Abstract
Carbon dioxide (CO2) is a key molecule in many biological processes; however, mechanisms by which organisms sense and respond to high CO2 levels remain largely unknown. Here we report that acute CO2 exposure leads to a rapid cessation in the contraction of the pharynx muscles in Caenorhabditis elegans. To uncover the molecular mechanisms underlying this response, we performed a forward genetic screen and found that hid-1, a key component in neuropeptide signaling, regulates this inhibition in muscle contraction. Surprisingly, we found that this hid-1-mediated pathway is independent of any previously known pathways controlling CO2 avoidance and oxygen sensing. In addition, animals with mutations in unc-31 and egl-21 (neuropeptide secretion and maturation components) show impaired inhibition of muscle contraction following acute exposure to high CO2 levels, in further support of our findings. Interestingly, the observed response in the pharynx muscle requires the BAG neurons, which also mediate CO2 avoidance. This novel hid-1-mediated pathway sheds new light on the physiological effects of high CO2 levels on animals at the organism-wide level.
Author Summary
Carbon dioxide (CO2) is a key molecule in many biological processes. High levels of CO2 in patients with pulmonary diseases are associated with worse outcomes. However, mechanisms by which organisms sense and respond to high CO2 levels remain largely unknown. Using Caenorhabditis elegans as a model system, we found that exposure to high CO2 levels leads to a very rapid cessation in the contraction of the pharynx muscles. Further analysis revealed that the pharynx muscle response is controlled by dense core vesicle secretion from the BAG neurons in a hid-1-mediated pathway. This novel hid-1 pathway sheds new light on the physiological effects of high CO2 levels on animals at the organism-wide level.
Introduction
One of the fundamental features shared by most, if not all, living organisms is the ability to maintain levels of carbon dioxide (CO2). Of particular importance is the ability of many animals to sense and respond to high levels of CO2 by either attraction or aversion [1]–[5]. In mammals, high levels of CO2 (hypercapnia) impair alveolar epithelial function of the lungs by activating the stress sensor AMPK, which leads to Na,K-ATPase endocytosis, impaired cell proliferation, and loss of distal lung epithelial function [6]–[10]. In addition, hypercapnia suppresses specific innate immune responses in Drosophila and mice, which increases mortality in a model of pneumonia and leads to changes in gene expression through the NF-kB pathway [11]–[14]. Cyclic AMP (cAMP) signaling also plays a role in the response of mammalian cells to elevated CO2 levels [15]–[17]. The molecular pathways mediating the responses to hypercapnia are the focus of intensive research (see [11], [18] and review in [19]).
High levels of CO2 quickly elicit an avoidance response in wild-type Caenorhabditis elegans animals via a cGMP signaling pathway [2], [4]. The cGMP-regulated avoidance response requires the CO2- and oxygen (O2)–sensing BAG neurons, in which the guanylyl cyclase receptor, gcy-9, controls the response to CO2 [20]–[22]. Interestingly, the response to hypercapnia requires the ETS-domain transcription factor, ETS-5, which controls the expression of gcy-9 in the BAG neurons and plays a role in BAG neuron differentiation [20]–[22]. Recently, the thermosensory AFD neurons and the salt-sensing ASE neurons were also shown to participate in CO2 sensing and avoidance [23]. These neurons, however, differ in their response kinetics to high levels of CO2; whereas BAG neurons reach maximal activation within 30 s, ASE neurons reach maximal activation only after 2 min, and AFD neurons show intricate dynamics in which Ca2+ levels first drop and then increase to maximal levels after 2 min [23]. Interestingly, starved C. elegans do not avoid high CO2 levels, nor do animals with defects in the daf-2 signaling pathway, which is an important regulator of the starvation response [2], [4].
In addition to avoidance, C. elegans exposed to high CO2 levels show specific phenotypes independent of pH [24]. These include a smaller brood size, delayed development, reduced motility coupled with deterioration of striated muscle, and a significant increase in lifespan that is independent of known life-extending pathways [24].
Here we report that exposure of C. elegans animals to short (10 s) hypercapnia-inducing levels of CO2 (≥5%) leads to a significant reduction in the rate of pharyngeal muscle contraction (pumping). Strikingly, this effect is independent of any currently known molecular pathways that regulate CO2 avoidance or O2 sensing. Specifically, through a forward genetic screen, we identified a novel participant in the response to CO2, hid-1, that plays a role in continued pumping in the presence of high CO2 levels. Moreover, we show that dense core vesicle secretion pathways in the BAG neurons contribute to the reduced pumping rate in response to high CO2 levels.
Results
High levels of CO2 significantly reduce the pumping rate of the pharynx
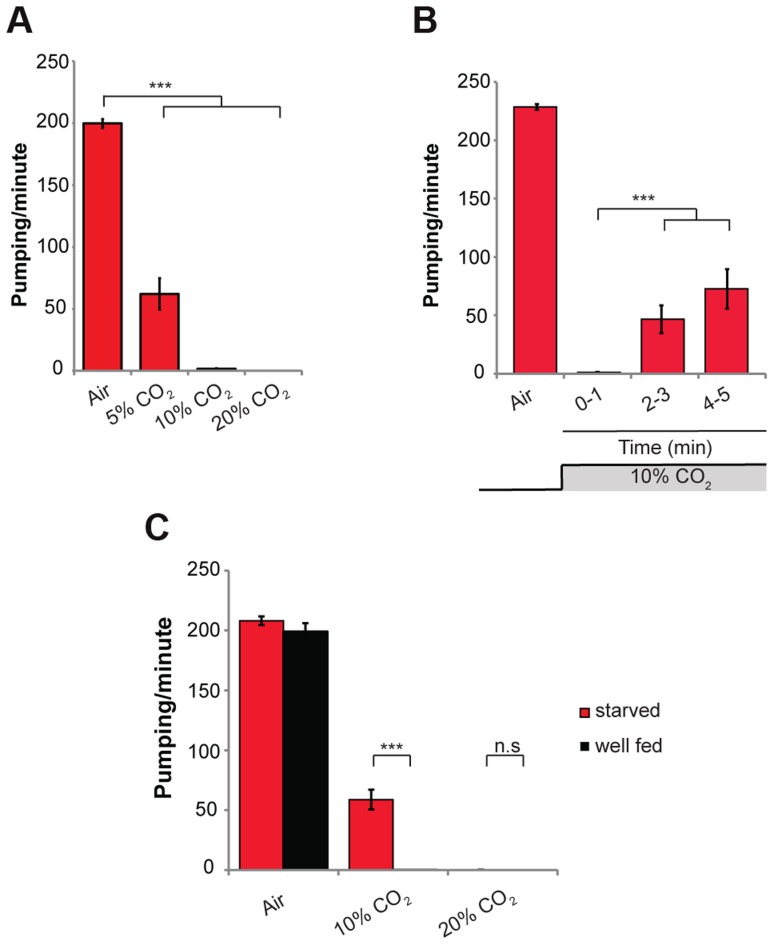
To investigate the effects of acute exposure of wild-type C. elegans (N2) to high levels of CO2, we exposed 1-day-old adult animals grown on standard NGM plates in a small chamber to gas mixtures containing 21% O2 and 5%, 10%, or 20% CO2 at 22°C. In normal air (0.0391% CO2), the rate of muscle contraction of the pharynx was ∼200 pumps/min. Within 10 s of exposure to 5% CO2 balanced with 21% O2 and 74% N2, the pumping rate of the pharynx was reduced from ∼200 to ∼60 pumps/min (Figure 1A). Exposure to 10% and 20% CO2 almost completely stopped the pumping of the pharynx (Figure 1A and Movie S1). After 2–3 min of continuous exposure to 10% CO2, pumping rate recovered partially to ∼40 pumps/min, and after 5 min of continuous exposure to 10% CO2 it recovered to ∼80 pumps/min (Figure 1B), suggesting a separate, existing mechanism that allows for a partial adaptation. Longer exposures of up to 30 min to 10% CO2 did not result in full recovery of the pumping rate (Figure S1A). To test whether the effect on the pumping is mediated by a change in the pH of the growth medium due to high CO2 levels, we measured the pumping rate of animals using NGM plates buffered to pH of 5.0 and 7.0 in addition to the normally used medium with a pH of 6.0. We did not find any differences between the animals in different growth mediums, both under normal air conditions and after exposure to 10% CO2, which suggests that the effect on the pumping is probably not mediated by changes in pH (Figure S1B). This conclusion is supported by a recent finding that activation of CO2-responsive neurons can occur independently of changes in extracellular or intracellular acidosis [25]. In addition, mutations in the carbonic anhydrase genes (cah-2, cah-5, and cah-6), which catalyze the conversion of CO2 into bicarbonate, had no effect on the pumping rate (Figure S1C). This suggests that the conversion of CO2 into bicarbonate is not necessary to induce the response of the pharynx. However, we cannot rule out the possibility of redundancy between the different carbonic anhydrase genes.
