Abstract
Rho GTPases are involved in the acquisition of all the hallmarks of cancer, which comprise 6 biological capabilities acquired during the development of human tumors. The hallmarks include proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis programs, as defined by Hanahan and Weinberg.1 Controlling these hallmarks are genome instability and inflammation. Emerging hallmarks are reprogramming of energy metabolism and evading immune destruction. To give a different view to the readers, we will not be focusing on invasion, metastasis, or cytoskeletal remodeling, but we will review here how Rho GTPases contribute to other hallmarks of cancer with a special emphasis on malignant transformation.
Keywords: Rho GTPases, transformation, tumor progression, oncogene, proliferation, senescence, metabolism, inflammation, survival
Introduction
Rho GTPases are a family of small G proteins that regulate the cytoskeleton dynamics, as well as other key cell functions such as cell cycle progression, cell migration, malignant transformation, cell polarity, invasion, and metastasis.2 Rho GTPases are molecular switches that cycle between a GDP-bound inactive form and a GTP-bound active form. Upon activation, Rho GTPases interact with downstream effectors that lead a signaling cascade to direct cellular responses.2
Tremendous efforts have been dedicated in the last years to elucidate and decipher the role of Rho GTPases on cytoskeletal remodeling.2,3 In this review, we will not address the contribution of Rho GTPases to cell migration. Instead, we will focus on how this family of proteins regulated other hallmarks of cancer, including cell cycle progression, oncogenic transformation, tumor suppression, cancer cell survival, senescence, inflammation, angiogenesis, and altered metabolism.
Aberrant Expression of Rho GTPases in Tumorigenesis
Several Rho GTPases have been found overexpressed in human tumors and, in some cases, this correlates with cancer progression4 (see Table 1 summarizing aberrant regulation of Rho GTPases). RhoA overexpression has been associated with progression in testicular cancer5 and breast cancer.6,7 Overexpression of RhoA has also been described in colon and lung cancers7 and in head and neck squamous cell carcinoma.8 RhoA expression and activity has also been found to be increased in liver cancer.9 RhoB has been shown to be overexpressed in breast cancer and correlate with progression of the disease,6 while other studies report a downregulation of RhoB expression in squamous cell carcinoma10 and loss of expression in lung cancer progression,11 suggesting a tumor suppressor role for RhoB (see “Rho GTPases regulating tumor suppressors” subheading below). Overexpression of RhoC has been identified in inflammatory breast cancer12 and colorectal carcinoma;13 and it correlates with progression and poor prognosis in melanoma14 and pancreatic adenocarcinoma.15 Rac1 is overexpressed in testicular cancer,5 breast cancer,7 and various leukemias.16 A self-activating splice variant of Rac1, Rac1b, was reported to be highly expressed in colon,17 breast,16 and non-small cell lung19 carcinomas; and it is thought to mediate the epithelial–mesenchymal transition in lung epithelial cells by upregulating the transcription factor Snail.17,20,21 Cdc42 has been shown to be overexpressed in several human cancers, such as non-small cell lung cancer,22 colorectal adenocarcinoma,23 melanoma,24 breast cancer,6,7 and testicular cancer.5 Overall, the frequent overexpression of Rho GTPases in cancer highlights the important role of Rho proteins in the different steps of tumorigenesis and the value of analyzing Rho protein levels as prognostic markers of cancer progression.
Table 1. Aberrant Rho GTPase expression in cancer.
| Rho GTPase | Deregulation | Tumor type |
|---|---|---|
| RhoA | Overexpression | Testicular cancer,5 breast,6,7 colon,7 lung,7 liver,9 head and neck squamous cell carcinoma,8 hepatocellular carcinoma,231 bladder,232 esophageal squamous cell carcinoma,233 ovarian carcinoma,234 gastric carcinoma235 |
| RhoB | Overexpression | Breast cancer6,7 |
| Downregulation | Squamous cell carcinoma10 | |
| Loss of expression | Lung cancer11 | |
| RhoC | Overexpression | Melanoma,14 inflammatory breast cancer,12 breast cancer,6,7,236 colorectal carcinoma,13 pancreatic ductal adenocarcinoma,15 non-small cell lung carcinoma,237 gastric cancer,238 hepatocellular carcinoma,239 prostate cancer,240 head and neck squamous cell carcinoma,241 squamous cell carcinoma of the skin,242 bladder cancer,232 esophageal squamous cell carcinoma,233 ovarian carcinoma234 |
| Rac1 | Overexpression | Testicular cancer,5 breast cancer,7 leukemia,16 gastric carcinoma,235 prostate cancer243 |
| Alternative splicing, Rac1b | Breast,18 colon carcinoma,17 non-small cell lung cancer19 | |
| Cdc42 | Overexpression | Non-small cell lung cancer,22 colorectal adenocarcinoma,23 melanoma,24 breast,6,7 testicular cancer5 |
Rho GTPases Mutated in Cancer
Unlike the Ras family of GTPases, which are mutated in 30% of human tumors,25,26 Rho GTPases had rarely been identified to be mutated in human cancers until recent advances on genome sequencing.27-29 Several studies in recent years have started to challenge the concept that Rho GTPases are not mutated in cancer30 (Table 2). Rac1 mutations located in the effector domain of the protein have been found in human brain tumors, which enhanced the activity of Rac1 and increased the survival of brain tumors.31 Recently, a human exome sequencing analysis allowed for the identification of a Rac1 mutation, Rac1P29S. This mutation was found in 5% of sun-exposed melanomas, making Rac1 the fourth most commonly mutated gene in melanoma after BRAF, NRAS and p53, and one of the most common mutations discovered in a Rho family GTPase.27-29 A mutation in a homologous residue in Rac2P29L has also been found in melanoma, along with a mutation in Cdc42G12D.27 Rac1P29S is a recurrent somatic missense mutation at codon 29 that results in substitution of a proline to a serine residue. This location is distinct from the commonly found oncogenic mutations in the Ras oncogenes and Rac1P29S has been reported to be a spontaneously activating cancer-associated GTPase.32 In contrast to Ras mutations, which affect the GTPase activity, Rac1P29S conserves intrinsic GTP hydrolysis and is activated by a fast-cycling GDP/GTP nucleotide exchange. Activated Rac1P29S has increased binding activity toward Rac1 effectors and mutated Rac1 induces increased cell proliferation, altered cell migration, and stimulates membrane ruffling and MAPK signaling.28 Other transforming Rac mutations (Rac1N92I, Rac1C157Y, Rac2P29L, Rac2P29Q) have also been found in human cancer cell lines.33
Table 2. Rho GTPase mutations in cancer.
| Rho GTPase | Mutation | Tumor type |
|---|---|---|
| Rac1 | Deletions, frame shift and point mutations | Brain cancer31 |
| P29S | Melanoma27,28 | |
| N92I | HT1080 fibrosarcoma cell line33 | |
| C157Y | Lung adenocarcinoma33 | |
| Rac2 | P29L | Melanoma27 HCC1143 breast cancer cell line33 |
| P29Q | KCL-22 chronic myeloid leukemia cell line33 | |
| Cdc42 | G12D | Melanoma27 |
| RhoA | G17V | Angioimmunoblastic T-cell lymphomas34,35 |
| RhoH | Rearrangement | Non-Hodgkin’s lymphomas, multiple myeloma37 |
| Point mutation | Diffuse large B-cell lymphoma36 |
Despite the oncogenic potential of RhoA in several human cancers (Table 1), very recent studies have unexpectedly identified a loss-of-function mutation on RhoA (RhoAG17V) in 50–68% of angioimmunoblastic T cell lymphomas.34,35 Whether activating mutations or aberrant expression of the other Rho GTPases contribute to the malignant progression of T-cell derived tumors remains to be elucidated.
RhoH gene is altered in tumors of myeloid origin. RhoH has been reported to be frequently rearranged in non-Hodgkin’s lymphomas and multiple myeloma, and the 5′untranslated region of RhoH gene is mutated in diffuse large cell lymphomas.36,37
These studies might just highlight the important advances that next-generation sequencing analysis will be able to provide in the near future. Identification of genetic alterations in Rho GTPases will help to understand the biological relevance of such mutations in cancer progression.
Rho GTPases and the Cell Cycle
In addition to their important role in regulating cytoskeletal dynamics,2,3 Rho GTPases are also important regulators of cell cycle progression and proliferation (Fig. 1). During cell proliferation, the accurate transition from G1 phase of the cell cycle to S phase is a critical step, which is controlled by the cyclin-dependent kinases (CDKs) as well as the cyclin regulatory subunits. Type D cyclins (cyclin D1, cyclin D2, and cyclin D3) associate with CDK4 or CDK6, whereas type E cyclins (cyclins E1 and cyclin E2) associate with CDK2. The activity of cyclin-CDK complexes is negatively regulated by CDK inhibitors (CDKIs), either by direct inhibition of CDK activity (the INK4 CDKI family of proteins) or by binding to the cyclin-CDK complexes (the Cip/Kip family of CDKIs). The Rho proteins have been reported to modulate G1 progression.38
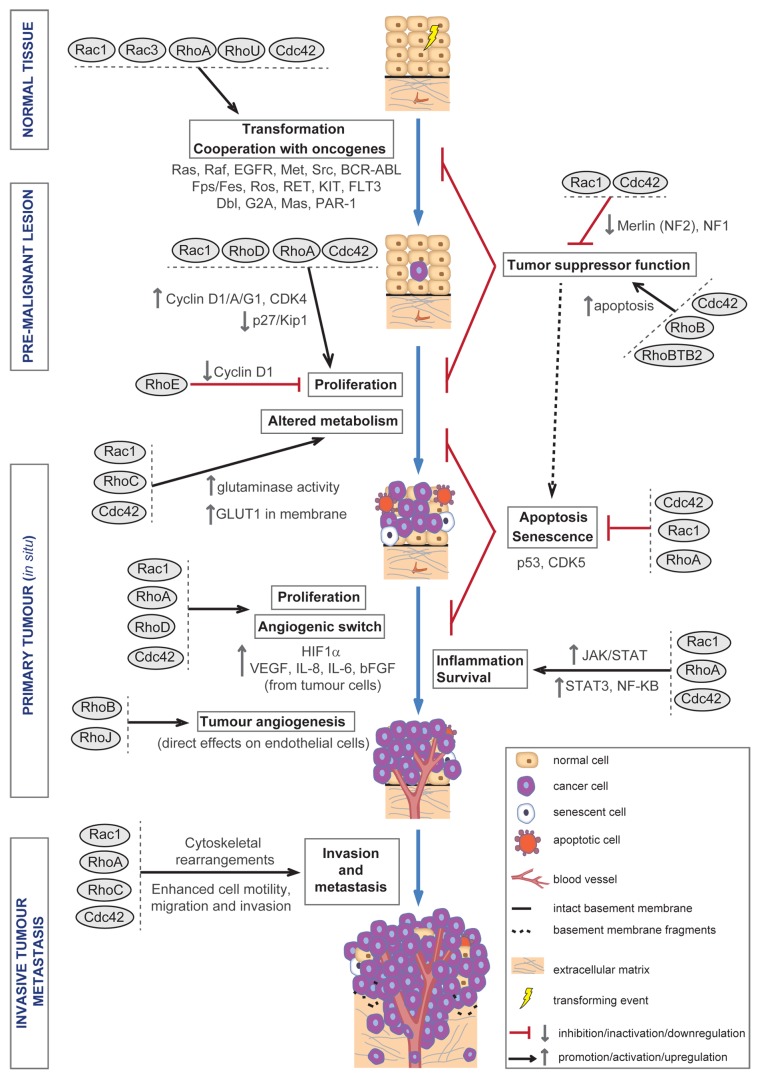
Figure 1. Roles of Rho GTPases during malignant transformation and tumor progression. Diagram showing different roles of Rho GTPases during cell transformation and tumor progression. Upon cell transformation, Rho GTPases contribute—alone or in cooperation with oncogenes—to aberrant proliferation, altered metabolism, increased survival, and evasion of senescence and apoptosis, which will sustain tumor proliferation. Later on, Rho GTPases also contribute to the development of an inflammatory environment, which increases cell survival and tumor progression; and to the induction of tumor angiogenesis, which will sustain tumor growth further and may also serve as an escape route for cancer cells to colonize distant organs. Rho GTPases are also essential for the cytoskeletal changes underlying cell motility and invasion, which allow cancer cells to migrate away from the primary tumor and invade surrounding and later distant tissues, ultimately developing metastasis.
