Abstract
The Bone Morphogenetic Protein antagonist Gremlin 2 (Grem2) is required for atrial differentiation and establishment of cardiac rhythm during embryonic development. A human Grem2 variant has been associated with familial atrial fibrillation, suggesting that abnormal Grem2 activity causes arrhythmias. However, it is not known how Grem2 integrates into signaling pathways to direct atrial cardiomyocyte differentiation. Here, we demonstrate that Grem2 expression is induced concurrently with the emergence of cardiovascular progenitor cells during differentiation of mouse embryonic (ES) stem cells. Grem2 exposure enhances the cardiogenic potential of ES cells by ~20–120 fold, preferentially inducing genes expressed in atrial myocytes such as Myl7, Nppa and Sarcolipin. We show that Grem2 acts upstream to upregulate pro-atrial transcriptional factors CoupTFII and Hey1 and downregulate atrial fate repressors Irx4 and Hey2. The molecular phenotype of Grem2-induced atrial cardiomyocytes was further supported by induction of ion channels encoded by Kcnj3, Kcnj5, and Cacna1D genes and establishment of atrial-like action potentials shown by electrophysiological recordings. We show that promotion of atrial-like cardiomyocyte is specific to the Gremlin subfamily of BMP antagonists. Grem2 pro-atrial differentiation activity is conveyed by non-canonical BMP signaling through phosphorylation of JNK and can be reversed by specific JNK inhibitors, but not by dorsomorphin, an inhibitor of canonical BMP signaling. Taken together, our data provide novel mechanistic insights into atrial cardiomyocyte differentiation from pluripotent stem cells and will assist the development of future approaches to study and treat arrhythmias.
INTRODUCTION
Embryonic stem (ES) cells differentiate to a wide range of cell types, offering a robust system to obtain cells to study developmental mechanisms and disease phenotypes [1, 2]. The ES cell model is particularly pertinent for generating cells of the cardiovascular system because these cells appear relatively early during development and ES cell differentiation [3–7]. A number of experimental protocols exist to promote the differentiation of ES cells toward cardiac cell fates [8–15]; however, how to direct ES cell-derived cardiac progenitors to cultures of specialized cell types, such as ventricular and atrial myocytes, pacemaker and conduction system cells, remains a major challenge [16].
Bone Morphogenetic Proteins (BMPs) exert pleiotropic effects on cardiac morphogenesis and cardiomyocyte maturation [17], including cardiac looping [18, 19], valve formation and ventricular development [20–26]. Besides forward BMP signaling, BMP antagonists such as Noggin are also necessary for cardiac development. Mice lacking Noggin have thicker myocardium than wild types [27]. This phenotype could be rescued by halving the gene dosage of Bmp4, indicating that fine-tuning the strength, or confining the zone of BMP activity is required for proper cardiac development [27].
In line with developmental data, modulation of BMP signaling influences the differentiation of ES cells to cardiomyocytes. BMP agonists stimulate expression of genes encoding cardiac contractile proteins, whereas transient inhibition of BMP signaling at the initial stages of ES cell differentiation enhances the cardiogenic potential of mesodermal progenitor cells [8, 12, 28, 29].
Gremlin 2 (Grem2), also called Protein Related to Dan and Cerberus (PRDC), is a secreted BMP antagonist that belongs to the CAN family, which includes Cerberus-like 1, Dan, Dante and Gremlin 1 [30, 31]. Grem2 inhibits Bmp2 and Bmp4 signaling in vitro, but not that of Tgfβ, Gdf9 or Activin [32]. In mice, Grem2 expression has been detected in commissural neurons of the developing spinal cord and in lung mesenchyme [33, 34]. In vivo studies in animal models have implicated Grem2 in follicle development, placode neurogenesis, osteogenic differentiation and craniofacial patterning [32, 35–37].
Our prior studies have shown that Grem2 is highly expressed in the eye, swim bladder and in the pharyngeal arch mesoderm adjacent to the developing heart of zebrafish embryos [38]. We determined that through regulation of BMP signaling, Grem2 is necessary for cardiac laterality and atrial differentiation during development [39]. In addition, we discovered that a human GREM2 variant is associated with familial atrial fibrillation, suggesting that abnormal Grem2 activity causes arrhythmia. Modeling of the human variant resulted in slower cardiac contraction rates, abnormal atrial contraction velocity and distorted wavefront propagation in zebrafish, supporting the idea that Grem2 regulates the establishment of proper cardiac rhythm in the atrium. Furthermore, we found that Grem2 overexpression during development led to ectopic contracting fields expressing atrial-specific genes; thus, Grem2 activity is necessary and sufficient for atrial differentiation [39].
Here, we show that Grem2 treatment shifts ES cell differentiation to cardiomyocytes with atrial molecular and electrophysiological properties. This Grem2 effect is driven by activation of the JNK signaling pathway. Our findings provide novel mechanistic insights into chamber-specific cardiomyocyte differentiation and the development of stem cell-based tools to study and treat atrial dysfunction.
MATERIALS AND METHODS
ES cell culture and embryoid body (EB) formation
Mouse CGR8 ES cells have been adapted to feeder-free culture conditions, facilitating molecular analyses of gene expression [7, 14, 39–41]. CGR8 cells were cultured in GMEM medium (Sigma) with 10% fetal bovine serum, 100 units/ml LIF (ESGRO-Millipore), 2 mM L-glutamine and 50 μM β-mercaptoethanol on 0.2% gelatin coated tissue culture plates. ES cells were kept at ≤ 70% confluence to preserve pluripotency.
ES cell differentiation was performed using the hanging-drop method [14, 42]. Briefly, ES cells were dissociated with 0.05% trypsin (Life Technologies) for 5 minutes and 500 cells in 20 μl drops were spotted on the inside lid surface of a 10 cm bacterial Petri dish. EBs were grown in differentiation medium [IMDM (Life Technologies) containing 20% FBS, 0.1 mM MEM essential amino acids, 2 mM-glutamine and 100 μM β-mercaptoethanol]. After 2 days, EBs were collected and cultured in suspension for an additional two days in bacterial Petri dishes to prevent attachment. At day 4, 30 EBs per well were allowed to attach onto 0.2% gelatin-coated 6-well plates for further differentiation.
Contracting areas as percentage of total culture surface were calculated in 6 EBs that were chosen at random from either control or Grem2-treated wells. Total area per EB was measured using the free selection tool of either Metamorph (Molecular Devices) or Nikon Elements software. All contracting areas within each EB were also measured using the same technique. The sum of contracting areas was divided by the total EB surface area to calculate percent values. Contraction rates were counted on at least 6 randomly selected contracting foci recorded for 2 minutes each.
Lyophilized Grem2 was purchased from R&D Systems (catalog #269-PR-050) and reconstituted according to supplier’s recommendations. Optimal concentration was between 1 and 5 μg/ml. All other BMP ligands and antagonists were also purchased from R&D Systems, reconstituted as directed on their respective product sheets, and used at concentrations (EC50) recommended by the supplier. The effectiveness of BMP signaling inhibition was monitored using ES cells stably transfected with the luciferase gene under the control of the BMP responsive element in the Id2 promoter (BRE2-Luc) [43]. JNK 2inhibitor II (SP600125, Tocris Biosciences) and JNK Inhibitor V (AS601245, EMD Millipore) were used at 10 μM.
Cloning of Myh6-DsRed2-Nuc and generation of CGR8 stable clones
The Myh6 promoter-polyA hGH cloning vector [44] (kindly provided by Dr. J. Robbins) was digested with SalI and HindIII. The DsRed2-Nuc open reading frame fragment was obtained by PCR from plasmid pDsRed2-Nuc (Clontech) using primers with attached SalI and HindIII restriction sites (underlined) as follows: 5′-GTGTCGACGGATGGC-CTCCTCCGAG-3′ and 5′-TGAAGCTTTTATCTAGATCCGGTGGATCCTACC-3′. The two fragments were ligated and successful cloning was confirmed by restriction enzyme digest mapping and sequencing. All experimental procedures followed National Institutes of Health guidelines.
Lipofectamine 2000 reagent (Invitrogen) was used to transfect CGR8 cells and generate stable clones expressing Myh6-DsRed2-Nuc [14]. ES cells at 50% confluence were cotransfected with 8 μg of linearized Myh6-DsRed2-Nuc plasmid and a plasmid carrying the neomycin resistance gene under the Phosphoglycerate kinase promoter (10:1 ratio) and selected with 200 μg/ml G418 for 10 days. Single colonies were picked and expanded to establish Myh6-DsRed2-Nuc ES cell lines.
PCR analysis
Total RNA was extracted from ES cells and EBs using the RNeasy kit (Qiagen) as described [14]. For quantitative PCR, an aliquot of reverse transcription product representing 1 ng of total RNA was amplified with the iQ SYBR Green Supermix kit on a CFX Thermocycler (BioRad). Primers were used at a final concentration of 0.25 μM each. β-actin and/or Gapdh were used as internal controls. Relative gene expression levels were quantified using the formula 2(−ΔΔCt) [45, 46]. Gene-specific primer sequences are included in supporting information Table S1.