Figure 1. High levels of CO2 reduce the pumping rate of the pharynx.
(A) One-day-old wild-type (N2) adult C. elegans were exposed to 5%, 10%, or 20% CO2 balanced with 21% O2 and N2. The pumping rate was measured under a dissecting microscope while the animals were exposed to different gas mixtures. A gas mixture of 21% O2 and 79% N2 was used as a normal air control. (B) One-day-old wild-type (N2) adult C. elegans were continuously exposed to 10% CO2 for 5 min. The pumping rate was measured during minutes 1, 3, and 5 of exposure to CO2. (C) One-day-old wild-type (N2) adult C. elegans were starved for 4 h and then the pumping rate was measured in 10% or 20% CO2. Well-fed worms were used as a control. In all experiments N≥30 animals. Different groups were compared by one-way ANOVA followed by t test. ***P<.001. Error bars indicate SEM.
The response of the pharynx to high CO2 levels was partially dependent on the nutritional state of the animal. Whereas “well fed” animals exposed to 10% CO2 stopped pumping, animals starved for 4 h continued pumping at a rate of ∼60 pumps/min (Figure 1C). These starved animals exposed to 20% CO2 stopped pumping, similar to wild-type animals (Figure 1C), suggesting a threshold effect of high CO2 levels. Together, these data demonstrate that high CO2 levels quickly affect muscle contraction of the pharynx, an effect that depends on the nutritional state of the animal.
The reduction in pumping is independent of CO2 avoidance and O2 sensing
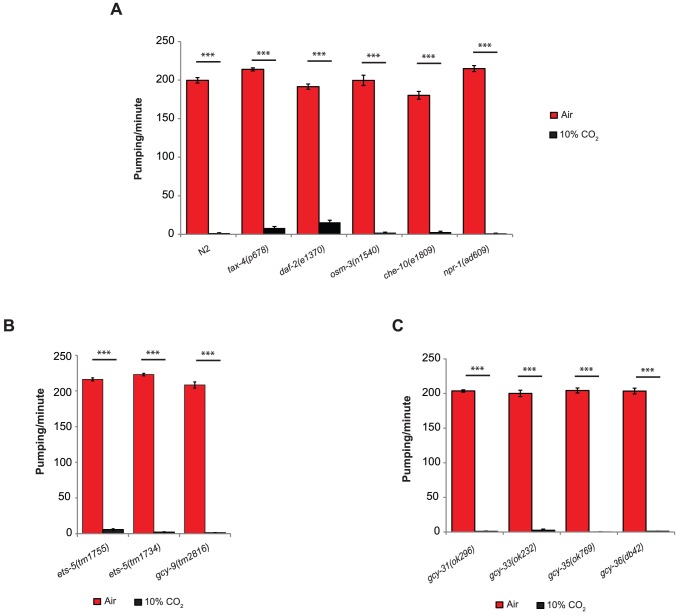
C. elegans animals quickly withdraw when acutely exposed to CO2. This response, known as CO2 avoidance, is regulated by cGMP signaling [2], [4]. TAX-2 and TAX-4 are two subunits of a cGMP-gated ion channel required for normal chemosensory and thermosensory responses. C. elegans null mutants for either TAX-2 (tax-2[p691]) or TAX-4 (tax-4[p678]) do not avoid high CO2. In addition, the insulin-IGF pathway mediates CO2 avoidance, as daf-2 mutants show reduced CO2 avoidance [2], [4]. The avoidance response also requires proper development of ciliated sensory neurons. Animals with mutations in osm-3 and che-10 have abnormal cilia as well as defective CO2 avoidance. In C. elegans strains carrying mutations in daf-2, osm-3, or che-10 the CO2 avoidance response is either reduced or absent [2], [4].
The effect of high CO2 levels on the C. elegans pharynx is quick and robust, similar to the avoidance response. However, rather than CO2 avoidance, tax-4(p678), daf-2(e1370), osm-3(n1540), and che-10(e1809) mutants show a significant reduction in pumping following exposure to 10% CO2, similar to the reduction observed in wild-type (N2) animals (Figure 2A). Loss-of-function mutation of the neuropeptide Y receptor, npr-1, completely abolishes the CO2 avoidance response by inhibiting the activity of the O2-sensing URX neurons [3]. In our assay, exposing npr-1(ad609) animals to 10% CO2 resulted in a response similar to that of wild-type animals (Figure 2A), suggesting that the high activity of the URX neurons in the animals with loss-of-function mutation in npr-1 does not regulate the pharynx response to CO2. The gcy-9 gene encodes a receptor-type guanylyl cyclase and is a target of the ETS domain ETS-5 transcription factor. Both the ets-5 and gcy-9 genes are required for the CO2 avoidance response [20]–[22]. When exposed to 10% CO2, the gcy-9(tm2816), ets-5(tm1734), and ets-5(tm1755) mutants stopped pumping, similar to wild-type animals (Figure 2B), thus further demonstrating that CO2 avoidance and acute CO2-dependent pumping inhibition are mediated through independent pathways.
Figure 2. The inhibition of pumping following exposure to high CO2 level is independent of molecular pathways that regulate CO2 avoidance and oxygen sensing.
One-day-old adult C. elegans strains containing mutations in genes that regulate CO2 avoidance (A, B) or O2 sensing (C) were exposed to 10% CO2. The pumping rate was measured under a dissecting microscope during the first minute of exposure to CO2. In all experiments N≥30 animals. Different groups were compared by one-way ANOVA followed by t test. ***P<.001. Error bars indicate SEM.
