Abstract
Overweight and obese individuals differ in their degree of hedonic eating. This may reflect adaptations in reward-related neural circuits, regulated in part by opioidergic activity. We examined an indirect, functional measure of central opioidergic activity by assessing cortisol and nausea responses to acute opioid blockade using the opioid antagonist naltrexone in overweight/obese women (mean BMI = 31.1 ± 4.8) prior to the start of a mindful eating intervention to reduce stress eating. In addition, we assessed indices of hedonic-related eating, including eating behaviors (binge eating, emotional eating, external eating, restraint) and intake of sweets/desserts and carbohydrates (Block Food Frequency); interoceptive awareness (which is associated with dysregulated eating behavior); and level of adiposity at baseline. Naltrexone-induced increases in cortisol were associated with greater emotional and restrained eating and lower interoceptive awareness. Naltrexone-induced nausea was associated with binge eating and higher adiposity. Furthermore, in a small exploratory analysis, naltrexone-induced nausea predicted treatment response to the mindful eating intervention, as participants with more severe nausea at baseline maintained weight whereas those without nausea responses tended to gain weight. These preliminary data suggest that naltrexone-induced cortisol release and nausea may help identify individuals who have greater underlying food reward dependence, which leads to an excessive drive to eat. Future research is needed to confirm this finding and to test if these markers of opioidergic tone might help predict success in certain types of weight management programs.
Keywords: naltrexone, hedonic eating, food addiction, cortisol, nausea, obesity
With the advent of the obesity epidemic and the abundance of palatable foods in the current food environment, the concept of hedonic eating has emerged. Hedonic eating refers to eating for the pleasurable, rewarding aspects of food, in contrast to homeostatic eating, which refers to eating for caloric need (Lowe & Butryn, 2007). Hedonic eating has been implicated in the concept of “food addiction,” the existence of which is being hotly debated in scientific and public discourses (Avena, Gearhardt, Gold, Wang, & Potenza, 2012; Ziauddeen, Farooqi, & Fletcher, 2012). Theorists propose that hedonic-driven eating can cause people to become addicted to food or its specific components in ways that resemble drug addiction (Davis, Zai, et al., 2011; Moreno & Tandon, 2011). In turn, these eating behaviors may lead to weight gain and obesity in a subset of individuals.
Correlative evidence supporting the concept of food addiction is accruing as neuroimaging studies reveal that both obese and drug addicted individuals have alterations in brain regions associated with reward sensitivity, incentive motivation, memory and learning, impulse control, stress reactivity, and interoceptive awareness (for a review, see Volkow, Wang, Fowler, Tomasi, & Baler, 2011). In animal studies, growing evidence indicates that palatable foods prevalent in our food supply (in particular, those containing high levels of sugar and fat) possess addictive properties. Rats given access to highly palatable foods display classic features of addiction, including binging, withdrawal, craving, and cross-sensitization as found in response to drugs of abuse (Avena, 2010).
The opioid system is in part contained within an important neural circuit involved in both substance use and food reward. Acute consumption of palatable food stimulates release of endogenous opioids, which mediate feelings of pleasure (Yeomans & Gray, 2002). However, repeated over-stimulation of post-synaptic opioid receptors due to chronic intake of palatable foods may elicit long-term changes in receptor function or transduction mechanisms that subsequently down-regulate opioid action (Kelley, Will, Steininger, Zhang, & Haber, 2003). For instance, rats given frequent access to chocolate or sucrose that elicit binge eating behaviors show reduced expression of enkephalins (an endogenous opioid) in the ventral striatum, a brain region involved in reward (Kelley et al., 2003; Spangler et al., 2004). The resulting opioidergic state may induce a state of withdrawal. Rats given chronic access to a high sucrose diet and then either abruptly taken off or treated with an opioid antagonist demonstrate behaviors consistent with opiate withdrawal (Colantuoni et al., 2002). A withdrawal state, in turn, can increase incentive salience for sugar, as found in alcohol abuse (Avena, Long, & Hoebel, 2005). The “wanting” of a food reward is mediated through μ-opioid signaling in the nucleus accumbens (Shin, Pistell, Phifer, & Berthoud, 2010). These various animal studies demonstrate that central opioid activity is involved in core addiction processes related to palatable foods, in particular, bingeing, withdrawal, and craving.
Despite compelling neurobiological models of addiction in animals, there is a paucity of direct evidence to validate the concept of hedonic-driven eating or food addiction in humans (Ziauddeen & Fletcher, 2013). There are no validated functional markers of central opioidergic activity in humans, short of positron-emission tomography (PET) scans to assess opioid receptor binding potential. However, as an indirect functional measure, the effects of opioid antagonists on the hypothalamic-pituitary-adrenal axis (HPA) have been studied to assess the role of endogenous opioidergic activity in alcohol and nicotine addictions (e.g., al'Absi, Wittmers, Hatsukami, & Westra, 2008; Ouwens, van Strien, van Leeuwe, & van der Staak, 2009; Wand, Mangold, El Deiry, McCaul, & Hoover, 1998; Wand et al., 2012). Endogenous opioids inhibit the HPA axis through two pathways. First, neurons in the arcuate nucleus containing β endorphin and enkephalin activate μ opioid receptors in the paraventricular nucleus to inhibit corticotropin releasing-hormone (CRH) release (Yajima et al., 1986). Opioids also inhibit the activity of norephinephrine-containing neurons in the locus coeruleus, which activate hypothalamic CRH neurons (Valentino, Rudoy, Saunders, Liu, & Van Bockstaele, 2001). Phamacologic blockade of opioid receptors releases the opioidergic inhibitory input to CRH neurons, stimulating pituitary adrenocorticotropic hormone (ACTH), and eventually cortisol from the adrenal gland. As a result, individual differences in central opioidergic activity can be detected by cortisol response to opioid antagonism. Greater increases in cortisol release to an opioid antagonist may indicate weaker endogenous opioid tone as a result of fewer endogenous opioids available to compete for binding sites, or a reduction in opioid receptor density resulting in a more complete blockade of inhibitory inputs to the hypothalamus (Roche, Childs, Epstein, & King, 2010; Wand et al., 1998). Thus far, one study found that patients with bulimia had higher cortisol levels in response to naloxone (an opioid antogonist) as compared to controls (Coiro et al., 1990).
While the exact mechanisms underlying the association between cortisol responses, central opioidergic activity, and opioid antagonists are unknown, we theorized that chronic overconsumption of highly palatable foods downregulates endogenous opioid peptide production or receptor density, which would be reflected by increased cortisol in response to an opioid antagonist. We also postulated that nausea responses to opioid antagonism may be a second indicator of central opioid activity, as those with low opioidergic tone may feel more nauseous after acute opioid blockade. Naltrexone therapy (primarily a μ opioid antagonist) in combination with bupropion results in clinically significant weight loss (Apovian et al., 2013) supporting the role of the opioid system in eating behavior and weight gain. Yet nausea is a common side effect of naltrexone, and a qualitative review suggests it may be increased in persons with obesity (Yeomans & Gray, 2002). In two large clinical trials that administered naltrexone to obese individuals, 30-34% reported nausea in the drug therapy condition compared to 5-11% in the placebo group (Katsiki, Hatzitolios, & Mikhailidis, 2011). Thus far, the relationship between naltrexone-induced nausea and hedonic-related eating remains unexplored.
