Abstract
An association of hepatitis C virus (HCV) infection with diabetes has been reported in many studies, but few have been population-based and applied standard criteria for diabetes diagnosis. We examined this relationship using recent population-based data from the U.S. National Health and Nutrition Examination Survey. 15,128 adult participants in the 1999–2010 surveys had data on diabetes status and serum HCV antibody (anti-HCV) or HCV RNA. Using American Diabetes Association criteria, diabetes was defined as a health care provider diagnosis, serum hemoglobin A1C (A1C) ≥6.5%, or fasting plasma glucose (FPG) ≥126 mg/dL; pre-diabetes as A1C 5.7%–<6.5% or FPG 100–<126 mg/dL; and normal glucose as A1C <5.7% and FPG <100 mg/dL. Odds ratios (OR) for diabetes and pre-diabetes, comparing persons with HCV infection to those without, were adjusted for demographics, BMI, C-reactive protein, smoking, drinking, and blood transfusion before 1992. Among participants without diabetes, we compared mean insulin resistance, estimated using homeostasis model assessment (HOMA-IR), by HCV status. The overall prevalence of anti-HCV+ was 1.7%, of HCV RNA+, 1.1%, of diabetes, 10.5%, and of pre-diabetes, 32.8%. The prevalence of diabetes and pre-diabetes did not differ by HCV status. In multivariate-adjusted analysis, diabetes remained unassociated with anti-HCV (OR=1.0, 95% confidence interval (CI), 0.6–1.7) or with HCV RNA (OR=1.1, 95% CI, 0.6–1.9). In contrast, elevated alanine aminotransferase and gamma glutamyltransferase activities were associated with diabetes regardless of HCV status. HOMA-IR was not associated with HCV markers in unadjusted or multivariate-adjusted analyses (p>0.05).
Conclusion
In the U.S. population, HCV was not associated with diabetes, or with insulin resistance among persons with normal glucose. Previously reported relationships of HCV with diabetes were possibly attributable to the effect of elevated liver enzymes.
Keywords: insulin resistance, alanine aminotransferase, gamma glutamyltransferase, National Health and Nutrition Examination Survey, epidemiology
Both hepatitis C virus (HCV) infection and diabetes are common conditions with an estimated 3.2 million persons chronically infected with HCV in the U.S.(1) and 25.8 million suffering from diabetes.(2) An association of HCV infection with type 2 diabetes has been reported since shortly after the discovery of HCV in 1989. For example, in two meta-analyses of a total of 47 unique studies, HCV was associated with diabetes with an adjusted odds ratio (OR) of 1.7 (95% confidence interval (CI), 1.2–2.2) in cross-sectional studies and an adjusted hazard ratio (HR) of 1.7 (95% CI, 1.3–2.1) in cohort studies in the first report(3) and an overall odds ratio of 1.7 (95% CI, 1.2–2.4) in the second report.(4) However, many of these studies were based on clinical series and may have suffered from ascertainment bias. Among the few population-based studies that have been conducted, results were inconsistent. In the third U.S. National Health and Nutrition Examination Survey (NHANES), 1988–1994, a non-statistically significant association was found overall, while a stronger relationship was reported among persons aged 40+ years.(5) In a U.S. community-based prospective study, persons with HCV infection had a non-statistically significant increased risk of diabetes overall.(6) An elevated HR was found among persons at high risk for diabetes, but was based on a small number of HCV positive cases. The strongest population-based association of HCV infection with diabetes risk was found in a Taiwanese community-based cohort study (HR, 1.7; 95% CI, 1.3–2.1).(7) In contrast, in a recent Italian population-based cohort study, HCV infection was unrelated to incident diabetes in multivariate-adjusted analysis.(8) An association of HCV infection with insulin resistance has also been reported in clinical series,(9) HCV treatment studies,(10–12), in comparison with hepatitis B virus (HBV)-infected controls,(13) and inconsistently in population-based studies.(6, 14, 15) We examined the association of HCV infection with diabetes using recent U.S. national population-based data. Additionally, we examined the relationship of HCV infection with insulin resistance among persons without diabetes.
MATERIALS AND METHODS
The NHANES is a cross-sectional study conducted in the United States by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) and since 1999 has been a continuous survey in 2-year cycles.(16) It consists of interview, examination, and laboratory data collected from a complex multistage, stratified, clustered probability sample representative of the civilian, non-institutionalized population with oversampling of persons age 60 years and older, African Americans, and Mexican Americans. The survey was approved by the CDC Institutional Review Board and all participants provided written informed consent to participate. The current analysis utilized data collected from 1999 through 2010.
A serum sample was collected and processed within 1 hour, frozen, and shipped weekly to testing laboratories. Specimens were screened for HCV by the Ortho HCV enzyme-linked immunosorbent assay (ELISA), version 3.0 (Ortho-Clinical Diagnostics, Raritan, NJ) in NHANES 1999–2008 or the VITROS Anti-HCV assay (Ortho-Clinical Diagnostics, Raritan, NJ) in NHANES 2009–2010. Positives were tested with recombinant immunoblot assay (RIBA) (Chiron RIBA HCV Strip Immunoblot Assay, version 3.0, Chiron Corporation, Inc., Emeryville, CA) at the CDC.(17, 18) Specimens classified as positive or indeterminate by RIBA were tested for HCV RNA with a quantitative nucleic acid amplification test (Roche Cobas 7 Amplicor HCV Monitor Test, version 2.0, Roche Molecular Diagnostics, Pleasanton, CA). If the result was below the level of detection, a qualitative assay (Amplicor HCV Test, version 2.0, Roche Molecular Diagnostics) was performed.(19) Participants with samples positive by quantitative or qualitative tests were considered to be infected with HCV. A positive test for HCV antibody (anti-HCV) indicates either past or current infection. A positive test for HCV RNA indicates current infection.