To determine whether molecular pathways shared by O2 sensing mediate the response of the pharynx to elevated CO2 levels, we tested strains with mutations in the gcy-31, gcy-33, gcy-35, or gcy-36 genes. These genes encode soluble guanylyl cyclases (sGC) that bind O2 and sense decreases (gcy-31 and gcy-33) or increases (gcy-35 and gcy-36) in O2 levels [26]. Exposing the C. elegans strains gcy-31(ok296), gcy-33(ok232), gcy-35(ok769), or gcy-36(db42) to 10% CO2 resulted in pumping inhibition similar to that observed in wild-type animals (Figure 2C). These results suggest that the effect of high CO2 levels on the pumping rate does not involve O2 sensing.
HID-1 is required for the CO2-dependent pumping response
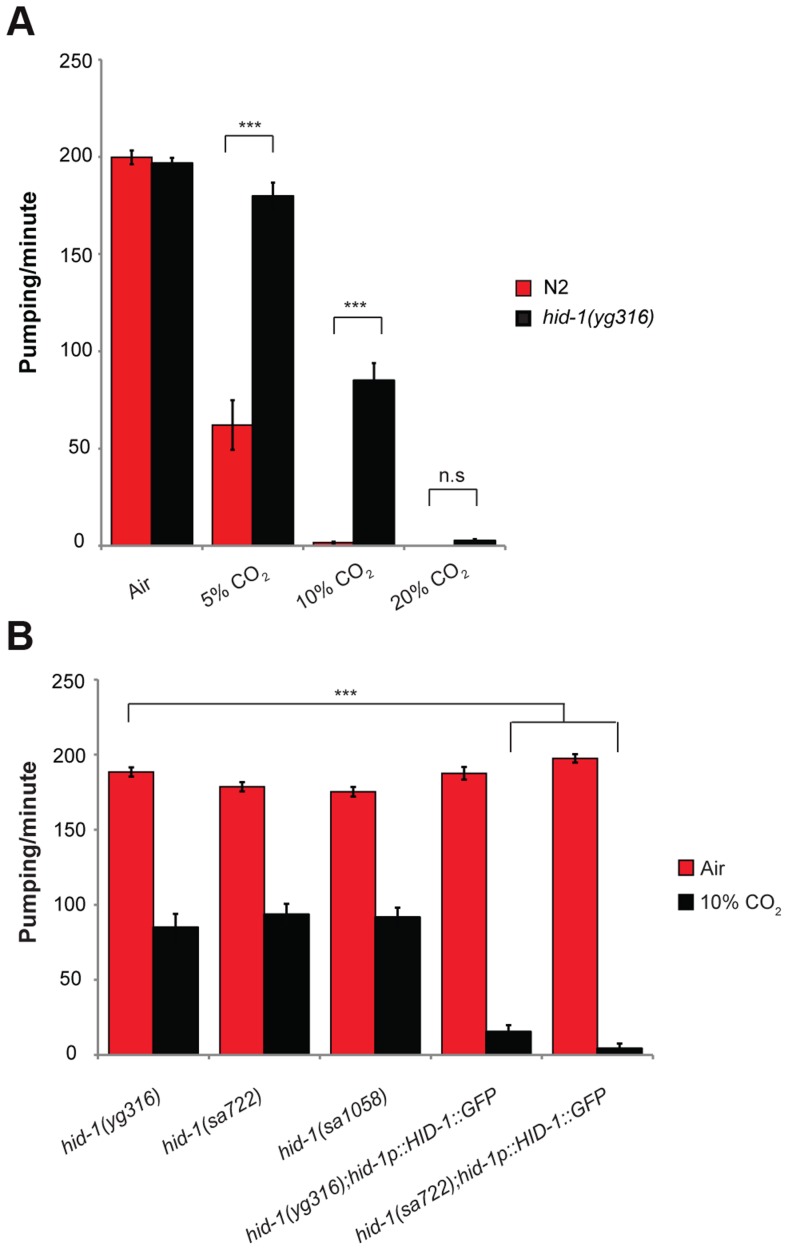
To identify genes that are involved in regulating the pharynx response to 10% CO2, we performed a forward genetic screen after ethyl methanesulfonate (EMS) mutagenesis. Specifically, we screened for mutant animals that do not stop pumping in response to 10% CO2 (Movie S2). We screened the progeny of ∼1200 F1 animals and found three strains that continued pumping when exposed to 10% CO2. One of these strains was further crossed to the Hawaiian strain, and deep sequencing was performed on DNA from recombinant F2 progeny. The region containing the mutant gene that enabled continuous pumping in 10% CO2 was identified by searching for a low number of Hawaiian single-nucleotide polymorphisms (SNPs), as described elsewhere [27]. This mutant strain has a premature stop codon in a previously characterized highly conserved gene, hid-1. In 5% CO2, unlike in wild-type animals, the pumping rate of animals with the isolated hid-1(yg316) allele was similar to the pumping rate in normal air conditions, and significant pumping continued after exposure to 10% CO2, whereas in 20% CO2 pumping was abolished (Figure 3A).
Figure 3. HID-1 is required for sensing CO2 level in the pharynx.
(A) One-day-old adult hid-1(yg316) and N2 worms were exposed to 5%, 10%, or 20% CO2 balanced with 21% O2 and N2. The pumping rate was measured under a dissecting microscope while the animals were exposed to the different gas mixtures. A gas mixture of 21% O2 and 79% N2 was used as a normal air control. (B) The inhibition of the pumping rate of the pharynx after exposure to high CO2 level in hid-1(yg316) allele mutants is significantly reduced. Similarly, the inhibition of the pumping rate of the pharynx after exposure to high CO2 level is reduced in other hid-1 allele mutants (sa772 and sa1058). Transgenic expression of HID-1 fused to eGFP in the sa722 or yg316 background (hid-1(sa722);HID-1::GFP or hid-1(yg316);HID-1::GFP) is sufficient to restore the effect of high CO2 level on the pumping rate back to the wild-type phenotype. In all experiments N≥30 animals. Different groups were compared by one-way ANOVA followed by t test. ***P<.001. Error bars indicate SEM.
The effect of HID-1 on the response to high levels of CO2 was specific to the pharynx, since hid-1 mutant animals still showed reduced egg laying (Figure S2) and a slower rate of development (data not shown), similar to wild-type animals exposed to high CO2 levels [24]. Two other alleles of hid-1, sa722 and sa1058 [28], also showed continuous pumping when exposed to 10% CO2 (Figure 3B). The change in pumping rate in response to CO2 is specific to HID-1, since transgenic expression of HID-1::GFP under its own promoter, in either hid-1(sa722) or hid-1(yg316) strains (Figure S3), was sufficient to restore the normal reduced pumping rate in 10% CO2 (Figure 3B). Together, these data suggest that hid-1 is required for the response of the pharynx to high levels of CO2.