In the current study, we assessed cortisol and nausea responses to a standardized naltrexone challenge among overweight and obese women. In cross-sectional analyses, we tested if these responses were associated with hedonic-related eating behaviors, including binge, emotional, and external-based eating. We also included dietary restraint because, although it does not explicitly measure hedonic eating, people high on restraint overeat in the face of stress or cognitive load (Lowe & Kral, 2006). Dietary restraint has also been recently re-conceptualized as reflecting a latent hedonic eating drive, with highly restrained individuals eating less than they want, rather than less than they need (Lowe & Butryn, 2007). We also assessed the relation between cortisol and nausea responses to naltrexone with dietary intake and adiposity. When given naltrexone, women reporting higher levels of hedonic-related eating behaviors may demonstrate a more severe opiate-like withdrawal state, similar to the rat model of high sugar intake (Colantuoni et al., 2002). Therefore, we predicted greater nausea and cortisol responses to naltrexone, presumably indicating weaker opioidergic activity, would be associated with higher levels of hedonic-related eating behaviors, greater intake of palatable foods, and excess adiposity.
We also explored the association of naltrexone responses with interoceptive awareness, the perception of sensations originating from inside the body. According to recent theories, interoceptive awareness is important for regulating homeostasis and may be altered as a result of addiction (Goldstein et al., 2009; Naqvi & Bechara, 2010; Paulus, Tapert, & Schulteis, 2009). Because addicted individuals chronically experience aversive bodily states either resulting from withdrawal symptoms or emotional distress, they may react more impulsively to sensations of craving or withdrawal either to satisfy urges or alleviate the aversive state (Paulus et al., 2009). As a first step towards understanding the potential relation between opioid-mediated food addictive processes and interoceptive awareness, we examined whether self-reported aspects of interoceptive awareness were related to naltrexone responses.
Lastly, responses to acute opioid blockade may have clinical utility by predicting individual differences in treatment response to interventions for overweight and obese individuals. We explored whether naltrexone responses at baseline predicted weight change among women enrolled in a randomized waitlist-control pilot study of a mindfulness-based program for stress eating (Daubenmier et al., 2011).
Methods
Participants
This paper reports on baseline data collected from a subset of women (N=33) who elected to participate in a substudy of a randomized waitlist control pilot trial of a mindfulness intervention for overeating and stress reduction (N=47), described previously (Daubenmier et al., 2011). Sample characteristics are reported in Table 1. The ethnic composition of the sample was 64% White, 18% Asian-American, 15% Hispanic/Latina, and 3% identified as another ethnicity. Five participants were on stable anti-depressant medication.
Table 1.
Sample Characteristics (N=33)
| Variable | Mean ± SD |
|---|---|
| Age | 40.9 ± 8.0 |
| Anthropometrics: | |
| Weight (kg) | 85.9 ± 15.5 |
| Body Mass Index | 31.1 ± 4.8 |
| Total Body Fat (%) | 45.7 ± 0.1 |
| Binge Eating Scale (BES) | 17.2 ± 7.9 |
| Dutch Eating Behavior Questionnaire: | |
| Emotional Eating | 3.4 ± 0.8 |
| Restrained Eating | 2.7 ± 0.5 |
| External-Based Eating | 3.5 ± 0.5 |
| Body Responsiveness Questionnaire: | |
| Importance of Interoceptive Awareness | 4.0 ± 1.4 |
| Perceived Incongruity | 4.1 ± 1.2 |
| Block Food Frequency | |
| Sweets and Desserts (% kcal) | 11.7 ± 6.5 |
| Carbohydrate (% kcal) | 45.5 ± 7.0 |
| Fat (% kcal) | 37.8 ± 5.6 |
The Institutional Review Board of the University of California, San Francisco (UCSF) approved this study and all participants provided informed consent. Briefly, adult female participants were recruited through media outlets with key eligibility criteria as follows: a body mass index (BMI) between 25 and 40; pre-menopausal; no history of diabetes or cardiovascular disease, or active endocrinologic disorder; not pregnant or less than one year postpartum; no prior or current meditation or yoga practice; not currently on a diet plan or taking medications that would affect weight; no current self-reported eating disorder or alcohol or drug addiction; not taking opiate pain medication, steroids, or antipsychotic medications; and English literate. Participants provided a urine sample to test for the presence of opioids or other drugs and pregnancy. All tests were negative. Eligible and interested participants completed two assessment visits at the UCSF Clinical Research Center (for eligibility and anthropometrics) and an on-line questionnaire battery at baseline. They were assessed again with a similar visit and questionnaire battery post-intervention.
Baseline Assessments
Cortisol and Nausea Reponses to Naltrexone
All baseline assessments were completed prior to randomization. Participants were instructed to complete home saliva sampling kits to assess cortisol levels on 4 days. The first three days were control days to assess diurnal cortisol rhythms upon waking, 30 minutes after waking (to capture morning rise), at 1pm, 2pm, 3pm, and 4pm. Participants were instructed to collect the first sample while in bed, and to not eat, drink, brush their teeth or engage in vigorous activity between the first two morning samples or for 20 minutes prior to all other samples.
On the fourth day, participants took a clinical dose of naltrexone (50 mg) after the 1pm saliva sample after lunch to control for cortisol responses to food intake. The 50 mg dose was chosen because it is the FDA-approved dosage for treatment of alcohol and opioid dependencies and it has been used in other studies (Roche et al., 2010). The timing of the saliva collection was determined based on studies showing evidence of peak levels of naltrexone and cortisol concentrations 2-3 hours after administration of naltrexone (King et al., 2002b). Participants were told about possible negative side effects including nausea and given a list of Frequently Asked Questions about naltrexone to take home with them that described the side effects. No placebo condition was administered. Each sample was collected by drooling into a straw in 2 mL SaliCaps tubes (IBL Hamburg, Germany). Cortisol analysis was performed at Dresden LabService at the Dresden University of Technology (Germany) using a commercial chemiluminescence immunoassay (CLIA; IBL Hamburg, Germany). Values greater than 100 nmol/L were excluded because they fell outside the range of the assay.
To assess nausea symptoms, participants completed a checklist of 14 symptoms, including nausea, using a 4-point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). Participants were asked to complete the checklist right before bedtime. Participants without a complete checklist were called by study staff to complete missing items.
Anthropometric Variables
A standard stadiometer (Perspective Enterprises, Portage, MI) was used to measure height to the nearest 1/8th inch. A digital scale (Wheelchair Scale 6002, Scale-Tronix, Carol Stream, IL) was used to measure weight to the nearest 0.1kg. Body mass index was calculated (kg/m2). Weight was reassessed post-intervention.
Body Fat
Whole-body dual energy X-ray absorptiometry (DEXA) scans were performed to assess total percent body fat. The DEXA densitometer (GE Healthcare Lunar Prodigy, Madison, WI, USA) was adjusted to the fan beam mode and EnCore software version 9.15 was used. The coefficient of variation in assessing fat mass from the UCSF General Clinical Research Center densitometer is 4%.
