Summary
Preterm birth and infectious diseases are the most common causes of neonatal and early childhood deaths worldwide. The rates of preterm birth have increased over recent decades and currently account for 11% of all births globally. Preterm infants are at significant risk of severe infection in early life and throughout childhood. Bacteraemia and/or inflammation during the neonatal period in preterm infants is associated with adverse outcomes, including death, chronic lung disease and neurodevelopmental impairment. Recent studies suggest that bacteraemia may trigger cerebral injury even without penetration of viable bacteria into the central nervous system. Here we review available evidence that supports the concept of a strong association between bacteraemia, inflammation and cerebral injury in preterm infants, with an emphasis on the underlying biological mechanisms, clinical correlates and translational opportunities.
Introduction
Globally, more than 15 million infants are born preterm(<37 completed weeks of gestation) each year,and over one million die. The rates of preterm birth have increased over the last several decades and affect approximately 11% of all pregnancies.1,2 Preterm birth now is the most common cause of neonatal mortality and will likely surpass pneumonia as the leading cause of death in early childhood by 2015.1,3Importantly, a significant proportion of the survivors of preterm birth suffer long-term neurological disabilities and evidence suggests that exposure to neonatal infection is a major contributor to cerebral injury in this population.4 Current treatment strategies for neonatal infections, however, largely focus on optimal antimicrobial activity without specifically targeting infection-induced inflammation. Accordingly, novel therapeutic approaches aimed at modulation of infection-related inflammatory responses may improve long-term outcomes. We conducted a literature search focused on chorioamnionitis, bacteraemia, sepsis, and necrotising enterocolitis in order to summarise the current state of the art with respect to mechanisms and potential mitigating agents for inflammation-induced preterm cerebral injury. Inflammation and cerebral injury caused by viral infections are beyond the scope of this review.
The burden of exposure to perinatal inflammation in preterm infants
The incidence, morbidity and mortality of neonatal infection
In developed countries, approximately 1% of all live births are affected by neonatal infections.5 Worldwide,infections account for two thirds of the 7.6 million annual deaths in children less than 5 years of age. The neonatal period carries the highest lifetime risk of serious infections, with an estimated 400,000 newborn deaths annually.3 Neonatal infections disproportionately (~80%) occur inthe minority of infants born preterm (8-12%), who have a several times higher risk of invasive bacterial infection than term infants.6,7 Depending on gestational age at birth, 25-60% of extremely preterm infants (<28 week gestation) develop at least one invasive bacterial infection during their birth-related hospital admission and recurrent neonatal infections are common.8Importantly, the heightened vulnerability to serious infection persists into later childhood and the infection-related morbidity and mortality is not limited to extremely preterm infants, but also affects the much largerproportion of moderate and late preterm infants.9-11
Globally, the majority of moderate and late preterm births occur in resource-poor settings, where dataare less easily collected and consequently less robust, but where the incidence of invasive infection is likely to be substantially higher than that reported for high-resource settings.1The surviving preterm infants in resource-poor settings are likely to be moderately preterm but of low birth weight, further increasing the risk for neonatal and childhood infection and infection-related mortality.12
The burden of exposure to perinatal inflammation
Chorioamnionitis (inflammation of the placental chorionic disc, extraplacental membranes, cord and/or amniotic fluid) affects 2-5% of all births, is intrinsically linked to premature rupture of membranes, spontaneous onset of preterm labour and is an important risk factor for early-onset neonatal infection.13 Large retrospective cohort studies demonstrate a strong inverse relationship between gestational age, birth weight and incidence of histologically diagnosed chorioamnionitis, which is present in approximately 65% of placentae at 23 to 24 weeks of gestational age, 30% of placentae at 29 weeks gestational age, and 2-14% at term.14,15 The clinical diagnosis of chorioamnionitis is unreliable and therefore studies withoutplacental histology are likely tounderestimate significantly the true incidence of chorioamnionitis and its biological effects.16 Chorioamnionitis is frequently caused by fastidious organisms that are not readily cultured with routine microbiological techniques. However, culture-independent methods, such as detection of conserved bacterial 16sRNA by polymerase-chain reaction, have demonstrated the presence of microorganisms in placental tissues and/or amniotic fluid in the majority of histologically confirmed chorioamnionitis.17Failure to recover fastidious organisms may also explain association of histological chorioamnionitis with adverse short- and long-term neonatal outcomes when routine placental bacterial culture is sterile.18 Thus even culture-negative, asymptomatic chorioamnionitis that does not result in early-onset neonatal infection may lead to persistent activation of the inflammatory response and have profound and pervasive effects by altering maturation of the neonatal immune system and longer term infection risk.19,20 Emerging data suggest that exposure to chorioamnionitis not only leads to increased neonatal morbidity, but also may have long-term effects on immune-related outcomes such as an increased risk of childhood asthma.21,22
The impact of infection and inflammation on cerebral injury and neurodevelopment in preterm infants
Human data
The potential link between perinatalinflammation, neonatal sepsis and cerebral injury was first noted over 30 years ago, when both autopsy data and subsequently cranial ultrasound studies showed an increased risk of periventricular leukomalacia in infants exposed to maternal infection or neonatal sepsis.23,24The association between maternal infection, chorioamnionitis and a several-fold increased risk of cerebral palsy was not limited to high-risk preterm infants, but was also observed in term infants.25-27
The commonest lesion associated with inflammation in the preterm infant is white matter injury, which is characterised by focal cystic periventricular leukomalacia and/or diffuse necrosis. White matter injury is defined by loss of immature preoligodendrocytes, which would normally mature to ensheath axons with myelin, but which are particularly susceptible to oxidative stress and inflammation.28,29 Further mechanisms of injury involve inhibition of neuronal precursor cell proliferation and activation of astrogliosis.30,31
Over the last decade, observational studies have provided more detail on the association between neonatal sepsis and adverse long-term neurological and neurocognitive outcomes.Data from a large US Neonatal Network demonstrates that any form of neonatal infection, including clinical infection, culture-proven sepsis, meningitis with or without sepsis, and necrotising enterolitis with or without sepsis,is associated with poor growth and increased risk of neurodevelopmental impairment.32,33 Similarly, severalstudies of preterm infants in Europeand Canada demonstrate an association between late-onset sepsis and adverse neurodevelopmental outcomes in childhood, with repeated infections and Gram-negative pathogens conferring the highest risk.34-39These findings are not limited to extremely preterm infants; ina brazilian study of moderately preterm infants, neonatal sepsis is also strongly and independently associated with increased risk of cerebral injury.40 Finally, in a US cohort, late-onset sepsis is independently associated with neurodevelopmental impairment in preterm infants with necrotising enterocolitis.41
Sophisticated magnetic resonance imaging (MRI) protocols allow increasingly detailed analyses of cerebral injury that may not be detected by cranial ultrasound examination, which is accurate for cystic periventricular leukomalacia, but has limited sensitivity for diffuse white matter injury. MRI analyses predict long-term neurological outcomes.42,43 In preterm infants who have had neonatal sepsis there is an increased risk for white matter injury on MRI and of motor impairment on clinical examination.44 In addition, recurrent neonatal culture-positive infection (without meningitis) is associated with a significantly greater risk of progressive white matter injury.45Furthermore, late-onset sepsis in preterm infants, both microbiologically proven and clinically diagnosed, is associated with acute alteration of cerebral function, indicated by acute changes in electrographic activity and burst suppression pattern.46
Gram-negative neonatal sepsis has a significantly higher mortality than sepsis caused by the most commonly isolated group of Gram-positive organisms, coagulase-negative staphylococci (which accounts for ~50-75%).8 However, the association between sepsis and cerebral injury appears to be largely independent of the bacterial species involved, indicating that a detrimental final common pathway can be activated by diverse initial host-microbe interactions.18,32,44,47 Neonatal clinical sepsis (i.e., signs of infection with negative microbial cultures) is a risk factor for preterm infant white matter injury in univariate analysis, whereas culture-positive infections (predominantly sepsis, but also cases of urinary tract infection and pneumonia in the absence of meningitis), are also a significant risk factor for white matter injury after adjustment for common confounders.48
Challenges in interpreting studies of the association between infection and brain injury include i) cohort variability, predominantly consisting of retrospective studies and clinical trials subject to selection bias, non-uniform definitions of ii) chorioamnionitis (clinical versus histological) and of iii) neonatal sepsis and iv) wide variations in clinical management (Table 1). Importantly, there is no universally accepted gold standard for diagnosing neonatal infection and hence definitions commonly include variable combinations of the following: a) positive culture from sterile site, predominantly blood, cerebrospinal fluid and urine, b) clinical signs such as respiratory distress, apneas, temperature instability, feed intolerance etc., which are both non-specific and insensitive), c) elevated inflammatory markers such as C-reactive protein whose use and cut-off values are variable, and d) intention-to-treat duration of antibiotic therapy which is highly variable between units.49 Furthermore various methods are used to quantify cerebral injury in neonates, including postmortem examination of the brain, cranial ultrasound, MRI and standardised clinical evaluations of neurodevelopmental outcome. Despite these methodological shortcomings, there is robust evidence for an heightened risk of cerebral injury and adverse effects on neurodevelopmental outcomes following a variety of perinatal inflammatory exposures, including maternal infection, maternal and fetal chorioamnionitis, and early and late neonatal infection.
