Abstract
Adult hippocampal progenitor cells (AHPCs) are generally maintained as a dispersed monolayer population of multipotent neural progenitors. To better understand cell-cell interactions among neural progenitors and their influences on cellular characteristics, we generated free-floating cellular aggregates, or neurospheres, from the adherent monolayer population of AHPCs. Results from in vitro analyses demonstrated that both populations of AHPCs were highly proliferative under maintenance conditions, but AHPCs formed in neurospheres favored differentiation along a glial lineage and displayed greater migrational activity, than the traditionally cultured AHPCs. To study the plasticity of AHPCs from both populations in vivo, we transplanted GFP-expressing AHPCs via intraocular injection into the developing rat eyes. Both AHPC populations were capable of surviving and integrating into the developing host central nervous system, but considerably more GFP-positive cells were observed in the retinas transplanted with neurosphere AHPCs, compared to adherent AHPCs. These results suggest that the culture configuration during maintenance for neural progenitor cells (NPCs) influences cell fate and motility in vitro as well as in vivo. Our findings have implication for understanding different cellular characteristics of NPCs according to distinct intercellular architectures and for developing cell-based therapeutic strategies using lineage-committed NPCs.
Keywords: Adult hippocampal neural progenitor cells, Neurosphere, Differentiation, Migration, Intravitreal transplantation
Introduction
Adult neural progenitor cells (NPCs) are capable of self-renewal and differentiation into neuronal and glial cells[1-4]. NPCs are particularly interesting due to their potential to provide cell-based therapies for CNS repair. To better understand the molecular and cellular properties of NPCs, culture systems have been established as simplified models for in vitro study[1, 3, 5-15]. In culture, multipotent NPCs can proliferate in the presence of mitogenic growth factors [1, 8, 13] and differentiate into neuronal and glial cell types following the removal of growth factors and/or addition of differentiation-inducing agents[1, 14].
During in vitro cell expansion, NPCs can be formed in a monolayer on purified extracellular matrix molecules or as free-floating aggregates called neurospheres[5, 7, 14-16]. For any given neurosphere, the NPCs are highly compact in a three-dimensional context, different from the monolayer of discrete, adherent cells. Studies have examined the plasticity and ability of NPCs to survive, proliferate, differentiate, and migrate in vivo, as either neurospheres or discrete, adherent cells in lesioned or diseased animal models[14]. However, little is known about different cellular characteristics between the NPCs cultured as single, discrete cells and the NPCs formed in neurospheres.
In this study, we investigated and directly compared the phenotypic differentiation and migrational activities of adult rat hippocampal progenitor cells (AHPCs; a gift from Dr. Fred H. Gage, Salk Institute, La Jolla, CA) maintained as an adherent population (AD-AHPCs) versus those maintained as neurospheres (NS-AHPCs). Our data show that both AHPCs were highly proliferative, but AHPCs formed in neurospheres preferentially differentiated toward a glial identity and migrated faster than adherent cells. Furthermore, cell plasticity as well as survival and integrating capabilities of both the AD-AHPC and the NS-AHPC populations in the developing central nervous system (CNS) were investigated in vivo.
Materials and Methods
Maintenance of adherent AHPCs (AD-AHPC)
AHPCs originally isolated from the brains of adult Fischer 344 rats and infected with retrovirus to express enhanced green fluorescent protein, were maintained as a monolayer of discrete, adherent cells as described previously[1, 7]. Briefly, AHPCs were maintained in 75 cm2 tissue culture flasks (T-75; Fisher Scientific, Pittsburgh, PA) coated with poly-L-ornithine (10 μg/mL; Sigma-Aldrich, St. Louis, MO) and purified mouse laminin I (5 μg/ml; R&D Systems, Inc., Minneapolis, MN) in Earle's balanced salt solution (EBSS; Invitrogen, Carlsbad, CA). The maintenance medium for AHPCs included Dulbecco's modified Eagle's medium/Ham's F-12 (DMEM/F-12, 1:1; Omega Scientific, Tarzana, CA), 2.5 mM L-glutamine, 1 × N2 supplement (Gibco BRL, Gaithersburg, MD), and 20 ng/mL basic fibroblast growth factor (human recombinant bFGF; Promega Corporation, Madison, WI). For propagation, the AHPCs were detached from a T-75 flask using 0.05% Trypsin-EDTA (Gibco BRL) and harvested by centrifugation at 800 rpm for 5 minutes. Resuspended cells were plated in two T-75 flasks coated with poly-L -ornithine and laminin I. Cells were maintained at 37°C in a 5% CO2 / 95% humidified air atmosphere. Culture media were replenished every other day.
For in vitro analysis, resuspended cells were plated on 12-mm glass coverslips coated with poly-L-ornithine (50 μg/ml) and laminin I (10 μg/ml) at initial densities of 100 cells/mm2. Cells were cultured in maintenance medium (MM) or differentiation medium (DM, which is maintenance medium without bFGF). Cultures used for the phenotypic characterization were maintained for 3 days or 6 days until being terminated for immunocytochemical analysis. Cells used for the migration studies were cultured in MM and DM for up to 5 days.
Generation of AHPC neurospheres (NS-AHPC)
AHPC neurospheres (designated as NS-AHPCs) were generated from the original adherent AHPCs (Figure 1, A). The adherent AHPCs (designated as AD-AHPCs) were cultured in uncoated 35-mm culture dishes under proliferation conditions (in MM). This resulted in AHPCs spontaneously aggregating and generating neurospheres that continued to proliferate. After seven days with regular feeding, the culture medium (i.e. conditioned medium which includes free-floating AHPC neurospheres) was collected into a 15-ml conical tube. Small neurospheres of AHPCs were collected by centrifugation at 500 rpm for 2 min, gently resuspended in 5 ml of fresh MM and cultured in an uncoated T-25 flask. The cultures were maintained in MM with regular feeding until being used for experiments.
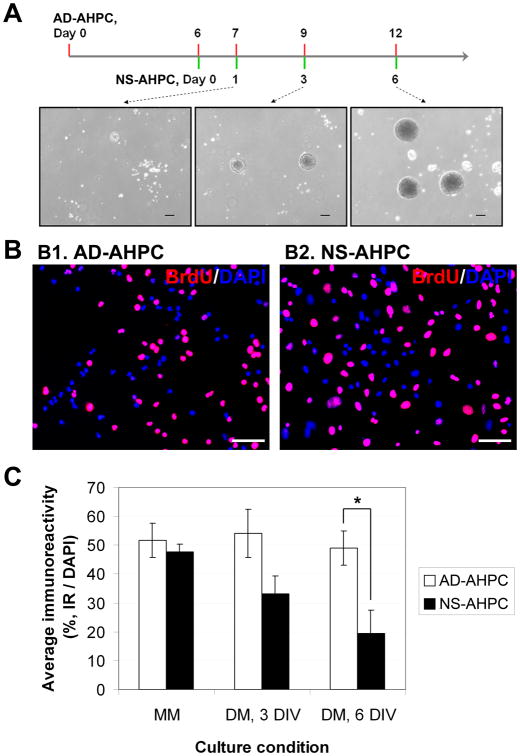
Figure 1.
Comparison of proliferating capacity of AHPCs, adherent and neurosphere. (A) Schematic time-line for generation of AHPC neurospheres. (B) Representative images of BrdU-incorporating adherent AHPCs (B1) and AHPCs in neurospheres (B2). (C) Quantitative data representing average percentage of BrdU-incorporating cells under proliferating or differentiating culture condition. N (number of independent experiments) = 3∼5. Scale bars in A, 200 μm; in B, 50 μm.
