Abstract
Although antibiotic treatment for Lyme disease is effective in the majority of cases, especially during the early phase of the disease, a minority of patients suffer from post-treatment Lyme disease syndrome (PTLDS). It is unclear what mechanisms drive this problem, and although slow or ineffective killing of Borrelia burgdorferi has been suggested as an explanation, there is a lack of evidence that viable organisms are present in PTLDS. Although not a clinical surrogate, insight may be gained by examining stationary-phase in vitro Borrelia burgdorferi persisters that survive treatment with the antibiotics doxycycline and amoxicillin. To identify drug candidates that can eliminate B. burgdorferi persisters more effectively, we screened an Food and Drug Administration (FDA)-approved drug library consisting of 1524 compounds against stationary-phase B. burgdorferi by using a newly developed high throughput SYBR Green I/propidium iodide (PI) assay. We identified 165 agents approved for use in other disease conditions that had more activity than doxycycline and amoxicillin against B. burgdorferi persisters. The top 27 drug candidates from the 165 hits were confirmed to have higher anti-persister activity than the current frontline antibiotics. Among the top 27 confirmed drug candidates from the 165 hits, daptomycin, clofazimine, carbomycin, sulfa drugs (e.g., sulfamethoxazole), and certain cephalosporins (e.g. cefoperazone) had the highest anti-persister activity. In addition, some drug candidates, such as daptomycin and clofazimine (which had the highest activity against non-growing persisters), had relatively poor activity or a high minimal inhibitory concentration (MIC) against growing B. burgdorferi. Our findings may have implications for the development of a more effective treatment for Lyme disease and for the relief of long-term symptoms that afflict some Lyme disease patients.
Keywords: Borrelia burgdorferi, drug discovery, FDA approved drug library, persisters, SYBR Green I
INTRODUCTION
Lyme disease is a multisystem disease that is caused by Borrelia burgdorferi sensu stricto in North America. The infection is transmitted to humans by tick vectors that normally feed upon rodents, reptiles, birds and deer.1 In 2012, Lyme disease was the most common vector-borne infection in the United States, with 22 014 confirmed and 8817 probable cases, although many cases are unreported to US state health departments.2
Antibiotic treatment is effective in the majority of Lyme disease cases. However, according to the Centers for Disease Control (CDC), approximately 10%–20% of patients treated for Lyme disease with the recommended 2–4 week antibiotic therapy still have lingering symptoms of fatigue, pain, or joint and muscle aches.3 In some cases, these symptoms can continue for 6 months or more after the initial diagnosis and treatment, a condition that is referred to as “post-treatment Lyme disease syndrome” (PTLDS). The actual numbers of PTLDS cases is unknown, but recruitment of these patients for clinical trials has been difficult.4,5,6 A greater percentage of patients experience symptoms that slowly resolve during the first few months after therapy.7 Why symptoms such as fatigue, musculoskeletal pain and subjective neurocognitive dysfunction slowly resolve or remain in certain patients is unclear. Considerations may include persisting immunological responses that may be independent of continued infection or possibly driven by the continued presence of antigenic debris,8 as well as persisting organisms. The question of whether B. burgdorferi may persist in some patients after antibiotic therapy and further evade host immune clearance has been raised by some researchers, but the idea is controversial.9,10
Although animals do not experience symptoms that might be judged to be PTLDS, in various animal models (mice, dogs and rhesus macaque monkeys), antibiotic therapy with doxycycline, ceftriaxone or tigecycline has not fully eradicated B. burgdorferi, as determined by methods including xenodiagnosis, although viable organisms have not been able to be cultured in conventional culture media.11,12,13,14 Others have raised concerns about such findings, including the use of high concentration inocula and the use of stationary-phase organisms for infection, insufficient antibiotic dosing and other methodological issues, including concerns that rodents are a natural reservoir of B. burgdorferi and that studies of persistence would not approximate human infections.15,16 Although a number of prospective, randomized clinical studies have demonstrated no significant beneficial effect of additional antibiotic therapy with conventionally employed antibiotic monotherapy and no evidence of the continued presence of B. burgdorferi in patients with long-term symptoms,6,17 other trials have reported improved fatigue symptoms after prolonged intravenous ceftriaxone treatment.18 Intriguingly, a recent study in humans demonstrated the recovery of B. burgdorferi DNA by xenodiagnoses in a patient with PTLDS despite antibiotic treatment.19 In addition, a recent mouse study observed a resurgence of B. burgdorferi DNA after 12 months treatment with Lyme antibiotics, and the RNA transcription of multiple B. burgdorferi genes was detected in mouse tissues despite a non-culturable state.13
Findings that suggest the continued presence of B. burgdorferi in some form indicate that current Lyme disease treatment may not sufficiently eliminate B. burgdorferi persisters or that the immune system fails to clear persisting organisms or bacterial debris, which may be the underlying cause for those who suffer from unresolved Lyme disease symptoms. These factors may also be responsible for antibiotic-refractory arthritis, as suggested in a murine model in which spirochetal antigens appeared to persist around cartilage.8 To date, there is no effective antibiotic treatment or preventative strategy for those who suffer from persistent symptoms after contracting Lyme disease.