Eating Behaviors
The Dutch Eating Behavior Questionnaire (DEBQ) (Van Strien, 1986) assesses restrained eating, emotional eating, and external-based eating. The Restrained Eating subscale evaluates intentions and behaviors to restrict food intake due to concerns about weight. Paradoxically, restrained eating predicts palatable food intake in response to non-stressful cognitive activities, suggesting that restrained eaters have a latent susceptibility to overconsume palatable foods (Leon, Fulkerson, Perry, & Early-Zald, 1995). The Emotional Eating subscale measures eating behaviors triggered by negative emotions, such as anger, boredom, anxiety, or fear. The External-based Eating subscale assesses eating in response to food-related stimuli, such as the smell or taste of food or presence of food in the environment. Responses were made on a 5-point scale from 1 = never to 5 = very often.
The Binge Eating Scale (BES) was used to assess the extent and severity of compulsive overeating patterns, including behavioral tendencies (e.g., eating large amounts of food) and negative feelings and thoughts related to binge eating episodes or one's body (Gormally, Black, Daston, & Rardin, 1982). It is a continuous measure sensitive to a wide range of concerns and patterns with overeating rather than diagnostic of binge eating disorder.
Interoceptive Awareness
The Body Responsiveness Questionnaire (BRQ) is a 7-item scale used to assess aspects of interoceptive awareness (Daubenmier, 2005; Mehling et al., 2009). A principal components factor analysis reveals two factors in past research (Daubenmier, unpublished analyses) as well as in the current study. The factor loadings were greater than .40 explaining 68% of the variance of the scale. The first subscale, “Importance of Interoceptive Awareness,” assesses the importance of using interoceptive information to consciously regulate behavior and self-awareness (sample items include: “It is important for me to know how my body is feeling throughout the day”; “I am confident that my body will let me know what is good for me”; “I enjoy becoming aware of how my body feels”). The second subscale, “Perceived Disconnection,” measures the extent of disconnection between psychological and physical states (sample items include: “My mind and my body often want to do different things”; “My bodily desires lead me to do things that I end up regretting”). Responses were measured on a 7-point scale ranging from 1 = not at all true about me to 7 = very true about me.
Dietary Intake
The Block 2005 Food Frequency Questionnaire, a semi-quantitative food frequency questionnaire, was used to assess food consumption of 110 food items over the past year (Block, 2005). Percent calories from carbohydrates, fat, and sweets/desserts were calculated according to analyses performed by NutritionQuest. Though widely used, it is somewhat insensitive to overeating or binge patterns as the largest quantity that can be indicated as typically consumed is limited for most food items.
Intervention Groups
All participants were randomized to the treatment or waitlist control group in a 1:1 ratio and stratified by BMI category (overweight: BMI 25 – 29.99 vs. obese: 30 - 39.99), age (≥ 40 years) and current anti-depressant medication use (n=7), as these factors may influence weight change. In the current substudy, 16 were randomized to the intervention and 17 to the control group.
Treatment Condition
A novel intervention was developed by integrating components from three empirically-validated programs, Mindfulness-Based Stress Reduction (MBSR) (Kabat-Zinn, 1990), Mindfulness-Based Cognitive Therapy for Depression, (Teasdale et al., 2000), and Mindfulness Based Eating Awareness Training (MB-EAT) (Kristeller & Hallett, 1999a; Kristeller & Wolever, 2011). Mindfulness meditation entails the systematic training of a focused state of awareness through repeated attendance to sensations of breath, other sensory experiences, thoughts, and emotions, as well as the development of a nonjudgmental attitude. MB-EAT, in particular, promotes awareness of physiological cues related to hunger, satiety, and taste satisfaction and emotional triggers for overeating. In the current study, the intervention program consisted of nine 2.5-hour classes and one 7-hour silent day of guided meditation practice during the sixth week of the program. Participants were encouraged to engage in daily home assignments that included up to 30 minutes per day of formal mindfulness meditation practices and to practice mindful eating during meals. More details regarding the intervention are described elsewhere (Daubenmier et al., 2011).
Control Condition
To provide guidelines for healthy eating and exercise during the intervention and to control for the effects of such information on study outcomes, both groups participated in a 2-hour nutrition and exercise information session aimed at moderate weight loss midway through the intervention, in which mindfulness was not discussed.
Statistical Analysis
Participants who had at least one day of control cortisol data were included in analyses. Paired samples t-tests using the least squares differences method were used to compare differences between cortisol concentrations at 1pm, 2pm, 3pm, and 4pm on the mean of the three control days and the naltrexone day, and to compare differences between times on the control days and the naltrexone day. We calculated two indicators of the cortisol response to naltrexone to explore the predictive utility of each measure. The first indicator was calculated by subtracting the peak cortisol response (at 4pm) from the cortisol level in the 1pm sample on the naltrexone day. The second indicator was calculated by subtracting the change in cortisol from 4pm-1pm on the naltrexone day from the mean difference from 4pm to 1pm on the control days to explore the added sensitivity of the measure when baseline cortisol concentrations were taken into account. Due to a skewed distribution of the cortisol response, Spearman's rank correlations were used to assess associations among cortisol responses to naltrexone and other measures.
Self-reported nausea was assessed by dividing participants into low (none or mild) and high (moderate or severe) symptom groups and independent sample t-tests were conducted to compare differences between groups on eating behavior, interoceptive awareness, and body fat measures. Levene's test for equality of variances was used to test for equality of variances between groups and degrees of freedom were adjusted for the independent sample t-tests if the test was significant (p < .05). To explore nausea as a predictor of weight change within the treatment group, a 2 × 2 ANCOVA was performed with treatment group (treatment vs. waitlist control group) and nausea group (low vs. high symptoms) as between-subjects factors with BMI and antidepressant medication used as covariates. The continuous variables of cortisol responses to naltrexone were examined as predictors of weight change by treatment group using multiple regression analysis. Baseline BMI, antidepressant medication use, treatment group, and cortisol response were entered on step 1 and the interaction term (treatment group × cortisol response) was entered in step 2 of the equation.
Results
Participants who elected to take part in the substudy had a significantly greater percentage of total adiposity compared to those who declined (45.7 ± 5.0 vs. 42.5 ± 3.7, p = .047). No other baseline differences (including sociodemographic or psychological variables) were significant between those who elected or declined to take part in the substudy. Three participants did not provide saliva samples or take naltrexone as prescribed, and were excluded from relevant analyses. Twenty-seven participants (82%) had complete cortisol data on all three control days and 30 participants (91%) had complete cortisol data on the naltrexone day. Twenty-seven participants (82%) had both complete cortisol data for a minimum of one control day and the naltrexone day. Three participants failed to answer the nausea question.