Immunofluorescence and in situ hybridization
Before staining, EBs were washed with 1X PBS twice, fixed with 4% paraformaldehyde for 15 minutes at 4°C, washed 3 times with 1x PBS, permeabilized with 0.2% TritonX-100 in 1xPBS for 30 minutes and incubated with blocking buffer (1 mg/ml BSA in 1X PBS) for 1 hour at room temperature (RT), or overnight (O/N) at 4 °C. After blocking, primary antibodies diluted in blocking buffer were added and incubated O/N at 4°C. Slides were washed 3 times with 1X PBS and incubated with secondary antibodies diluted in blocking buffer for 1 hour at RT. After repeat washes with 1X PBS, slides were sealed with mounting medium (Vector Laboratories). Images were captured with a Zeiss LSM 510 inverted confocal microscope. Primary antibodies were goat anti-Nkx2.5 polyclonal antibody (Santa Cruz), 1:50 dilution; monoclonal mouse anti-CoupTFII antibody (R&D Systems), 1:100. Secondary antibodies used were donkey anti-goat and donkey anti-mouse IgG coupled to Alexa Fluor 488 (Life Technologies), 1:200. 4–6 EBs from each condition were analyzed and divided into six optical fields covering the entire EB. The fields were photographed and Nkx2.5, DsRed, or DsRed/CoupTFII double positive cells in Control and Grem2-treated samples were quantified by counting the number of labeled nuclei in each field.
Mouse E8.0 embryos were prepared and analyzed for in situ hybridization analysis using Grem2-specific riboprobes using standard protocols [38, 39].
Flow cytometry analysis
EBs were washed once with 1X PBS, incubated with 1 mg/ml Collagenase for 10 minutes at 37°C and dissociated using gentle pipetting. Single cell suspensions were transferred in Eppendorf tubes and spun for 5 minutes, 3,000 rpm at 4°C. Cells were then fixed with 2% paraformaldehyde at 4°C for 10 minutes, spun and washed with 1X PBS and resuspended in 1 ml FACS buffer (1X PBS containing 5% BSA and 2mM EDTA), spun and resuspended in 100 μl 1X FACS buffer containing 0.2% Saponin. We then added mouse monoclonal antibodies recognizing α-Actinin (Sigma, 1:2,000 dilution) or Tnnt2 (Thermo Scientific, 1:1000), and incubated samples for 60 minutes at RT, washed twice with 1X FACS buffer with 0.2% Saponin and resuspended in 1X FACS buffer containing 0.2% Saponin and secondary antibody goat anti-mouse IgG Alexa Fluor 488 (Life Technologies, 1:200). Cells were incubated in the dark at RT for 30 minutes, washed 3 times with 1X FACS buffer and analyzed using the LSRII flow cytometer (BD Biosciences) in the Vanderbilt Flow Cytometry Core Lab.
Western blotting
Western blotting was performed on EB protein samples prepared in NP40 cell lysis buffer (Life Technologies) containing Protease Inhibitor Cocktail Set III and Phosphatase Inhibitor Cocktail Set II (Calbiochem), both diluted 1:100. Insoluble material was removed by centrifugation, and protein concentration was determined by Bio-Rad protein assay. We then added 1X reducing agent (Life Technologies) and 1X LDS Sample buffer (Life Technologies), denatured proteins by heating for 10 minutes at 95°C and placed samples on ice. Twenty micrograms of protein was size fractionated using SDS-PAGE and electro-transferred onto nitrocellulose membranes (Life Technologies). Membranes were blocked with 5% dry milk in Tris-buffered saline containing 0.1% Tween-20 (1X TBST) for 1 hour at RT and then incubated with specific antibodies. Rabbit polyclonal antibodies recognizing phosphorylated or total Smad 1/5/8 (Cell Signaling, 1:1,000 dilution) and phosphorylated or total JNK 1&2 (Life Technologies, 1:1,000 dilution) were added and incubated O/N at 4°C. Membranes were then washed 5 times, 10 minutes each with TBST and incubated for 1 hour at RT with secondary antibody goat anti rabbit IgG linked to Horse Radish Peroxidase (HRP) (Promega, 1:10,000 dilution) in blocking buffer. Membranes were again washed 5 times, 10 minutes each with TBST and bound HRP was detected using the ECL Western Blotting Detection Reagents (Thermo Scientific) and imaged using Thermo Scientific CL-XPosure Film. Films were scanned and analyzed using Biorad Image Lab software (version 4.1). Western blots were repeated four times. Two independent experiments were used for quantification.
Electrophysiology
Myh6-DsRed2-Nuc CGR8 EBs were dissociated with collagenase treatment (see above) at differentiation days 10–12 and individual cardiomyocytes marked with red fluorescence were chosen for action potential recordings using the whole-cell patch clamp technique in the current-clamp mode with glass pipettes of 2–5 MΩ resistance. The standard intracellular (pipette) recording solution contained (in mmol/L): 110 K-glutamate, 10 NaCl, 10 KCl, 2 EGTA, 10 HEPES and 5 MgATP, adjusted to pH 7.2 with NaOH. The extracellular (bath) recording solution contained (in mmol/L): 137 NaCl, 5.4 KCl, 1 MgCl2, 2 HEPES, 10 glucose, 2 CaCl2, adjusted to pH 7.4 with NaOH. All patch-clamp experiments were performed at 37°C. The seal and the whole-cell configuration were obtained under voltage-clamp mode and capacity transients were compensated before switching to the current-clamp mode. Using an Axopatch 200B amplifier, the series resistance was compensated by ~70%. Traces were acquired using p-clamp 9 software, recordings were filtered at 5 kHz and sampled at a rate of 20 kHz using a Digidata 1200 series interface (Axon Instruments). Action potentials were evoked using 4 msec depolarizing current injections. Data were analyzed using pCLAMP 10.0 or Microsoft Excel 2010.
Statistical analysis
Data are shown as mean ± SEM from at least three separate experiments, each performed with triplicate samples. Differences between groups were analyzed for statistical significance using unpaired two-tailed Student’s t-test or one-way ANOVA with Tukey post-hoc test (SPSS software). Patch-clamp measurements are presented as the means ± SE. Statistical comparisons were made using Student’s t-test. P-values <0.05 were accepted as statistically significant.
RESULTS
Grem2 is expressed during early differentiation of cardiac progenitor cells
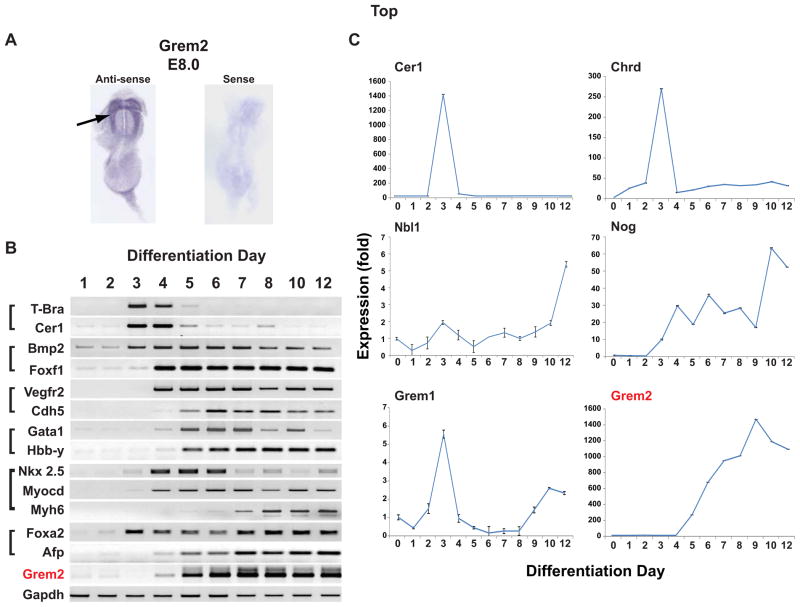
In zebrafish embryos, grem2 expression first appears during the early stages of organogenesis after the formation of the three germ layers [38]. During heart development, the grem2 expression domain is juxtaposed to the cardiac field at the time that cardiac progenitor cells migrate from the midline to the left side of the embryo and begin to differentiate [39]. To determine Grem2 expression during early cardiac development in a mammalian organism, we stained mouse embryos by in situ hybridization using Grem2 riboprobes. The results showed that Grem2 transcripts were present at E8.0 in the developing heart area, suggesting that both the timing and initial domain of Grem2 expression have been conserved during evolution (Fig. 1A).
Figure 1. Grem2 expression during development and ES cell differentiation.
(A) In situ hybridization analysis of E8.0 mouse embryos using anti-sense and sense Grem2 riboprobes shows Grem2 expression in the cardiac field area (arrow). (B) Gene expression analysis at days 1–12 of CGR8 ES cell differentiation by RT-PCR. Differentiating CGR8 cells closely recapitulate events of early development regarding the timing of expression of gastrulation (T-bra, Cer1), mesodermal (Bmp2, Foxf1), endothelial (Vegfr2, Cdh5), hematopoietic (Gata1, Hbb-Y), cardiac (Nkx2.5, Myocd, Myh6) and endodermal (Foxa2, Afp) genes. Grem2 expression starts around day 4 and increases thereafter. Gapdh served as a control. (C) CGR8 ES cells were allowed to differentiate, RNA samples were collected at successive differentiation days and expression of BMP antagonists was analyzed by quantitative PCR. Gene abbreviations for panels (B) and (C): Afp: α Fetoprotein; Bmp2: Bone morphogenetic protein 2; Cer1, Cerberus-like 1; Cdh5: Vascular endothelial cadherin; Chrd: Chordin; Foxa2: Forkhead box A2; Foxf1: Forkhead box F1; Gapdh: Glyceraldehyde-3-phosphate dehydrogenase; Gata1: GATA binding protein 1; Grem1: Gremlin 1; Grem2: Gremlin 2 (PRDC); Hbb-Y; Hemoglobin Y, beta-like embryonic chain; Myocd: Myocardin; Myh6: Myosin Heavy chain 6, or α Myosin heavy chain; Nkx2.5: NK2 Homeobox 5; Nog: Noggin; Nbl1: Neuroblastoma suppressor of tumorigenicity 1 precursor (Dan); T-bra: T-brachyury; Vegfr2: Vascular endothelial growth factor receptor 2 (Flk-1).