RhoA is required for G1 to S progression in mammary epithelial cells, in response to normal or oncogenic signaling, by repression of p21 (Waf1/Cip1)39 and the induction of cyclin D1.40 RhoA also downregulates CDK inhibitor p27 (Kip1) during the G1 phase of the cell cycle41 and contributes to maintaining the correct timing of cyclin D1 expression in G1 phase by sustaining ERK signaling.42 In the same lines, RhoA-mediated activation of ROCK and mDia promotes G1 progression via Skp2-mediated degradation of p27 (Kip1).43
RhoC has been shown to promote proliferation of gastric cells by recruitment of IQ-domain GTPase-activating protein 1 (IQGAP1) and increased expression of cyclin E and cyclin D1.44 In normal hepatocytes, RhoC overexpression induced proliferation and anchorage-independent growth by increasing the expression of cell cycle-related genes, such as cyclin G1, cyclin A, cyclin D1, and CDK4, and downregulating the mRNA expression of G1 cyclin-CDK inhibitor p27 (Kip1) and the formation of tumors in nude mice.45
Rac1 and p21-activated kinase (PAK) have been shown to induce cyclin D1 expression primarily through activation of nuclear factor of kappa light polypeptide gene enhancer in B-cells (NF-κB) to activate the cyclin D1 promoter.46 Rac1 can also directly activate cyclin D1 expression47 and mediate integrin-induced cyclin D1 translation.48 Rac1 and ERK are required for the mid-G1 phase induction of cyclin D1 and S phase entry in both epithelial and mesenchymal cells.49 On the other hand, in mouse embryonic fibroblasts, cyclin D1 expression is induced by Rac1 independently of NF-κB signaling pathway.50 The Rac effector WAVE3 (Wiskott-Aldrich syndrome protein) has also been shown to promote tumor growth in orthotopic breast cancer mouse models.51 Adenocarcinoma cells overexpressing or lacking Rac1 and orthotopically injected into mice showed that Rac1 is important for tumor progression.52 Cdc42 induces cyclin E expression and promotes G1 progression through p70 S6 kinase53 and regulates cyclin D1 expression in bovine tracheal myocytes.54 Therefore, inhibition of Rac1 and Cdc42, using a novel small molecule inhibitor targeting both Rho GTPases, caused a decrease in tumor growth of primary human prostate cancer xenografts and prolonged survival in mice. Treatment with this inhibitor downregulated cyclin D1 expression and PAK and Akt activity in prostate cancer cells.55
Other less characterized Rho GTPases, such as RhoD, can extert a positive regulation of G1/S-phase progression and increased proliferation via its novel effector Diaph1.56 Among the atypical Rho GTPases, the putative tumor suppressor RhoBTB2 might also negatively regulate cell cycle progression and induce apoptosis.57 On the other hand, RhoE overexpression inhibits cell cycle progression by decreasing cyclin D1 expression.58
Further to their role in cell cycle regulation as mediators of G1 progression, Rho GTPases can also contribute to mitosis and/or cytokinesis. These processes need to be carefully regulated in order to maintain genomic stability and to prevent tumorigenesis.59 RhoA regulates centrosome duplication and positioning.60 The Rho effector Citron-K has been shown to have a role in G2/M progression of hepatocytes.61 A dominant negative Rac1 expressed in Rat2 fibroblasts resulted in accumulation of the G2/M phase.62 In late G2 phase, expression of a mutant of Rac1 that is localized in the nucleus increased the mitotic rate.63 During metaphase, Cdc42 and mDia3 regulate spindle microtubule attachment to kinetochores and control mitosis, while RhoJ/TCL and RhoQ/TC10 enhance this effect when suppressed in conjunction with Cdc42.64 Cdc42 and Ect2 have been reported to regulate spindle assembly in mitosis in Xenopus embryogenesis.65,66
The process of the cytoplasmic division or cytokinesis, which terminates cell division, requires the formation and contraction of a cortical ring containing actin and myosin, resulting in cell body cleavage.67 RhoA and its downstream effectors, such as ROCK and Citron-K, contribute to different aspects of this process.67 Active RhoA promotes cytokinesis by coordinating the diaphanous-related formins (DRF)-mediated formation of linear filamentous actin with contractile force driven by myosin-II motor activity to promote the assembly and constriction of a contractile ring.68-70 ROCK contributes to the progression of cytokinesis through the phosphorylation of myosin light chain at the cleavage furrow,71 whereas Citron-K associates with the central spindle kinesin family member 14 (KIF14), and these two proteins depend on each other for proper localization during cytokinesis.72,73 Further roles of Rho GTPases in mitosis and/or cytokinesis have been reviewed in detail elsewhere.59,74
These studies reveal that Rho proteins are key players in promoting uncontrolled cell proliferation by stimulating positive regulators and decreasing negative regulators of cell cycle progression.
Rho GTPases Mediate Oncogenic Transformation
Rho GTPases are required for Ras GTPase-mediated oncogenesis and also for aberrant growth induced by other oncoproteins that will be discussed below, such as polyomavirus middle T antigen;75,76 tyrosine kinases such as Abl, Met, Fps, BCR-Abl, RET, epidermal growth factor receptor and insulin-like growth factor receptor; G protein-coupled receptors (Mas, G2A, M1-muscarinic receptor, and PAR-1), and Dbl family proteins (Fig. 1).77-88
Ras-promoted oncogenic transformation
Ras GTPase family of proteins are the most frequently mutated genes in cancer.25,26 Several studies reported that Ras transformation of rodent fibroblasts is prevented by inhibitory mutants of RhoA,79,82,85,86,89 RhoB,80 RhoG,86 Rac1,79,84,86 TC10,81and Cdc42;83 whereas constitutively activated mutants of these proteins can lead to cellular transformation of rodent fibroblasts. Rac1 has also been shown to suppress Ras-induced apoptosis by a mechanism linked with NF-κB activation contributing to Ras oncogenic transformation.90
Activated Rac1 can cooperate with activated membrane-targeted Raf-1 (Raf-CAAX)84 to promote malignant transformation, while Rac1 and Raf induce E1A-dependent transformation of primary BRK rat epithelial cells.91 Activated Rac1 also cooperates with MEK1 to promote growth transformation of FRTL-5 rat thyroid epithelial cells.92 In many cells, PAK signaling downstream of Ras and PI3 kinase sustains cell transformation.93-95 Furthermore, the less characterized Rho GTPase Wrch1/RhoU is able to induce transformation of fibroblasts when overexpressed.96,97
Several lines of evidence suggest that each GTPase alone can confer an advantage in the growth-promoting actions of Ras-mediated oncogenesis. Activated RhoA or Rac1 stably expressed in NIH-3T3 cells or constitutively active Cdc42 expressed in Rat-1 fibroblasts promote anchorage-independent growth and the formation of tumors in nude mice.79,83,98 Nevertheless, the oncogenic potential of Rho GTPases is not comparable to the morphologic transformation induced by activated Ras, as measured by formation of foci or growth in soft agar.83-85,99
More recent studies using in vivo models have shown that Rac1 contributes to tumorigenesis in K-RasG12D-driven lung tumors100 and oral papillomas.101 Rac1 also cooperates with N-RasQ61K to promote dermal melanocyte survival in vivo and to increase invasiveness on primary melanocytes in vitro and in vivo.102 Inhibition of Rac1 in melanoma tumors harboring the mutation N-RasQ61K suppresses tumor growth and lymph node spread.102 In a colorectal carcinoma model, Rac1-overexpressing adenocarcinoma cells orthotopically injected into mice accelerated tumor formation.52 The alternative splice variant of Rac1, Rac1b, has been found to be upregulated in a significant fraction of lung tumors, correlating with mutational status of K-Ras. Moreover, expression of Rac1b promotes K-Ras-induced lung adenocarcinoma in vivo.19
These studies infer that Ras oncogenes in different cancers will take advantage of specific Rho GTPase signaling cascades to further promote tumorigenesis.
Tyrosine kinase oncoprotein transformation
Epidermal growth factor receptor (EGFR)
EGFR is an oncoprotein that is overexpressed, mutated, and/or aberrantly activated in several human cancers.103 Inappropriate EGFR signaling contributes to tumor progression through activation of mitogenic signaling pathways.104 Therefore, EGFR activity must be tightly regulated by different mechanisms, such as ligand-mediated receptor activation and through endocytosis and recycling/degradation.105 Both active RhoA106 and/or Rac1107 are required for EGFR-induced mitogenesis and cell transformation. In addition, Rho GTPases also contribute to the disruption of the normal EGFR endocytosis and degradation cycles, which leads to aberrant and sustained mitogenic signal. RhoA and ROCK negatively regulate EGFR endocytosis through activation and recruitment of endophilin A1 to the EGFR-c-Cbl-CIN85 complex, which reduces EGFR endocytosis.108,109 Activated RhoB110 and Cdc42111 can also delay EGFR endocytic trafficking. On the other hand, activation of Cdc42 prevents EGFR degradation through sequestering the ubiquitin ligase c-Cbl, which contributes to the initiation of EGFR degradation.112,113
Met/Hepatocyte growth factor (HGF) receptor
Met is a receptor tyrosine kinase involved in cell transformation and which is overexpressed and mutated in a variety of tumors.114 Several studies using dominant negative mutants of Rho GTPases have shown the implication of Rac1 and Cdc42 in mediating the transforming ability of Met in fibroblasts and epithelial cells.115,116
Insulin-like growth factor-I receptor (IGFR)
IGFR is a tyrosine kinase receptor that plays an important role in the maintenance of the transformed phenotype. Upon activation with several ligands, IGFR leads to cell type-dependent proliferation, differentiation or inhibition of apoptosis.117 Transformation by IGFR requires Rho GTPase activity, since dominant negative mutants of RhoA, Rac1, and Cdc42 inhibited IGFR-induced fibroblast transformation, as measured by focus and colony forming ability. In particular RhoA was suggested to promote escape of contact inhibition, whereas Cdc42 seems to be more important for anchorage-independent growth.87
Src
The cytoplasmic tyrosine kinase Src was initially identified as the protein product of the viral oncogene v-Src, responsible for the transforming ability of the Rous sarcoma virus.118-120 Its normal cellular counterpart, the product of the proto-oncogene c-Src, plays a key role in signal transduction processes by acting downstream from several cell surface receptors, such as EGFR, and it is amplified in a variety of cancers.120 RhoA, Rac1, and Cdc42 play roles in cellular transformation mediated by Src.121 Rac1 activation by the GEFs Vav2 and Tiam1 was shown to be required for Src-induced cell transformation.122 The Rac1 effector WAVE was found to be activated in v-Src-transformed cells through phosphorylation downstream of MAPK signaling, suggesting its potential role in cell transformation.123 The Rho effector mDia1 is also essential for v-Src-induced cellular transformation, since this process is inhibited in mDia1-deficient cells.124 Src kinases, both v-Src and c-Src, need to be directed to a specific subcellular structure(s) to induce transformation. Deficiency of mDia decreases the levels of several tyrosine-phosphorylated proteins in v-Src-transformed cells, which impairs the translocation of v-Src from the perinuclear region to the cell periphery. Such impairment leads to the downregulation of downstream signaling pathways and suppression of v-Src-induced cell transformation.124 Likewise, RhoB also regulates the peripheral translocation of Src and ultimately its activity via endosomal transport.125,126
Abl and BCR
The viral oncogene v-Abl of the Abelson murine lymphosarcoma virus127 has been shown to require Rac1 to induce mitogenesis.128 The human ortholog of Abl was identified as part of a fusion oncoprotein with breakpoint cluster region (BCR), BCR-ABL, common in chronic myeloid leukemia (CML) patients,129 and which can be found as the p190- and p210-BCR-ABL isoforms. The p210-BCR-ABL isoform displays GEF activity through the double homology (DH) domain and can activate Rac1, RhoA, and Cdc42.130 Rac1 and Rac2 deficiency was described to impair myeloid leukemogenesis induced by p210-BCR-ABL expression in hematopoietic stem and progenitor cells.131,132 It has been proposed that Rac2-induced reactive oxygen species (ROS) generation133 could lead to DNA damage and genetic instability in BCR-ABL leukemias.134 The p190-BCR-ABL lacks a DH domain but can activate Rac1,130 suggesting activation of alternative GEFs. For example, Vav3 Dbl family protein (see below) was required for p190-BCR-ABL-induced leukemogenesis, since Vav3 deficiency decreased p190-BCR-ABL-induced Rac activation and cell proliferation.135 In vivo studies of Rac3-null mice have reported that Rac3, but not Rac1 or Rac2, specifically contributes to the development of BCR-ABL-induced lymphomas.136
Rho GTPases have also been implicated in other tyrosine kinase oncogenic pathways.137 Cell transformation by Fps/Fes tyrosine kinases requires Rac and Cdc42 (along with Ras), which then in turn activate c-Jun N-terminal kinases (JNKs) to sustain proliferation.138 Rac, Cdc42 and RhoA were shown to be necessary for anchorage-independent growth after Ros receptor tyrosine kinase-induced transformation.139 RhoA was also found to facilitate evasion of apoptosis upon transformation with oncogenic RET tyrosine kinase.140 The Vav/Rac/PAK pathway plays an essential role in regulating transformation via an oncogenic form of KIT (KITD814V) associated with myeloproliferative neoplasms and acute myeloid leukemia.141 It has also been reported that the Rac/PAK pathway is vital to ErbB2-mediated transformation of human breast epithelial cancer cells.142 Finally, RhoA-ROCK signaling regulates the survival and transformation of cells harboring oncogenic forms of KIT, FLT3, and BCR-ABL.143
G-protein coupled receptor (GPCR) transformation
GPCR are the largest family of cell surface receptors, and some of them, when aberrantly activated, can lead to oncogenic transformation through Rho GTPase.88 Mas transformation appears to be mediated by Rac activation,144 whereas transformation by G2A,145,146 PAR-1,147 and m1 muscarinic acetylcholine receptor148,149 involves the activation of RhoA.
