Abstract
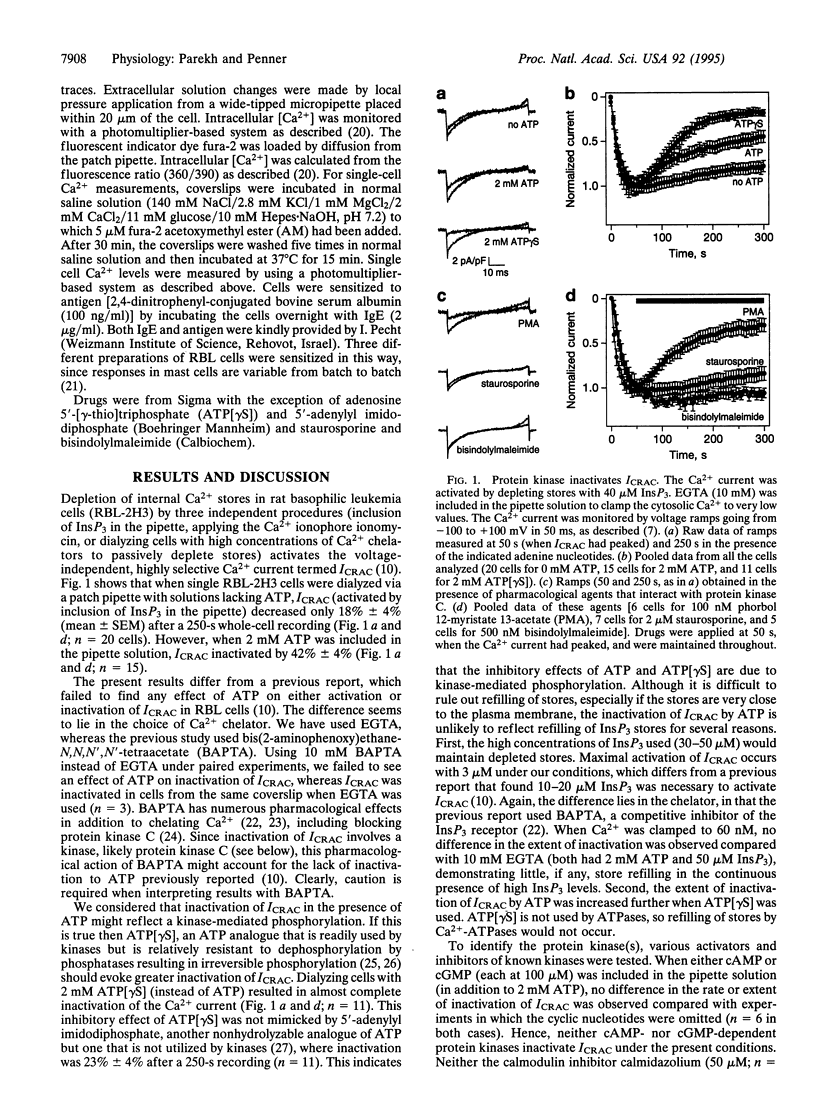
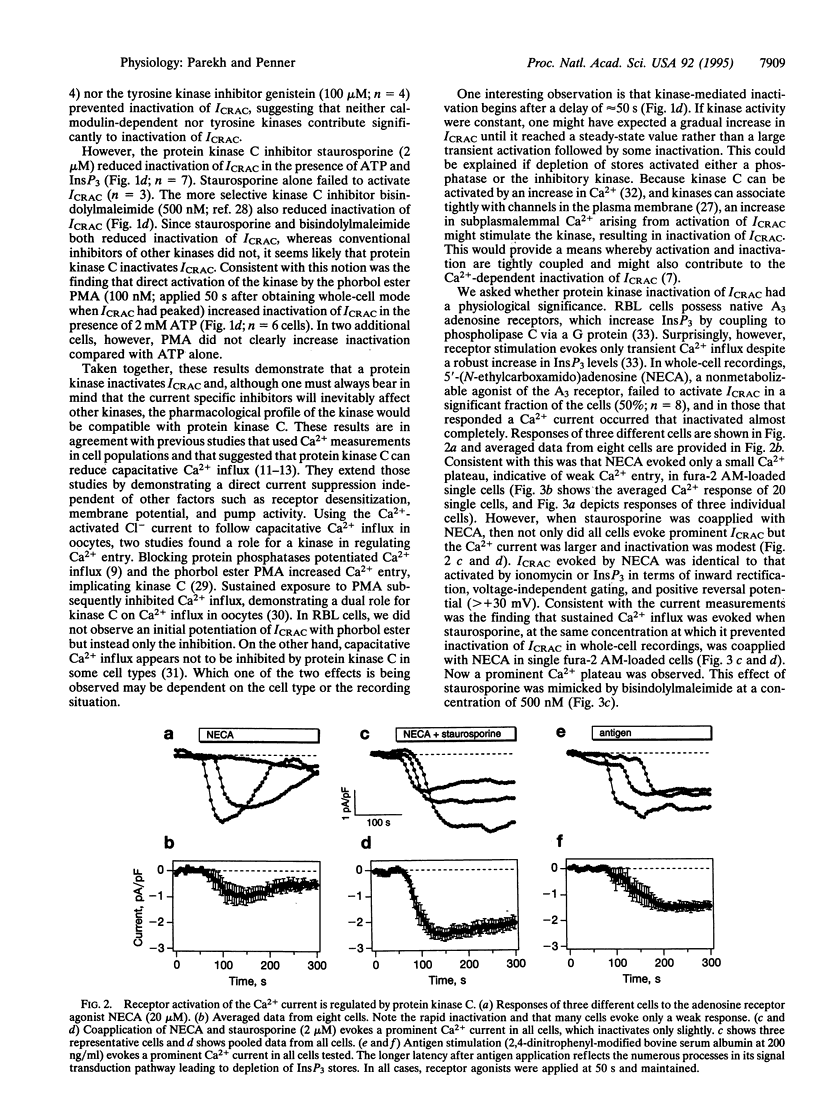
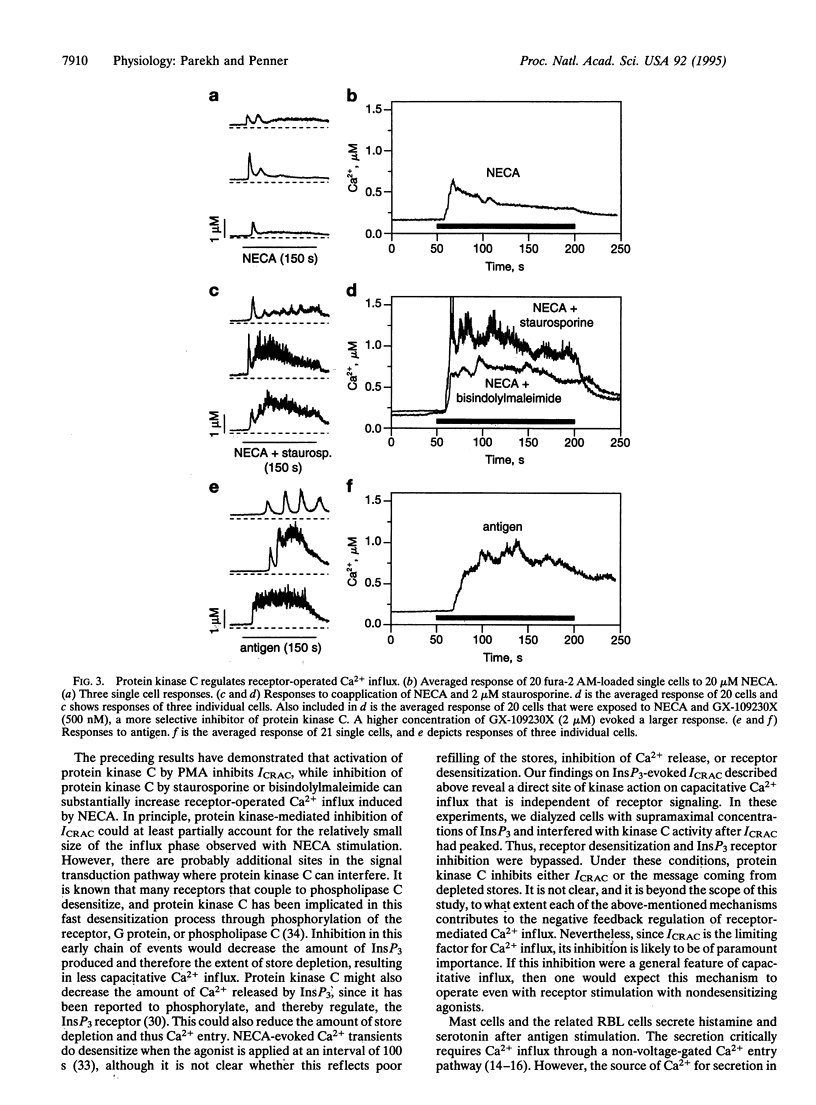
Whole-cell patch-clamp recordings and single-cell Ca2+ measurements were used to study the control of Ca2+ entry through the Ca2+ release-activated Ca2+ influx pathway (ICRAC) in rat basophilic leukemia cells. When intracellular inositol 1,4,5-trisphosphate (InsP3)-sensitive stores were depleted by dialyzing cells with high concentrations of InsP3, ICRAC inactivated only slightly in the absence of ATP. Inclusion of ATP accelerated inactivation 2-fold. The inactivation was increased further by the ATP analogue adenosine 5'-[gamma-thio]triphosphate, which is readily used by protein kinases, but not by 5'-adenylyl imidodiphosphate, another ATP analogue that is not used by kinases. Neither cyclic nucleotides nor inhibition of calmodulin or tyrosine kinase prevented the inactivation. Staurosporine and bisindolylmaleimide, protein kinase C inhibitors, reduced inactivation of ICRAC, whereas phorbol ester accelerated inactivation of the current. These results demonstrate that a protein kinase-mediated phosphorylation, probably through protein kinase C, inactivates ICRAC. Activation of the adenosine receptor (A3 type) in RBL cells did not evoke much Ca2+ influx or systematic activation of ICRAC. After protein kinase C was blocked, however, large ICRAC was observed in all cells and this was accompanied by large Ca2+ influx. The ability of a receptor to evoke Ca2+ entry is determined, at least in part, by protein kinase C. Antigen stimulation, which triggers secretion through a process that requires Ca2+ influx, activated ICRAC. The regulation of ICRAC by protein kinase will therefore have important consequences on cell functioning.