For in vitro analyses and comparison with the adherent population, neurosphere cultures were always established together with adherent cell cultures side by side. Neurospheres used for phenotypic characterization were dissociated and plated on poly-L-ornithine/laminin-coated 12mm coverslips. Cultures were kept in MM or DM for 3 or 6 days with regular feeding. For migration studies, three to four neurospheres were placed on a coated 12-mm coverslip or in an O-ring chamber with a PTFE (Teflon®) O-ring (inner diameters of 9/16 in, outer diameters of 3/4 in and widths of 3/32 in; Small Parts, Inc., Miami Lakes, FL) attached to a glass coverslip (22 × 22 mm square; Corning, Corning, NY) by SylGard® (Dow Corning Corp., Midland, MI). Neurospheres used in the migration studies were cultured up to 5 days.
Immunocytochemistry and antibodies
After cultures were terminated, AHPCs were fixed in 4% paraformaldehyde in 0.1 M phosphate (PO4) buffer, and rinsed in phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KCl, 8.1 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.4). Cultured cells were incubated in blocking solution [2.5% normal donkey serum (Jackson ImmunoResearch, West Grove, PA), 0.4% bovine serum albumin (Sigma) and 0.2% Triton X-100 (Fisher Scientific, Houston, TX) dissolved in PO4 buffer] for 1.5 hours. Cells were then incubated with primary antibodies against phenotypic markers (see below) overnight at 4°C. After rinsing in PBS, cells were incubated with secondary antibody (Donkey anti-Mouse IgG, Cy3-conjugated (Jackson ImmunoResearch)) at a dilution of 1:500. Cell nuclei were stained with 1 μM of 4′, 6-diamidino-2-phenylindole, dilactate (DAPI, Invitrogen Life Technologies, Carlsbad, CA). Preparations were then mounted onto microscope slides using an anti-fade mounting medium (Fluoro-Gel; Fisher Scientific).
To analyze proliferation capacity, the AHPCs were treated with 5 μM of 5′-bromo-2′-deoxyuridine (BrdU, Sigma-Aldrich) for 12 hours prior to fixation. To visualize BrdU-incorporation, an antibody against BrdU (anti-BrdU, rat monoclonal IgG, Abcam Inc., Cambridge, MA) was used at a 1:200 dilution. To label early neurons, anti-βIII tubulin (TuJ1, mouse monoclonal IgG; R&D systems, Inc., Minneapolis, MN) was used at a dilution of 1:200. To label oligodendrocytes and astrocytes, anti-receptor interacting protein (RIP, mouse monoclonal IgG; Developmental Studies Hybridoma Bank, Iowa City, IA) diluted at 1:1,000 and anti-glial fibrillary acidic protein (GFAP, mouse monoclonal IgG; Lab Vision Corp., Fremont, CA) diluted at 1:1,000 were used, respectively.
Quantitative analysis of immunocytochemistry
Following immunocytochemical procedures, the preparations were imaged using a fluorescence microscope (Nikon Microphot FXA, Tokyo, Japan) equipped with a Retiga 2000R digital camera controlled by QCapture software (QImaging, Surrey, British Columbia, Canada). A total of 8 to 10 microscope fields per coverslip were taken using a 20×objective. To calculate the percentage of cells immunoreactive (IR) for anti-TuJ1, RIP and GFAP, the number of phenotypic marker-IR cells was divided by the total number of cells (DAPI-stained nuclei).
Time-lapse imaging
To characterize the migration properties of the AHPCs, both adherent and neurosphere populations were plated on 50-mm glass coverslips coated with poly-l-ornithine and laminin I. Cells were allowed to adhere to the coverslip for an hour. The coverslips with the cells were then placed into an FCS2 Focht Live-Cell Chamber System for time-lapse imaging (BIOPTECHS Inc., Butler, PA), which was mounted onto the heated (37°C) stage of a Leica DMIRE2 inverted microscope (Leica Microsystems, Bannockburn, IL) powered by the modular imaging program OpenLab (Improvision, Bentham, MA). Micrographs at 20× magnification (frame size: 663 μm × 530 μm) were captured every 5 minutes for a period of 24 hours. Culture medium was maintained at 37°C and 5% CO2 and perfused at a rate of 5.5 mL/hr. using a Instech P720 Peristaltic Pump (Instech, Plymouth Meeting, PA).
Analysis of migration
From each time-lapse imaging session, the positions of 20 to 24 randomly selected cells in each frame were recorded. The total distance, displacement, and average speed of each cell were determined over three time intervals: the first 12 hours, the next 12 hours, and the entire 24 hours. The total distance (in μm) for each interval was found by taking the sum of the distance traveled between each consecutive frame in that interval. Displacement was the straight-line distance between the initial and final positions in the interval. The average speed (in μm/hour) was the total distance divided by the duration of that interval. The mean total distance, displacement, and speed for each time-lapse was calculated.
Displacement was chosen as a general measurement of the “directed” movement of a cell - that is, the portion of the migration that actually contributed to the final position of a single cell. The average speed or distance, on the other hand, represented the total movement of a single cell, regardless of whether it had significant movement in any one direction. In this study, many cells with high total distance had small displacements, so it was crucial to differentiate between displacement and total distance.
Animals
All animal studies were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, and procedures were approved by the Iowa State University Institutional Animal Care and Use Committee. Un-timed pregnant Fischer 344 rats were purchased from Harlan Laboratories (Madison, WI) and postnatal (PN) day 1 Fischer 344 rat pups (a litter of 10 pups) were used for transplantation experiments. Rats were kept under a 12-hour light/12-hour dark regimen and provided food and water ad libitum.
Intraocular transplantation of AHPC
Cultured cells (both AD-AHPC and NS-AHPC populations) were harvested and resuspended in EBSS. Prior to transplantation rat pups (1 PN) were initially anesthetized via hypothermia and then placed on a chilled platform under a dissecting microscope. Five pups were assigned at random to receive injections of AD-AHPCs (50,000 cells/μl), and the other five pups were designated to receive NS-AHPCs (one neurosphere/μl; estimated at 30,000∼60,000 cells included in a neurosphere with a diameter of approximately 600∼800 μm). Following injection, pups were allowed to recover on a heating pad, and subsequently returned to their dam.
Tissue processing
Following transplantation, animals were euthanized at 7, 14 and 28 days post-transplantation (DPT). Two pups, one per cell population (either AD- or NS-AHPCs), were euthanized at 7 DPT. Four pups, two per cell population, were euthanized at 14 DPT, and the remaining four pups were euthanized at 28 DPT (two per cell population). Both eyes were harvested and fixed in 4% paraformaldehyde in 0.1 M PO4 buffer for three days. Fixed tissue samples were cryoprotected in a graded sucrose series (10%, 20%, and 30% sucrose dissolved in 0.1 M PO4 buffer). The tissue samples were then embedded in optimal cutting temperature (OCT) compound (Tissue-Tek; VWR International, West Chester, PA), frozen and stored at -80°C until ready for sectioning. Frozen tissues were sectioned coronally using a cryostat (HM 550 VP, Thermo Fisher Scientific) at 16 μm for eyes. Sections were mounted on warmed SuperFrost microscope slides (Fisher Scientific), dried on slide warmers, and stored at -20°C until used for immunohistochemistry.