Dbl family oncoproteins
Many Dbl family proteins were initially identified as oncoproteins before being characterized as Rho GEFs, such as Dbl, Vav, Ect2, Tim, Net1, Lfc, Lsc, or Tiam1.150,151 Fast cycling mutants of RhoA, Cdc42 and Rac1 can mediate cell transformation induced by the Dbl oncoprotein.152,153 Some Dbl family members were initially identified as rearranged in human leukemias, and these fusion proteins also require Rho GTPase activity for promoting tumorigenesis.137 For example, the oncogenic fusion protein BCR-ABL (see above Abl and BCR subheading) activates Rac1 through the Vav Dbl family protein.135,154,155 In addition, leukemia-associated RhoGEF (LARG) is also rearranged in mixed lineage leukemia (MLL), encoding a chimeric fusion protein with MLL.156 LARG has been shown to cause cell transformation via activation of RhoA.157,158
In summary, Rho GTPases play a crucial role in transformation mediated by a variety of oncogenic pathways (Fig. 1), suggesting their potential use as therapeutic targets in cancers driven by those oncogenes. Targeting specific GEFs or GTPase effectors (ROCKs, PAKs), alone or in combination with drugs against certain oncogenes (i.e., Ras, Raf, Src), might provide effective therapeutic opportunities.
Rho GTPases Regulating Tumor Suppressors
In addition to their cooperation with oncogenic signaling pathways, Rho GTPases have also been associated with either promotion or inhibition of tumor suppressor function, which could depend on cell type, stage of tumor progression, and differentiation status (Fig. 1).
Overexpression of the tumor suppressor Merlin (Nf2) was described to block Rac1-induced transformation, whereas Merlin deficiency enhances Rac1 activity and aberrant cell growth. In addition, Rac1 promoted Merlin phosphorylation, rendering it inactive.159,160 Moreover, the hematopoietic-specific Rac2 was described to cooperate with Ras to promote the proliferation of mast cells deficient for the tumor suppressor neurofibromin (NF1).161 Therefore, Rac impairs tumor suppressor function, and unsurprisingly its activity is negatively regulated by tumor suppressors.
On the other hand, RhoB has been proposed as a tumor suppressor, since it is activated in response to several stress stimuli such as DNA damage or hypoxia, and it was shown to promote apoptosis and inhibit tumor growth, cell migration and invasion.162-164 In addition, RhoB knockout mice have enhanced carcinogen-induced skin tumor formation,164 also in agreement with its putative role as a tumor suppressor.
The association between Cdc42 and tumor suppression appears to be more complex and context-dependent. In agreement with its oncogenic abilities described in previous sections, Cdc42 can control cell transformation upon loss of tumor suppressor function signal (e.g., loss of p53, PTEN, or NF1). In fact, increased cell growth in p53- or p19ARF-deficient fibroblasts was partially dependent on regulation of NF-κB and cyclin D1 by Cdc42.165 Cdc42 and Rho can cooperate to regulate PTEN localization and activity at the cell membrane, ultimately controlling PTEN-mediated tumor suppression activity.166 Cdc42 was also shown to inactivate the tumor suppressor merlin;159 and the Cdc42 effector PAK was required for transformation upon loss of NF1.94 A possible oncogenic role on human liver tumors has also been suggested for Cdc42, as a study on liver regeneration showed elevated Cdc42 levels correlating with hepatocyte proliferation after partial hepatectomy,167 and Cdc42 upregulation was described in human hepatocellular carcinoma (HCC).168,169
However, other lines of evidence suggest a tumor suppressor function for Cdc42. Contrary to the aforementioned studies on liver tumors; loss of Cdc42 in the liver was reported to lead to development of HCC,170 which warrant further studies to elucidate the complex role Cdc42 has on hepatocellular carcinoma. Further evidence on the putative tumor suppressor function of Cdc42 comes from studies on hematopoietic and neuroblastoma models. Specific deletion of Cdc42 in murine bone marrow hematopoietic progenitor cells caused loss of hematopoietic stem cell quiescence, skewed differentiation from erythroid to a myeloid fate and hyperproliferation of blood progenitors, leading to lethality in mice due to myeloproliferative disease.171 Most neuroblastomas with N-myc amplifications harbor deletions in the chromosomal region encompassing the Cdc42 gene, leading to loss of one gene copy in these cancers.172 N-myc overexpression in neuroblastoma cells was also shown to decrease Cdc42 expression, while exogenous activated Cdc42 promoted neuroblastoma cell differentiation. Therefore, these studies taken together suggest that Cdc42 could be suppressing tumor development by promoting cell differentiation and tightly controlling progenitor differentiation.173
An additional mechanism of tumor suppression mediated by Cdc42 could be through maintenance of cell polarity, which is critical to the correct function of cells in multicellular environments such as epithelia, but also in cell migration, directional cell growth and asymmetrical cell division, among other cell functions.173,174 Cdc42 can regulate the establishment of cell polarity through modulation of intracellular vesicle trafficking to the apical surface,175 orientation of the cell division spindle,176 and through strengthening of cell-cell junctions.177 Therefore, absence of Cdc42 could result in disruption of cell junctions and loss of epithelial cell polarity associated with cell transformation and tumorigenicity.173,178
These observations support the notion that the effect of Cdc42 signaling in tumor progression is complex, displaying pro-tumorigenic or tumor suppressive functions that seem to depend on cell type, differentiation status, and stage in malignant progression.
Rho GTPases and Tumor Senescence
Normal cells, unlike cancer cells, have a finite proliferative capacity in vitro that leads to a stable and long-term cell cycle arrest, termed senescence, which is characterized by lack of response to growth factors, sustained metabolic activity and morphological changes.179 Interestingly, aberrant oncogene signaling can trigger senescence and, in fact, senescence is prevalent in pre-malignant lesions but absent in malignant tumors. Therefore, senescence has been proposed as a barrier that tumor cells need to evade in order to sustaining aberrant proliferation.180 The characteristic morphologic changes that senescent cells undergo—flattening and enlarged cell shapes—hint toward a role of Rho GTPases in this cytoskeletal reorganization. Nevertheless, Rho GTPases could eitherbe drivers of the process or activated as a consequence of senescence. For example, cyclin-dependent kinase 5 (CDK5) activation in senescing cells reduced Rac1 activity and PAK activation, leading to actin polymerization and change in cell shape.181 In line with this, the membrane linker ezrin was shown to be phosphorylated by CDK5 in senescing cells after retinoblastoma protein transfection, causing the dissociation of Rho GDI from an ezrin/Rho-GDI complex. The release of Rho-GDI resulted in increased interaction with Rac1 and posterior inhibition of Rac1 activity, contributing to the flattened cell morphology.182
However, several studies favor a direct role of Rho GTPases in the regulation of cell senescence (Fig. 1). Rac1-deficient embryonic fibroblasts display decreased cell growth, increased apoptosis and higher ROS levels due to Rac3 upregulation, which leads to increased DNA damage and subsequent cell senescence through augmented p53 activity.183 Therefore, Rac1 could serve as a regulator of cell senescence through modulation of ROS, genomic stability and p53 activity. A role in bypassing senescence has been suggested for RhoA. Interestingly, enhanced expression of LPA2 or Dbs bypassed senescence in a Rho-dependent fashion, since dominant negative mutant RhoA blocked proliferation in LPA2- or Dbs-immortalized cells.184 Cdc42 has been implicated in p53-induced changes in cell morphology and increased apoptosis.185 In addition, activation of Cdc42 upon loss of Cdc42GAP was shown to be sufficient to promote a premature p53-dependent cell senescent phenotype.186
Further investigations are warranted in order to elucidate if Rho GTPases could have also a direct role in senescence in the context of aging.
Rho GTPases, Cancer Cell Survival, and Inflammation
Proinflammatory cytokines are frequently observed in the tumor microenvironment, and chronic inflammation is involved in cancer initiation and progression. The inflammatory tumor microenvironment includes leukocytes, cytokines, and complement components; and are orchestrated by transcription factors, such as signal transducer of activator of transcription 3 (STAT3) and NF-κB. Inflammation in the tumor microenvironment promotes proliferation and survival of malignant cells, angiogenesis, metastasis, subversion of adaptive immunity, response to hormones, and chemotherapeutic agents.187
The JAK-STAT pathway is an important oncogenic signaling cascade that consists of the Janus kinase (JAK) family of non-receptor tyrosine kinases and the STAT family of transcription factors.188 However, in most malignancies, STAT proteins and particularly STAT3, are aberrantly activated (tyrosine phosphorylation).189,190 Some of the tumor-intrinsic functions of activated STAT3 include: inhibition of differentiation, cancer stem cell expansion and/or survival (WNT5A, CD44, and jagged), proliferation (stimulation of transcription of cyclin D1, cdc2, c-myc, cyclin B1, c-jun, c-fos, and greb1), evasion of apoptosis (upregulation of the expression of the pro-survival Bcl-2, Bcl-xL, Mcl-1, Bcl-w, and Survivin transcripts) and response to hypoxia and cellular metabolism (HIF1α, Glut1, Hsp70, Hsp90, p21, and Cdc2).190-192 The JAK-STAT3 pathway also regulates tumor-extrinsic aspects of tumorigenesis including: angiogenesis (regulates transcription of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1 α (HIF1α)), endothelial cell survival and neo-vascularization, immune cell infiltration, mesenchymal cell activation and finally progression to metastasis.193,194 Therefore JAK-STAT3 signaling is crucial for tumor initiation and progression, and cross-talk with Rho GTPase signaling has now started to be evident.195
Rac1, acting in a complex with the MgcRacGAP (male germ cell RacGAP), promotes tyrosine phosphorylation of STAT3 by the interleukin-6 (IL6)-receptor family and/or Jak kinase complex, as well as its translocation to the nucleus.196 Interestingly, evidence also shows that the engagement of cadherins -cell to cell adhesion molecules- specifically induces a striking increase in Rac1 and Cdc42 protein levels and activity, which in turn results in STAT3 activation.197 Furthermore, JAK1/2 and Rho-ROCK signaling have been placed upstream and downstream of each other; therefore JAKs and ROCKs may mutually enhance each other in tumor cells to propagate pro-tumorigenic signals.198,199 In addition, NF-κB is a transcription factor which functions as a master regulator of genes implicated in inflammation, immune response, cell proliferation, apoptosis and invasion. There is evidence that NF-κB regulates STAT3 activation through control of IL-6.200 Connections between Rho-GTPase signaling and NF-κB activity have also been reported, that could further amplify STAT3 mediated protumorigenic functions. All three GTPases, Rho, Rac, and Cdc42 are strong activators of transcription factor NF-κB,201 but specific functions for each of them have been reported. For example, Rhotekin (RTKN), the gene coding for the Rho effector RTKN, was shown to be overexpressed in human gastric cancer and reducing RTKN expression by small interfering RNAs greatly sensitized cells to apoptosis, and this was dependent on NF-κB regulation of antiapoptotic genes.202 Rac1/PAK1 activation downstream of α6β4 integrin leads to NF-κB-mediated resistance of mammary epithelial cells to apoptosis in 3-dimensional cultures.203
Therefore Rho GTPase-regulated signaling pathways can impinge on transcriptional regulators that promote tumorigenesis through cytokine mediated inflammatory signals allowing cancer cells to evade apoptosis, bypass senescence and induce malignant transformation (Fig. 1). These inflammatory mediators and their regulated pathways emerge as very attractive therapeutic targets for cancers of inflammatory origin.
