic strategy for these patients [29]. Concerns still exist about unexpected interactions between antiretroviral drugs and CRT because HIV-infected patients tend to be excluded from clinical trials.
Hodgkin Lymphoma
The incidence rate of HL is 22–82 new cases per 100,000 HIV-infected people per year [15–17], corresponding to a 10- to 20-fold excess risk in comparison with the general population [7, 8, 16, 18] (Table 1).
In the HAART era, the gap in survival after HL between PWHA and the general population remains wide. In the period 1996–2000, 55% of PHWA survived 24 months after diagnosis compared with 89% for HIV-negative persons [30]. The relative risk of death for HL patients among PWHA was estimated at >100 in the period 1999–2006 [11]. The pathology of HIV-associated HL markedly differs from that recorded in the general population. In fact, a greater proportion of mixed cellularity and lymphocyte-depletion subtypes are specifically related to immunosuppression. During HAART, however, the partial restoration of the immune system seemed to be paralleled by an increased risk of developing the nodular sclerosis subtype of HL [31]. However, this suggestion was rebutted by further work [32]. The Epstein–Barr virus (EBV) coinfection is documented in >80% of HL cases seen in HIV-positive persons compared with 40% in the general population [33]. The expression of LMP-1 viral protein suggests that EBV plays a pathogenetic role by mimicking an active CD40 receptor [34, 35].
The association between the degree of immunodeficiency and HL occurrence is controversial. In the Swiss HIV Cohort Study, a lower ratio of CD4+ to CD8+ at 1–2 years before HL diagnosis was significantly associated with increased HL risk, and HL risk did not appear to increase in recent years or among HIV patients using combination antiretroviral therapy in Switzerland. Moreover, there was no evidence that HL risk should be increased in the setting of improved immunity [32].
The sequestration of lymphocytes at the tumor site makes difficult to interpret the role of CD4 cell counts preceding HL onset. Accordingly, the definition of the pathogenetic effects of immunoreconstitution, immune activation and inflammation during HAART emerged as a major challenge in the last few years [36, 37].
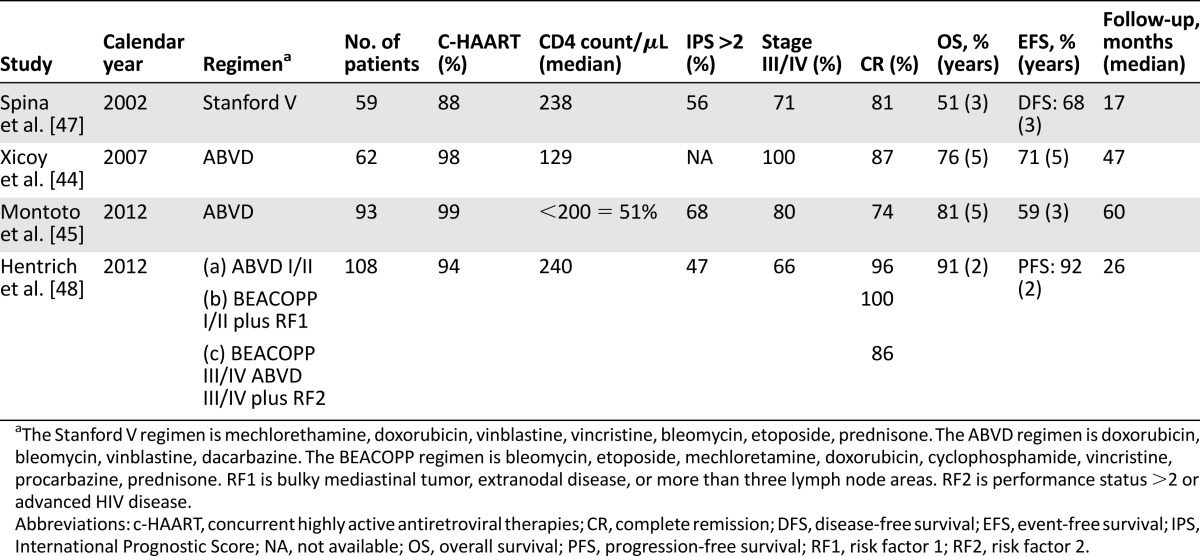
At diagnosis, the majority of HIV-HL patients present unfavorable clinical characteristics, such as B symptoms and advanced stages of disease with involvement of extranodal sites, bone marrow, liver, and spleen [38–41]. Primary bone marrow HL was found in 3%–14% of cases and was characterized by aggressive clinical course [41, 42]. Standard ABVD chemotherapy (doxorubicin, bleomycin, vinblastine, dacarbazine) without HAART resulted in a low complete response (CR) rate (43%) and poor outcome [43]. The concomitant use of HAART caused significant improvement in CR rate (74%–87%), OS (76%–81% at 5 years), and event-free survival (EFS; 59%–72% at 5 years) [44, 45]. The use of granulocyte-colony stimulating factor plus prophylaxis for major opportunistic infections made intensive chemotherapy protocols feasible in HIV-HL. The Stanford V regimen (mechlorethamine, doxorubicin, vinblastine, vincristine, bleomycin, etoposide, prednisone) followed by RT resulted in a CR rate of 81% and estimated 5-year OS of 59% [46]. The VEBEP regimen (epirubicin, bleomycin, vinorelbine, cyclophosphamide, prednisone) was