Sectioned tissues were rinsed in PBS, permeabilized in 0.1% Triton X-100 in PBS for 10 min, and then incubated in a blocking solution (10 % normal donkey serum in PBS) for 1 hour at room temperature. Sections were then incubated with primary antibodies against neural and retinal specific phenotypic markers (Table 1) for 12-36 hours at 4°C. After rinsing in PBS, sections were incubated with secondary antibody, Cy3-conjugated (Jackson ImmunoResearch) at a dilution of 1:500 (for anti-mouse IgG) or 1:250 (for anti-rabbit IgG or anti-rat IgG). Cell nuclei were stained with 1 μM of DAPI. Preparations were then mounted using 24 × 60 mm coverslips (Corning) using Fluoro-Gel (Fisher Scientific).
Table 1. Primary antibody information.
| Antibody | Cell type specific for | Source and type of antibody | Vendor |
|---|---|---|---|
| BrdU (bromodeoxyuridine) | Proliferating cells | Rat monoclonal | abcam |
| Ki-67 | Rabbit polyclonal | DAKO | |
| Nestin | Neural progenitors | Mouse monoclonal | DSHB |
| TuJ1 (βIII-tubulin) | Young neurons | Mouse monoclonal | R&D Systems |
| MAP2ab (microtubule associated protein 2ab) | Mature neurons | Mouse monoclonal | Sigma |
| RIP (receptor interacting protein) | Oligodendrocytes | Mouse monoclonal | DSHB |
| GFAP (glial fibrillary acidic protein) | Astrocytes | Mouse monoclonal | Lab Vision |
| Brn3a (brain-specific hoeobox/POU domain protein 3a) | Ganglion cells | Mouse monoclonal | Millipore |
| Calretinin | Neurons in INL | Rabbit polyclonal | Millipore |
| PKCα (protein kinase C alpha) | Rod bipolar cells | Mouse monoclonal | DSHB |
| Pax6 (paired box gene 6) | Amacrine cells | Mouse monoclonal | DSHB |
| Recoverin | Photoreceptors | Rabbit polyclonal | Millipore |
| Rho1D4 (rhodopsin 1D4) | Photoreceptors | Mouse monoclonal | abcam |
| CRALBP (cellular retinaldehyde-binding protein) | Retinal glial cells | Rabbit polyclonal | Thermo Scientific |
| GS (Glutamine synthetase) | Retinal glial cells | Mouse monoclonal | Millipore |
The preparations were imaged using a fluorescence microscope (Nikon Microphot FXA, Nikon Inc., Garden City, NY) equipped with a Retiga 2000R digital camera controlled by QCapture software (QImaging, Surrey, BC, Canada).
Results
Proliferation capacity of AHPCs in neurospheres compared to adherent AHPCs
To compare the proliferating capacity of AHPCs formed in free-floating neurospheres (NS-AHPC) to the adherent AHPCs (AD-AHPC), AHPCs grown as neurospheres were dissociated by gentle trituration before being plated onto poly-L-ornithine/laminin-coated coverslips. Both populations were cultured under either proliferating (for 3 days) or differentiating conditions (for 3 or 6 days). To detect the cells engaged in DNA synthesis, cultures were incubated in BrdU (5 μM) for 12 hours prior to fixation. Proliferating capacity was defined as the percent of BrdU-immunoreactive cells (Fig. 1, B and C).
Proliferation of AD-AHPCs did not seem to be affected by culturing in differentiation conditions. In contrast, the proliferating capacity of NS-AHPCs significantly decreased under differentiation condition at 6 days in vitro (DIV) compared to that under the maintenance media condition. This result demonstrates that AHPCs formed in neurospheres were more susceptible to the differentiation-inducing culture condition than the adherent population.
Phenotypic differentiation of AHPCs in neurospheres compared to adherent AHPCs
To investigate possible differences in the differentiation capacities of AD-AHPCs and NS-AHPCs, both populations were cultured in parallel under proliferating (MM) or differentiating (DM) conditions for up to 6 days. AHPC differentiation was defined as the percentage of cells immunoreactive for specific phenotypic markers: TuJ1 for neurons, RIP for oligodendrocytes and GFAP for astrocytes (representative images shown in Fig. 2, A: A1 for AD-AHPC; A2 for NS-AHPC).
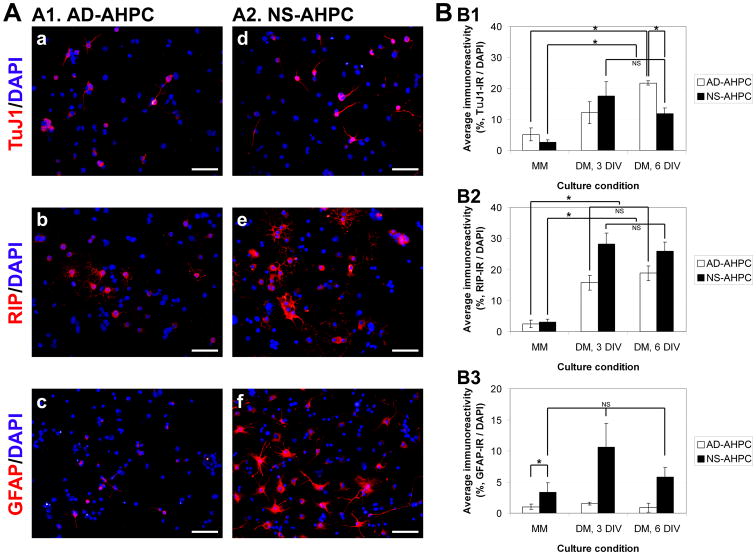
Figure 2.
Comparison of differentiating capacity of AHPCs, adherent and neurosphere. (A) Representative images of TuJ1-, RIP- and GFAP-immunoreactive adherent AHPCs (A1, a-c) and AHPCs in neurospheres (A2, d-f). (B) Quantitative data representing average percentages of phenotypic marker-immunoreactive cells under proliferating or differentiating culture conditions. TuJ1, a marker for neurons (B1); RIP, a marker for oligodendrocytes (B2); GFAP, a marker for astrocytes (B3). N = 3∼5 independent experiments. Scale bars in A, 50 μm.
When the AD-AHPCs and NS-AHPCs were cultured under proliferation conditions (in MM) very little differentiation was observed, as the percentages of TuJ1-, RIP- or GFAP-immunoreactive cells were relatively low and no significant differences were noted between the two different AHPC populations (varying from 1∼5%; Fig. 2, B1∼B3). For neuronal differentiation, under differentiation conditions (in DM) both populations of AHPCs (adherent and neurospheres) displayed significant increases in the percentages of TuJ1-immunoreactive cells compared to the MM culture conditions (Fig. 2, B1). For oligodendrocytic differentiation, when cultured in DM, both populations of AHPCs showed significantly higher percentages of RIP-immunoreactive cells compared to those in MM. In addition, NS-AHPC population in DM had a greater fraction of RIP-immunoreactive cells compared to AD-AHPC population (Fig. 2, B2). For astrocytic differentiation, NS-AHPCs had a greater fraction of GFAP-immunoreactive cells compared to AD-AHPCs cultured in MM as well as in DM (Fig. 2, B3). These results suggest that AHPCs maintained as neurospheres were prone to differentiate toward glial lineages (RIP- and GFAP-immunoreactive) when compared to AHPCs maintained as an adherent, discrete monolayer, though these differences were not statistically significant.
Migration properties of adherent AHPCs versus neurosphere AHPCs
To examine migration rates of AHPCs, we performed time-lapse imaging and measured migration speed (calculated from total distance of migration divided by imaging period) and displacement from point of origin. For time-lapse imaging, cells (AD-AHPCs or NS-AHPCs) were plated onto laminin-coated substrates in DM. Preparations were equilibrated in the imaging chamber for 1 hour, and then time-lapse imaged every 5 minutes over a 24 hour period (Fig. 3, A and B).
