Abstract
The accumulation of DNA damage is thought to contribute to the physiological decay associated with the aging process. Here, we report the results of a large-scale study examining longevity in various mouse models defective in the repair of DNA alkylation damage, or defective in the DNA damage response. We find that the repair of spontaneous DNA damage by alkyladenine DNA glycosylase (Aag/Mpg)-initiated base excision repair and O6-methylguanine DNA methyltransferase (Mgmt)-mediated direct reversal contributes to maximum life span in the laboratory mouse. We also uncovered important genetic interactions between Aag, which excises a wide variety of damaged DNA bases, and the DNA damage sensor and signaling protein, Atm. We show that Atm plays a role in mediating survival in the face of both spontaneous and induced DNA damage, and that Aag deficiency not only promotes overall survival, but also alters the tumor spectrum in Atm−/− mice. Further, the reversal of spontaneous alkylation damage by Mgmt interacts with the DNA mismatch repair pathway to modulate survival and tumor spectrum. Since these aging studies were performed without treatment with DNA damaging agents, our results indicate that the DNA damage that is generated endogenously accumulates with age, and that DNA alkylation repair proteins play a role in influencing longevity.
Keywords: AAG/MPG, Mgmt, DNA adducts, DNA glycosylase, aging, base excision repair
1 INTRODUCTION
Aging can be thought of as a progressive decline in function at the cellular, tissue, and organismal level, possibly resulting from cumulative damage to important biomolecules [1]. The reasons why we age and modulators of the aging process have been intensively studied for decades (reviewed in [1]). One predominant but recently contested school of thought [2, 3], is described in the mitochondrial free radical theory of aging. In 1956, Harman first proposed that mitochondrially-generated reactive oxygen and nitrogen species (RONS), along with other harmful environmental physical and chemical agents, result in accumulating damage in numerous biomolecules critical for proper cell function [4]. Genetic experiments in various model organisms have pinpointed a variety of genes and pathways that influence how an organism ages (reviewed in [5–8]); however, it has become clear that additional random events also play an important role in the determination of longevity. In fact, the accumulation of unrepaired DNA damage causing decreased genomic integrity, has long been proposed as a major source of stochastic changes that can influence aging (reviewed in [9, 10]). Accordingly, animals with genetic deficiencies in double-strand-break repair or telomere maintenance have much shorter lifespans than wild-type (WT) mice [11, 12]. Further demonstrating the importance of unrepaired DNA damage in aging, mice or patients carrying mutations in the transcription-coupled branch of nucleotide excision repair (NER) suffer from a premature onset of aging-related symptoms and consequent shortening of lifespan, but interestingly, with the exception of skin cancers, the decreased longevity occurs in the absence of increased cancer development (reviewed in [13, 14][10]).
Inactivating mutations that disrupt the maintenance of genome stability can decrease longevity through either increasing cancer predisposition or causing more general premature aging and progeroid-like characteristics (reviewed in [10, 15, 16]). Indeed, multiple important DNA damage response proteins were originally identified through the investigation of cancer-prone patients. Cancer-prone Li-Fraumeni and ataxia telangiectasia (AT) patients exhibit germline mutations in two important DNA damage response proteins, namely p53 and ataxia telangiectasia mutated (ATM), respectively [17]. p53 (i.e., Trp53) is a stress sensor and transcription factor responsible for inducing cell cycle checkpoints, apoptosis or senescence upon exposure to DNA damage, hypoxia, and oncogene activation among other stimuli; p53 was originally identified as a tumor suppressor and is known as the “guardian” of the genome [17–19]. p53 also appears to have additional roles in modulating aging, independent of its role in tumor suppression, presumably related to its role in cellular senescence [17, 20]. ATM, an integral DNA damage signaling protein, is a serine/threonine protein kinase that is activated in response to double-strand DNA breaks; ATM’s activation initiates important signaling pathways, some of which involve p53, responsible for cell cycle checkpoint activation, apoptosis and DNA repair (reviewed in [21]).
DNA is exposed to a wide-range of damaging agents, not only from exogenous, but also from endogenous sources. DNA base alkylation is one common consequence of multiple endogenous metabolic processes (reviewed in [22]). For example, alkylation can occur as a consequence of the non-enzymatic transfer of methyl groups to DNA from the universal methyl donor, S-adenosylmethionine. Additionally, RONS, inevitable byproducts of aerobic metabolism and also an important component of the innate immune response, are highly reactive chemical species that produce numerous types of DNA damage. Furthermore, RONS can indirectly induce alkylation DNA damage as a result of lipid peroxidation reactions that generate reactive alkylating agents that react to produce etheno (ε) and other DNA base adducts [23–27]. Livers of aged animals exhibit an accumulation of ε adducts, specifically εA, suggesting a possible role for these lipid peroxidation reactions in aging organisms [28]. DNA base lesions are also increased under conditions of chronic inflammation, and are believed to contribute to the increased risk of carcinogenesis observed in patients with chronic inflammation [29–32]. Therefore, various types of alkylation damage arise in cells as a function of normal metabolic functions, and the role of such endogenous DNA damage in influencing longevity has yet to be determined.
The pathways primarily responsible for the repair of alkylated DNA base lesions are base excision repair (BER) and direct reversal (reviewed in [33].) BER is initiated when a damaged DNA base is recognized and excised by a DNA glycosylase; alkyladenine DNA glycosylase (Aag, a.k.a Mpg) recognizes numerous alkylated DNA base lesions, including 3-methyladenine (3meA) and 7-methylguanine (7meG) in mammals. Aag also recognizes many lesions induced by RONS and lipid peroxidation products including hypoxanthine and εA respectively [34–36]. Other alkylated DNA bases are subject to direct reversal repair, either by oxidative demethylation catalyzed by the AlkB homolog (Alkbh) family of proteins (reviewed in [33]), or by the efficient transfer of the unwanted methyl group on O6-methylguanine (O6MeG) lesions to a cysteine residue in the O6MeG DNA methyltransferase (Mgmt) in a suicide reaction [37]. Unrepaired O6MeG lesions pair with thymine during replication. The mismatch repair (MMR) pathway recognizes O6MeG:thymine (O6MeG:T) mismatches and subsequent MMR processing plays an essential role in alkylation-induced cytotoxicity. MutSα, a heterodimer of Msh2 and Msh6 MMR proteins, recognizes O6MeG:T mispairs, and this recognition is required for the induction of apoptosis [38–40]. The MMR pathway then engages in futile cycles of Exonuclease 1 (Exo1)-mediated excision and DNA polymerase resynthesis at O6MeG:T mismatches, with excision and reinsertion of the thymine opposite O6MeG; this results in persistent strand breaks that ultimately culminate in the formation of double-strand DNA breaks at collapsed replication forks (reviewed in [33, 37]). This alkylation hypersensitivity observed in the absence of Mgmt is dependent on the presence of a functional MMR pathway [40–43].
The contribution of endogenous DNA alkylation damage to longevity has not been rigorously examined. It is well-documented that Mgmt expression protects mice from tumors induced by treatment with exogenous alkylating agents and therefore prolongs survival following treatment with alkylating agents [44–47]. Intriguingly, transgenic C3HeB/FeJ male mice overexpressing Mgmt are protected against spontaneous hepatocellular carcinomas in a susceptible mouse strain [48], suggesting a role for O6MeG lesions in spontaneous tumors in a predisposed background and perhaps enhanced overall survival. It has been more challenging to investigate the role for BER in longevity due to the embryonic lethality observed upon deletion of many BER proteins [49–51]. However, studies in heterozygous mice have provided insight into the importance of BER proteins in modulating survival; for example, Polb+/− mice exhibit an acceleration in the age-dependent mortality rate as well as increased tumorigenesis [52]. Finally, we and others have illustrated the importance of Mgmt- and Aag-initiated DNA repair in chronic inflammation-associated cancer, a frequent aging-associated phenomenon; Aag−/− and Mgmt−/− mice are more susceptible to chronic inflammation-associated colon carcinogenesis [31, 32, 53, 54]. However, the contribution of Aag and Mgmt to overall longevity was, heretofore, not specifically investigated.
Here we exploit mouse model systems to determine whether DNA alkylation repair proteins acting on spontaneous DNA damage contribute to aging and longevity. We performed a large-scale study to assess whether two major DNA alkylation repair pathways, namely Aag-initiated BER and Mgmt-mediated direct reversal, promote longevity. Supplemental Table 1 lists the mouse genotypes used in this study. We compared the longevity of Aag−/−, Mgmt−/− and Aag−/−/Mgmt−/− mice with that of WT mice. We also investigated putative genetic interactions that Aag and Mgmt might have with the DNA damage response pathways controlled by p53 and Atm. Finally, because the MMR pathway is an important modulator of cellular responses to O MeG, we investigated possible genetic interactions between Mgmt and MMR by examining Mgmt −/−/Msh6 −/− and Mgmt −/−/Exo1 −/− mice. Together, our comprehensive study illustrates that the repair of spontaneous DNA base damage, likely to be primarily alkylation damage, influences the longevity of mice, and provides information about potential interactions between DNA alkylation repair proteins and downstream DNA damage response mediators.
