Abstract
Over 95% of patients with small-cell lung cancer (SCLC) die within five years of diagnosis. The standard of care and the dismal prognosis for this disease have not changed significantly over the past 25 years. Some of the characteristics of SCLC that have defined it as a particularly virulent form of cancer – rapid proliferation, excessive metabolic and angiogenic dependence, apoptotic imbalance and genetic instability – are now being pursued as tumor-specific targets for intervention both in preclinical and early phase clinical studies. Here, we summarize areas of ongoing anti-cancer drug development, including classes of agents that target essential pathways regulating proliferation, angiogenesis, apoptotic resistance, chromosomal and protein stability, and cell–cell and cell–matrix interaction.
Introduction: new molecular targets in a refractory disease
It is estimated that lung cancer accounts for over 170 000 new diagnoses and over 160 000 deaths in 2006, making it the leading cause of cancer-related deaths in USA [1]. Small-cell lung cancer (SCLC) represents 15–20% of lung cancer cases [2]. Clinically, SCLC is highly aggressive with a rapid doubling time and early metastatic behavior; the majority of SCLC patients present with advanced disease. Newly diagnosed SCLC is typically sensitive to cytotoxic chemotherapy with reported response rates of up to 80%. Most patients relapse, however, and eventually die with chemotherapy resistant disease. With a median survival of 18 months and ten months for limited-stage (LS) and extensive-stage (ES) disease, respectively, there have only been incremental changes in the prognosis for patients with SCLC over the past 20 years.
Current recommendations for the treatment of LS SCLC, defined as a disease that is confined within the ipsilateral hemithorax that can be encompassed within a single radiation port, includes combination chemotherapy (typically etoposide and a platinum agent) and external beam radiation. A subset of LS SCLC patients with very early stage disease might also benefit from surgical resection. The recommended treatment for ES SCLC, defined as a disease that extends beyond the ipsilateral hemithorax or that cannot be encompassed within a single radiation port, is doublet chemotherapy consisting of a platinum agent and either etoposide or irinotecan, or triple therapy with cyclophosphamide, doxorubicin and etoposide (or vincristine) [3]. For recurrent disease, recommended therapy includes topotecan monotherapy, which yields response rates of 20–30%.
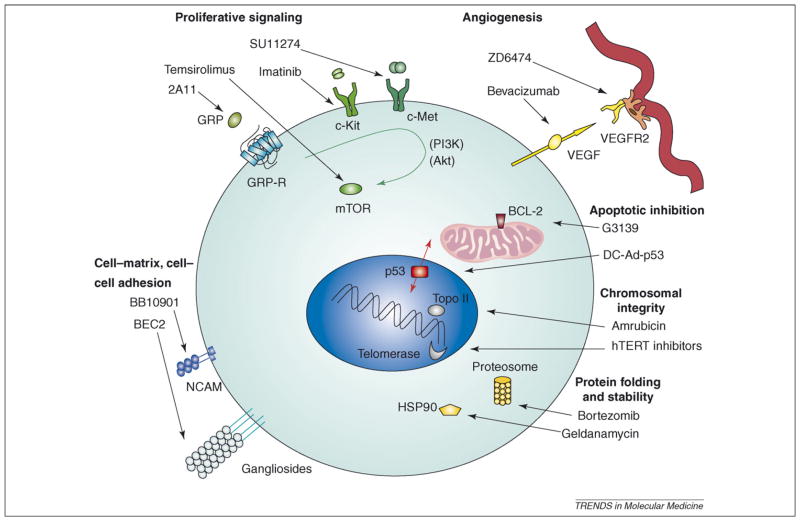
Although various combinations of cytotoxic agents continue to be investigated, much clinical research on SCLC has shifted towards the identification of specific molecular changes that can be used as targets for therapy. Key features of malignant transformation include: alterations in proliferative signaling pathways, tumor-associated angiogenesis, cell-survival pathways, chromosomal stability and replication, protein folding and stability, and cell–cell and cell–matrix interactions (Figure 1). Paralleling the diversity of targets is the diversity of approaches: classes of agents currently under preclinical and clinical evaluation include antisense therapies, small-molecule inhibitors, peptide antagonists, immunotherapies and gene therapies. Here, we focus on agents under active clinical and laboratory investigation for the treatment of SCLC.
Figure 1.
Diverse cellular processes that contribute to malignant transformation are being targeted through the development of new therapeutics for SCLC. Key components of proliferative signaling pathways are shown here, including tumor-associated angiogenesis, cell-survival pathways, mediators of chromosomal stability and replication, protein folding and stability, and cell–cell and cell–matrix interactions.
Cell proliferation: targeting growth-factor signaling pathways
Targeted inhibition of cell-surface growth-factor receptors and their downstream signaling pathways that drive cancer-cell proliferation has been a major focus of anticancer drug development over the past decade. Several receptor tyrosine kinases, including c-Kit and c-Met, are highly expressed and have been implicated in malignant transformation in SCLC. Also, a G-protein-coupled receptor –the gastrin-releasing peptide (GRP) receptor – is almost universally active in SCLC. Several of these growth-factor receptors act upstream of shared proliferative signaling pathways, including the phosphatidylinositol 3-kinase (PI 3-K)–Akt (protein kinase B)–mammalian target of rapamycin (mTOR) pathway and the RAS–RAF–MEK (MAPK–ERK) pathway. These growth-factor receptors and some of the downstream second messengers are amenable to targeted inhibition.
c-Kit
c-Kit gene encodes a transmembrane receptor tyrosine kinase of the platelet-derived growth-factor (PDGF) receptor subfamily. Upon binding of its ligand steel factor [SLF, also known as stem cell factor (SCF)], c-Kit homodimerizes and initiates an intracellular signaling cascade. Up to 70% of SCLCs have been reported to express c-Kit and SCF, although the prognostic value of this finding in unclear [4–6]. Imatinib (STI-571, Gleevec™) is a small-molecule inhibitor of ABL tyrosine kinase, PDGF receptor and c-Kit. Imatinib inhibits SCLC cell lines and xenografts in a dose-dependent manner [7,8]. In phase II clinical trials, however, imatinib did not show activity against de novo or recurrent SCLC [9], as maintenance therapy after induction with etoposide and irinotecan in patients with ES SCLC [10] or in pre-screened patients with c-Kit-positive recurrent SCLC [11]. These entirely negative trials in SCLC, in contrast to the dramatic activity of this agent against gastrointestinal stromal tumors bearing c-Kit-activating mutations [12], was an early indication that, in human cancer patients, small-molecule tyrosine kinase inhibitors might be most effective when directed against mutated receptors rather than an upregulated and active receptor.