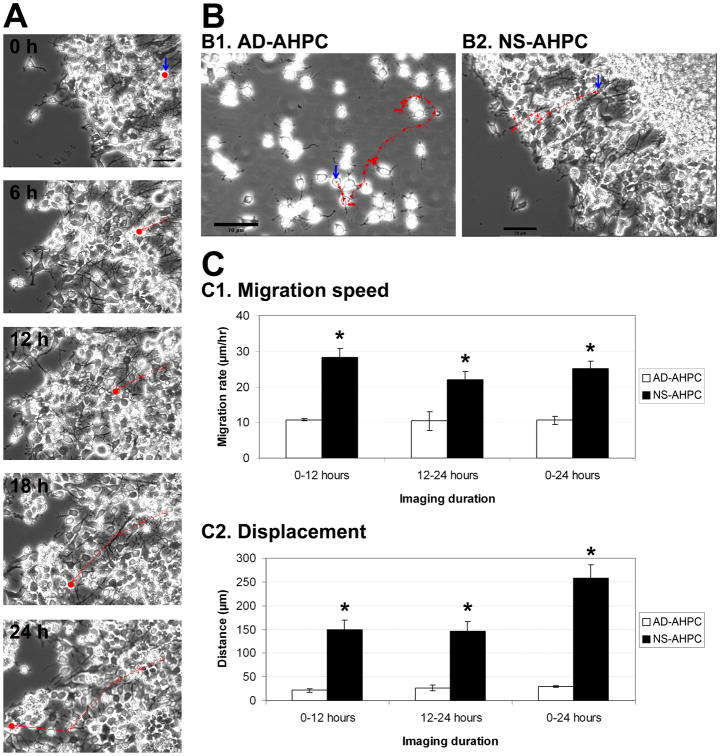
Figure 3.
Comparison of migrating properties of AHPCs, adherent and neurosphere. (A) Sample image frames of a single cell in the same microscopic field from a time-lapse series at 0, 6, 12, 18 and 24 hours. The solid red dot in each image represents the position of the cell at the time indicated. The solid red line shows migration that occurred during the 6-hour interval. The dotted red line indicates migration accumulated from previous intervals. (B) Representative images of an adherent AHPC (B1) and an AHPC from a neurosphere (B2) traced to examine the migration rate. The red arrows represent the migrating path of a single AHPC for a 24-hour period; each individual arrow represents migrational movement during a 2-hour time period. (C) Quantitative data illustrating average migration speed (C1) and average displacement (C2) for AHPCs from neurospheres compared to adherent AHPCs. Three independent experiments were performed; 20∼25 cells per cell type were analyzed from each experimental session. Blue arrows in A and B indicate the initial position of a single cell (at 0 h for time-lapse imaging) traced for total 24 hours. Scale bars in A, 50 μm; in B, 70 μm. Asterisks indicate significant differences in comparison of NS-AHPC to AD-AHPC.
Figure 3 illustrates that NS-AHPCs migrating out of the neurosphere had a faster rate of migration (an average speed of 25 μm/hr); migrated farther (an average total migration distance of 603 μm); and had a greater displacement from their point of origin (an average displacement of 258 μm) compared to the AD-AHPCs (average speed: 12 μm/hr; average distance: 143 μm; average displacement: 27 μm) (Fig. 3, C). Over 24 hours, AD-AHPCs showed a more meandering movement compared to the NS-AHPCs, as supported by a lower displacement-to-distance ratio as well (data not shown).
Transplantation of AHPCs (NS-AHPCs and AD-AHPCs) into the developing retina: Survival, differentiation and morphological integration
The retina is anatomically and developmentally an extension of the CNS, and its peripheral location provides easy accessibility for transplants. For these reasons, a number of studies have used the developing retina as an in vivo CNS location to test the plasticity of brain-derived neural progenitor/stem cells[17-30]. To investigate and compare the ability of the different forms of the AHPCs (neurosphere versus adherent) to survive, differentiate and integrate into the mammalian CNS, we transplanted the cells into the developing retina. Approximately equal quantities of the GFP-expressing AHPCs were transplanted via intraocular injection into the eyes of postnatal day 1 Fischer 344 rats. Cell survival, differentiation and morphological integration were examined at 7, 14 and 28 days post-transplantation. The transplanted cells were identified based upon GFP-expression, and differentiation was assessed by examining their morphology and immunolabeling with a panel of cell-type specific antibody markers (see Table 1).
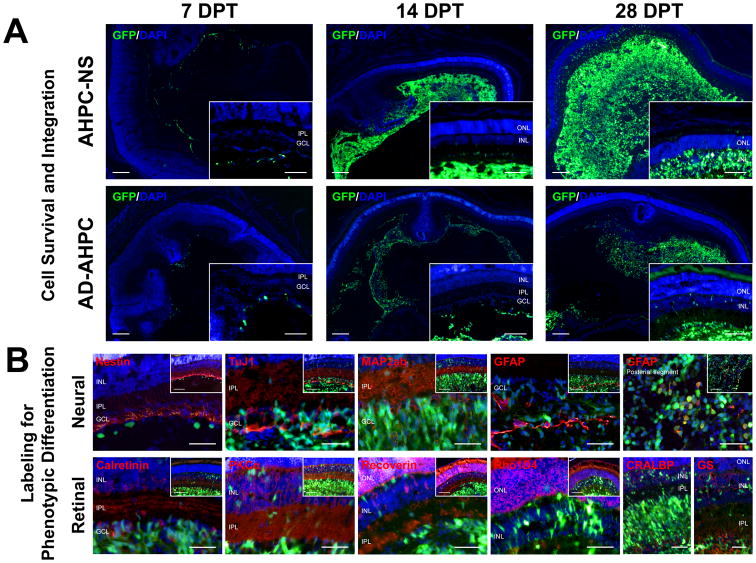
From 7 to 28 DPT, AHPCs were observed within the vitreous body and/or posterior segment, indicating survival for both NS- and AD-AHPC populations. There were generally fewer AHPCs found at 7 DPT, gradually increasing at 14 and 28 DPT for both populations. The general number of NS-AHPCs observed at 7 DPT was comparable with that of AD-AHPCs at 7 DPT. At 14 and 28 DPT, considerably more GFP-positive cells were present in the eyes receiving NS-AHPCs compared to those receiving AD-AHPCs. Furthermore, at 28 DPT (some cases of 14 DPT) for both populations, many AHPCs were found in the inner nuclear layer (INL) and a few in the outer nuclear layer (ONL). Morphologically, AHPCs found in the retinal layers seemed to be well differentiated by displaying nicely arborized processes in the nuclear layers as well as inner plexiform layer (IPL). In addition, the more AHPCs present within the vitreous body, the more likely AHPCs were found in the retinal layers. These observations, i.e. the gradual increase in abundance of AHPCs present in the transplanted eyes, particularly by 28 DPT, suggested that both AHPC populations were presumably capable of proliferating after transplantation into the developing eyes. Proliferation marker, Ki-67, was used to immunolabel mitotic cells at the time of sacrifice; however, due to labeling issues immunoreactivity was either not present or individual labeled cells were not discernable, as such the extent of proliferation was not determined.