Rho GTPases and Tumor Cell Metabolism
Bioenergetics and cellular metabolism are known to play an essential role in cancer development. After the seminal observations that tumor cells display elevated glycolytic activity (the Warburg effect),204 recent studies are providing insights into how tumor cells establish this altered metabolic phenotype and its role in tumorigenesis.205-207 The tricarboxylic acid cycle (TCA, also citric acid cycle) is essential in proliferating cells since it provides key precursors needed for macromolecule biosynthesis. Recently, a mutant version of the tumor suppressor protein p53 has been described to contribute to the Warburg effect.208 Using human cancer cells and mouse models, the authors showed that mutant p53 stimulates aerobic glycolysis by promoting the translocation of the glucose transporter GLUT1 to the plasma membrane, which is mediated by active RhoA and ROCK. Importantly, disruption of glycolysis in tumor cells impaired promotion of tumorigenesis by mutant p53.208 Therefore, targeting altered glucose metabolism driven by Rho-ROCK could be a feasible therapeutic strategy for mutant p53-bearing tumors. The Rac and Cdc42 effector PAK1 affects the glycolytic pathway by inhibition of phosphoglycerate mutase (PGAM)-B, an important regulatory enzyme in cellular glucose utilization and energy homeostasis.209,210
Apart from increased glycolytic activity, tumor cells also sustain enhanced glutamine consumption, which fosters proliferation by replenishing TCA cycle intermediates, allowing a sustained biogenesis of lipids, aminoacids/proteins and nucleic acids.211 A novel mechanism was provided by which Rho GTPases could contribute to cell transformation by means of alteration of glutamine metabolism.212 Wang and coworkers showed that the transforming capability of Cdc42, Rac1 and RhoC requires glutaminase activity, which is elevated in transformed and/or cancer cells.207 Glutaminase is critical for sustained glutamine metabolism, since it hydrolyzes glutamine to glutamate and ammonia.213 Suppression of glutaminase activity by small molecule inhibitors diminished cell growth and transformation induced by the three GTPases fast cycling mutants (Cdc42F28L, RacF28L, and RhoCF30L)212 (Fig. 1). Whether wild type Rho GTPases cooperate with glutaminase remains to be determined.
Therefore, the ability of Rho GTPases to induce cell transformation could be associated not only with their ability to impinge on the cell cycle, but also with their ability to enhance metabolic activity of cancer cells to fulfill fast growing demands. Targeting Rho GTPase signaling could provide novel therapeutic avenues to halt tumor growth facilitated by high metabolic rate.
Rho GTPases and Tumor Angiogenesis
In order to grow beyond a certain size, solid tumors need to induce the formation of new blood vessels, or angiogenesis, which will maintain a supply of oxygen and nutrients, and eventually may also serve as an escape route to colonize distant organs.1 Tumour angiogenesis is controlled by a balance of pro- and anti-angiogenic factors, and during tumor progression cancer cells need to shift the balance toward increased pro-angiogenic factors (angiogenic switch),214,215 which in turn activate endothelial cells. In response to pro-angiogenic factors Rho GTPases regulate different processes in endothelial cells during angiogenesis, including proliferation, survival, and migration.216 In particular, Rho GTPase actions on endothelial cell survival and sprouting during angiogenesis require low ROCK activity and high ERK-MAPK signaling.217 In addition, RhoJ has been recently shown to promote tumor angiogenesis. Using mouse models, Kim and coworkers described how RhoJ blockade inhibits tumor angiogenesis and disrupts the preformed tumor vessels through activation of RhoA-ROCK signaling pathway in tumor endothelial cells, eventually resulting in a functional failure of tumor vasculature.218 RhoB has also been implicated in the regulation of angiogenesis through direct actions on endothelial cells. After the angiogenic switch in a breast cancer model, RhoB promotes tumor angiogenesis through enhanced Akt signaling, growth and survival of endothelial cells.219 In a mouse model of ischemia the loss of RhoB decreased pathological angiogenesis in the ischemic retina and reduced angiogenesis in response to cutaneous wounding.220
PAKs are also important in controlling key cellular events required for angiogenesis, including endothelial cell proliferation, survival, attachment and migration.221 In endothelial cells, the ERK pathway regulates cellular proliferation and migration downstream the activation of Rac/PAK pathway.222 PAK1 and PAK4 also protect endothelial cells against apoptotic stimuli by phosphorylation of Bad and CRAF, which induces the translocation of CRAF to mitochondria and the displacement of Bad/Bcl-2 complexes.221,223
Regarding Rho GTPase actions on cancer cells, some Rho GTPases have been shown to contribute to angiogenesis by regulating the hypoxia-triggered induction of HIF1α224 or the production of angiogenic factors,225 which would lead to increased tumor vascularization. Hypoxia increases the expression and activity of Cdc42, Rac1 and RhoA in cancer cells.224 Then, RhoA,224 Rac1,226,227,228 and Cdc42228 have been shown to be required for the accumulation of HIF1α under hypoxic conditions (Fig. 1). RhoC-mediated transformation of mammary epithelial cells increases expression and/or secretion of proangiogenic factors such as VEGF, basic fibroblast growth factor (bFGF), IL-6, and interleukin-8 (IL-8).229 Rac1 has also been shown to promote angiogenesis through augmented expression and secretion of VEGF via HIF1α stabilization in hepatocellular carcinoma cells (Fig. 1).227 The Rac effector WAVE3 has been described to modulate tumor angiogenesis. As such, WAVE3-knockdown in breast cancer cells reduces microvessel density in orthothopic tumors in mice, possibly due to reduced VEGF levels from WAVE3-knockdown cells.51
Therefore, Rho GTPases are essential for tumor angiogenesis since they play key roles in both the endothelial and tumor cell compartments, which make them appealing targets to improve current antitumor and antiangiogenic therapies (Fig. 1). Apart from targeting Rho GTPase effectors (PAK, N-WASP) or GEFs (Trio) with small molecule inhibitors to impair tumor angiogenesis, other approaches are being considered such as the use post-translational modification inhibitors (statins) in combination with conventional anticancer therapies.230
Concluding Remarks
Rho GTPases emerge as key molecular machinery involved in every single step during cancer progression (Fig. 1). Strong efforts have recently focused on studying the role of Rho GTPases in the later stages of tumor progression such as invasion and metastatic dissemination. We have reviewed here evidence supporting the role of Rho GTPase signaling in the initial steps leading to malignant transformation, providing a rationale for targeting Rho signaling to block not only tumor dissemination, but initial tumor growth as well.
Rho GTPase functions are in many cases redundant for tumor development (Fig. 1). It appears that cancer cells manage to direct Rho GTPase signaling to achieve full transformation potential driven by oncogenes and, even in some cases, Rho GTPases act as oncogenes themselves. Therefore even when some Rho GTPase activity is lost, cancer cells will overexpress or activate another family member to compensate. In some other cases, the role of individual Rho GTPases is completely different or even opposed between members of the family. How specificity and selection is achieved in different tumor contexts, either through engagement of GEFs, GAPs, or effectors, remains a matter of active study in the field.
On the other hand, the recent findings regarding Rho GTPases mutated in cancer may change current views on Rho GTPase regulation of tumor progression. Future studies dissecting the biological meaning of these mutations will be of the utmost importance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Cancer Research UK Grant C33043/A12065 and Royal Society RG110591. V.S.M. is a CRUK Career Development Fellow and C.H. holds a post-doctoral FEBS fellowship.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 3.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 5.Kamai T, Yamanishi T, Shirataki H, Takagi K, Asami H, Ito Y, Yoshida K. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 2004;10:4799–805. doi: 10.1158/1078-0432.CCR-0436-03. [DOI] [PubMed] [Google Scholar]
- 6.Fritz G, Brachetti C, Bahlmann F, Schmidt M, Kaina B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br J Cancer. 2002;87:635–44. doi: 10.1038/sj.bjc.6600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int J Cancer. 1999;81:682–7. doi: 10.1002/(SICI)1097-0215(19990531)81:5<682::AID-IJC2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Abraham MT, Kuriakose MA, Sacks PG, Yee H, Chiriboga L, Bearer EL, Delacure MD. Motility-related proteins as markers for head and neck squamous cell cancer. Laryngoscope. 2001;111:1285–9. doi: 10.1097/00005537-200107000-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gómez del Pulgar T, Benitah SA, Valerón PF, Espina C, Lacal JC. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays. 2005;27:602–13. doi: 10.1002/bies.20238. [DOI] [PubMed] [Google Scholar]
- 10.Adnane J, Muro-Cacho C, Mathews L, Sebti SM, Muñoz-Antonia T. Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin Cancer Res. 2002;8:2225–32. [PubMed] [Google Scholar]
- 11.Mazieres J, Antonia T, Daste G, Muro-Cacho C, Berchery D, Tillement V, Pradines A, Sebti S, Favre G. Loss of RhoB expression in human lung cancer progression. Clin Cancer Res. 2004;10:2742–50. doi: 10.1158/1078-0432.CCR-03-0149. [DOI] [PubMed] [Google Scholar]
- 12.van Golen KL, Wu ZF, Qiao XT, Bao LW, Merajver SD, Rho C. RhoC GTPase, a novel transforming oncogene for human mammary epithelial cells that partially recapitulates the inflammatory breast cancer phenotype. Cancer Res. 2000;60:5832–8. [PubMed] [Google Scholar]
- 13.Wang HB, Liu XP, Liang J, Yang K, Sui AH, Liu YJ. Expression of RhoA and RhoC in colorectal carcinoma and its relations with clinicopathological parameters. Clin Chem Lab Med. 2009;47:811–7. doi: 10.1515/CCLM.2009.186. [DOI] [PubMed] [Google Scholar]
- 14.Boone B, Van Gele M, Lambert J, Haspeslagh M, Brochez L. The role of RhoC in growth and metastatic capacity of melanoma. J Cutan Pathol. 2009;36:629–36. doi: 10.1111/j.1600-0560.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 15.Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, Narumiya S, Hiai H, Fukumoto M. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77:147–52. doi: 10.1038/bjc.1998.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Rao Q, Wang M, Wei H, Xing H, Liu H, Wang Y, Tang K, Peng L, Tian Z, et al. Overexpression of Rac1 in leukemia patients and its role in leukemia cell migration and growth. Biochem Biophys Res Commun. 2009;386:769–74. doi: 10.1016/j.bbrc.2009.06.125. [DOI] [PubMed] [Google Scholar]
- 17.Jordan P, Brazåo R, Boavida MG, Gespach C, Chastre E. Cloning of a novel human Rac1b splice variant with increased expression in colorectal tumors. Oncogene. 1999;18:6835–9. doi: 10.1038/sj.onc.1203233. [DOI] [PubMed] [Google Scholar]
- 18.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–20. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Licciulli S, Avila JL, Cho M, Troutman S, Jiang P, Kossenkov AV, Showe LC, Liu Q, Vachani A, et al. The Rac1 splice form Rac1b promotes K-ras-induced lung tumorigenesis. Oncogene. 2013;32:903–9. doi: 10.1038/onc.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiegen D, Haeusler LC, Blumenstein L, Herbrand U, Dvorsky R, Vetter IR, Ahmadian MR. Alternative splicing of Rac1 generates Rac1b, a self-activating GTPase. J Biol Chem. 2004;279:4743–9. doi: 10.1074/jbc.M310281200. [DOI] [PubMed] [Google Scholar]