less toxic than Stanford V, but the percentages of CR, OS, and EFS were less favorable [47]. The very intensive BEACOPP regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) has been tested recently in a stage- and risk-adapted treatment study [48]. The outcomes of patients treated with ABVD or BEACOPP followed by RT in the presence of bulky or residual disease were similar in all patients. Treatment-related mortality was close to 6% for all but one of the deaths occurring in patients who received BEACOPP [48]. Data from clinical trials are reported in Table 3. ABVD plus HAART should thus be considered as standard treatment for HIV-HL. Despite these impressive improvements, unresolved concerns related to treatment modalities of HL in HIV-positive patients still exist. In particular, drug-drug interactions cause increased frequency of peripheral neuropathy and hematologic toxicity [49]. To overcome the lack of guidelines for dose adjustment, a HAART regimen including raltegravir, an integrase inhibitor with lower potential for drug interaction than protein inhibitors, and/or sequential toxicity monitoring may be used to reduce side effects in patients on chemotherapy plus HAART [50].
Table 3.
Major clinical trials on HIV-Hodgkin lymphoma in the HAART era
High-dose chemotherapy and autologous stem cell transplantation (ASCT) may represent a possibility for HIV-HL patients after relapse or lymphoma progression [51]. A recent study provided further evidence that HIV status does not affect the long-term outcome of ASCT for lymphomas; therefore, these patients should be included in transplant clinical trials [52].
Hepatocellular Carcinoma
HIV-infected people are at greater risk of acquiring and dying of HCC. In the HAART period, the incidence of liver cancer was 10–36 new cases per 100,000 HIV-infected people per year. This corresponded to threefold to sixfold excess risk in comparison with the general population (Table 1). Moreover, between 1999 and 2006, it was reported that liver cancer was among the most common causes of death for people with AIDS [11, 53, 54].
In persons coinfected with HIV and hepatitis B virus (HBV), HCC pathogenesis presents peculiar characteristics. A mutation in the precore or core region of HBV, common in HIV-HBV coinfected persons, is associated with higher HBV DNA levels than in HBV-monoinfected persons [55], but it is not clear whether this contributes to oncogenicity [56]. HIV may have a direct oncogenic effect on hepatocytes through the Tat protein [57, 58] or an indirect effect through the modulation of the anti-HBV immune response [59]. The pathogenetic mechanisms involved in the development of HCC in persons infected with hepatitis C virus (HCV) and HIV are less defined. For instance, the high levels of intrahepatic HCV detected in HIV-positive patients with ongoing HCV replication may increase inflammation or skew immune response [60]. The relationship between HCC development and immune dysregulation is still under scrutiny. Immune deficiency may favor carcinogenesis [61, 62], but immune activation caused by microbial translocation in the gut may accelerate tumor development [37, 63].
The relationship between HCC development and immune dysregulation is still under scrutiny. Immune deficiency may favor carcinogenesis, but immune activation caused by microbial translocation in the gut may accelerate tumor development.