Immunolabeling of transplanted AHPCs for neural- and retinal-specific markers
To determine if transplanted cells expressed general neural markers, tissue sections were immunolabeled with various antibodies (refer to Table 1). Cells constructing the host retinal structure were labeled for neural- and retinal-specific markers. However, both NS-AHPCs and AD-AHPCs that migrated into the retinal layers were not co-labeled with any neural- or retinal-specific markers, except co-labeling with GFAP within the mass of AHPCs found in the vitreous body and posterior segment (Fig. 4, B). This result suggests that AHPCs intravitreously transplanted into the developing eyes may be able to morphologically integrate into the host neural network even as early as 14 DPT. However, it is unlikely that these AHPCs had phenotypically differentiated even up to 28 DPT which was the longest time point that was examined in this study.
Figure 4.
Representative images of retinal sections following transplantation. (A) Cell survival and integration are shown at 7, 14, and 28 days post-transplant (DPT) for both NS-AHPC and AD-AHPC populations. General presence of AHPCs was increased from 7 to 28 DPT. Scale bars, 100 μm. (B) Images for phenotypic labeling were from NS-AHPC populations at 14 and 28 DPT. Scale bars, 50 μm; 100 μm in insets.
Discussion
Adherent AHPCs growing as a monolayer (two-dimensional system, 2-D) are capable of differentiating into neuronal or glial lineages under differentiation culture conditions[1, 3, 5-15, 31, 32] . To investigate the possibility that more complex cellular interactions might impact progenitor cell differentiation we took advantage of the formation of neurospheres, which are highly compacted and grown in a three-dimensional (3-D) environment. AHPC neurospheres were generated by culturing the adherent AHPCs on a less adhesive substrate thus favoring cell-to-cell interactions rather than cell-to-substrate adhesion. To understand the cellular characteristics of AHPCs formed in neurospheres, we examined in vitro proliferating capacity, differentiating capability and migrating properties of AHPCs in neurospheres (NS-AHPCs), and compared those characteristics with the adherent AHPCs (AD-AHPCs). Our in vitro results demonstrated that: (1) the majority of NS-AHPCs were proliferative, but more susceptible to bFGF-exclusion culturing conditions compared to AD-AHPCs, (2) NS-AHPCs retained multipotency, and a greater fraction of cells were differentiated along glial lineages compared to AD-AHPCs, and (3) migration of NS-AHPCs was more directed and faster compared to AD-AHPCs. To study plasticity of AHPCs in vivo, we transplanted NS-AHPC and AD-AHPC populations into the developing eyes and examined cell survival, differentiation and morphological integration of the transplanted cells. Our in vivo results demonstrated that (1) retinas transplanted with NS-AHPCs generally had more cells present and a greater level of morphological differentiation and integration compared to AD-AHPCs, and (2) AHPCs integrated into the retinas emanated arborized processes into plexiform layers; however no evidence for differentiation into retinal specific cell types was observed.
Proliferation capacity and its potential effects on differentiation
BrdU-incorporation is indicative of proliferation capacity[1, 5, 6, 8, 31]. Our results showed that both NS-AHPCs and AD-AHPCs were highly proliferative in the presence of bFGF, a mitogenic growth factor which is essential for the viability and self-renewing of AHPCs[1, 8, 13, 33]. When AHPCs are cultured in the absence of bFGF, it is expected that the number of BrdU-incorporating cells is decreased. In our culture system, however, AD-AHPCs appeared to retain proliferating capacity as they were cultured in the absence of bFGF (which was defined as differentiation condition in this study) up to 6 days. In contrast, NS-AHPCs rapidly lose their proliferative features when cultured under differentiation conditions. This greater decline in proliferation experienced by NS-AHPCs compared to AD-AHPCs suggests a potential influence of the neurosphere structure (extremely proximal distance among the cells in a neurosphere) on proliferating capacity.
It has been reported that cells in the core of neurosphere tend to be differentiating in culture[34, 35]. It is likely that the physical proximity among the cells in the neurosphere may lead to the interaction among the NPCs undergoing differentiation. It is speculated that the interacting cells in the core of the neurosphere may be exposed to paracrine or juxtacrine factors that promote differentiation, rather than to a plethora of exogenous growth factors, including bFGF, provided in the maintenance medium. Indeed, when treating neurospheres with BrdU in the maintenance medium, sectioning with 20 μm-thickness, and immunostaining for BrdU incorporation, we observed that, in the core region of AHPC neurospheres, fewer cells were BrdU-immunostained than the cells in the peripheral regions (Suppl. Fig. 1). However, when the neurospheres were dissociated into single cells, cultured on purified extracellular matrix and maintain in maintenance condition, proliferating capability of NS-AHPCs was similar to that of AD-AHPC population. These results suggest that AHPCs in the core area of neurosphere may retain proliferating capability, even though they had limitations in being exposed to the extrinsic growth factors and physical stress was given from the neighboring cells within the neurosphere.
Furthermore, under differentiation condition (culturing without bFGF for up to 6 days), many AD-AHPCs continued to maintain a proliferative capacity. This suggests that removal of bFGF, by itself was insufficient to induce AHPCs to exit the cell cycle. On the other hand, NS-AHPCs appeared to cease dividing at earlier time points than AD-AHPCs when maintained under differentiation conditions. This suggests that physical contact among the cells in the neurosphere together with removal of the growth factor can presumably facilitate AHPCs to exit mitotic stages. Taken together, it is possible that under proliferation (same as maintenance) condition, AHPCs in the core region of neurosphere may still be proliferative but not yet committed to differentiate into specific cell lineages.
Neuronal versus glial differentiation of NS-AHPCs compared to AD-AHPCs
Phenotypic differentiation was examined by immunocytochemical analysis and quantified for neuronal, oligodendrocytic and astrocytic differentiation for both NS- and AD-AHPCs. Both AHPC populations were maintained in maintenance medium. For in vitro analysis they were cultured under proliferation or differentiation conditions. Our results demonstrated that NS-AHPCs were biased to differentiate into glial phenotypes, rather than a neuronal phenotype, under differentiation conditions.
Under proliferation conditions, both AHPC populations showed very small portions of cells (less than 5%) were neuronal or glial marker-immunoreactive. Interestingly, under proliferation conditions, more AD-AHPCs were TuJ1-positive and more NS-AHPCs were GFAP-positive, compared to each other. Since AHPCs are hippocampus-derived progenitor cells, it is not reasonable to conclude that those GFAP-expressing cells are undifferentiated neural stem cells which are typically found in the subventricular zone in vivo (called ‘Type B’ cells)[36]. Thus, more GFAP-immunoreactive cells observed in NS-AHPC population compared to the AD-AHPC population may be indicative of greater astrocytic differentiation of AHPCs occurring in the structure of the neurospheres.
Furthermore, under differentiation conditions, a greater fraction of NS-AHPCs differentiated along glial lineages (significantly higher percentages of RIP- and GFAP-immunoreactive cells), rather than neuronal lineage (significantly lower percentage in TuJ1-immunoreactive cells when cultured for 6 days). These results suggest that certain factors were present which increased glial differentiation during the process of neurosphere formation or maintenance of the spheres in culture. A possible condition is a limitation of the cells in highly compacted neurospheres to interact with growth factors which may be essential for AHPC proliferation and extrinsically provided in the maintenance medium[1]. In addition, extracellular matrix molecules bound to the receptors on NPCs can influence differentiative properties of NPCs[37, 38]. Lastly, close physical proximity among NS-AHPCs may induce glial differentiation. Juxtacrine interaction of Notch receptor with its specific ligand is known to control NPC fates in vitro as well as in vivo[38, 39]. Notch activation was thought to maintain neural stem cells in an undifferentiated state by inhibiting their differentiation[40]. However recent studies show that transient activation of Notch signaling actively directs stem cell differentiation into glial lineages[40, 41]. Tanigaki et al. reported that Notch signaling activation induced glial (especially astrocytic) differentiation from hippocampus-derived NPCs[13]. Thus in the formation of neurosphere, AHPCs are physically very close to each other, which may essentially cause cell-cell contact. Because of physical proximity of the cells in neurospheres, Notch receptor may possibly be activated by juxtacrine interaction with its ligand. Activated Notch signaling perhaps promotes glial differentiation of AHPCs in the neurospheres. Further studies will provide more detailed information on the signaling pathways by which AHPCs are differentiated into specific cell lineages.