Serum hemoglobin A1C (A1C) was measured using a Primus Automated HPLC system (1999–2004), Tosoh A1C 2.2 Plus Glycohemoglobin Analyzer (2005–2006), or Tosoh G7 Automated HPLC Analyzer (2007–2010), which had reportable ranges of 2.0–20%, 3.4–18.8%, and 3.0–19.0%, respectively. The inter-assay coefficient of variation ranged from 0.7–2.0%. Fasting glucose was measured in plasma by a hexokinase method using a Roche Cobas Mira Analyzer (1999–2004), Roche/Hitachi 911 Analyzer (2005–2006), or Roche Modular P Chemistry Analyzer (2007–2010), which had an analytical measurement range of 2–600 mg/dL, 2–750 mg/dL, and 0–750 mg/dL, respectively. The inter-assay coefficient of variation ranged from 0.8–3.0%. In accordance with American Diabetes Association criteria, diagnosed diabetes was defined as a health care provider diagnosis; persons without diagnosed diabetes were defined as having undiagnosed diabetes by A1C ≥6.5% or fasting plasma glucose ≥126 mg/dL; pre-diabetes by A1C ≥5.7%–<6.5% or fasting glucose ≥100–<126 mg/dL; and normal glucose by A1C <5.7% and fasting glucose <100 mg/dL. Fasting insulin was measured in serum by a radioimmunoassay using a Berthold Multi-Crystal Gamma Counter (1999–2002) or two-site immunoenzymometric assay using a Tosoh AIA-PACK IRI (2003–2004), Beckman Coulter Biomek 2000 (2005–2008), or Roche Elecsys 2010 (2009–2010). The inter-assay coefficient of variation ranged from 2.0–9.1%. Insulin resistance was estimated by homeostasis model assessment (HOMA-IR) using the formula: fasting serum insulin (μU/mL) * fasting plasma glucose (mmol/L) / 22.5.(20)
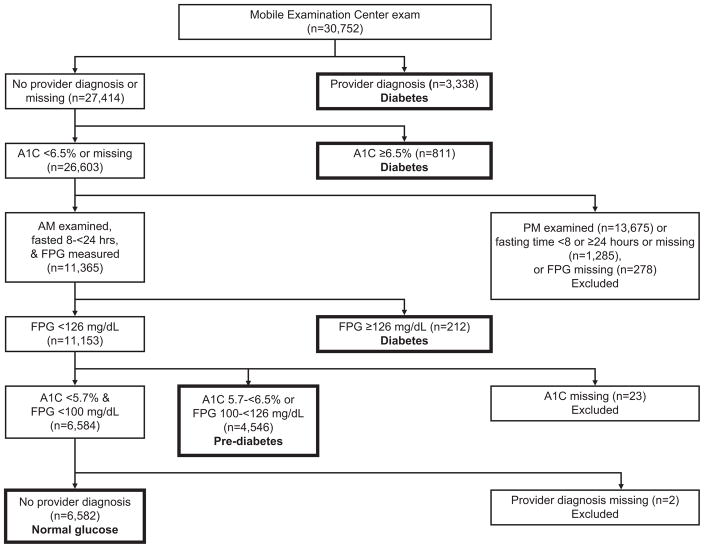
Of 43,426 sampled persons aged 20 years and older, 30,752 (71%) attended an examination at a mobile examination center. One-half of participants were randomly assigned to a morning examination and asked to fast and the other half were assigned to an afternoon or evening examination. Because persons assigned to the PM group were not asked to fast, this analysis was limited to the morning sample and to PM participants with diabetes based on a provider diagnosis or an elevated A1C. Diabetes status could be determined on 15,489 persons after the following exclusions (Figure 1): examined in the afternoon or evening (n=13,675), fasted < 8 or ≥24 hours or fasting time missing (n=1,285), or data missing on fasting plasma glucose (n=278), A1C (n=23), or provider diagnosis (n=2). We also excluded participants for whom anti-HCV was indeterminate (n=59) or missing (n=305), resulting in a sample of 15,125 for analyses of anti-HCV. HCV RNA analyses excluded 363 persons for whom RNA was missing and 90 who were RNA negative, but either anti-HCV positive or indeterminate, resulting in a sample of 15,036. Therefore, the comparison group for both markers consisted of 14,848 anti-HCV negative participants. There were 277 participants positive for anti-HCV and 188 positive for HCV RNA. The total analysis sample consisted of 15,128 participants with known glucose and HCV status (15,125 with data on anti-HCV and 3 RNA+ persons who were anti-HCV indeterminate). Included in this sample were 3,082 cases with diagnosed diabetes, 1,006 with undiagnosed diabetes, 4,511 with pre-diabetes, and 6,529 controls with normal glucose. Because the glucose status of almost half the examined sample could not be determined with certainty (primarily because participants randomly assigned to an afternoon or evening examination were not asked to fast), excluded participants were analyzed as a separate category compared to persons with normal glucose and found to have a similar prevalence of HCV markers and risk factors for diabetes (data not shown). Analyses of HCV and HOMA-IR excluded participants with diabetes or who were missing data on serum insulin level, leaving 11,962 persons for anti-HCV analyses and 11,896 persons for HCV RNA analyses.
Figure 1.
Derivation of the analysis sample with known diabetes status. A1C, hemoglobin A1C; FPG, fasting plasma glucose.
Data were collected on factors known or thought to be related to HCV or diabetes and included as covariates in multivariate analyses: age (years), sex, race-ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, other), education (years; <12, 12, >12), body mass index (BMI; weight [kg]/height [m2]), waist circumference (cm), serum triglycerides (mg/dL) and high-density lipoprotein (HDL) cholesterol (mg/dL), systolic and diastolic blood pressure (mmHg), C-reactive protein (mg/dL; 0–0.3, >0.3), cigarette smoking (never, former, <1 pack/day, ≥1 pack/day), alcohol consumption (drinks/day; 0, <1, 1–2, >2), and blood transfusion before 1992. Additional HCV-related factors only available in a subgroup of participants included previous use of illegal injection drugs (asked of persons 20–59 years), and age at first sexual intercourse (years; <18, ≥18) and lifetime number of sexual partners (≤20, >20) (asked of persons 20–59 years from 1999–2006 or 20–69 years from 2007–2010). Alanine aminotransferase (ALT) and gamma glutamyltransferase (GGT) activities (IU/L) were measured(21–24) and categorized as normal or elevated using cut-points of >43 for men or >31 for women for ALT and >58 for men or >35 for women for GGT. Liver enzyme cut-points were the 95th percentiles among persons at low risk for liver injury (i.e. negative for HCV antibody and hepatitis B virus surface antigen, alcohol consumption ≤2 drinks/day for men and ≤1 drink/day for women, BMI <25 kg/m2, waist circumference ≤102 cm for men and ≤88 cm for women, no provider-diagnosed diabetes, and A1C <6.5%).