- 21.Stallings-Mann ML, Waldmann J, Zhang Y, Miller E, Gauthier ML, Visscher DW, Downey GP, Radisky ES, Fields AP, Radisky DC. Matrix metalloproteinase induction of Rac1b, a key effector of lung cancer progression. Sci Transl Med. 2012;4:42ra95. doi: 10.1126/scitranslmed.3004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Wang Y, Zhang Y, Miao Y, Zhao Y, Zhang PX, Jiang GY, Zhang JY, Han Y, Lin XY, et al. Abnormal expression of p120-catenin, E-cadherin, and small GTPases is significantly associated with malignant phenotype of human lung cancer. Lung Cancer. 2009;63:375–82. doi: 10.1016/j.lungcan.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Gómez Del Pulgar T, Valdés-Mora F, Bandrés E, Pérez-Palacios R, Espina C, Cejas P, García-Cabezas MA, Nistal M, Casado E, González-Barón M, et al. Cdc42 is highly expressed in colorectal adenocarcinoma and downregulates ID4 through an epigenetic mechanism. Int J Oncol. 2008;33:185–93. [PubMed] [Google Scholar]
- 24.Tucci MG, Lucarini G, Brancorsini D, Zizzi A, Pugnaloni A, Giacchetti A, Ricotti G, Biagini G. Involvement of E-cadherin, beta-catenin, Cdc42 and CXCR4 in the progression and prognosis of cutaneous melanoma. Br J Dermatol. 2007;157:1212–6. doi: 10.1111/j.1365-2133.2007.08246.x. [DOI] [PubMed] [Google Scholar]
- 25.Fernández-Medarde A, Santos E. Ras in cancer and developmental diseases. Genes Cancer. 2011;2:344–58. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945–50. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–63. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet. 2012;44:1006–14. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machesky LM, Sansom OJ. Rac1 in the driver’s seat for melanoma. Pigment Cell Melanoma Res. 2012;25:762–4. doi: 10.1111/pcmr.12004. [DOI] [PubMed] [Google Scholar]
- 30.Alan JK, Lundquist EA. Mutationally activated Rho GTPases in cancer. Small GTPases. 2013;4:159–63. doi: 10.4161/sgtp.26530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang SL, Hong YR, Sy WD, Lieu AS, Lin CL, Lee KS, Howng SL. Rac1 gene mutations in human brain tumours. Eur J Surg Oncol. 2004;30:68–72. doi: 10.1016/j.ejso.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Davis MJ, Ha BH, Holman EC, Halaban R, Schlessinger J, Boggon TJ. RAC1P29S is a spontaneously activating cancer-associated GTPase. Proc Natl Acad Sci U S A. 2013;110:912–7. doi: 10.1073/pnas.1220895110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawazu M, Ueno T, Kontani K, Ogita Y, Ando M, Fukumura K, Yamato A, Soda M, Takeuchi K, Miki Y, et al. Transforming mutations of RAC guanosine triphosphatases in human cancers. Proc Natl Acad Sci U S A. 2013;110:3029–34. doi: 10.1073/pnas.1216141110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakata-Yanagimoto M, Enami T, Yoshida K, Shiraishi Y, Ishii R, Miyake Y, Muto H, Tsuyama N, Sato-Otsubo A, Okuno Y, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:171–5. doi: 10.1038/ng.2872. [DOI] [PubMed] [Google Scholar]
- 35.Yoo HY, Sung MK, Lee SH, Kim S, Lee H, Park S, Kim SC, Lee B, Rho K, Lee JE, et al. A recurrent inactivating mutation in RHOA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46:371–5. doi: 10.1038/ng.2916. [DOI] [PubMed] [Google Scholar]
- 36.Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Küppers R, Dalla-Favera R. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–6. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 37.Preudhomme C, Roumier C, Hildebrand MP, Dallery-Prudhomme E, Lantoine D, Laï JL, Daudignon A, Adenis C, Bauters F, Fenaux P, et al. Nonrandom 4p13 rearrangements of the RhoH/TTF gene, encoding a GTP-binding protein, in non-Hodgkin’s lymphoma and multiple myeloma. Oncogene. 2000;19:2023–32. doi: 10.1038/sj.onc.1203521. [DOI] [PubMed] [Google Scholar]
- 38.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–2. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 39.Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–9. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 40.Liberto M, Cobrinik D, Minden A. Rho regulates p21(CIP1), cyclin D1, and checkpoint control in mammary epithelial cells. Oncogene. 2002;21:1590–9. doi: 10.1038/sj.onc.1205242. [DOI] [PubMed] [Google Scholar]
- 41.Weber JD, Hu W, Jefcoat SC, Jr., Raben DM, Baldassare JJ. Ras-stimulated extracellular signal-related kinase 1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27. J Biol Chem. 1997;272:32966–71. doi: 10.1074/jbc.272.52.32966. [DOI] [PubMed] [Google Scholar]
- 42.Welsh CF, Roovers K, Villanueva J, Liu Y, Schwartz MA, Assoian RK. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat Cell Biol. 2001;3:950–7. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- 43.Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–30. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y, Tao Y, Chen Y, Xu W. RhoC regulates the proliferation of gastric cancer cells through interaction with IQGAP1. PLoS One. 2012;7:e48917. doi: 10.1371/journal.pone.0048917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie S, Zhu M, Lv G, Geng Y, Chen G, Ma J, Wang G. Overexpression of Ras homologous C (RhoC) induces malignant transformation of hepatocytes in vitro and in nude mouse xenografts. PLoS One. 2013;8:e54493. doi: 10.1371/journal.pone.0054493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westwick JK, Lambert QT, Clark GJ, Symons M, Van Aelst L, Pestell RG, Der CJ. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–35. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Page K, Li J, Hodge JA, Liu PT, Vanden Hoek TL, Becker LB, Pestell RG, Rosner MR, Hershenson MB. Characterization of a Rac1 signaling pathway to cyclin D(1) expression in airway smooth muscle cells. J Biol Chem. 1999;274:22065–71. doi: 10.1074/jbc.274.31.22065. [DOI] [PubMed] [Google Scholar]
- 48.Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–27. doi: 10.1016/S1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- 49.Klein EA, Campbell LE, Kothapalli D, Fournier AK, Assoian RK. Joint requirement for Rac and ERK activities underlies the mid-G1 phase induction of cyclin D1 and S phase entry in both epithelial and mesenchymal cells. J Biol Chem. 2008;283:30911–8. doi: 10.1074/jbc.M804537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein EA, Yang C, Kazanietz MG, Assoian RK. NFkappaB-independent signaling to the cyclin D1 gene by Rac. Cell Cycle. 2007;6:1115–21. doi: 10.4161/cc.6.9.4147. [DOI] [PubMed] [Google Scholar]
- 51.Sossey-Alaoui K, Safina A, Li X, Vaughan MM, Hicks DG, Bakin AV, Cowell JK. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am J Pathol. 2007;170:2112–21. doi: 10.2353/ajpath.2007.060975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espina C, Céspedes MV, García-Cabezas MA, Gómez del Pulgar MT, Boluda A, Oroz LG, Benitah SA, Cejas P, Nistal M, Mangues R, et al. A critical role for Rac1 in tumor progression of human colorectal adenocarcinoma cells. Am J Pathol. 2008;172:156–66. doi: 10.2353/ajpath.2008.070561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chou MM, Masuda-Robens JM, Gupta ML. Cdc42 promotes G1 progression through p70 S6 kinase-mediated induction of cyclin E expression. J Biol Chem. 2003;278:35241–7. doi: 10.1074/jbc.M305246200. [DOI] [PubMed] [Google Scholar]
- 54.Bauerfeld CP, Hershenson MB, Page K. Cdc42, but not RhoA, regulates cyclin D1 expression in bovine tracheal myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L974–82. doi: 10.1152/ajplung.2001.280.5.L974. [DOI] [PubMed] [Google Scholar]
- 55.Zins K, Lucas T, Reichl P, Abraham D, Aharinejad S. A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice. PLoS One. 2013;8:e74924. doi: 10.1371/journal.pone.0074924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyrkou A, Soufi M, Bahtz R, Ferguson C, Bai M, Parton RG, Hoffmann I, Zerial M, Fotsis T, Murphy C. RhoD participates in the regulation of cell-cycle progression and centrosome duplication. Oncogene. 2013;32:1831–42. doi: 10.1038/onc.2012.195. [DOI] [PubMed] [Google Scholar]
- 57.Freeman SN, Ma Y, Cress WD. RhoBTB2 (DBC2) is a mitotic E2F1 target gene with a novel role in apoptosis. J Biol Chem. 2008;283:2353–62. doi: 10.1074/jbc.M705986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villalonga P, Guasch RM, Riento K, Ridley AJ. RhoE inhibits cell cycle progression and Ras-induced transformation. Mol Cell Biol. 2004;24:7829–40. doi: 10.1128/MCB.24.18.7829-7840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jordan SN, Canman JC. Rho GTPases in animal cell cytokinesis: an occupation by the one percent. Cytoskeleton (Hoboken) 2012;69:919–30. doi: 10.1002/cm.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chevrier V, Piel M, Collomb N, Saoudi Y, Frank R, Paintrand M, Narumiya S, Bornens M, Job D. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002;157:807–17. doi: 10.1083/jcb.200203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu H, Di Cunto F, Imarisio S, Reid LM. Citron kinase is a cell cycle-dependent, nuclear protein required for G2/M transition of hepatocytes. J Biol Chem. 2003;278:2541–8. doi: 10.1074/jbc.M210391200. [DOI] [PubMed] [Google Scholar]
- 62.Moore KA, Sethi R, Doanes AM, Johnson TM, Pracyk JB, Kirby M, Irani K, Goldschmidt-Clermont PJ, Finkel T. Rac1 is required for cell proliferation and G2/M progression. Biochem J. 1997;326:17–20. doi: 10.1042/bj3260017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michaelson D, Abidi W, Guardavaccaro D, Zhou M, Ahearn I, Pagano M, Philips MR. Rac1 accumulates in the nucleus during the G2 phase of the cell cycle and promotes cell division. J Cell Biol. 2008;181:485–96. doi: 10.1083/jcb.200801047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, Ishizaki T, Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature. 2004;428:767–71. doi: 10.1038/nature02452. [DOI] [PubMed] [Google Scholar]
- 65.Oceguera-Yanez F, Kimura K, Yasuda S, Higashida C, Kitamura T, Hiraoka Y, Haraguchi T, Narumiya S. Ect2 and MgcRacGAP regulate the activation and function of Cdc42 in mitosis. J Cell Biol. 2005;168:221–32. doi: 10.1083/jcb.200408085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tatsumoto T, Sakata H, Dasso M, Miki T. Potential roles of the nucleotide exchange factor ECT2 and Cdc42 GTPase in spindle assembly in Xenopus egg cell-free extracts. J Cell Biochem. 2003;90:892–900. doi: 10.1002/jcb.10750. [DOI] [PubMed] [Google Scholar]
- 67.Normand G, King RW. Understanding cytokinesis failure. Adv Exp Med Biol. 2010;676:27–55. doi: 10.1007/978-1-4419-6199-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18:273–81. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–8. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe S, Okawa K, Miki T, Sakamoto S, Morinaga T, Segawa K, Arakawa T, Kinoshita M, Ishizaki T, Narumiya S. Rho and anillin-dependent control of mDia2 localization and function in cytokinesis. Mol Biol Cell. 2010;21:3193–204. doi: 10.1091/mbc.E10-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–64. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 72.Bassi ZI, Audusseau M, Riparbelli MG, Callaini G, D’Avino PP. Citron kinase controls a molecular network required for midbody formation in cytokinesis. Proc Natl Acad Sci U S A. 2013;110:9782–7. doi: 10.1073/pnas.1301328110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gruneberg U, Neef R, Li X, Chan EH, Chalamalasetty RB, Nigg EA, Barr FA. KIF14 and citron kinase act together to promote efficient cytokinesis. J Cell Biol. 2006;172:363–72. doi: 10.1083/jcb.200511061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Narumiya S, Yasuda S. Rho GTPases in animal cell mitosis. Curr Opin Cell Biol. 2006;18:199–205. doi: 10.1016/j.ceb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 75.Connolly JO, Soga N, Guo XL, Alvarez U, Hruska KA. Rac is essential in the transformation of endothelial cells by polyoma middle T. Cell Adhes Commun. 2000;7:409–22. doi: 10.3109/15419060009109022. [DOI] [PubMed] [Google Scholar]
- 76.Urich M, Senften M, Shaw PE, Ballmer-Hofer K. A role for the small GTPase Rac in polyomavirus middle-T antigen-mediated activation of the serum response element and in cell transformation. Oncogene. 1997;14:1235–41. doi: 10.1038/sj.onc.1200982. [DOI] [PubMed] [Google Scholar]
- 77.Boerner JL, McManus MJ, Martin GS, Maihle NJ. Ras-independent oncogenic transformation by an EGF-receptor mutant. J Cell Sci. 2000;113:935–42. doi: 10.1242/jcs.113.6.935. [DOI] [PubMed] [Google Scholar]