To position the Grem2 expression profile within the emergence of various cell lineages during ES cell differentiation, we used the pluripotent, germline competent mouse CGR8 line [7, 14, 39–41]. For this experiment, we dissociated CGR8 ES cells and allowed them to form EBs in hanging-drops. EBs differentiated in suspension for 4 days, before attaching to gelatin-coated culture dishes for further differentiation [14, 42]. Subsequently, RNA samples were prepared at successive days of differentiation for gene expression analyses by conventional PCR analysis. Our results show genes that typically appear during the gastrulation phase of development, such as Cerberus-like 1 (Cer1) and T-brachyury (T-bra), are transiently induced around day 3–4 of differentiation, whereas early mesendoderm markers, including Bmp2, Foxf1 and Foxa2, begin to express at day 4 (Fig. 1B). Shortly thereafter, the first hematopoietic and vascular-specific genes such as Gata1 and Vegfr2 appear, followed by the early cardiac-specific genes Nkx2.5 and Myocardin (Myocd). Markers of mature cells, i.e., Hemoglobin Hbb-Y, Vascular Endothelial cadherin (VE-cadherin, or Cdh5), αMyosin heavy chain (Myh6) and alpha Fetoprotein (Afp) are initially detected between days 5–7 (Fig. 1B). Grem2 expression starts around day 4 and becomes prominent from day 5 onwards, thus appearing during the early differentiation stages of mesodermal and endodermal progenitor cells, marked by expression of Foxf1 and Foxa2 respectively, and early cardiac progenitor cells marked by Nkx2.5 and Myocd (Fig. 1B). The sequential appearance of genes specific for primitive endoderm and mesoderm, hemopoiesis, vasculogenesis and cardiogenesis in differentiating EBs follows the chronological order of comparable developmental stages in the mouse embryo [7, 47].
To establish the expression pattern of Grem2 in relation to other BMP antagonists during the differentiation of specific cell lineages, we analyzed expression profiles by quantitative PCR. We found that except for Dante, whose expression levels are very low throughout the examined differentiation time (not shown), the remaining BMP antagonists are expressed at varying levels and patterns during the first 10 days of ES cell differentiation (Fig. 1C). Specifically, Cerberus-like 1 and Chordin, and to lesser extent Grem1, appear transiently around the time of germ layer formation (day 3 of EB differentiation), followed by Noggin (Nog), Dan (Nbl1) and Grem2.
The expression profiles of BMP ligands and receptors were assembled using the FunGenES database (http://biit.cs.ut.ee/fungenes) [7]. The heatmaps show that BMP type I and type II receptors are initially expressed at low levels, but increase from day 2 of differentiation (supporting information Fig. S1). With the exception of Bmp4, which is present at low levels in undifferentiated ES cells, expression of the remaining BMP ligands starts around days 3–4 and becomes prominent during formation and subsequent differentiation of lineage-specific progenitor cells, and overlaps primarily with Nog and Grem2 (days 4–10; Fig. 1C).
In summary, our data show that Grem2 expression is induced around the time that lineage-specific progenitor cells for the heart, blood, endothelium and endoderm become specified and begin to differentiate, and is consistent with its pattern during embryonic development. Moreover, the co-expression of BMP agonists and antagonists such as Nog and Grem2 suggests that BMP signaling is tightly regulated throughout the ES cell differentiation process.
Grem2 promotes cardiac differentiation
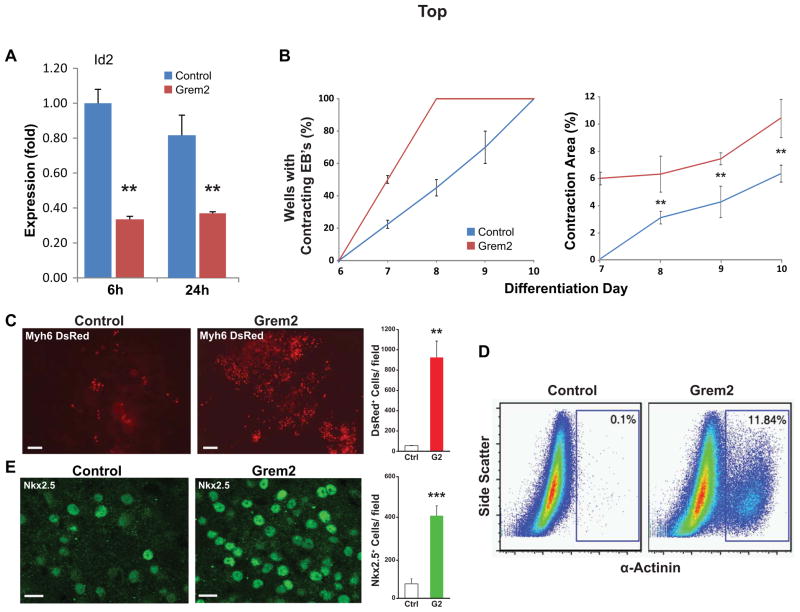
We treated EBs with Grem2 protein starting at differentiation day 4, the time point that Grem2 transcripts appear in differentiating ES cells, to enhance and thus reveal the Grem2 effect on the differentiation of specific cell lineages. Grem2 addition suppressed Id2 expression, a known BMP target, indicating that Grem2 inhibited BMP signaling (Fig. 2A).
Figure 2. Grem2 stimulates cardiac differentiation.
CGR8 ES cells were allowed to form EBs with or without addition of Grem2 starting at differentiation day 4. (A) Quantitative PCR analysis shows that Grem2 suppresses expression of the BMP signaling target gene Id2 at 6 and 24 hours (h) after addition. Id2 expression at different time points was compared to day 6 levels in control, untreated EBs, that were arbitrarily set as 1. ** P < 0.01. Id2: Inhibitor of DNA binding 2. (B) Grem2 accelerates the appearance (left graph) and expands the size of contracting areas (as percent of the total culture area) in differentiating EBs (right). ** P < 0.01. (C) CGR8 ES cells were stably engineered to express the DsRed2 fluorescent protein gene fused to a nuclear localization signal under the Myh6 promoter (Myh6-DsRed) in order to specifically mark cardiomyocytes. Grem2 increases 20-fold the yield of cells with nuclear fluorescence compared to control. Scale bar is 50 μm. ** P < 0.01. (D) Grem2 increases 120-fold the number of α-Actinin+ cardiomyocytes, quantified by flow cytometry analysis. (E) Immunofluorescence analysis using an antibody recognizing Nkx2.5 shows a 6-fold increase of positive nuclei in Grem2 treated EBs at day 12 of differentiation compared to control. Scale bar is 10 μm. *** P < 0.001.
Visual inspection of differentiating EB cultures revealed that Grem2 treatment enhanced differentiation of cardiomyocytes, as evidenced by both the accelerated appearance of contracting cells and the larger size of contracting areas compared to untreated cultures (Fig. 2B). To enable cardiomyocyte quantification per EB, we engineered CGR8 cells to express the DsRed fluorescent protein fused to a nuclear localization signal (DsRed-Nuc) under a promoter fragment of the cardiac-specific a Myosin heavy chain gene Myh6 [14, 44]. As a result, cardiomyocytes marked with nuclear red fluorescence can be easily identified and measured independently of contractility. The Myh6 promoter was chosen because expression of Myh6 is detected in the mouse in all myocardial cells as the heart tube is forming. As chamber formation begins, Myh6 expression is downregulated in the ventricles, reaching a low point around E16, while expression remains strong in the atria. After E16, Myh6 levels in the ventricles rise, replacing Myh7 after birth. Thus, except for a brief developmental period where it is mostly detected in atrial tissue, Myh6 generally shows pan-cardiac expression [48, 49], allowing us to fluorescently label cardiac myocytes.
Treating CGR8-Myh6-DsRed cells with Grem2 led to 20-fold increase of fluorescently-labeled cells, which coincided with contracting areas indicating they were cardiomyocytes (Fig. 2C). To independently quantify the Grem2 effect on cardiac cells, we dissociated EBs to single cell suspensions and stained with antibodies recognizing the cardiac-specific α-Actinin and Troponin T2 (Tnnt2) proteins. Using flow cytometry, we found that at day 10 of EB differentiation Grem2 treatment led to a 20-fold and 120-fold increase of Tnnt2+ and α-Actinin+ cells, respectively, compared to control cultures (Fig. 2D and supporting information Fig. S2). Immunofluorecence staining of EBs at day 6 with antibodies recognizing Nkx2.5 showed a 6-fold increase of Nkx2.5+ cells in Grem2-treated cultures compared to controls (Fig. 2E).