c-Met
Activation of the c-Met tyrosine kinase by its natural ligand hepatocyte growth factor (HGF, also known as scatter factor) leads to multiple cellular activities, including proliferation, angiogenesis, invasion into the extracellular matrix and wound healing [13]. c-Met is expressed in a large percentage of SCLCs [14], and tumor-specific mutations of the c-Met receptor have been described in SCLC [15]. The HGF–c-Met signaling network is targeted by various agents in preclinical and early phase clinical development, including blocking monoclonal antibodies against the ligand and the receptor, in addition to small-molecule receptor tyrosine kinase inhibitors. Two small-molecule inhibitors of c-Met, SU011274 and PHA665752, have antitumor activity in xenografts that are derived from various human tumors [16,17], similar to humanized monoclonal antibodies against HGF [18,19]. Clinical activity of c-Met inhibitors in SCLC is currently being explored in early phase clinical trials but has not yet been reported.
Gastrin-releasing peptide
SCLC cells produce several neuropeptides, including GRP and neuromedin B [20,21]. Up to 85% of SCLCs express the related GRP receptor, neuromedin B (NMB) receptor and bombesin receptor subtype 3 (BBR-3), therefore creating an autocrine activation loop [22]. Binding of these G-protein-coupled receptors to their respective ligands results in the activation of multiple proliferative signaling cascades [23,24]. GRP also seems to have angiogenic properties, inducing endothelial-cell migration and cord formation in vitro, and promotion of angiogenesis in vivo [25].
This pathway has been targeted with small-molecule inhibitors, peptide antagonists and monoclonal antibodies. A small-molecule inhibitor of the GRP receptor, 77427, prevented angiogenesis in an in vivo model; this effect was specific to GRP because 77427 had no effect on VEGF-induced angiogenesis [25]. Preclinical studies have also demonstrated that treatment with bombesin-like peptide antagonists or neutralizing antibodies results in inhibition of SCLC tumor growth in vitro and in vivo [23,26,27]. In a phase I trial of 13 relapsed SCLC patients, treatment with the anti-GRP 2A11 murine monoclonal antibody caused one complete response [28,29].
Farnesylation and receptor-associated second messengers
Several signal-transduction molecules that associate with growth-factor receptors and participate in downstream signaling are recruited to the cell membrane by linkage to lipids via farnesyl transferase. Several specific inhibitors of farnesyl transferase have been developed. Farnesyl transferase inhibitors have shown efficacy in preclinical models of SCLC, presumably through effects on the large number of farnesylated second messengers that regulate growth and survival [30]. This observation has not yet successfully translated into the clinic; in an initial phase II clinical trial of 22 patients with relapsed SCLC, the farnesyl transferase inhibitor tipifarnib (Zarnestra™, R115777) failed to show clinical activity as a single agent [31].
mTOR
The PI3K–Akt–mTOR pathway is a signaling cascade that mediates proliferation, cell growth, anchorage independence and apoptosis (reviewed in [32,33]). Activation of this pathway promotes tumorigenesis and therapeutic resistance, whereas its inhibition can lead to chemotherapy sensitization of SCLC cell lines [34,35]. Temsirolimus (CCI-779) is a mTOR inhibitor that has cytotoxic and antitumor activity in several preclinical models. In a phase II clinical trial in ES SCLC, 87 patients were randomized to receive two doses of temsirolimus after standard first-line chemotherapy. Patients treated with either dose showed no improvement in median survival compared with reported outcomes in earlier studies [36]. Additional studies to evaluate mTOR inhibitor activity in SCLC are ongoing.
Angiogenesis: targeting tumor-associated vascular growth
Angiogenesis, the stimulation of new blood vessel growth, is fundamental to normal tissue development, wound healing and reproduction. It has also been definitively associated with tumorigenesis. One of the most important promoters of angiogenesis, produced in different amounts by many human tumors, is the vascular endothelial growth factor (VEGF), which binds to specific receptors including the VEGF-receptor 2 to stimulate blood vessel growth. Clinical correlative studies have established that microvessel density and tissue VEGF expression portend a poorer prognosis in non-small cell lung cancer (NSCLC). This clinical correlation has not been definitively studied in SCLC, due in part to difficulty in obtaining SCLC biopsy samples (reviewed in [37]). Although the prognostic value of serum VEGF levels in SCLC patients in unclear [38,39], the role of angiogenesis in tumor progression and the success of anti-angiogenic agents across various tumor types has prompted investigation of angiogenic targeting in SCLC.
Vascular endothelial growth factor
Bevacizumab (Avastin™), a monoclonal antibody directed against VEGF, has demonstrated clear activity in colorectal cancer, NSCLC and breast cancer. Bevacizumab is currently in a phase II study as maintenance therapy for LS SCLC after treatment with irinotecan and carboplatin. Early analysis suggests that bevacizumab might improve progression-free survival compared with standard therapy [40].
ZD6474 is an orally bioavailable inhibitor of VEGF-receptor 2, which also inhibits other receptor tyrosine kinases that are implicated in angiogenesis and cell proliferation. In preclinical models of SCLC, ZD6474 reduced metastatic rate and induced tumor-cell apoptosis [41]. ZD6474 is currently being evaluated in the adjuvant setting of LS and ES SCLC in a phase II cooperative group trial led by the National Cancer Institute of Canada.