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Asaoka Y., Nakamura S., Yoshida K., Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992 Oct;17(10):414–417. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Cunha-Melo J. R. Membrane phosphoinositide-activated signals in mast cells and basophils. Prog Allergy. 1988;42:123–184. [PubMed] [Google Scholar]
- Beaven M. A., Rogers J., Moore J. P., Hesketh T. R., Smith G. A., Metcalfe J. C. The mechanism of the calcium signal and correlation with histamine release in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7129–7136. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Obie J. F., Putney J. W., Jr Sinusoidal oscillations in intracellular calcium requiring negative feedback by protein kinase C. J Biol Chem. 1993 Apr 25;268(12):8425–8428. [PubMed] [Google Scholar]
- Chad J. E., Eckert R. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J Physiol. 1986 Sep;378:31–51. doi: 10.1113/jphysiol.1986.sp016206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. K., Reinhart P. H., Martin B. L., Brautigan D., Levitan I. B. Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science. 1991 Aug 2;253(5019):560–562. doi: 10.1126/science.1857986. [DOI] [PubMed] [Google Scholar]
- Dieter P., Fitzke E., Duyster J. BAPTA induces a decrease of intracellular free calcium and a translocation and inactivation of protein kinase C in macrophages. Biol Chem Hoppe Seyler. 1993 Mar;374(3):171–174. doi: 10.1515/bchm3.1993.374.1-6.171. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Hoth M., Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem. 1993 Oct 5;268(28):20737–20740. [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Bredt D. S., Cameron A. M., Snyder S. H. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke M., Dar O., Droogmans G., Pecht I., Nilius B. Immunological stimulation of single rat basophilic leukemia RBL-2H3 cells co-activates Ca(2+)-entry and K(+)-channels. Cell Calcium. 1995 Jan;17(1):71–83. doi: 10.1016/0143-4160(95)90104-3. [DOI] [PubMed] [Google Scholar]
- Girard S., Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993 Apr 9;260(5105):229–232. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- McCloskey M. A., Cahalan M. D. G protein control of potassium channel activity in a mast cell line. J Gen Physiol. 1990 Feb;95(2):205–227. doi: 10.1085/jgp.95.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol. 1987 Mar;104(3):783–792. doi: 10.1083/jcb.104.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., Garcia-Sancho J., Alvarez J. Transient inhibition by chemotactic peptide of a store-operated Ca2+ entry pathway in human neutrophils. J Biol Chem. 1993 Jun 25;268(18):13055–13061. [PubMed] [Google Scholar]
- Montero M., García-Sancho J., Alvarez J. Phosphorylation down-regulates the store-operated Ca2+ entry pathway of human neutrophils. J Biol Chem. 1994 Feb 11;269(6):3963–3967. [PubMed] [Google Scholar]
- Neher E. Ion influx as a transduction signal in mast cells. Int Arch Allergy Appl Immunol. 1991;94(1-4):47–50. doi: 10.1159/000235322. [DOI] [PubMed] [Google Scholar]
- Parekh A. B., Foguet M., Lübbert H., Stühmer W. Ca2+ oscillations and Ca2+ influx in Xenopus oocytes expressing a novel 5-hydroxytryptamine receptor. J Physiol. 1993 Sep;469:653–671. doi: 10.1113/jphysiol.1993.sp019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E. Secretory responses of rat peritoneal mast cells to high intracellular calcium. FEBS Lett. 1988 Jan 4;226(2):307–313. doi: 10.1016/0014-5793(88)81445-5. [DOI] [PubMed] [Google Scholar]