2 RESULTS
2.1 Deficiency in alkylation repair alters long-term survival
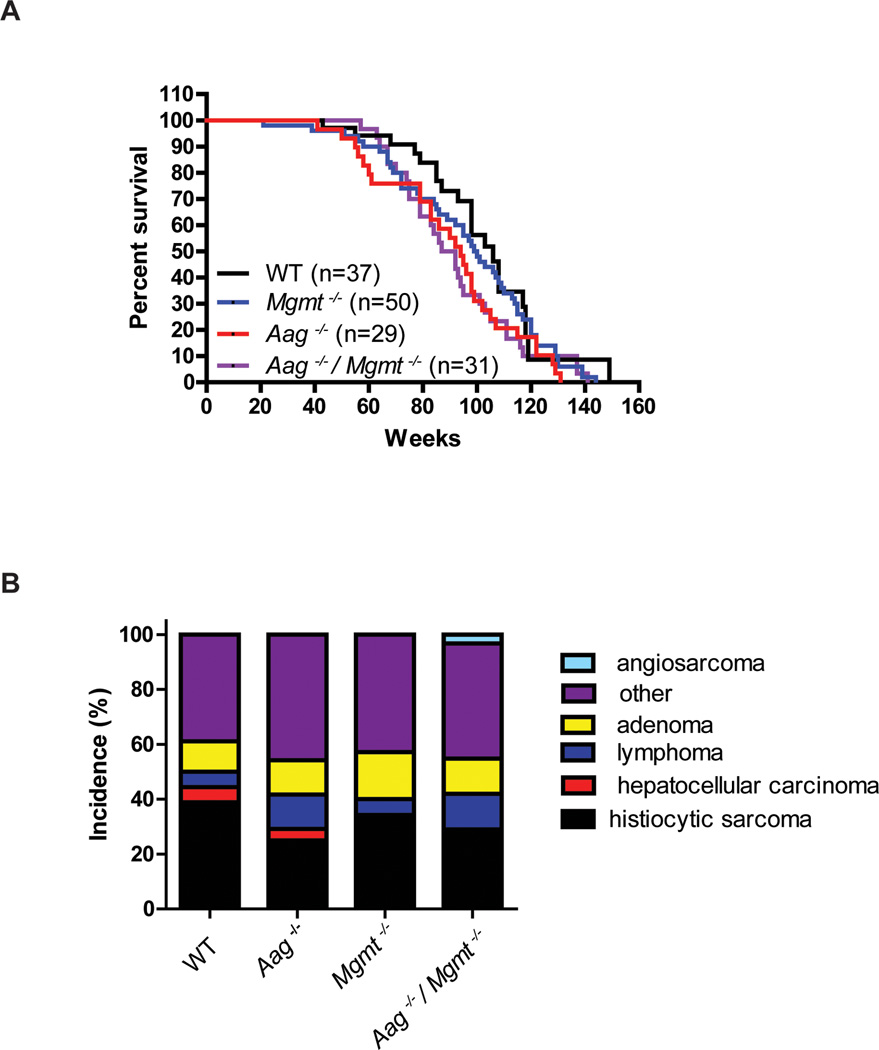
Given that the endogenous generation of DNA damage is ubiquitous and continuous, we determined whether repair of spontaneous DNA base damage, primarily alkylation damage, contributes to longevity in mammals by assessing the long-term survival of mice deficient in genes for the repair of alkylated DNA bases, i.e, Aag−/− and Mgmt−/− mice. Large cohorts of WT, Aag−/−, Mgmt−/− and Aag−/−/Mgmt−/− mice were established and carefully monitored for up to three years. Unlike mice deficient in NER [10, 14], none of the genotypes exhibited any signs of premature aging. As mice became moribund, survival and histological data were collected. Compared to WT mice, Aag−/− and Mgmt−/− mice exhibit a trend toward decreased longevity, which did not reach statistical significance (Figure 1A). However, the Aag−/−/Mgmt−/− animals display a significantly shorter life-span compared to WT (p=0.04); the median survival of Aag−/−/Mgmt−/−mice was 89.5 weeks, more than 15 weeks shorter than the median survival observed in WT mice (Figure 1A). These data indicate the importance of repairing spontaneous DNA base lesions for attaining maximum longevity.
Figure 1. DNA alkylation repair contributes to longevity.
A) Survival curves of wild type (black lines, n=37), Aag−/− (red line, n=29), Mgmt−/− (blue line, n=50) and Aag−/− Mgmt−/− (purple line, n=31). Pair-wise comparisons: Aag−/− to wild type, p=0.1003; Mgmt−/− to wild type, p=0.4752; Aag−/− Mgmt−/− to wild type, p=0.04, all Log-rank (Mantel-Cox) test. B) Histopathological classification of pathologies found in WT (n=18), Aag−/− (n=24), Mgmt−/− (n=35), and Aag−/− Mgmt−/− (n=31) mice.
The large-scale aging studies included detailed histopathological examination to identify pathological features in the mice, including classification of any tumors, to determine whether modulating DNA repair altered tumor incidence and/or tumor spectrum. In our study, the most prevalent tumor type in WT, Aag−/−, Mgmt−/−, and Aag−/− /Mgmt−/− mice was histiocytic sarcoma, a macrophage neoplasm and the most common tumor classification in the C57Bl/6 strain [55]. We find that the absence of either Aag or Mgmt activity (or both) did not significantly alter the tumor incidence or spectrum when compared to the WT mice (Figure 1B); in other words, although the repair-deficient mice succumb earlier than WT, the spectrum of disease and cause of death remains similar.
2.1 Influence of DNA damage response proteins on responses to endogenous DNA alkylation damage
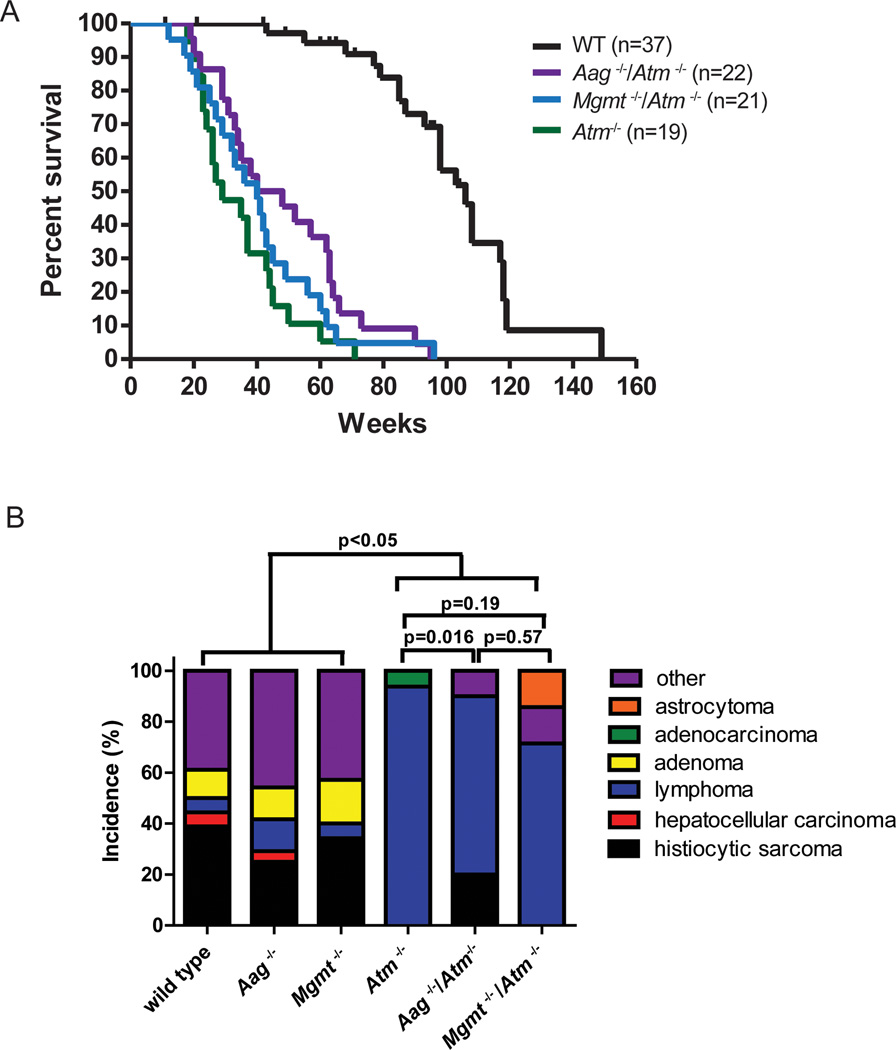
The p53 and Atm proteins are important stress mediators that respond to DNA damage. Mice deficient in these proteins exhibit drastically reduced longevity, developing thymic lymphoma within the first year of life [56, 57]. We sought to determine whether accumulating unrepaired spontaneous DNA base damage (again, primarily alkylation damage) may contribute to lymphomagenesis and diminished longevity in Atm−/− and p53−/− mice. Although it is well established that Atm−/− mice exhibit significantly shortened life spans [56, 58], detailed longevity, studies have not been reported for the C57Bl/6 strain background. Figure 2A shows Kaplan-Meier survival curves for Atm−/− mice, both alone and in combination with the Aag or Mgmt null alleles. Atm−/−, Aag−/−/Atm−/−, and Mgmt−/−/Atm−/− mice all exhibit decreased survival when compared to the WT mice (all pair-wise comparisons to wild type, p<0.0001). However, in contrast to previous studies in mixed background mice, we find that Atm−/− C57Bl/6 mice survive significantly longer than Atm−/− mixed background mice [56, 58]. In fact, in our aging study, 20% of Atm−/− C57Bl/6 mice survive longer than one year (Figure 2A), whereas most Atm−/− mice on a mixed background succumbed to thymic lymphoma by 4.5 months [56, 58]. We find that the addition of the Mgmt null allele does not significantly change the survival of Atm−/− animals (p=0.3423), suggesting that endogenously formed O6MeG lesions are not determinants of survival in Atm−/− mice. Surprisingly, Aag−/−/Atm−/− C57Bl/6 mice live significantly longer than Atm −/− C57Bl/6 mice (pair-wise comparison between Atm−/− and Aag−/−/Atm−/−, p=0.0193) (Figure 2A). These results indicate that, in contrast to Aag-mediated repair of endogenous DNA base damage extending longevity, Aag activity in Atm−/− mice actually decreases longevity. This counterintuitive finding is considered further in the discussion.