Basic fibroblast growth factor
Thalidomide, an immunosuppressant and anti-inflammatory drug, also has potent anti-angiogenesis activity associated with decreased tumor production of both VEGF and a second key mediator of angiogenesis, basic fibroblast growth factor (bFGF) [42]. Thalidomide was well tolerated in combination with carboplatin and etoposide in a phase II clinical trial [43]. In a phase III study, which was stopped early due to poor accrual, 92 ES SCLC patients with chemotherapy responsive disease were randomized to receive cisplatin, etoposide, epirubicin and cyclophosphamide, with or without thalidomide. Despite the small size of the study, a statistically significant difference in survival was observed in favor of the thalidomide arm: 11.7 versus 8.7 months (hazard ratio: 0.48; 95% confidence interval: 0.24–0.93; p = 0.03) [44]. More patients on the thalidomide treatment arm withdrew from the study due to toxicity (33% versus 19%), notably neuropathy and constipation. Nevertheless, this result is encouraging and supports further investigation of targeted antiangiogenic therapy in SCLC. Receptor tyrosine kinase inhibitors that are active against both VEGF-receptor 2 and bFGF receptor are under development and might represent a better tolerated alternative.
Matrix metalloproteinases
An early step in angiogenesis and tumor invasion is the breakdown of the extracellular matrix mediated by matrix metalloproteinases (MMPs). Both MMP-2 and MMP-9 expression have been associated with poor prognosis in lung cancer [45]. Marimastat, an inhibitor of MMP-2 and MMP-9, was evaluated in a phase III clinical trial of ES SCLC patients who responded to first-line chemotherapy. No survival improvement was observed, and significant musculoskeletal toxicity resulted in discontinuation of therapy in 20% of patients [46]. Unfortunately, this result mirrored that in other disease types.
Apoptotic inhibition: targeting aberrant cell-survival pathways
Apoptosis is crucial for the development and homeostasis of multicellular organisms. These processes are dysregulated in tumors, promoting cancer cell survival when programmed cell death would normally be triggered. Apoptotic inhibition has key roles in both tumorigenesis and therapeutic resistance. Among the central regulators of apoptotic signaling are p53, which translates responses to DNA damage and other stresses into activation of an intrinsic apoptotic pathway, and the B-cell CLL/lymphoma 2 (BCL-2) family of proteins that regulate an important step in apoptotic commitment – that is, permeability transition of the mitochondrial membrane. Both of these regulators are currently being targeted in SCLC in preclinical models and early phase clinical trials.
BCL-2
BCL-2 is a key regulator of the apoptotic cascade and is overexpressed in most SCLCs [47,48]. Aberrant expression of BCL-2 has been correlated with therapeutic resistance [49,50]. Antisense oligonucleotides against BCL-2 mRNA have shown antitumor activity in preclinical SCLC models [51]. Initial clinical trials, however, combining standard chemotherapy with the BCL-2 antisense oligonucleotide G3139 in patients with SCLC did not result in suppression of BCL-2 protein levels in vivo [52]. Consistent with inadequate target suppression, outcome in early phase clinical trials was similar to that of patients treated with chemotherapy alone [52,53]. A randomized phase II study of carboplatin and etoposide with or without G3139 suggests that G3139 does not increase response rate to standard chemotherapy [54].
A more promising alternative strategy for inhibiting BCL-2 function is the use of small-molecule inhibitors. Stabilized α-helix of BCL-2 domains (SAHBA) is a peptide-mimetic modeled after the naturally occurring BCL-2 inhibitor BH3interacting domain death agonist (BID). SAHBA has antitumor activity against human leukemia xenografts [55]. Nonpeptide-mimetics based on the same strategy include ABT-737, AT101 (gossypol) and GX15–070. ABT-737 exhibits remarkable cytotoxicity as a single agent or in combination with chemotherapeutic agents or γ-radiation in cell lines, and activity against human leukemia, lymphoma and SCLC xenografts [56]. Clinical trials to evaluate the effects of these agents or related derivatives are currently starting in SCLC.
p53
The p53 gene encodes a crucial factor that regulates cell survival and damage response pathways. p53 orchestrates a series of responses to perceived DNA damage or aberrant cell-cycle progression, triggering cell-cycle arrest, DNA damage response pathways and the apoptotic cascade [57]. p53 is mutated in over 90% of SCLCs [58]. Preclinical studies demonstrate that adenoviral or retroviral expression of wild-type p53 restores chemotherapy and radiation sensitivity and induces apoptosis in tumor cell lines, including SCLC [59,60].
The prolonged half-life and typically higher expression of mutant p53 in cancer cells enable its use as an antigenic target for vaccine therapy, an approach that is supported by several preclinical studies [61,62]. A vaccine composed of dendritic cells that are transduced with an adenoviral vector expressing p53 (DC-Ad-p53) has been used in combination with chemotherapy in ES SCLC patients [63]. In this study, p53-specific T-cell responses were observed in >50% of patients and vaccination was associated with a high rate of objective response to chemotherapy. A phase I and II dose-escalation clinical trial of the DC-Ad-p53 vaccine in SCLC is underway.
Chromosomal stability: targeting genomic integrity and replication
An important finding in SCLC and many other solid tumors is the loss of genomic stability, ranging from individual gene mutation to gross chromosomal aberration and alterations of ploidy. Almost all SCLCs have deletions that affect multiple chromosomal sites (reviewed in [64]). Common regions of chromosome loss include 3p, 4p, 5q, 13q, 16q and 17p regions, which are loci with well-known tumor suppressor genes such as retinoblastoma (RB) and p53. Deletion of the short arm of chromosome 3 alone has been reported in up to 90% of SCLC cases. Genes that are located on 3p include retinoic acid receptor-β (RAR-β), fusion 1 (FUS1) and fragile histidine triad (FHIT), the last of which is altered in up to 80% of SCLC cases. Comparative genomic hybridization studies have reiterated findings from earlier genomic studies that showed gene amplification at multiple loci in SCLC, including 1p, 2q, 3q, 5p, 6p12, 6p15, 6p22–23, 7q, 8q, 11q, 12p, 14q and 18q. These regions encode oncogenes, including MYC, K-RAS, mixed lineage leukemia (MLL) and BCL-2.