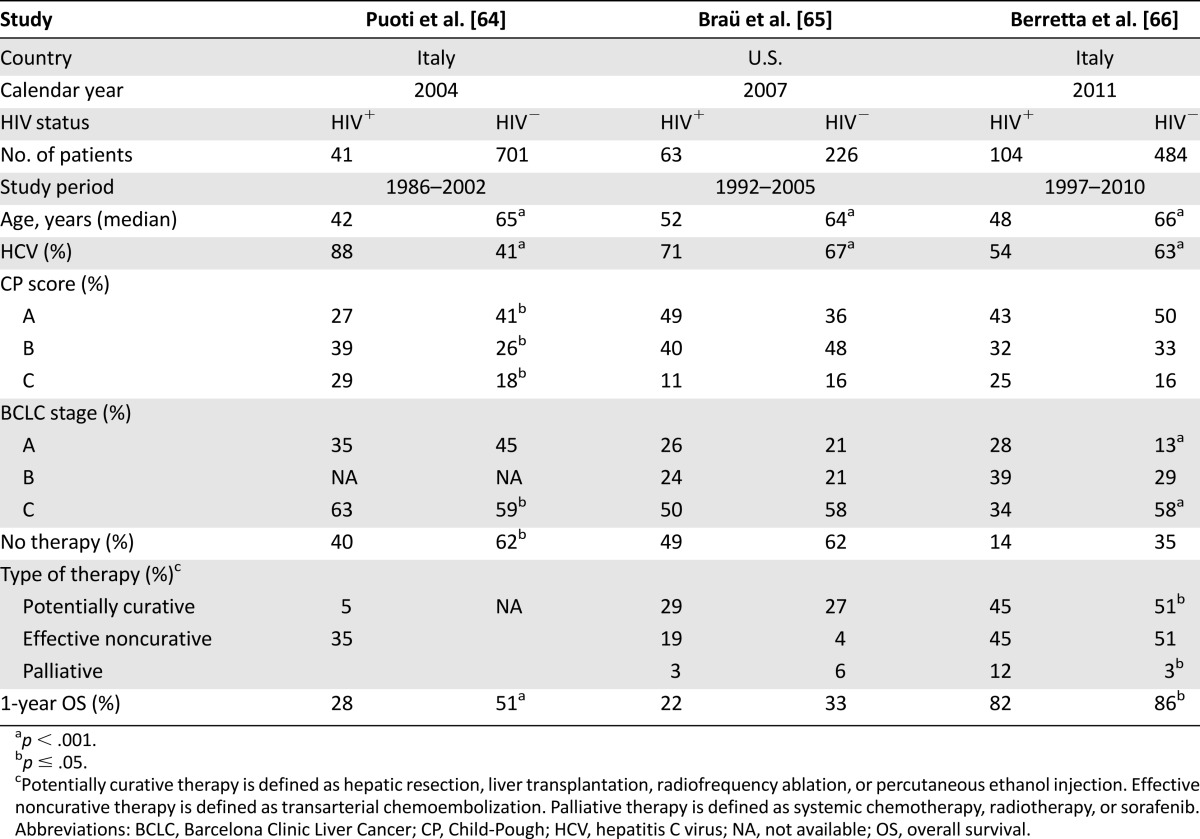
Clinically, most HIV-infected patients present advanced tumor stage with multifocal invasive or extrahepatic lesions (Table 4) [64, 65]. The majority of them received no treatment or received supportive therapy only, whereas one-third underwent nonsurgical locoregional treatment and <10% received hepatic resection. In a recent series, despite HCC diagnosed at an early stage, survival was significantly shorter in HIV-infected persons compared with uninfected persons (median: 35 months vs. 59 months; p = .05). Prognostic factors for survival were retreatment at recurrence and tumor characteristics but not HIV status [66]. Currently, liver transplantation represents another therapeutic option for HIV-infected patients with HCC. However, direct drug-drug interactions and the combination of HAART with antirejection immunosuppressive treatments for liver transplant are concerns and pose challenging questions. Pharmacokinetic interactions between HAART and immunosuppressive drugs can be reduced by the use of new antiretrovirals with low pharmacologic interference. Triple anti-HCV therapy, including the new protease inhibitors telaprevir or boceprevir, in addition to pegylated interferon and ribavirin has shown increase sustained viral clearance in HIV-HCV coinfected patients [61–67]. As previously demonstrated for other NADCs, a multidisciplinary and standardized care approach in combination with effective antiretroviral therapy may ensure the same outcome as that recorded among HIV-negative patients with HCC [68].
Table 4.
Major retrospective studies on hepatocellular carcinoma by HIV
Lung Cancer
In the HAART era, lung cancer was the most frequent NADC diagnosed in PWHA (34–93 new cases per 100,000 HIV-infected people per year). More than twofold excess risk, in comparison with the general population, was reported by almost all studies (Table 1).
The relationship between HIV-related immunosuppression and lung cancer is controversial and strongly influenced by cigarette smoking. In high-resource countries, where >70% of PWHA are smokers [69], these neoplasms are mostly attributable to smoking habits [70, 71], although not completely accounting for the lung cancer increase observed in PWHA during the HAART era [72]. Aging of the HIV-infected population has minimal effects on lung cancer risk [17].
The role of HIV as a cofactor in the development of lung cancer is still controversial. Wistuba et al. [73] found elevated frequencies of microsatellite alterations and of loss of heterozygosity in tissues from HIV-positive patients with lung cancer. Given that viral sequences were absent in neoplastic cells, an indirect effect of HIV was supposed. Conversely, HIV-1 Tat protein modulates proto-oncogene expression in bronchoalveolar carcinoma cell lines, suggesting a direct oncogenic role for HIV [74]. Lung cancer in HIV-positive subjects does not seem closely related to CD4+ cell count or HIV viral load [72, 75], but conflicting data have been reported [21, 76].