Statistical analysis
Characteristics of participants with normal glucose, pre-diabetes, undiagnosed diabetes, and diagnosed diabetes were examined by comparing means (standard deviations) of continuous variables using a t-test and percentages of categorical variables using a χ2 test. The unadjusted prevalence of diabetes and pre-diabetes by HCV infection status was compared using a χ2 test. OR estimates and 95% CI for diabetes and pre-diabetes, comparing persons with HCV infection to those without, were calculated using polychotomous logistic regression analysis (SUDAAN, PROC MULTILOG, SUDAAN User’s Manual, Release 10.0, 2008; RTI, Research Triangle Park, NC). Models were adjusted for age alone and for demographics, BMI, C-reactive protein, smoking, drinking, and blood transfusion before 1992. Because liver enzyme elevation is strongly associated with both HCV infection and diabetes,(25) we also conducted analyses among strata cross-classified by both HCV status and normal or elevated activity of ALT or GGT. The consistency of the relationship of HCV with diabetes was evaluated across age (years; 20–39, 40–59, 60+) and BMI (kg/m2; <25, 25–<30, 30+) strata, and interaction between HCV status and age or BMI subgroups was tested by adding interaction terms individually to regression models. Finally, we conducted analyses to compare mean insulin resistance of participants with HCV infection to those without HCV infection among the subgroup without diabetes using linear regression analysis (PROC REGRESS) to calculate adjusted (least squares) mean estimates and 95% CI. Multivariate analyses were adjusted for the same factors as in the main analysis above. All multivariate analyses excluded persons with missing values for any factor included in the model. P-values were 2-sided and P<0.05 was considered to indicate statistical significance. Analyses used sample weights that accounted for unequal selection probabilities and nonresponse. Variance calculations accounted for the design effects of the survey using Taylor series linearization.(26)
RESULTS
When the data on the 15,128 participants were weighted to be representative of the U.S. population, the overall prevalence of anti-HCV+ was 1.7% and of HCV RNA+ was 1.1%. Diagnosed diabetes was found in 7.3%, undiagnosed diabetes in 3.2%, and pre-diabetes in 32.8%. Increasing impairment of glucose metabolism was associated with older age, non-Hispanic black ethnicity, less education, higher BMI and waist circumference, higher triglycerides, lower HDL cholesterol, higher systolic blood pressure, elevated C-reactive protein, having received a blood transfusion before 1992, a past history of smoking, lower alcohol consumption, and higher insulin, HOMA-IR, and ALT and GGT activities (Table 1). For most factors, diagnosed and undiagnosed diabetes did not differ greatly from each other. Therefore, for the remainder of analyses, results are shown with all diabetes cases combined to increase statistical power. Supplementary Online Table 1 provides results for individual analyses of diagnosed and undiagnosed diabetes.
Table 1.
Characteristics of participants by diabetes status (N=15,128)
| Characteristic | Normal glucose | Pre-diabetes | Undiagnosed diabetes | Diagnosed diabetes |
|---|---|---|---|---|
| Prevalence of glucose categories (%)* | 56.7 | 32.8 | 3.2 | 7.3 |
| Age (years; mean (SD)) | 40.8 (15.2) | 51.9 (16.1)1 | 58.9 (14.4)1, 2 | 59.2 (13.9)1, 2 |
| % women | 57.5 | 43.41 | 38.81 | 51.11, 2,3 |
| Race-ethnicity (%) | ||||
| Non-Hispanic white | 71.9 | 71.3 | 68.3 | 63.21, 2,3 |
| Non-Hispanic black | 10.2 | 10.5 | 12.71 | 15.91, 2,3 |
| Mexican American | 7.6 | 8.1 | 9.0 | 8.1 |
| Education (years; %) | ||||
| <12 | 15.6 | 22.61 | 29.81, 2 | 31.31, 2 |
| 12 | 23.8 | 26.61 | 29.71 | 24.73 |
| >12 | 60.5 | 50.81 | 40.41, 2 | 44.01, 2 |
| BMI (kg/m2; mean (SD)) | 26.8 (5.7) | 29.7 (6.7)1 | 32.7 (7.5)1, 2 | 32.4 (7.5)1, 2 |
| Waist circumference (cm; mean (SD)) | 92.3 (14.3) | 101.7 (15.0)1 | 110.5 (15.9)1, 2 | 109.2 (16.2)1, 2 |
| Triglycerides (mg/dL; mean (SD))† | 123.7 (101.1) | 153.9 (126.0)1 | 208.6 (265.0)1, 2 | 184.2 (169.3)1, 2 |
| HDL cholesterol (mg/dL; mean (SD)) | 55.5 (16.1) | 50.9 (15.3)1 | 46.3 (13.5)1, 2 | 48.0 (14.1)1, 2,3 |
| Systolic blood pressure (mmHg; mean (SD)) | 117.0 (16.0) | 125.1 (17.7)1 | 131.6 (20.8)1, 2 | 130.3 (20.4)1, 2 |
| Diastolic blood pressure (mmHg; mean (SD)) | 69.8 (11.5) | 71.1 (13.0)1 | 71.5 (14.7)1 | 68.3 (15.0)1, 2,3 |
| C-reactive protein >0.3 mg/dL (%) | 29.9 | 40.61 | 56.01, 2 | 50.31, 2,3 |
| Blood transfusion before 1992 (%) | 5.5 | 8.61 | 11.21 | 12.21, 2 |
| Smoking (%) | ||||
| Never | 54.6 | 47.91 | 44.31 | 48.61,3 |
| Former | 21.4 | 30.01 | 37.31, 2 | 33.61, 2 |
| <1 pack/day | 14.8 | 11.91 | 9.21, 2 | 9.41, 2 |
| ≥1 pack/day | 9.2 | 10.1 | 9.1 | 8.42 |
| Alcohol (drinks/day; %) | ||||
| 0 | 24.3 | 30.01 | 39.11, 2 | 52.61, 2,3 |
| <1 | 60.0 | 53.21 | 46.31, 2 | 39.81, 2,3 |
| 1–2 | 9.8 | 10.4 | 8.4 | 5.21, 2,3 |
| >2 | 6.0 | 6.4 | 6.1 | 2.51, 2,3 |
| Illegal injection drug use ever (%)‡ | 2.0 | 2.1 | 0.61, 2 | 1.6 |
| Age at first sexual intercourse <18 years (%)§ | 56.0 | 52.21 | 47.61 | 51.81 |
| Sexual partners in lifetime >20 (%)§ | 13.4 | 13.0 | 10.9 | 12.8 |
| Hemoglobin A1C (%; mean (SD)) | 5.2 (0.28) | 5.6 (0.35)1 | 6.9 (1.6)1, 2 | 7.3 (1.8)1, 2,3 |
| Glucose (mg/dL; mean (SD))† | 90.2 (6.2) | 104.4 (8.1)1 | 154.7 (54.6)1, 2 | 151.4 (63.9)1, 2 |
| Insulin (pmol/L; mean (SD))† | 56.1 (43.8) | 85.0 (66.8)1 | 129.7 (100.2)1, 2 | -- |
| HOMA-IR (mean (SD))† | 2.1 (1.7) | 3.7 (3.0)1 | 8.2 (7.0)1, 2 | -- |
| ALT (IU/L; mean (SD)) | 24.1 (18.9) | 28.8 (44.3)1 | 32.3 (24.1)1, 2 | 26.5 (23.8)1, 2,3 |
| ALT elevated (%)|| | 9.4 | 13.41 | 23.21, 2 | 12.71,3 |
| GGT (IU/L; mean (SD)) | 24.4 (29.9) | 32.2 (36.4)1 | 54.2 (125.7)1, 2 | 36.5 (51.2)1, 2,3 |
| GGT elevated (%)¶ | 7.6 | 12.81 | 24.31, 2 | 19.81, 2,3 |
| HCV antibody positive (%) | 1.6 | 1.8 | 1.7 | 1.6 |
| HCV RNA positive (%) | 0.9 | 1.3 | 1.3 | 1.2 |
SD, standard deviation; BMI, body mass index; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; HCV, hepatitis C virus.