Some experimental studies have observed at least three morphologic forms of persistent B. burgdorferi: spirochete, spheroplast (or L-form), and cystic or round-body forms.10,20,21 There have been reports of spheroplast or cystic forms in humans, but it is unclear whether such morphologic variants exist with any frequency in vivo, and no study has yet evaluated a link with clinical disease or determined the effect of antibiotic treatment in humans.22 These morphological variants have altered antibiotic susceptibilities.23 Frontline drugs such as doxycycline and amoxicillin kill the replicating spirochetal form of B. burgdorferi quite effectively, but they exhibit little activity against non-replicating persisters that are enriched in the stationary phase or in biofilm-like aggregates of B. burgdorferi.23 Although some antibiotics have been tested for their activity against B. burgdorferi, the full spectrum of antibiotic susceptibility for B. burgdorferi has not been determined.24 In addition, there has been no study to systematically identify or assess drugs targeting B. burgdorferi persisters.
Because Food and Drug Administration (FDA)-approved drugs have relatively clear safety and pharmacokinetic profiles in patients, a study examining whether existing drugs effectively eliminate Borrelia burgdorferi may lead to quicker implementation than the development of novel compounds. We recently developed a new SYBR Green I/propidium iodide (PI) assay for rapid viability assessment of B. burgdorferi in a 96-well plate format that is superior to the current commercially available LIVE/DEAD BacLight viability assay (Feng et al., unpublished data). Using this new assay, we screened an FDA-approved drug library on stationary-phase B. burgdorferi persisters and identified a number of interesting drug candidates that have excellent activity against in vitro B. burgdorferi persisters.
MATERIALS AND METHODS
Bacterial strain, media and culture
Borrelia burgdorferi strain B31 (ATCC 35210) was obtained from the American Type Tissue Collection (Manassas, VA, USA). B. burgdorferi was cultured in BSK-H medium (HiMedia Laboratories Pvt. Ltd., Mumbai, India) with 6% rabbit serum (Sigma-Aldrich, St. Louis, MO, USA). All culture media were filter-sterilized using a 0.2 µm filter. Cultures were incubated in sterile 50 mL closed conical tubes (BD Biosciences, CA, USA) at 33 °C without antibiotics. After 6–7 days, the B. burgdorferi reached stationary phase in the culture system (Figure 1A). Then, 7-day-old stationary-phase B. burgdorferi cultures were transferred to 96-well tissue culture microplates for drug screening.
Figure 1.
(A) Growth curve of B. burgdorferi strain B31 in vitro. (B) Representative images of the log phase (3-day culture) and stationary phase of B. burgdorferi B31 strain (7-day culture), observed with fluorescent microscopy using the SYBR Green I/PI stain (×400 magnification). The arrows indicate multiple morphological forms of B. burgdorferi in stationary phase.
Microscopy techniques
Specimens were examined using a Nikon Eclipse E800 microscope equipped with differential interference contrast and epifluorescent illumination and recorded with a SPOT slider color camera. Cell proliferation assays were performed by direct counting using a bacterial counting chamber (Hausser Scientific Partnership, PA, USA) and differential interference contrast microscopy. To assay the viability of B. burgdorferi, the SYBR Green I/PI assay or LIVE/DEAD BacLight bacterial viability assay was performed. The ratio of live (green) and dead (red) B. burgdorferi was calculated by counting these cells using a bacterial counting chamber and epifluorescence microscopy.
Antibiotics and the FDA drug library
Antibiotics, including doxycycline, amoxicillin, metronidazole, clofazimine, and sulfamethoxazole (SMX), were purchased from Sigma and dissolved in appropriate solvents25 to form stock solutions. All antibiotic stocks were filter-sterilized using a 0.2 µm filter.
The FDA-approved drugs were assembled according to the Johns Hopkins Clinical Compound Library (JHCCL) version 1.3.26 The FDA drug library was prepared as 10 mM stock solutions in dimethyl sulfoxide and was arrayed in a total of 24 96-well plates, leaving the first and last columns in each plate for controls. Each drug solution in these master plates was diluted with phosphate buffer solution to produce 500 µM pre-diluted plates. The first and last columns in each pre-diluted plate included a blank control, doxycycline control, and amoxicillin control. The pre-diluted drug plates were sealed and stored at −20°C.
Screening of FDA-approved drug library in the B. burgdorferi stationary-phase persister model
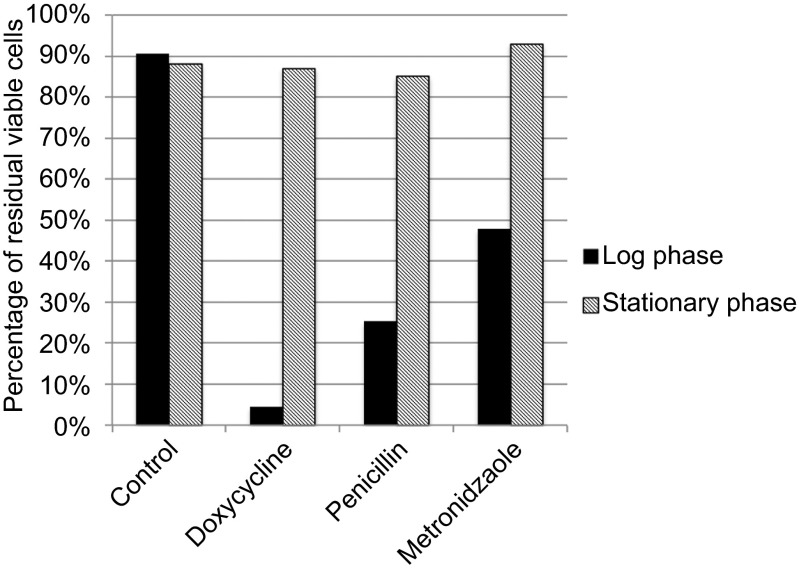
In our preliminary studies, we determined that stationary-phase B. burgdorferi were refractory to killing by the frontline drugs, doxycycline or amoxicillin (Figure 2) and could thus serve as a persister model for drug screens. To qualitatively determine the effect of FDA-approved drugs on B. burgdorferi persisters, each compound (10 µL) from the pre-diluted stock was added to 7-day-old B. burgdorferi stationary-phase culture in the screening plate. The final volume per well was adjusted to 100 µL to achieve a final drug library concentration of 50 µM in the drug screen. The plates were sealed and placed in a 33°C incubator for 7 days.