Common characteristics of lung cancer in HIV-positive persons include younger age at diagnosis, non-small cell histotype (88%–100%), adenocarcinoma morphology (up to 70%), and late-stage diagnosis, with >75% of cases being stage III or IV [39, 71, 76–80]. Treated and untreated HIV-positive patients have higher mortality rates than HIV-uninfected patients. In the general population, 5-year OS ranges from 80% for stage I to <1% for stage IV disease [81], whereas it is significantly shorter for HIV-infected patients in almost all case-control studies (median: 2–9 months vs. 7–9.4 months, respectively). Major unfavorable prognostic factors for survival were advanced stage of cancer, poor performance status, and lack of appropriate therapy, as in the general population [76–80]. It has been estimated that 55%–90% of HIV-positive lung cancer patients were on HAART, with median CD4+ cell counts not exceeding 300–350 cells per μL [39, 71, 76–80, 82]. The beneficial effects of first-generation HAART regimens were limited, whereas encouraging findings have emerged for modern drug regimens. In an updated series from Italy, the OS rate was significantly better for HAART compared with pre-HAART patient groups (3.8 months vs. 7,0 months; p = .01) [83]. Two other recent series showed that HAART use after non-small cell lung cancer diagnosis significantly increased survival by 74% (hazard ratio [HR]: 0.26; 95% CI: 0.09–0.74) and by 60% (HR: 0.40; 95% CI: 0.20–0.90) [79, 82]. Between 1995 and 2009, patients with local stage were less likely to receive the standard of care treatment, defined as either surgery or radiation [84]. In addition, two-thirds of patients were deemed ineligible for surgery based on advanced cancer stages at presentation, poor performance status, or AIDS-related comorbidities [84]. However, when early stage patients underwent surgery, survival was similar in HIV-infected and uninfected patients [85]. Presently, data from retrospective studies provide evidence that therapeutic decision making for HIV-infected persons with lung cancer must consider HIV disease, HAART use, and comorbidities.
Discussion
In this review we described evidence accumulated in the modern HAART era on NADCs, with respect to epidemiology, etiology, pathogenic characteristics, and treatment strategies. Although population-based studies [30, 86] showed improved survival of PHWA with cancer in recent years, NADCs were still a major cause of death in the HAART era, accounting for approximately 10%–15% of all deaths [54, 86–88].
The bulk of the evidence did not support claims of an adverse influence of HAART per se or of HAART-related immune reconstitution on cancer risk. In addition, the observed pattern of neoplasms in PWHA did not change for at least 10 years after AIDS diagnosis, with a narrowed but persisting gap in OS between PHWA with cancer and persons with cancer alone [30]. Prevention and screening programs for HIV-infected persons need to be mandated, particularly those aimed at smoking and alcohol cessation and treatment of HCV infection and preneoplastic HPV-related lesions.
The mechanisms that HIV may use to promote the development of NADCs are presently unclear; immunologic mechanisms, either immunodeficiency and/or immunoactivation, may play a role. The use of more specific, clinically applicable, immunologic markers is needed to clarify this aspect [2]. Because infectious agents play a prominent role in the development of NADCs, screening and prevention campaigns and early initiation of HAART are expected to have an impact on different virally associated cancers.
Because infectious agents play a prominent role in the development of NADCs, screening and prevention campaigns and early initiation of HAART are expected to have an impact on different virally associated cancers.
HAART is a key component of treatment of all HIV-infected patients with cancer, including NADCs. Studies have suggested that equivalent treatment is feasible and outcomes can be similar in HIV-infected patients on effective HAART compared with uninfected patients, although patients with advanced HIV disease and/or aging-related comorbidities have poorer tolerance for therapy and are likely to experience worse outcomes. Unique strategies for these patients need to be urgently evaluated in prospective clinical trials, including drug-drug interaction studies.
Acknowledgments
This work was supported by the Italian Ministry of Health, Ricerca Corrente Istituto di Ricovero e Cura a Carattere Scientifico, Centro di Riferimento Oncologico, Aviano. We thank Luigina Mei for editorial assistance.
Author Contributions
Conception/Design: Emanuela Vaccher, Diego Serraino, Antonino Carbone, Paolo De Paoli
Provision of study material or patients: Emanuela Vaccher, Diego Serraino, Antonino Carbone, Paolo De Paoli
Collection and/or assembly of data: Emanuela Vaccher, Diego Serraino, Antonino Carbone, Paolo De Paoli
Data analysis and interpretation: Emanuela Vaccher, Diego Serraino, Antonino Carbone, Paolo De Paoli
Manuscript writing: Emanuela Vaccher, Diego Serraino, Antonino Carbone, Paolo De Paoli
Final approval of manuscript: Emanuela Vaccher, Diego Serraino, Antonino Carbone, Paolo De Paoli
Disclosures
The authors indicated no financial relationships.