Table 1.
Key animal studies linking Bacteria-induced Inflammation with Cerebral Injury.
| Author/year | Animal species |
Age at intervention |
Intervention | Clinical/anatomical outcomes | Biomarkers | Reference |
|---|---|---|---|---|---|---|
| Mallard et al., 2003 |
Fetal sheep |
Days 93-96 of gestation |
Intravenous E. coli LPS | Focal inflammatory infiltrates and cystic lesions in periventricular white matter, microglial activation, astrocyte damage, loss of oligodendrocytes. |
None | 150 |
| Svedin et al., 2005 |
Fetal sheep |
Median days 89 or 121 of gestation |
Intravenous E. coli LPS | Microglial activation and loss of neurofilament indicative of white matter injury. |
None | 67 |
| Garnier et al., 2006 |
Fetal sheep |
Median day 107 of gestation |
Intravenous E. coli LPS | Inflammatory infiltrates and cystic lesions in periventricular white matter. |
Increase S100B blood levels |
69 |
| Stolp et al., 2007 |
Opossums | Postnatal day 35 |
Intraperitoneal E. coli LPS |
Single LPS injection induced short-lasting blood brain barrier dysfunction. Repeated LPS exposure resulted in more profound and prolongated blood brain barrier impairment. |
None | 90 |
| Orihuela et al., 2006 |
Mice | 4-5 weeks old | Intranasal or intravenous live or heat-killed whole S. pneumoniae or purified cell wall preparation |
Both whole bacteria and cell wall preparations induce hippocampal neuronal injury. This was partially mitigated in TLR2- /−, NOD2−/− and IL10-overexpressing mice |
None | 89 |
| Gavilanes et al., 2009 |
Fetal sheep |
Day 111 or 123 of gestation |
Intra-amniotic E. coli LPS with 2d or 14d survival |
Fetuses with long-term survival displayed apoptosis, microglial activation and astrogliosis. Loss of mature oligodendrocytes and neurons were decreased in some regions of the brain |
None | 68 |
| Du et al., 2011 |
Newborn mice |
Postnatal days 3-11 |
Once daily intraperitoneal TLR2 agonist (Pam3CSK4) |
Decreased volume of gray and white matter and activation of microglia |
Elevated levels of IL1, IL6, CCL2 in brain homogenates after 1st injection |
81 |
| Dean et al., 2011 |
Fetal sheep |
Day 102 of gestation |
Single intravenous E. coli LPS injection |
Reduced grey and white matter volume, including loss of oligodendtrocytes and cortical neurons. Loss of normal maturation of EEG |
None | 66 |
| Keogh et al., 2012 |
Fetal sheep |
Days 103-108 of gestation |
Intravenous E. coli LPS infusion |
Loss of EEG maturation, increased cerebral inflammation and caspase 3 positive cells in white matter, but no loss of oligodendrocytes and cortical neurons |
Transient rise in plasma cortisol and IL-6 |
79 |
Humoral mediators, including pro-inflammatory cytokines such as interleukin (IL)-1, IL-6 and chemokines such as CXCL-8 (formerly named IL-8) as well as tumour necrosis factor-α (TNF), type I and II interferons and reactive oxygen species are likely to be key mediators in the pathogenesis of cerebral injury.29,50-53Levels of these cytokines/chemokines are elevated in amniotic fluid, cord blood, cerebrospinal fluidand cerebral tissue of infants diagnosed with inflammation-related white matter lesions, predominantly periventricular leukomalacia.54-57 Detrimental neurotoxic effects are not only induced by direct host-microbe interaction, but may also be generated by exposure to perinatal inflammation; activation of fetal/neonatal immune cells triggered by bacterial products that activate pattern recognition receptors and/or maternal pro-inflammatory mediators that cross the placenta.58 For example, exposure to intrauterine inflammation results in a fetal inflammatory response, characterised by activation of CD45RO+ T-cells and elevated levels of pro-inflammatory cytokines, which are associated with cerebral injury on MRI.59 Therefore, an active fetal response rather than exclusively passive transfer of maternal mediators may be a key pathogenic mechanism for cerebral damage in preterm infants. Furthermore, high plasma levels of pro-inflammatory cytokines in preterm infants with sepsis or necrotising enterocolitis are associated with increased risk of ultrasound-detected white matter injury.60 However, although both blood and cerebrospinal fluid cytokine levels are associated with white matter injury, plasma cytokine levels may not reflect local cytokine production in the brain and imbalance of pro- and anti-inflammatory mediators may be at least as importantas absolute levels of individual cytokines.61
In the newborn, cytokines can be released from activated immune cells, mainly monocytes, macrophages and T-cells. Activation can occur systemically in the blood compartment or at sites of infection; in the brain, this is predominantly by resident microglia or activated macrophages infliltrating the brain, and both mechanisms may be active simultaneously.54,62 In addition, there is increasing appreciation of the immunological implications of brain immaturity in the preterm infant, particularly with respect to the immaturity of central nervous system immune cell regulation, which may render the preterm brain exquisitely vulnerable to damage by poorly controlled and pervasive inflammation.58,63 These data also support the observation that direct bacterial infections of the neonatal brain, such as meningitis, only cause a small proportion of cerebral injuriesand that significant neonatal brain injury can occur without entry of bacteria into the cerebrospinal fluid.48
In the following section we review recent data from in vitro and animal models that have significantly advanced the understanding of mechanisms underlying the relationship between bacteraemia, inflammation and cerebral injury in preterm infants.48,64
Experimental models
The vulnerability of the newborn brain to infection/inflammation was first described by Gilles in 1976 when systemic administration of bacterial lipopolysaccharide (LPS), that activates cells via Toll-like receptor 4 (TLR4), caused leukencephalopathy in neonatal kittens, but not in mature cats.65 Furthermore, the newborn feline brain is particularly vulnerable to the effects of LPS, whereas other organs were comparatively resistant. In fetal sheep, systemic intrauterine LPS exposure leads to activation of microglia, the resident macrophage-like cells of the central nervous system and to loss of neurofilament and myelin basic protein, changes that are associated with white matter injury, specifically periventricular leukomalacia.66-68 Furthermore, these changes are associated with acute increase in blood levels of S100B protein, a marker of cerebral injury.69 In mice, microglia and neurons express TLR1-9 and TLR2, -3, -4 and -8, respectively, and stimulation of quiescient microglia with TLR agonists initiates rapid upregulation of cytokines and chemokines, indicating their importance in mediating responses to infectious organisms.70,71In addition, microglia are the principal central nervous system cell population responsive to peripherally administered TLR agonists. Microglia-mediated neuronal injury was critically dependent on intact expression of TLR2 and 4, respectively, highlighting the biological relevance of the TLR pathways in the central nervous system.72-75 There are no studies specifically investigating leukocyte entry into the immature brain after peripheral administration of TLR agonists. However data from adult animals suggest active transport of some cytokines across the blood brain barrier or production by endothelial cells with subsequent release into adjacent cerebral tissue(Figure 1).76,77
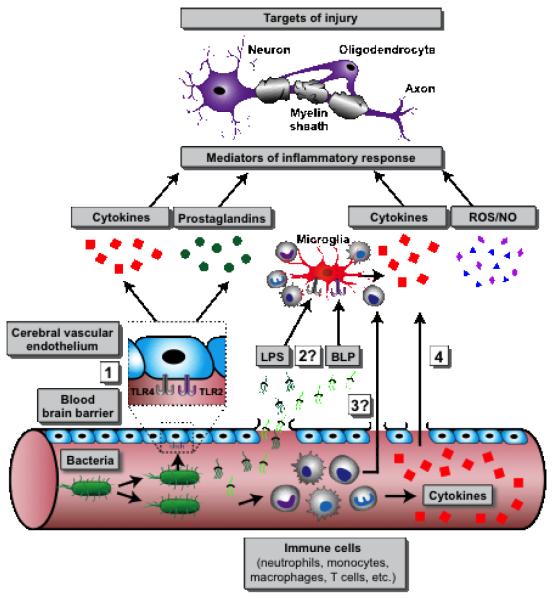
Figure 1.
This figure summarizes known and hypothetical pathways of bacteraemia-induced neuronal damage: (1) bacterial products on or shed from bacteria in the bloodstream activate endothelial pattern recognition receptors such TLRs triggering release of inflammatory mediators into the CNS; (2) Leak of bacterial products such as lipopolysaccharide (LPS) and bacterial lipopeptide (BLP) across the blood brain barrier that activate microglia to release inflammatory mediators; (3) Entry of leukocytes into the CNS; and (4) direct diffusion of cytokines/chemokines from the peripheral circulation across the blood-brain barrier. Hypothetical mechanisms for which there has not yet been published evidence are marked with a question mark (“?”).