p<0.05 compared with normal glucose,
p<0.05 compared with pre-diabetes,
p<0.05 compared with undiagnosed diabetes.
Weighted to be representative of the general U.S. population.
AM fasting sample.
Persons 20–59 years.
Persons 20–59 years from 1999–2006 or 20–69 years from 2007–2010.
ALT (IU/L) >43 for men or >31 for women.
GGT (IU/L) >58 for men or >35 for women.
Relationship of HCV infection with diabetes
The unadjusted prevalence of diabetes and pre-diabetes did not differ among participants positive for anti-HCV or for HCV RNA compared to persons without HCV infection (Table 2). After adjusting for age or for multiple factors, diabetes and pre-diabetes remained unassociated with HCV infection (Table 2). Substituting waist circumference for BMI, adding history of illegal injection drug use and first sexual intercourse before age 18 years, or adding lipid and blood pressure measures had little effect on results (data not shown). In some previous reports the relationship of HCV infection and diabetes varied by age or BMI subgroup; therefore, we conducted analyses stratified by these factors. No association of HCV with diabetes was found in any age or BMI subgroup (p>0.05) and interaction terms for HCV and age or BMI were not statistically significant (data not shown).
Table 2.
Unadjusted prevalence and age-adjusted and multivariate-adjusted odds ratios for diabetes and pre-diabetes by HCV status (N=15,125 for anti-HCV analysis and 15,036 for HCV RNA analysis.)
| Diabetes status | Unadjusted prevalence (%) | Age-adjusted
|
Multivariate-adjusted*
|
||||
|---|---|---|---|---|---|---|---|
| HCV status | OR† | 95% CI | p-value | OR | 95% CI | p-value | |
| Anti-HCV | |||||||
| Diabetes | 0.72 | 0.99 | |||||
| Anti-HCV− | 10.5 | 1.0 | 1.0 | ||||
| Anti-HCV+ | 10.2 | 1.10 | 0.64 – 1.88 | 1.00 | 0.59 – 1.70 | ||
| Pre-diabetes | 0.52 | 0.79 | |||||
| Anti-HCV− | 32.7 | 1.0 | 1.0 | ||||
| Anti-HCV+ | 36.4 | 1.12 | 0.79 – 1.61 | 1.06 | 0.68 – 1.65 | ||
| HCV RNA | |||||||
| Diabetes | 0.44 | 0.84 | |||||
| HCV RNA− | 10.5 | 1.0 | 1.0 | ||||
| HCV RNA+ | 12.0 | 1.30 | 0.67 – 2.52 | 1.06 | 0.59 – 1.90 | ||
| Pre-diabetes | 0.22 | 0.53 | |||||
| HCV RNA− | 32.7 | 1.0 | 1.0 | ||||
| HCV RNA+ | 39.6 | 1.27 | 0.86 – 1.87 | 1.17 | 0.72 – 1.89 | ||
HCV, hepatitis C virus; anti-HCV, HCV antibody; OR, odds ratio; CI, confidence interval.
Adjusted for age, sex, race-ethnicity, education, BMI, elevated C-reactive protein, smoking, alcohol use, and blood transfusion before 1992. N=13,630 for anti-HCV analysis and N=13,554 for HCV RNA analysis.
From logistic regression analysis.
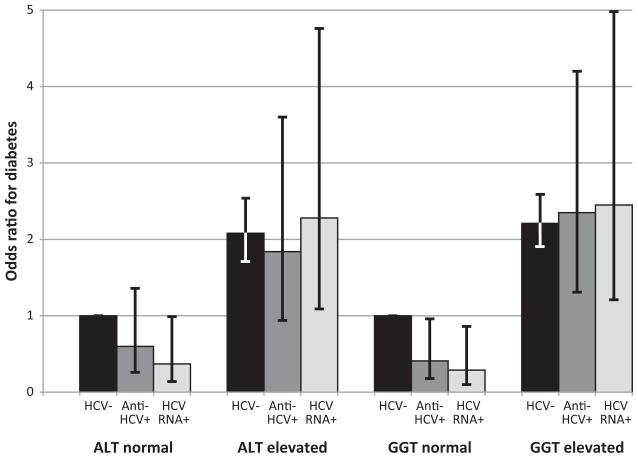
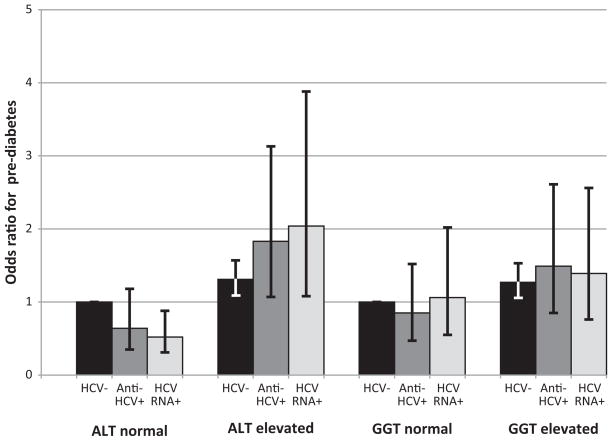
Because elevation of liver enzymes (ALT and GGT) has been found to increase the risk of diabetes without regard to underlying liver disease, we hypothesized that the association of HCV and diabetes found in clinical studies might be attributable to nonspecific elevation of liver enzyme activity rather than to an effect of HCV infection itself. Therefore, we examined the combined effect of HCV markers and activities of ALT or GGT on the odds of diabetes and pre-diabetes. The prevalence of elevated enzymes was much higher among participants with HCV markers (Table 3). In multivariate-adjusted analyses, elevated enzymes were associated with increased odds of diabetes irrespective of HCV status (Figure 2a). In contrast, the presence of HCV markers did not increase the odds of diabetes. Similar relationships were seen when separate analyses were performed for diagnosed and undiagnosed diabetes (data not shown). For pre-diabetes, there was also a positive association with elevated enzyme activity (Figure 2b). Beyond the effect of elevated enzymes, a positive association of pre-diabetes with HCV markers was evident for ALT, but not GGT.