Figure 2. Atm plays a role in the response to endogenous/spontaneous DNA alkylation damage.
A) Survival curves of wild type (black lines, n=37), Atm−/− (green line, n=19), Aag−/− Atm−/− (green/red line, n=22) and Mgmt−/− Atm−/− (cyan line, n=21). All pair-wise comparisons to wild type, p<0.0001. Pair-wise comparison between Atm−/− and Aag−/− Atm−/−, p=0.0193, and between Atm−/− and Mgmt−/− Atm−/−, p=0.3423, all comparisons Log-rank (Mantel-Cox) test. B) Aag deficiency protects against lymphoma in Atm−/− animals. Graph shows the incidence of age-related pathologies observed in Aag and Atm genotypic combinations. Wild-type (n=18); Aag−/− (n=24); Mgmt−/− (n=35); Atm−/− (n=15); Mgmt−/− Atm−/− (n=7); Aag−/− Atm−/− (n=10).
We also monitored disease incidence and tumor spectrum in the aged Atm−/−, Mgmt−/−/Atm−/− or Aag−/−/Atm−/− mice; Figure 2B shows the incidence of spontaneous pathology. WT, Mgmt−/− and Aag−/− mice exhibit a remarkably similar spectrum of tumors, but this spectrum is significantly different from that in the Atm deficient genotypes (Atm−/−, Mgmt−/−/Atm−/−, and Aag−/−/Atm−/−) that exhibit a predominance of lymphoma [56, 58]; 94% of the Atm−/− mice in our study presented with lymphomas at the time of death. Aag−/−/Atm−/− animals exhibit a decreased incidence of lymphoma (70%), and the overall difference in tumor spectrum between Atm−/− and Aag−/−/Atm−/− mice is statistically significant (p<0.016). This suggests that one mechanism by which Aag deficiency increases longevity in Atm−/− animals may be by decreasing the development of aggressive lymphomas. Although Mgmt−/−/Atm−/− also display a decrease in lymphoma incidence (71.5%), it did not alter the overall tumor spectrum (p=0.19) (Figure 2B) or longevity (Figure 1B), suggesting that the tumors that arose instead of lymphoma in Mgmt−/−/Atm−/− mice were as aggressive as lymphoma.
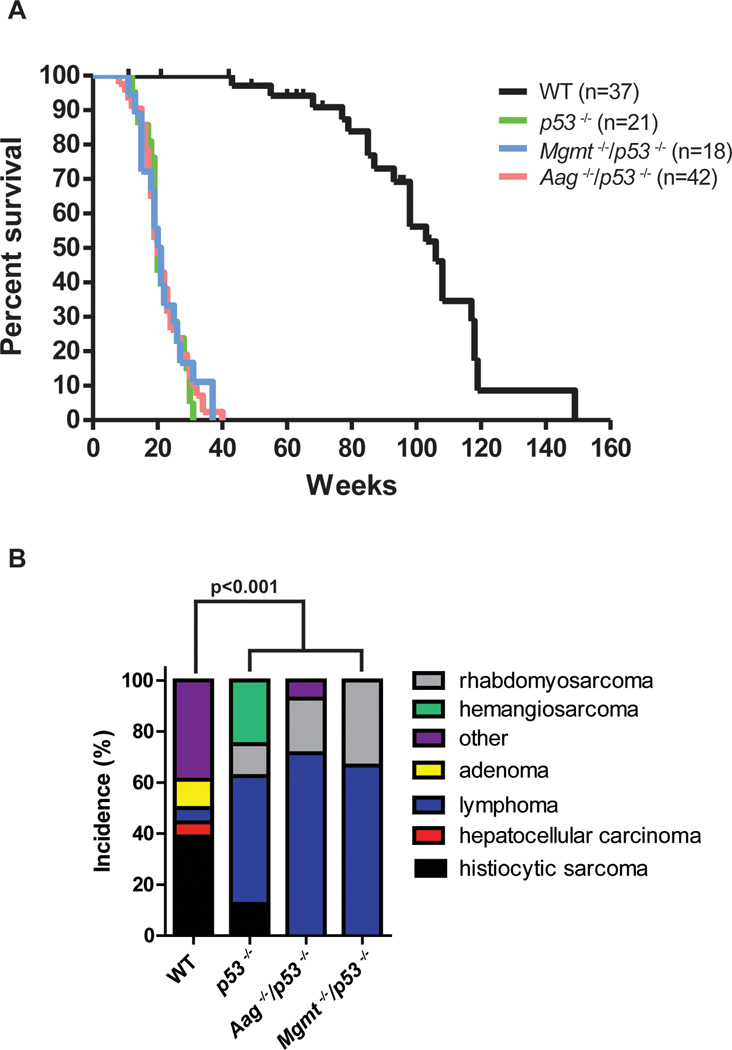
Detailed survival studies have been published for p53−/− mice [57], and here we set out to determine whether decreased repair of primarily alkylated DNA bases would affect longevity in p53−/− mice. In stark contrast to the effect of combining Aag−/− or Mgmt−/− with the Atm−/− genotype, we observe virtually identical survival in p53−/−, Aag−/−/p53−/−, and Mgmt−/−/p53−/− mice; all three genotypes exhibited significant and similarly-decreased survival compared to WT mice (p<0.0001) (Figure 3A). All p53 deficient genotypes exhibited an altered tumor spectrum compared to WT mice, but there was no difference in tumor spectrum between p53−/−, Aag−/−/p53−/−, and Mgmt−/−/p53−/− mice (Figure 3B).
Figure 3. Aag and Mgmt mutations do not affect longevity of p53 mutant animals.
A) Survival curves of wild type (black lines, n=37), p53−/− (light green line, n=21), Aag−/− p53−/− (light red line, n=42), and Mgmt−/− p53−/− (light blue line, n=18). All pair-wise comparisons to wild type, p<0.0001, Log-rank (Mantel-Cox) test. B) Aag or Mgmt deficiency does not shift tumor spectrum in p53−/− mice. Graph shows the incidence of age-related pathologies observed in Aag and p53 genotypic combinations. Wild-type (n=18); p53−/− (n=8); Aag−/− p53−/− (n=14); Mgmt−/− p53−/− (n=9).
2.3 Genetic interaction between Mgmt and the MMR pathway
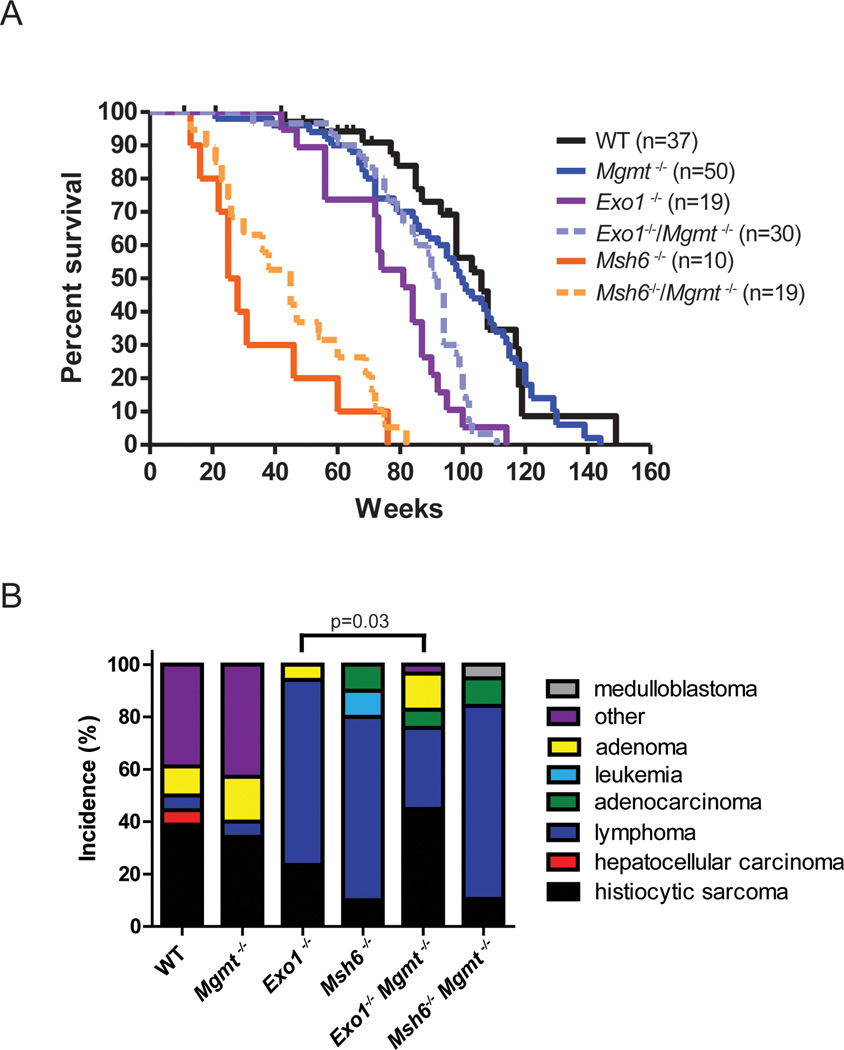
Given the established link between Mgmt and MMR in modulating alkylation-induced cytotoxicity [37, 40, 41, 59, 60], we investigated whether deficiency of both Mgmt and MMR proteins may cooperate to alter longevity. The effect of eliminating mismatch recognition and excisions steps of MMR in combination with Mgmt was investigated. As described, Mgmt deficiency does not significantly alter long-term survival in vivo (Figure 1A). Figure 4A presents survival data for WT, Mgmt−/−, Msh6−/−, Exo1−/−, Mgmt−/−/Msh6−/− and Mgmt−/−/Exo1−/− mice. Similar to previous reports, we observe significantly decreased survival in Msh6−/− and Exo1−/−mice compared to WT mice (p<0.0001) [61, 62]. We observed a trend toward increased longevity in Mgmt−/−/Msh6−/−, which did not reach statistical significance (pairwise comparison between Msh6−/−and Mgmt−/− Msh6−/−, p<0.3388). Similarly, the trend toward increased survival in Mgmt−/−/Exo1−/− versus Exo1−/− mice did not reach statistical significance (pairwise comparison between Exo1−/−and Mgmt−/− Exo1−/−, p=0.1352) (Figure 4A). We infer that Mgmt substrates do not significantly impact whole-animal survival, even in the absence of functional MMR. Although a genetic interaction was observed between Mgmt and Msh6 or Exo1 in terms of mediating alkylation cytotoxicity upon treatment with exogenous alkylating agents in vivo [63], this does not appear to translate to effects from endogenous alkylation arising in vivo.