Arterial hypotension is common in both neonatal sepsis and experimental models, especially in Gram-negative infection and necrotising enterocolitis, and via cerebral ischemia and reperfusion can potentiate the risk of white matter injury.78 However, cerebral damage can be induced by systemic inflammation in the absence of systemic cardiovascular impairment and in the human preterm infant the relationship between infection, hypotension and white matter damage is often inadequately documented and thus incompletely understood.79
Most neonatal bacterial infections are caused by Gram-positive organisms that do not express LPS and predominantly signal via TLR2 and other pattern recognition receptors. In vitro experiments and in vivostudies show that administration of TLR2 agonists (Pam3CSK4 and FSL1) as well as inactivated whole bacteria result in inhibition of neural progenitor cell proliferation and consequently in perinatal brain injury (Table 2).80-82Cell wall preparations and secreted factors of one of the principal early-onset sepsis pathogens and most common cause of neonatal meningitis, Group B streptococcus (GBS), induce neuronal cell death in vitro, which is dependent on the presence of microglia expressing TLR2 and the TLR-adapter protein MyD88.83 In addition, GBS-mediated activation induces microglial apopotosis via caspase-8, a potentially autoregulatory mechanism limiting ongoing innate immune activation and inflammatory damage in the brain.84
Table 2.
ClinicalStudies Linking Bacterial infection and Cerebral Injury in Preterm Infants.
| Author | Number of infants |
Study population |
Definition of infection | Central nervous system involvement |
Key outcomes | Reference |
|---|---|---|---|---|---|---|
| Stoll et al., 2004 |
N=6093 | Birth weight 401-1500g |
Positive blood culture plus ≥5 days of antibiotics. Clinical infection: culture negative, but ≥5 days of antibiotics Positive (cerebrospinal fluid , CSF) culture plus ≥5 days of antibiotics |
192 cases of meningitis | Any postnatal infection associated with poor growth and neurodevelopmental impairment. Strongest effect for sepsis/NEC |
32 |
| Glass et al., 2008 |
N=133 | <34 weeks | Intention-to-treat plus positive culture from blood, endotracheal tube, urine, skin lesion, or CSF (plus suggestive CSF findings) |
Cases of meningoencephalitis excluded from analysis Due to small numbers, meningoencephalitis was not significantly associated with adverse outcomes |
Recurrent postnatal infections are associated with increased risk of progressive white matter injury |
45 |
| Shah et al., 2008 |
N=192 | <30 weeks | Positive blood culture plus abnormal I/T ratio, CRP or platelet count plus ≥5 days of antibiotics Meningitis: CSF contains >20 cells/mm3 plus therapeutic course of antibiotics |
3 cases of culture-negative meningitis; 2 in proven sepsis group, 1 in clinical sepsis group |
Postnatal infection and/or NEC associated with white matter injury increased risk of motor impairment at 2 years of age |
44 |
| Chau et al., 2009 |
N=96 | 24-32 weeks | Any positive culture from blood, CSF, urine; in tracheal aspirate if associated with >4 leukocytes and clinical pneumonia |
No case of meningitis mentioned |
Postnatal infection, but not histological chorioamnionitis is associated with white matter injury |
78 |
| Bassler et al., 2009 |
N=944 | Birth weight 500-999g |
Sepsis: positive blood culture; Meningitisi: positive CSF culture, no further information give |
22 cases of meningitis. Meningitis with or without sepsis showed strongest association with aderse outcome |
Any postnatal infection or NEC increased the risk of late death or survival with neurosensory impairment |
33 |
| Martin et al., 2010 |
N=1155 | 23-27 weeks | Positive blood culture (taken weekly as routine part of ELGAN study) |
Meningitis not mentioned | Increased risk of impaired neurodevelopment at 24 months of age in infants with surgical NEC, esp. when accompanied by sepsis |
41 |
| Helderman et al., 2010 |
N=108 | 24-26.6 weeks |
Positive blood culture plus clinical signs plus ≥5 days of antibiotics. Culture-negative infection: negative culture plus clinical signs plus ≥5 days of antibiotics |
No cases of meningitis | Culture-positive and clinical sepsis are associated with acute encephalopathy, but normal rate of brain maturation |
46 |
| Silveira et al., 2011 |
N=88 | Birth weight 500-1500g |
Clinical signs plus positive culture from blood or CSF |
No case of meningitis | Postnatal sepsis associated with increased risk of periventricular leukomalacia |
40 |
| Chau et al., 2012 |
N=117 | 24-32 weeks | Any positive culture from blood, urine or CSF; in tracheal aspirate if associated with >4 leukocytes and clinical pneumonia |
4 cases of meningitis. Results unchanged when meningitis exluded from analysis |
Postnatal infections (proven and clinical) are associated with abnormalities in metabolic and structural brain development |
48 |
Direct bacterial cytotoxicity and activation of the local host response with production of various inflammator mediators, such as cytokines, prostaglandins and reactive oxygen species are the main detrimental mechanisms of bacterial infection of the newborn central nervous system.51,52,85 In contrast, it is largely unknown how cerebral injury is mediated in the absence of meningitis. Systemic inflammation can exert rapid negative impact on cerebral function that precedes peripheral organ dysfunction and can occur without bacterial invasion of the central nervous system.46,86 This is presumably augmented in part by blood-brain barrier dysfunction during infection, as exposure to bacterial cell wall components directly increases blood-brain barrier permeability.87 Additional potential mechanisms, such as direct transfer of bacterial components or inflammatory mediators across endothelial cells, remain incompletely characterised (Figure 1).75,88Neuronal apoptosis occurs within hours of pneumococcal bacteremia or systemic challenge with pneumococcal cell wall preparations in mice, and importantly, this effect does not require binding of bacterial components to the endothelium and is independent of bacteria or leukocytes entering the cerebrospinal fluid.89 In newborn marsupial opossums, in whom the majority of brain maturation occurs postnatally (akin to very preterm infants) LPS administration, especially repeated exposure, results in significant and sustained increases in blood brain barrier permeability, microglial activation and white matter injury.90 In several animal models, intraperitoneal LPS injection induces activation of microglia, intracerebral expression of TLR2, IL-1 and IL-6 and decreased hippocampal neurogenesis and cerebral damage can occur within hours of pneumococcal bacteraemia without meningitis.91-94 The pathogenic mechanisms appear to be at least partially independent of direct cytokine-mediated inflammation as anti-TNF antibodies do not reduce neuronal injury. However, overexpression of IL-10 is protective (Figure 1).89 Preterm infants not only show gestational age-dependent impairment of inflammatory responses, but importantly, have profoundly reduced capacity to produce anti-inflammatory cytokines, such as IL-10.95-98 The quality and quantity of anti-inflammatory responses of central nervous system cells to systemic inflammation are not defined, particularly in human newborn infants.
Exposure to bacterial pattern recognition receptor agonists increases vulnerability of the preterm brain
Bacterial infection increases the vulnerability of the preterm brain to non-inflammatory insults: in human infants the combination of maternal infection and asphyxia amplifies the risk of cerebral palsy.99-101 Administration of LPS to rat fetuses or newborn rat pups induces cerebral expression of CD14 and TLR4 and sensitises the immature brain to subsequent hypoxic-ischaemic injury.102,103In mice pre-treatment with LPS converts a sub-threshold hypoxic insult to a critical one.72 Furthermore, the potentiation of cerebral injury induced by LPS exposure is dependent on intact expression of MyD88. MyD88-deficient animals displayed significantly reduced activation of nuclear factor kappa-B, inflammatory cytokines and chemokines and reduced white and gray matter injury.104 Deletion of the TNF gene cluster in mice results in lack of LPS-induced activation of microglia and endothelial cells and abolishes the sensitisation to hypoxic-ischaemic insult following LPS-exposure.105 Cerebral sensitisation after LPS exposure of fetal mice is pervasive even if the postnatal hypoxic-ischemic insult occurs as late as day 70.106Of note, in rats, these sequential insults resulted in anatomical grey and white matter damage as well as behavioural and motor deficits similar to those observed in human infants.107
The association between exposure to bacterial components, hypoxic-ischaemic insult and cerebral injury is not uniform, but appears to be sensitive to timing and dosage of the insults, context and, importantly, the maturity of the animal. LPS pre-treatment in adult mice actually reduced loss of cerebral tissue upon hypoxic-ischaemic insult, indicating that in mature animals LPS may have a protective preconditioning effect.106 In neonatal rats, the sensitisation afforded by LPS is time-dependent: LPS given either 6 hours or 72 hours prior to hypoxic-ischaemic insult results in increased cerebral injury, wheras injury size is markedly reduced with a 24 hour intervall.108 In addition, increasing brain maturity (postnatal age) and the concurrent rise in cerebral expression of TLR4 is critical for the protective preconditoning effects of LPS.109
Detection of cerebral injury in preterm infants with bacteraemia
The relationship between bacteraemia and cerebral injury is complicated by the multiple pathways that may contribute to an increased risk of brain injury in preterm infants. In addition, clinical outcomes associated with bacteraemia, such as requirement for indwelling plastic devices (e.g., respiratory and feeding tubes, intravascular catheters) and total parenteral nutrition may themselves be related to alteration of cerebral development.