Table 3.
Serum liver enzyme activities by HCV status
| HCV negative (N=14,848) | Anti-HCV+ (N=277) | HCV RNA+ (N=188) | |
|---|---|---|---|
| ALT (IU/L) | |||
| Elevated (%)* | 10.8 | 48.0 | 59.8 |
| Mean (SD) | 25.6 (29.6) | 52.3 (46.0) | 60.5 (47.3) |
| GGT (IU/L) | |||
| Elevated (%)† | 10.2 | 45.1 | 50.3 |
| Mean (SD) | 28.0 (38.7) | 80.3 (101.2) | 89.5 (109.9) |
HCV, hepatitis C virus; anti-HCV, HCV antibody; ALT, alanine aminotransferase; SD, standard deviation; GGT, gamma glutamyltransferase.
P<0.001 for all comparisons of anti-HCV+ or HCV RNA+ to HCV negative. Means were compared using a t test and percentages using a χ2 test.
ALT (IU/L) >43 for men or >31 for women.
GGT (IU/L) >58 for men or >35 for women.
Figure 2.
Figure 2a. Odds ratio for diabetes according to enzyme activities (ALT and GGT) and presence of markers of HCV infection (anti-HCV+ or HCV RNA+). Odds ratios were adjusted for age, sex, race-ethnicity, education, BMI, elevated C-reactive protein, smoking, alcohol use, and blood transfusion before 1992. ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; HCV, hepatitis C virus; anti-HCV, HCV antibody.
Figure 2b. Odds ratio for pre-diabetes according to enzyme activities (ALT and GGT) and presence of markers of HCV infection (anti-HCV+ or HCV RNA+). Odds ratios were adjusted for age, sex, race-ethnicity, education, BMI, elevated C-reactive protein, smoking, alcohol use, and blood transfusion before 1992. ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; HCV, hepatitis C virus; anti-HCV, HCV antibody.
Relationship of HCV infection with insulin resistance
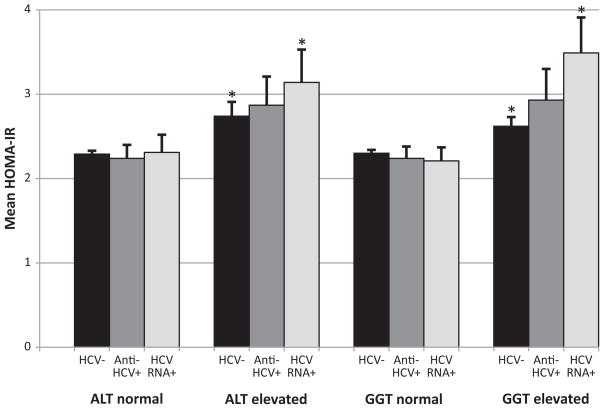
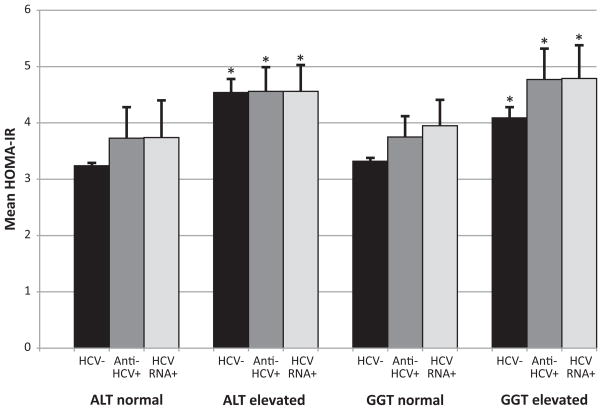
Among participants with normal glucose, mean HOMA-IR did not differ in persons negative compared to positive for anti-HCV or for HCV RNA (p>0.05 for both) (Table 4). HCV markers remained unassociated with age-adjusted and multivariate-adjusted HOMA-IR (p>0.05) (Table 4). Among participants with pre-diabetes, HOMA-IR was higher in persons positive than persons negative for anti-HCV and HCV RNA, but these differences were not statistically significant (except the multivariate-adjusted analysis among persons with pre-diabetes (p<0.05 for both anti-HCV and HCV RNA)) (Table 4). We further examined the relationship of HCV status to HOMA-IR according to enzyme activity. Elevated ALT and GGT activities were associated with increased HOMA-IR regardless of HCV status among persons with either normal glucose (Figure 3a) or with pre-diabetes (Figure 3b). Neither anti-HCV nor HCV RNA positivity was associated with increased HOMA-IR among persons with normal enzyme activities. Only among persons with elevated enzyme activity could a relationship be seen of markers of HCV with HOMA-IR.
Table 4.
Mean (SE) insulin resistance* comparing persons by HCV status within diabetes categories
| HCV negative | Anti-HCV+ | HCV RNA+ | |
|---|---|---|---|
| Normal | |||
| Unadjusted | 2.10 (0.03) | 1.80 (0.16) | 1.97 (0.23) |
| Age-adjusted | 1.98 (0.03) | 1.78 (0.17) | 2.00 (0.23) |
| Multivariate-adjusted† | 2.33 (0.04) | 2.48 (0.18) | 2.70 (0.22) |
| Pre-diabetes | |||
| Unadjusted | 3.67 (0.06) | 4.23 (0.36) | 4.19 (0.43) |
| Age-adjusted | 3.80 (0.07) | 4.30 (0.36) | 4.28 (0.43) |
| Multivariate-adjusted† | 3.41 (0.06) | 4.25 (0.33)‡ | 4.36 (0.38)‡ |
SE, standard error; HCV, hepatitis C virus; anti-HCV, HCV antibody.