Cortisol and Nausea Responses
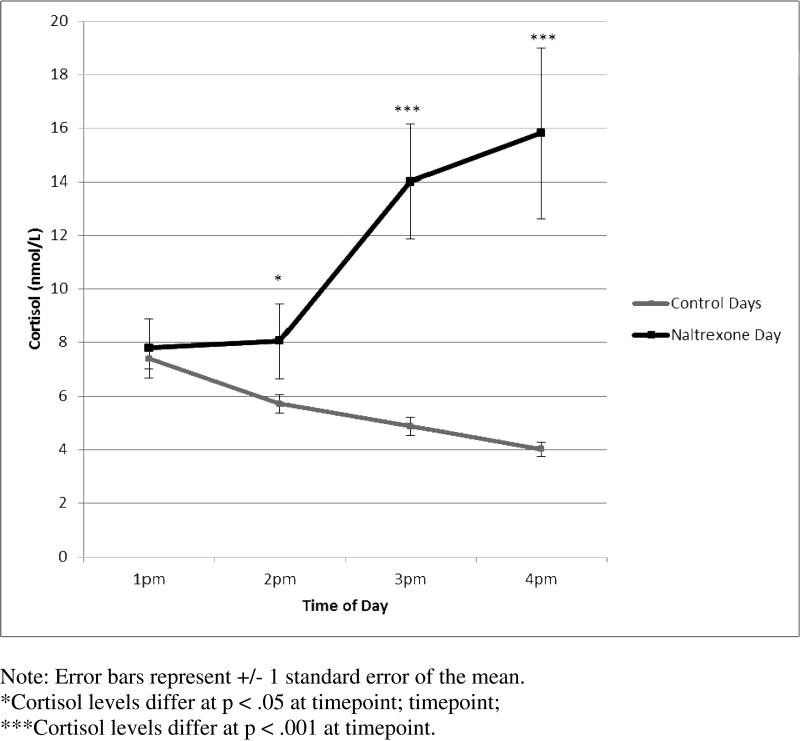
Cortisol decreased by 3.6 ± 2.2 nmol/L between 1pm and 4pm on the control days (95% CI: 2.8 – 4.4; t (32) = 9.4, p < .001) and increased on the naltrexone day by 8.0 ± 17.4 nmol/L (95% CI: 1.5 – 14.5; t (29) = 2.53, p = .02) between 1pm and 4pm (see Figure 1). Cortisol concentrations did not differ significantly between control days versus the naltrexone day at the baseline timepoint of 1pm [t(30) = 0.80; p = .43)]. By 2pm (one hour after taking naltrexone) cortisol values were 3.3 ± 8.1 nmol/L (95% CI: 0.2 – 6.4) higher than the average on control days at 2pm [t(28)=2.2, p = .04]. By 3pm (two hours after taking naltrexone) cortisol values were 9.0 ± 12.5 nmol/L (95% CI: 4.4 – 13.6) higher than the average on control days at 2pm [t(30)=4.0, p < .001]. This difference increased by 4pm, with mean cortisol values on the naltrexone day that were 11.5 ± 17.9 nmol/L (95% CI: 5.1 – 18.0) higher than at 4 pm on control days [t(31)= 3.6, p = .001].
Figure 1.
Cortisol Responses on Control Days and Naltrexone Day
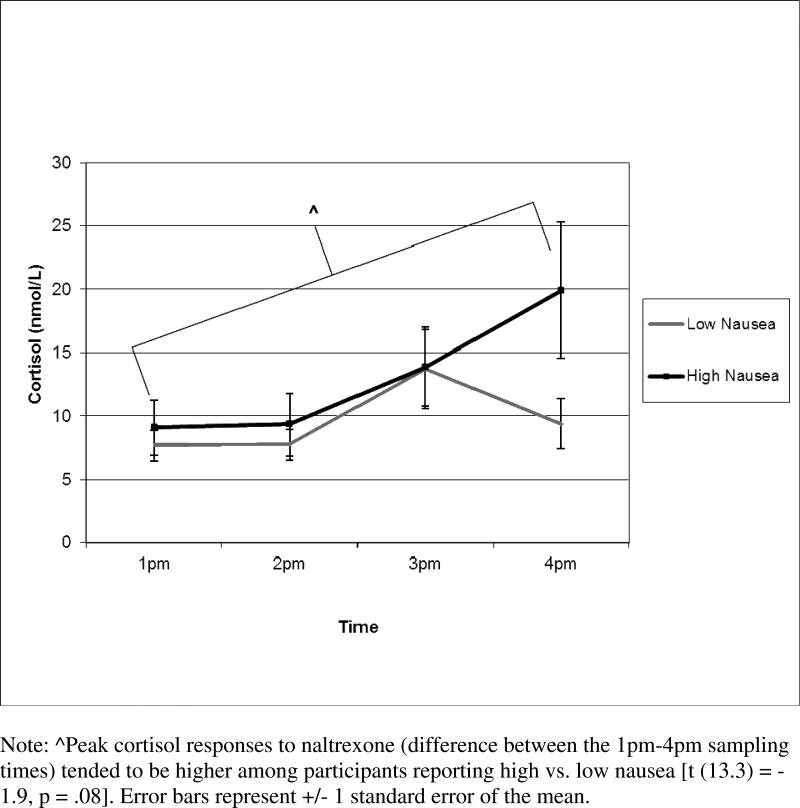
The mean level of nausea severity was 1.23 ± 1.3. Due to a skewed distribution, participants were divided into low vs. high nausea groups, with 60% of participants (n=18) reporting none to mild nausea and 40% reporting moderate to severe levels (n=12). Peak cortisol responses to naltrexone (i.e., difference between the 4pm – 1pm) tended to be higher among participants reporting more severe nausea (13.4 ± 17.3 nmol/L) compared to those with low nausea [2.0 ± 10.9 nmol/L; t (13.3 = −1.9, p = .08, see Figure 2].
Figure 2.
Cortisol Responses to Naltrexone by Low and High Nausea Groups
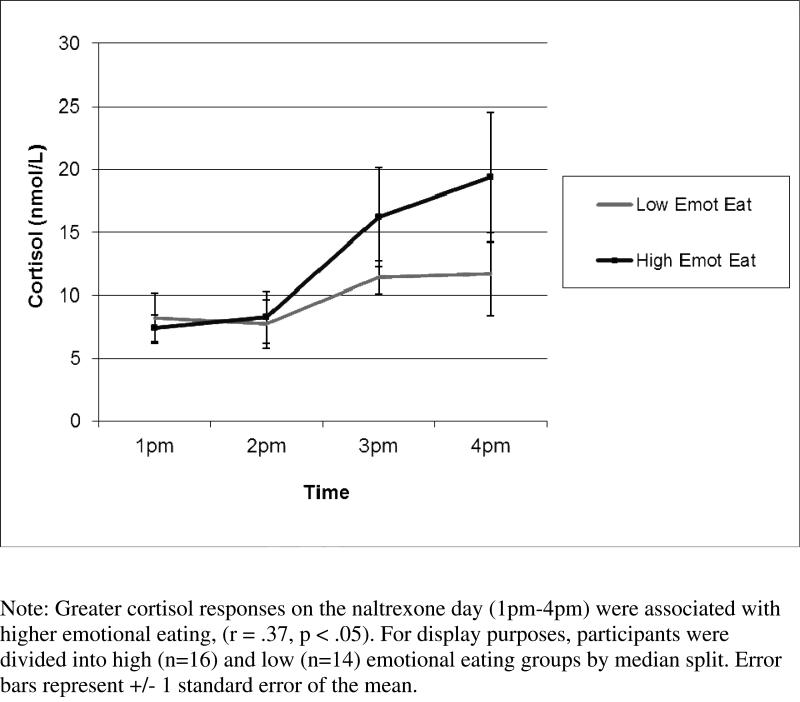
Correlations between cortisol naltrexone responses and adiposity, hedonic eating behaviors, and interoceptive awareness are shown in Table 2. Greater peak cortisol responses on the naltrexone day were significantly associated with higher emotional and restrained eating and lower importance of interoceptive awareness. To illustrate the finding in high vs. low emotional eaters see Figure 3. Greater peak cortisol responses to naltrexone relative to control days was significantly related to greater restrained eating, lower scores on importance of interoceptive awareness, greater carbohydrate intake, and marginally related to greater intake of sweets and desserts.