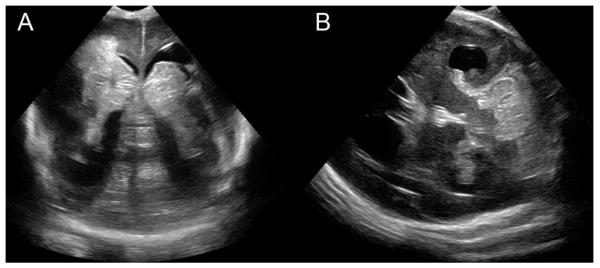
Intraventricular haemorrhage is the most common form of cerebral injury in very preterm infants currently detected with conventional neuroimaging, such as cranial ultrasound. Preterm infants with early-onset bactaeremia have a significantly elevated risk (up to 20%) for high grade intraventricular haemorrhage (Figure 2A).110Cerebellar haemorrhage may occur in up to 20% of preterm infants, but the incidence is often underestimated by cranial ultrasound (Figure 2B). In preterm infants, the association between neonatal sepsis andincreased risk for cerebellar haemorrhage further supports the concept ofbacteremia and exacerbation of neonatal brain injury.111
Figure 2.
Intracranial haemorrhage in a preterm with bacteraemia. A preterm infant born at 24 weeks gestation following onset of maternal fever grew E. coli from blood cultures obtained at 45 minutes of age. He had septic shock with coagulopathy, thrombocytopaenia and hypotension prompting inotropic support. His day 2 cranial ultrasound revealed left grade III and right grade IV intraventricular haemorrhage (A) with severe cerebellar haemorrhage (B). Note that areas of echodensity (brightness) indicate haemorrhage.
The incidence of periventricular leukomalaciaand white matter injury varies with the method of detection. Preliminary studies suggest that MRI is more sensitive for white matter injury than either computed tomography or ultrasound.112,113Human MRI studies document a strong association between infection and white matter lesions in the preterm brain.44,45Abnormalities have also been reported on white matter microstructural integrity, measured by white matter diffusion, supporting an adverse impact of infection or inflammation on the cerebral white matter.48White matter injury isparticularly severe in the setting of necrotising enterocolitis, presumably relating to the distinct and long-lasting systemic inflammation inherent to this disease, and may be further aggravated by associated systemic hypotension (Figure 3).114
Figure 3.
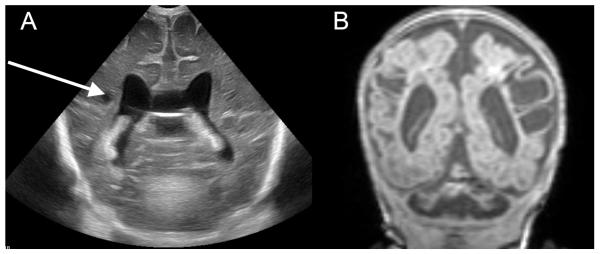
White matter injury in a preterm infant with necrotizing enterocolitis. A preterm infant was born at 24 weeks gestation with germinal matrix hemorrhage developed severe necrotising enterocolitis at 6 weeks of age requiring surgery. (A) ultrasound 4 weeks post- surgery revealed small cystic white matter echolucencies (arrow). (B) An MRI scan 2 weeks later at 36 weeks post-menstrual age demonstrated extensive white matter injury with periventricular gliosis and extensive encephaloclastic changes.
Finally, MRI can define alterations in cerebral development including reductions in cerebral growth both globally and regionally, as well as alterations in cerebral biochemistry (spectroscopy). Bacteremia is associated with alterations in brain biochemistry and reductions in cerebral growth, consistent with the known adverse effect of neonatal infection on somatic growth.32,48,115An understanding of alterations in the sequence of normal cerebral development, combined with or independent of cerebral injury, will provide a betterappreciatiation of the impact of bacteraemia and associated complications on the central nervous system.
In summar neuroimaging has given a clear delineation of the breadth of impact of neonatal bacteremia from exacerbation of brain injury to impairments in brain development.
Potential future interventions to reduce bacteraemia-associated cerebral injury
The incidence of preterm birth has risen over the past thirty years and interventions aimed at reducing this trend have largely been disappointing.116 Furthermore, a significant proportion of infants born preterm will have been exposed to inflammation from well before birth, and attempts at ameliorating the postnatal consequences will be challenging. Therefore, new strategies for the prevention and treatment of perinatal inflammation and neonatal sepsis are urgently needed. Interventions that reduce infection and inflammation-induced cerebral injury are of particular relevance in the context of extreme prematurity, as the detrimental effects of neonatal infection on white matter injury and long-term neurological outcomes appear intimately related to the release and circulation of pro-inflammatory bacteria-derived molecules that induce systemic inflammation. Consequently, antibiotic therapy alone does not ameliorate the risk of white matter injury, neurodevelopmental impairment and cerebral palsy associated with neonatal sepsis.47Based on emerging evidence discussed above, novel protective interventions might include those targeting free-radical generation or accumulation, anti-apoptotic agents, and anti-inflammatory agents and compounds targeted at blunting the host inflammatory response to microbial products, e.g. bacterial lipopeptides and lipoteichoic acids (TLR2), LPS (TLR4), peptidogycans (nucleotide oligomerization domain 1, NOD1) and others.
Corticosteroids are potent anti-inflammatory agents and dexamethasone in particular has long been used in preterm infants, primarily for the treatment and prevention of chronic lung disease. Both in experimental models of perinatal inflammation and in chorioamnionitis-exposed human infants, corticosteroids can modulate inflammationand ameliorate related lung disease.117,118However,dexamethasone hashighly significant adverse long-term neurodevelopmental consequences, including an increased risk of cerebral palsy, precluding its universal use as an anti-inflammatory agent.119-121Hydrocortisonemay be equally effective for chronic lung disease without the detrimental neurodevelopmental outcomes associated with dexamethasone, but also has acute adverse effects, such as gastrointestinal perforation.117,122 Importantly, in addition to the significant side effects, postnatal steroids have not been evaluated systematicallyas immunomodulators in preterm infant infection, and thus their effects on survival and long-term outcomes in this contextremain unknown.
The history of neonatology includes serious, unexpected long-term side effects of well-intended interventions with apparent short-term benefits. Any new intervention will need to be evaluated carefully with an emphasis on safetyprior to being advocated for routine use, particularly at this critical period of immunological development.
N-acetylcysteine
The free radical scavenging/anti-oxidant agent N-acetylcysteinereadily crosses the placenta and is considered safe during pregnancy and in the preterm neonate.123 N-acetylcysteine may prevent LPS-induced degeneration of oligodendrocyte progenitors and hypomyelination in the developing rat brain, an effect associated with attenuation of the intracerebral inflammatory reaction, including reduced levels of TNF, IL-1, and expression of inducible nitric oxide synthase.124In mice, N-acetylcysteine administration to the pregnant dam attenuates the maternal and fetal pro-inflammatory response to intrauterine LPS administration, resulting in fewer preterm births and reduced neonatal white matter injury.125,126In addition, N-acetylcysteine provides substantial neuroprotection against brain injury caused by the combination of LPS exposure and hypoxic-ischaemic insult in neonatal rats, suggesting that N-acetylcysteine has potential therapeutic value, especially considering the protective effects of antenatal administration to the pregnant animal with chorioamnionitis – a scenario where postnatal intervention may be of limited benefit.127 However, there are some concerns that N-acetylcysteine may compromise fetal cardiovascular stability and to date, no large clinical trials have evaluated systemic N-acetylcysteine administration in newborn infants - or their mothers - prior to delivery.128
Erythropoietin
Erythropoietin, previously appreciatedexclusively as a kidney-derived haemopoietic growth factor, is neuroprotective in a range of experimental models of cerebral injury. The protective mechanisms are not completely understood, but may include anti-oxidative, anti-apoptotic as well as significant anti-inflammatory action, both systemically and in the brain.129,130Importantly, erythropoietincrosses the blood-brain barrier and interacts with cerebral erythopoietin receptors that are expressed from early in gestation.131,132 Clinical trialsdemonstrate that repeated administraiton ofrecombinant human erythropoietin is well tolerated and results in plasma levels that are neuroprotective in animal studies. Furthermore, pilot datasuggest that erythropoietin improves neurodevelopmental outcomes in infants with hypoxicischaemic encephalopathy and is associated with superior developmental outcomes at 10-13 years of age in extremely preterm infants with intraventricular hemorrhage treated prophylactically with erythropoietin for anaemia of prematurity.133-135 However, recent meta-analyses confirm that early routine administration of erythropoietin increases the risk of retinopathy of prematurity in extremely preterm infants. This is of concern particularly in the context of late-onset sepsis, which also is an independent risk factor for the development of retinopathy.136,137 Large-scale clinical trials to evaluate erythropoietin for neuroprotection in preterm infants and in term infants with hypoxic-ischaemic encephalopathy are ongoing.