It is also known that cell differentiation can be influenced by various factors related to cell geometry, as recently reviewed by Yao et al.[42]. Based on the unique micropatterning technique [43], Fudan group successfully localized single and multiple cells on adhesive microislands [44] and examined cell differentiation on microislands as well as on free plates [45]. The systematic studies by the Jiandong group illustrated unambiguously that cell shape [46, 47], cell size[48], cell density[48] and cell-cell contact[48, 49] regulate cell differentiations. These regulations on patterned surfaces can interpret many experiments of cells beyond on patterned surfaces. In the present study, the cells in neurospheres might exhibit different cell shape, size, density and cell-cell contacts from those on monolayers. So, it is not unreasonable that the corresponding cell differentiation and other cell behaviors of AHPCs maintained in distinct structures may be different.
Migration of NS-AHPCs compared to AD-AHPCs
Our time-lapse imaging results showed that NS-AHPCs migrated faster and farther over time than AD-AHPCs both in proliferation and differentiation conditions. Moreover, AHPCs migrating away from the neurospheres exhibited more ‘directional’ movement (indicated by overall displacement from the point of origin; i.e. more of the distance traveled was along the direction of the final displacement) compared to AD-AHPCs. These results suggest that the more directed migration of NS-AHPCs may be cell density-dependent. In other words, when the distance between cells increases, cell migratory speed decreases. It is supported by the observations that (1) AHPCs from neurosphere generally migrated radially outward with having little means to migrate inward where cells were more densely populated, (2) over 24 hours, the distance and speed in the first 12 hours were greater than the distance traveled in the last 12 hours, and (3) the adherent cells, which were grown at a lower density, migrated at a considerably slower rate compared to the cells from neurospheres. These results strongly suggest that forces of repulsion may exist among the AHPCs when they are placed at an extremely close distance on a cell adhesion molecule (purified extracellular matrix molecule; mouse laminin-1 was used in this study).
A possible mechanism of cell repulsion for the AHPC migration is through Eph-related signaling. Eph (receptor)-ephrin (ligand) interaction is a well-known cue that regulates cell-cell contact-mediated attractive and repulsive responses during neural development[50, 51] as well as adult neurogenesis[52, 53]. Eph signaling influences cytoskeletal organization and cell adhesion, thereby enabling to affect cellular migratory behavior or dynamics of cellular protrusions[50, 54]. Especially, Chumley et al. reported that EphB1 receptor is expressed in the hippocampal NPCs and that mice lacking EphB1 receptor or its ligand, ephrin-B3, showed the disruption in hippocampal neurogenesis (fewer progenitors found, newly generated cells mis-positioned, and cell polarity impaired)[55]. They suggested that EphB1-ephrin-B3 complex functions as a positive regulator of migration and proliferation of hippocampal NPCs in vivo[55]. However, how Eph signaling can modulate AHPC migration in cellular and molecular levels remains to be elucidated.
Considering the formation of neurospheres, it is conceivable that there may be non-hydrogenic interactions, such as hydrophobic, electrostatic interactions and van der Waals forces, present between the plasma membranes of AHPCs. In addition, cells within the neurosphere may possibly be affected by mechanical pressure which is generated by their neighboring cells. These biochemical and physical stresses can be transmitted via integrin receptors that AHPCs normally express[56]. Since integrins are correlated with cytoskeletal filaments, it is presumed that the physical pressure provided by neighboring cells within the neurosphere can effect cytoarchitecture as well as nuclear morphology of AHPCs[57]. Another possible stressor is the extracellular matrix deprivation removed for migration studies (laminin was not given for the maintenance of AHPC neurospheres, but it was provided for migration studies). Integrins binding to laminin substrates may quickly activate signaling pathways in the AHPCs which migrated away from the neurosphere, compared to the adherent cells which were maintained as well as analyzed in the presence of laminin. To understand how these various factors and stressors impact neurospheres where cells are highly-compacted and influenced by integrin signaling(s) associated with cell motility, further intensive studies using biochemical and biophysical approaches need to be undertaken.
Cell survival and expansion of NS-AHPCs and AD-AHPCs post-transplantation
At birth the rat retina is in a relatively immature state and can serve as a receptive host environment for neural progenitor cell transplants[17-23]. In developing eyes, transplanted NS-or AD-AHPCs were able to survive, and seemed to be extensively expanded from 7 DPT to 28 DPT. This observation suggests that some of the AHPCs retain a proliferative capacity after transplantation, and that the host microenvironment within the developing eyes can support progenitor/stem cell proliferation. In the eyes of neonatal rats, rod photoreceptors, Müller glia cells, and bipolar cells are still being generated until a week after birth[58]. In addition, proliferation and fate determination of retinal progenitor cells are controlled by intrinsic[59, 60] and extrinsic regulators[60, 61] during retinal development. For example, cyclin-dependent kinases and numerous neurotransmitters are reported as extrinsic regulators for retinal development and histogenesis[58, 62]. Thus, the retinal microenvironment of newborn rats is likely to include factors which can promote AHPC proliferation.
From our retinal transplantations, NS-AHPCs exhibited a relatively greater expansion of cells in the posterior segment compared to AD-AHPCs, although a general number of cells observed in the eye samples for both populations were apparently similar. However, our in vitro results demonstrated that proliferating capabilities of both populations (assessed by the percentages of BrdU-incorporating cells) were comparable in the presence of bFGF. Thus a greater cell expansion of NS-AHPCs than AD-AHPCs in the developing eyes of newborn rats maybe ascribed to the greater fraction of AHPCs committed to glial lineages (especially astrocytic) in the formation of neurosphere compared to adherent cells, presuming that all experimental procedures for transplantation and retinal microenvironment from a litter of rats examined were similar. Insulin-like growth factor-1 (IGF-1), tumor necrosis factor alpha (TNF-α), leukemia inhibitory factor (LIF), and ciliary neurotrophic factor (CNTF), are considered critical regulators for NPC survival and proliferation[63]. Astroglial cells are known to produce these regulatory factors[64]. Since NS-AHPCs are likely to differentiate into glial lineages compared to AD-AHPCs as our in vitro results showed, it is feasible that more glial cells which can produce neurogenic factors may be delivered into the vitreous body when NS-AHPCs were injected. Thus differentiating properties of AHPCs may possibly influence cell proliferation in the microenvironment of the developing retina.
Cell plasticity of NS-AHPCs and AD-AHPCs post-transplantation
AHPCs transplanted into the eyes of newborn rats were well integrated into the retina in 4 weeks and showed morphological differentiation with arborized processes, which are consistent with the results shown by Takahashi et al.[17]. However, phenotypic differentiation of integrated AHPCs was not apparent. Our results from immunohistochemical analyses on the transplanted tissues showed that the GFP-expressing AHPCs that migrated into the retinal layers were not labeled for any neural or retinal markers, except some AHPCs within the vitreous cavity which were labeled for GFAP (an astrocytic marker) or nestin (a neural progenitor marker). To determine if the AHPC transplants are capable of differentiating phenotypically after being morphologically integrated into the retina, it will be necessary to examine the expression of phenotypic markers for retinal specific neurons in the transplanted and integrated AHPCs following a longer survival period. Overall, the intercellular architecture of AHPC populations for maintenance – neurosphere vs monolayer – influences lineage commitment of AHPCs, and in vivo microenvironment plays a crucial role on differentiation of those AHPCs following transplantation.