Several agents that are currently being explored are intended to increase chromosomal instability or to interfere with cellular responses to chromosomal instability. These include a novel derivative of a traditional cytotoxic class, the topoisomerase II inhibitor amrubicin, and agents that target telomerase, the enzyme that maintains the integrity of chromosomal ends.
DNA topoisomerase II
The DNA topoisomerase II inhibitors etoposide and doxorubicin are among the most-active single agents against SCLC. Amrubicin, a synthetic anthracycline, and its 13-hydroxy metabolite amrubicinol represent a newer generation of potent topoisomerase II inhibitors [65]. A phase II study of 33 patients with previously untreated SCLC reported a response rate of 76% for amrubicin [66], and a 44-patient phase I and II study reported an 88% response rate in combination with cisplatin [67]. The latter combination was associated with a median survival time of 13.6 months.
Two recent phase II trials of amrubicin monotherapy for recurrent SCLC have also reported encouraging activity. In a phase II trial of 60 SCLC patients that were pretreated with at least one platinum-containing regimen, Seto et al. [68] reported response rates of 52% for chemosensitive relapse, and 50% in 16 patients with chemorefractory relapse, defined as progression within 60 days of completion of first-line therapy. Median survival was 11.7 and 10.9 months for patients with chemosensitive and chemorefractory disease, respectively [68]. These results were supported by another phase II study of SCLC patients that were treated with one or two prior therapies, in which single agent amrubicin was associated with a response rate of 53%, median survival of 8.8 months and one-year survival of 26% [69]. The results of these early phase clinical trials indicate improvements upon reported objective response rate, overall survival and one-year survival data from studies of topotecan monotherapy in recurrent SCLC, particularly for chemorefractory relapse [70]. It is unclear whether the pretreated patients in these amrubicin trials received prior therapy with a DNA topoisomerase II inhibitor (e.g. etoposide or doxorubicin), and whether the toxicities of this agent are significant (grade 3–4 neutropenia was reported in 83–97% of patients and febrile neutropenia in 5–35%).
Further questions to be addressed include the efficacy of amrubicin in more-diverse patient populations and in randomized comparisons with standard therapy. These questions might be addressed with a phase II trial that compares amrubicin monotherapy with that of topotecan in relapsed SCLC, which is currently underway in the USA. Trials of amrubicin combination therapy that have currently started in Japan include a phase II trial of amrubicin and irinotecan in patients with relapsed disease, and a phase III trial of amrubicin and carboplatin in chemotherapy naïve elderly patients with ES SCLC.
Telomerase
Chromosome ends (telomeres) are capped by structures containing defined nucleotide repeat sequences that distinguish telomeres from DNA breaks (reviewed in [71]). Critically short or uncapped telomeres can trigger a DNA damage response pathway, leading to cell-cycle arrest and apoptosis. Telomeric repeats are synthesized by the ribo-nucleoprotein telomerase to preserve telomere length in replicating cells. Telomerase expression is silenced in terminally differentiated cells, but is frequently reactivated in the process of malignant transformation, facilitating unlimited proliferative capacity of cancer cells [72]. Telomerase is reactivated in most (up to 98%) SCLCs, and can serve both as a target of directed inhibition and as a tumor antigen. Immune responses and one clinical complete response have been observed in a NSCLC study of two synthetic peptide vaccines: GV1001 (human telomerase reverse transcriptase, hTERT; amino acids 611–626) and HR2882 (hTERT; amino acids 540–548) [73]. A telomerase-specific oncolytic virus, telomelysin (OBP-301), and a telomerase inhibitor, CKD601, are currently undergoing preclinical evaluation.
Retinoblastoma
RB is a crucial negative regulator of cell-cycle entry. Deletion or alteration of the RB gene has been reported in >90% of SCLC cases [74]. The relationship between loss of p53 and RB and development of SCLC is supported by the development of a SCLC mouse model in which p53 and RB could be conditionally lost [75]. Despite extensive characterization of RB pathway disruption in several tumor types, there have been few attempts to target this key factor in oncogenesis. An oncolytic adenovirus developed by ONYX Pharmaceuticals demonstrated preclinical evidence of specific antitumor activity against RB-deficient tumors, but has not been explored clinically.
Protein folding and stability: targeting metastable and mutant oncoproteins
Many of the key factors in malignant transformation are aberrantly expressed and/or stabilized derivatives of normal regulators of cell proliferation and cell survival. Intracellular levels of these important oncoproteins and tumor suppressors are normally tightly regulated by control both of gene transcription and translation, and of protein degradation. Homeostatic disruption either by inhibition of protein folding or of protein degradation has been shown to have selective antitumor activity.
26S proteasome
The 26S proteasome is a multi-enzyme unit that regulates intracellular protein homeostasis via proteolytic degradation. It has several substrates that are important in cell-cycle regulation and apoptosis including cyclins, IκB and p53 (reviewed in [76]). Proteasomal inhibition in lung cancer cell lines causes cell-cycle arrest and apoptosis associated with increased levels of p21 and decreased levels of BCL-2 [77,78]. Bortezomib (Velcade™), a reversible proteasome inhibitor, has received Food and Drug Administration (FDA) approval for the treatment of multiple myeloma, and can induce apoptosis in SCLC cell lines [78]. A phase I clinical trial of bortezomib in combination with topotecan for relapsed SCLC is currently being conducted at the University of California Davis.
HSP90
Heat shock protein 90 (HSP90) is a molecular chaperone, required for efficient folding of nascent ‘client’ proteins into fully functional tertiary structures. HSP90 inhibition is associated with disruption of multiple signaling pathways implicated in cancer, including the I-κB kinase–nuclear factor-κB (IKK–NF-κB), PI 3-K–Akt and RAS–RAF–MEK pathways [79]. Treatment of SCLC lines in vitro with geldanamycin, an HSP90 inhibitor, inhibits growth and viability of multiple SCLC lines associated with apoptotic induction [80]. Several potent and specific inhibitors of HSP90 have been developed and are being actively evaluated in early phase clinical trials [81].
