Key Points
Heme-bound iron activates placenta growth factor expression in erythroid cells via EKLF, a crucial erythroid-specific transcription factor.
Markers of iron burden predict mortality in adults with sickle cell disease.
Abstract
In adults with sickle cell disease (SCD), markers of iron burden are associated with excessive production of the angiogenic protein placenta growth factor (PlGF) and high estimated pulmonary artery pressure. Enforced PlGF expression in mice stimulates production of the potent vasoconstrictor endothelin-1, producing pulmonary hypertension. We now demonstrate heme-bound iron (hemin) induces PlGF mRNA >200-fold in a dose- and time-dependent fashion. In murine and human erythroid cells, expression of erythroid Krüppel-like factor (EKLF) precedes PlGF, and its enforced expression in human erythroid progenitor cells induces PlGF mRNA. Hemin-induced expression of PlGF is abolished in EKLF-deficient murine erythroid cells but rescued by conditional expression of EKLF. Chromatin immunoprecipitation reveals that EKLF binds to the PlGF promoter region. SCD patients show higher level expression of both EKLF and PlGF mRNA in circulating blood cells, and markers of iron overload are associated with high PlGF and early mortality. Finally, PlGF association with iron burden generalizes to other human diseases of iron overload. Our results demonstrate a specific mechanistic pathway induced by excess iron that is linked in humans with SCD and in mice to markers of vasculopathy and pulmonary hypertension. These trials were registered at www.clinicaltrials.gov as #NCT00007150, #NCT00023296, #NCT00081523, and #NCT00352430.
Introduction
Several clinical trials have demonstrated that catheterization-proven pulmonary arterial hypertension occurs in 4% to 6% of adults with sickle cell disease (SCD), a rate that is 1000- to 3000-fold greater than that in the general population.1-4 However, the mechanism of pulmonary hypertension is not completely understood.
Placenta growth factor (PlGF) is a member of the vascular endothelial growth factor (VEGF) family5-9 and plays important roles under physiological and pathological conditions.10-20 We recently demonstrated that patients with SCD have elevated levels of PlGF and endothelin-1 (ET-1) and that enforced expression of PlGF in mice induces ET-1.21 This causes pulmonary vasoconstriction, right ventricular hypertrophy, and histological evidence of pulmonary hypertension. PlGF is also associated in adults with SCD with high tricuspid regurgitation peak velocity, an echocardiographic marker of risk for high pulmonary artery pressure. We further showed that PlGF levels are significantly correlated with levels of the iron overload markers ferritin and transferrin, which in turn are reproducibly associated with high tricuspid regurgitation peak velocity in patients with SCD.21 These results led us to hypothesize that iron overload increases the expression of PlGF in erythroid cells, an important nonplacental tissue source of PlGF.22 In adults, human erythroblast cells, and not other mature hematopoietic cells, produce PlGF.22 It is known that SCD patients manifest severe chronic hemolytic anemia, stimulating erythroid hyperplasia and providing a basis for increased erythroblast expression of PlGF.
Investigation of iron overload and PlGF expression was undertaken to provide insight to the mechanism of pulmonary hypertension development and the treatment of the disease. In this study, we explore the role of iron-containing hemin stimulating PlGF expression in human erythroid cells. We find that human primary erythroid progenitor cells express EKLF immediately prior to PlGF and that EKLF is required for PlGF expression using EKLF deficient murine erythroid cells. We demonstrate that SCD patients have both elevated EKLF and PlGF and that patients with other known iron overload diseases, especially hereditary hemochromatosis (HH), have higher level of PlGF, which decrease after relief of iron overload. This provides the first evidence linking iron overload and PlGF expression, and pulmonary hypertension, suggesting chelation therapy for iron overload may have a role in reducing the risk of clinical complications such as pulmonary hypertension.
Materials and methods
Materials
Hemin, mesoporphyrin, and Fe(III) mesoporphyrin are from Frontier Scientific (Logan, UT). Anti-PlGF was from Acris (San Diego, CA). Anti-EKLF was from Active Motif (Carlsbad, CA). The PlGF enzyme-linked immunosorbent assay kit was from R & D Systems (Minneapolis, MN). All the other chemicals were purchased from Sigma-Aldrich except where indicated. Oligonucleotides used for mouse erythroid Krüppel-like factor (EKLF) knockout genotyping are 5′-AAGGCCACTTCCAGCTCTTTCGCG, 5′-TTGGAGTAGCTCTTCCCGCAGCCT for wild-type and 5′-AGGGTTGGTGACTGGGCCTTTGGG, 5′-CCGCTATCAGGACATAGCGTTGGCTA for knockout. Oligonucleotide pairs for the human PlGF expression reverse transcriptase-polymerase chain reaction (RT-PCR) are 5′-CGAGTACCCCAGCGAGGTG and 5′-GGAGTCACTGAAGAGTGTGACGG, 5′-AAGATGCCGGTCATGAGGC and 5′-CACACTTCCTGGAAGGGTAC, and 5′-AAGATGCCGGTCATGAGGC and 5′-CCTCTCCAGCGCCCGGCAGTAGCTGCG. Oligonucleotides for human actin are 5′-AGCGAGCATCCCCCAAAGTT and 5′-GGGCACGAAGGCTCATCATT.