Estimated using Homeostasis Model Assessment (HOMA).
Adjusted for age, sex, race-ethnicity, education, alcohol use, smoking status, BMI, elevated C-reactive protein, and blood transfusion before 1992.
p-value <0.05 compared to HCV negative.
Figure 3.
Figure 3a. Mean HOMA-IR according to enzyme activities (ALT and GGT) and presence of markers of HCV infection (anti-HCV+ or HCV RNA+) among participants with normal glucose. Error bars represent standard errors. Insulin resistance was estimated using homeostasis model assessment (HOMA). Means were adjusted for age, sex, race-ethnicity, education, alcohol use, smoking status, BMI, elevated C-reactive protein, and blood transfusion before 1992. *indicates p<0.05 compared to normal liver enzymes and HCV−. HOMA-IR, homeostasis model assessment of insulin resistance; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; HCV, hepatitis C virus; anti-HCV, HCV antibody.
Figure 3b. Mean HOMA-IR according to enzyme activities (ALT and GGT) and presence of markers of HCV infection (anti-HCV+ or HCV RNA+) among participants with pre-diabetes. Error bars represent standard errors. Insulin resistance was estimated using homeostasis model assessment (HOMA). Means were adjusted for age, sex, race-ethnicity, education, alcohol use, smoking status, BMI, elevated C-reactive protein, and blood transfusion before 1992. *indicates p<0.05 compared to normal liver enzymes and HCV−. HOMA-IR, homeostasis model assessment of insulin resistance; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; HCV, hepatitis C virus; anti-HCV, HCV antibody.
DISCUSSION
In this large U.S. national population-based study, HCV infection was not associated with diabetes. No relationship was found in unadjusted analyses or in analyses adjusted for multiple factors associated with HCV or diabetes. Neither ever having had HCV infection nor current HCV infection was related to diabetes in overall analysis.
An association of HCV infection with diabetes has been found in comparison with non-infected controls in mostly clinical studies of patient samples.(3, 4) A similar relationship has been reported in comparison with HBV-infected controls with adjusted ORs of 1.8 (95% CI, 1.2–2.4) in one meta-analysis(3) and 1.9 (95% CI, 1.4–2.6) in a second meta-analysis.(4) While some studies matched HCV− and HBV-infected patients on various factors including disease severity, the association with diabetes may have been influenced by the greater prevalence of liver enzyme elevation with HCV infection compared with HBV infection.(27) Many studies that used HBV-infected controls did not conduct multivariate-adjusted analyses or did not include liver enzymes in adjusted analyses. In one study that did adjust for ALT, HCV infection was no longer statistically significantly associated with diabetes in adjusted analysis, while ALT was an independent predictor.(28) In another study, insulin resistance in non-diabetic individuals was not independently associated with HCV infection after adjustment for ALT and GGT elevation, while ALT and GGT elevation were independent predictors.(29) In a third study, both HCV infection and ALT elevation were independently associated with diabetes.(30) A decreased incidence of diabetes following successful treatment of HCV (sustained virological response) has also been reported.(31) ALT elevation at the beginning of follow-up was not an independent predictor of diabetes development, but the relationship of treatment outcome with liver enzyme levels was not presented.
Among the few previous population-based studies of the relationship of HCV infection and diabetes, findings were inconsistent. A positive association was found in a Taiwanese community-based cohort study in which anti-HCV positive persons had a higher risk of incident diabetes (based on fasting glucose ≥126 mg/dL or casual glucose ≥200 mg/dL).(7) The association was strongest at a younger age and higher BMI. In the U.S. community-based prospective Atherosclerosis Risk in Communities (ARIC) Study, anti-HCV positive persons were at increased risk for incident diabetes (based on self-report or fasting glucose), but the association was not statistically significant (HR, 1.9, 95% CI, 0.6–6.2).(6) An elevated HR was found among persons at high risk for diabetes (defined by older age and higher BMI), but was based on a small number of anti-HCV positive cases.
Several previous U.S. studies have utilized NHANES data to examine the association of HCV and diabetes. In an analysis of the third NHANES, 1988–1994, a non-statistically significant association with diabetes (based on self-report or fasting glucose) was found overall, while a stronger relationship was reported among persons aged 40+ years (OR, 3.8; 95% CI, 1.8–7.9).(5) In a study that used later NHANES cycles, no association was found in NHANES 1999–2004 (OR, 0.9; 95% CI, 0.6–1.6) or NHANES 2005–2008 (OR, 1.3; 95% CI, 0.7–2.3).(14) In a second study by the same group using NHANES 1999–2010, persons with chronic HCV infection were reported to be over twice as likely to have diabetes (OR, 2.3; 95% CI, 1.2–4.5).(32) However, the control group was limited to persons with no evidence of chronic liver disease, for which no liver enzyme elevation was a criterion. We believe those criteria were overly restrictive, resulting in a control group at low risk of diabetes. As we have shown in the current study, diabetes was associated with liver enzyme elevation, rather than with HCV infection per se. Also differing from other studies, we applied recognized standard diabetes criteria to rule out diabetes and pre-diabetes among controls. Previous studies, including those that analyzed NHANES data, have most often employed less precise definitions of diabetes and normal glucose and have not examined the relationship with pre-diabetes at all.
An association of HCV with diabetes was found only in the presence of increased liver enzyme activities. Increased ALT and GGT activities are markers of fatty liver, which itself is associated with diabetes. The causal direction of this association is unclear. Fatty liver may increase the risk of diabetes, but diabetes might also increase the risk of fatty liver, or there are common factors that might increase the risk of both. In any case, it was not surprising in the current study that elevated ALT and GGT activities were associated with diabetes, a relationship documented (for ALT) by a recent meta-analysis of incident type 2 diabetes.(25) On the other hand, it is not entirely clear why diabetes was found to be relatively underrepresented among participants with HCV infection and normal enzyme activities (Figures 2a and 2b). It may be that persons infected with HCV who have normal enzyme activity are at particularly low risk of fatty liver. However, this is a side issue to the major findings and one that we were not able to pursue further in the current analysis. In a recent Italian population-based prospective study in which there was no overall association of HCV infection with incident diabetes, a higher risk was found among persons with abnormal ALT.(8) With cross-classification by both ALT elevation and HCV status, increased diabetes risk with HCV positivity was seen only with ALT elevation. We are unaware of other studies that have considered specifically the effect of liver enzyme elevations in their reports of associations of HCV and diabetes. Most studies have relied on patients undergoing clinical care for HCV. It would be expected that the large majority of such patients would have elevated liver enzymes. Additionally, patients under clinical observation and treatment for HCV likely have more advanced liver disease than persons in the general population with unrecognized HCV. Because advanced liver disease is associated with diabetes, regardless of cause,(33, 34) clinical series that over-represent patients with advanced HCV would be expected to show a stronger association than that found in the current study.