Cell–matrix and cell–cell adhesion: targeting the cancer-cell environment
Another important determinant of malignant transformation concerns tumor-cell interaction with adjacent cells and surrounding extracellular matrix. Cell–cell and cell–matrix interactions are key factors that regulate cell survival and proliferation through activation of transmembrane complexes, including cadherins and integrins, in addition to associated intracellular membrane signaling molecules such as the focal adhesion kinase. Oncogenic transformation is associated with aberrant production or structure of cell–cell and cell–matrix complexes. These tumor-specific abnormalities represent exposed cell-surface targets and have been exploited as putative antigens for antibody-based anti-cancer immunotherapies.
CD56
Neural cell adhesion molecule (NCAM) modulates neuroendocrine cell growth, migration and differentiation [82]. The NCAM gene encodes three distinct isoforms, one of which is CD56 [83]. NCAM is expressed in most SCLCs [84]. A humanized anti-CD56 monoclonal antibody conjugated to a cytotoxic maytansinoid drug, DM-1, (BB10901; huN901-DM1) has been developed. Early reports of a phase II clinical trial in SCLC patients with relapsed, CD56+ disease indicated that BB10901 was well tolerated, and clinical activity was observed in three of the first ten patients enrolled [85]. Two clinical trials of BB10901 in SCLC are currently underway.
Gangliosides
SCLC cells produce tumor-specific gangliosides on their cell surface, including GM2, GD2, GD3 and 9-O-acteyl-CD3 [86,87]. BEC2 (mitumomab) isa mouse monoclonal antibody against CD3 combined with Bacillus Calmette-Guerin (BCG), which is used as an immune adjuvant. In a pilot study, 15 SCLC patients received a combination of anti-BEC2 and BCG in the adjuvant setting after standard chemotherapy. All patients developed anti-BEC2antibodies and median survival in this cohort was 21 months [88]. These initially promising results did not hold up in a subsequent phase III trial of 515 patients with LS SCLC randomized to receive BEC2 and BCG or best supportive care after major response to chemotherapy and radiation; the vaccine did not improve survival in LS SCLC patients [89]. Subset analysis suggested a trend towards prolonged survival in patients who developed a humoral response to the vaccine.
Concluding remarks: challenges in SCLC therapeutic development
Current therapeutic research in SCLC represents a convergence of tumor biology, offering a detailed view of the essential components of SCLC malignant transformation and growth, and molecular pharmacology, offering a spectrum of targeted inhibitors of these important pathways. SCLC is a notoriously rapidly growing tumor with aggressive metastatic potential. Many of the key alterations that lead to constitutive activation of proliferative and metabolic pathways, loss of cell–cell and cell–matrix adhesion, disruption of apoptotic control and stimulation of angiogenic support have been defined. These same alterations that are essential to SCLC survival, growth and spread are being specifically targeted by a new generation of targeted therapeutics.
One of the lessons to be learned from the past decades of clinical research in SCLC is the need for better and more-representative preclinical models of disease. The promises of many seemingly successful strategies, showing high-level activity in vitro and apparent cures in immunosuppressed mice bearing xenograft tumors, have failed in clinical studies. Although cancer-cell-line-based xenograft models represent a standard testing ground for new antic-ancer strategies in this and other cancers, these models are an imperfect reflection of the disease. Cancer cell lines that are maintained in tissue culture are selected for properties that are optimal for ex vivo propagation, including growth as monolayers on plastic, in high-glucose media and in high-oxygen tension. Human cancers, by contrast, arise and are maintained in hypoxic and nutrient-poor complex environments in which selection for angiogenic drive and other survival strategies are operant. Reflective of these different selective pressures, cell-line-based SCLC xenografts tend to grow as non-invasive tumor nodules with low metastatic potential, in marked contrast to the human disease that is characterized by aggressive local invasion and early and widespread metastasis.
Multiple strategies for developing more-representative preclinical models of SCLC are being explored. One strategy involves genetic manipulation of mice to produce murine tumors with features that are similar to human disease. A prime example of this for SCLC is based on concomitant interruption of RB and p53 loci, resulting in mice that develop tumors with several features in common with human SCLC. An alternative strategy, avoiding the inherent biological differences between genetically derived murine tumors and a human cancer demonstrating complex genetic and genomic alterations, is to optimize human tumor xenograft models. One approach, which has shown great promise in multiple tumor types and is now being applied in SCLC, involves the transfer of cancer cells from the patient directly into the mouse with no intervening ex vivoculture. Recent data, from glioblastoma in particular, suggest that such primary heterotransplants might retain biological similarity to human disease that is lost in cancer cell lines ex vivo [90]. Use of these models as preclinical platforms for innovative therapeutic development is just beginning. Our own laboratory has been using such a model to evaluate the efficacy of highly selective BCL-2 inhibitors, a strategy that we think holds promise for improved treatment of SCLC.
Despite active and ongoing research into novel approaches to treat SCLC, there has been minimal progress in the clinical treatment of this disease over the past 25 years. The basis of cytotoxic intervention for SCLC in the early 1980s remains a standard of care at present. Advances in supportive care and technical advances in radiation therapy have led to the application of therapies with less toxicity and greater safety in most patients. Many strategies for molecularly targeted intervention are now being explored in both late preclinical and early phase clinical development. Referral of patients with SCLC to active clinical research centers for participation in clinical trials is of primary importance. Recent insights into the underlying biology of malignant transformation in SCLC and development of more-representative laboratory models of disease will facilitate continued focused therapeutic testing of novel anticancer strategies. The challenge and the promise of the upcoming years will be translating these insights into more-effective therapies for patients with SCLC.
Acknowledgments
This work was supported by an American Society of Clinical Oncology Young Investigator Award (CLH), the Flight Attendant Medical Research Institute (CMR) and the Burroughs Wellcome Fund (CMR).