References
- 1.Wada N, Jacobson LP, Cohen M, et al. Cause specific life expectancies after 35 years of age for HIV-infected and human immunodeficiency syndrome negative individuals followed simultaneously in long term cohort studies, 1984-2008. Am J Epidemiol. 2013;177:116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engels EA. Non-AIDS-defining malignancies in HIV-infected persons: Etiologic puzzles, epidemiologic perils, prevention opportunities. AIDS. 2009;23:875–885. doi: 10.1097/QAD.0b013e328329216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grulich AE, Jin F, Poynten IM, et al. HIV, cancer, and aging. Sex Health. 2011;8:521–525. doi: 10.1071/SH11048. [DOI] [PubMed] [Google Scholar]
- 4.Greene M, Justice A, Lampiris H, et al. Management of human immunodeficiency virus infection in advanced age. JAMA. 2013;309:1397–1405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powles T, Robinson D, Stebbing J, et al. Highly active antiretroviral therapy and the incidence of non-AIDS-defining cancers in people with HIV infection. J Clin Oncol. 2009;27:884–890. doi: 10.1200/JCO.2008.19.6626. [DOI] [PubMed] [Google Scholar]
- 6.Deeken JF, Tjen-A-Looi A, Rudek MA, et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis. 2012;55:1228–1235. doi: 10.1093/cid/cis613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dal Maso L, Polesel J, Serraino D, et al. Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer. 2009;100:840–847. doi: 10.1038/sj.bjc.6604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.A Review of Human Carcinogens: Biological Agents. Vol 100B. Lyon, France: International Agency for Research on Cancer; 2012. [Google Scholar]
- 9.Silverberg MJ, Chao C, Leyden WA, et al. HIV infection and the risk of cancers with and without a known infectious cause. AIDS. 2009;23:2337–2345. doi: 10.1097/QAD.0b013e3283319184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achenbach CJ, Cole SR, Kitahata MM, et al. Mortality after cancer diagnosis in HIV-infected individuals treated with antiretroviral therapy. AIDS. 2011;25:691–700. doi: 10.1097/QAD.0b013e3283437f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zucchetto A, Suligoi B, De Paoli A, et al. Excess mortality for non–AIDS-defining cancers among people with AIDS. Clin Infect Dis. 2010;51:1099–1101. doi: 10.1086/656629. [DOI] [PubMed] [Google Scholar]
- 12.Serraino D, Piselli P, Busnach G, et al. Risk of cancer following immunosuppression in organ transplant recipients and in HIV-positive individuals in southern Europe. Eur J Cancer. 2007;43:2117–2123. doi: 10.1016/j.ejca.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 14.Shiels MS, Cole SR, Kirk GD, et al. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 16.Franceschi S, Lise M, Clifford GM, et al. Changing patterns of cancer incidence in the early- and late-HAART periods: The Swiss HIV Cohort Study. Br J Cancer. 2010;103:416–422. doi: 10.1038/sj.bjc.6605756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polesel J, Franceschi S, Suligoi B, et al. Cancer incidence in people with AIDS in Italy. Int J Cancer. 2010;127:1437–1445. doi: 10.1002/ijc.25153. [DOI] [PubMed] [Google Scholar]
- 18.Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 19.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: A systematic review and meta analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 20.Hoots BE, Palefsky J, Pimenta J, et al. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124:2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- 21.Guiguet M, Boué F, Cadranel J, et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): A prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 22.Bertisch B, Franceschi S, Lise M, et al. Risk factors for anal cancer in persons infected with HIV: A nested case-control study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013;178:877–884. doi: 10.1093/aje/kwt153. [DOI] [PubMed] [Google Scholar]
- 23.Engels EA, Madeleine MM. Invited commentary: Biological and clinical insights from epidemiologic research into HIV, HPV, and anal cancer. Am J Epidemiol. 2013;178:885–887. doi: 10.1093/aje/kwt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oehler-Jänne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: A multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol. 2008;26:2550–2557. doi: 10.1200/JCO.2007.15.2348. [DOI] [PubMed] [Google Scholar]
- 25.Fraunholz I, Rabeneck D, Gerstein J, et al. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for anal carcinoma: Are there differences between HIV-positive and HIV-negative patients in the era of highly active antiretroviral therapy? Radiother Oncol. 2011;98:99–104. doi: 10.1016/j.radonc.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Abramowitz L, Mathieu N, Roudot-Thoraval F, et al. Epidermoid anal cancer prognosis comparison among HIV+ and HIV- patients. Aliment Pharmacol Ther. 2009;30:414–421. doi: 10.1111/j.1365-2036.2009.04026.x. [DOI] [PubMed] [Google Scholar]