We also studied the relationship of HCV infection with insulin resistance (as estimated by HOMA-IR) among persons with normal glucose and with pre-diabetes and did not find associations. As with diabetes, we did observe a relationship of elevated enzyme activities with insulin resistance. We are unaware of other studies that adequately considered the influence of enzyme elevation on insulin resistance. For example, treatment studies that have demonstrated improvement in HOMA-IR following a sustained virological response have not shown that the improvement was independent of improvement in liver enzymes. (10–12).
NHANES is a cross-sectional study, which is the most important limitation of our work. Thus we cannot, for example, rule out the possibility that persons with both HCV and diabetes may have been under-represented because increased morbidity and mortality prevented them from participating. Such a potential problem could be addressed in a cohort study that followed persons with HCV from time of infection for a long enough time to determine if the incidence of diabetes was greater than among uninfected but otherwise comparable persons. Although the participation rate was relatively high, sampled persons who did not come to the mobile examination center represent a potential source of bias in studies using NHANES data because they may be less healthy than examined persons. An additional source of bias is the restriction of the NHANES sample to the civilian non-institutionalized population. Some non-sampled groups, particularly incarcerated persons, are known to have a high prevalence of HCV.(35, 36) However, we are unaware of any literature on the relationship of HCV and diabetes among the incarcerated, and have no reason to believe that the prevalence of diabetes would be particularly high among HCV+ incarcerated persons. There were other less significant limitations. The number of participants with HCV infection was relatively small, despite utilizing 12 years of data. The prevalence of diabetes was lower than if oral glucose tolerance test (OGTT) results had been included in the definition,(37) however OGTT data were not available for all survey years. In addition, we were unable to distinguish type 1 diabetes, which is not thought to be increased with HCV infection,(38) from type 2 diabetes. However, 90–95 percent of diabetes cases in this adult cohort would be expected to have type 2 diabetes.(2) Finally, analyses of the relationship of HCV infection with insulin resistance used HOMA-IR as a surrogate measure of insulin resistance.(39) While this is a limitation, HOMA-IR is a commonly used marker for insulin resistance in epidemiologic studies in which more direct measurement cannot be performed. An important strength of our study was the precise case definitions for diabetes status, as discussed above. Other strengths of this large, national, population-based sample include the avoidance of ascertainment bias that occurs in clinical studies of selected patients and the ability to generalize the results to the U.S. population.
In conclusion, in the U.S. population, we were unable to demonstrate an association of HCV infection with diabetes or with insulin resistance. Elevated liver enzyme activities were associated with both diabetes and with insulin resistance. We suggest that previous reports of relationships of HCV with diabetes may in large measure have been due to this effect of elevated liver enzymes.
Supplementary Material
Acknowledgments
Financial support: This work was supported by a contract from the National Institute of Diabetes and Digestive and Kidney Diseases (HHSN276201200161U).
Abbreviations
- HCV
hepatitis C virus
- anti-HCV
HCV antibody
- A1C
hemoglobin A1C
- FPG
fasting plasma glucose
- OR
odds ratio
- HOMA-IR
homeostasis model assessment of insulin resistance
- CI
confidence interval
- HR
hazard ratio
- NHANES
National Health and Nutrition Examination Survey
- HBV
hepatitis B virus
- NCHS
National Center for Health Statistics
- CDC
Centers for Disease Control and Prevention
- RIBA
recombinant immunoblot assay
- BMI
body mass index
- HDL
high-density lipoprotein
- ALT
alanine aminotransferase
- GGT
gamma glutamyltransferase
- OGTT
oral glucose tolerance test
Contributor Information
Constance E. Ruhl, Email: cruhl@s-3.com, Social & Scientific Systems, Inc., 8757 Georgia Avenue, 12thfloor, Silver Spring, MD 20910, 301-628-3272 (phone), 301-628-3201 (fax)
Andy Menke, Email: amenke@s-3.com, Social & Scientific Systems, Inc., 8757 Georgia Avenue, 12thfloor, Silver Spring, MD 20910
Catherine C. Cowie, Email: CowieC@extra.niddk.nih.gov, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services, 2 Democracy Plaza, Room 691, 6707 Democracy Boulevard MSC 5460, Bethesda, MD 20892-5450
James E. Everhart, Email: EverhartJ@extra.niddk.nih.gov, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services, 2 Democracy Plaza, Room 642F, 6707 Democracy Boulevard MSC 5450, Bethesda, MD 20892-5450
References
- 1.CDC. [Accessed December 2013];Viral Hepatitis Surveillance - United States. 2010 http://www.cdc.gov/hepatitis/Statistics/2010Surveillance/Commentary.htm.
- 2.CDC. [Accessed December 2013];National Diabetes Fact Sheet. 2011 http://www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 3.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naing C, Mak JW, Ahmed SI, Maung M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World J Gastroenterol. 2012;18:1642–1651. doi: 10.3748/wjg.v18.i14.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 6.Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 7.Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am J Epidemiol. 2007;166:196–203. doi: 10.1093/aje/kwm061. [DOI] [PubMed] [Google Scholar]
- 8.Montenegro L, De Michina A, Misciagna G, Guerra V, Di Leo A. Virus C hepatitis and type 2 diabetes: a cohort study in southern Italy. Am J Gastroenterol. 2013;108:1108–1111. doi: 10.1038/ajg.2013.90. [DOI] [PubMed] [Google Scholar]
- 9.Yoneda M, Saito S, Ikeda T, Fujita K, Mawatari H, Kirikoshi H, et al. Hepatitis C virus directly associates with insulin resistance independent of the visceral fat area in nonobese and nondiabetic patients. J Viral Hepat. 2007;14:600–607. doi: 10.1111/j.1365-2893.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 10.Conjeevaram HS, Wahed AS, Afdhal N, Howell CD, Everhart JE, Hoofnagle JH. Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology. 2011;140:469–477. doi: 10.1053/j.gastro.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570–576. doi: 10.1111/j.1572-0241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 12.Brandman D, Bacchetti P, Ayala CE, Maher JJ, Khalili M. Impact of insulin resistance on HCV treatment response and impact of HCV treatment on insulin sensitivity using direct measurements of insulin action. Diabetes Care. 2012;35:1090–1094. doi: 10.2337/dc11-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moucari R, Asselah T, Cazals-Hatem D, Voitot H, Boyer N, Ripault MP, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134:416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Stepanova M, Lam B, Younossi Y, Srishord MK, Younossi ZM. Association of hepatitis C with insulin resistance and type 2 diabetes in US general population: the impact of the epidemic of obesity. J Viral Hepat. 2012;19:341–345. doi: 10.1111/j.1365-2893.2011.01554.x. [DOI] [PubMed] [Google Scholar]
- 15.Miyajima I, Kawaguchi T, Fukami A, Nagao Y, Adachi H, Sasaki S, et al. Chronic HCV infection was associated with severe insulin resistance and mild atherosclerosis: a population-based study in an HCV hyperendemic area. J Gastroenterol. 2013;48:93–100. doi: 10.1007/s00535-012-0610-3. [DOI] [PubMed] [Google Scholar]
- 16.NCHS. [Accessed December 2013];National Health and Nutrition Examination Survey (NHANES) http://www.cdc.gov/nchs/nhanes.htm.