References
- 1.American Cancer Society. Cancer Facts and Figures 2006. American Cancer Society; 2006. [Google Scholar]
- 2.Navada S, et al. Temporal trends in small cell lung cancer: analysis of the national surveillance, epidemiology, and end-results (SEER) database. J Clin Oncol 2006 ASCO Annual Meeting Proceedings Part I. 2006;24 (18S):7082. [Google Scholar]
- 3.Johnson BE, et al. Small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2006;4:602–622. doi: 10.6004/jnccn.2006.0050. [DOI] [PubMed] [Google Scholar]
- 4.Micke P, et al. Characterization of c-kit expression in small cell lung cancer: prognostic and therapeutic implications. Clin Cancer Res. 2003;9:188–194. [PubMed] [Google Scholar]
- 5.Blackhall FH, et al. Expression and prognostic significance of kit, protein kinase B, and mitogen-activated protein kinase in patients with small cell lung cancer. Clin Cancer Res. 2003;9:2241–2247. [PubMed] [Google Scholar]
- 6.Potti A, et al. CD117 (c-KIT) overexpression in patients with extensive-stage small-cell lung carcinoma. Ann Oncol. 2003;14:894–897. doi: 10.1093/annonc/mdg253. [DOI] [PubMed] [Google Scholar]
- 7.Wang WL, et al. Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI 571. Oncogene. 2000;19:3521–3528. doi: 10.1038/sj.onc.1203698. [DOI] [PubMed] [Google Scholar]
- 8.Decaudin D, et al. In vivo efficacy of STI571 in xenografted human small cell lung cancer alone or combined with chemotherapy. Int J Cancer. 2005;113:849–856. doi: 10.1002/ijc.20652. [DOI] [PubMed] [Google Scholar]
- 9.Johnson BE, et al. Phase II study of imatinib in patients with small cell lung cancer. Clin Cancer Res. 2003;9:5880–5887. [PubMed] [Google Scholar]
- 10.Schneider BJ, et al. Phase II trial of imatinib maintenance therapy after irinotecan and cisplatin in patients with c-kit positive extensive-stage small cell lung cancer (ES SCLC) J Clin Oncol 2006 ASCO Annual Meeting Proceedings Part I. 2006;24 (18S):17089. doi: 10.3816/CLC.2010.n.028. [DOI] [PubMed] [Google Scholar]
- 11.Krug LM, et al. Imatinib mesylate lacks activity in small cell lung carcinoma expressing c-kit protein: a phase II clinical trial. Cancer. 2005;103:2128–2131. doi: 10.1002/cncr.21000. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich MC, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 13.Stella MC, Comoglio PM. HGF: a multifunctional growth factor controllingcell scattering. Int J Biochem Cell Biol. 1999;31:1357–1362. doi: 10.1016/s1357-2725(99)00089-8. [DOI] [PubMed] [Google Scholar]
- 14.Rygaard K, et al. Expression of the proto-oncogenes c-met and c-kit and their ligands, hepatocyte growth factor/scatter factor and stem cell factor, in SCLC cell lines and xenografts. Br J Cancer. 1993;67:37–46. doi: 10.1038/bjc.1993.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma PC, et al. c-MET mutational analysis in small cell lung cancer: novel juxtamembrane domain mutations regulating cytoskeletal functions. Cancer Res. 2003;63:6272–6281. [PubMed] [Google Scholar]
- 16.Christensen JG, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- 17.Smolen GA, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A. 2006;103:2316–2321. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgess T, et al. Fully human monoclonal antibodies to hepatocyte growth factor with therapeutic potential against hepatocyte growth factor/c-Met-dependent human tumors. Cancer Res. 2006;66:1721–1729. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- 19.Kim KJ, et al. Systemic anti-hepatocyte growth factor monoclonal antibody therapy induces the regression of intracranial glioma xenografts. Clin Cancer Res. 2006;12:1292–1298. doi: 10.1158/1078-0432.CCR-05-1793. [DOI] [PubMed] [Google Scholar]
- 20.Sausville EA, et al. Expression of the gastrin-releasing peptide gene in human small cell lung cancer. Evidence for alternative processing resulting in three distinct mRNAs. J Biol Chem. 1986;261:2451–2457. [PubMed] [Google Scholar]
- 21.Cardona C, et al. Production of neuromedin B and neuromedin B gene expression in human lung tumor cell lines. Cancer Res. 1991;51:5205–5211. [PubMed] [Google Scholar]
- 22.Corjay MH, et al. Two distinct bombesin receptor subtypes are expressed and functional in human lung carcinoma cells. J Biol Chem. 1991;266:18771–18779. [PubMed] [Google Scholar]
- 23.Cuttitta F, et al. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 24.Hellmich MR, et al. Multiple protein kinase pathways are involved in gastrin-releasing peptide receptor-regulated secretion. J Biol Chem. 1999;274:23901–23909. doi: 10.1074/jbc.274.34.23901. [DOI] [PubMed] [Google Scholar]
- 25.Martinez A, et al. Gastrin-releasing peptide (GRP) induces angiogenesis and the specific GRP blocker 77427 inhibits tumor growth in vitro and in vivo. Oncogene. 2005;24:4106–4113. doi: 10.1038/sj.onc.1208581. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoud S, et al. [Psi 13,14] bombesin analogues inhibit growth of small cell lung cancer in vitro and in vivo. Cancer Res. 1991;51:1798–1802. [PubMed] [Google Scholar]
- 27.Halmos G, Schally AV. Reduction in receptors for bombesin and epidermal growth factor in xenografts of human small-cell lung cancer after treatment with bombesin antagonist RC-3095. Proc Natl Acad Sci U S A. 1997;94:956–960. doi: 10.1073/pnas.94.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelley MJ, et al. Antitumor activity of a monoclonal antibody directed against gastrin-releasing peptide in patients with small cell lung cancer. Chest. 1997;112:256–261. doi: 10.1378/chest.112.1.256. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhry A, et al. Phase I and imaging trial of a monoclonal antibody directed against gastrin-releasing peptide in patients with lung cancer. Clin Cancer Res. 1999;5:3385–3393. [PubMed] [Google Scholar]