- 27.Munoz-Bongrand N, Poghosyan T, Zohar S, et al. Anal carcinoma in HIV-infected patients in the era of antiretroviral therapy: A comparative study. Dis Colon Rectum. 2011;54:729–735. doi: 10.1007/DCR.0b013e3182137de9. [DOI] [PubMed] [Google Scholar]
- 28.Chiao EY, Giordano TP, Richardson P, et al. Human immunodeficiency virus-associated squamous cell cancer of the anus: Epidemiology and outcomes in the highly active antiretroviral therapy era. J Clin Oncol. 2008;26:474–479. doi: 10.1200/JCO.2007.14.2810. [DOI] [PubMed] [Google Scholar]
- 29.Salama JK, Mell LK, Schomas DA, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: A multicenter experience. J Clin Oncol. 2007;25:4581–4586. doi: 10.1200/JCO.2007.12.0170. [DOI] [PubMed] [Google Scholar]
- 30.Biggar RJ, Engels EA, Ly S, et al. Survival after cancer diagnosis in persons with AIDS. J Acquir Immune Defic Syndr. 2005;39:293–299. doi: 10.1097/01.qai.0000164033.02947.e3. [DOI] [PubMed] [Google Scholar]
- 31.Biggar RJ, Jaffe ES, Goedert JJ, et al. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108:3786–3791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clifford GM, Rickenbach M, Lise M, et al. Hodgkin lymphoma in the Swiss HIV Cohort Study. Blood. 2009;113:5737–5742. doi: 10.1182/blood-2009-02-204172. [DOI] [PubMed] [Google Scholar]
- 33.Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 34.Grogg KL, Miller RF, Dogan A. HIV infection and lymphoma. J Clin Pathol. 2007;60:1365–1372. doi: 10.1136/jcp.2007.051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carbone A, Cabras A, Gloghini A. HIV-associated Hodgkin’s lymphoma. Antiapoptotic pathways and mechanisms for immune escape by tumor cells in the setting of improved immunity. Int J Biol Markers. 2007;22:161–163. doi: 10.1177/172460080702200211. [DOI] [PubMed] [Google Scholar]
- 36.Novak RM, Richardson JT, Buchacz K, et al. Immune reconstitution inflammatory syndrome: Incidence and implications for mortality. AIDS. 2012;26:721–730. doi: 10.1097/QAD.0b013e3283511e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajasuriar R, Khoury G, Kamarulzaman A, et al. Persistent immune activation in chronic HIV infection: Do any interventions work? AIDS. 2013;27:1199–1208. doi: 10.1097/QAD.0b013e32835ecb8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tirelli U, Errante D, Dolcetti R, et al. Hodgkin’s disease and human immunodeficiency virus infection: Clinicopathologic and virologic features of 114 patients from the Italian Cooperative Group on AIDS and Tumors. J Clin Oncol. 1995;13:1758–1767. doi: 10.1200/JCO.1995.13.7.1758. [DOI] [PubMed] [Google Scholar]
- 39.Vaccher E, Spina M, Tirelli U. Clinical aspects and management of Hodgkin’s disease and other tumours in HIV-infected individuals. Eur J Cancer. 2001;37:1306–1315. doi: 10.1016/s0959-8049(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 40.Carbone A, Spina M, Gloghini A, et al. Classical Hodgkin’s lymphoma arising in different host’s conditions: Pathobiology parameters, therapeutic options, and outcome. Am J Hematol. 2011;86:170–179. doi: 10.1002/ajh.21910. [DOI] [PubMed] [Google Scholar]
- 41.Martis N, Mounier N. Hodgkin lymphoma in patients with HIV infection: A review. Curr Hematol Malig Rep. 2012;7:228–234. doi: 10.1007/s11899-012-0125-2. [DOI] [PubMed] [Google Scholar]
- 42.van Schalkwyk WA, Opie J, Novitzky N. The diagnostic utility of bone marrow biopsies performed for the investigation of fever and/or cytopenias in HIV-infected adults at Groote Schuur Hospital, Western Cape, South Africa. Int J Lab Hematol. 2011;33:258–266. doi: 10.1111/j.1751-553X.2010.01280.x. [DOI] [PubMed] [Google Scholar]
- 43.Levine AM, Li P, Cheung T, et al. Chemotherapy consisting of doxorubicin, bleomycin, vinblastine, and dacarbazine with granulocyte-colony-stimulating factor in HIV-infected patients with newly diagnosed Hodgkin’s disease: A prospective, multi-institutional AIDS clinical trials group study (ACTG 149) J Acquir Immune Defic Syndr. 2000;24:444–450. doi: 10.1097/00126334-200008150-00009. [DOI] [PubMed] [Google Scholar]
- 44.Xicoy B, Ribera JM, Miralles P, et al. Results of treatment with doxorubicin, bleomycin, vinblastine and dacarbazine and highly active antiretroviral therapy in advanced stage, human immunodeficiency virus-related Hodgkin’s lymphoma. Haematologica. 2007;92:191–198. doi: 10.3324/haematol.10479. [DOI] [PubMed] [Google Scholar]