- 78.Karnoub AE, Symons M, Campbell SL, Der CJ. Molecular basis for Rho GTPase signaling specificity. Breast Cancer Res Treat. 2004;84:61–71. doi: 10.1023/B:BREA.0000018427.84929.5c. [DOI] [PubMed] [Google Scholar]
- 79.Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–53. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lebowitz PF, Du W, Prendergast GC. Prenylation of RhoB is required for its cell transforming function but not its ability to activate serum response element-dependent transcription. J Biol Chem. 1997;272:16093–5. doi: 10.1074/jbc.272.26.16093. [DOI] [PubMed] [Google Scholar]
- 81.Murphy GA, Solski PA, Jillian SA, Pérez de la Ossa P, D’Eustachio P, Der CJ, Rush MG. Cellular functions of TC10, a Rho family GTPase: regulation of morphology, signal transduction and cell growth. Oncogene. 1999;18:3831–45. doi: 10.1038/sj.onc.1202758. [DOI] [PubMed] [Google Scholar]
- 82.Prendergast GC, Khosravi-Far R, Solski PA, Kurzawa H, Lebowitz PF, Der CJ. Critical role of Rho in cell transformation by oncogenic Ras. Oncogene. 1995;10:2289–96. [PubMed] [Google Scholar]
- 83.Qiu RG, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol. 1997;17:3449–58. doi: 10.1128/mcb.17.6.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–9. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 85.Qiu RG, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci U S A. 1995;92:11781–5. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roux P, Gauthier-Rouvière C, Doucet-Brutin S, Fort P. The small GTPases Cdc42Hs, Rac1 and RhoG delineate Raf-independent pathways that cooperate to transform NIH3T3 cells. Curr Biol. 1997;7:629–37. doi: 10.1016/S0960-9822(06)00289-2. [DOI] [PubMed] [Google Scholar]
- 87.Sachdev P, Jiang YX, Li W, Miki T, Maruta H, Nur-E-Kamal MS, Wang LH. Differential requirement for Rho family GTPases in an oncogenic insulin-like growth factor-I receptor-induced cell transformation. J Biol Chem. 2001;276:26461–71. doi: 10.1074/jbc.M010995200. [DOI] [PubMed] [Google Scholar]
- 88.Whitehead IP, Zohn IE, Der CJ. Rho GTPase-dependent transformation by G protein-coupled receptors. Oncogene. 2001;20:1547–55. doi: 10.1038/sj.onc.1204188. [DOI] [PubMed] [Google Scholar]
- 89.Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001;20:755–66. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Joneson T, Bar-Sagi D. Suppression of Ras-induced apoptosis by the Rac GTPase. Mol Cell Biol. 1999;19:5892–901. doi: 10.1128/mcb.19.9.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fischer RS, Zheng Y, Quinlan MP. Rac1 and extracellularly regulated kinase activation are sufficient for E1A-dependent cooperative transformation of primary epithelial cells, but progression can only be modulated by E1A or Rac1. Cell Growth Differ. 1998;9:209–21. [PubMed] [Google Scholar]
- 92.Cobellis G, Missero C, Di Lauro R. Concomitant activation of MEK-1 and Rac-1 increases the proliferative potential of thyroid epithelial cells, without affecting their differentiation. Oncogene. 1998;17:2047–57. doi: 10.1038/sj.onc.1202130. [DOI] [PubMed] [Google Scholar]
- 93.Tang Y, Chen Z, Ambrose D, Liu J, Gibbs JB, Chernoff J, Field J. Kinase-deficient Pak1 mutants inhibit Ras transformation of Rat-1 fibroblasts. Mol Cell Biol. 1997;17:4454–64. doi: 10.1128/mcb.17.8.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tang Y, Marwaha S, Rutkowski JL, Tennekoon GI, Phillips PC, Field J. A role for Pak protein kinases in Schwann cell transformation. Proc Natl Acad Sci U S A. 1998;95:5139–44. doi: 10.1073/pnas.95.9.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang Y, Yu J, Field J. Signals from the Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol Cell Biol. 1999;19:1881–91. doi: 10.1128/mcb.19.3.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shutes A, Berzat AC, Cox AD, Der CJ. Atypical mechanism of regulation of the Wrch-1 Rho family small GTPase. Curr Biol. 2004;14:2052–6. doi: 10.1016/j.cub.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 97.Tao W, Pennica D, Xu L, Kalejta RF, Levine AJ. Wrch-1, a novel member of the Rho gene family that is regulated by Wnt-1. Genes Dev. 2001;15:1796–807. doi: 10.1101/gad.894301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin R, Bagrodia S, Cerione R, Manor D. A novel Cdc42Hs mutant induces cellular transformation. Curr Biol. 1997;7:794–7. doi: 10.1016/S0960-9822(06)00338-1. [DOI] [PubMed] [Google Scholar]
- 99.Jaffe AB, Hall A. Rho GTPases in transformation and metastasis. Adv Cancer Res. 2002;84:57–80. doi: 10.1016/S0065-230X(02)84003-9. [DOI] [PubMed] [Google Scholar]
- 100.Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, Sweet-Cordero A, Eckman MS, Tuveson DA, Capobianco AJ, Tybulewicz VL, et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–94. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- 101.Samuel MS, Lourenço FC, Olson MF. K-Ras mediated murine epidermal tumorigenesis is dependent upon and associated with elevated Rac1 activity. PLoS One. 2011;6:e17143. doi: 10.1371/journal.pone.0017143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li A, Ma Y, Jin M, Mason S, Mort RL, Blyth K, Larue L, Sansom OJ, Machesky LM. Activated mutant NRas(Q61K) drives aberrant melanocyte signaling, survival, and invasiveness via a Rac1-dependent mechanism. J Invest Dermatol. 2012;132:2610–21. doi: 10.1038/jid.2012.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rowinsky EK. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med. 2004;55:433–57. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]
- 104.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 105.Cerione RA. Cdc42: new roads to travel. Trends Cell Biol. 2004;14:127–32. doi: 10.1016/j.tcb.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 106.Boerner JL, Danielsen A, McManus MJ, Maihle NJ. Activation of Rho is required for ligand-independent oncogenic signaling by a mutant epidermal growth factor receptor. J Biol Chem. 2001;276:3691–5. doi: 10.1074/jbc.M003801200. [DOI] [PubMed] [Google Scholar]
- 107.Kim BC, Yi JY, Yi SJ, Shin IC, Ha KS, Jhun BH, Hwang SB, Kim JH. Rac GTPase activity is essential for EGF-induced mitogenesis. Mol Cells. 1998;8:90–5. [PubMed] [Google Scholar]
- 108.Kaneko T, Maeda A, Takefuji M, Aoyama H, Nakayama M, Kawabata S, Kawano Y, Iwamatsu A, Amano M, Kaibuchi K. Rho mediates endocytosis of epidermal growth factor receptor through phosphorylation of endophilin A1 by Rho-kinase. Genes Cells. 2005;10:973–87. doi: 10.1111/j.1365-2443.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- 109.Ung CY, Li H, Ma XH, Jia J, Li BW, Low BC, Chen YZ. Simulation of the regulation of EGFR endocytosis and EGFR-ERK signaling by endophilin-mediated RhoA-EGFR crosstalk. FEBS Lett. 2008;582:2283–90. doi: 10.1016/j.febslet.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 110.Wherlock M, Gampel A, Futter C, Mellor H. Farnesyltransferase inhibitors disrupt EGF receptor traffic through modulation of the RhoB GTPase. J Cell Sci. 2004;117:3221–31. doi: 10.1242/jcs.01193. [DOI] [PubMed] [Google Scholar]
- 111.Wu WJ, Erickson JW, Lin R, Cerione RA. The gamma-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature. 2000;405:800–4. doi: 10.1038/35015585. [DOI] [PubMed] [Google Scholar]
- 112.Hirsch DS, Shen Y, Wu WJ. Growth and motility inhibition of breast cancer cells by epidermal growth factor receptor degradation is correlated with inactivation of Cdc42. Cancer Res. 2006;66:3523–30. doi: 10.1158/0008-5472.CAN-05-1547. [DOI] [PubMed] [Google Scholar]
- 113.Wu WJ, Tu S, Cerione RA. Activated Cdc42 sequesters c-Cbl and prevents EGF receptor degradation. Cell. 2003;114:715–25. doi: 10.1016/S0092-8674(03)00688-3. [DOI] [PubMed] [Google Scholar]
- 114.Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi: 10.1038/nrc3205. [DOI] [PubMed] [Google Scholar]
- 115.Rodrigues GA, Park M, Schlessinger J. Activation of the JNK pathway is essential for transformation by the Met oncogene. EMBO J. 1997;16:2634–45. doi: 10.1093/emboj/16.10.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–25. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–12. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bjorge JD, Jakymiw A, Fujita DJ. Selected glimpses into the activation and function of Src kinase. Oncogene. 2000;19:5620–35. doi: 10.1038/sj.onc.1203923. [DOI] [PubMed] [Google Scholar]
- 119.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–75. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 120.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 121.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 122.Servitja JM, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS. Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J Biol Chem. 2003;278:34339–46. doi: 10.1074/jbc.M302960200. [DOI] [PubMed] [Google Scholar]
- 123.Miki H, Fukuda M, Nishida E, Takenawa T. Phosphorylation of WAVE downstream of mitogen-activated protein kinase signaling. J Biol Chem. 1999;274:27605–9. doi: 10.1074/jbc.274.39.27605. [DOI] [PubMed] [Google Scholar]
- 124.Tanji M, Ishizaki T, Ebrahimi S, Tsuboguchi Y, Sukezane T, Akagi T, Frame MC, Hashimoto N, Miyamoto S, Narumiya S. mDia1 targets v-Src to the cell periphery and facilitates cell transformation, tumorigenesis, and invasion. Mol Cell Biol. 2010;30:4604–15. doi: 10.1128/MCB.00197-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaplan KB, Swedlow JR, Varmus HE, Morgan DO. Association of p60c-src with endosomal membranes in mammalian fibroblasts. J Cell Biol. 1992;118:321–33. doi: 10.1083/jcb.118.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell. 2004;7:855–69. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 127.Colicelli J. ABL tyrosine kinases: evolution of function, regulation, and specificity. Sci Signal. 2010;3:re6. doi: 10.1126/scisignal.3139re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Renshaw MW, Lea-Chou E, Wang JY. Rac is required for v-Abl tyrosine kinase to activate mitogenesis. Curr Biol. 1996;6:76–83. doi: 10.1016/S0960-9822(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 129.Ben-Neriah Y, Daley GQ, Mes-Masson AM, Witte ON, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr/abl hybrid gene. Science. 1986;233:212–4. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- 130.Harnois T, Constantin B, Rioux A, Grenioux E, Kitzis A, Bourmeyster N. Differential interaction and activation of Rho family GTPases by p210bcr-abl and p190bcr-abl. Oncogene. 2003;22:6445–54. doi: 10.1038/sj.onc.1206626. [DOI] [PubMed] [Google Scholar]
- 131.Sengupta A, Arnett J, Dunn S, Williams DA, Cancelas JA. Rac2 GTPase deficiency depletes BCR-ABL+ leukemic stem cells and progenitors in vivo. Blood. 2010;116:81–4. doi: 10.1182/blood-2009-10-247437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, Neviani P, Druker BJ, Setchell KD, Zheng Y, et al. Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell. 2007;12:467–78. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 133.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2:211–5. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 134.Muvarak N, Nagaria P, Rassool FV. Genomic instability in chronic myeloid leukemia: targets for therapy? Curr Hematol Malig Rep. 2012;7:94–102. doi: 10.1007/s11899-012-0119-0. [DOI] [PubMed] [Google Scholar]
- 135.Chang KH, Sanchez-Aguilera A, Shen S, Sengupta A, Madhu MN, Ficker AM, Dunn SK, Kuenzi AM, Arnett JL, Santho RA, et al. Vav3 collaborates with p190-BCR-ABL in lymphoid progenitor leukemogenesis, proliferation, and survival. Blood. 2012;120:800–11. doi: 10.1182/blood-2011-06-361709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cho YJ, Zhang B, Kaartinen V, Haataja L, de Curtis I, Groffen J, Heisterkamp N. Generation of rac3 null mutant mice: role of Rac3 in Bcr/Abl-caused lymphoblastic leukemia. Mol Cell Biol. 2005;25:5777–85. doi: 10.1128/MCB.25.13.5777-5785.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karnoub A, Der C. Rho Family GTPases and Cellular Transformation. Madame Curie Bioscience Database [Internet]. Austin (TX): Available from: http://www.ncbi.nlm.nih.gov/books/NBK6594/, 2000.