Figure 4. Interaction between Mgmt deficiency and the mismatch repair pathway.
Survival curves of wild type (black lines, n=37), Mgmt−/− (blue line, n=50), Exo1−/− (purple line, n=19), Mgmt−/− Exo1−/− (dashed lilac line, n=30), Msh6−/− (orange line, n=10), and Mgmt−/− Msh6−/− (dashed light orange line, n=19). Pair-wise comparison between Mgmt−/− and Mgmt−/− Exo1−/−, p=0.0008 and between Mgmt−/− and Mgmt−/− Msh6−/−, p<0.0001, Log-rank (Mantel-Cox) test. B) The Mgmt null mutation leads to a decrease in the incidence of lymphomas in Exo1−/− animals but not in Msh6−/− animals. Wild-type (n=18); Mgmt−/− (n=33), Exo1−/− (n=17), Msh6−/− (n=10), Mgmt−/− Msh6−/− (n=19), Mgmt−/− Exo1−/− (n=29).
Detailed histological examination of the aged animals showed that the trends toward increased survival were accompanied by differences in pathology. Figure 4B shows the incidence of spontaneous pathology in animals with combinations of the Mgmt null allele with either Msh6 or Exo1 null alleles, namely Mgmt−/−, Msh6−/−, Exo1−/−, Mgmt−/−/Msh6−/−, and Mgmt−/−/Exo1−/− mice. The majority of the MMR defective animals exhibit lymphomas at the time of death; 70% of Msh6−/−animals and 70.5% of Exo1−/− animals present with lymphoma, consistent with the published literature (Figure 4B) [61, 62]. The additional inactivation of the Mgmt gene does not significantly alter the tumor spectrum in Msh6 mutant background; 73% of Mgmt−/−/Msh6−/− mice develop lymphoma (Figure 4B). Remarkably, Mgmt deficiency results in a greater than 50% reduction in the incidence of lymphoma in Exo1−/− mice; 70.5% in Exo1−/− mice develop lymphoma whereas only 31% of Mgmt−/− /Exo1−/− mice present with this pathology. The reduction of lymphoma in Mgmt−/− /Exo1−/− mice coincides with a two-fold increase in histiocytic sarcoma, the predominant pathology observed in Mgmt−/− mice. The change in tumor spectrum between the Exo1−/− and the Mgmt−/−/Exo1−/− is significant (p=0.03).
2.4 The contribution of Atm to cellular responses following exogenous DNA alkylation damage
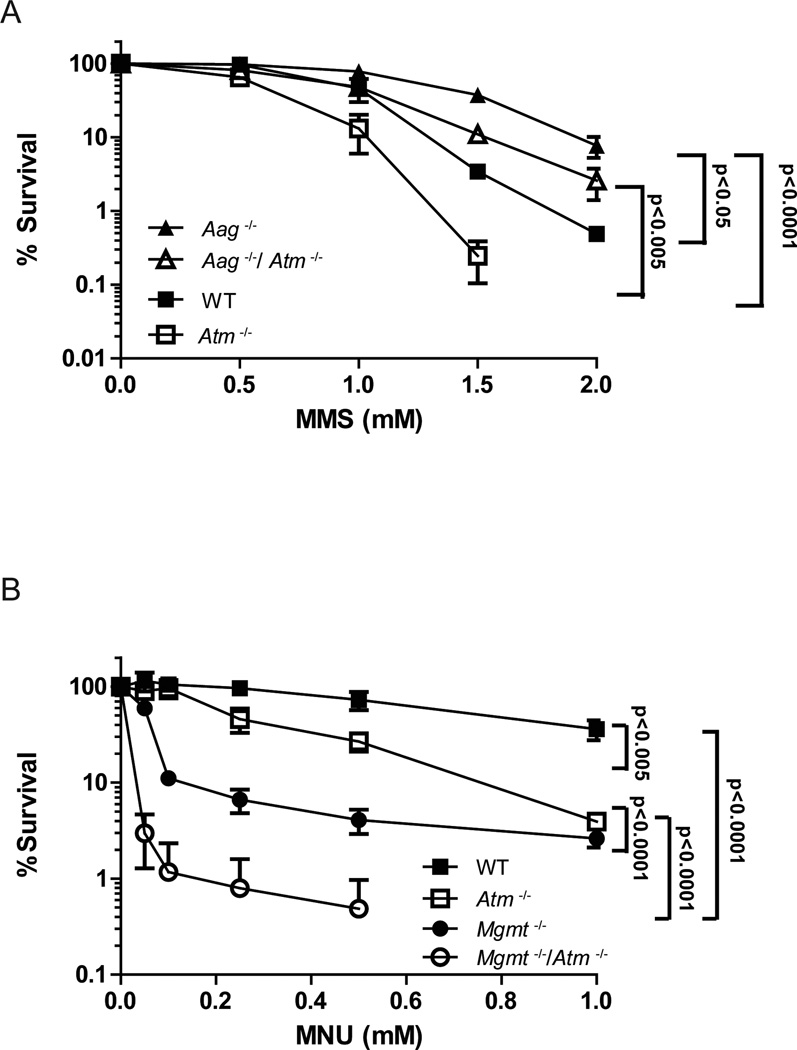
Aag deficiency resulted in a counter-intuitive increase in longevity in Atm−/− deficient mice, accompanied by alterations in the tumor spectrum (Figures 2A and 2B). To further examine this genetic interaction between Atm and Aag, we used the tractable bone marrow (BM) ex vivo clonogenic survival assay to determine whether, as seems to be the case for endogenous DNA damage, Atm modulates Aag-mediated alkylation-induced cytotoxicity. BM cells were treated ex vivo with the alkylating agent methyl methane sulfonate (MMS) and then plated on semisolid media to allow formation of hematopoietic myeloid progenitor colonies. MMS is an SN2 alkylating agent that induces predominantly 7MeG and 3MeA DNA lesions, known Aag substrates [34]. Consistent with a previous report [64], we show here that Aag−/− BM cells are resistant to MMS (Figure 5). This is consistent with multiple recent reports showing that initiation of BER by DNA glycosylases generates repair intermediates (AP-sites, and single-strand breaks (SSBs)) that, if accumulate due to downstream BER enzymes being limited, are more toxic than the original DNA base lesions (reviewed in [33]). This is supported by evidence indicating that alkylation sensitivity is dependent on Aag-initiated BER both in cultured cells and in animals [65–67]. Interestingly, Atm−/− BM cells display increased MMS sensitivity in comparison to all other genotypes, indicating that Atm signaling is an important mediator of MMS-mediated toxicity. The increased sensitivity observed in Atm−/− BM cells is almost totally suppressed in Aag−/−/Atm−/− BM cells, suggesting that much of the alkylation sensitivity observed in the Atm−/− cells is due to Aag-initiated BER of MMS-induced base damage followed by the accumulation of toxic BER intermediates that are ultimately sensed by Atm (Figure 5A). Together, these ex vivo assays illustrate that Atm is an integral modulator of toxicity induced by Aag-initiated BER, and pinpoints a role for the Atm DNA damage response protein in signaling downstream of toxic BER intermediates.
Figure 5. Atm and Aag interact in response to induced alkylation damage.
A) Ex vivo alkylation sensitivity of BM cells to methyl methanesulfonate (MMS). BM cells were derived from wild type (closed squares), Aag−/− (closed triangle), Atm−/− (open squares) and Aag−/− Atm−/− (open triangle) mice. Experiments were done a minimum of three times each, data are mean ± SEM.. B) Synergistic interaction between Mgmt and Atm in response to MNU treatment. Ex vivo alkylation sensitivity of BM cells to methyl nitrosourea (MNU). BM cells were derived from wild type (closed squares), Mgmt−/− (closed circles), Atm−/− (open squares) and Mgmt−/− Atm−/− (open circles) mice. Experiments were done a minimum of three times each, data are mean ± SEM.