Other dense core vesicle secretion and maturation mutants are also involved in the inhibition of pumping by CO2
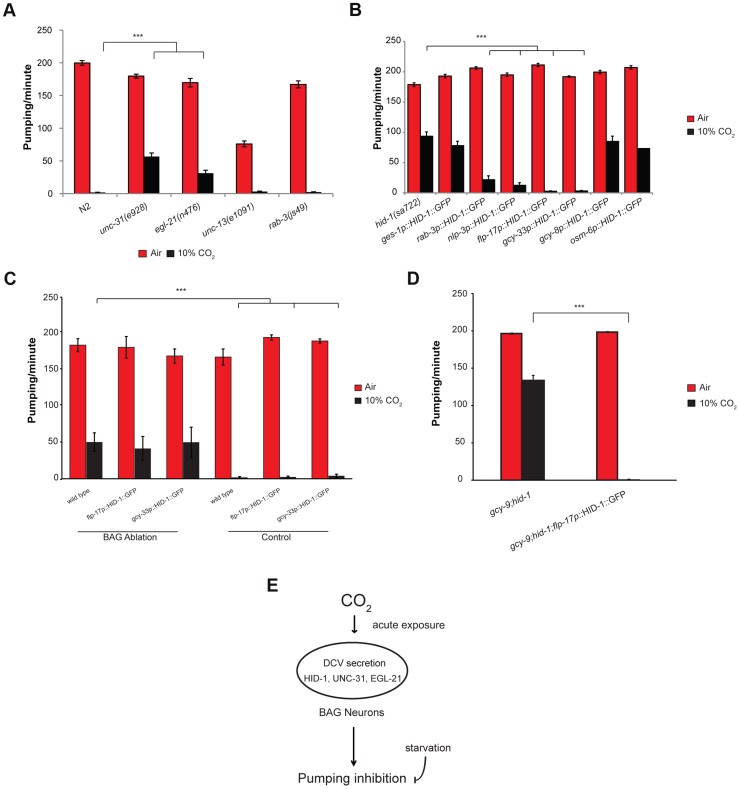
Dense core vesicles (DCVs) secrete neuropeptides in peptidergic neurons [29]. HID-1 is associated with Golgi membranes by way of N-terminal myristoylation and is required for the sorting of DCVs, where it prevents sorting of peptide cargoes to lysosomes for degradation [30]–[32]. We hypothesized that HID-1 plays a role in the response of the pharynx to high CO2 by regulating neuropeptide secretion. We tested this hypothesis by scoring pumping response to 10% CO2 in mutants defective in other genes involved in neuropeptide secretion. The gene unc-31 encodes the C. elegans ortholog of CAPS (calcium-dependent activator protein for secretion), an important component of DCV exocytosis [33]. The gene egl-21 encodes the C. elegans ortholog of carboxypeptidase E, an important component in neuropeptide maturation [34]. Following exposure of unc-31(e928) or egl-21(n476) deletion strains to 10% CO2, the pumping rate of the pharynx was significantly higher compared with that in wild-type animals exposed to the same concentration of CO2 (Figure 4A). We also tested the role of synaptic vesicle secretion on the pumping response to 10% CO2. The unc-13 gene is involved in synaptic vesicle secretion of neurotransmitters [35], [36]. The rab-3 gene is a Rab GTPase that affects the distribution of synaptic vesicle populations [37]. Exposure of unc-13(e1091) or rab-3(js49) mutant strains to 10% CO2 showed pumping behavior similar to that of wild-type strains (Figure 4A). These data suggest that DCVs play an important role in mediating the response of the pharynx to high CO2 levels and that compromising DCV secretion probably impairs the pumping response to high CO2 levels.
Figure 4. The effect of high CO2 level on the pharynx requires HID-1 activity in the BAG neurons.
(A) One-day-old adult C. elegans strains containing mutations in unc-31 or egl-21 genes, which are important for proper neuropeptide secretion and maturation, show a significant rescue of the pumping rate after exposure to 10% CO2. In contrast, strains with mutations in unc-13 or rab-3, which promote synaptic vesicle secretion, do not show a changed pharynx response to 10% CO2. (B) Transgenic expression of HID-1 in the gut using the gut-specific ges-1 promoter (ges-1p-HID-1::GFP) was not sufficient to restore pumping phenotype to wild type after exposure to 10% CO2. In contrast, transgenic expression of HID-1 in neurons using the rab-3 promoter (rab-3p-HID-1::GFP) was sufficient to restore pumping rate after exposure to 10% CO2 almost back to wild-type levels. Cell-specific expression of HID-1 in the AFD neurons (gcy-8p-HID-1::GFP) or in the amphid and tail ciliated neurons, including ASE neurons (osm-6p-HID-1::GFP), did not restore the CO2 effect on the pumping back to wild-type levels, whereas cell-specific expression of HID-1 in sensory and pharyngeal neurons (nlp-3p-HID-1::GFP) or in BAG neurons (flp-17p-HID-1::GFP and gcy-33p-HID-1::GFP) was sufficient to restore the effect of high CO2 level back to the wild-type phenotype. (C) The BAG neurons of wild-type C. elegans expressing gcy-33::GFP were laser ablated and the pharyngeal pumping rate was measured in normal air and 10% CO2. Similarly, the BAG neurons of flp-17p::HID-1::GFP and gcy-33p::HID-1::GFP strains were laser ablated and the pharyngeal pumping rate subsequently measured. Controls include measurement of the pumping rate in the same C. elegans strains without ablation. (D) Transgenic expression of HID-1 in the BAG neurons of hid-1(sa722);gcy-9(tm2816) animals restores the suppression of pumping in the presence of high CO2 level. (E) Schematic model of CO2 response of pharyngeal muscle contraction. The inhibition of muscle contraction in the pharynx is mediated by neuropeptide secretion via dense core vesicles (DCVs) in BAG neurons. The CO2 response is decreased after starvation. In all experiments N≥30 animals, except in panel C in flp-17p::HID-1::GFP (N = 5) and gcy-33p::HID-1::GFP (N = 10). Different groups were compared by one-way ANOVA followed by t test. ***P<.001. Error bars indicate SEM.
Expression of HID-1 in the BAG neurons is sufficient to restore wild-type CO2 response in hid-1 mutant strains
HID-1 is expressed in all neuron and gut cells of C. elegans [30]. To test whether inhibition of pharynx pumping in response to 10% CO2 requires expression of hid-1 in the gut, neurons, or both, we used transgenic lines that express HID-1 fused to GFP driven by either the pan-neuronal promoter rab-3 or the gut-specific promoter ges-1. Expression of HID-1 under the rab-3 promoter in neurons of hid-1(sa722) background was sufficient to restore inhibition of the pharynx pumping almost to the levels shown by wild-type animals (Figure 4B). In contrast, expression of HID-1 under the ges-1 promoter in the gut of hid-1(sa722) had no significant effect on the response of the pharynx to 10% CO2.