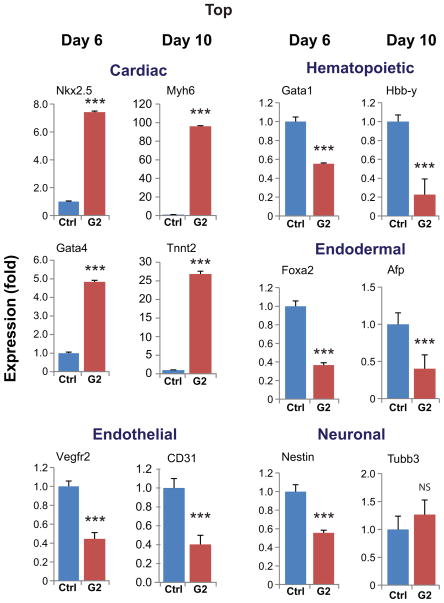
We analyzed RNA samples of control (untreated) and Grem2-treated cells by quantitative PCR with a number of gene-specific primers at day 6 during the early stages of cardiovascular differentiation and day 10, when differentiation of various cardiovascular lineages has taken place [7, 14]. In agreement with the visual observations and the flow cytometry data, Grem2 treatment induced both early and late cardiomyocyte-specific markers such as Nkx2.5 (7-fold), Gata4 (5-fold), Tnnt2 (27-fold) and Myh6 (96-fold) (Fig. 3 and supporting information Fig. S3). To the contrary, Grem2 had a statistically significant inhibitory effect (2–5 fold) on the expression levels of both early and late hematopoietic- (Gata1, Hbb-y), endothelial- (Vegfr2, CD31) and endodermal- (Foxa2, Afp) specific genes. In addition, there were minor changes in the expression of neuronal markers (Nestin, Tubb3) (Fig. 3).
Figure 3. Grem2 specifically expands cardiac differentiation.
CGR8 ES cells were allowed to differentiate untreated (Control, Ctrl) or treated with Grem2 (G2) between differentiation days 4–10. Quantitative PCR analysis of RNA samples at day 6 or 10 of differentiation shows induction of cardiac genes (Nkx2.5, Gata4, Myh6, Tnnt2) and decrease in the expression levels of hematopoietic (Gata1, Hbb-y), endothelial (Vegfr2, CD31) and endodermal (Foxa2, Afp), as well as a subset of neuronal (Nestin, Tubb3) genes. Bars represent standard error of at least 3 independent biological repeats in triplicates. Abbreviations as in Figure 1 and CD31: Cluster of differentiation 31, or Platelet Endothelial Cell Adhesion Molecule-1; Gata4: GATA binding protein 4; Tnnt2: Troponin T type 2; Tubb3: tubulin, beta 3 class III. *** P < 0.001; NS, not significant.
Taken together, the cellular and molecular data suggest that Grem2 promotes cardiac differentiation, while suppressing hematopoietic, endothelial and endodermal cell lineage markers. Depending on detection method, time point, and gene analyzed, our results show early cardiac differentiation is increased 5–7 fold (day 6), whereas late differentiation is increased 20–120 fold (day 10).
Grem2 preferentially induces atrial-like cardiomyocytes
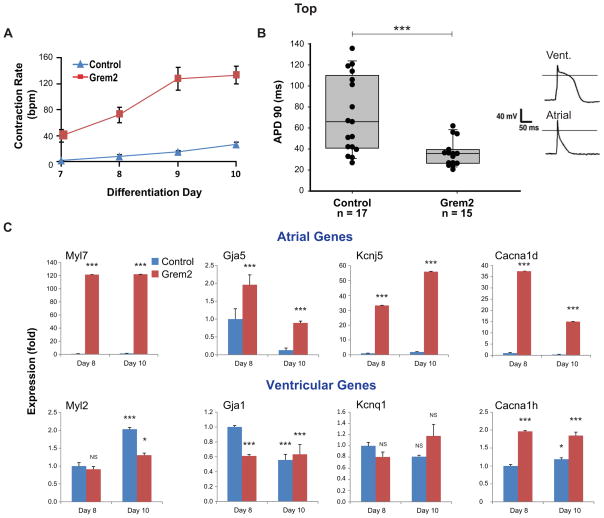
We observed that cardiomyocytes derived from Grem2-treated EB cultures contracted at higher rates than untreated cells, suggesting a distinct electrophysiological phenotype (Fig. 4A). To test this possibility, we isolated DsRed+ cardiomyocytes from differentiated Myh6-DsRed CGR8 cultures and measured action potential in individual cells using patch-clamp electrophysiology. Untreated, control cells displayed a wide range of action potentials, either short lasting, typical of atrial cells, or with a long plateau phase, characteristic of ventricular cells [50–52]. In contrast, Grem2-treated differentiating ES cells gave rise to a relatively uniform cardiomyocyte population with short, atrial-like, action potentials (Fig. 4B).
Figure 4. Grem2 promotes atrial differentiation.
CGR8 ES cells were allowed to differentiate untreated (Control) or treated with Grem2 between differentiation days 4–10. (A) Grem2 treatment yields cardiomyocytes with higher contraction rates averaging approximately 120 beats per minute (bpm) at day 10 as compared to untreated controls that contract at 30 bmp on average. Bars represent standard error of 3 repeats. (B) Action potential duration (APD) recorded from single ES cell-derived cardiomyocytes using patch-clamp analysis in the current clamp mode. Data points represent APD measured from individual cells (n=17 control, n=15 Grem2-treated cells) at 90% repolarization (APD90) in milliseconds (ms). Control EB cultures contain a collection of cardiac cell types showing a wide range of APD90. Grem2-treated cells show short APD typical of atrial cells. Top right panels show representative recorded long and short APD. A line is drawn at 0 mV. *** P < 0.001. (C) Quantitative PCR analysis of RNA samples isolated at days 8 and 10 of differentiation shows that Grem2 preferentially induces atrial-specific genes (Myl7, Gja5, Kcnj5, Cacna1d), but either does not affect, or suppresses the expression levels of ventricular-specific genes (Myl2, Gja1, Kcnq1, Cacna1h). Bars represent standard error of 3 repeats. * P < 0.05; *** P < 0.001; NS, not significant, as compared to Day 8 control expression levels that were arbitrarily set at value 1. Abbreviations: Canca1d: Calcium channel, voltage-dependent, L type, alpha 1D subunit; Canca1h: Calcium channel, voltage-dependent, T type, alpha 1H subunit; Gja1: Connexin-43: Gja5: Connexin-40; Kcnj5: Potassium inwardly-rectifying channel, subfamily J, member 5; Kcnq1: Potassium voltage-gated channel, KQT-like subfamily, member 1; Myl2: Myosin regulatory light chain 2v (ventricular): Myl7: Myosin regulatory light chain 2a (atrial).
The electrophysiology results indicate that Grem2 preferentially drives differentiation of atrial cardiomyocytes. To confirm the electrophysiological phenotype at the molecular level, we analyzed RNA samples from control and Grem2-treated cells at differentiation days 8 and 10. The results showed that Grem2 induced genes that encode atrial contractile proteins (Myl7 or Mlc2a) [53], gap junction proteins (Gja5) [54] and ion channels (Kcnj5, Cacna1d) [55, 56], as well as Nppa and Sarcolipin [57, 58] (Fig. 4C and supporting information Fig. S3). In contrast, Grem2 treatment did not have a significant effect (less than 2-fold up or down regulation) on ventricular markers for contractile proteins (Myl2 or Mlc2v) [59, 60], gap junction proteins (Gja1) [61] and ion channels (Kcnq1, Cacna1h) [62, 63].
In summary, our data indicate that Grem2 robustly stimulates cardiomyocyte differentiation. This is primarily due to expansion of the atrial lineage, rather than specification changes in cardiac progenitor cells, since differentiation of ventricular myocytes is moderately suppressed but proceeds in typical fashion.
Grem2 induces atrial- and suppresses ventricular-specific transcriptional regulators
Factors regulating atrial differentiation are poorly understood. One exception is the orphan nuclear receptor CoupTFII (or Nr2f2), the expression of which is restricted to the sinus venosus area of the primitive heart tube and later is present in developing atria, but not in ventricles [64, 65]. In line with the expression profile data, CoupTFII inactivation by homologous recombination leads to atrial malformations whereas ventricular development is unaffected [64]. Furthermore, the Notch signaling activated transcription factor Hey1 is also atrial-specific and required for atria development [66, 67]. Conversely, the expression of transcription factors Irx4 and Hey2 is confined to the developing ventricles, where they promote ventricular-specific and suppress atrial-specific genes [66, 67, 68–71].
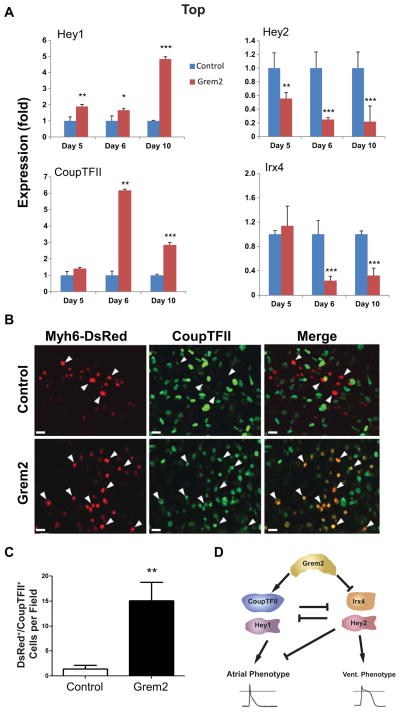
To determine whether Grem2 promotes atrial gene expression by induction of early transcriptional regulators of atrial fate, suppression of regulators of ventricular fate, or both, we analyzed RNA samples from untreated (control) and Grem2-treated cells. The results showed that Grem2 upregulates Hey1 and CoupTFII and suppresses the expression of Hey2 and Irx4 (Fig. 5A).
Figure 5. Grem2 induces atrial and suppresses ventricular specific transcriptional regulators.