Figure 2.
Susceptibility of log phase (3 days) and stationary-phase (7 days) B. burgdorferi to 50 µM drugs after a 5-day treatment. The percentages of residual live cells were determined using the SYBR Green I/PI assay.
To assay live and dead cells in the screening plates, a SYBR Green I/PI assay was used as described in our previous study (Feng et al., unpublished data). Briefly, SYBR Green I (10 000×stock, Invitrogen, Grand Island, NY, USA) (10 µL) was mixed with 30 µL propidium iodide (20 mM, Sigma) into 1.0 mL of sterilized distilled water. Staining mixture (10 µL) was added to each well and mixed thoroughly. The plates were incubated in the dark for 15 min at room temperature. With the excitation wavelength set at 485 nm, the fluorescence intensities at 535 nm (green emission) and 635 nm (red emission) were measured for each well of the screening plate using an HTS 7000 plus Bio Assay Reader (PerkinElmer Inc., Waltham, MA, USA). Meanwhile, B. burgdorferi suspensions (live and 70% isopropyl alcohol killed) at five different proportions of live: dead cells (0:10, 2:8, 5:5, 8:2, 10:0) were mixed and added to the wells of the 96-well plate. Then, SYBR Green I/PI reagent was added to each of the five samples, and the green/red fluorescence ratios for each proportion of live/dead B. burgdorferi were measured using the HTS 7000 plus as above. Using least-square fitting analysis, the regression equation and regression curve of the relationship between percentage of live bacteria and green/red fluorescence ratios were obtained. The regression equation was used to calculate the percentage of live cells in each well of the screening plate. Based on the green fluorescence to red fluorescence ratio, we determined that the SYBR Green I/PI assay is superior to all other assays for measuring the viability of B. burgdorferi in terms of reliability, sensitivity, and speed. The BSK-H medium, which produced a high background for the BacLight viability assay, did not affect the SYBR Green I/PI assay, and the viability of B. burgdorferi cultures could be directly measured using a microtiter plate reader. Thus, the SYBR Green I/PI assay provides a convenient and more sensitive assay for evaluating the antibiotic susceptibility of B. burgdorferi than other currently used viability assays, such as the LIVE/DEAD BacLight method, and it can be used for high-throughput screening of new drugs. Some effective candidates were further confirmed by epifluorescence microscope counting.
Minimum inhibitory concentration determination
The standard microdilution method was used to determine the antibiotic minimum inhibitory concentration (MIC) that would inhibit visible growth of B. burgdorferi after a 72 h incubation period.23,27,28 B. burgdorferi cells (1×105) were inoculated into each well of a 96-well microplate containing 90 µL fresh BSK-H medium per well. Each diluted antibiotic (10 µL) was added to the culture. All experiments were run in triplicate. The 96-well plate was sealed and placed in an incubator at 33°C for 5 days. Cell proliferation was assessed using the SYBR Green I/PI assay and a bacterial counting chamber after the incubation.
RESULTS
Morphology of B. burgdorferi during different growth phases
B. burgdorferi was grown in BSK-H medium for up to 11 days, and cell numbers were determined by microscopy at various time points (Figure 1A). Based on the cell number increase, 2–5-day cultures were considered log-phase cultures, whereas 6–11-day cultures were considered stationary phase because B. burgdorferi growth reached its peak (5×107 spirochetes/mL) after 5–6 days, and the cell density remained relatively constant from 6–11 days of incubation (Figure 1A). Multiple morphological forms of B. burgdorferi, i.e., a spirochetal form, round bodies (cysts), and biofilms, have been observed and have different antibiotic susceptibilities.20,23 We observed that B. burgdorferi cultures were primarily in the spirochetal form during the log phase (Figure 1B, left panel), but variant forms such as coccoid or round-body forms and micro-colonies were significantly more abundant in stationary-phase cultures (Figure 1B, right panel).
Stationary-phase B. burgdorferi bacteria are tolerant to antibiotics and are used as a persister model for drug screens
Previous studies and clinical experiences have demonstrated that high doses of doxycycline and amoxicillin used for Lyme disease treatment exhibit bactericidal activity against B. burgdorferi.24 These antibiotics do not kill the cystic or round-body forms of B. burgdorferi, but metronidazole does have activity against the cystic form of B. burgdorferi.29 Here, we tested the efficacy of commonly used drugs (doxycycline, amoxicillin and metronidazole) against log phase and stationary-phase B. burgdorferi and evaluated their susceptibility using the SYBR Green I/PI assay, as described in the MATERIALS AND METHODS section. The results demonstrated that the current antibiotics, doxycycline and amoxicillin, were highly active against log-phase B. burgdorferi but had little activity against stationary-phase B. burgdorferi (Figure 2). Metronidazole had some activity against log-phase B. burgdorferi but had little activity against stationary-phase B. burgdorferi (Figure 2). These findings suggest that the current antibiotics used to treat Lyme disease would have little or no activity on B. burgdorferi persisters, if existing, in vivo. Thus, we chose the B. burgdorferi stationary-phase culture at 7 days as a persister model to screen for drugs targeting B. burgdorferi persisters as described below.