We also assessed the contribution of Atm to O6meG-mediated cytotoxicity following exposure to the SN1 alkylating agent, N-methyl-N-nitrosourea (MNU), which generates toxic and mutagenic O6MeG, in addition to 7meG and 3meA DNA base lesions. Ex vivo clonogenic survival assays were performed with BM from WT, Mgmt−/−, Atm−/− and Mgmt−/−/Atm−/−mice. In contrast to MMS, Atm−/− BM cells exhibit no difference in MNU sensitivity compared to WT cells at the doses used (Figure 5B), presumably because both WT and Atm−/− cells express Mgmt to reverse the toxic
O6meG lesions. Accordingly, Mgmt−/− cells exhibit increased sensitivity to MNU compared to both WT and Atm−/− cells. Strikingly, we observe a massively synergistic interaction between Mgmt and Atm; Mgmt−/−/Atm−/− cells exhibit dramatically increased sensitivity to MNU when compared to Mgmt−/− or Atm−/− cells (Figure 5B). We infer that when O6MeG base lesions are unrepaired (as in the Mgmt−/− cells), Atm plays a pivotal role in modulating the toxicity induced by MMR processing of DNA containing O6MeG DNA lesions.
3 DISCUSSION
Here we describe a large-scale aging study of numerous mouse models defective in several DNA repair genes and DNA damage response genes (Supplemental Table 1). Essential for a study of this magnitude, all animals were backcrossed to the C57Bl/6 genetic background for at least 10 generations to ensure that any differences observed could not be attributed to differences in strain background.
Accumulating toxic and mutagenic damage in mitochondrial and nuclear DNA is known to affect the aging process in model organisms [68–71]. We show here that endogenously damaged DNA bases that are substrates for two DNA alkylation repair pathways contribute to long-term survival; mice deficient in both Aag and Mgmt activity exhibit decreased life-span that is statistically significant. The accumulation of unrepaired DNA damage and mutations associated with aging may simply arise due to long-term exposure to endogenous metabolites that damage DNA, and may be exacerbated by an age-related decline in the DNA repair capacity. The capacity to perform BER, NER and double-strand break (DSB) repair have, in fact, all been shown to decline with age (reviewed in [72–74]). Further, studies in mice indicate that certain tissues or anatomical sites may be more susceptible to such age-related DNA repair decline [69] perhaps due to differing exposure to RONS [75, 76]. All of these possibilities are not necessarily mutually exclusive and likely cooperate as contributing factors in influencing longevity. Indeed, in the worst case scenario, aging tissues could have both decreased DNA repair and DNA damage responses accompanied by increased levels of endogenous metabolites that damage DNA.
We and others have demonstrated that for certain cell types Aag-mediated initiation of BER can lead to cell death, and that Aag deficiency can actually be protective [65, 66]. Here, we find that Aag deficiency protects Atm−/− mice both in terms of increasing overall longevity and in reducing the development of lymphoma; this protection is consistent with a role for Aag in generating toxic BER intermediates that trigger the DNA damage response orchestrated by Atm. Further, Aag deficiency provided protection against MMS-induced toxicity in Atm−/− BM cells, ex vivo. Together, this indicates that Atm is required for protection against Aag-mediated alkylation-induced toxicity, and that endogenously-generated Aag substrates can influence organismal longevity. This may not be surprising given that Aag acts on a wide range of endogenously-generated base lesions including 7meG, 3meA, deaminated adenine, oxidized guanine and etheno-base lesions [26, 34, 77, 78]. A link between Atm and BER has been implicated in numerous reports [79–81], but the data here provide in vivo evidence that Atm plays a key role in protecting against the detrimental effects of Aag-mediated BER intermediate formation at sites of spontaneous DNA base damage.
Interestingly, although ATM is known to phosphorylate, stabilize and activate p53 [82], there is no change in survival in Aag−/−/p53−/− mice, in contrast to enhanced survival in Aag−/−/Atm−/− mice. The protection in Aag−/−/Atm−/− mice compared to Aag−/−/p53−/− mice may be explained by the numerous p53-independent functions of Atm [82]. Alternatively, it has been shown previously that Aag physically interacts with and represses p53 [83]; therefore genetic deletion of both Aag and p53 would be epistatic and not alter overall survival compared to p53−/− mice.
Mgmt deficiency does not affect survival or tumor spectrum in Atm−/− mice. However, a clear genetic interaction between Mgmt and Atm was observed in the ex vivo BM clonogenic survival assays. Predictably, Mgmt−/− BM cells exhibit alkylation hypersensitivity but surprisingly, Mgmt−/−/Atm−/− BM cells exhibit a synergistic increase in alkylation sensitivity. We propose that in the absence of Mgmt, futile cycling of MMR at O6meG:T mispairs results in the generation of DSBs that activate Atm [83–86]. Without Atm and Mgmt, MMR-mediated futile cycling continues without the Atm-mediated signaling pathways, further exacerbating cell death. Together, this illustrates that Atm contributes to the cellular response to O6meG induced by exogenous alkylating agents, but implies that spontaneous O6meG lesions are not relevant in the development of morbidity in Atm−/−/Mgmt−/− mice, although it is possible that the decreased lifespan of Atm−/− mice precludes any potential cumulative detrimental effects of endogenous O6meG lesions in Atm−/−/Mgmt−/− mice.
It is intriguing that Mgmt deficiency protected Exo1−/− mice against the development of lymphoma; instead Mgmt−/−/Exo1−/− mice developed histiocytic sarcoma, the prevalent disease in C57Bl/6 mice. Although Mgmt−/−/Exo1−/− mice exhibited decreased incidence of lymphoma, there was only a trend toward increased longevity, indicating that the protection against lymphoma and the overall shift in tumor spectrum did not prolong lifespan. Mgmt−/− mice develop histiocytic sarcoma at an average latency of 25.5 months, whereas in Mgmt−/−/Exo1−/− mice, the onset of histiocytic sarcoma is significantly earlier (p=0.0035), with an average latency of 21.7 months. Although Mgmt deficiency altered tumor penetrance in Exo1−/− mice, this was not observed in Msh6−/− mice. Msh6 deficiency results in a strong predisposition to lymphomagenesis, which occurs significantly earlier than in Exo1−/− mice (p=0.0002). The constitutive MMR deficiency (CMMRD) cancer syndrome in humans substantiates the role for Msh6 in preventing hematological malignancies and other cancers [87–89] and reinforces the finding that Msh6 deficiency is a strong inducer of lymphoma in mice [61]. EXO1 deficiency is not causative of CMMRD, but EXO1 mutations have been found in diffuse B-cell lymphoma [87]. The strong association between MSH6 mutations and lymphoma may explain why Mgmt deficiency was insufficient to change tumor spectrum in Msh6−/− mice, whereas Mgmt−/−/Exo1−/− mice exhibited a shift in tumor spectrum towards histiocytic sarcoma.
Although the link between accumulating DNA damage and aging has been clearly established, the consequences of lifestyle interventions that increase longevity and their role on altering DNA repair capacity remain unresolved. One proven strategy demonstrated to enhance longevity is calorie restriction (CR); the consequence of CR on DNA repair remains controversial. CR has been shown to reduce the age-dependent decline in non-homologous end joining activity [90], whereas other studies show a decrease in DNA repair transcript levels following CR [91]. Additionally, it is well-known that habitual endurance exercise improves health-span [92, 93], and although endurance exercise is associated with an increase in oxidative DNA damage [94], exercise-induced RONS are thought to induce DNA repair and other molecular systems to cope with increased RONS damage [95–98]. Finally, resveratrol, a polyphenol found in red wine and an activator of the NAD-dependent deacetylase sirtuin-1 (Sirt1), has been hypothesised to increase longevity. Resveratrol increases the formation of APE/XRCC1 complex during BER [99], but also reduces the activity or expression of other DNA repair proteins [100, 101]. These few examples underscore the fact that much remains to be learned regarding the relationship between DNA repair and lifestyle interventions that may modulate longevity.
Significant progress has been made regarding the pathways and factors that modulate longevity [1, 102, 103], and yet many questions remain unanswered. Several theories of aging have been proposed including: the mitochondrial free radical theory of aging, telomere attrition, mitochondrial dysfunction, and more recently, the functional decline of stem cells (aging theories reviewed in [104–106]). It is likely that many of the proposed mechanisms of aging interact with each other to influence the longevity of an organism. Here, using long-term lifespan studies in DNA repair- and DNA damage-response deficient mouse models, we establish that the repair of DNA base alkylation damage arising from endogenous sources is at least one contributing factor to longevity.
4 METHODS
4.1 Mice
The Aag−/− mice [107] and Mgmt−/− mice [108] have been described. Trp53−/− mice (B6.129S2-Trp53tm1Tyj, former name C57BL/6J-Trp53tm1Tyj) and Atm−/− mice (129S6/SvEvTac-Atmtm1Awb/J) were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). Exo1−/− and Msh6−/− mice have been described previously [61, 62]. All mice were backcrossed at least 10 times to the C57BL/6 background. Mice were fed standard diet ad libitum and housed in an AAALAC accredited facility. Animals were sacrificed by CO2 asphyxiation. All animal procedures were approved by the MIT Committee on Animal Care.