CGR8 ES cells were allowed to differentiate untreated (Control) or treated with Grem2 between differentiation days 4–10. (A) Quantitative PCR analysis of RNA samples isolated at day 5, 6, and 10 of differentiation shows induction of Hey1 and CoupTFII and suppression of Hey2 and Irx4 after Grem2 treatment. * P < 0.05; ** P < 0.01; *** P < 0.001, comparing Grem2 samples to Controls of the same time point, which were arbitrarily set at value 1. (B) Immunofluorescence analysis of EBs generated using ES cells engineered with the Myh6-DsRed construct and stained with an antibody recognizing CoupTFII at day 12 of differentiation. Grem2-treated cultures contain cardiomyocytes (red nuclear fluorescence) that express nuclear CoupTFII in green (arrowheads), whereas Control cultures display fewer CoupTFII+ cardiomyocytes. Scale bar is 10 μm. (C) Quantification of immunofluorescence in panel B shows 11-fold increase in DsRED/CoupTFII double positive cardiomyocytes by Grem2. ** P < 0.01. (D) Schematic model of Grem2 effects on cardiomyocyte differentiation. CoupTFII and Hey 1 promote atrial differentiation, whereas Hey2 and Irx4 enhance ventricular and suppress atrial differentiation. Grem2 directly or indirectly induces CoupTFII and Hey1, and blocks Irx4 and Hey2 expression. The net result is an increase of atrial differentiation.
Because CoupTFII is found in a number of cell types in other tissues including venous endothelium, somites and brain [64], we tested whether Grem2 induces expression of CoupTFII protein in cardiomyocytes by staining Myh6-DsRed expressing CGR8 cells with antibodies against CoupTFII. Confocal images show that the majority of the DsRed+ cardiomyocytes co-stain with the CoupTFII antibody after Grem2 treatment, whereas there is less overlap between the two markers in control cultures (Fig. 5B). Quantification of single DsRed+ and double DsRed+/CoupTFII+ cells showed an 11-fold enrichment of cardiomyocytes with nuclear expression of the atrial regulatory factor CoupTFII (Fig. 5C). In summary, Grem2 concurrently stimulates expression of atrial differentiation-promoting transcription factors and downregulates transcriptional repressors of atrial fate (Fig. 5D).
Promotion of cardiac differentiation is a specific property of the gremlin subfamily of BMP antagonists
To test whether enhancement of cardiomyocytic differentiation is specific to Grem2, or a general property of BMP signaling inhibition, we compared the effects of Grem2 to those of other BMP antagonists. In this experiment, we treated EBs from differentiation days 4 to 10 with a panel of BMP ligands and BMP antagonists, isolated RNA samples at days 6 and 10, and analyzed gene expression by quantitative PCR.
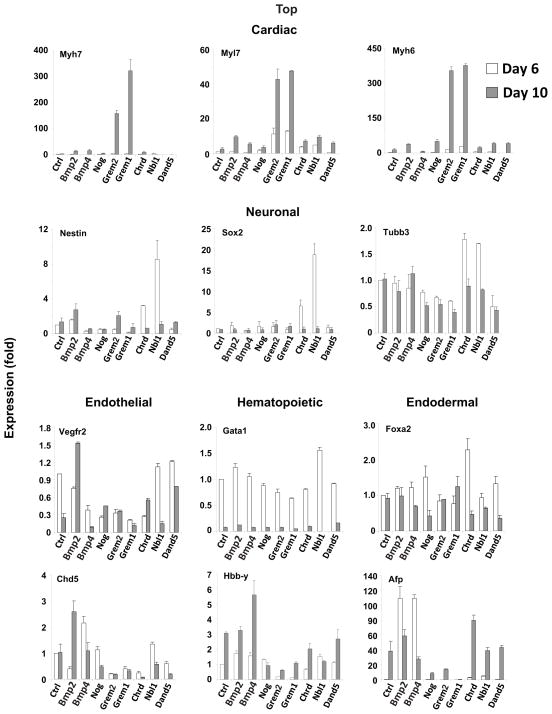
The results showed that induction of cardiac-specific markers Myl7, Myh6 and Myh7 is a unique property of Grem2 and its close paralog Gremlin 1 (Fig. 6). Grem1 had moderate negative influence on the differentiation of endothelial, hematopoietic and endodermal cells, similar to Grem2. Conversely, the rest of the tested BMP ligands and antagonists had minor effects on cardiac differentiation when applied at the same time points (i.e., differentiation days 4–10). BMP2 and BMP4 had moderate effects on endothelial, hematopoietic and endodermal gene expression, with the exception of an early, robust induction of Afp. Dan, also known as “Neuroblastoma suppressor of tumorigenicity 1 precursor (Nbl1)”, led to induction of neuronal-stem cell specific markers Nestin and Sox2, whereas Chordin treatment resulted in an increase of endoderm-specific genes, in agreement with the roles of Dan and Chordin in vivo (Fig. 6) [72, 73].
Figure 6. Induction of cardiac genes is a specific property of the Gremlin subfamily of BMP antagonists.
CGR8 ES cells were allowed to differentiate untreated (Control, Ctrl) or treated between day 4 to 10 with Bmp2, Bmp4, Noggin (Nog), Gremlin 2 (Grem2), Gremlin 1 (Grem1), Chordin (Chrd), Dan (Nbl1) and Dante (Dand5). The graphs depict quantitative PCR analysis results of RNA samples at differentiation days 6 and 10 using gene-specific primers for cardiac (Myh7, Myl7, Myh6), neuronal (Nestin, Sox2, Tubb3), endothelial (Vegfr2, Cdh5), hematopoietic (Gata1, Hbb-Y) and endodermal (Foxa2, Afp) markers. Bars represent standard error of at least 4 repeats. Day 6 control expression levels were arbitrarily set at value 1. Gene name abbreviations as in earlier figures and Sox2: Sry box binding protein 2.
Taken together, our results indicate that manipulation of EBs with individual BMP antagonists can lead to enrichment of various cell types; however, only Grem2 and Grem1 trigger a specific and robust induction in cardiac-specific genes.
JNK signaling mediates Grem2-induced atrial differentiation
BMP signaling is induced when BMP ligands bind to Type II receptors forming a complex that engages the Type I receptors, which subsequently undergo transphosphorylation by the constitutively active kinase domain of Type II receptors. This leads to recruitment and phosphorylation of cytoplasmic Smads 1/5/8 (P-Smads), which then heterodimerize with Smad4 and translocate to the nucleus, acting as transcriptional regulators of BMP target genes such as Id2. Besides canonical, Smad-mediated signaling, activated BMP receptors can also stimulate several other signaling cascades involving p38, JNK and other MAP kinases [74].
The selective induction of cardiac-specific genes by Grem2 might be due to inhibition of particular BMP ligands, or to a specific effect on intracellular BMP signaling. To test the first possibility, we examined the effect of blocking BMP2 and BMP4 by different antagonists. ES cells were stably transfected with a construct carrying the Luciferase reporter gene under the control of the BMP Responsive Element of the Id2 gene (BRE2-Luc), acting as an indicator of Smad-mediated BMP signaling [43]. The results showed that all DAN family antagonists, as well as Chordin and Noggin, effectively inhibit BMP signaling at the concentrations used in the experiments described above (supporting information Fig. S4). Moreover, we found that Grem2 is equally effective at inhibiting a wide range of BMP ligands including BMP 2, 4, 5, 6, 7 and 10, suggesting that it does not preferentially antagonize select BMP ligands (supporting information Fig. S5).
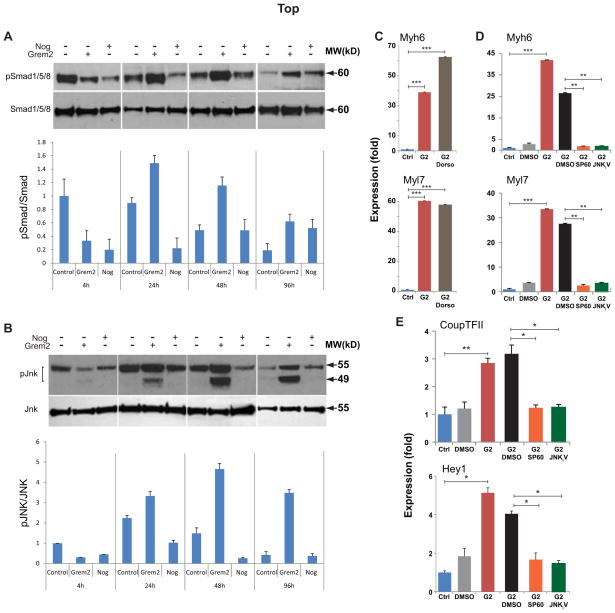
Next, we compared Grem2 and Noggin effects on canonical and non-canonical BMP signaling in EBs treated starting at differentiation day 4. Western blot analysis of protein samples isolated 4, 24, 48 and 96 hours after Grem2 and Noggin treatment using antibodies against activated phospho-Smad1/5/8 and phospho-JNK proteins showed that, initially (4 hours), both Grem2 and Noggin effectively blocked Smad phosphorylation (Fig. 7A). However, Grem2 led to a subsequent strong upregulation of both Smad1/5/8 and JNK phosphorylation, possibly through a delayed feedback response (Fig. 7A,B).
Figure 7. JNK signaling activation by Grem2 is required for atrial cardiogenesis.