FDA drug library screen to identify drugs that are effective against dormant B. burgdorferi persisters
In our previous study, we observed that the number of green and red fluorescent organisms counted by microscopy correlated well with the number obtained by the SYBR Green I/PI plate assay, which can be used as a high-throughput screening method for rapid viability assessment of B. burgdorferi (Feng et al., unpublished data). To identify drugs that have activity against B. burgdorferi persisters, we used stationary-phase B. burgdorferi as a persistence model (see above section) to screen an FDA-approved drug library. The currently used Lyme disease treatment antibiotics, doxycycline and amoxicillin, were included in each plate as control drugs. Consistent with the above results (Figure 2), doxycycline and amoxicillin had poor activity against stationary-phase B. burgdorferi persisters, and wells treated with each of these two antibiotics contained 75% and 76% viable stationary cells, respectively, compared with 93% viable cells in the drug-free control (Table 1). Of the 1524 drugs in the FDA-approved drug library tested, 165 had higher activity against B. burgdorferi persisters than doxycycline and amoxicillin.
Table 1. Activity of top 27 active hits with better activity than the current Lyme disease antibiotics against stationary-phase B. burgdorferi persisters a.
| Drugs (50 μM) | Residual viable cellsb | Residual viable cellsc | Ratio of green/red fluoresce | |||
|---|---|---|---|---|---|---|
| Primary screening | Rescreening | Rescreening | P-valued | |||
| Control | 93% | 94% | 8.67 | 8.38 | 8.59 | - |
| Amoxicilline | 76% | 76% | 7.98 | 7.86 | 7.82 | 1.000000 |
| Doxycyclinee | 75% | 67% | 7.62 | 7.35 | 7.58 | 0.233596 |
| Penicillin Ge | 75% | 68% | 7.41 | 7.68 | 7.92 | 0.699416 |
| Tetracyclinee | 54% | 50% | 7.59 | 6.14 | 7.18 | 0.102366 |
| Ceftriaxonee | 50% | 44% | 6.74 | 6.89 | 6.78 | 0.000182 |
| Cefuroximee | 49% | 43% | 6.59 | 6.84 | 6.67 | 0.000317 |
| Clarithromycine | 70% | 65% | 7.70 | 7.36 | 7.59 | 0.038775 |
| Azithromycine | 77% | 80% | 8.33 | 8.10 | 7.92 | 0.071492 |
| Daptomycin | 35% | 28% | 6.10 | 6.20 | 6.09 | 0.000008 |
| Clofazimine | 45% | 32% | 6.56 | 6.23 | 6.02 | 0.000599 |
| Cefoperazone | 37% | 34% | 6.54 | 6.32 | 6.23 | 0.000126 |
| Carbomycin | 41% | 37% | 6.37 | 6.81 | 6.32 | 0.001045 |
| Vancomycin | 48% | 38% | 6.65 | 6.58 | 6.37 | 0.000152 |
| Cephalothin | 49% | 40% | 6.74 | 6.49 | 6.55 | 0.000133 |
| Cefotiam | 42% | 43% | 6.41 | 7.55 | 6.21 | 0.000503 |
| Cefmetazole | - | 43% | 6.80 | 7.38 | 6.00 | 0.045064 |
| Cefepime | - | 44% | 6.67 | 7.16 | 6.45 | 0.006368 |
| Amodiaquin | - | 45% | 6.79 | 6.44 | 6.85 | 0.000946 |
| Streptomycin | - | 45% | 6.72 | 6.93 | 6.76 | 0.000175 |
| Ticarcillin | - | 46% | 6.82 | 6.72 | 6.93 | 0.000163 |
| Cefonicid | - | 46% | 6.86 | 7.54 | 6.07 | 0.067661 |
| Piperacillin-tazobactam | 47% | 47% | 7.18 | 6.47 | 6.98 | 0.009594 |
| Cefdinir | - | 48% | 6.88 | 7.51 | 6.29 | 0.049107 |
| Ceforanide | - | 48% | 6.89 | 7.49 | 6.33 | 0.043847 |
| Cefmenoxime | - | 48% | 6.82 | 7.59 | 6.32 | 0.058674 |
| Bismuth | - | 48% | 6.94 | 6.82 | 6.92 | 0.000082 |
| Ceftizoxime | - | 49% | 6.94 | 6.83 | 7.03 | 0.000223 |
| Ceftibuten | 51% | 49% | 6.81 | 6.78 | 7.27 | 0.004888 |
| Amphotericin B | - | 50% | 7.14 | 6.88 | 6.87 | 0.000783 |
| Cefamandole | - | 50% | 6.71 | 7.73 | 6.52 | 0.076304 |
| Quinine hydrobromide | - | 50% | 7.00 | 6.85 | 6.88 | 0.000124 |
| Cyclacillin | 51% | 53% | 6.81 | 6.88 | 7.64 | 0.045210 |
| Colistin | 50% | 54% | 7.15 | 7.26 | 7.23 | 0.000319 |
| Sulfameter | 54% | 7.13 | 7.46 | 6.98 | 0.009635 | |
| Tigecycline | 58% | 51% | 6.98 | 7.06 | 6.96 | 0.001557 |
Stationary-phase B. burgdorferi (7-day old) cells were treated with drugs for 7 days. The line above clarithromycin refers to antibiotics used to treat Lyme disease.
Residual viable B. burgdorferi was assayed by epifluorescence microscope counting.