We next asked which subset of neurons is required for mediating the effect of high CO2 levels on the pharynx. The nlp-3 gene is expressed in sensory neurons (ADF, ASE, ASH, AWB, ASJ, and BAG) as well as in pharyngeal neurons (I1, I2, I3, I4, M1, M3, and NSMR) (Figure S3) [38]. Transgenic expression of HID-1::GFP under the nlp-3 promoter in hid-1(sa722) background was sufficient to restore pharynx pumping inhibition after exposure to 10% CO2 (Figure 4B). High levels of CO2 activate the AFD neurons [23]. Surprisingly, transgenic expression of HID-1::GFP driven by a gcy-8 promoter in the thermosensory AFD of hid-1(sa722) background did not restore the CO2-mediated pumping inhibition (Figure 4B), which suggests that the activation of the AFD neurons by high CO2 levels is not sufficient to induce the peptidergic signaling that mediates the effect of high CO2 levels on the pharynx. Among the sensory neurons expressing nlp-3 are the BAG and the ASE neurons, which are also known to respond to high CO2 levels [23]. Transgenic expression of HID-1::GFP driven by the osm-6 promoter, which was expressed in ASE neurons (undetected in BAG neurons), did not restore the CO2-mediated pumping inhibition (Figure 4B). We next tested the role of BAG neurons in the CO2-dependent pumping inhibition of the pharynx. Transgenic lines expressing HID-1::GFP under the promoter of flp-17 showed expression in the BAG neurons (Figure S3). This expression was sufficient to fully restore the CO2-dependent pumping inhibition (Figure 4B). Similarly, the expression of HID-1::GFP under the gcy-33 promoter in hid-1(sa722) background was specific to the BAG neurons (Figure S3) [21]. This expression was sufficient to fully restore the CO2-dependent pumping inhibition (Figure 4B). Next, we ablated the BAG neurons in transgenic worms expressing HID-1::GFP under flp-17 and gcy-33 promoters in hid-1(sa722) background. We found that following the removal of the HID-1::GFP-expressing BAG neurons, the pumping in 10% CO2 was similar to that of hid-1(sa722) animals (Figure 4C). These results suggest that the specific expression of HID-1 in the BAG neurons is sufficient to induce the CO2-dependent pumping inhibition. We also ablated the BAG neurons in wild-type background using GFP driven by gcy-33 promoter as a marker. We found that following ablation of the BAG neurons, the pumping in response to 10% CO2 was similar to that in HID-1-null animals (Figure 4C). These results suggest that the BAG neurons are required for the pumping inhibition. In addition, to test possible cross talk between the neuropeptide secretion pathway and the guanylyl cyclase receptor pathway, which is required for CO2 avoidance, we measured the pharyngeal pumping rate of animals carrying both hid-1(sa722) and gcy-9(tm2816) mutations. The pumping rate was similar to that of hid-1(sa722) animals (Figure 4D). Moreover, in the same genetic background, transgenic expression of HID-1 in the BAG neurons, using the flp-17 promoter, restored the suppression of pumping in the presence of high CO2 level (Figure 4D), further demonstrating that the response to CO2 mediated by hid-1 is independent of the response to CO2 mediated by gcy-9. We conclude that proper hid-1 activity in the BAG neurons is important to mediate the pumping inhibition by CO2.
Discussion
In humans, high CO2 levels have diverse effects on the lung epithelium, immunity, and muscle function. However, the effects of acute exposure of muscle cells to high CO2 levels were unknown. In addition, recent studies suggest that mammals, like C. elegans, are able to sense elevated CO2 levels, which is of broad physiologic significance.
CO2 avoidance and CO2-dependent reduced pharyngeal pumping are probably regulated via different pathways
Acute exposure of well-fed adult C. elegans animals to high CO2 levels quickly reduces the pumping rate of the pharynx. This effect depends in part on the nutritional status of the animal, since starved animals exposed to 10% CO2 in air continue to pump, albeit at a significantly slower rate. Our genetic data suggest that the effect of acute exposure to high CO2 levels on the pumping rate is independent of the avoidance responses of C. elegans to high CO2 levels. First, cGMP signaling is required for mediating the avoidance response, as mutations in the cGMP gated ion channel encoded by tax-2 and tax-4 completely disrupt the avoidance response [2], [4]. In contrast, the same mutation in tax-4 does not completely rescue the immediate response of the pumping rate to high CO2 levels. Second, mutation in the insulin-like receptor encoded by daf-2 also disrupts the avoidance response [2], [4]. The pumping rate of daf-2 mutants under exposure to 10% CO2 is dramatically reduced, like in the wild-type animals. The limited recovery of the pumping rate in daf-2 mutants at 10% CO2 could be due to the effect of daf-2 on starvation regulating pathways [39], [40]. Third, interference with proper function of ciliated sensory neurons by mutations in osm-3 and che-10 also significantly changes the avoidance response. Again, in the pumping assay, similar mutations in these genes did not change the response of C. elegans to high CO2 levels. In addition, mutations in ets-5 and gcy-9, which were previously shown to be required for the calcium response of the BAG neurons to CO2, did not change the response of the pharynx to high CO2 levels. Finally, the rescue of pumping by hid-1 in the BAG neurons was not affected by gcy-9 mutations.
C. elegans animals presumably interpret high CO2 levels as a harmful cue that leads to avoidance and pumping inhibition. The ability of the animal to stop eating for several minutes probably allows it to avoid undesirable food. Surprisingly, although both the avoidance and the pumping responses to the same stressful cue are immediate, our genetic data suggest that different molecular pathways mediate the two responses to high CO2.
The potential role of neuropeptides in the response of the pharynx to high levels of CO2
Our genetic screen identified hid-1 as a regulator of the pumping response to high CO2 levels, as mutations in the hid-1 gene blunted the response of the pharynx to high CO2 levels. HID-1 is required for the neuropeptide secretion pathway [30], [32]. Indeed, mutations in other known genes in peptidergic signaling, unc-31 and egl-21, could also partially suppress the pharyngeal pumping suppression upon exposure to 10% CO2. Neuropeptides are important signaling molecules in many physiological responses both in C. elegans and in other organisms. In C. elegans there are more than 250 neuropeptides that play a role in feeding and metabolism, and most neurons in C. elegans secrete neuropeptides [41]. Neuropeptides are also secreted from the intestine [38], and hid-1, an important peptidergic signaling gene, is expressed both in the nervous system and in the intestine [30], [32]. Neuropeptide signaling was previously shown to regulate pumping inhibition in the absence of food [42]. Specifically, unc-31 mutants demonstrate continuous pumping in the absence of food, unlike wild-type animals [42]. Since hid-1 also partially suppresses the inhibition of pumping in the absence of food (data not shown), we cannot completely rule out the possibility that hid-1 generally inhibits pumping and acts in parallel with CO2.
The pharynx response to 10% CO2 is probably mediated by several different neuropeptides, since pumping inhibition could not be inhibited by deletion of individual neuropeptide genes known to be overexpressed in the BAG neurons, including flp-10, flp-16, flp-27, nlp-1, flp-17, and nlp-14 (Figure S4). Neuropeptide secretion can only partially explain the response of the pharynx to high levels of CO2, since none of the peptidergic signaling mutants we examined at 10% CO2 (hid-1, unc-31, and egl-21) exhibited the pumping rate seen at normal air levels (Figure 4). Thus we cannot completely rule out the possibility that the effects of unc-31 and egl-21 are due to the other pathway(s) that must be acting in parallel with hid-1. This implies the existence of other, HID-1-independent mechanisms that must regulate the response of the pharynx to CO2 levels. For example, it is possible that high CO2 levels trigger other presynaptic inputs that mediate the effect on the pharynx in parallel with the peptidergic signaling, or that CO2 has also a direct postsynaptic effect on the pharyngeal muscles that inhibits their normal function. Interestingly, such parallel pathways depend on the CO2 levels, since hid-1 completely rescues the pumping inhibition at 5% CO2 and fails to rescue the pumping at 20% CO2 (Figure 3A).