4.2 Longevity studies
Mice were allowed to age and observed for development of disease and subject to full necropsy when diseased or deceased. Tissues were fixed in Bouin’s fixative, paraffin embedded, sectioned at 5 µm and stained with haematoxylin and eosin (H&E). Tissues harvested include: brain, eyes, salivary gland, thymus, heart, lung, liver, kidney, spleen, intestine, reproductive organs, and femur. All H&E stained slides were analyzed blind by a pathologist (R.T.B) for the cause of death as well as for identification of any tumors/lesions. Examples of lesions classified as other include: dermatitis, cystic endometrium/uterus, emphysema, kidney disease and osteoarthritis.
4.3 Bone marrow clonogenic survival assay
BM clonogenic survival assays were performed as described in [64]. Briefly, cells were harvested from the femurs of mice, treated ex vivo with MMS (Sigma-Aldrich Co, St. Louis, MO) or MNU (Sigma-Aldrich Co, St. Louis, MO) and plated in methylcellulose-containing media (Stem Cell Technologies, Vancouver, BC, Canada), and plated in duplicate. After approximately 2 weeks, colonies containing > 50 cells were scored. Experiments were performed at least three times.
4.4 Statistical analysis
GraphPad Prism was used to generate Kaplan-Meier plots for survival and to calculate significance using Log-rank (Mantel-Cox) test. Fisher’s exact, programmed in R, was used to establish whether the differences in tumor spectra between genotypes were significant.
Supplementary Material
Table 1.
Median survival of all mice, median survival of mice with tumors, and tumor penetrance in aging study.
| Genotype | Median Survival, weeks |
Median Survival (tumor), weeks |
% tumor at termination |
|---|---|---|---|
| Wildtype | 106 | 98 | 61.1 |
| Aag−/− | 94 | 99 | 54.2 |
| Mgmt−/− | 99.5 | 103.5 | 57.1 |
| Atm−/− | 29 | 35 | 100 |
| p53−/− | 20 | 21.5 | 100 |
| Exo1−/− | 81 | 82.3 | 94.1 |
| Msh6−/− | 26.5 | 25 | 100 |
| Aag−/−Mgmt−/− | 89.5 | 94.5 | 58 |
| Aag−/−Atm−/− | 44 | 63 | 90 |
| Aag−/−p53−/− | 20 | 22 | 92.8 |
| Mgmt−/−Atm−/− | 40 | 49 | 100 |
| Mgmt−/−p53−/− | 20.5 | 22 | 100 |
| Mgmt−/−Exo1−/− | 91.5 | 92 | 96.5 |
| Mgmt−/−Msh6−/− | 45 | 45 | 100 |
Highlights.
Large-scale mouse aging study examines role for DNA repair and DNA damage response.
Repair of endogenous alkylation damage plays a role in determining longevity.
Atm plays a key role in protecting against detrimental effects of Aag-mediated BER.
ACKNOWLEDGMENTS
The authors thank Jonathan Brasher for help in the initial stages of this project. We thank Axel Nohturfft for assistance in writing the R code for the Fisher’s exact test. We thank Dr. Edelman for the Exo1−/− and Msh6−/− mice. We acknowledge The Hope Babette Tang Histology Facility at MIT’s David H. Koch Institute for Integrative Cancer Research, especially Alicia Caron (NCI P30-CA14051). LDS is an ACS Research Professor and the research was supported by NIH grants R01-CA075576, R01-ES022872, R01-CA149261, and P30-ES002109.
Abbreviations
- 3meA
3-methyladenine
- Aag/Mpg
alkyladenine glycosylase
- AP
abasic
- Atm
ataxia telangiectasia mutated
- BER
base excision repair
- BM
bone marrow
- CR
calorie restriction
- Exo1
exonuclease 1
- Mgmt
O6MeG DNA methyltransferase
- MMR
mismatch repair
- MMS
methyl methanesulfonate
- MNU
N-methyl-N-nitrosourea
- Msh6
MutS homolog 6
- NER
nucleotide excision repair
- O6MeG
O6-methylguanine
- RONS
reactive oxygen nitrogen species
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
6 REFERENCES
- 1.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 2.Alexeyev MF. Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS Journal. 2009;276:5768–5787. doi: 10.1111/j.1742-4658.2009.07269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lapointe J, Hekimi S. When a theory of aging ages badly. Cellular and Molecular Life Sciences. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 5.Kenyon J, Gerson SL. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Research. 2007;35:7557–7565. doi: 10.1093/nar/gkm1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burhans WC, Weinberger M. DNA replication stress, genome instability and aging. Nucleic Acids Research. 2007;35:7545–7556. doi: 10.1093/nar/gkm1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. doi: 10.1111/j.1474-9726.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 8.Laplante M, Sabatini David M. mTOR Signaling in Growth Control and Disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoeijmakers J. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher B, Garinis GA. J.H. Hoeijmakers, Age to survive: DNA damage and aging. Trends Genet. 2008;24:77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Martínez P, Thanasoula M, Muñoz P, Liao C, Tejera A, McNees C, Flores JM, Fernández-Capetillo O, Tarsounas M, Blasco MA. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes & Development. 2009;23:2060–2075. doi: 10.1101/gad.543509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee H-W, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boer J, Hoeijmakers JHJ. Cancer from the outside, aging from the inside: Mouse models to study the consequences of defective nucleotide excision repair. Biochimie. 1999;81:127–137. doi: 10.1016/s0300-9084(99)80045-5. [DOI] [PubMed] [Google Scholar]
- 14.Niedernhofer LJ. Tissue-specific accelerated aging in nucleotide excision repair deficiency. Mech Ageing Dev. 2008;129:408–415. doi: 10.1016/j.mad.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers CJ, Nigg A, van Steeg H, Bootsma D, Hoeijmakers JH. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr Biol. 1997;7:427–439. doi: 10.1016/s0960-9822(06)00190-4. [DOI] [PubMed] [Google Scholar]
- 16.Vogel H, Lim D-S, Karsenty G, Finegold M, Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proceedings of the National Academy of Sciences. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhardt HC, Schumacher B. The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends in Genetics. 2012;28:128–136. doi: 10.1016/j.tig.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vousden KH LD. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 19.Murray-Zmijewski F, Slee EA, X L. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- 20.Tucci P. Caloric restriction: is mammalian life extension linked to p53? Aging. 2012;4:525–534. doi: 10.18632/aging.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tannenbaum Steven R, Tamir S, de Rojas-Walker T, Wishnok John S. DNA Damage and Cytotoxicity Caused by Nitric Oxide, in: Nitrosamines and Related N-Nitroso Compounds. American Chemical Society. 1994:120–135. [Google Scholar]
- 22.De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 23.Pang B, Zhou X, Yu H, Dong M, Taghizadeh K, Wishnok JS, Tannenbaum SR, Dedon PC. Lipid peroxidation dominates the chemistry of DNA adduct formation in a mouse model of inflammation. Carcinogenesis. 2007 doi: 10.1093/carcin/bgm037. [DOI] [PubMed] [Google Scholar]
- 24.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 25.Marnett LJ, Burcham PC. Endogenous DNA adducts: potential and paradox. Chem Res Toxicol. 1993;6:771–785. doi: 10.1021/tx00036a005. [DOI] [PubMed] [Google Scholar]
- 26.Bartsch H. Hunting for electrophiles that harm human DNA: Frits Sobels Award Lecture. Mutagenesis. 2002;17:281–287. doi: 10.1093/mutage/17.4.281. [DOI] [PubMed] [Google Scholar]
- 27.Bartsch H, Nair J. Potential role of lipid peroxidation derived DNA damage in human colon carcinogenesis: studies on exocyclic base adducts as stable oxidative stress markers. Cancer Detect Prev. 2002;26:308–312. doi: 10.1016/s0361-090x(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 28.Ringvoll J, Moen MN, Nordstrand LM, Meira LB, Pang B, Bekkelund A, Dedon PC, Bjelland S, Samson LD, Falnes PO, Klungland A. AlkB homologue 2-mediated repair of ethenoadenine lesions in mammalian DNA. Cancer Res. 2008;68:4142–4149. doi: 10.1158/0008-5472.CAN-08-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartsch H, Nair J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat Res. 2005;591:34–44. doi: 10.1016/j.mrfmmm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: a review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–1120. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Bacalini MG, Tavolaro S, Peragine N, Marinelli M, Santangelo S, Del Giudice I, Mauro FR, Di Maio V, Ricciardi MR, Caiafa P, Chiaretti S, Foà R, Guarini A, Reale A. A subset of chronic lymphocytic leukemia patients display reduced levels of PARP1 expression coupled with a defective irradiation-induced apoptosis. Experimental Hematology. 2012;40:197–206. doi: 10.1016/j.exphem.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, Schauer DB, Dedon PC, Fox JG, Samson LD. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lockett KL, Hall MC, Xu J, Zheng SL, Berwick M, Chuang SC, Clark PE, Cramer SD, Lohman K, Hu JJ. The ADPRT V762A genetic variant contributes to prostate cancer susceptibility and deficient enzyme function. Cancer Res. 2004;64:6344–6348. doi: 10.1158/0008-5472.CAN-04-0338. [DOI] [PubMed] [Google Scholar]