Residual viable B. burgdorferi was calculated according to the regression equation and ratio of Green/Red fluorescence obtained by SYBR Green I/PI assay.
P-values of the standard t-test for the treated group versus a control group treated with amoxicillin, which is known to have poor activity against stationary-phase persisters.
Currently recommended antibiotics for Lyme disease.5
Based on the results of the primary screen, we selected some active candidates for rescreening using the SYBR Green I/PI assay and microscope counting. Microscope counting further validated the effective drug candidates identified using the SYBR Green I/PI assay with good overall agreement, as the largest difference was less than 20%. From the rescreens and confirmation by microscopy, we were able to confirm the 27 top active hits that had a significant difference in anti-persister activity over the current Lyme disease antibiotic amoxicillin (P<0.05) (Table 1). The top 27 hits remained the same when doxycycline was used as a control drug for comparison.
We identified several FDA-approved drugs that had good activity against stationary-phase B. burgdorferi. The anti-persister activities of some drugs were significantly higher than the frontline antibiotics, doxycycline and amoxicillin (Table 1). For example, daptomycin, clofazimine, carbomycin and some cephalosporin antibiotics (such as cefoperazone, cephalothin, cefotiam and cefuroxime) had among the highest activities against stationary-phase B. burgdorferi persisters. Antimalarial antibiotics(amodiaquine and quinine), aminoglycoside streptomycin, bismuth, tetracycline, and sulfa drugs also had relatively high activity against B. burgdorferi persisters (Table 1). We also included the currently used Lyme disease treatment antibiotics for comparison with the new active hits. It is interesting to note that cephalosporin antibiotics, ceftriaxone and cefuroxime, and tigecycline had some activity against persisters, but their anti-persister activities were not as strong as cefoperazone, daptomycin, clofazimine or carbomycin (Table 1). Doxycycline, amoxicillin, penicillin G, macrolide antibiotics, azithromycin and clarithromycin had relatively poor activity against B. burgdorferi persisters (Table 1).
Although most drugs did not affect the SYBR Green I/PI assay, some colored compounds caused interference in the SYBR Green I/PI assay. For example, pyrvinium pamoate and doxorubicin were identified by the SYBR Green I/PI assay as having activity, but microscopic counting proved otherwise. We found that these red compounds contributed to the background, causing false positive results. Thus, validation by other methods, such as microscopic counting, is necessary to confirm the SYBR Green I/PI data.
Relationship between MIC values and anti-persister activity
Antibiotics that have good activity against growing bacteria (a low MIC) may not have good activity against non-replicating persisters, and vice versa.30 However, some antibiotics, such as the new tuberculosis drug candidates TMC207 and PA-824, have good activity for growing bacteria and non-growing persisters.31 Thus, we sought to determine the MICs of some antibiotics with good anti-persister activity against B. burgdorferi using the new SYBR Green I/PI assay and microscope counting. The results obtained by the two methods had good overall concordance. The MIC values (Table 2) of doxycycline, amoxicillin, vancomycin, and metronidazole were in agreement with previous studies,23,24 and these antibiotics had low activity against B. burgdorferi persisters. We also observed that the macrolide carbomycin, cephalosporins, cefoperazone and cefotiam, and sulfamethoxazole, were highly active against log-phase replicating B. burgdorferi, having low MICs (Table 2) in addition to having good activity for stationary-phase B. burgdorferi persisters. Conversely, daptomycin and clofazimine were less potent against replicating B. burgdorferi, having relatively high MICs, 12.5–25 µg/mL and 6.25 µg/mL, respectively, but had excellent anti-persister activity (Table 2, Figures 3D and 3G). With the exception of clofazimine and metronidazole, the Cmax values of the drug candidates were generally higher than the MIC values (Table 2).
Table 2. Comparison of the MIC values and anti-persister activity of selected antibiotics against B. burgdorferi.
| Antibiotics | MIC (μg/mL) | Cmax (μg/mL)a | Activity against persisters |
|---|---|---|---|
| Doxycycline | ≤0.25 | 3.6–4.6 | − |
| Amoxicillin | ≤0.25 | 1.5–13.8 | − |
| Metronidazole | 25 | 12.5–19.4 | − |
| Daptomycin | 12.5–25 | 57.8–93.9 | ++++ |
| Clofazimine | 6.25 | 0.47–0.7 | +++ |
| Carbomycin | ≤0.25 | 0.625 | +++ |
| Cefoperazone | ≤0.25 | 111–375 | +++ |
| Cefotiam | ≤0.25 | 30–170 | ++ |
| Vancomycin | 0.2–0.4 | 19–23 | + |
| Tazobactam | 12.5 | 14.8–33.8 | − |
| Sulfamethoxazole | ≤0.25 | 46.3 | − |
Cmax values are derived from the literature.
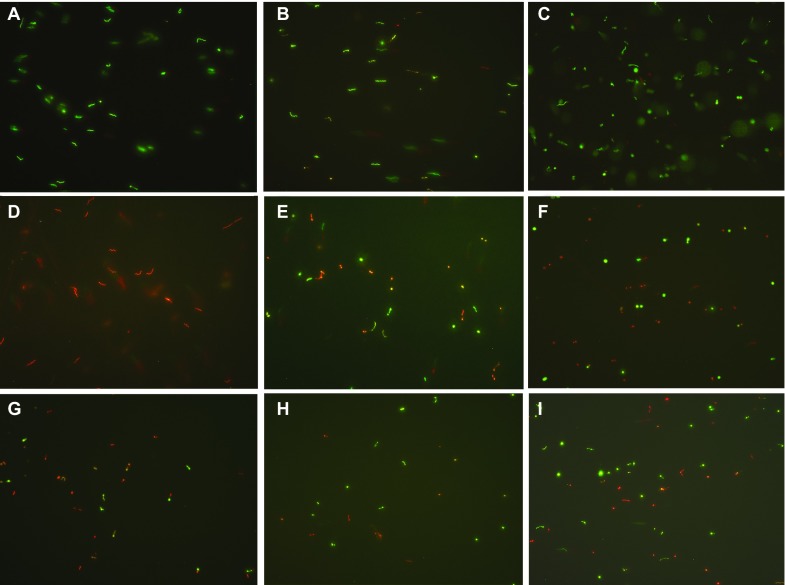
Figure 3.