The presence of HID-1 is specifically required in the BAG neurons
Using transgenic lines that express HID-1 either in the gut or in the nervous system we have determined that the hid-1 activity is specifically required in neurons to mediate the effect of high CO2 levels on the pharynx. We used an AFD-specific promoter to show that hid-1 activity in the AFD neurons, which are activated by high CO2 levels [23], is not sufficient to mediate the effect of high CO2 levels on the pharynx. In contrast, transgenic expression of HID-1::GFP under the nlp-3 promoter is sufficient to restore CO2-mediated pharynx pumping inhibition. Using the BAG-specific promoters flp-17 and gcy-33 and performing ablation experiments on the BAG neurons, we have further narrowed the CO2 effect on the pharynx to the BAG neurons. Paradoxically, our genetic data (Figures 2 and 4) suggest the existence of different molecular pathways for the avoidance and the pharynx responses. However, the BAG neurons in the pharynx response are the same neurons that control the avoidance response. Interestingly, mutant animals that block CO2-mediated calcium response in the BAG neurons still show normal pumping inhibition. The existence of such a pathway is especially surprising given that DCV secretion is expected to depend on an increase in calcium levels.
The physiological and molecular effects of high CO2 levels, in both vertebrates and invertebrates, have been the focus of several recent studies [2], [4], [6], [7], [12], [14], [22], [24], [43]. However, the sensing mechanism of cells to high CO2 levels is yet largely unknown. Soluble adenylyl cyclases are bicarbonate sensors in several organisms including mammals [15], [44], [45]. In C. elegans, which do not have soluble adenylyl cyclases, the soluble guanylyl cyclases GCY-31 and GCY-33 are important for eliciting CO2 avoidance in the BAG neurons [23]. However, it is yet unknown whether the gcy genes are directly activated by either CO2 or HCO3 − . Our results show that neither GCY-31 nor GCY-33 are required for mediating the effect of high CO2 levels on the pharynx (Figure 2C).
Our study sheds new light on the response of C. elegans to high CO2 levels. It also shows that the CO2-induced response is differentially regulated across different tissues. Furthermore, different levels of CO2 lead to various outcomes in the same tissue. Deciphering the mechanisms underlying these fundamental pathways will hopefully help us to better understand the CO2-induced responses that are activated in human diseases.
Materials and Methods
Strains
Worms were handled as described elsewhere [46]. The following strains were used in this study: N2 (wild type); CF1041, daf-2(e1370); CB3329, che-10(e1809); CX2948, tax-4(p678); PR802, osm-3(n1540); DA609, npr-1(ad609); CZ3714, gcy-31(ok296); CZ3715, gcy-33(ok232); CX6448, gcy-35(ok769); AX1296, gcy-36(db42); JT722, hid-1(sa722); JT1058, hid-1(sa1058); YG316, hid-1(yg316); YG2310, hid-1(yg316); jsEx896 [hid-1p::HID-1::GFP]; NM3017, hid-1(sa722) and lin-15(n765); jsEx896[hid-1p::HID-1::GFP]; NM3053, hid-1(sa722) and lin-15(n765); jsEx897[rab-3p::HID-1::GFP]; NM3139, hid-1(sa722) and lin-15(n765); jsEx909[ges-1p::HID-1::GFP]; YG2313, hid-1(sa722); ygEx317 [gcy-8p::HID-1::GFP]; YG2318, hid-1(sa722); ygEx318 [nlp-3p::HID-1::GFP]; YG2319, hid-1(sa722); ygEx319 [flp-17-p::HID-1::GFP]; YG2340, hid-1(sa722); ygEx320 [gcy-33-p::HID-1::GFP]; DA509, unc-31(e928); KP2018, egl-21(n476); YG2302, unc-13(e1091); YG2320, ets-5(tm1734); YG2321, ets-5 (tm1755); YG2322, gcy-9(tm2816); YG2323, gcy-9(tm2816) and hid-1(sa722); YG2324, gcy-9(tm2816) and hid-1(sa722); ygEx321 [flp-17-p::HID-1::GFP]; RB1340, nlp-1(ok1469); RB2575, flp-19(ok3587); VC2012, flp-27(gk3331); VC1108, nlp-14(ok1517)/szT1 X; RB1989, flp-10(ok2624); RB2275, flp-16(ok3085). All strains were obtained from the C. elegans Genome Center (CGC) or the National BioResourse Project (NBRP), except for CX2948, which was kindly provided by the De-Bono laboratory, and NM3017, NM3053, and NM3139, which were kindly provided by the Nonet laboratory [2], [30].
Measurement of pumping rate
A standard NGM plate covered with a lid-shaped chamber with inlet and outlet holes to allow gas flow was used to measure the pumping rate under different concentrations of CO2 in air. The chamber was connected to a mechanical valve that controlled the humidified gas mixture entering the chamber. For all pumping assays, NGM plates were seeded with 20 µL of OP50 5 h before the experiment to allow normal feeding and to keep worms in a restricted area. A single 1-day-old adult worm was seeded on a plate just before the start of the experiment. Initially, normal air mixture (21% O2, 79% N2) flowed into the chamber and worms were allowed to adjust for 1 min. The number of pharynx muscle contractions was subsequently measured for 1 min under normal air conditions. Then the airflow was switched to a high-CO2 gas mixture, and after 10 s the pharynx muscle contraction rate was measured again. To measure pumping rate after starvation, well-fed wild-type 1-day-old adult worms were collected using M9 buffer and washed four or five times in M9 buffer. Worms were then seeded on either NGM plates with no bacteria or NGM plates seeded with OP50, for 4 h prior to measurements. All pumping assays were performed at 22°C.
EMS screen and SNP mapping
The EMS mutagenesis was performed essentially as described elsewhere [46]. Briefly, wild-type (N2) worms in the L4 stage were exposed to 50 mM EMS in M9 buffer for 4 h and then transferred to fresh plates for 2–3 h (P0) for recovery. After recovery, five P0 animals were transferred again to an NGM plate and allowed to lay F1 progeny. Adult F1 animals were cloned onto individual NGM plates and their L4-adult F2 progeny where exposed to 10% CO2. F2 worms that continued the pumping of the pharynx even after exposure to 10% CO2 were isolated. In total, we scored the progeny of ∼1200 F1 animals. The isolated strains were outcrossed three times. The mutation was mapped as described elsewhere [27]. Mutant worms were crossed with the Hawaiian strain and F1 progeny were isolated. Then 44 F2 recombinants that continued the pumping after exposure to 10% CO2 were isolated, and the DNA of their F3 and F4 offspring was extracted using a Gentra Puregene kit (Qiagen, cat. no. 158667). Whole genome sequencing was performed using the Applied Biosystems SOLiD 3 deep sequencing apparatus. The positions of the Hawaiian SNPs were mapped on the DNA of the yg316 strain. A 1.2-MB region in chromosome X that did not contain any Hawaiian SNP was found. Within this region a premature stop codon (W625X) in the coding sequence of hid-1 was found to cause the phenotype as described in the text.
Plasmid constructs, transgenes, and laser ablation
The NM1699 construct, which contains the hid-1 promoter driving the genomic hid-1 coding region fused to eGFP, was a kind gift from the Nonet laboratory [30]. The NM1699 construct was digested with KpnI and AatII to replace the native hid-1 promoter with various neuron-specific promoters. To drive AFD-specific expression an 800-bp fragment upstream of the gcy-8 start codon was amplified and subsequently digested with KpnI-AatII to generate pKS10. Similarly, to drive sensory and pharyngeal specific expression, a 700-bp fragment upstream of the nlp-3 start codon was amplified and digested with KpnI-AatII to generate pKS20. To drive BAG-specific expression a 3.4-kb fragment upstream of the flp-17 start codon was amplified and fused by PCR to HID-1::GFP from NM1699. In addition, to drive BAG-specific expression a 980-bp fragment upstream of the gcy-33 start codon was amplified and subsequently digested with KpnI-AatII to generate pKS30. All plasmids were verified by sequencing and microinjected to either JT722 or YG316 with an elt-2::GFP marker as described elsewhere [47].