Table 2.
Associations Among Cortisol and Nausea Responses to Naltrexone and Indicators Hedonic Eating and Adiposity
| Naltrexone Cortisol Responsea | Naltrexone Cortisol Response - Average of Control Daysa | |
|---|---|---|
| Adiposity | ||
| BMI | −.13 | .08 |
| Total body fat (%) | −.05 | .16 |
| Binge Eating Scale | .03 | .03 |
| Dutch Eating Behavior Questionnaire | ||
| Emotional Eating | .37* | .29 |
| External Eating | .04 | −.08 |
| Restrained Eating | .40* | .36* |
| Body Responsiveness Questionnaire | ||
| Importance | −.40* | −.48** |
| Perceived Disconnection | −.08 | −.11 |
| Food Frequency Questionnaire | ||
| Sweets and Desserts (% kcal) | .24 | .32^ |
| Carbohydrate (% kcal) | .28 | .37* |
| Fat (% kcal) | −.12 | −.13 |
Results of Spearman's rho correlations are presented
p < .01
p≤ .05
p≤ .10.
Figure 3.
Cortisol Responses After Naltrexone by Emotional Eating Group
As shown in Table 3, the high nausea group had significantly greater percent body fat, reported greater binge eating symptoms, and tended to have higher BMISs, and report more emotional eating and less importance of interoceptive awareness compared to the low nausea group, with these last three differences of marginal statistical significance. The means of percent caloric intake from sweets and desserts were in the predicted direction, with higher intake among the high nausea group, but the difference did not reach statistical significance.
Table 3.
Means and Standard Deviations of Adiposity, Hedonic Eating, and Interoceptive Awareness by Nausea Group
| Low Nausea (n=18) (M ± SD) | High Nausea (n=12) (M ± SD) | Mean Diff (H – L) (SE) | 95% CI of Mean Diff | P | |
|---|---|---|---|---|---|
| Adiposity | |||||
| BMI | 30.0 ± 4.4 | 33.2 ± 4.4 | 3.3 (1.6) | −0.1–6.1 | .056 |
| Body fat (%) | 44.4 ± 5.0 | 48.1 ± 4.0 | 3.7 (1.7) | −7.1–−0.1 | .04 |
| Binge Eating Scale | 14.9 ± 6.6 | 20.9 ± 9.4 | 6.0 (2.9) | 0.1–12.0 | .048 |
| Dutch Eating Behavior Questionnaire | |||||
| Emotional Eating | 3.1 ± 0.6 | 3.6 ± 0.9 | 0.5 (0.3) | −0.1–1.1 | .08 |
| External Eating | 3.4 ± 0.5 | 3.6 ± 0.4 | 0.2 (0.2) | −0.1–0.6 | .23 |
| Restrained Eating | 2.7 ± 0.4 | 2.9 ± 0.5 | 0.2 (0.2) | −0.1–0.5 | .23 |
| Body Responsiveness Questionnaire | |||||
| Importance | 4.4 ± 1.4 | 3.4 ± 1.5 | −1.0 (0.5) | −2.1–0.7 | .07 |
| Perceived Disconnection | 4.0 ± 1.4 | 4.1 ± 1.0 | 0.1 (0.4) | −0.7–1.0 | .76 |
| Food Frequency Questionnaire | |||||
| Sweets and Desserts (% kcal) | 9.7 ± 5.8 | 13.1 ± 7.0 | 3.4 (2.5) | −1.4–8.2 | .16 |
| Carbohydrate (% kcal) | 45.7 ± 8.1 | 45.1 ± 6.1 | −0.7 (2.8) | −6.3–5.0 | .81 |
| Fat (% kcal) | 37.1 ± 6.2 | 38.5 ± 5.3 | 1.4 (2.2) | −3.1–5.9 | .54 |
Exploratory analysis
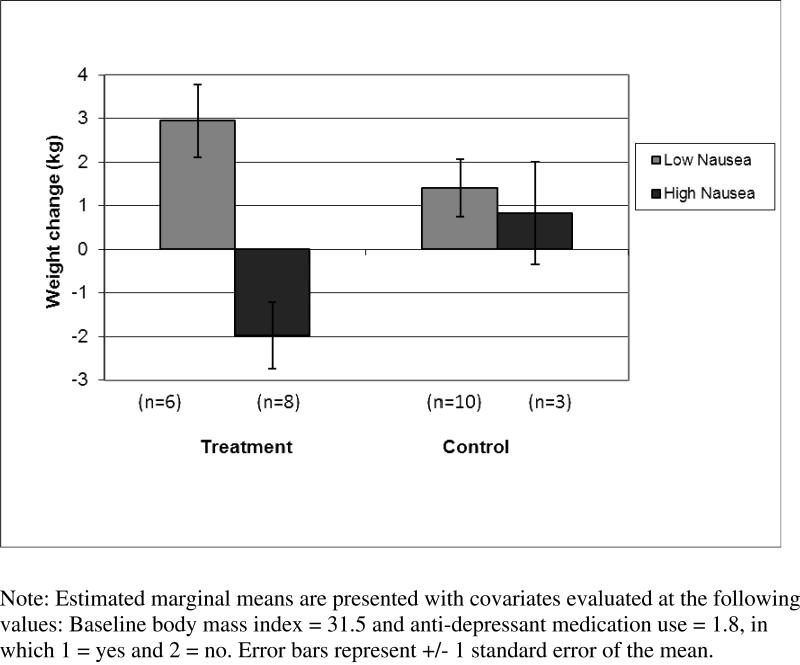
In terms of predicting treatment response to the mindfulness intervention, results of the ANCOVA revealed a significant treatment group x nausea interaction on weight change [F (1, 21) = 6.1, p = .02; see Figure 4]. Follow-up ANCOVAs indicated that the more severe nausea group maintained weight on average (−1.2 ± 2.9 kg) compared to the low nausea group in the treatment group who gained weight on average (2.7 ± 1.7 kg) [F (1, 10) = 14.4, p = .004] but with no significant differences by nausea group in the waitlist condition [F (1, 9) = 0.3, p = .58]. Multiple regression analyses examining cortisol responses to naltrexone as a predictor of weight change by treatment group and across groups were not significant (p > .76).
Figure 4.