(A) CGR8 ES cells were allowed to differentiate untreated (Control) or treated between day 4 to 10 with Noggin (Nog), or Grem2. Western blot analysis of EB protein lysates collected at sequential differentiation time points and blotted with antibodies recognizing total and phosphorylated forms of Smad1/5/8 [expected Molecular Weight (MW) 60 kD] and pJNK isoforms (49 and 55 kD, respectively). Both Noggin and Grem2 effectively inhibit Smad phosphorylation. However, in both Noggin- and Grem2- treated samples Smad phosphorylation recovers at later time points. In contrast, only Grem2 treatment leads to strong upregulation of JNK phosphorylation. Graphs below the blots represent quantification of phosphorylated Smad and JNK forms relative to total protein levels. (C, D) CGR8 ES cells were allowed to differentiate untreated (Control, Ctrl) or treated between days 4 to 10 with Grem2 (G2). Grem2 samples were also treated with the Smad1/5/8 inhibitor dorsomorphin (Dorso) and the JNK inhibitors SP600125 (SP60) and JNKi V. The JNK inhibitors’ vehicle (DMSO) was also used as a control. Quantitative PCR analysis of Myh6 and Myl7 expression at differentiation day 10 shows that JNK inhibition suppresses Grem2-induced cardiac and atrial genes, whereas dorsomorphin has no effect. Control values were arbitrarily set at 1. (E) Quantitative PCR analysis at D10 shows that JNK inhibition suppresses Grem2-induced activation of atrial regulatory genes CoupTFII and Hey1. * P < 0.05; ** P < 0.01; *** P < 0.001, comparing samples as indicated by horizontal bars.
To determine whether Grem2 promotion of atrial fate during initial cardiac differentiation depends on the counter activation of Smad1/5/8 or JNK 1&2 signaling, we co-incubated Grem2-treated EBs with the specific Smad1/5/8 inhibitor dorsomorphin [12] or the JNK inhibitor SP600125 [14]. The results showed that JNK signaling inhibition of Grem2-treated EBs between days 4–6 completely abolished induction of cardiac genes, whereas dorsomorphin had no significant effect (Fig. 7C, D). We obtained comparable attenuation of the effects of Grem2 on cardiac differentiation using JNKi V (AS601245), a structurally distinct inhibitor of the JNK signaling pathway (Fig. 7D) [75]. To test the role of JNK signaling in the induction of early transcriptional regulators of atrial differentiation, we analyzed Hey1 and CoupTFII expression in RNA samples of Grem2-treated EBs in the presence or absence of SP600125 and JNKi V. The results showed that both JNK inhibitors blocked induction of Hey1 and CoupTFII (Fig. 7E).
In conclusion, although Grem2 inhibits canonical, i.e., Smad-mediated BMP signaling as effectively as other BMP antagonists such as Noggin, Grem2 treatment of differentiating ES cells selectively stimulates JNK signaling. JNK signaling acts upstream to activate transcriptional regulators of atrial differentiation such as CoupTFII and Hey1.
DISCUSSION
Our data demonstrate that the Grem2 morphogen, which regulates atrial formation and establishment of cardiac rhythm during embryonic development, can be employed to promote differentiation of pluripotent stem cells to cardiomyocytes with electrophysiological and molecular properties characteristic of atrial cells. Grem2 treatment leads to ~20–120-fold expansion of myocytes and comparable induction levels in the expression of genes encoding atrial-specific contractile, gap junction and ion channel proteins such as Myl7, Gja5, Cacna1d and Kcnj5. Interestingly, Grem2 has no major effects on ventricular myocyte differentiation and the expression levels of corresponding ventricular-specific genes such as Myl2, Gja1, Cacna1h and Kcnq1. Consistent with these findings, electrophysiology data show that Grem2-induced, ES-cell derived cardiomyocytes have action potentials typical of atrial myocytes. In contrast, cardiomyocytes in control samples display wide heterogeneity of electrical properties.
Activation or suppression of downstream signaling gene networks conferring chamber identity mechanisms could explain the Grem2 effects. A recent elegant analysis of cardiac-specific CoupTFII knockout mice demonstrated that inactivation of CoupTFII leads to ventricularized atria with loss of atrial-specific genes Hey1, Myl7, Gja5, Kcnj3, and Kcnj5 and gain of ventricular-specific genes Hey2, Irx4 and Myl2 [65]. In complementary fashion, overexpression of CoupTFII in ventricles leads to increased Myl7 and decreased Myl2 expression, further suggesting a critical role of CoupTFII in regulating atrial identity. Our data show that Grem2 treatment of differentiating ES cells leads to induction of CoupTFII and has similar effects as gain of CoupTFII (and opposite effects to loss of CoupTFII) function in mouse hearts, suggesting that Grem2 acts upstream of CoupTFII to confer atrial identity.
The Notch signaling pathway promotes cardiac cell proliferation and differentiation and thus, is required for multiple aspects of cardiovascular development. Hairy related transcription factors Hey1 and Hey2 are downstream targets of Notch signaling. Hey2 is specifically expressed in ventricular cardiomyocytes and is known to suppress atrial-specific gene expression, including CoupTFII [66, 67]. Therefore, it is also possible that Grem2 induces atrial phenotype through direct Hey2 downregulation, thus, removing the suppression of CoupTFII [70, 71, 76].
Our data further show that stimulation of atrial cardiomyocyte differentiation is confined to the gremlin subfamily of BMP antagonists and does not depend singularly on BMP inhibition. Other tested BMP antagonists such as Noggin, DAN, Chordin and Dante do not induce atrial, nor cardiac differentiation at this stage of ES cell differentiation. Instead, our results indicate that Grem2 leads to JNK signaling activation, which is necessary for atrial differentiation. Interestingly, JNK signaling activation by non-canonical Wnt ligands such as Wnt11 is required for cardiac induction during development [77] and differentiation of ES-derived cardiac progenitor cells to cardiomyocytes [14]. However, unlike non-canonical Wnt signaling, Grem2-mediated JNK activation specifically induces atrial cardiomyocytes, acting upstream of CoupTFII and Hey1.
The molecular mechanisms of Grem2 function at the protein structure level are currently being discovered. Unlike BMP ligands that have similar structures, BMP antagonists are structurally diverse, suggesting that their mode of BMP signaling inhibition varies. Consistent with this possibility, the Grem2 structure has been recently resolved and shows that Grem2 forms head-to-tail dimers unlike the head-to-head pairing in Noggin complexes [78]. Although the Grem2/BMP complex structure has not yet been resolved, this arrangement predicts that Grem2 does not completely surround BMP complexes as Noggin does. As a result, it is likely that Grem2 selectively hinders the interaction of BMP ligands with their receptors, allowing stimulation of downstream signaling through counter activation of compensatory feedback loop mechanisms. Additionally, Grem2, unlike Noggin, shows structural characteristics of growth factors. Therefore, it is possible that Grem2 binds and activates yet to be determined receptors that lead to JNK phosphorylation, independently of BMP signaling. Resolution of the BMP/Grem2 structural complexes and their interaction with BMP receptors may shed light on these different scenarios.
SUMMARY
Atrial and ventricular cardiomyocytes display distinct phenotypic characteristics, including expression of particular sets of contractile proteins and ion channels as well as different responses to hormonal stimuli [79–81]. However, adult cardiac cells do not grow well in culture and lose tissue-specific characteristics after isolation, thus hindering investigations on the molecular and cellular bases of various cardiomyopathies or arrhythmias. Moreover, the adult heart has limited regenerative potential necessitating the development of exogenous sources of cells for possible repair of cardiac tissue after acute ischemic injury or chronic heart failure [82].
Our data demonstrate that the atrial morphogen Grem2 can significantly increase both the cardiogenic output of mouse ES cells and their differentiation to cardiomyocytes with atrial characteristics. The capacity of Grem2 to guide differentiation of ES cells toward atrial fates will assist in the development of novel strategies to enrich the yield of cardiomyocytes with specific electrical and contractile properties for experimental and clinical applications in order to study and treat atrial disorders. To this end, we are currently investigating the expression profile of Grem2 during differentiation of human pluripotent stem cells and the effects of Grem2 on the differentiation of human atrial cardiomyocytes.
Supplementary Material
Figure S1. Expression of BMP signaling components during ES cell differentiation. CGR8 ES cells were allowed to differentiate and RNA samples were collected at successive differentiation days. (A) Gene expression analysis of BMP receptors and ligands by quantitative PCR. Gene name abbreviations as in the manuscript text and Bmpr: BMP receptor. (B) Expression profile heatmaps of genes encoding BMP signaling receptors, ligands and antagonists at consecutive days of CGR8 ES cell differentiation obtained using the analytical tools of the FunGenES mouse ES cell database. The color scale represents fold changes from −3 (green) to +3 (red color).
Figure S2. Grem2 promotes differentiation of cardiomyocytes. CGR8 ES cells were allowed to form EBs with or without addition of Grem2 between differentiation days 4–10. Flow cytometry analysis using the cardiac specific type 2 Troponin T (Tnnt2) antibody shows 21-fold increase in number of Tnnt2+ cardiomyocytes at day 10 of differentiation in Grem2-treated cultures compared to control.
Figure S3. Grem2 stimulates expression of cardiac-specific genes during ES cell differentiation. CGR8 ES cells were allowed to differentiate and Grem2 was added between differentiation days 4–10. RNA samples were isolated at day 10 and analyzed by quantitative RCR. Grem2 treatment leads to induction of cardiac and atrial genes compared to untreated, control samples. Gene name abbreviations: Isl1: Insulin gene enhancer protein 1; Kcne2: Potassium voltage-gated channel, Isk-related family, member 2; Kcne4: Potassium voltage-gated channel, Isk-related family, member 4; Kcnj3: Potassium inwardly-rectifying channel, subfamily J, member 3; Myh7: Myosin heavy chain 7 or β Myosin heavy chain; Mybpc3: Myosin binding protein C, cardiac; Nppa: Natriuretic peptide A; Sln: Sarcolipin. ** P< 0.01, *** P<0.001, compared to the corresponding controls.