- 45.Montoto S, Shaw K, Okosun J, et al. HIV status does not influence outcome in patients with classical Hodgkin lymphoma treated with chemotherapy using doxorubicin, bleomycin, vinblastine, and dacarbazine in the highly active antiretroviral therapy era. J Clin Oncol. 2012;30:4111–4116. doi: 10.1200/JCO.2011.41.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spina M, Gabarre J, Rossi G, et al. Stanford V regimen and concomitant HAART in 59 patients with Hodgkin disease and HIV infection. Blood. 2002;100:1984–1988. doi: 10.1182/blood-2002-03-0989. [DOI] [PubMed] [Google Scholar]
- 47.Spina M, Rossi G, Antinori A, et al. VEBEP regimen and highly active antiretroviral therapy (HAART) in patients (PTS) with HD and HIV infection (HD-HIV) Ann Oncol. 2008;19(suppl 4):iv152. Abstract 227. [Google Scholar]
- 48.Hentrich M, Berger M, Wyen C, et al. Stage-adapted treatment of HIV-associated Hodgkin lymphoma: Results of a prospective multicenter study. J Clin Oncol. 2012;30:4117–4123. doi: 10.1200/JCO.2012.41.8137. [DOI] [PubMed] [Google Scholar]
- 49.Corona G, Vaccher E, Spina M, et al. Potential hazard drug-drug interaction between boosted protease inhibitors and vinblastine in HIV patients with Hodgkin’s lymphoma. AIDS. 2013;27:1033–1035. doi: 10.1097/QAD.0b013e32835e0777. [DOI] [PubMed] [Google Scholar]
- 50.Rudek MA, Flexner C, Ambinder RF. Use of antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet Oncol. 2011;12:905–912. doi: 10.1016/S1470-2045(11)70056-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michieli M, Mazzucato M, Tirelli U, et al. Stem cell transplantation for lymphoma patients with HIV infection. Cell Transplant. 2011;20:351–370. doi: 10.3727/096368910X528076. [DOI] [PubMed] [Google Scholar]
- 52.Spitzer TR, Ambinder RF, Lee JY, et al. Dose-reduced busulfan, cyclophosphamide, and autologous stem cell transplantation for human immunodeficiency virus-associated lymphoma: AIDS Malignancy Consortium study 020. Biol Blood Marrow Transplant. 2008;14:59–66. doi: 10.1016/j.bbmt.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hessamfar-Bonarek M, Morlat P, Salmon D, et al. Causes of death in HIV-infected women: Persistent role of AIDS. The ‘Mortalité 2000 & 2005’ Surveys (ANRS EN19) Int J Epidemiol. 2010;39:135–146. doi: 10.1093/ije/dyp300. [DOI] [PubMed] [Google Scholar]
- 54.Antiretroviral Therapy Cohort Collaboration Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996-2006: Collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Revill PA, Littlejohn M, Ayres A, et al. Identification of a novel hepatitis B virus precore/core deletion mutant in HIV/hepatitis B virus co-infected individuals. AIDS. 2007;21:1701–1710. doi: 10.1097/QAD.0b013e32826fb305. [DOI] [PubMed] [Google Scholar]
- 56.Cabuang LM, Shaw T, Littlejohn M, et al. In vitro replication phenotype of a novel (-1G) hepatitis B virus variant associated with HIV co-infection. J Med Virol. 2012;84:1166–1176. doi: 10.1002/jmv.23328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel BE, Lee SJ, Hildebrand A, et al. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol. 1993;121:461–468. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altavilla G, Caputo A, Trabanelli C, et al. Prevalence of liver tumours in HIV-1 tat-transgenic mice treated with urethane. Eur J Cancer. 2004;40:275–283. doi: 10.1016/j.ejca.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 59.Thio C. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49:S138–S145. doi: 10.1002/hep.22883. [DOI] [PubMed] [Google Scholar]
- 60.Tedeschi R, Pivetta E, Zanussi S, et al. Quantification of hepatitis C virus (HCV) in liver specimens and sera from patients with human immunodeficiency virus coinfection by using the Versant HCV RNA 3.0 (branched DNA-based) DNA assay. J Clin Microbiol. 2003;41:3046–3050. doi: 10.1128/JCM.41.7.3046-3050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ioannou GN, Bryson CL, Weiss NS, et al. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57:249–257. doi: 10.1002/hep.25800. [DOI] [PubMed] [Google Scholar]
- 62.MacDonald D, Nelson M, Bower M, et al. Hepatocellular carcinoma, human immunodeficiency virus and viral hepatitis in the HAART era. World J Gastroenterol. 2008;14:1657–1663. doi: 10.3748/wjg.14.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puoti M, Bruno R, Soriano V, et al. Hepatocellular carcinoma in HIV-infected patients: Epidemiological features, clinical presentation and outcome. AIDS. 2004;18:2285–2293. doi: 10.1097/00002030-200411190-00009. [DOI] [PubMed] [Google Scholar]