Conclusions
To study different phenotypic characteristics and migratory properties of NPCs maintained in a 2-D or 3-D environment, we compared proliferation and differentiation capabilities and migration rates of AHPCs cultured as an adherent population to those of AHPCs cultured as free-floating cell aggregates, termed ‘neurospheres’. From in vitro study, we observed three main differences between the two populations. (1) Under maintenance condition, both adherent and neurosphere populations were highly proliferative. However, when cultured under differentiation condition, AHPCs formed in neurospheres lost their proliferation capability faster than adherent AHPCs. (2) AHPCs in neurospheres preferred to differentiate into glial lineages under differentiation conditions, compared to adherent cells. (3) AHPCs from the neurospheres migrated much faster and in a more directed manner than adherent cells. To further study cell plasticity, we transplanted both populations of AHPCs (AHPCs formed in neurospheres versus adherent AHPCs) into the developing retina and compared their capacities of cell survival, differentiation, and integration. Our in vivo results demonstrated that (1) retinas transplanted with AHPCs in neurosphere generally had more cells present and a greater level of morphological differentiation and integration, compared to adherent AHPCs, and (2) AHPCs morphologically differentiated and migrated into the retina were observed; however there was no evidence for phenotypic differentiation into retinal specific cell types. Overall, our results in this study suggest that the intercellular architecture of AHPCs during maintenance in culture influences lineage commitment of AHPCs, and in vivo microenvironment in the developing retina of neonatal rats plays an important role on differentiation of the transplanted AHPCs.
Supplementary Material
Acknowledgments
Financial support was provided by the National Eye Institute (1R01EY019294), the National Science Foundation-Research Experience for Undergraduates (DBI-0552371), the Stem Cell Research Fund, and the Department of Genetics, Development and Cell Biology, Iowa State University. Donald S. Sakaguchi was funded by the NIH (NIGMS 1 RO1 GM072005). The authors would like to thank Dr. Fred H. Gage at the Salk Institute, La Jolla, CA, for the gift of the AHPCs.
Abbreviations
- 2-D
two-dimensional
- 3-D
three-dimensional
- AD-AHPC
adherent AHPCs
- AHPC
adult hippocampal progenitor cell
- bFGF
basic fibroblast growth factor or fibroblast growth factor-2
- BrdU
5′-bromo-2′-deoxyuridine
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- DAPI
4′, 6-diamidino-2-phenylindole, dilactate
- DIV
days in vitro
- DM
differentiation medium, which is maintenance medium without bFGF
- DMEM/F-12
Dulbecco's modified Eagle's medium/Ham's F-12
- DPT
days post-transplantation
- EBSS
Earle's balanced salt solution
- GFAP
glial fibrillary acidic protein, a phenotypic marker for astrocytes
- GFP
green fluorescent protein
- IGF-1
insulin-like growth factor-1
- INL
inner nuclear layer of the retina
- IPL
inner plexiform layer of the retina
- LIF
leukemia inhibitory factor
- MM
maintenance medium
- NPC
adult neural progenitor cell
- NS-AHPC
AHPCs formed in neurospheres
- OCT
optimal cutting temperature
- ONL
outer nuclear layer of the retina
- PBS
phosphate-buffered saline
- PN
postnatal (i.e. 1 PN, postnatal day 1)
- RIP
receptor interacting protein, a phenotypic marker for oligodendrocytes
- T-75
75 cm2 tissue culture flasks
- TNF-α
tumor necrosis factor alpha
- TuJ1
βIII tubulin, a phenotypic marker for early neurons
- X-IR
immunoreactive for a phenotypic marker, X
Footnotes
The authors have declared no conflict of interest.
References
- 1.Gage FH, Coates PW, Palmer TD, Kuhn HG, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 3.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5:438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Bez A, Corsini E, Curti D, Biggiogera M, et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003;993:18–29. doi: 10.1016/j.brainres.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 6.Jessberger S, Clemenson GD, Jr, Gage FH. Spontaneous fusion and nonclonal growth of adult neural stem cells. Stem Cells. 2007;25:871–874. doi: 10.1634/stemcells.2006-0620. [DOI] [PubMed] [Google Scholar]
- 7.Oh J, Recknor JB, Recknor JC, Mallapragada SK, et al. Soluble factors from neocortical astrocytes enhance neuronal differentiation of neural progenitor cells from adult rat hippocampus on micropatterned polymer substrates. J Biomed Mater Res A. 2009;91:575–585. doi: 10.1002/jbm.a.32242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oishi K, Kamakura S, Isazawa Y, Yoshimatsu T, et al. Notch promotes survival of neural precursor cells via mechanisms distinct from those regulating neurogenesis. Dev Biol. 2004;276:172–184. doi: 10.1016/j.ydbio.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 10.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 11.Ray J, Peterson DA, Schinstine M, Gage FH. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci U S A. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recknor JB, Sakaguchi DS, Mallapragada SK. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials. 2006;27:4098–4108. doi: 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Tanigaki K, Nogaki F, Takahashi J, Tashiro K, et al. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29:45–55. doi: 10.1016/s0896-6273(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Suzuki Y, Kitada M, Kitaura M, et al. Migration, integration, and differentiation of hippocampus-derived neurosphere cells after transplantation into injured rat spinal cord. Neurosci Lett. 2001;312:173–176. doi: 10.1016/s0304-3940(01)02219-4. [DOI] [PubMed] [Google Scholar]
- 15.Vukicevic V, Jauch A, Dinger TC, Gebauer L, et al. Genetic instability and diminished differentiation capacity in long-term cultured mouse neurosphere cells. Mech Ageing Dev. 2010;131:124–132. doi: 10.1016/j.mad.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Mothe AJ, Kulbatski I, Parr A, Mohareb M, et al. Adult spinal cord stem/progenitor cells transplanted as neurospheres preferentially differentiate into oligodendrocytes in the adult rat spinal cord. Cell Transplant. 2008;17:735–751. doi: 10.3727/096368908786516756. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M, Palmer TD, Takahashi J, Gage FH. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol Cell Neurosci. 1998;12:340–348. doi: 10.1006/mcne.1998.0721. [DOI] [PubMed] [Google Scholar]
- 18.Van Hoffelen SJ, Young MJ, Shatos MA, Sakaguchi DS. Incorporation of murine brain progenitor cells into the developing mammalian retina. Invest Ophthalmol Vis Sci. 2003;44:426–434. doi: 10.1167/iovs.02-0269. [DOI] [PubMed] [Google Scholar]
- 19.Young MJ, Ray J, Whiteley SJ, Klassen H, et al. Neuronal differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16:197–205. doi: 10.1006/mcne.2000.0869. [DOI] [PubMed] [Google Scholar]
- 20.Blong CC, Jeon CJ, Yeo JY, Ye EA, et al. Differentiation and behavior of human neural progenitors on micropatterned substrates and in the developing retina. J Neurosci Res. 2010;88:1445–1456. doi: 10.1002/jnr.22324. [DOI] [PubMed] [Google Scholar]