- 138.Li J, Smithgall TE. Fibroblast transformation by Fps/Fes tyrosine kinases requires Ras, Rac, and Cdc42 and induces extracellular signal-regulated and c-Jun N-terminal kinase activation. J Biol Chem. 1998;273:13828–34. doi: 10.1074/jbc.273.22.13828. [DOI] [PubMed] [Google Scholar]
- 139.Nguyen KT, Zong CS, Uttamsingh S, Sachdev P, Bhanot M, Le MT, Chan JL, Wang LH. The role of phosphatidylinositol 3-kinase, rho family GTPases, and STAT3 in Ros-induced cell transformation. J Biol Chem. 2002;277:11107–15. doi: 10.1074/jbc.M108166200. [DOI] [PubMed] [Google Scholar]
- 140.Barone MV, Sepe L, Melillo RM, Mineo A, Santelli G, Monaco C, Castellone MD, Tramontano D, Fusco A, Santoro M. RET/PTC1 oncogene signaling in PC Cl 3 thyroid cells requires the small GTP-binding protein Rho. Oncogene. 2001;20:6973–82. doi: 10.1038/sj.onc.1204886. [DOI] [PubMed] [Google Scholar]
- 141.Martin H, Mali RS, Ma P, Chatterjee A, Ramdas B, Sims E, Munugalavadla V, Ghosh J, Mattingly RR, Visconte V, et al. Pak and Rac GTPases promote oncogenic KIT-induced neoplasms. J Clin Invest. 2013;123:4449–63. doi: 10.1172/JCI67509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, Chernoff J. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene. 2010;29:5839–49. doi: 10.1038/onc.2010.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mali RS, Ramdas B, Ma P, Shi J, Munugalavadla V, Sims E, Wei L, Vemula S, Nabinger SC, Goodwin CB, et al. Rho kinase regulates the survival and transformation of cells bearing oncogenic forms of KIT, FLT3, and BCR-ABL. Cancer Cell. 2011;20:357–69. doi: 10.1016/j.ccr.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zohn IE, Symons M, Chrzanowska-Wodnicka M, Westwick JK, Der CJ. Mas oncogene signaling and transformation require the small GTP-binding protein Rac. Mol Cell Biol. 1998;18:1225–35. doi: 10.1128/mcb.18.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kabarowski JH, Feramisco JD, Le LQ, Gu JL, Luoh SW, Simon MI, Witte ON. Direct genetic demonstration of G alpha 13 coupling to the orphan G protein-coupled receptor G2A leading to RhoA-dependent actin rearrangement. Proc Natl Acad Sci U S A. 2000;97:12109–14. doi: 10.1073/pnas.97.22.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zohn IE, Klinger M, Karp X, Kirk H, Symons M, Chrzanowska-Wodnicka M, Der CJ, Kay RJ. G2A is an oncogenic G protein-coupled receptor. Oncogene. 2000;19:3866–77. doi: 10.1038/sj.onc.1203731. [DOI] [PubMed] [Google Scholar]
- 147.Martin CB, Mahon GM, Klinger MB, Kay RJ, Symons M, Der CJ, Whitehead IP. The thrombin receptor, PAR-1, causes transformation by activation of Rho-mediated signaling pathways. Oncogene. 2001;20:1953–63. doi: 10.1038/sj.onc.1204281. [DOI] [PubMed] [Google Scholar]
- 148.Fromm C, Coso OA, Montaner S, Xu N, Gutkind JS. The small GTP-binding protein Rho links G protein-coupled receptors and Galpha12 to the serum response element and to cellular transformation. Proc Natl Acad Sci U S A. 1997;94:10098–103. doi: 10.1073/pnas.94.19.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gutkind JS, Novotny EA, Brann MR, Robbins KC. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc Natl Acad Sci U S A. 1991;88:4703–7. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Whitehead IP, Campbell S, Rossman KL, Der CJ. Dbl family proteins. Biochim Biophys Acta. 1997;1332:F1–23. doi: 10.1016/s0304-419x(96)00040-6. [DOI] [PubMed] [Google Scholar]
- 151.Zheng Y. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci. 2001;26:724–32. doi: 10.1016/S0968-0004(01)01973-9. [DOI] [PubMed] [Google Scholar]
- 152.Hart MJ, Eva A, Evans T, Aaronson SA, Cerione RA. Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature. 1991;354:311–4. doi: 10.1038/354311a0. [DOI] [PubMed] [Google Scholar]
- 153.Lin R, Cerione RA, Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J Biol Chem. 1999;274:23633–41. doi: 10.1074/jbc.274.33.23633. [DOI] [PubMed] [Google Scholar]
- 154.Bassermann F, Jahn T, Miething C, Seipel P, Bai RY, Coutinho S, Tybulewicz VL, Peschel C, Duyster J. Association of Bcr-Abl with the proto-oncogene Vav is implicated in activation of the Rac-1 pathway. J Biol Chem. 2002;277:12437–45. doi: 10.1074/jbc.M112397200. [DOI] [PubMed] [Google Scholar]
- 155.Skorski T, Wlodarski P, Daheron L, Salomoni P, Nieborowska-Skorska M, Majewski M, Wasik M, Calabretta B. BCR/ABL-mediated leukemogenesis requires the activity of the small GTP-binding protein Rac. Proc Natl Acad Sci U S A. 1998;95:11858–62. doi: 10.1073/pnas.95.20.11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kourlas PJ, Strout MP, Becknell B, Veronese ML, Croce CM, Theil KS, Krahe R, Ruutu T, Knuutila S, Bloomfield CD, et al. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: evidence for its fusion with MLL in acute myeloid leukemia. Proc Natl Acad Sci U S A. 2000;97:2145–50. doi: 10.1073/pnas.040569197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 2000;485:183–8. doi: 10.1016/S0014-5793(00)02224-9. [DOI] [PubMed] [Google Scholar]
- 158.Reuther GW, Lambert QT, Booden MA, Wennerberg K, Becknell B, Marcucci G, Sondek J, Caligiuri MA, Der CJ. Leukemia-associated Rho guanine nucleotide exchange factor, a Dbl family protein found mutated in leukemia, causes transformation by activation of RhoA. J Biol Chem. 2001;276:27145–51. doi: 10.1074/jbc.M103565200. [DOI] [PubMed] [Google Scholar]
- 159.Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, O’Bryan JP, Gupta V, Ratner N, Der CJ, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/S1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 160.Sherman LS, Gutmann DH. Merlin: hanging tumor suppression on the Rac. Trends Cell Biol. 2001;11:442–4. doi: 10.1016/S0962-8924(01)81334-9. [DOI] [PubMed] [Google Scholar]
- 161.Ingram DA, Hiatt K, King AJ, Fisher L, Shivakumar R, Derstine C, Wenning MJ, Diaz B, Travers JB, Hood A, et al. Hyperactivation of p21(ras) and the hematopoietic-specific Rho GTPase, Rac2, cooperate to alter the proliferation of neurofibromin-deficient mast cells in vivo and in vitro. J Exp Med. 2001;194:57–69. doi: 10.1084/jem.194.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang M, Prendergast GC. RhoB in cancer suppression. Histol Histopathol. 2006;21:213–8. doi: 10.14670/HH-21.213. [DOI] [PubMed] [Google Scholar]
- 163.Liu Ax, Cerniglia GJ, Bernhard EJ, Prendergast GC. RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc Natl Acad Sci U S A. 2001;98:6192–7. doi: 10.1073/pnas.111137198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu AX, Rane N, Liu JP, Prendergast GC. RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol. 2001;21:6906–12. doi: 10.1128/MCB.21.20.6906-6912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guo F, Zheng Y. Involvement of Rho family GTPases in p19Arf- and p53-mediated proliferation of primary mouse embryonic fibroblasts. Mol Cell Biol. 2004;24:1426–38. doi: 10.1128/MCB.24.3.1426-1438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 167.Cimica V, Batusic D, Chen Y, Hollemann T, Pieler T, Ramadori G. Transcriptome analysis of rat liver regeneration in a model of oval hepatic stem cells. Genomics. 2005;86:352–64. doi: 10.1016/j.ygeno.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 168.Chang CS, Huang SM, Lin HH, Wu CC, Wang CJ. Different expression of apoptotic proteins between HBV-infected and non-HBV-infected hepatocellular carcinoma. Hepatogastroenterology. 2007;54:2061–8. [PubMed] [Google Scholar]
- 169.Grise F, Bidaud A, Moreau V. Rho GTPases in hepatocellular carcinoma. Biochim Biophys Acta. 2009;1795:137–51. doi: 10.1016/j.bbcan.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 170.van Hengel J, D’Hooge P, Hooghe B, Wu X, Libbrecht L, De Vos R, Quondamatteo F, Klempt M, Brakebusch C, van Roy F. Continuous cell injury promotes hepatic tumorigenesis in cdc42-deficient mouse liver. Gastroenterology. 2008;134:781–92. doi: 10.1053/j.gastro.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 171.Yang L, Wang L, Kalfa TA, Cancelas JA, Shang X, Pushkaran S, Mo J, Williams DA, Zheng Y. Cdc42 critically regulates the balance between myelopoiesis and erythropoiesis. Blood. 2007;110:3853–61. doi: 10.1182/blood-2007-03-079582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Valentijn LJ, Koppen A, van Asperen R, Root HA, Haneveld F, Versteeg R. Inhibition of a new differentiation pathway in neuroblastoma by copy number defects of N-myc, Cdc42, and nm23 genes. Cancer Res. 2005;65:3136–45. doi: 10.1158/0008-5472.CAN-04-2469. [DOI] [PubMed] [Google Scholar]
- 173.Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal. 2011;23:1415–23. doi: 10.1016/j.cellsig.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Goehring NW, Grill SW. Cell polarity: mechanochemical patterning. Trends Cell Biol. 2013;23:72–80. doi: 10.1016/j.tcb.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 175.Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–45. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–33. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–94. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2012;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- 179.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 180.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–7. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alexander K, Yang HS, Hinds PW. Cellular senescence requires CDK5 repression of Rac1 activity. Mol Cell Biol. 2004;24:2808–19. doi: 10.1128/MCB.24.7.2808-2819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang HS, Hinds PW. Phosphorylation of ezrin by cyclin-dependent kinase 5 induces the release of Rho GDP dissociation inhibitor to inhibit Rac1 activity in senescent cells. Cancer Res. 2006;66:2708–15. doi: 10.1158/0008-5472.CAN-05-3141. [DOI] [PubMed] [Google Scholar]
- 183.Guo F, Debidda M, Yang L, Williams DA, Zheng Y. Genetic deletion of Rac1 GTPase reveals its critical role in actin stress fiber formation and focal adhesion complex assembly. J Biol Chem. 2006;281:18652–9. doi: 10.1074/jbc.M603508200. [DOI] [PubMed] [Google Scholar]
- 184.Kortlever RM, Brummelkamp TR, van Meeteren LA, Moolenaar WH, Bernards R. Suppression of the p53-dependent replicative senescence response by lysophosphatidic acid signaling. Mol Cancer Res. 2008;6:1452–60. doi: 10.1158/1541-7786.MCR-08-0066. [DOI] [PubMed] [Google Scholar]
- 185.Gadéa G, Lapasset L, Gauthier-Rouvière C, Roux P. Regulation of Cdc42-mediated morphological effects: a novel function for p53. EMBO J. 2002;21:2373–82. doi: 10.1093/emboj/21.10.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang L, Yang L, Debidda M, Witte D, Zheng Y. Cdc42 GTPase-activating protein deficiency promotes genomic instability and premature aging-like phenotypes. Proc Natl Acad Sci U S A. 2007;104:1248–53. doi: 10.1073/pnas.0609149104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mantovani A. Molecular pathways linking inflammation and cancer. Curr Mol Med. 2010;10:369–73. doi: 10.2174/156652410791316968. [DOI] [PubMed] [Google Scholar]