Weight Change in Treatment vs. Control Groups by Nausea Group
Discussion
To our knowledge, this is the first study to investigate an indirect measure of central opioidergic activity in relation to hedonic-related eating behaviors among overweight and obese adults. First, we established that the clinical paradigm of response to naltrexone was working as expected. We tested acute effects of a single, clinical dose of the opioid antagonist naltrexone on cortisol concentrations and nausea severity. Cortisol concentrations increased 103% on average in response to naltrexone over a 3-hour period, whereas they decreased 48% on average across three control days without naltrexone during the same time period. These findings replicate those of prior studies showing reliable naltrexone-induced increases in HPA activity (al'Absi et al., 2008; King et al., 2002a; Roche et al., 2010). We also found a wide range of individual variation in nausea severity in response to naltrexone, with a subgroup of 40% showing a meaningful (moderate to severe) level of nausea. We then tested whether these differential responses in cortisol and nausea predicted indices of hedonic-related eating.
In line with our hypotheses, individual differences in naltrexone-induced cortisol and nausea responses were associated with greater hedonic-related eating behaviors, intake of carbohydrates, adiposity, a trend for increased palatable food intake, and lower interoceptive awareness. It is not clear in this cross-sectional study whether hedonic eating behavior contributed to low opioid activity, or whether pre-existing low activity lead to drive to eat, or both. Animal studies suggest that binge eating on palatable foods down-regulates opioidergic activity (Kelley et al., 2003; Spangler et al., 2004), whereas genetically-driven low opioidergic activity may induce hedonic overeating as a way to compensate for low basal levels of pleasure based on studies of the μ opioid receptor OPRMI genotype (Davis, Curtis, et al., 2011).
Although causality is unclear, the positive associations of naltrexone-induced cortisol responses with emotional and restrained eating are consistent with recent models of stress eating. People high on restrained or emotional eating tend to overeat sweet and fatty foods in response to stress or cognitively demanding tasks (Wallis & Hetherington, 2004). Consumption of palatable foods due to emotional or disinhibited eating stemming from restrained eating attitudes may produce surges in opioidergic activity and serve to reduce acute stress responses. Support for this model comes from animal studies which show that rats eating a diet high in fat and sugar have reduced HPA responses to acute stressors compared to rats eating chow (Dallman, Pecoraro, & la Fleur, 2005). If emotional or restrained eating becomes chronic, this may down-regulate opioidergic activity and increasingly require greater consumption of palatable foods to regulate the feeling of stress or even maintain feelings of well-being, fostering dependency and addictive–like behaviors. Thus, greater naltrexone-induced cortisol responses, potentially reflecting low opioid activity, may in part reflect overconsumption of palatable foods to dampen HPA stress responses.
An alternative explanation is that high naltrexone-induced cortisol responses do not reflect opioid sensitivity but merely reflect general hyperactivity of the HPA. If this were the case, one might expect to find a strong positive correlation between cortisol responses on the naltrexone day and on control days when no drug was administered; however this was not the case (Spearman's rho = .22, p = .25) suggesting that hypersensitivity of the HPA axis alone does not account for the present findings. However, a further test would be to establish whether cortisol levels in response to some other mild stressor or challenge (e.g., ACTH) fully account for the findings. It is important to note though that chronically low endogenous opioidergic activity may also result in greater cortisol reactivity to stressors due to opioidergic inhibitory input in the hypothalamus.
Higher cortisol responses to naltrexone were also positively related to greater dietary intake of carbohydrates and, marginally, to greater intake of sweets and desserts, but were not related to fat intake. These findings are congruent with those of animal studies suggesting that sugar binging leads to down-regulation of the endogenous opioid system (Corwin, Avena, & Boggiano, 2011), but binging on fatty foods does not have addictive effects, as fatty foods do not produce somatic or anxiety symptoms of opiate-like withdrawal (Bocarsly, Berner, Hoebel, & Avena, 2011). One possible explanation for the inability of fat to alter the opioid system involves the neuropeptide galanin (GAL), which is stimulated in reward areas in response to a high-fat meal. GAL may inhibit opiate reward, as peripheral injections of galnon, a synthetic GAL agonist, decrease opiate withdrawal signs in morphine-addicted mice (as reviewed in Avena, Rada, & Hoebel, 2009). Thus, bingeing on high-fat foods may attenuate opioid reward due to increases in GAL. Our findings are consistent with the theory that carbohydrate-rich sugary rather than fatty foods have addictive properties mediated by the opioid system (Garber & Lustig, 2011).
Nausea severity was positively associated with total adiposity. This finding confirms qualitative observations in the literature that reports of nausea increase with BMI (Yeomans & Gray, 2002). In addition, nausea severity was associated with higher scores on the Binge Eating Scale, an indicator of a general pattern of compulsive overeating behavior. Nausea severity also tended to be related to greater emotional eating. These findings are analogous to those from a rat study, when after bingeing on a high-sucrose diet, rats show greater withdrawal symptoms following naltrexone administration compared to control rats (Colantuoni et al., 2002). More severe nausea may be a type of withdrawal symptom due to low levels of opioidergic activity. As suggested by animal studies, chronic intermittent intake of large amounts of palatable foods may down-regulate opioidergic activity. Thus, individuals who binge eat may have lower opioidergic activity.
One outstanding question regarding the overall results concerns the different pattern of associations between the two markers of opioidergic activity. Here we assume that both nausea and cortisol increases to opioid blockade reflect underlying low opioidergic activity and thus could be characterized as withdrawal symptoms from blockade. Indeed, the high nausea group tended to have higher cortisol responses compared to the low nausea group. However, cortisol response is more associated with emotional eating and dietary restraint, whereas nausea response is more related to binge eating and adiposity. Cortisol concentrations increase as a result of decreased opioidergic inhibitory input on the HPA axis, whereas the subjective reports of nausea are a result of complex phenomena involving central and peripheral processing, as well as primitive and higher order cognitions and emotional responses. Therefore, it is not surprising that cortisol reactivity and subjective nausea are not highly coordinated responses (show some independence) and operate differently. Further, cortisol increases were clearly in response to naltrexone, whereas our measure of nausea may be more trait-like, as we did not assess changes in nausea across the naltrexone response period or on the control days. In more controlled studies, future work is needed to understand how cortisol and nausea responses may underlie unique and common mechanisms of naltrexone responses associated with hedonic-related eating patterns.
Low interoceptive awareness has been found to predict hedonic eating behavior and disordered eating (Leon et al., 1995; Ouwens et al., 2009). It is also thought that interoceptive awareness is dysregulated in addiction (Goldstein et al., 2009; Naqvi & Bechara, 2010; Paulus et al., 2009). We found that lower interoceptive awareness, specifically, placing less importance on interoceptive awareness to regulate conscious self-awareness and decision-making, was associated with greater cortisol responses. Greater nausea tended to be related to less interoceptive awareness as well. These novel findings offer preliminary support for the theory that interoceptive awareness as a form of self-awareness that facilitates insight and self-control is reduced in addiction (Goldstein et al., 2009). Further research is warranted to understand the involvement of interoceptive awareness in the syndrome of reward-based eating.