Representative images of stationary-phase B. burgdorferi strain B31 treated with different antibiotics (50 µM) followed by staining in the SYBR Green I/PI assay (×400 magnification). (A) Drug-free control, (B) Doxycycline, (C) Amoxicillin, (D) Daptomycin, (E) Cefoperazone, (F) Clofazimine, (G) Carbomycin, (H) Cefotiam, and (I) Tetracycline.
DISCUSSION
The current antibiotics used to treat Lyme disease, doxycycline and amoxicillin, have little or no activity against B. burgdorferi persisters in vitro (Table 1, Figure 3). There has been significant interest in identifying drugs that target persisters in general,32 and B. burgdorferi persisters in particular.20 However, because of the technical challenge of the current B. burgdorferi culture system, it has not been possible to apply the high throughput methodology that has been used to identify antibiotics active against persisters of other bacteria to B. burgdorferi. We have recently developed a rapid and convenient SYBR Green I/PI viability assay amenable to high-throughput screening to identify new drugs targeting B. burgdorferi persisters (Feng et al., unpublished data). Using this rapid method, we screened the FDA approved drug library for activity against non-replicating persisters of B. burgdorferi and identified a number of interesting drug candidates that have excellent anti-persister activity (Table 1). These include daptomycin, clofazimine, carbomycin, certain cephalosporins, and some sulfa drugs. Most of the candidate drugs are used for treatment of infections other than Lyme disease and would represent a novel use of old drugs for anti-persister activity.
Daptomycin is a lipopeptide antibiotic that has been used in the treatment of severe infections caused by antibiotic resistant gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE). Daptomycin can disrupt bacterial cell membrane function, including insertion into the membrane, creating pores that allow cells to leak ions, which results in rapid membrane depolarization, loss of membrane potential and bacterial cell death.33 We determined that daptomycin had the highest activity against B. burgdorferi persisters among all the active hits (Table 1, Figure 3D) but had a high MIC (12.5–25 µg/mL) against log-phase organisms (Table 2). The B. burgdorferi stationary-phase cells treated by daptomycin had almost all red fluorescence as spirochetes (Figure 3D). This result indicated the daptomycin could similarly disrupt the cell membrane of B. burgdorferi, causing PI dye to permeate the cell, leading to cell death. Microscope examination revealed spirochetal shaped remnants after daptomycin treatment (Figure 3A), suggesting that the cells were dead and did not change form to coccoid shape, which occurred following cefoperazone and tetracycline treatment (Figures 3E and 3I).
Macrolides and ketolides have been studied in vitro for activity against B. burgdorferi.24 Here, we observed that carbomycin (Table 1, Figure 3G), a 16-membered macrolide, had higher bactericidal activity against stationary-phase B. burgdorferi than the classic macrolides such as azithromycin, clarithromycin, and erythromycin. The MIC data (Table 2) demonstrated that carbomycin was also effective against multiplying B. burgdorferi. Macrolides that can penetrate B. burgdorferi cells may have greater activity than penicillin or ceftriaxone. Carbomycin may be a candidate for further investigation to enhance monotherapy treatment of Lyme disease.
β-Lactam antibiotics such as amoxicillin and cefuroxime are commonly used in the treatment of Lyme disease in clinical settings.24 β-Lactams induce the round-body form of B. burgdorferi by disrupting the synthesis of the peptidoglycan layer of cell walls.20,34 Microscopic examination determined that the round-body forms were the majority of cells in cefoperazone treated stationary-phase B. burgdorferi cultures (Figure 3E). According to the MICs measured by previous studies and in our experiments, all β-lactams had good activity against replicating B. burgdorferi, but the effects of the β-lactam antibiotics differed greatly on stationary-phase cells. Cefoperazone (a third generation cephalosporin) appears to be the best β-lactam antibiotic against stationary-phase B. burgdorferi, followed by some second generation cephalosporins such as cefotiam (Figure 3H), cefmetazole and cefonicid. Consistent with previous studies,24 we also observed that first generation cephalosporins had very limited activity against stationary-phase B. burgdorferi. It is likely that the side chain linked to the β-lactam ring in the cephalosporin may be responsible for the preferential activity of certain cephalosporins against B. burgdorferi persisters. Future structure activity relationship studies are needed to confirm this possibility.