Pentoxifylline
Pentoxifylline is a synthetic xanthine-derived phosphodiesterase inhibitor that raises cellular concentrations of cyclic adenosine monophosphaste thereby inhibiting production of inflammatory mediators such as TNF.Pentoxyfilline has beneficial effects in models of neonatal inflammatory conditions, including sepsis and necrotising enterocolitis.138,139Pilot studies ofpentoxifylline as adjunct therapy for neonatal sepsis show that it is safe and well-tolerated with a favourable side-effect profile, and meta-analysis of the available data (a total of 227 patients from four studies) conclude that pentoxifylline reduced sepsis-related mortality.140,141 However, although pentoxifylline has also been implicated as a potential adjunct agent for treatment of hypoxic-ischaemic encephalopathy based on animal studies, there are no neonatal clinical data to date. Given its anti-inflammatory properties and promising clinical profile, pentoxifylline warrants evaluation in larger clinical trials as adjunct therapy in neonatal sepsis, including assessment of long-term neurodevelopmental outcomes.
Minocycline
The tetracycline antibiotic minocycline has shown promising anti-apoptotic, anti-oxidant and anti-inflammatory effects in animal models, especially inhibiting microglial activation after cerebral insults, as well as protective effects on blood-brain barrier integrity in systemic inflammation.90,142 However, the use of tetracyclines in neonates and infants is contentious, particularly because of concerns of disruption of normal formation of bone and tooth enamel. Furthermore, while there is experimental evidence in animal models for neuroprotective effects of minocycline, even when administered after the cerebral insult, there is a lack of supportive data from human clinical trials to date.
Immununological interventions
Our understanding of neonatal and infant immune function and its maturation in early childhood remains incomplete.Over the past decade, a number of immune interventions aimed at preventing or improving the outcome of neonatal sepsis, such as colony-stimulating growth factors and intravenous immunoglobulin, have been unsuccessful.143,144 The mechanisms underpinning the unique susceptibility of preterm infantsto invasive infections are the subject of ongoing research efforts.
Antimicrobial proteins and peptides (APPs), a group of naturally occuring molecules expressed in leukocytes and on mucosal epithelial cells possess anti-infective and immunomodulatory properties and have shown promise in animal and/or early human clinical studies.145 These cationic molecules kill microbes, but unlike conventional antibiotics are also able to bind microbial components and reduce their inflammatory activity by preventing their interaction with bacterial products. For example, adminstration of an endotoxin-neutralising recombinant 21 kDa fragment of bactericidal/permeability-increasing protein (rBPI21) in addition to a fluoroquinolone antibiotic improves survival in mice exposed to lethal radiation and reduces systemic inflammation and morbidity when given with conventional antibiotics to children with meningococcal sepsis.146,147 In human preterm infants, oral supplementation with lactoferrin, an iron-binding glycoprotein with anti-infective activities, reduces the incidence of bacterial and fungal late-onset sepsisand large international clinical trials further evaluating this approach are ongoing.148Systemic administration of the synthetic immune defense regulator peptide 1018, a derivative of the human cathelicidin LL-37, resulted in marked mitigation ofLPS and hypoxia-ischemia-induced cerebral injury in mice.149
Overall, accumulating evidence indicates that bacterial infection triggers inflammatory pathways that damage the preterm brain even in the absence of direct bacterial entry to the central nervous system. Given the high global rate of preterm birth, the frequency of preterm brain injury, and its long term morbidities, translational research directed at defining the underlying mechanisms and adjunctive therapies is urgently needed to provide novel approaches to mitigate severe long-term neurodevelopmental consequences for this highly susceptible population.
Search strategy and selection criteria.
References for this review were identified through searches of PubMed for articles published from January, 1973, to February 2014, by use of terms “sepsis”, “bacteremia”, “necrotising enterocolitis”, “chorioamnionitis”, “newborn”, “preterm infant”, “brain injury”, “long-term outcome”, “neurodevelopment” and “intervention”. Articles resulting from these searches and relevant references cited in those articles were reviewed.
Acknowledgments
OL’s laboratory is funded by the National Institutes of Health R01-AI100135-01 and by Bill & Melinda Gates Foundation Global Health Grants OPPGH5284 and OPP1035192. OL is an inventor on a patent for the use of recombinant bactericidal/permability-increasing protein (rBPI) in radiation injury. DB is supported by a National Health and Medical Research Council of Australia Career Development Fellowship (572504). XW is supported by grants from the Swedish Medical Research Council (VR K2009-54X-21119-01-4), VINNMER—Marie Curie international qualification (VINNOVA, 2011-03458), and Bill & Melinda Gates Foundation—Grand Challenges Explorations (OPP1036135).CM’s research is supported by the Swedish Research Council (VR2012-2992), Government grant in Public Health Service at the Sahlgrenska University Hospital (ALFGBG-142881), European Union grant FP7, (Neurobid, HEALTHF2-2009-241778), the Leducq foundation (DSRR_P34404), Åhlén Foundation and the Swedish Brain Foundation (FO2013-095). TI is supported by the Doris Duke Charitable Foundation and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Born too soon: the global action report on preterm birth. World Health Organisation; Geneva, Switzerland: 2012. [Google Scholar]
- 2.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 4.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 5.Vergnano S, Menson E, Kennea N, et al. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F9–F14. doi: 10.1136/adc.2009.178798. [DOI] [PubMed] [Google Scholar]
- 6.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88(Suppl 2):S69–74. doi: 10.1016/S0378-3782(12)70019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berardi A, Rossi C, Lugli L, et al. Group B streptococcus late-onset disease: 2003-2010. Pediatrics. 2013;131(2):e361–8. doi: 10.1542/peds.2012-1231. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle LW, Ford G, Davis N. Health and hospitalistions after discharge in extremely low birth weight infants. Semin Neonatol. 2003;8(2):137–45. doi: 10.1016/S1084-2756(02)00221-X. [DOI] [PubMed] [Google Scholar]
- 10.Carbonell-Estrany X, Figueras-Aloy J, Law BJ. Identifying risk factors for severe respiratory syncytial virus among infants born after 33 through 35 completed weeks of gestation: different methodologies yield consistent findings. Pediatr Infect Dis J. 2004;23(11 Suppl):S193–201. doi: 10.1097/01.inf.0000144664.31888.53. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MS, Demissie K, Yang H, Platt RW, Sauve R, Liston R. The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA. 2000;284(7):843–9. doi: 10.1001/jama.284.7.843. [DOI] [PubMed] [Google Scholar]
- 12.Hviid A, Melbye M. The impact of birth weight on infectious disease hospitalization in childhood. Am J Epidemiol. 2007;165(7):756–61. doi: 10.1093/aje/kwk064. [DOI] [PubMed] [Google Scholar]
- 13.Martius JA, Roos T, Gora B, et al. Risk factors associated with early-onset sepsis in premature infants. Eur J Obstet Gynecol Reprod Biol. 1999;85(2):151–8. doi: 10.1016/s0301-2115(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 14.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol. 2004;190(1):147–51. doi: 10.1016/j.ajog.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Becroft DM, Thompson JM, Mitchell EA. Placental chorioamnionitis at term: epidemiology and follow-up in childhood. Pediatr Dev Pathol. 2010;13(4):282–90. doi: 10.2350/09-06-0659-OA.1. [DOI] [PubMed] [Google Scholar]
- 16.Curtin WM, Katzman PJ, Florescue H, Metlay LA. Accuracy of signs of clinical chorioamnionitis in the term parturient. J Perinatol. 2013;33(6):422–8. doi: 10.1038/jp.2012.135. [DOI] [PubMed] [Google Scholar]
- 17.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47(1):38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leviton A, Allred EN, Kuban KC, et al. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res. 2010;67(1):95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leviton A, Hecht JL, Allred EN, Yamamoto H, Fichorova RN, Dammann O. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol. 2011;90(2):235–43. doi: 10.1016/j.jri.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Strunk T, Doherty D, Jacques A, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. 2012;129(1):e134–41. doi: 10.1542/peds.2010-3493. [DOI] [PubMed] [Google Scholar]