Lastly, we examined whether cortisol or nausea responses predicted treatment response for women enrolled in a mindfulness intervention for stress eating. Our analysis was exploratory, given the small sample size and lack of specific predictions. On one hand, women demonstrating a greater indication of opioid-mediated hedonic eating may be more resistant to treatment compared to women with less indication. On the other hand, mindfulness training has shown promise for treating substance use and binge eating disorders and may be particularly apt to improve self-regulation and eating in response to cravings and negative emotions (Bowen et al., 2009; Kristeller & Hallett, 1999b; Kristeller & Wolever, 2011). Interestingly, we found that participants with more severe nausea at baseline, presumably indicating lower opioidergic activity, had better weight maintenance following the mindfulness intervention compared to participants with less nausea who gained weight. No differences in weight maintenance were found between the low and high nausea individuals in the waitlist group. Our sample was small and conclusions should be held tentatively. Yet, with this limitation in mind, these results suggest that mindfulness potentially may be an effective treatment for overweight to obese adults with high levels of hedonic eating or features of food addiction.
We examined two indicators of cortisol responses: the peak rise in cortisol three hours after naltrexone administration and the peak rise relative to a mean change when naltrexone was not administered. Response on the same day (not compared to a control day) was a stronger predictor of drive to eat, suggesting a one day assessment may be a sufficient biomarker for opioidergic activity, although this finding demands replication.
A significant limitation of the present study is the lack of a placebo condition. In addition, participants were given, in advance, a list of numerous possible side effects, of which nausea was one, and nausea responses may reflect individual differences in suggestibility. Also, some participants recalled their level of nausea retrospectively over the phone. However, the percentage of participants reporting at least moderate nausea in this study (40%) is similar to the percentage of obese patients reporting nausea in large scale placebo-controlled clinical trials of naltrexone (30-34%) (Katsiki et al., 2011). Even if participant reports of nausea involved suggestibility to some extent, 30% of participants reported severe nausea (and five reported vomiting), which is unlikely the result of suggestibility. Suggestibility may influence nausea ratings to some extent, but would not likely also induce greater adiposity and hedonic eating drive. In other words, it is unlikely that suggestibility is causing both nausea and signs of dysregulated eating, or causing the relationship observed between the two. Future research will need to address this limitation by including a double-blind placebo condition. Another limitation is the small sample, and it could be argued that the levels of dysregulated eating observed in this sample were moderate. Nevertheless, the variability within the sample is clearly meaningful in regards to underlying neurophysiological regulatory processes. Lastly, our study was limited to women. Women have been shown to have stronger cortisol responses to naltrexone than men (Roche et al., 2010). Future work would need to replicate this study in men.
It is currently not clear what increased cortisol responses to acute opioid blockade indicate about central opioidergic activity in the context of hedonic eating or among individuals with features of food addiction. Based on prior work of this probe and animal studies showing down-regulation of the opioid system in response to palatable food (Spangler et al., 2004), we theorized that greater increases in cortisol release indicates either weaker endogenous opioidergic activity as a result of fewer endogenous opioids available to compete for binding sites with an opioid antagonist, or a reduction in opioid receptor density resulting in a more complete blockade of inhibitory inputs to the hypothalamus (Roche et al., 2010; Wand et al., 1998). PET studies demonstrate that greater cortisol responses to naloxone, a non-specific opioid receptor antagonist, are associated with lower μ and δ opioid-receptor binding potential in several brain regions (including the hypothalamus) among healthy controls, but not among acutely abstinent alcohol-dependent participants (Wand et al., 2011; Wand et al., 2012). While we may have expected that cortisol responses would be positively associated with opioid receptor binding potential, it is not clear what PET studies of binding potential indicate, as lower binding potential may reflect increased endogenous opioid release, down-regulation of receptors, or loss of neurons with opioid receptors (Sprenger, Berthele, Platzer, Boecker, & Tolle, 2005). A consistent pattern of findings of cortisol responses to acute opioid blockade in alcohol addiction have not been observed either. Specifically, cortisol response to opioid antagonists are higher in those at risk for alcoholism based on a positive family history (King et al., 2002a; Wand, Mangold, Ali, & Giggey, 1999; Wand et al., 1998; Wand, McCaul, Gotjen, Reynolds, & Lee, 2001), but not all have found this association (Lovallo et al., 2012). Furthermore, among alcohol-dependent participants HPA activity appears to be blunted compared to controls (Inder et al., 1995; Kemper et al., 1990) although not in all studies (Wand et al., 2012). Thus, the significance of what cortisol responses to opioid antagonists indicates about opioid signaling within and across addictions is not clear.
To gain a better understanding of these mechanisms, future research could examine naltrexone-induced cortisol and nausea responses in relation to PET assessments of opioid receptor binding potential in individuals with high levels of hedonic eating or features of food addiction and controls. These responses could also be examined in relation to variations in genes that regulate opioid receptors. Some evidence suggests that the opioid-receptor polymorphism A118G predicts cortisol responses to naloxone (Chong et al., 2006).
In summary, individuals with high levels of hedonic-related eating, such as emotional and binge eating, may have a down-regulated opioidergic system. Results of the present study suggest that opioid tone can be measured in a relatively unobtrusive way, at home, in overweight and obese adults. Although these findings need to be replicated in future studies, this study suggests that cortisol and nausea responses to acute opioid blockade may serve as biomarkers of hedonic-related eating and potentially food addiction.
Highlights.
Cortisol and nausea responses to acute opioid blockade were examined.
Responses were related to emotional, binge, and restrained eating, and adiposity.
Nausea predicted weight maintenance in a mindfulness intervention for overeating.
Cortisol and nausea responses may identify people with food reward dependence.