- 21.Grozdanic SD, Ast AM, Lazic T, Kwon YH, et al. Morphological integration and functional assessment of transplanted neural progenitor cells in healthy and acute ischemic rat eyes. Exp Eye Res. 2006;82:597–607. doi: 10.1016/j.exer.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Saloupis P, Shaw SJ, Rickman DW. Engraftment of adult neural progenitor cells transplanted to rat retina injured by transient ischemia. Invest Ophthalmol Vis Sci. 2003;44:3194–3201. doi: 10.1167/iovs.02-0875. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi DS, Van Hoffelen SJ, Theusch E, Parker E, et al. Transplantation of neural progenitor cells into the developing retina of the Brazilian opossum: an in vivo system for studying stem/progenitor cell plasticity. Dev Neurosci. 2004;26:336–345. doi: 10.1159/000082275. [DOI] [PubMed] [Google Scholar]
- 24.Lu B, Kwan T, Kurimoto Y, Shatos M, et al. Transplantation of EGF-responsive neurospheres from GFP transgenic mice into the eyes of rd mice. Brain Res. 2002;943:292–300. doi: 10.1016/s0006-8993(02)02906-2. [DOI] [PubMed] [Google Scholar]
- 25.Wojciechowski AB, Englund U, Lundberg C, Wictorin K, et al. Subretinal transplantation of brain-derived precursor cells to young RCS rats promotes photoreceptor cell survival. Exp Eye Res. 2002;75:23–37. doi: 10.1006/exer.2001.1172. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi DS, Van Hoffelen SJ, Young MJ. Differentiation and morphological integration of neural progenitor cells transplanted into the developing mammalian eye. Ann N Y Acad Sci. 2003;995:127–139. doi: 10.1111/j.1749-6632.2003.tb03216.x. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi DS, Van Hoffelen SJ, Grozdanic SD, Kwon YH, et al. Neural progenitor cell transplants into the developing and mature central nervous system. Ann N Y Acad Sci. 2005;1049:118–134. doi: 10.1196/annals.1334.012. [DOI] [PubMed] [Google Scholar]
- 28.Gamm DM, Wang S, Lu B, Girman S, et al. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS One. 2007;2:e338. doi: 10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Girman S, Lu B, Bischoff N, et al. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2008;49:3201–3206. doi: 10.1167/iovs.08-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis PJ, Wang S, Zhang Y, Brown A, et al. Subretinal transplantation of forebrain progenitor cells in nonhuman primates: survival and intact retinal function. Invest Ophthalmol Vis Sci. 2009;50:3425–3431. doi: 10.1167/iovs.08-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gage FH, Kempermann G, Palmer TD, Peterson DA, et al. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Qian X, Shen Q, Goderie SK, He W, et al. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 33.Vescovi AL, Gritti A, Galli R, Parati EA. Isolation and intracerebral grafting of nontransformed multipotential embryonic human CNS stem cells. J Neurotrauma. 1999;16:689–693. doi: 10.1089/neu.1999.16.689. [DOI] [PubMed] [Google Scholar]
- 34.Campos LS. Neurospheres: insights into neural stem cell biology. J Neurosci Res. 2004;78:761–769. doi: 10.1002/jnr.20333. [DOI] [PubMed] [Google Scholar]
- 35.Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11:176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- 36.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ariza CA, McHugh KP, White SJ, Sakaguchi DS, et al. Extracellular matrix proteins and astrocyte-derived soluble factors influence the differentiation and proliferation of adult neural progenitor cells. J Biomed Mater Res A. 2010;94:816–824. doi: 10.1002/jbm.a.32741. [DOI] [PubMed] [Google Scholar]
- 38.Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol. 2002;4:E75–76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- 39.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 40.Gaiano N, Fishell G. The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 41.Morrison SJ, Perez SE, Qiao Z, Verdi JM, et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 42.Yao X, Peng R, Ding J. Cell-material interactions revealed via material techniques of surface patterning. Adv Mater. 2013;25:5257–5286. doi: 10.1002/adma.201301762. [DOI] [PubMed] [Google Scholar]
- 43.Sun J, Graeter SV, Yu L, Duan S, et al. Technique of surface modification of a cell-adhesion-resistant hydrogel by a cell-adhesion-available inorganic microarray. Biomacromolecules. 2008;9:2569–2572. doi: 10.1021/bm800477s. [DOI] [PubMed] [Google Scholar]
- 44.Yan C, Sun J, Ding J. Critical areas of cell adhesion on micropatterned surfaces. Biomaterials. 2011;32:3931–3938. doi: 10.1016/j.biomaterials.2011.01.078. [DOI] [PubMed] [Google Scholar]
- 45.Yao X, Hu Y, Cao B, Peng R, et al. Effects of surface molecular chirality on adhesion and differentiation of stem cells. Biomaterials. 2013;34:9001–9009. doi: 10.1016/j.biomaterials.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Peng R, Yao X, Ding J. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion. Biomaterials. 2011;32:8048–8057. doi: 10.1016/j.biomaterials.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 47.Yao X, Peng R, Ding J. Effects of aspect ratios of stem cells on lineage commitments with and without induction media. Biomaterials. 2013;34:930–939. doi: 10.1016/j.biomaterials.2012.10.052. [DOI] [PubMed] [Google Scholar]
- 48.Peng R, Yao X, Cao B, Tang J, et al. The effect of culture conditions on the adipogenic and osteogenic inductions of mesenchymal stem cells on micropatterned surfaces. Biomaterials. 2012;33:6008–6019. doi: 10.1016/j.biomaterials.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Tang J, Peng R, Ding J. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials. 2010;31:2470–2476. doi: 10.1016/j.biomaterials.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6:462–475. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 51.Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2:155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- 52.Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, et al. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi Y, Pasquale EB. Eph receptors in the adult brain. Curr Opin Neurobiol. 2004;14:288–296. doi: 10.1016/j.conb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 55.Chumley MJ, Catchpole T, Silvany RE, Kernie SG, et al. EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. J Neurosci. 2007;27:13481–13490. doi: 10.1523/JNEUROSCI.4158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harper MM, Ye EA, Blong CC, Jacobson ML, et al. Integrins contribute to initial morphological development and process outgrowth in rat adult hippocampal progenitor cells. J Mol Neurosci. 2010;40:269–283. doi: 10.1007/s12031-009-9211-x. [DOI] [PubMed] [Google Scholar]
- 57.Zhu C, Bao G, Wang N. Cell mechanics: mechanical response, cell adhesion, and molecular deformation. Annu Rev Biomed Eng. 2000;2:189–226. doi: 10.1146/annurev.bioeng.2.1.189. [DOI] [PubMed] [Google Scholar]
- 58.Martins RA, Pearson RA. Control of cell proliferation by neurotransmitters in the developing vertebrate retina. Brain Res. 2008;1192:37–60. doi: 10.1016/j.brainres.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 59.Levine EM, Green ES. Cell-intrinsic regulators of proliferation in vertebrate retinal progenitors. Semin Cell Dev Biol. 2004;15:63–74. doi: 10.1016/j.semcdb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 61.Dyer MA, Cepko CL. Regulating proliferation during retinal development. Nat Rev Neurosci. 2001;2:333–342. doi: 10.1038/35072555. [DOI] [PubMed] [Google Scholar]
- 62.Cepko CL, Austin CP, Yang X, Alexiades M, et al. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez-Perez O, Quinones-Hinojosa A, Garcia-Verdugo JM. Immunological control of adult neural stem cells. J Stem Cells. 2010;5:23–31. [PMC free article] [PubMed] [Google Scholar]
- 64.Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65:2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.