Laser ablation was performed using an Andor Revolution XD confocal spinning disk system with a Nikon TiE inverted microscope equipped with a nitrogen pulsed laser and a 365-nm Micropoint dye cell. The microscope and laser were controlled by means of IQ software and the Micropoint Mosaic I System 85-75, respectively. The region of interest was set according to the size of the neuron cell body as revealed by the GFP marker. We used a frequency of 15 Hz, an energy range of 80%–90%, and 3–5 repeats in order to completely ablate the GFP marker in the neuron cell body.
Supporting Information
Pumping inhibition is not rescued by either 30 min of exposure to 10% CO2, pH of 5.0 or 7.0, or mutations in the carbonic anhydrase genes. (A) One-day-old wild-type (N2) adult C. elegans were continuously exposed to 10% CO2 for 30 min and pumping rate was measured at different time points. (B) One-day-old wild-type (N2) adult C. elegans were transferred to NGM plates buffered at pH of 5.0, 6.0, or 7.0 followed by exposure to 10% CO2 and measurements of the pharyngeal pumping. (C) One-day-old adult worms with mutations in cah-2, cah-5, or cah-6 genes exposed to 10% CO2 showed pharyngeal pumping rate similar to that of wild-type animals.
(PDF)
The egg-laying rate of hid-1(yg316) animals exposed to 10% CO2 is similar to that of wild-type animals. Gravid animals were exposed to either normal air conditions or air containing 19% or 10% CO2 for 6 h. The number of embryos laid during this period was measured.
(PDF)
Transgenic expression of HID-1::GFP. HID-1 fused to eGFP was expressed under its own promoter in the background of yg316 or under gcy-8, nlp-3, osm-6, flp-17, or gcy-33 promoters in the background of sa722. Arrows indicate the AFD neurons (gcy-8p) and BAG neurons (flp-17p and gcy-33p).The expression of hid-1p, nlp-3p and osm-6p was detected in several neurons. Scale bar, 10 µm.
(PDF)
Animals with deletions in neuropeptide genes expressed in the BAG neurons still show strong CO2-mediated pumping inhibition. One-day-old animals with mutations in neuropeptide genes, which are known to be overexpressed in the BAG neurons, were exposed to 10% CO2 and pumping rate was measured. The pumping rate of nlp-1, nlp-14, and flp-16 mutants in 10% CO2 (but not in normal air conditions) was significantly different from that of the wild-type (N2) animals and showed small but significant rescue. *P<.01. Error bars indicate SEM.
(PDF)
Pumping of wild-type C. elegans exposed to 10% CO2. Pumping rate of wild-type animal under dissecting microscope is presented. Worms are first exposed to normal air and then exposed to 10% CO2.
(AVI)
Pumping of hid-1(yg316) mutant exposed to 10% CO2. Pumping rate of hid-1(yg316) mutant animal under dissecting microscope is presented. Worms are first exposed to normal air and then exposed to 10% CO2.
(AVI)
Acknowledgments
We thank Tamar Gattegno, Veronika Kravtsov, and Benjamin Podbilewicz for their help with the ablation experiments, Valery Zayat for helping to create the gcy-33-HID-1::GFP strains, Jennifer Davis for editing the manuscript, and the Nonet and DeBono laboratories for providing strains and constructs.
Funding Statement
This work was supported by the Arian Solis Ostrosky and Sydney Dwyer Davis foundation to YG and the NIH RO1-HL85534 to JIS and YG. Funding was also received from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant N° 336803 to AZ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bowen MF (1991) The Sensory Physiology of Host-Seeking Behavior in Mosquitoes. Annual Review of Entomology 36: 139–158. [DOI] [PubMed] [Google Scholar]
- 2. Bretscher AJ, Busch KE, de Bono M (2008) A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans . Proceedings of the National Academy of Sciences 105: 8044–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carrillo M, Guillermin M, Rengarajan S, Okubo R, Hallem E (2013) O2-Sensing Neurons Control CO2 Response in C. elegans. The Journal of neuroscience: the official journal of the Society for Neuroscience 33: 9675–9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hallem EA, Sternberg PW (2008) Acute carbon dioxide avoidance in Caenorhabditis elegans . Proceedings of the National Academy of Sciences 105: 8038–8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suh GSB, Wong AM, Hergarden AC, Wang JW, Simon AF, et al. (2004) A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431: 854–859. [DOI] [PubMed] [Google Scholar]
- 6. Briva A, Vadász I, Lecuona E, Welch LC, Chen J, et al. (2007) High CO2 Levels Impair Alveolar Epithelial Function Independently of pH. PLoS ONE 2: e1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vadász I, Dada LA, Briva A, Trejo HE, Welch LC, et al. (2008) AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. The Journal of Clinical Investigation 118: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadász I, et al. (2011) Elevated CO2 Levels Cause Mitochondrial Dysfunction and Impair Cell Proliferation. Journal of Biological Chemistry 286: 37067–37076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lecuona E, Sun H, Chen J, Trejo HE, Baker MA, et al. (2013) Protein kinase A-Ialpha regulates Na,K-ATPase endocytosis in alveolar epithelial cells exposed to high CO(2) concentrations. Am J Respir Cell Mol Biol 48: 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vadasz I, Dada LA, Briva A, Helenius IT, Sharabi K, et al. (2012) Evolutionary conserved role of c-Jun-N-terminal kinase in CO2-induced epithelial dysfunction. PLoS ONE 7: e46696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cummins EP, Oliver KM, Lenihan CR, Fitzpatrick SF, Bruning U, et al. (2010) NF-κB Links CO2 Sensing to Innate Immunity and Inflammation in Mammalian Cells. The Journal of Immunology 185: 4439–4445. [DOI] [PubMed] [Google Scholar]
- 12. Helenius IT, Krupinski T, Turnbull DW, Gruenbaum Y, Silverman N, et al. (2009) Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proceedings of the National Academy of Sciences 106: 18710–18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oliver KM, Lenihan CR, Bruning U, Cheong A, Laffey JG, et al. (2012) Hypercapnia Induces Cleavage and Nuclear Localization of RelB Protein, Giving Insight into CO2 Sensing and Signaling. Journal of Biological Chemistry 287: 14004–14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang N, Gates KL, Trejo H, Favoreto S, Schleimer RP, et al. (2010) Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. The FASEB Journal 24: 2178–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, et al. (2000) Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628. [DOI] [PubMed] [Google Scholar]