- 21.Adams-Chapman I. Long-term impact of infection on the preterm neonate. Semin Perinatol. 2012;36(6):462–70. doi: 10.1053/j.semperi.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Getahun D, Strickland D, Zeiger RS, et al. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med. 2010;164(2):187–92. doi: 10.1001/archpediatrics.2009.238. [DOI] [PubMed] [Google Scholar]
- 23.Faix RG, Donn SM. Association of septic shock caused by early-onset group B streptococcal sepsis and periventricular leukomalacia in the preterm infant. Pediatrics. 1985;76(3):415–9. [PubMed] [Google Scholar]
- 24.Leviton A, Gilles F, Neff R, Yaney P. Multivariate analysis of risk of perinatal telencephalic leucoencephalopathy. Am J Epidemiol. 1976;104(6):621–6. doi: 10.1093/oxfordjournals.aje.a112340. [DOI] [PubMed] [Google Scholar]
- 25.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278(3):207–11. [PubMed] [Google Scholar]
- 26.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284(11):1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 27.Soraisham AS, Trevenen C, Wood S, Singhal N, Sauve R. Histological chorioamnionitis and neurodevelopmental outcome in preterm infants. J Perinatol. 2013;33(1):70–5. doi: 10.1038/jp.2012.49. [DOI] [PubMed] [Google Scholar]
- 28.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2008;93(2):F153–F61. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haynes RL, Folkerth RD, Trachtenberg FL, Volpe JJ, Kinney HC. Nitrosative stress and inducible nitric oxide synthase expression in periventricular leukomalacia. Acta Neuropathol. 2009;118(3):391–9. doi: 10.1007/s00401-009-0540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology : official journal of the Japanese Society of Neuropathology. 2002;22(3):106–32. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 31.Buser JR, Maire J, Riddle A, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71(1):93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–65. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 33.Bassler D, Stoll BJ, Schmidt B, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123(1):313–8. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, Reiter G, Trawoger R. Adverse neurodevelopmental outcome in preterm infants: risk factor profiles for different gestational ages. Acta Paediatr. 2009;98(5):792–6. doi: 10.1111/j.1651-2227.2009.01219.x. [DOI] [PubMed] [Google Scholar]
- 35.van der Ree M, Tanis JC, Van Braeckel KN, Bos AF, Roze E. Functional impairments at school age of preterm born children with late-onset sepsis. Early Hum Dev. 2011;87(12):821–6. doi: 10.1016/j.earlhumdev.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Schlapbach LJ, Aebischer M, Adams M, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128(2):e348–57. doi: 10.1542/peds.2010-3338. [DOI] [PubMed] [Google Scholar]
- 37.Alshaikh B, Yee W, Lodha A, Henderson E, Yusuf K, Sauve R. Coagulase-negative staphylococcus sepsis in preterm infants and long-term neurodevelopmental outcome. J Perinatol. 2014;34(2):125–9. doi: 10.1038/jp.2013.155. [DOI] [PubMed] [Google Scholar]
- 38.Mitha A, Foix-L’Helias L, Arnaud C, et al. Neonatal infection and 5-year neurodevelopmental outcome of very preterm infants. Pediatrics. 2013;132(2):e372–80. doi: 10.1542/peds.2012-3979. [DOI] [PubMed] [Google Scholar]
- 39.de Haan TR, Beckers L, de Jonge RC, et al. Neonatal gram negative and Candida sepsis survival and neurodevelopmental outcome at the corrected age of 24 months. PLoS One. 2013;8(3):e59214. doi: 10.1371/journal.pone.0059214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silveira RC, Procianoy RS, Dill JC, da Costa CS. Periventricular leukomalacia in very low birth weight preterm neonates with high risk for neonatal sepsis. Jornal de pediatria. 2008;84(3):211–6. doi: 10.2223/JPED.1777. [DOI] [PubMed] [Google Scholar]
- 41.Martin CR, Dammann O, Allred EN, et al. Neurodevelopment of extremely preterm infants who had necrotizing enterocolitis with or without late bacteremia. J Pediatr. 2010;157(5):751–6. e1. doi: 10.1016/j.jpeds.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathur AM, Neil JJ, Inder TE. Understanding brain injury and neurodevelopmental disabilities in the preterm infant: the evolving role of advanced magnetic resonance imaging. Semin Perinatol. 2010;34(1):57–66. doi: 10.1053/j.semperi.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–94. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 44.Shah DK, Doyle LW, Anderson PJ, et al. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153(2):170–5. 5 e1. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 45.Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122(2):299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 46.Helderman JB, Welch CD, Leng X, O’Shea TM. Sepsis-associated electroencephalographic changes in extremely low gestational age neonates. Early Hum Dev. 2010;86(8):509–13. doi: 10.1016/j.earlhumdev.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 47.Volpe JJ. Postnatal Sepsis, Necrotizing Entercolitis, and the Critical Role of Systemic Inflammation in White Matter Injury in Premature Infants. J Pediatr. 2008;153(2):160–3. doi: 10.1016/j.jpeds.2008.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chau V, Brant R, Poskitt KJ, Tam EW, Synnes A, Miller SP. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res. 2012;71(3):274–9. doi: 10.1038/pr.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shane AL, Stoll BJ. Recent Developments and Current Issues in the Epidemiology, Diagnosis, and Management of Bacterial and Fungal Neonatal Sepsis. Am J Perinatol. 2013 doi: 10.1055/s-0032-1333413. [DOI] [PubMed] [Google Scholar]
- 50.Grether JK, Nelson KB, Dambrosia JM, Phillips TM. Interferons and cerebral palsy. J Pediatr. 1999;134(3):324–32. doi: 10.1016/s0022-3476(99)70458-0. [DOI] [PubMed] [Google Scholar]
- 51.Bogdan I, Leib SL, Bergeron M, Chow L, Tauber MG. Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J Infect Dis. 1997;176(3):693–7. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 52.Leib SL, Kim YS, Chow LL, Sheldon RA, Tauber MG. Reactive oxygen intermediates contribute to necrotic and apoptotic neuronal injury in an infant rat model of bacterial meningitis due to group B streptococci. J Clin Invest. 1996;98(11):2632–9. doi: 10.1172/JCI119084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75(3):376–80. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoon BH, Romero R, Kim CJ, et al. High expression of tumor necrosis factor-alpha and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997;177(2):406–11. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- 55.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177(1):19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 56.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174(5):1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 57.Martinez E, Figueroa R, Garry D, et al. Elevated Amniotic Fluid Interleukin-6 as a Predictor of Neonatal Periventricular Leukomalacia and Intraventricular Hemorrhage. Journal of maternal-fetal investigation : the official journal of French Society of Ultrasound in Medicine and Biology [et al] 1998;8(3):101–7. [PubMed] [Google Scholar]
- 58.Girard S, Kadhim H, Roy M, et al. Role of perinatal inflammation in cerebral palsy. Pediatric neurology. 2009;40(3):168–74. doi: 10.1016/j.pediatrneurol.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 59.Duggan PJ, Maalouf EF, Watts TL, et al. Intrauterine T-cell activation and increased proinflammatory cytokine concentrations in preterm infants with cerebral lesions. Lancet. 2001;358(9294):1699–700. doi: 10.1016/s0140-6736(01)06723-x. [DOI] [PubMed] [Google Scholar]
- 60.Procianoy RS, Silveira RC. Association between high cytokine levels with white matter injury in preterm infants with sepsis. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012;13(2):183–7. doi: 10.1097/PCC.0b013e3182231074. [DOI] [PubMed] [Google Scholar]
- 61.Ellison VJ, Mocatta TJ, Winterbourn CC, Darlow BA, Volpe JJ, Inder TE. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005;57(2):282–6. doi: 10.1203/01.PDR.0000148286.53572.95. [DOI] [PubMed] [Google Scholar]
- 62.Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56(10):1278–84. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 63.Edwards AD, Tan S. Perinatal infections, prematurity and brain injury. Curr Opin Pediatr. 2006;18(2):119–24. doi: 10.1097/01.mop.0000193290.02270.30. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Mallard C, Levy O. Potential role of Coagulase-negative staphylococcus infection in preterm brain injury. Advances in Neuroimmune Biology. 2012;3(1):41–8. [Google Scholar]
- 65.Gilles FH, Leviton A, Kerr CS. Endotoxin leucoencephalopathy in the telencephalon of the newborn kitten. J Neurol Sci. 1976;27(2):183–91. doi: 10.1016/0022-510x(76)90060-5. [DOI] [PubMed] [Google Scholar]
- 66.Dean JM, van de Looij Y, Sizonenko SV, et al. Delayed cortical impairment following lipopolysaccharide exposure in preterm fetal sheep. Ann Neurol. 2011;70(5):846–56. doi: 10.1002/ana.22480. [DOI] [PubMed] [Google Scholar]