Figure S4. BMP antagonists inhibit signaling of BMP2 and BMP4. ES cells were stably transfected with the BRE2-Luciferase plasmid that carries the reporter luciferase gene under the control of the BMP-Responsive Element in the Id2 gene promoter. The reporter ES cell line was treated for 6 hours with BMP2 or BMP4 and increasing concentrations of specific antagonists as indicated. Luciferase activity analysis of protein lysates shows that all tested BMP antagonists effectively inhibit Smad-mediated BMP signaling.
Figure S5. Grem2 inhibits a wide range of BMP ligands. ES cells, stably transfected with the BRE2-Luciferase plasmid were exposed for 6 hrs to BMP ligands in the absence or presence of increasing amounts of Grem2 as indicated. Cells were lysed and Luciferase activity was measured. Grem2 effectively inhibits BMP signaling by all tested BMP ligands.
Acknowledgments
We’d like to thank Dr. Jeffrey Robbins for providing the Myh6 gene promoter plasmid, Kevin Tompkins, Leshana Saint-Jean in Dr. Scott Baldwin’s lab for assistance with mouse embryo isolation, and Daniel Levic for help with in situ hybridization. We are grateful to Bryan Fioret for critical reading of the manuscript. This work was supported by NIH grants HL083958 and HL100398 (A. K. H.), DE018477 (E. W. K), and 2T32HL007411-33 “Program in Cardiovascular Mechanisms: Training in Investigation” (J. B.).
Footnotes
Author contributions: V.T., J.B.B. and J.Y., conception and design, collection and data assembly, data analysis and interpretation, manuscript writing; J.H., J.M.W., A.M., W.H.H., W-D.W., F.P. and M.R. data collection, data analysis and interpretation; S.K., data analysis and interpretation; E.W.K and A.K.H., conception and design, data analysis and interpretation, manuscript writing.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors have no potential conflicts of interest.
References
- 1.Doetschman TC, Eistetter H, Katz M, et al. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 2.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 3.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Moretti A, Caron L, Nakano A, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Wu SM, Fujiwara Y, Cibulsky SM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 7.Schulz H, Kolde R, Adler P, et al. The FunGenES database: a genomics resource for mouse embryonic stem cell differentiation. PLoS ONE. 2009;4:e6804. doi: 10.1371/journal.pone.0006804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuasa S, Itabashi Y, Koshimizu U, et al. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J, Han P, Zhang Q, et al. Cardiac differentiation of human pluripotent stem cells. J Cell Mol Med. 2012;16:1663–1668. doi: 10.1111/j.1582-4934.2012.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mummery CL, Zhang J, Ng ES, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344–358. doi: 10.1161/CIRCRESAHA.110.227512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onizuka T, Yuasa S, Kusumoto D, et al. Wnt2 accelerates cardiac myocyte differentiation from ES-cell derived mesodermal cells via non-canonical pathway. Mol Cell Cardiol. 2012;52:650–659. doi: 10.1016/j.yjmcc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Hao J, Daleo MA, Murphy CK, et al. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni TT, Rellinger EJ, Mukherjee A, et al. Discovering small molecules that promote cardiomyocyte generation by modulating Wnt signaling. Chem Biol. 2011;18:1658–1668. doi: 10.1016/j.chembiol.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rai M, Walthall JM, Hu J, et al. Continuous antagonism by Dkk1 counter activates canonical Wnt signaling and promotes cardiomyocyte differentiation of embryonic stem cells. Stem Cells Dev. 2012;21:54–66. doi: 10.1089/scd.2011.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turbendian HK, Gordillo M, Tsai SY, et al. GATA factors efficiently direct cardiac fate from embryonic stem cells. Development. 2013;140:1639–44. doi: 10.1242/dev.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pater E, de Ciampricotti M, Priller F, et al. Bmp signaling exerts opposite effects on cardiac differentiation. Circ Res. 2012;110:578–587. doi: 10.1161/CIRCRESAHA.111.261172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breckenridge RA, Mohun TJ, Amaya E. A role for BMP signalling in heart looping morphogenesis in Xenopus. Dev Biol. 2001;232:191–203. doi: 10.1006/dbio.2001.0164. [DOI] [PubMed] [Google Scholar]
- 19.Chocron S, Verhoeven MC, Rentzsch F, et al. Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Dev Biol. 2007;305:577–588. doi: 10.1016/j.ydbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 21.Ma L, Lu MF, Schwartz RJ, et al. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- 22.Liu W, Selever J, Wang D, et al. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci USA. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCulley DJ, Kang JO, Martin JF, et al. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5:Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- 25.Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Shi S, Acosta L, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development. 2004;131:2219–2231. doi: 10.1242/dev.01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi M, Stottmann RW, Yang YP, et al. The bone morphogenetic protein antagonist noggin regulates mammalian cardiac morphogenesis. Circ Res. 2007;100:220–228. doi: 10.1161/01.RES.0000257780.60484.6a. [DOI] [PubMed] [Google Scholar]
- 28.Monzen K, Hiroi Y, Kudoh S, et al. Smads, TAK1, and their common target ATF-2 play a critical role in cardiomyocyte differentiation. J Cell Biol. 2001;153:687–698. doi: 10.1083/jcb.153.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Pearce JJ, Penny G, Rossant J. A mouse cerberus/Dan-related gene family. Dev Biol. 1999;209:98–110. doi: 10.1006/dbio.1999.9240. [DOI] [PubMed] [Google Scholar]
- 31.Avsian-Kretchmer O, Hsueh AJ. Comparative genomic analysis of the eight-membered ring cystine knot-containing bone morphogenetic protein antagonists. Mol Endocrinol. 2004;18:1–12. doi: 10.1210/me.2003-0227. [DOI] [PubMed] [Google Scholar]
- 32.Sudo S, Avsian-Kretchmer O, Wang LS, et al. Protein related to DAN and cerberus is a bone morphogenetic protein antagonist that participates in ovarian paracrine regulation. J Biol Chem. 2004;279:23134–23141. doi: 10.1074/jbc.M402376200. [DOI] [PubMed] [Google Scholar]
- 33.Minabe-Saegusa C, Saegusa H, Tsukahara M, et al. Sequence and expression of a novel mouse gene PRDC (protein related to DAN and cerberus) identified by a gene trap approach. Dev Growth Differ. 1998;40:343–353. doi: 10.1046/j.1440-169x.1998.t01-1-00010.x. [DOI] [PubMed] [Google Scholar]
- 34.Lu MM, Yang H, Zhang L, et al. The bone morphogenic protein antagonist gremlin regulates proximal-distal patterning of the lung. Dev Dyn. 2001;222:667–680. doi: 10.1002/dvdy.1231. [DOI] [PubMed] [Google Scholar]
- 35.Kriebitz NN, Kiecker C, McCormick L, et al. PRDC regulates placode neurogenesis in chick by modulating BMP signalling. Dev Biol. 2009;336:280–292. doi: 10.1016/j.ydbio.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Ideno H, Takanabe R, Shimada A, et al. Protein related to DAN and cerberus (PRDC) inhibits osteoblastic differentiation and its suppression promotes osteogenesis in vitro. Exp Cell Res. 2009;315:474–484. doi: 10.1016/j.yexcr.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 37.Zuniga E, Rippen M, Alexander C, et al. Gremlin 2 regulates distinct roles of BMP and Endothelin 1 signaling in dorsoventral patterning of the facial skeleton. Development. 2011;138:5147–5156. doi: 10.1242/dev.067785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller II, Knapik EW, Hatzopoulos AK. Expression of the protein related to Dan and Cerberus gene--prdc--during eye, pharyngeal arch, somite, and swim bladder development in zebrafish. Dev Dyn. 2006;235:2881–2888. doi: 10.1002/dvdy.20925. [DOI] [PubMed] [Google Scholar]
- 39.Müller II, Melville DB, Tanwar V, et al. Functional modeling in zebrafish demonstrates that the atrial-fibrillation-associated gene GREM2 regulates cardiac laterality, cardiomyocyte differentiation and atrial rhythm. Dis Model Mech. 2013;6:332–341. doi: 10.1242/dmm.010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mountford P, Zevnik B, Duwel A, et al. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer N, Jaconi M, Landopoulou A, et al. A fluorescent reporter gene as a marker for ventricular specification in ES-derived cardiac cells. FEBS Lett. 2000;478:151–158. doi: 10.1016/s0014-5793(00)01839-1. [DOI] [PubMed] [Google Scholar]
- 42.Sachinidis A, Fleischmann BK, Kolossov E, et al. Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc Res. 2003;58:278–291. doi: 10.1016/s0008-6363(03)00248-7. [DOI] [PubMed] [Google Scholar]
- 43.Monteiro RM, de Sousa Lope SM, Korchynskyi O, et al. Spatio-temporal activation of Smad1 and Smad5 in vivo: monitoring transcriptional activity of Smad proteins. J Cell Sci. 2004;117:4653–4663. doi: 10.1242/jcs.01337. [DOI] [PubMed] [Google Scholar]
- 44.Subramaniam A, Jones WK, Gulick J, et al. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. J Biol Chem. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 45.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Beck H, Semisch M, Culmsee C, et al. Egr-1 regulates expression of the glial scar component phosphacan in astrocytes after experimental stroke. Am J Pathol. 2008;173:77–92. doi: 10.2353/ajpath.2008.070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Lyons GE, Schiaffino S, Sassoon D, et al. Developmental regulation of myosin gene expression in mouse cardiac muscle. J Cell Biol. 1990;111:2427–2436. doi: 10.1083/jcb.111.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rindt H, Subramaniam A, Robbins J. An in vivo analysis of transcriptional elements in the mouse α-myosin heavy chain gene promoter. Trans Res. 1995;4:397–405. doi: 10.1007/BF01973758. [DOI] [PubMed] [Google Scholar]