- 34.Lee C, Delaney J, Kartalou M, Lingaraju G, Essigmann J, Maor-Shoshani A, Samson LD. Recognition and Processing of a New Repertoire of DNA Substrates by Human 3-Methyladenine DNA Glycosylase (AAG) Biochemistry. 2009;48 doi: 10.1021/bi8018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarini AA, Lang KS, Verschoor A, Recher M, Zinkernagel AS, Nizet V, Odermatt B, Hengartner H, Zinkernagel RM. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proceedings of the National Academy of Sciences. 2009;106:7107–7112. doi: 10.1073/pnas.0901162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien PJ, Ellenberger T. Dissecting the broad substrate specificity of human 3-methyladenine-DNA glycosylase. J Biol Chem. 2004;279:9750–9757. doi: 10.1074/jbc.M312232200. [DOI] [PubMed] [Google Scholar]
- 37.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Duckett DR, Drummond JT, Murchie AI, Reardon JT, Sancar A, Lilley DM, Modrich P. Human MutSalpha recognizes damaged DNA base pairs containing O6-methylguanine, O4-methylthymine, or the cisplatin-d(GpG) adduct. Proc Natl Acad Sci U S A. 1996;93:6443–6447. doi: 10.1073/pnas.93.13.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meikrantz W, Bergom MA, Memisoglu A, Samson L. O6-alkylguanine DNA lesions trigger apoptosis. Carcinogenesis. 1998;19:369–372. doi: 10.1093/carcin/19.2.369. [DOI] [PubMed] [Google Scholar]
- 40.Hickman MJ, Samson LD. Role of DNA mismatch repair and p53 in signaling induction of apoptosis by alkylating agents. Proc Natl Acad Sci U S A. 1999;96:10764–10769. doi: 10.1073/pnas.96.19.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klapacz J, Meira LB, Luchetti DG, Calvo JA, Bronson RT, Edelmann W, Samson LD. O6-methylguanine-induced cell death involves exonuclease 1 as well as DNA mismatch recognition in vivo. Proc Natl Acad Sci U S A. 2009;106:576–581. doi: 10.1073/pnas.0811991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dosch J, Christmann M, Kaina B. Mismatch G-T binding activity and MSH2 expression is quantitatively related to sensitivity of cells to methylating agents. Carcinogenesis. 1998;19:567–573. doi: 10.1093/carcin/19.4.567. [DOI] [PubMed] [Google Scholar]
- 43.Mojas N, Lopes M, Jiricny J. Mismatch repair-dependent processing of methylation damage gives rise to persistent single-stranded gaps in newly replicated DNA. Genes & Development. 2007;21:3342–3355. doi: 10.1101/gad.455407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakatsuru Y, Matsukuma S, Nemoto N, Sugano H, Sekiguchi M, Ishikawa T. O6-methylguanine-DNA methyltransferase protects against nitrosamine-induced hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1993;90:6468–6472. doi: 10.1073/pnas.90.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumenco LL, Allay E, Norton K, Gerson SL. The prevention of thymic lymphomas in transgenic mice by human O6-alkylguanine-DNA alkyltransferase. Science. 1993;259:219–222. doi: 10.1126/science.8421782. [DOI] [PubMed] [Google Scholar]
- 46.Becker K, Dosch J, Gregel CM, Martin BA, Kaina B. Targeted expression of human O(6)-methylguanine-DNA methyltransferase (MGMT) in transgenic mice protects against tumor initiation in two-stage skin carcinogenesis. Cancer Res. 1996;56:3244–3249. [PubMed] [Google Scholar]
- 47.Liu L, Allay E, Dumenco LL, Gerson SL. Rapid repair of O6-methylguanine-DNA adducts protects transgenic mice from N-methylnitrosourea-induced thymic lymphomas. Cancer Res. 1994;54:4648–4652. [PubMed] [Google Scholar]
- 48.Zhou ZQ, Manguino D, Kewitt K, Intano GW, McMahan CA, Herbert DC, Hanes M, Reddick R, Ikeno Y, Walter CA. Spontaneous hepatocellular carcinoma is reduced in transgenic mice overexpressing human O6- methylguanine-DNA methyltransferase. Proc Natl Acad Sci U S A. 2001;98:12566–12571. doi: 10.1073/pnas.221232998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu G, Herzig M, Rotrekl V, Walter CA. Base excision repair, aging and health span. Mech Ageing Dev. 2008;129:366–382. doi: 10.1016/j.mad.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedberg EC, Meira LB. Database of mouse strains carrying targeted mutations in genes affecting biological responses to DNA damage Version 7. DNA Repair. 2006;5:189–209. doi: 10.1016/j.dnarep.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Meira LB, Burgis NE, Samson LD. Base excision repair. Adv Exp Med Biol. 2005;570:125–173. doi: 10.1007/1-4020-3764-3_5. [DOI] [PubMed] [Google Scholar]
- 52.Cabelof DC, Ikeno Y, Nyska A, Busuttil RA, Anyangwe N, Vijg J, Matherly LH, Tucker JD, Wilson SH, Richardson A, Heydari AR. Haploinsufficiency in DNA polymerase beta increases cancer risk with age and alters mortality rate. Cancer Res. 2006;66:7460–7465. doi: 10.1158/0008-5472.CAN-06-1177. [DOI] [PubMed] [Google Scholar]
- 53.Wirtz S, Nagel G, Eshkind L, Neurath MF, Samson LD, Kaina B. Both base excision repair and O6-methylguanine-DNA methyltransferase protect against methylation-induced colon carcinogenesis. Carcinogenesis. 2010;31:2111–2117. doi: 10.1093/carcin/bgq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bugni JM, Meira LB, Samson LD. Alkylation-induced colon tumorigenesis in mice deficient in the Mgmt and Msh6 proteins. Oncogene. 2009;28:734–741. doi: 10.1038/onc.2008.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blackwell BN, Bucci TJ, Hart RW, Turturro A. Longevity, body weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol. 1995;23:570–582. doi: 10.1177/019262339502300503. [DOI] [PubMed] [Google Scholar]
- 56.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-Deficient Mice: A Paradigm of Ataxia Telangiectasia. Cell. 1996;86:159–171. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 57.Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Current Biology. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 58.Xu Y, Ashley T, Brainerd EE, Bronson RT, Meyn MS, Baltimore D. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes & Development. 1996;10:2411–2422. doi: 10.1101/gad.10.19.2411. [DOI] [PubMed] [Google Scholar]
- 59.Hickman MJ, Samson LD. Apoptotic signaling in response to a single type of DNA lesion, O(6)-methylguanine. Mol Cell. 2004;14:105–116. doi: 10.1016/s1097-2765(04)00162-5. [DOI] [PubMed] [Google Scholar]
- 60.Jiricny J. The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol. 2006;7:335–346. doi: 10.1038/nrm1907. [DOI] [PubMed] [Google Scholar]
- 61.Edelmann W, Yang K, Umar A, Heyer J, Lau K, Fan K, Liedtke W, Cohen PE, Kane MF, Lipford JR, Yu N, Crouse GF, Pollard JW, Kunkel T, Lipkin M, Kolodner R, Kucherlapati R. Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell. 1997;91:467–477. doi: 10.1016/s0092-8674(00)80433-x. [DOI] [PubMed] [Google Scholar]
- 62.Wei K, Clark AB, Wong E, Kane MF, Mazur DJ, Parris T, Kolas NK, Russell R, Hou H, Jr, Kneitz B, Yang G, Kunkel TA, Kolodner RD, Cohen PE, Edelmann W. Inactivation of Exonuclease 1 in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes Dev. 2003;17:603–614. doi: 10.1101/gad.1060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung C, Chanock S. Current status of genome-wide association studies in cancer. Human Genetics. 2011;130:59–78. doi: 10.1007/s00439-011-1030-9. [DOI] [PubMed] [Google Scholar]
- 64.Roth RB, Samson LD. 3-Methyladenine DNA glycosylase-deficient Aag null mice display unexpected bone marrow alkylation resistance. Cancer Res. 2002;62:656–660. [PubMed] [Google Scholar]
- 65.Meira L, Moroski-Erkul C, Green S, Calvo J, Bronson R, Shah D, Samson L. Aag-initiated base excision repair drives alkylation-induced retinal degeneration in mice. Proc Natl Acad Sci U S A. 2009;106:888–893. doi: 10.1073/pnas.0807030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Domagala P, Huzarski T, Lubinski J, Gugala K, Domagala W. PARP-1 expression in breast cancer including <i>BRCA1</i>-associated, triple negative and basal-like tumors: possible implications for PARP-1 inhibitor therapy. Breast Cancer Research and Treatment. 2011;127:861–869. doi: 10.1007/s10549-011-1441-2. [DOI] [PubMed] [Google Scholar]
- 67.Sobol RW, Kartalou M, Almeida KH, Joyce DF, Engelward BP, Horton JK, Prasad R, Samson LD, Wilson SH. Base excision repair intermediates induce p53-independent cytotoxic and genotoxic responses. J Biol Chem. 2003;278:39951–39959. doi: 10.1074/jbc.M306592200. [DOI] [PubMed] [Google Scholar]
- 68.Schlotterer A, Hamann A, Kukudov G, Ibrahim Y, Heckmann B, Bozorgmehr F, Pfeiffer M, Hutter H, Stern D, Du X, Brownlee M, Bierhaus A, Nawroth P, Morcos M. Apurinic/apyrimidinic endonuclease 1, p53, and thioredoxin are linked in control of aging in C. elegans. Aging Cell. 2010;9:420–432. doi: 10.1111/j.1474-9726.2010.00572.x. [DOI] [PubMed] [Google Scholar]