- 30.Sepp-Lorenzino L, et al. A peptidomimetic inhibitor of farnesyl:protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- 31.Heymach JV, et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with sensitive relapse small-cell lung cancer. Ann Oncol. 2004;15:1187–1193. doi: 10.1093/annonc/mdh315. [DOI] [PubMed] [Google Scholar]
- 32.Dancey JE. Clinical development of mammalian target of rapamycin inhibitors. Hematol Oncol Clin North Am. 2002;16:1101–1114. doi: 10.1016/s0889-8588(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 33.Moore SM, et al. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer Res. 1998;58:5239–5247. [PubMed] [Google Scholar]
- 34.Kraus AC, et al. In vitro chemo- and radio-resistance in small cell lung cancer correlates with cell adhesion and constitutive activation of AKT and MAP kinase pathways. Oncogene. 2002;21:8683–8695. doi: 10.1038/sj.onc.1205939. [DOI] [PubMed] [Google Scholar]
- 35.Krystal GW, et al. Inhibition of phosphatidylinositol 3-kinase-Akt signaling blocks growth, promotes apoptosis, and enhances sensitivity of small cell lung cancer cells to chemotherapy. Mol Cancer Ther. 2002;1:913–922. [PubMed] [Google Scholar]
- 36.Pandya KJ, et al. A randomized, phase II ECOG trial of two dose levels of temsirolimus (CCI-779) in patients with extensive stage small cell lung cancer in remission after induction chemotherapy. A preliminary report. J Clin Oncol 2005 ASCO Annual Meeting Proceedings. 2005;23 (16S):7005. [Google Scholar]
- 37.Blackhall FH, Shepherd FA. Angiogenesis inhibitors in the treatment of small cell and non-small cell lung cancer. Hematol Oncol Clin North Am. 2004;18:1121–1141. doi: 10.1016/j.hoc.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa Y, et al. Vascular endothelial growth factor level as a prognostic determinant of small cell lung cancer in Japanese patients. Intern Med. 2005;44:26–34. doi: 10.2169/internalmedicine.44.26. [DOI] [PubMed] [Google Scholar]
- 39.Tas F, et al. Serum vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) levels in small cell lung cancer. Cancer Invest. 2006;24:492–496. doi: 10.1080/07357900600814771. [DOI] [PubMed] [Google Scholar]
- 40.Patton JF, et al. Irinotecan (I), carboplatin (C), and radiotherapy (RT) followed by maintenance bevacizumab (B) in the treatment (tx) of limited-stage small cell lung cancer (LS-SCLC): update of a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2006 ASCO Annual Meeting Proceedings, Part 1. 2006;24 (18S):7085. [Google Scholar]
- 41.Yano S, et al. Antitumor vascular strategy for controlling experimental metastatic spread of human small-cell lung cancer cells with ZD6474 in natural killer cell-depleted severe combined immunodeficient mice. Clin Cancer Res. 2005;11:8789–8798. doi: 10.1158/1078-0432.CCR-05-0674. [DOI] [PubMed] [Google Scholar]
- 42.D’Amato RJ, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SM, et al. A phase II study of carboplatin/etoposide with thalidomide in small cell lung cancer (SCLC) Proceedings of the 38th Annual Meeting of the American Society of Clinical Oncology. 2002;21:313a. [Google Scholar]
- 44.Pujol JL, et al. A prospective randomized phase III, double-blind, placebo-controlled study of thalidomide in extended-disease (ED) SCLC patients after response to chemotherapy (CT): An intergroup study FNCLCC Cleo04 - IFCT 00-01. J Clin Oncol 2006 ASCO Annual Meeting Proceedings Part I. 2006;24 (18S):7057. [Google Scholar]
- 45.Shou Y, et al. Influence of angiogenetic factors and matrix metalloproteinases upon tumour progression in non-small-cell lung cancer. Br J Cancer. 2001;85:1706–1712. doi: 10.1054/bjoc.2001.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shepherd FA, et al. Prospective, randomized, double-blind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J Clin Oncol. 2002;20:4434–4439. doi: 10.1200/JCO.2002.02.108. [DOI] [PubMed] [Google Scholar]
- 47.Higashiyama M, et al. High prevalence of bcl-2 oncoprotein expression in small cell lung cancer. Anticancer Res. 1995;15:503–505. [PubMed] [Google Scholar]
- 48.Jiang SX, et al. Expression of bcl-2 oncogene protein is prevalent in small cell lung carcinomas. J Pathol. 1995;177:135–138. doi: 10.1002/path.1711770206. [DOI] [PubMed] [Google Scholar]
- 49.Luo D, et al. Effects of Bcl-2 and Bcl-XL protein levels on chemoresistance of hepatoblastoma HepG2 cell line. Biochem Cell Biol. 2000;78:119–126. [PubMed] [Google Scholar]
- 50.Guensberg P, et al. Bcl-xL antisense oligonucleotides chemosensitize human glioblastoma cells. Chemotherapy. 2002;48:189–195. doi: 10.1159/000063873. [DOI] [PubMed] [Google Scholar]
- 51.Ziegler A, et al. Induction of apoptosis in small-cell lung cancer cells by an antisense oligodeoxynucleotide targeting the Bcl-2 coding sequence. J Natl Cancer Inst. 1997;89:1027–1036. doi: 10.1093/jnci/89.14.1027. [DOI] [PubMed] [Google Scholar]
- 52.Rudin CM, et al. Phase I study of G3139, a bcl-2 antisense oligonucleotide, combined with carboplatin and etoposide in patients with small-cell lung cancer. J Clin Oncol. 2004;22:1110–1117. doi: 10.1200/JCO.2004.10.148. [DOI] [PubMed] [Google Scholar]
- 53.Rudin CM, et al. Phase I study of G3139, a Bcl-2 antisense oligonucleotide, combined with carboplatin and etoposide in patients with previously untreated extensive stage small cell lung cancer. Eur J Cancer. 2002;38:S7, 147. doi: 10.1200/JCO.2004.10.148. [DOI] [PubMed] [Google Scholar]
- 54.Rudin CM, et al. CALGB 30103: a randomized phase II study of carboplatin and etoposide with or without G3139 in patients with extensive stage small cell lung cancer. J Clin Oncol. 2005;22:662s. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walensky LD, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 57.Sidransky D, Hollstein M. Clinical implications of the p53 gene. Annu Rev Med. 1996;47:285–301. doi: 10.1146/annurev.med.47.1.285. [DOI] [PubMed] [Google Scholar]