- 16. Cook ZC, Gray MA, Cann MJ (2012) Elevated Carbon Dioxide Blunts Mammalian cAMP Signaling Dependent on Inositol 1,4,5-Triphosphate Receptor-mediated Ca2+ Release. Journal of Biological Chemistry 287: 26291–26301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Townsend PD, Holliday PM, Fenyk S, Hess KC, Gray MA, et al. (2009) Stimulation of Mammalian G-protein-responsive Adenylyl Cyclases by Carbon Dioxide. Journal of Biological Chemistry 284: 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gates KL, Howell HA, Nair A, Vohwinkel CU, Welch LC, et al. (2013) Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am J Respir Cell Mol Biol 49: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharabi K, Lecuona E, Helenius IT, Beitel GJ, Sznajder JI, et al. (2009) Sensing, physiological effects and molecular response to elevated CO2 levels in eukaryotes. Journal of Cellular and Molecular Medicine 13: 4304–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandt JP, Aziz-Zaman S, Juozaityte V, Martinez-Velazquez LA, Petersen JG, et al. (2012) A Single Gene Target of an ETS-Family Transcription Factor Determines Neuronal CO2 Chemosensitivity. PLoS ONE 7: e34014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guillermin ML, Castelletto ML, Hallem EA (2011) Differentiation of Carbon Dioxide-Sensing Neurons in Caenorhabditis elegans Requires the ETS-5 Transcription Factor. Genetics 189: 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hallem EA, Spencer WC, McWhirter RD, Zeller G, Henz SR, et al. (2011) Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans . Proceedings of the National Academy of Sciences 108: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, et al. (2011) Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron 69: 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharabi K, Hurwitz A, Simon AJ, Beitel GJ, Morimoto RI, et al. (2009) Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans . Proceedings of the National Academy of Sciences 106: 4024–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith ES, Martinez-Velazquez L, Ringstad N (2013) A chemoreceptor that detects molecular carbon dioxide. J Biol Chem 288: 37071–37081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zimmer M, Gray JM, Pokala N, Chang AJ, Karow DS, et al. (2009) Neurons Detect Increases and Decreases in Oxygen Levels Using Distinct Guanylate Cyclases. Neuron 61: 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doitsidou M, Poole RJ, Sarin S, Bigelow H, Hobert O (2010) C. elegans Mutant Identification with a One-Step Whole-Genome-Sequencing and SNP Mapping Strategy. PLoS ONE 5: e15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ailion M, Thomas JH (2003) Isolation and Characterization of High-Temperature-Induced Dauer Formation Mutants in Caenorhabditis elegans . Genetics 165: 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burgoyne RD, Morgan A (2003) Secretory Granule Exocytosis. Physiological Reviews 83: 581–632. [DOI] [PubMed] [Google Scholar]
- 30. Mesa R, Luo S, Hoover CM, Miller K, Minniti A, et al. (2011) HID-1, a new component of the peptidergic signaling pathway. Genetics 187: 467–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L, Zhan Y, Song E, Yu Y, Jiu Y, et al. (2011) HID-1 is a peripheral membrane protein primarily associated with the medial- and trans- Golgi apparatus. Protein Cell 2: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu Y, Wang L, Jiu Y, Zhan Y, Liu L, et al. (2011) HID-1 is a novel player in the regulation of neuropeptide sorting. Biochem J 434: 383–390. [DOI] [PubMed] [Google Scholar]
- 33. Hammarlund M, Watanabe S, Schuske K, Jorgensen EM (2008) CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. The Journal of Cell Biology 180: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacob TC, Kaplan JM (2003) The EGL-21 Carboxypeptidase E Facilitates Acetylcholine Release at Caenorhabditis elegans Neuromuscular Junctions. The Journal of Neuroscience 23: 2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richmond JE, Davis WS, Jorgensen EM (1999) UNC-13 is required for synaptic vesicle fusion in C. elegans . Nat Neurosci 2: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Richmond JE, Weimer RM, Jorgensen EM (2001) An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature 412: 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gracheva EO, Hadwiger G, Nonet ML, Richmond JE (2008) Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci Lett 444: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nathoo AN, Moeller RA, Westlund BA, Hart AC (2001) Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proceedings of the National Academy of Sciences 98: 14000–14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henderson STBM, Johnson TE (2006) daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci 61: 444–460. [DOI] [PubMed] [Google Scholar]
- 40. Kimura KD, Riddle DL, Ruvkun G (2011) The C. elegans DAF-2 insulin-like receptor is abundantly expressed in the nervous system and regulated by nutritional status. Cold Spring Harb Symp Quant Biol 76: 113–120. [DOI] [PubMed] [Google Scholar]
- 41. Holden-Dye L, Walker RJ (2013) The roles of neuropeptides in Caenorhabditis elegans including their importance in the regulation of feeding and metabolism. Protein Pept Lett 20: 636–646. [DOI] [PubMed] [Google Scholar]
- 42. Avery L, Bargmann CI, Horvitz HR (1993) The Caenorhabditis elegans unc-31 gene affects multiple nervous system-controlled functions. Genetics 134: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sun L, Wang H, Hu J, Han J, Matsunami H, et al. (2009) Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proceedings of the National Academy of Sciences 106: 2041–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schroppel K, et al. (2005) Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15: 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mogensen EG, Janbon G, Chaloupka J, Steegborn C, Fu MS, et al. (2006) Cryptococcus neoformans Senses CO2 through the Carbonic Anhydrase Can2 and the Adenylyl Cyclase Cac1. Eukaryotic Cell 5: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brenner S (1974) The Genetics of Caenorhabditis elegans . Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pumping inhibition is not rescued by either 30 min of exposure to 10% CO2, pH of 5.0 or 7.0, or mutations in the carbonic anhydrase genes. (A) One-day-old wild-type (N2) adult C. elegans were continuously exposed to 10% CO2 for 30 min and pumping rate was measured at different time points. (B) One-day-old wild-type (N2) adult C. elegans were transferred to NGM plates buffered at pH of 5.0, 6.0, or 7.0 followed by exposure to 10% CO2 and measurements of the pharyngeal pumping. (C) One-day-old adult worms with mutations in cah-2, cah-5, or cah-6 genes exposed to 10% CO2 showed pharyngeal pumping rate similar to that of wild-type animals.
(PDF)
The egg-laying rate of hid-1(yg316) animals exposed to 10% CO2 is similar to that of wild-type animals. Gravid animals were exposed to either normal air conditions or air containing 19% or 10% CO2 for 6 h. The number of embryos laid during this period was measured.
(PDF)
Transgenic expression of HID-1::GFP. HID-1 fused to eGFP was expressed under its own promoter in the background of yg316 or under gcy-8, nlp-3, osm-6, flp-17, or gcy-33 promoters in the background of sa722. Arrows indicate the AFD neurons (gcy-8p) and BAG neurons (flp-17p and gcy-33p).The expression of hid-1p, nlp-3p and osm-6p was detected in several neurons. Scale bar, 10 µm.
(PDF)
Animals with deletions in neuropeptide genes expressed in the BAG neurons still show strong CO2-mediated pumping inhibition. One-day-old animals with mutations in neuropeptide genes, which are known to be overexpressed in the BAG neurons, were exposed to 10% CO2 and pumping rate was measured. The pumping rate of nlp-1, nlp-14, and flp-16 mutants in 10% CO2 (but not in normal air conditions) was significantly different from that of the wild-type (N2) animals and showed small but significant rescue. *P<.01. Error bars indicate SEM.
(PDF)
Pumping of wild-type C. elegans exposed to 10% CO2. Pumping rate of wild-type animal under dissecting microscope is presented. Worms are first exposed to normal air and then exposed to 10% CO2.
(AVI)
Pumping of hid-1(yg316) mutant exposed to 10% CO2. Pumping rate of hid-1(yg316) mutant animal under dissecting microscope is presented. Worms are first exposed to normal air and then exposed to 10% CO2.
(AVI)