- 69.Szczesny B, Tann AW, Mitra S. Age- and tissue-specific changes in mitochondrial and nuclear DNA base excision repair activity in mice: Susceptibility of skeletal muscles to oxidative injury. Mechanisms of Ageing and Development. 2010;131:330–337. doi: 10.1016/j.mad.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maslov AY, Ganapathi S, Westerhof M, Quispe-Tintaya W, White RR, Van Houten B, Reiling E, Dollé MET, van Steeg H, Hasty P, Hoeijmakers JHJ, Vijg J. DNA damage in normally and prematurely aged mice. Aging Cell. 2013;12:467–477. doi: 10.1111/acel.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444:1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 72.Simon K, Mukundan A, Dewundara S, Van Remmen H, Dombkowski AA, Cabelof DC. Transcriptional profiling of the age-related response to genotoxic stress points to differential DNA damage response with age. Mechanisms of Ageing and Development. 2009;130:637–647. doi: 10.1016/j.mad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer J, Boyd W, Azzam G, Haugen A, Freedman J, Van Houten B. Decline of nucleotide excision repair capacity in aging Caenorhabditis elegans. Genome biology. 2007;8:R70. doi: 10.1186/gb-2007-8-5-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gorbunova V, Seluanov A, Mao Z, Hine C. Changes in DNA repair during aging. Nucleic Acids Research. 2007;35:7466–7474. doi: 10.1093/nar/gkm756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kozlov AV, Szalay L, Umar F, Kropik K, Staniek K, Niedermüller H, Bahrami S, Nohl H. Skeletal muscles, heart, and lung are the main sources of oxygen radicals in old rats. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2005;1740:382–389. doi: 10.1016/j.bbadis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Kozlov AV, Szalay L, Umar F, Fink B, Kropik K, Nohl H, Redl H, Bahrami S. Epr analysis reveals three tissues responding to endotoxin by increased formation of reactive oxygen and nitrogen species. Free Radical Biology and Medicine. 2003;34:1555–1562. doi: 10.1016/s0891-5849(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 77.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006 doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 78.Zhao C, Hemminki K. The in vivo levels of DNA alkylation products in human lymphocytes are not age dependent: an assay of 7-methyl- and 7-(2-hydroxyethyl)-guanine DNA adducts. Carcinogenesis. 2002;23:307–310. doi: 10.1093/carcin/23.2.307. [DOI] [PubMed] [Google Scholar]
- 79.Brem R, Fernet M, Chapot B, Hall J. The methyl methanesulfonate induced S-phase delay in XRCC1-deficient cells requires ATM and ATR. DNA Repair. 2008;7:849–857. doi: 10.1016/j.dnarep.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Barfknecht TR, Little JB. Hypersensitivity of ataxia telangiectasia skin fibroblasts to DNA alkylating agents. Mutation Research. 1982;94:369–382. doi: 10.1016/0027-5107(82)90299-8. [DOI] [PubMed] [Google Scholar]
- 81.Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO Journal. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 83.Song S, Xing G, Yuan L, Wang J, Wang S, Yin Y, Tian C, He F, Zhang L. N-methylpurine DNA glycosylase inhibits p53-mediated cell cycle arrest and coordinates with p53 to determine sensitivity to alkylating agents. Cell Res. 2012;22:1285–1303. doi: 10.1038/cr.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Noonan EM, Shah D, Yaffe MB, Lauffenburger DA, Samson LD. O 6-Methylguanine DNA lesions induce an intra-S-phase arrest from which cells exit into apoptosis governed by early and late multi-pathway signaling network activation. Integrative Biology. 2012;4:1237–1255. doi: 10.1039/c2ib20091k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaina B. Mechanisms and consequences of methylating agent-induced SCEs and chromosomal aberrations: a long road traveled and still a far way to go. Cytogenet Genome Res. 2004;104:77–86. doi: 10.1159/000077469. [DOI] [PubMed] [Google Scholar]
- 86.York SJ, Modrich P. Mismatch repair-dependent iterative excision at irreparable O6-methylguanine lesions in human nuclear extracts. J Biol Chem. 2006;281:22674–22683. doi: 10.1074/jbc.M603667200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Miranda NF, Peng R, Georgiou K, Wu C, Sörqvist EF, Berglund M, Chen L, Gao Z, Lagerstedt K, Lisboa S, Roos F, van Wezel T, Teixeira MR, Rosenquist R, Sundström C, Enblad G, Nilsson M, Zeng Y, Kipling D, Pan-Hammarström Q. DNA repair genes are selectively mutated in diffuse large B cell lymphomas. The Journal of Experimental Medicine. 2013;210:1729–1742. doi: 10.1084/jem.20122842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ripperger T, Beger C, Rahner N, Sykora KW, Bockmeyer CL, Lehmann U, Kreipe HH, Schlegelberger B. Constitutional mismatch repair deficiency and childhood leukemia/lymphoma – report on a novel biallelic MSH6 mutation. Haematologica. 2010;95:841–844. doi: 10.3324/haematol.2009.015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Couronné L, Ruminy P, Waultier-Rascalou A, Rainville V, Cornic M, Picquenot J-M, Figeac M, Bastard C, Tilly H, Jardin F. Mutation mismatch repair gene deletions in diffuse large B-cell lymphoma. Leukemia & Lymphoma. 2013;54:1079–1086. doi: 10.3109/10428194.2012.739687. [DOI] [PubMed] [Google Scholar]
- 90.Lee J-E, Heo J-I, Park S-H, Kim J-H, Kho Y-J, Kang H-J, Chung HY, Yoon J-L, Lee J-Y. Calorie restriction (CR) reduces age-dependent decline of non-homologous end joining (NHEJ) activity in rat tissues. Experimental Gerontology. 2011;46:891–896. doi: 10.1016/j.exger.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 91.Weindruch R, Kayo T, Lee C-K, Prolla TA. Microarray Profiling of Gene Expression in Aging and Its Alteration by Caloric Restriction in Mice. The Journal of Nutrition. 2001;131:918S–923S. doi: 10.1093/jn/131.3.918S. [DOI] [PubMed] [Google Scholar]
- 92.Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Research Reviews. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Radak Z, Chung H, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–75. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- 94.Wagner K-H, Reichhold S, Neubauer O. Impact of endurance and ultraendurance exercise on DNA damage. Annals of the New York Academy of Sciences. 2011;1229:115–123. doi: 10.1111/j.1749-6632.2011.06106.x. [DOI] [PubMed] [Google Scholar]
- 95.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radical Biology and Medicine. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 96.Radák Z, Naito H, Kaneko T, Tahara S, Nakamoto H, Takahashi R, Cardozo-Pelaez F, Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch - Eur J Physiol. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 97.Siu PM, Pei XM, Teng BT, Benzie IF, Ying M, Wong SH. Habitual exercise increases resistance of lymphocytes to oxidant-induced DNA damage by upregulating expression of antioxidant and DNA repairing enzymes. Experimental Physiology. 2011;96:889–906. doi: 10.1113/expphysiol.2011.058396. [DOI] [PubMed] [Google Scholar]
- 98.Marosi K, Bori Z, Hart N, Sárga L, Koltai E, Radák Z, Nyakas C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience. 2012;226:21–28. doi: 10.1016/j.neuroscience.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 99.Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, Jung S-B, Kim C-S, Irani K. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic Acids Research. 2010;38:832–845. doi: 10.1093/nar/gkp1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gatz SA, Keimling M, Baumann C, Dörk T, Debatin K-M, Fulda S, Wiesmüller L. Resveratrol modulates DNA double-strand break repair pathways in an ATM/ATR–p53- and – Nbs1-dependent manner. Carcinogenesis. 2008;29:519–527. doi: 10.1093/carcin/bgm283. [DOI] [PubMed] [Google Scholar]
- 101.Leon-Galicia I, Diaz-Chavez J, Garcia-Villa E, Uribe-Figueroa L, Hidalgo-Miranda A, Herrera LA, Alvarez-Rios E, Garcia-Mena J, Gariglio P. Resveratrol induces downregulation of DNA repair genes in MCF-7 human breast cancer cells. European Journal of Cancer Prevention. 2013;22:11–20. doi: 10.1097/CEJ.0b013e328353edcb. [DOI] [PubMed] [Google Scholar]
- 102.Martin GM. The biology of aging: 1985–2010 and beyond. The FASEB Journal. 2011;25:3756–3762. doi: 10.1096/fj.11-1102.ufm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Campisi J. Aging, Cellular Senescence, and Cancer. Annual Review of Physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sahin E, DePinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bratic A, Larsson N-G, xF ran. The role of mitochondria in aging. The Journal of Clinical Investigation. 2013;123:951–957. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Engelward BP, Weeda G, Wyatt MD, Broekhof JL, de Wit J, Donker I, Allan JM, Gold B, Hoeijmakers JH, Samson LD. Base excision repair deficient mice lacking the Aag alkyladenine DNA glycosylase. Proc Natl Acad Sci U S A. 1997;94:13087–13092. doi: 10.1073/pnas.94.24.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Glassner BJ, Weeda G, Allan JM, Broekhof JL, Carls NH, Donker I, Engelward BP, Hampson RJ, Hersmus R, Hickman MJ, Roth RB, Warren HB, Wu MM, Hoeijmakers JH, Samson LD. DNA repair methyltransferase (Mgmt) knockout mice are sensitive to the lethal effects of chemotherapeutic alkylating agents. Mutagenesis. 1999;14:339–347. doi: 10.1093/mutage/14.3.339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.