- 58.Wistuba II, et al. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28 (2, Suppl 4):3–13. [PubMed] [Google Scholar]
- 59.Fujiwara T, et al. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Res. 1994;54:2287–2291. [PubMed] [Google Scholar]
- 60.Wills KN, et al. Development and characterization of recombinant adenoviruses encoding human p53 for gene therapy of cancer. Hum Gene Ther. 1994;5:1079–1088. doi: 10.1089/hum.1994.5.9-1079. [DOI] [PubMed] [Google Scholar]
- 61.Ishida T, et al. Dendritic cells transduced with wild-type p53 gene elicit potent anti-tumour immune responses. Clin Exp Immunol. 1999;117:244–251. doi: 10.1046/j.1365-2249.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikitina EY, et al. An effective immunization and cancer treatment with activated dendritic cells transduced with full-length wild-type p53. Gene Ther. 2002;9:345–352. doi: 10.1038/sj.gt.3301670. [DOI] [PubMed] [Google Scholar]
- 63.Antonia SJ, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 64.Balsara BR, Testa JR. Chromosomal imbalances in human lung cancer. Oncogene. 2002;21:6877–6883. doi: 10.1038/sj.onc.1205836. [DOI] [PubMed] [Google Scholar]
- 65.Hanada M, et al. A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II–DNA complex. Jpn J Cancer Res. 1998;89:1229–1238. doi: 10.1111/j.1349-7006.1998.tb00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yana T, et al. Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJTOG) study. Invest New Drugs. 2006 doi: 10.1007/s10637-006-9012-9. [DOI] [PubMed] [Google Scholar]
- 67.Ohe Y, et al. Phase I–II study of amrubicin and cisplatin in previously untreated patients with extensive-stage small-cell lung cancer. Ann Oncol. 2005;16:430–436. doi: 10.1093/annonc/mdi081. [DOI] [PubMed] [Google Scholar]
- 68.Seto T, et al. Phase II study of amrubicin, a new active drug in refractory or relapsed small-cell lung cancer (SCLC): Thoracic Oncology Research Group Trial 0301. J Clin Oncol 2006 ASCO Annual Meeting Proceedings Part I. 2006;24 (18S):7060. doi: 10.1200/JCO.2006.08.4145. [DOI] [PubMed] [Google Scholar]
- 69.Kato T, et al. Phase II trial of amrubicin in patients with previously treated small cell lung cancer (SCLC) J Clin Oncol 2006 ASCO Annual Meeting Proceedings Part I. 2006;24 (18S):7061. [Google Scholar]
- 70.von Pawel J, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 71.Armanios M, Greider CW. Telomerase and cancer stem cells. Cold Spring Harb Symp Quant Biol. 2005;70:205–208. doi: 10.1101/sqb.2005.70.030. [DOI] [PubMed] [Google Scholar]
- 72.Harley CB, et al. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 73.Brunsvig PF, et al. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55:1553–1564. doi: 10.1007/s00262-006-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cagle PT, et al. Differential retinoblastoma protein expression in neuroendocrine tumors of the lung. Potential diagnostic implications. Am J Pathol. 1997;150:393–400. [PMC free article] [PubMed] [Google Scholar]
- 75.Meuwissen R, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 76.Richardson PG, et al. Proteasome inhibition in the treatment of cancer. Cell Cycle. 2005;4:290–296. [PubMed] [Google Scholar]
- 77.Ling YH, et al. Mechanisms of proteasome inhibitor PS-341-induced G(2)–M-phase arrest and apoptosis in human non-small cell lung cancer cell lines. Clin Cancer Res. 2003;9:1145–1154. [PubMed] [Google Scholar]
- 78.Mortenson MM, et al. Reduction in BCL-2 levels by 26S proteasome inhibition with bortezomib is associated with induction of apoptosis in small cell lung cancer. Lung Cancer. 2005;49:163–170. doi: 10.1016/j.lungcan.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 79.Mitsiades CS, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107:1092–1100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maulik G, et al. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 2002;8:620–627. [PubMed] [Google Scholar]
- 81.Cullinan SB, Whitesell L. Heat shock protein 90: a unique chemotherapeutic target. Semin Oncol. 2006;33:457–465. doi: 10.1053/j.seminoncol.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 82.Christofori G. Changing neighbours, changing behaviour: cell adhesion molecule-mediated signalling during tumour progression. EMBO J. 2003;22:2318–2323. doi: 10.1093/emboj/cdg228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lanier LL, et al. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233–2238. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doria MI, Jr, et al. Immunophenotype of small cell lung carcinoma. Expression of NKH-1 and transferrin receptor and absence of most myeloid antigens. Cancer. 1988;62:1939–1945. doi: 10.1002/1097-0142(19881101)62:9<1939::aid-cncr2820620912>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 85.Fossella F, et al. Phase II trial of BB-10901 (huN901-DM1) given weekly for four consecutive weeks every 6 weeks in patients with relapsed SCLC and CD56-positive small cell carcinoma. J Clin Oncol 2005 ASCO Annual Meeting Proceedings. 2005;23 (16S):7159. [Google Scholar]
- 86.Brezicka T, et al. Reactivity of monoclonal antibodies with ganglioside antigens in human small cell lung cancer tissues. Lung Cancer. 2000;28:29–36. doi: 10.1016/s0169-5002(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 87.Fuentes R, et al. Ganglioside expression in lung cancer cell lines. Lung Cancer. 1997;18:21–33. doi: 10.1016/s0169-5002(97)00049-4. [DOI] [PubMed] [Google Scholar]
- 88.Grant SC, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guerin. Clin Cancer Res. 1999;5:1319–1323. [PubMed] [Google Scholar]
- 89.Giaccone G, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study) J Clin Oncol. 2005;23:6854–6864. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 90.Lee J, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]