- 50.Hescheler J, Fleischmann BK, Lentini S, et al. Embryonic stem cells: a model to study structural and functional properties in cardiomyogenesis. Cardiovasc Res. 1997;36:149–162. doi: 10.1016/s0008-6363(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 51.He J, Ma Q, Lee Y, et al. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Klos M, Wilson GF, et al. Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res. 2012;111:1125–1136. doi: 10.1161/CIRCRESAHA.112.273144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kubalak SW, Miller-Hance WC, O’Brien TX, et al. Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis. J Biol Chem. 1994;269:16961–16970. [PubMed] [Google Scholar]
- 54.Leaf DE, Feig JE, Vasquez C, et al. Connexin 40 imparts conduction heterogeneity to atrial tissue. Circ Res. 2008;103:1001–1008. doi: 10.1161/CIRCRESAHA.107.168997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleischmann BK, Duan Y, Fan Y, et al. Differential subunit composition of the G protein-activated inward-rectifier potassium channel during cardiac development. J Clin Invest. 2004;114:994–1001. doi: 10.1172/JCI15925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z, He Y, Tuteja D, et al. Functional roles of Cav1. 3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation. 2005;112:1936–1944. doi: 10.1161/CIRCULATIONAHA.105.540070. [DOI] [PubMed] [Google Scholar]
- 57.Houweling AC, Somi S, Van Den Hoff MJ, et al. Developmental pattern of ANF gene expression reveals a strict localization of cardiac chamber formation in chicken. Anat Rec. 2002;266:93–102. doi: 10.1002/ar.10042. [DOI] [PubMed] [Google Scholar]
- 58.Minamisawa S, Wang Y, Chen J, et al. Atrial chamber-specific expression of sarcolipin is regulated during development and hypertrophic remodeling. J Biol Chem. 2003;278:9570–9575. doi: 10.1074/jbc.m213132200. [DOI] [PubMed] [Google Scholar]
- 59.O’Brien TX, Lee KJ, Chien KR. Positional specification of ventricular myosin light chain 2 expression in the primitive murine heart tube. Proc Natl Acad Sci USA. 1993;90:5157–5161. doi: 10.1073/pnas.90.11.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee MY, Sun B, Schliffke S, et al. Derivation of functional ventricular cardiomyocytes using endogenous promoter sequence from murine embryonic stem cells. Stem Cell Res. 2012;8:49–57. doi: 10.1016/j.scr.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gutstein DE, Morley GE, Vaidya D, et al. Heterogeneous expression of Gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 62.Péréon Y, Demolombe S, Baró I, et al. Differential expression of KvLQT1 isoforms across the human ventricular wall. Am J Physiol Heart Circ Physiol. 2000;278:H1908–1915. doi: 10.1152/ajpheart.2000.278.6.H1908. [DOI] [PubMed] [Google Scholar]
- 63.Markandeya YS, Fahey JM, Pluteanu F, et al. Caveolin-3 regulates protein kinase A modulation of the Ca(V)3. 2 (alpha1H) T-type Ca2+ channels. J Biol Chem. 2011;286:2433–2444. doi: 10.1074/jbc.M110.182550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira FA, Qiu Y, Zhou G, et al. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu SP, Cheng CM, Lanz RB, et al. Atrial identity is determined by a COUP-TFII regulatory network. Dev Cell. 2013;25:417–426. doi: 10.1016/j.devcel.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakagawa O, Nakagawa M, Richardson JA, et al. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol. 1999;216:72–84. doi: 10.1006/dbio.1999.9454. [DOI] [PubMed] [Google Scholar]
- 67.Kokubo H, Tomita-Miyagawa S, Hamada Y, et al. Hesr1 and Hesr2 regulate atrioventricular boundary formation in the developing heart through the repression of Tbx2. Development. 2007;134:747–755. doi: 10.1242/dev.02777. [DOI] [PubMed] [Google Scholar]
- 68.Bao ZZ, Bruneau BG, Seidman JG, et al. Regulation of chamber-specific gene expression in the developing heart by Irx4. Science. 1999;283:1161–1164. doi: 10.1126/science.283.5405.1161. [DOI] [PubMed] [Google Scholar]
- 69.Bruneau BG, Bao ZZ, Fatkin D, et al. Cardiomyopathy in Irx4-deficient mice is preceded by abnormal ventricular gene expression. Mol Cell Biol. 2001;21:1730–1736. doi: 10.1128/MCB.21.5.1730-1736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koibuchi N, Chin MT. CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ Res. 2007;100:850–855. doi: 10.1161/01.RES.0000261693.13269.bf. [DOI] [PubMed] [Google Scholar]
- 71.Xin M, Small EM, van Rooij E, et al. Essential roles of the bHLH transcription factor Hrt2 in repression of atrial gene expression and maintenance of postnatal cardiac function. Proc Natl Acad Sci USA. 2007;104:7975–7980. doi: 10.1073/pnas.0702447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sasai Y, Lu B, Piccolo S, et al. Endoderm induction by the organizer-secreted factors chordin and noggin in Xenopus animal caps. EMBO J. 1996;15:4547–4555. [PMC free article] [PubMed] [Google Scholar]
- 73.Krizhanovsky V, Ben-Arie N. A novel role for the choroid plexus in BMP-mediated inhibition of differentiation of cerebellar neural progenitors. Mech Dev. 2006;123:67–75. doi: 10.1016/j.mod.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 74.Sieber C, Kopf J, Hiepen C, et al. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Gaillard P, Jeanclaude-Etter I, Ardisonne V, et al. Design and Synthesis of the First Generation of Novel Potent, Selective, and in Vivo Active (Benzothiazol–2-yl)acetonitrile Inhibitors of the c-Jun N-Terminal Kinase. J Med Chem. 2005;48:4596–4607. doi: 10.1021/jm0310986. [DOI] [PubMed] [Google Scholar]
- 76.Diez H, Fischer A, Winkler A, et al. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res. 2007;313:1–9. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Pandur P, Läsche M, Eisenberg LM, et al. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 78.Nolan K, Kattamuri C, Luedeke DM, et al. Structure of protein related to dan and cerberus: insights into the mechanism of bone morphogenetic protein antagonism. Structure. 2013;21:1417–1429. doi: 10.1016/j.str.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Evans SM, Yelon D, Conlon FL, et al. Myocardial lineage development. Circ Res. 2010;107:1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gaborit N, Le Bouter S, Szuts V, et al. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–693. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ellinghaus P, Scheubel RJ, Dobrev D, et al. Comparing the global mRNA expression profile of human atrial and ventricular myocardium with high-density oligonucleotide arrays. Thorac Cardiovasc Surg. 2005;129:1383–1390. doi: 10.1016/j.jtcvs.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 82.Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: the Rubik’s cube of cell therapy for heart disease. Dis Model Mech. 2009;2:344–358. doi: 10.1242/dmm.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Expression of BMP signaling components during ES cell differentiation. CGR8 ES cells were allowed to differentiate and RNA samples were collected at successive differentiation days. (A) Gene expression analysis of BMP receptors and ligands by quantitative PCR. Gene name abbreviations as in the manuscript text and Bmpr: BMP receptor. (B) Expression profile heatmaps of genes encoding BMP signaling receptors, ligands and antagonists at consecutive days of CGR8 ES cell differentiation obtained using the analytical tools of the FunGenES mouse ES cell database. The color scale represents fold changes from −3 (green) to +3 (red color).
Figure S2. Grem2 promotes differentiation of cardiomyocytes. CGR8 ES cells were allowed to form EBs with or without addition of Grem2 between differentiation days 4–10. Flow cytometry analysis using the cardiac specific type 2 Troponin T (Tnnt2) antibody shows 21-fold increase in number of Tnnt2+ cardiomyocytes at day 10 of differentiation in Grem2-treated cultures compared to control.
Figure S3. Grem2 stimulates expression of cardiac-specific genes during ES cell differentiation. CGR8 ES cells were allowed to differentiate and Grem2 was added between differentiation days 4–10. RNA samples were isolated at day 10 and analyzed by quantitative RCR. Grem2 treatment leads to induction of cardiac and atrial genes compared to untreated, control samples. Gene name abbreviations: Isl1: Insulin gene enhancer protein 1; Kcne2: Potassium voltage-gated channel, Isk-related family, member 2; Kcne4: Potassium voltage-gated channel, Isk-related family, member 4; Kcnj3: Potassium inwardly-rectifying channel, subfamily J, member 3; Myh7: Myosin heavy chain 7 or β Myosin heavy chain; Mybpc3: Myosin binding protein C, cardiac; Nppa: Natriuretic peptide A; Sln: Sarcolipin. ** P< 0.01, *** P<0.001, compared to the corresponding controls.
Figure S4. BMP antagonists inhibit signaling of BMP2 and BMP4. ES cells were stably transfected with the BRE2-Luciferase plasmid that carries the reporter luciferase gene under the control of the BMP-Responsive Element in the Id2 gene promoter. The reporter ES cell line was treated for 6 hours with BMP2 or BMP4 and increasing concentrations of specific antagonists as indicated. Luciferase activity analysis of protein lysates shows that all tested BMP antagonists effectively inhibit Smad-mediated BMP signaling.
Figure S5. Grem2 inhibits a wide range of BMP ligands. ES cells, stably transfected with the BRE2-Luciferase plasmid were exposed for 6 hrs to BMP ligands in the absence or presence of increasing amounts of Grem2 as indicated. Cells were lysed and Luciferase activity was measured. Grem2 effectively inhibits BMP signaling by all tested BMP ligands.