- Petersen C. C., Berridge M. J. The regulation of capacitative calcium entry by calcium and protein kinase C in Xenopus oocytes. J Biol Chem. 1994 Dec 23;269(51):32246–32253. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Qian Y. X., McCloskey M. A. Activation of mast cell K+ channels through multiple G protein-linked receptors. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7844–7848. doi: 10.1073/pnas.90.16.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A., Taylor C. W. Effects of Ca2+ chelators on purified inositol 1,4,5-trisphosphate (InsP3) receptors and InsP3-stimulated Ca2+ mobilization. J Biol Chem. 1993 Jun 5;268(16):11528–11533. [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Törnquist K. Modulatory effect of protein kinase C on thapsigargin-induced calcium entry in thyroid FRTL-5 cells. Biochem J. 1993 Mar 1;290(Pt 2):443–447. doi: 10.1042/bj2900443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz R. J., Tobin A. B., Nahorski S. R. Desensitization of cell signalling mediated by phosphoinositidase C. Trends Pharmacol Sci. 1993 Jul;14(7):279–285. doi: 10.1016/0165-6147(93)90131-3. [DOI] [PubMed] [Google Scholar]
- Yao Y., Parker I. Inositol trisphosphate-mediated Ca2+ influx into Xenopus oocytes triggers Ca2+ liberation from intracellular stores. J Physiol. 1993 Aug;468:275–295. doi: 10.1113/jphysiol.1993.sp019771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., McCloskey M. A. Immunoglobulin E receptor-activated calcium conductance in rat mast cells. J Physiol. 1995 Feb 15;483(Pt 1):59–66. doi: 10.1113/jphysiol.1995.sp020567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]