Acknowledgements
This research was supported by the Mt Zion Health Fund; The William Bowes, Jr., Fund; the Robert Deidrick Fund; and NIH grant K01AT004199 awarded to JD from the National Center For Complementary & Alternative Medicine and and the National Institutes of Health/National Center for Research Resources UCSF-CTSI Grant no. ULI RR024131. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Alternative Medicine or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al'Absi M, Wittmers LE, Hatsukami D, Westra R. Blunted opiate modulation of hypothalamic-pituitary-adrenocortical activity in men and women who smoke. Psychosom Med. 2008;70(8):928–935. doi: 10.1097/PSY.0b013e31818434ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, Dunayevich E. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring) 2013;21(5):935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM. The study of food addiction using animal models of binge eating. Appetite. 2010;55(3):734–737. doi: 10.1016/j.appet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Gearhardt AN, Gold MS, Wang GJ, Potenza MN. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nat Rev Neurosci. 2012;13(7):514. doi: 10.1038/nrn3212-c1. author reply 514. [DOI] [PubMed] [Google Scholar]
- Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84(3):359–362. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139(3):623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block G. Block 2005 Food Frequency Questionnaire. NutritionQuest/Block Dietary Data Systems; Berkeley, CA: 2005. [Google Scholar]
- Bocarsly ME, Berner LA, Hoebel BG, Avena NM. Rats that binge eat fat-rich food do not show somatic signs or anxiety associated with opiate-like withdrawal: implications for nutrient-specific food addiction behaviors. Physiol Behav. 2011;104(5):865–872. doi: 10.1016/j.physbeh.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S, Chawla N, Collins SE, Witkiewitz K, Hsu S, Grow J, Marlatt A. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30(4):295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31(1):204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Coiro V, d'Amato L, Marchesi C, Capretti L, Volpi R, Roberti G, Chiodera P. Luteinizing hormone and cortisol responses to naloxone in normal weight women with bulimia. Psychoneuroendocrinology. 1990;15(5-6):463–470. doi: 10.1016/0306-4530(90)90069-l. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10(6):478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Avena NM, Boggiano MM. Feeding and reward: perspectives from three rat models of binge eating. Physiol Behav. 2011;104(1):87–97. doi: 10.1016/j.physbeh.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19(4):275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, Epel E. Mindfulness Intervention for Stress Eating to Reduce Cortisol and Abdominal Fat among Overweight and Obese Women: An Exploratory Randomized Controlled Study. J Obes. 2011;2011:651936. doi: 10.1155/2011/651936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier JJ. The Relationship of Yoga, Body Awareness, And Body Responsiveness To Self-Objectification And Disordered Eating. Psychology of Women Quarterly. 2005;29(2):207–219. [Google Scholar]
- Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that ‘food addiction’ is a valid phenotype of obesity. Appetite. 2011;57(3):711–717. doi: 10.1016/j.appet.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Davis C, Zai C, Levitan RD, Kaplan AS, Carter JC, Reid-Westoby C, Kennedy JL. Opiates, overeating and obesity: a psychogenetic analysis. Int J Obes (Lond) 2011;35(10):1347–1354. doi: 10.1038/ijo.2010.276. [DOI] [PubMed] [Google Scholar]
- Garber AK, Lustig RH. Is fast food addictive? Curr Drug Abuse Rev. 2011;4(3):146–162. doi: 10.2174/1874473711104030146. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13(9):372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive Behaviors. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Joyce PR, Ellis MJ, Evans MJ, Livesey JH, Donald RA. The effects of alcoholism on the hypothalamic-pituitary-adrenal axis: interaction with endogenous opioid peptides. Clin Endocrinol (Oxf) 1995;43(3):283–290. doi: 10.1111/j.1365-2265.1995.tb02033.x. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living. Dell Publishing; New York: 1990. [Google Scholar]
- Katsiki N, Hatzitolios AI, Mikhailidis DP. Naltrexone sustained-release (SR) + bupropion SR combination therapy for the treatment of obesity: ‘a new kid on the block’? Ann Med. 2011;43(4):249–258. doi: 10.3109/07853890.2010.541490. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18(9):2592–2598. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- Kemper A, Koalick F, Thiele H, Retzow A, Rathsack R, Nickel B. Cortisol and beta-endorphin response in alcoholics and alcohol abusers following a high naloxone dosage. Drug Alcohol Depend. 1990;25(3):319–326. doi: 10.1016/0376-8716(90)90158-b. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: Preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002a;26:778–788. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002b;26(6):778–788. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- Kristeller J, Hallett C. An exploratory study of a meditation-based intervention for binge eating disorder. Journal of Health Psychology. 1999a;4:357–363. doi: 10.1177/135910539900400305. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, Hallett CB. An Exploratory Study of a Meditation-based Intervention for Binge Eating Disorder. J Health Psychol. 1999b;4(3):357–363. doi: 10.1177/135910539900400305. [DOI] [PubMed] [Google Scholar]
- Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eat Disord. 2011;19(1):49–61. doi: 10.1080/10640266.2011.533605. [DOI] [PubMed] [Google Scholar]
- Leon GR, Fulkerson JA, Perry CL, Early-Zald MB. Prospective analysis of personality and behavioral vulnerabilities and gender influences in the later development of disordered eating. J Abnorm Psychol. 1995;104(1):140–149. doi: 10.1037//0021-843x.104.1.140. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012;37(12):1922–1928. doi: 10.1016/j.psyneuen.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav. 2007;91(4):432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Kral TV. Stress-induced eating in restrained eaters may not be caused by stress or restraint. Appetite. 2006;46(1):16–21. doi: 10.1016/j.appet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Mehling WE, Gopisetty V, Daubenmier J, Price CJ, Hecht FM, Stewart A. Body awareness: construct and self-report measures. PLoS ONE. 2009;4(5):e5614. doi: 10.1371/journal.pone.0005614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C, Tandon R. Should overeating and obesity be classified as an addictive disorder in DSM-5? Curr Pharm Des. 2011;17(12):1128–1131. doi: 10.2174/138161211795656701. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214(5-6):435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwens MA, van Strien T, van Leeuwe JF, van der Staak CP. The dual pathway model of overeating. Replication and extension with actual food consumption. Appetite. 2009;52(1):234–237. doi: 10.1016/j.appet.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav. 2009;94(1):1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Childs E, Epstein AM, King AC. Acute HPA axis response to naltrexone differs in female vs. male smokers. Psychoneuroendocrinology. 2010;35(4):596–606. doi: 10.1016/j.psyneuen.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Pistell PJ, Phifer CB, Berthoud HR. Reversible suppression of food reward behavior by chronic mu-opioid receptor antagonism in the nucleus accumbens. Neuroscience. 2010;170(2):580–588. doi: 10.1016/j.neuroscience.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124(2):134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Sprenger T, Berthele A, Platzer S, Boecker H, Tolle TR. What to learn from in vivo opioidergic brain imaging? Eur J Pain. 2005;9(2):117–121. doi: 10.1016/j.ejpain.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Rudoy C, Saunders A, Liu XB, Van Bockstaele EJ. Corticotropin-releasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience. 2001;106(2):375–384. doi: 10.1016/s0306-4522(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters J, Bergersm GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and Drug Reward: Overlapping Circuits in Human Obesity and Addiction. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- Wallis DJ, Hetherington MM. Stress and eating: the effects of ego-threat and cognitive demand on food intake in restrained and emotional eaters. Appetite. 2004;43(1):39–46. doi: 10.1016/j.appet.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M, Giggey P. Adrenocortical responses and family history of alcoholism. Alcohol Clin Exp Res. 1999;23(7):1185–1190. [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55(12):1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Gotjen D, Reynolds J, Lee S. Confirmation that offspring from families with alcohol dependent individuals have greater HPA axis activation-induced by naloxone compared to offspring without a family history of alcohol dependence. Alcohol Clin Exp Res. 2001;25:1134–1139. [PubMed] [Google Scholar]
- Wand GS, Weerts EM, Kuwabara H, Frost JJ, Xu X, McCaul ME. Naloxone-induced cortisol predicts mu opioid receptor binding potential in specific brain regions of healthy subjects. Psychoneuroendocrinology. 2011;36(10):1453–1459. doi: 10.1016/j.psyneuen.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Weerts EM, Kuwabara H, Wong DF, Xu X, McCaul ME. The relationship between naloxone-induced cortisol and delta opioid receptor availability in mesolimbic structures is disrupted in alcohol-dependent subjects. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2011.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima F, Suda T, Tomori N, Sumitomo T, Nakagami Y, Ushiyama T, Shizume K. Effects of opioid peptides on immunoreactive corticotropin-releasing factor release from the rat hypothalamus in vitro. Life Sci. 1986;39(2):181–186. doi: 10.1016/0024-3205(86)90453-4. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26(6):713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci. 2012;13(4):279–286. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obes Rev. 2013;14(1):19–28. doi: 10.1111/j.1467-789X.2012.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]