- 17.NCHS. [Accessed December 2013];NHANES 1999–2000 Laboratory Procedure Manual - Hepatitis C Antibody / Hepatitis C Confirmatory. http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab02_met_hepatitis_c_%20eia_riba.pdf.
- 18.NCHS. [Accessed December 2013];NHANES 2009–2010 Laboratory Procedure Manual - Hepatitis C Antibody / Hepatitis C Confirmatory Test (Anti-HCV) http://www.cdc.gov/NCHS/data/nhanes/nhanes_09_10/HEPC_F_met_hepc.pdf.
- 19.NCHS. [Accessed December 2013];NHANES 2005–2006 Laboratory Procedure Manual - HCV RNA Quantification Assay for Hepatitis C Virus. http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/HEPC_D_met_LBXHCR.pdf.
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.NCHS. [Accessed December 2013];NHANES 1999–2000 Laboratory Procedure Manual - Lab 18 Biochemistry Profile. http://www.cdc.gov/nchs/data/nhanes/nhanes_99_00/lab18_met_biochemistry_profile.pdf.
- 22.NCHS. [Accessed December 2013];NHANES 2001–2002 Laboratory Procedure Manual - Lab 18 Biochemistry Profile. http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l18_b_met_biochemistry_profile.pdf.
- 23.NCHS. [Accessed December 2013];NHANES 2001–2002 Laboratory Procedure Manual - Lab 40 ALT. http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l40_b_met_alanine_amino_transferase.pdf.
- 24.NCHS. [Accessed December 2013];NHANES 2001–2002 Laboratory Procedure Manual - Lab 40 GGT. http://www.cdc.gov/nchs/data/nhanes/nhanes_01_02/l40_b_met_gamma_glutanyl_transferase_sum.pdf.
- 25.Kunutsor SK, Apekey TA, Walley J. Liver aminotransferases and risk of incident type 2 diabetes: a systematic review and meta-analysis. Am J Epidemiol. 2013;178:159–171. doi: 10.1093/aje/kws469. [DOI] [PubMed] [Google Scholar]
- 26.Breslow NE, Day NE. Statistical Methods in Cancer Research: the Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer; 1987. pp. 48–79. [PubMed] [Google Scholar]
- 27.Helsper C, van Essen G, Frijling BD, de Wit NJ. Follow-up of mild alanine aminotransferase elevation identifies hidden hepatitis C in primary care. Br J Gen Pract. 2012;62:e212–216. doi: 10.3399/bjgp12X630115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arao M, Murase K, Kusakabe A, Yoshioka K, Fukuzawa Y, Ishikawa T, et al. Prevalence of diabetes mellitus in Japanese patients infected chronically with hepatitis C virus. J Gastroenterol. 2003;38:355–360. doi: 10.1007/s005350300063. [DOI] [PubMed] [Google Scholar]
- 29.Imazeki F, Yokosuka O, Fukai K, Kanda T, Kojima H, Saisho H. Prevalence of diabetes mellitus and insulin resistance in patients with chronic hepatitis C: comparison with hepatitis B virus-infected and hepatitis C virus-cleared patients. Liver Int. 2008;28:355–362. doi: 10.1111/j.1478-3231.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 30.Rouabhia S, Malek R, Bounecer H, Dekaken A, Bendali Amor F, Sadelaoud M, et al. Prevalence of type 2 diabetes in Algerian patients with hepatitis C virus infection. World J Gastroenterol. 2010;16:3427–3431. doi: 10.3748/wjg.v16.i27.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744. doi: 10.1002/hep.22703. [DOI] [PubMed] [Google Scholar]
- 32.Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37:647–652. doi: 10.1111/apt.12234. [DOI] [PubMed] [Google Scholar]
- 33.Holstein A, Hinze S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677–681. doi: 10.1046/j.1440-1746.2002.02755.x. [DOI] [PubMed] [Google Scholar]
- 34.Gentile S, Loguercio C, Marmo R, Carbone L, Del Vecchio Blanco C. Incidence of altered glucose tolerance in liver cirrhosis. Diabetes Res Clin Pract. 1993;22:37–44. doi: 10.1016/0168-8227(93)90130-w. [DOI] [PubMed] [Google Scholar]
- 35.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 36.Binswanger IA, Krueger PM, Steiner JF. Prevalence of chronic medical conditions among jail and prison inmates in the USA compared with the general population. J Epidemiol Community Health. 2009;63:912–919. doi: 10.1136/jech.2009.090662. [DOI] [PubMed] [Google Scholar]
- 37.Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33:562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabris P, Floreani A, Tositti G, Vergani D, De Lalla F, Betterle C. Type 1 diabetes mellitus in patients with chronic hepatitis C before and after interferon therapy. Aliment Pharmacol Ther. 2003;18:549–558. doi: 10.1046/j.1365-2036.2003.01681.x. [DOI] [PubMed] [Google Scholar]
- 39.Lam KD, Bacchetti P, Abbasi F, Ayala CE, Loeb SM, Shah V, et al. Comparison of surrogate and direct measurement of insulin resistance in chronic hepatitis C virus infection: impact of obesity and ethnicity. Hepatology. 2010;52:38–46. doi: 10.1002/hep.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.