- 188.Darnell JE., Jr. STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 189.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–42. doi: 10.1172/JCI0215617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 191.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/S0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 192.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–77. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 195.Bournazou E, Bromberg J. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT. 2013;2:e23828. doi: 10.4161/jkst.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tonozuka Y, Minoshima Y, Bao YC, Moon Y, Tsubono Y, Hatori T, Nakajima H, Nosaka T, Kawashima T, Kitamura T. A GTPase-activating protein binds STAT3 and is required for IL-6-induced STAT3 activation and for differentiation of a leukemic cell line. Blood. 2004;104:3550–7. doi: 10.1182/blood-2004-03-1066. [DOI] [PubMed] [Google Scholar]
- 197.Raptis L, Arulanandam R, Geletu M, Turkson J. The R(h)oads to Stat3: Stat3 activation by the Rho GTPases. Exp Cell Res. 2011;317:1787–95. doi: 10.1016/j.yexcr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Peelman F, Tavernier J. ROCKing the JAKs. JAKSTAT. 2013;2:e24074. doi: 10.4161/jkst.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sanz-Moreno V, Gaggioli C, Yeo M, Albrengues J, Wallberg F, Viros A, Hooper S, Mitter R, Féral CC, Cook M, et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20:229–45. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 200.Sriuranpong V, Park JI, Amornphimoltham P, Patel V, Nelkin BD, Gutkind JS. Epidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/gp130 cytokine system. Cancer Res. 2003;63:2948–56. [PubMed] [Google Scholar]
- 201.Cammarano MS, Minden A. Dbl and the Rho GTPases activate NF kappa B by I kappa B kinase (IKK)-dependent and IKK-independent pathways. J Biol Chem. 2001;276:25876–82. doi: 10.1074/jbc.M011345200. [DOI] [PubMed] [Google Scholar]
- 202.Liu CA, Wang MJ, Chi CW, Wu CW, Chen JY. Rho/Rhotekin-mediated NF-kappaB activation confers resistance to apoptosis. Oncogene. 2004;23:8731–42. doi: 10.1038/sj.onc.1208106. [DOI] [PubMed] [Google Scholar]
- 203.Friedland JC, Lakins JN, Kazanietz MG, Chernoff J, Boettiger D, Weaver VM. alpha6beta4 integrin activates Rac-dependent p21-activated kinase 1 to drive NF-kappaB-dependent resistance to apoptosis in 3D mammary acini. J Cell Sci. 2007;120:3700–12. doi: 10.1242/jcs.03484. [DOI] [PubMed] [Google Scholar]
- 204.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 205.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 206.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–6. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 207.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang C, Liu J, Liang Y, Wu R, Zhao Y, Hong X, Lin M, Yu H, Liu L, Levine AJ, et al. Tumour-associated mutant p53 drives the Warburg effect. Nat Commun. 2013;4:2935. doi: 10.1038/ncomms3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gururaj A, Barnes CJ, Vadlamudi RK, Kumar R. Regulation of phosphoglucomutase 1 phosphorylation and activity by a signaling kinase. Oncogene. 2004;23:8118–27. doi: 10.1038/sj.onc.1207969. [DOI] [PubMed] [Google Scholar]
- 210.Shalom-Barak T, Knaus UG. A p21-activated kinase-controlled metabolic switch up-regulates phagocyte NADPH oxidase. J Biol Chem. 2002;277:40659–65. doi: 10.1074/jbc.M206650200. [DOI] [PubMed] [Google Scholar]
- 211.DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–24. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang JB, Erickson JW, Fuji R, Ramachandran S, Gao P, Dinavahi R, Wilson KF, Ambrosio AL, Dias SM, Dang CV, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell. 2010;18:207–19. doi: 10.1016/j.ccr.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr. 1995;15:133–59. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- 214.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 215.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 216.Bryan BA, D’Amore PA. What tangled webs they weave: Rho-GTPase control of angiogenesis. Cell Mol Life Sci. 2007;64:2053–65. doi: 10.1007/s00018-007-7008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell. 2006;9:33–44. doi: 10.1016/j.ccr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 218.Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park JS, Park I, Jung J, Kataoka H, Lee D, et al. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer Cell. 2014;25:102–17. doi: 10.1016/j.ccr.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 219.Kazerounian S, Gerald D, Huang M, Chin YR, Udayakumar D, Zheng N, O’Donnell RK, Perruzzi C, Mangiante L, Pourat J, et al. RhoB differentially controls Akt function in tumor cells and stromal endothelial cells during breast tumorigenesis. Cancer Res. 2013;73:50–61. doi: 10.1158/0008-5472.CAN-11-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gerald D, Adini I, Shechter S, Perruzzi C, Varnau J, Hopkins B, Kazerounian S, Kurschat P, Blachon S, Khedkar S, et al. RhoB controls coordination of adult angiogenesis and lymphangiogenesis following injury by regulating VEZF1-mediated transcription. Nat Commun. 2013;4:2824. doi: 10.1038/ncomms3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Radu M, Semenova G, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer. 2014;14:13–25. doi: 10.1038/nrc3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Eblen ST, Slack JK, Weber MJ, Catling AD. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol Cell Biol. 2002;22:6023–33. doi: 10.1128/MCB.22.17.6023-6033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. 2003;301:94–6. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- 224.Turcotte S, Desrosiers RR, Béliveau R. HIF-1alpha mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. J Cell Sci. 2003;116:2247–60. doi: 10.1242/jcs.00427. [DOI] [PubMed] [Google Scholar]
- 225.Merajver SD, Usmani SZ. Multifaceted role of Rho proteins in angiogenesis. J Mammary Gland Biol Neoplasia. 2005;10:291–8. doi: 10.1007/s10911-006-9002-8. [DOI] [PubMed] [Google Scholar]
- 226.Hirota K, Semenza GL. Rac1 activity is required for the activation of hypoxia-inducible factor 1. J Biol Chem. 2001;276:21166–72. doi: 10.1074/jbc.M100677200. [DOI] [PubMed] [Google Scholar]
- 227.Lee TK, Poon RT, Yuen AP, Man K, Yang ZF, Guan XY, Fan ST. Rac activation is associated with hepatocellular carcinoma metastasis by up-regulation of vascular endothelial growth factor expression. Clin Cancer Res. 2006;12:5082–9. doi: 10.1158/1078-0432.CCR-05-2794. [DOI] [PubMed] [Google Scholar]
- 228.Xue Y, Bi F, Zhang X, Zhang S, Pan Y, Liu N, Shi Y, Yao X, Zheng Y, Fan D. Role of Rac1 and Cdc42 in hypoxia induced p53 and von Hippel-Lindau suppression and HIF1alpha activation. Int J Cancer. 2006;118:2965–72. doi: 10.1002/ijc.21763. [DOI] [PubMed] [Google Scholar]
- 229.van Golen KL, Wu ZF, Qiao XT, Bao L, Merajver SD, Rho C. RhoC GTPase overexpression modulates induction of angiogenic factors in breast cells. Neoplasia. 2000;2:418–25. doi: 10.1038/sj.neo.7900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.van der Meel R, Symons MH, Kudernatsch R, Kok RJ, Schiffelers RM, Storm G, Gallagher WM, Byrne AT. The VEGF/Rho GTPase signalling pathway: a promising target for anti-angiogenic/anti-invasion therapy. Drug Discov Today. 2011;16:219–28. doi: 10.1016/j.drudis.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 231.Li XR, Ji F, Ouyang J, Wu W, Qian LY, Yang KY. Overexpression of RhoA is associated with poor prognosis in hepatocellular carcinoma. Eur J Surg Oncol. 2006;32:1130–4. doi: 10.1016/j.ejso.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 232.Kamai T, Tsujii T, Arai K, Takagi K, Asami H, Ito Y, Oshima H. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin Cancer Res. 2003;9:2632–41. [PubMed] [Google Scholar]
- 233.Faried A, Faried LS, Usman N, Kato H, Kuwano H. Clinical and prognostic significance of RhoA and RhoC gene expression in esophageal squamous cell carcinoma. Ann Surg Oncol. 2007;14:3593–601. doi: 10.1245/s10434-007-9562-x. [DOI] [PubMed] [Google Scholar]
- 234.Horiuchi A, Imai T, Wang C, Ohira S, Feng Y, Nikaido T, Konishi I. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–70. doi: 10.1097/01.LAB.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 235.Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y, Fan D. Expression of seven main Rho family members in gastric carcinoma. Biochem Biophys Res Commun. 2004;315:686–91. doi: 10.1016/j.bbrc.2004.01.108. [DOI] [PubMed] [Google Scholar]
- 236.Jiang WG, Watkins G, Lane J, Cunnick GH, Douglas-Jones A, Mokbel K, Mansel RE. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003;9:6432–40. [PubMed] [Google Scholar]
- 237.Shikada Y, Yoshino I, Okamoto T, Fukuyama S, Kameyama T, Maehara Y. Higher expression of RhoC is related to invasiveness in non-small cell lung carcinoma. Clin Cancer Res. 2003;9:5282–6. [PubMed] [Google Scholar]
- 238.Kondo T, Sentani K, Oue N, Yoshida K, Nakayama H, Yasui W. Expression of RHOC is associated with metastasis of gastric carcinomas. Pathobiology. 2004;71:19–25. doi: 10.1159/000072958. [DOI] [PubMed] [Google Scholar]
- 239.Wang W, Yang LY, Huang GW, Lu WQ, Yang ZL, Yang JQ, Liu HL. Genomic analysis reveals RhoC as a potential marker in hepatocellular carcinoma with poor prognosis. Br J Cancer. 2004;90:2349–55. doi: 10.1038/sj.bjc.6601749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Furuta E, et al. RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res. 2008;68:7613–20. doi: 10.1158/0008-5472.CAN-07-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kleer CG, Teknos TN, Islam M, Marcus B, Lee JS, Pan Q, Merajver SD, Rho C. RhoC GTPase expression as a potential marker of lymph node metastasis in squamous cell carcinomas of the head and neck. Clin Cancer Res. 2006;12:4485–90. doi: 10.1158/1078-0432.CCR-06-0376. [DOI] [PubMed] [Google Scholar]
- 242.Marionnet C, Lalou C, Mollier K, Chazal M, Delestaing G, Compan D, Verola O, Vilmer C, Cuminet J, Dubertret L, et al. Differential molecular profiling between skin carcinomas reveals four newly reported genes potentially implicated in squamous cell carcinoma development. Oncogene. 2003;22:3500–5. doi: 10.1038/sj.onc.1206571. [DOI] [PubMed] [Google Scholar]
- 243.Engers R, Ziegler S, Mueller M, Walter A, Willers R, Gabbert HE. Prognostic relevance of increased Rac GTPase expression in prostate carcinomas. Endocr Relat Cancer. 2007;14:245–56. doi: 10.1677/ERC-06-0036. [DOI] [PubMed] [Google Scholar]