Doxycycline is used clinically as a frontline drug for treating Lyme disease. Doxycycline has low MIC values24 and good activity on multiplying B. burgdorferi. Interestingly, we observed that tetracycline had higher activity against stationary-phase B. burgdorferi persisters than doxycycline (Table 1). This result is consistent with a previous study, which determined that tetracycline is more active than doxycycline in a minimum bactericidal concentration test.35 Consistent with this finding, we observed that most cells in the stationary-phase B. burgdorferi cultures treated with tetracycline had round-body forms (Figure 3I), in contrast to stationary-phase B. burgdorferi cultures treated with doxycycline, which remained in spirochetal form (Figure 3B). B. burgdorferi can form different morphological shapes in the stationary phase or under adverse conditions, and tetracycline may be ineffective against some cell morphologies, such as round bodies (cysts).23
Clofazimine was originally developed for the treatment of tuberculosis and currently is commonly used for the treatment of leprosy.36 Additionally, with the increasing drug-resistant TB problem, it has been used for the treatment of MDR-TB.37 Clofazimine is thought to act through different mechanisms on mycobacteria, including membrane destabilization, the production of reactive oxygen species and the inhibition of membrane energy production.37 Here, we observed that clofazimine was highly active against stationary-phase B. burgdorferi persisters (Figure 3F), although the MIC of clofazimine was relatively high (6.25 µg/mL). The preferential activity of clofazimine against B. burgdorferi persisters may be due to its high lipophilicity and its effects on the membrane. It is of interest to note that clofazimine is known to accumulate in host tissues,37 and this property may allow clofazimine to accumulate to high concentrations and act preferentially against B. burgdorferi persisters if they occur in human tissues. Future studies are needed to test this possibility.
Amphotericin B is a powerful antifungal drug that targets sterols in fungal cell membranes, forming a transmembrane channel that leads to ion leakage.38 Because sterol mainly exists in the membranes of eukaryotes, amphotericin B is not a good agent to control prokaryotes. Because B. burgdorferi is one of the rare prokaryotes that possesses cholesterol and cholesterol glycolipids in its membranes,39 it is not surprising that amphotericin B had some activity against stationary-phase B. burgdorferi persisters (Table 1). Its activity was comparable to colistin, but it was less active than daptomycin (Table 1). Amphotericin B may also target the sterol lipid rafts in the membrane of B. burgdorferi.
In addition, some sulfa drugs such as sulfameter and sulfisoxazole were observed to be effective against stationary-phase B. burgdorferi, while sulfamethoxazole exhibited low MIC values (≤0.2 µg/mL). These effective antibiotics may be evaluated as candidates for further drug combination studies in animal models and potentially for clinical investigations.
The problem of persistent B. burgdorferi infection has been difficult to study for several reasons, including difficulty culturing the persisting organisms after antibiotic treatment; inability of the current antibiotics, doxycycline and amoxicillin, to kill the persister organisms, as demonstrated in various animal models; and a lack of antibiotics that are effective against B. burgdorferi persisters. While concern remains whether PTLDS is due to persisting organisms, identification of antibiotics that have activity against B. burgdorferi persisters we feel should prompt testing of some antibiotic combinations that could impact either persisters if they exist or presence of antigenic debris, and by whatever mechanisms, study whether such an approach may lead to improved clinical outcomes in Lyme disease including Lyme arthritis or patients with PTLDS.
In summary, this study represents the first high throughput screen against B. burgdorferi in vitro persisters and identified a number of interesting FDA-approved drugs that have excellent anti-persister activity. Further studies are needed to evaluate these drug candidates in animal models of B. burgdorferi persistence and determine whether they can break the persistence phenomenon the current Lyme disease antibiotics failed to eliminate. Although the question of B. burgdorferi persistence in humans has been raised, there is no high-quality evidence to support this concept or the idea that additional antibiotic therapy is helpful for patients for PTLDS. Whether earlier resolution of B. burgdorferi infection, either alone or in combination with current Lyme disease antibiotics, will decrease long term symptoms of fatigue or PTLDS is unknown and will require further study.
Acknowledgments
We thank Lyme Research Alliance and LymeDisease.org for support of this work.
References
- Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Lyme disease data. Atlanta: CDC; 2012. Available at http://www.cdc.gov/lyme/stats/index.html (accessed 12 April 2014). [Google Scholar]
- Centers for Disease Control and Prevention Post-Treatment Lyme Disease Syndrome Atlanta; CDC; 2014. Available at: http://www.cdc.gov/lyme/postLDS/index.html (accessed 13 Apirl 2014). [Google Scholar]
- Marques A.Chronic Lyme diseaseIn: Halperin JJ (ed.)Lyme disease: an evidence based approach Oxfordshire: CABI; 2011248–258. [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- Klempner MS, Baker PJ, Shapiro ED, et al. Treatment trials for post-Lyme disease symptoms revisited. Am J Med. 2013;126:665–669. doi: 10.1016/j.amjmed.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski J, Nadelman RB, Sell R, et al. Long-term follow-up of patients with culture-confirmed Lyme disease. Am J Med. 2003;115:91–96. doi: 10.1016/s0002-9343(03)00308-5. [DOI] [PubMed] [Google Scholar]
- Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J Clin Invest. 2012;122:2652–2660. doi: 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E, Feng S, Holden K, Freet KJ, Barthold SW. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob Agents Chemother. 2008;52:1728–1736. doi: 10.1128/AAC.01050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diterich I, Rauter C, Kirschning CJ, Hartung T. Borrelia burgdorferi-induced tolerance as a model of persistence via immunosuppression. Infect Immun. 2003;71:3979–3987. doi: 10.1128/IAI.71.7.3979-3987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Hodzic E, Imai DM, Feng S, Yang X, Luft BJ. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob Agents Chemother. 2010;54:643–651. doi: 10.1128/AAC.00788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers ME, Barthold SW, Borda JT, et al. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One. 2012;7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E, Imai D, Feng S, Barthold SW. Resurgence of Persisting Non-Cultivable Borrelia burgdorferi following Antibiotic Treatment in Mice. PLoS One. 2014;9:e86907. doi: 10.1371/journal.pone.0086907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger RK, Summers BA, Chang YF, Appel MJ. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Clin Microbiol. 1997;35:111–116. doi: 10.1128/jcm.35.1.111-116.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Baker PJ, O'Connell S, Pachner AR, Schwartz I, Shapiro ED. Critical analysis of treatment trials of rhesus macaques infected with Borrelia burgdorferi reveals important flaws in experimental design. Vector Borne Zoonotic Dis. 2012;12:535–538. doi: 10.1089/vbz.2012.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Schwartz I. Antibiotic treatment of animals infected with Borrelia burgdorferi. Clin Microbiol Rev. 2009;22:387–395. doi: 10.1128/CMR.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon BA, Keilp JG, Corbera KM, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. [DOI] [PubMed] [Google Scholar]
- Krupp LB, Hyman LG, Grimson R, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60:1923–1930. doi: 10.1212/01.wnl.0000071227.23769.9e. [DOI] [PubMed] [Google Scholar]
- Marques A, Telford SR, 3rd, Turk SP.et al. Xenodiagnosis to Detect Borrelia burgdorferi Infection: A First-in-Human Study Clin Infect Dis 201458937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson O, Brorson SH, Scythes J, MacAllister J, Wier A, Margulis L. Destruction of spirochete Borrelia burgdorferi round-body propagules (RBs) by the antibiotic tigecycline. Proc Natl Acad Sci USA. 2009;106:18656–18661. doi: 10.1073/pnas.0908236106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Kasas S, Zurn AD, McCall S, Yu S, McGeer PL. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreliosis. J Neuroinflammation. 2008;5:40. doi: 10.1186/1742-2094-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos PM, Auwaerter PG, Wormser GP. A systematic review of Borrelia burgdorferi morphologic variants does not support a role in chronic Lyme disease. Clin Infect Dis. 2014;58:663–671. doi: 10.1093/cid/cit810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapi E, Kaur N, Anyanwu S, et al. Evaluation of in-vitro antibiotic susceptibility of different morphological forms of Borrelia burgdorferi. Infect Drug Resist. 2011;4:97–113. doi: 10.2147/IDR.S19201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunfeld KP, Brade V. Antimicrobial susceptibility of Borrelia burgdorferi sensu lato: what we know, what we don't know, and what we need to know. Wien Klin Wochenschr. 2006;118:659–668. doi: 10.1007/s00508-006-0693-z. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. Wayne: CLSI; 2007. [Google Scholar]
- Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ., Jr A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol. 2006;2:415–416. doi: 10.1038/nchembio806. [DOI] [PubMed] [Google Scholar]
- Dever LL, Jorgensen JH, Barbour AG. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies. J Clin Microbiol. 1992;30:2692–2697. doi: 10.1128/jcm.30.10.2692-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner J, Failing K, Wittenbrink MM. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: influence of test conditions on minimal inhibitory concentration (MIC) values. Zentralbl Bakteriol. 1995;283:49–60. doi: 10.1016/s0934-8840(11)80890-x. [DOI] [PubMed] [Google Scholar]
- Brorson O, Brorson SH. An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to metronidazole. Apmis. 1999;107:566–576. doi: 10.1111/j.1699-0463.1999.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y. The magic bullets and tuberculosis drug targets. Annu Rev Pharmacol Toxicol. 2005;45:529–564. doi: 10.1146/annurev.pharmtox.45.120403.100120. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yew WW, Barer MR. Targeting persisters for tuberculosis control. Antimicrob Agents Chemother. 2012;56:2223–2230. doi: 10.1128/AAC.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Persisters, Persistent Infections and the Yin-Yang Model. Emerg Microbes Infect. 2014;3:e3. doi: 10.1038/emi.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogliano J, Pogliano N, Silverman JA. Daptomycin-mediated reorganization of membrane architecture causes mislocalization of essential cell division proteins. J Bacteriol. 2012;194:4494–4504. doi: 10.1128/JB.00011-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten A, Poitschek C, Rauch S, Aberer E. Effects of penicillin, ceftriaxone, and doxycycline on morphology of Borrelia burgdorferi. Antimicrob Agents Chemother. 1995;39:1127–1133. doi: 10.1128/aac.39.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates L, Hanssen-Hubner C, Norris DE, Richter D, Kraiczy P, Hunfeld KP. Comparison of in vitro activities of tigecycline, doxycycline, and tetracycline against the spirochete Borrelia burgdorferi. Ticks Tick Borne Dis. 2010;1:30–34. doi: 10.1016/j.ttbdis.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Moschella SL. Clofazimine: a review of its medical uses and mechanisms of action. J Am Acad Dermatol. 1995;32:241–247. doi: 10.1016/0190-9622(95)90134-5. [DOI] [PubMed] [Google Scholar]
- Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R. Clofazimine: current status and future prospects. J Antimicrob Chemother. 2012;67:290–298. doi: 10.1093/jac/dkr444. [DOI] [PubMed] [Google Scholar]
- Baginski MCJ. Amphotericin B and its new derivatives - mode of action. Curr Drug Metab. 2009;10:459–469. doi: 10.2174/138920009788898019. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Pathak P, Chiantia S, et al. Proving lipid rafts exist: membrane domains in the prokaryote Borrelia burgdorferi have the same properties as eukaryotic lipid rafts. PLoS Pathog. 2013;9:e1003353. doi: 10.1371/journal.ppat.1003353. [DOI] [PMC free article] [PubMed] [Google Scholar]