- 65.Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: A U.S.-Canadian multicenter study. J Hepatol. 2007;47:527–537. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 66.Berretta M, Garlassi E, Cacopardo B, et al. Hepatocellular carcinoma in HIV-infected patients: Check early, treat hard. The Oncologist. 2011;16:1258–1269. doi: 10.1634/theoncologist.2010-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sulkowski MS. Current management of hepatitis C virus infection in patients with HIV co-infection. J Infect Dis. 2013;207(suppl 1):S26–S32. doi: 10.1093/infdis/jis764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim C, Goutte N, Gervais A, et al. Standardized care management ensures similar survival rates in HIV-positive and HIV-negative patients with hepatocellular carcinoma. J Acquir Immune Defic Syndr. 2012;61:581–587. doi: 10.1097/QAI.0b013e31826ebdc7. [DOI] [PubMed] [Google Scholar]
- 69.Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV Cohort Study: Associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 70.Tobacco Smoke and Involuntary Smoking. Vol 83. Lyon, France: International Agency for Research on Cancer; 2004. [Google Scholar]
- 71.Tirelli U, Spina M, Sandri S, et al. Lung carcinoma in 36 patients with human immunodeficiency virus infection. Cancer. 2000;88:563–569. doi: 10.1002/(sici)1097-0142(20000201)88:3<563::aid-cncr11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 72.Chaturvedi AK, Pfeiffer RM, Chang L, et al. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21:207–213. doi: 10.1097/QAD.0b013e3280118fca. [DOI] [PubMed] [Google Scholar]
- 73.Wistuba II, Behrens C, Milchgrub S, et al. Comparison of molecular changes in lung cancers in HIV-positive and HIV-indeterminate subjects. JAMA. 1998;279:1554–1559. doi: 10.1001/jama.279.19.1554. [DOI] [PubMed] [Google Scholar]
- 74.el-Solh A, Kumar NM, Nair MP, et al. An RGD containing peptide from HIV-1 Tat-(65-80) modulates protooncogene expression in human bronchoalveolar carcinoma cell line, A549. Immunol Invest. 1997;26:351–370. doi: 10.3109/08820139709022692. [DOI] [PubMed] [Google Scholar]
- 75.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS. 2012;26:1017–1025. doi: 10.1097/QAD.0b013e328352d1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pakkala S, Chen Z, Rimland D, et al. Human immunodeficiency virus-associated lung cancer in the era of highly active antiretroviral therapy. Cancer. 2012;118:164–172. doi: 10.1002/cncr.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brock MV, Hooker CM, Engels EA, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: Implications for patient care. J Acquir Immune Defic Syndr. 2006;43:47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 78.Hakimian R, Fang H, Thomas L, et al. Lung cancer in HIV-infected patients in the era of highly active antiretroviral therapy. J Thorac Oncol. 2007;2:268–272. doi: 10.1097/01.JTO.0000263707.31202.d7. [DOI] [PubMed] [Google Scholar]
- 79.Lavolé A, Chouaïd C, Baudrin L, et al. Effect of highly active antiretroviral therapy on survival of HIV infected patients with non-small-cell lung cancer. Lung Cancer. 2009;65:345–350. doi: 10.1016/j.lungcan.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 80.D’Jaen GA, Pantanowitz L, Bower M, et al. Human immunodeficiency virus-associated primary lung cancer in the era of highly active antiretroviral therapy: A multi-institutional collaboration. Clin Lung Cancer. 2010;11:396–404. doi: 10.3816/CLC.2010.n.051. [DOI] [PubMed] [Google Scholar]
- 81.Goldstraw P, Crowley J. The International Association for the Study of Lung Cancer international staging project on lung cancer. J Thorac Oncol. 2006;1:281–286. [Google Scholar]
- 82.Makinson A, Tenon JC, Eymard-Duvernay S, et al. Human immunodeficiency virus infection and non-small cell lung cancer: Survival and toxicity of antineoplastic chemotherapy in a cohort study. J Thorac Oncol. 2011;6:1022–1029. doi: 10.1097/JTO.0b013e318217b6e0. [DOI] [PubMed] [Google Scholar]
- 83.Bearz A, Vaccher E, Talamini R, et al. Comment on ‘Lung cancer in the Swiss HIV Cohort Study: Role of smoking, immunodeficiency and pulmonary infection’. Br J Cancer. 2012;106:1899–1900. doi: 10.1038/bjc.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suneja G, Shiels MS, Melville SK, et al. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS. 2013;27:459–468. doi: 10.1097/QAD.0b013e32835ad56e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rengan R, Mitra N, Liao K, et al. Effect of HIV on survival in patients with non-small-cell lung cancer in the era of highly active antiretroviral therapy: A population-based study. Lancet Oncol. 2012;13:1203–1209. doi: 10.1016/S1470-2045(12)70466-7. [DOI] [PubMed] [Google Scholar]
- 86.Serraino D, Zucchetto A, Suligoi B, et al. Survival after AIDS diagnosis in Italy, 1999-2006: A population-based study. J Acquir Immune Defic Syndr. 2009;52:99–105. doi: 10.1097/QAI.0b013e3181a4f663. [DOI] [PubMed] [Google Scholar]
- 87.Lewden C, May T, Rosenthal E, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalité 2000 and 2005” surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 88.Marin B, Thiébaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]