- 67.Svedin P, Kjellmer I, Welin AK, Blad S, Mallard C. Maturational effects of lipopolysaccharide on white-matter injury in fetal sheep. Journal of child neurology. 2005;20(12):960–4. doi: 10.1177/08830738050200120501. [DOI] [PubMed] [Google Scholar]
- 68.Gavilanes AW, Strackx E, Kramer BW, et al. Chorioamnionitis induced by intraamniotic lipopolysaccharide resulted in an interval-dependent increase in central nervous system injury in the fetal sheep. Am J Obstet Gynecol. 2009;200(4):437, e1–8. doi: 10.1016/j.ajog.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Garnier Y, Berger R, Alm S, et al. Systemic endotoxin administration results in increased S100B protein blood levels and periventricular brain white matter injury in the preterm fetal sheep. Eur J Obstet Gynecol Reprod Biol. 2006;124(1):15–22. doi: 10.1016/j.ejogrb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 70.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173(6):3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 71.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain research reviews. 2009;59(2):278–92. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lehnardt S, Massillon L, Follett P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100(14):8514–9. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffmann O, Braun JS, Becker D, et al. TLR2 mediates neuroinflammation and neuronal damage. J Immunol. 2007;178(10):6476–81. doi: 10.4049/jimmunol.178.10.6476. [DOI] [PubMed] [Google Scholar]
- 74.Eklind S, Hagberg H, Wang X, et al. Effect of lipopolysaccharide on global gene expression in the immature rat brain. Pediatr Res. 2006;60(2):161–8. doi: 10.1203/01.pdr.0000228323.32445.7d. [DOI] [PubMed] [Google Scholar]
- 75.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25(7):1788–96. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain, behavior, and immunity. 2006;20(5):449–55. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Current pharmaceutical design. 2005;11(8):973–84. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- 78.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66(2):155–64. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 79.Keogh MJ, Bennet L, Drury PP, et al. Subclinical exposure to low-dose endotoxin impairs EEG maturation in preterm fetal sheep. American journal of physiology Regulatory, integrative and comparative physiology. 2012;303(3):R270–8. doi: 10.1152/ajpregu.00216.2012. [DOI] [PubMed] [Google Scholar]
- 80.Okun E, Griffioen KJ, Son TG, et al. TLR2 activation inhibits embryonic neural progenitor cell proliferation. Journal of neurochemistry. 2010;114(2):462–74. doi: 10.1111/j.1471-4159.2010.06778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Du X, Fleiss B, Li H, et al. Systemic stimulation of TLR2 impairs neonatal mouse brain development. PLoS One. 2011;6(5):e19583. doi: 10.1371/journal.pone.0019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bennet L, Cowie RV, Stone PR, et al. The neural and vascular effects of killed Su-Streptococcus pyogenes (OK-432) in preterm fetal sheep. American journal of physiology Regulatory, integrative and comparative physiology. 2010;299(2):R664–72. doi: 10.1152/ajpregu.00116.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lehnardt S, Henneke P, Lien E, et al. A mechanism for neurodegeneration induced by group B streptococci through activation of the TLR2/MyD88 pathway in microglia. J Immunol. 2006;177(1):583–92. doi: 10.4049/jimmunol.177.1.583. [DOI] [PubMed] [Google Scholar]
- 84.Lehnardt S, Wennekamp J, Freyer D, et al. TLR2 and caspase-8 are essential for group B Streptococcus-induced apoptosis in microglia. J Immunol. 2007;179(9):6134–43. doi: 10.4049/jimmunol.179.9.6134. [DOI] [PubMed] [Google Scholar]
- 85.Toti P, De Felice C, Schürfeld K, et al. Cyclooxygenase-2 immunoreactivity in the ischemic neonatal human brain. An autopsy study. J Submicrosc Cytol Pathol. 2001;33(3):245–9. [PubMed] [Google Scholar]
- 86.Sprung CL, Peduzzi PN, Shatney CH, et al. Impact of encephalopathy on mortality in the sepsis syndrome. The Veterans Administration Systemic Sepsis Cooperative Study Group. Crit Care Med. 1990;18(8):801–6. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 87.Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY) 2000;223(1):22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- 88.Spellerberg B, Prasad S, Cabellos C, Burroughs M, Cahill P, Tuomanen E. Penetration of the blood-brain barrier: enhancement of drug delivery and imaging by bacterial glycopeptides. J Exp Med. 1995;182(4):1037–43. doi: 10.1084/jem.182.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orihuela CJ, Fillon S, Smith-Sielicki SH, et al. Cell wall-mediated neuronal damage in early sepsis. Infect Immun. 2006;74(7):3783–9. doi: 10.1128/IAI.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stolp HB, Ek CJ, Johansson PA, et al. Effect of minocycline on inflammation-induced damage to the blood-brain barrier and white matter during development. The European journal of neuroscience. 2007;26(12):3465–74. doi: 10.1111/j.1460-9568.2007.05973.x. [DOI] [PubMed] [Google Scholar]
- 91.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 92.Laflamme N, Soucy G, Rivest S. Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. Journal of neurochemistry. 2001;79(3):648–57. doi: 10.1046/j.1471-4159.2001.00603.x. [DOI] [PubMed] [Google Scholar]
- 93.Turrin NP, Gayle D, Ilyin SE, et al. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain research bulletin. 2001;54(4):443–53. doi: 10.1016/s0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- 94.Vallieres L, Rivest S. Regulation of the genes encoding interleukin-6, its receptor, and gp130 in the rat brain in response to the immune activator lipopolysaccharide and the proinflammatory cytokine interleukin-1beta. Journal of neurochemistry. 1997;69(4):1668–83. doi: 10.1046/j.1471-4159.1997.69041668.x. [DOI] [PubMed] [Google Scholar]
- 95.Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: prematurity means more than immaturity. J Matern Fetal Neonatal Med. 2011;24(1):25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- 96.Strunk T, Prosser A, Levy O, et al. Responsiveness of human monocytes to the commensal bacterium Staphylococcus epidermidis develops late in gestation. Pediatr Res. 2012;72(1):10–8. doi: 10.1038/pr.2012.48. [DOI] [PubMed] [Google Scholar]
- 97.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7(5):379–90. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 98.Schultz C, Temming P, Bucsky P, Gopel W, Strunk T, Hartel C. Immature anti-inflammatory response in neonates. Clin Exp Immunol. 2004;135(1):130–6. doi: 10.1111/j.1365-2249.2004.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179(2):507–13. doi: 10.1016/s0002-9378(98)70387-4. [DOI] [PubMed] [Google Scholar]
- 100.Bracci R, Buonocore G. Chorioamnionitis: a risk factor for fetal and neonatal morbidity. Biol Neonate. 2003;83(2):85–96. doi: 10.1159/000067956. [DOI] [PubMed] [Google Scholar]
- 101.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290(20):2677–84. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 102.Larouche A, Roy M, Kadhim H, Tsanaclis AM, Fortin D, Sebire G. Neuronal injuries induced by perinatal hypoxic-ischemic insults are potentiated by prenatal exposure to lipopolysaccharide: animal model for perinatally acquired encephalopathy. Developmental neuroscience. 2005;27(2-4):134–42. doi: 10.1159/000085985. [DOI] [PubMed] [Google Scholar]
- 103.Coumans AB, Middelanis JS, Garnier Y, et al. Intracisternal application of endotoxin enhances the susceptibility to subsequent hypoxic-ischemic brain damage in neonatal rats. Pediatr Res. 2003;53(5):770–5. doi: 10.1203/01.PDR.0000059221.40073.82. [DOI] [PubMed] [Google Scholar]
- 104.Wang X, Stridh L, Li W, et al. Lipopolysaccharide sensitizes neonatal hypoxicischemic brain injury in a MyD88-dependent manner. J Immunol. 2009;183(11):7471–7. doi: 10.4049/jimmunol.0900762. [DOI] [PubMed] [Google Scholar]
- 105.Kendall GS, Hirstova M, Horn S, et al. TNF gene cluster deletion abolishes lipopolysaccharide-mediated sensitization of the neonatal brain to hypoxic ischemic insult. Laboratory investigation; a journal of technical methods and pathology. 2011;91(3):328–41. doi: 10.1038/labinvest.2010.192. [DOI] [PubMed] [Google Scholar]
- 106.Wang X, Hagberg H, Nie C, Zhu C, Ikeda T, Mallard C. Dual role of intrauterine immune challenge on neonatal and adult brain vulnerability to hypoxiaischemia. Journal of neuropathology and experimental neurology. 2007;66(6):552–61. doi: 10.1097/01.jnen.0000263870.91811.6f. [DOI] [PubMed] [Google Scholar]
- 107.Girard S, Kadhim H, Beaudet N, Sarret P, Sebire G. Developmental motor deficits induced by combined fetal exposure to lipopolysaccharide and early neonatal hypoxia/ischemia: a novel animal model for cerebral palsy in very premature infants. Neuroscience. 2009;158(2):673–82. doi: 10.1016/j.neuroscience.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 108.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58(1):112–6. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 109.Hickey E, Shi H, Van Arsdell G, Askalan R. Lipopolysaccharide-induced preconditioning against ischemic injury is associated with changes in toll-like receptor 4 expression in the rat developing brain. Pediatr Res. 2011;70(1):10–4. doi: 10.1203/PDR.0b013e31821d02aa. [DOI] [PubMed] [Google Scholar]
- 110.Sarkar S, Bhagat I, Dechert R, Schumacher RE, Donn SM. Severe intraventricular hemorrhage in preterm infants: comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Am J Perinatol. 2009;26(6):419–24. doi: 10.1055/s-0029-1214237. [DOI] [PubMed] [Google Scholar]
- 111.Sehgal A, El-Naggar W, Glanc P, Asztalos E. Risk factors and ultrasonographic profile of posterior fossa haemorrhages in preterm infants. J Paediatr Child Health. 2009;45(4):215–8. doi: 10.1111/j.1440-1754.2008.01456.x. [DOI] [PubMed] [Google Scholar]
- 112.Inder TE, Anderson NJ, Spencer C, Wells S, Volpe JJ. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR American journal of neuroradiology. 2003;24(5):805–9. [PMC free article] [PubMed] [Google Scholar]
- 113.Miller SP, Cozzio CC, Goldstein RB, et al. Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR American journal of neuroradiology. 2003;24(8):1661–9. [PMC free article] [PubMed] [Google Scholar]
- 114.Filan PM, Hunt RW, Anderson PJ, Doyle LW, Inder TE. Neurologic outcomes in very preterm infants undergoing surgery. J Pediatr. 2012;160(3):409–14. doi: 10.1016/j.jpeds.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 115.Nguyen The Tich S. Anderson PJ, Shimony JS, Hunt RW, Doyle LW, Inder TE. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR American journal of neuroradiology. 2009;30(1):125–31. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–75. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 117.Watterberg KL, Gerdes JS, Cole CH, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. 2004;114(6):1649–57. doi: 10.1542/peds.2004-1159. [DOI] [PubMed] [Google Scholar]
- 118.Wolfe KB, Snyder CC, Gisslen T, et al. Modulation of lipopolysaccharide-induced chorioamnionitis in fetal sheep by maternal betamethasone. Reproductive sciences. 2013;20(12):1447–54. doi: 10.1177/1933719113488445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wilson-Costello D, Walsh MC, Langer JC, et al. Impact of postnatal corticosteroid use on neurodevelopment at 18 to 22 months’ adjusted age: effects of dose, timing, and risk of bronchopulmonary dysplasia in extremely low birth weight infants. Pediatrics. 2009;123(3):e430–7. doi: 10.1542/peds.2008-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment after the first week of life for bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98(4):289–96. doi: 10.1159/000286212. [DOI] [PubMed] [Google Scholar]
- 121.Doyle LW, Ehrenkranz RA, Halliday HL. Dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology. 2010;98(3):217–24. doi: 10.1159/000286210. [DOI] [PubMed] [Google Scholar]
- 122.Rademaker KJ, de Vries WB. Long-term effects of neonatal hydrocortisone treatment for chronic lung disease on the developing brain and heart. Semin Fetal Neonatal Med. 2009;14(3):171–7. doi: 10.1016/j.siny.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 123.Sandberg K, Fellman V, Stigson L, Thiringer K, Hjalmarson O. N-acetylcysteine administration during the first week of life does not improve lung function in extremely low birth weight infants. Biol Neonate. 2004;86(4):275–9. doi: 10.1159/000080089. [DOI] [PubMed] [Google Scholar]
- 124.Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. Journal of neuroscience research. 2004;78(3):347–61. doi: 10.1002/jnr.20261. [DOI] [PubMed] [Google Scholar]
- 125.Chang EY, Zhang J, Sullivan S, Newman R, Singh I. N-acetylcysteine attenuates the maternal and fetal proinflammatory response to intrauterine LPS injection in an animal model for preterm birth and brain injury. J Matern Fetal Neonatal Med. 2011;24(5):732–40. doi: 10.3109/14767058.2010.528089. [DOI] [PubMed] [Google Scholar]
- 126.Beloosesky R, Ginsberg Y, Khatib N, et al. Prophylactic maternal N-acetylcysteine in rats prevents maternal inflammation-induced offspring cerebral injury shown on magnetic resonance imaging. Am J Obstet Gynecol. 2013;208(3):213, e1–6. doi: 10.1016/j.ajog.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 127.Wang X, Svedin P, Nie C, et al. N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann Neurol. 2007;61(3):263–71. doi: 10.1002/ana.21066. [DOI] [PubMed] [Google Scholar]
- 128.Probyn ME, Cock ML, Duncan JR, et al. The anti-inflammatory agent N-acetyl cysteine exacerbates endotoxin-induced hypoxemia and hypotension and induces polycythemia in the ovine fetus. Neonatology. 2010;98(2):118–27. doi: 10.1159/000280385. [DOI] [PubMed] [Google Scholar]
- 129.Strunk T, Hartel C, Temming P, Matzke N, Zimmer J, Schultz C. Erythropoietin inhibits cytokine production of neonatal and adult leukocytes. Acta Paediatr. 2008;97(1):16–20. doi: 10.1111/j.1651-2227.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 130.Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 2009;65(5):485–92. doi: 10.1203/PDR.0b013e31819d90c8. [DOI] [PubMed] [Google Scholar]
- 131.Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. Journal of neuropathology and experimental neurology. 2001;60(4):386–92. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 132.Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, Gleason CA. Erytropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate. 2004;85(2):138–44. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- 133.Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125(5):e1135–42. doi: 10.1542/peds.2009-2268. [DOI] [PubMed] [Google Scholar]
- 134.Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012;130(4):683–91. doi: 10.1542/peds.2012-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67(5):657–66. doi: 10.1002/ana.21977. [DOI] [PubMed] [Google Scholar]
- 136.Tolsma KW, Allred EN, Chen ML, Duker J, Leviton A, Dammann O. Neonatal bacteremia and retinopathy of prematurity: the ELGAN study. Archives of ophthalmology. 2011;129(12):1555–63. doi: 10.1001/archophthalmol.2011.319. [DOI] [PubMed] [Google Scholar]
- 137.Aher SM, Ohlsson A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2012;10 doi: 10.1002/14651858.CD004865.pub3. CD004865. [DOI] [PubMed] [Google Scholar]
- 138.Travadi J, Patole S, Charles A, Dvorak B, Doherty D, Simmer K. Pentoxifylline reduces the incidence and severity of necrotizing enterocolitis in a neonatal rat model. Pediatr Res. 2006;60(2):185–9. doi: 10.1203/01.pdr.0000228325.24945.ac. [DOI] [PubMed] [Google Scholar]
- 139.Dilek M, Kumral A, Okyay E, et al. Protective effects of pentoxifylline on lipopolysaccharide-induced white matter injury in a rat model of periventricular leukomalasia. J Matern Fetal Neonatal Med. 2013;26(18):1865–71. doi: 10.3109/14767058.2013.798290. [DOI] [PubMed] [Google Scholar]
- 140.Harris E, Schulzke SM, Patole SK. Pentoxifylline in preterm neonates: a systematic review. Paediatr Drugs. 2010;12(5):301–11. doi: 10.2165/11532600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 141.Haque KN, Pammi M. Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev. 2011;(10) doi: 10.1002/14651858.CD004205.pub2. CD004205. [DOI] [PubMed] [Google Scholar]
- 142.Buller KM, Carty ML, Reinebrant HE, Wixey JA. Minocycline: a neuroprotective agent for hypoxic-ischemic brain injury in the neonate? Journal of neuroscience research. 2009;87(3):599–608. doi: 10.1002/jnr.21890. [DOI] [PubMed] [Google Scholar]
- 143.Carr R, Brocklehurst P, Dore CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet. 2009;373(9659):226–33. doi: 10.1016/S0140-6736(09)60071-4. [DOI] [PubMed] [Google Scholar]
- 144.Brocklehurst P, Farrell B, King A, et al. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. 2011;365(13):1201–11. doi: 10.1056/NEJMoa1100441. [DOI] [PubMed] [Google Scholar]
- 145.Levy O. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood. 2000;96(8):2664–72. [PubMed] [Google Scholar]
- 146.Guinan EC, Barbon CM, Kalish LA, et al. Bactericidal/Permeability-Increasing Protein (rBPI21) and Fluoroquinolone Mitigate Radiation-Induced Bone Marrow Aplasia and Death. Sci Transl Med. 2011;3(110):110ra8. doi: 10.1126/scitranslmed.3003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Levin M, Quint PA, Goldstein B, et al. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. rBPI21 Meningococcal Sepsis Study Group. Lancet. 2000;356(9234):961–7. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 148.Manzoni P, Stolfi I, Messner H, et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics. 2012;129(1):116–23. doi: 10.1542/peds.2011-0279. [DOI] [PubMed] [Google Scholar]
- 149.Bolouri H, Savman K, Wang W, et al. Innate defence regulator peptide 1018 protects against perinatal brain injury. Ann Neurol. 2013 doi: 10.1002/ana.24087. [DOI] [PubMed] [Google Scholar]
- 150.Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28(2):215–23. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]