The primers for quantitating precipitated DNA from mouse EKLF chromatin immunoprecipitation (ChIP) are 5′-AGGTCTCACCGCCAACCGGCTCTCA and 5′-AGCGATGTGAAAGGATCTTAG, and the probe is 5′-FAM-GTTTCCTTCTCAGACTGCAGCTT-3′-TamSP.
Patient samples
Adult patients ≥18 years of age with the SCD genotype were enrolled in a clinical protocol approved by the Institutional Review Board of the National Heart, Lung and Blood Institute. Thirty patients with HH characterized by the HFE C282Y/C282Y genotype and 10 controls were enrolled on protocol NCT00007150. All subjects provided written informed consent in accordance with the Declaration of Helsinki. Normal erythroid progenitor cultures were prepared from buffy coat cells (leukocytes) harvested as a byproduct of volunteer-donor blood units and were distributed in an anonymized manner. They meet the criteria for exemption from need for informed consent and Institutional Review Board review as defined in 45CFR46, and their distribution abides by all National Institutes of Health guidelines for human subjects research.
Cell culture
K562 human erythroleukemia cells are from the American Type Culture Collection (ATCC, Manassas, VA). K562 cells were grown and maintained in RPMI 1640 medium with 10% fetal bovine serum and 2 mM glutamine at 37°C in an incubator with 5% CO2. The B1 cell is EKLF−/−, and the EK-1 cell is EKLF−/− with inducible expression of the EKLF–estrogen receptor fusion, as described previously.23,24 Both cells were maintained in Dulbecco’s modified Eagle medium with 10% fetal bovine serum and 2 mM glutamine at 37°C, 5% CO2. Erythroid progenitor cells were isolated and cultured in a 2-phase approach, supplemented in the second phase with erythropoietin (EPO; 1 U/mL) and human holo-transferrin (0.3 mg/mL) as previously described.25,26 Erythroid-containing cells were isolated by gradient centrifugation with Ficoll-Hypaque and washed. The isolated cells were cultured by the 2-phase culture method. Where indicated, plasmid DNA was introduced into the cells by electroporation.
Quantitative PCR
RNA was isolated using the RNeasy kit from Qiagen (Valencia, CA) and reverse transcribed to cDNA with a high-capacity cDNA reverse transcription kit (Applied Biosystems). Quantitative PCR was performed with ABI 7900HT (Applied Biosystems). Gene expression assays were from Applied Biosystems: Hs99999903_m1 for human actin, Hs00610592_m1 for human EKLF, Hs00157965_m1 for human HO-1, Hs01119262_m1 for human PlGF, Mm99999915_g1 for mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and Mm00435610_m1 for mouse PlGF. The expression levels were normalized to the reference gene, actin, or GAPDH and expressed relative to the expressions in control samples. The 2−ΔΔCT formula was used for the threshold cycle (CT) calculations: ΔΔCT = [CT(target) − CT(actin or GAPDH)] experimental sample − [CT(target) − CT(actin or GAPDH)] control sample.
Microarray
K562 cells were treated as indicated for 24 hours. Total RNA samples from 4 independent biological replicates were extracted using the RNeasy kit from Qiagen. The microarray assay were performed and analyzed using the Affymetrix microarray platform according to the manufacturer’s recommended protocol and workflow (Santa Clara, CA). The selected genes expression profiles were validated by quantitative PCR. The microarray data were submitted to Gene Expression Omnibus with accession number GSE52087 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=izetsgmsfzgnbot&acc).
ChIP
ChIP was performed using the Upstate ChIP kit (Upstate Biotechnology, Lake Placid, NY). Anti-EKLF antibody 7B2 was obtained from Active Motif (Carlsbad, CA). Ten micrograms of anti-EKLF was included in each immunoprecipitation. Protein A/G agarose beads were from Pierce Biotechnology (Rockford, IL). Briefly, EK-1 cells were induced with 100 nM 4-hydroxytamoxifen for 2.5 hours and cross-linked in 1% formaldehyde at room temperature for 10 minutes with agitation, and the fixation was stopped with 0.125 M glycine for 5 minutes. Activation of PlGF expression was confirmed by real-time PCR with PlGF primers and a fluorescence-labeled probe. The cells were collected at 4°C and washed with cold PBS. The treated cells were sonicated by a Covaris sonicator (S-series SonoLAB single; Covaris, Woburn, MA) for 15 minutes and 15 cycles, at power 8, 5% duty, 4% intensity, and 200 cycles/burst. Lysates were cleared by centrifugation, and the fragmented DNA of 200 to 1000 bp was confirmed by agarose gel electrophoresis after dissociation between proteins and DNA. Chromatin occupancy levels were quantified by real-time PCR using a fluorescence-labeled probe and primers as indicated. The results are relative to a titrated chromatin input standard curve. Primers were verified by showing that each primer pair generated a single amplicon and dissociation curve. PCR was performed with an ABI Prism 7900HT sequence detection system.
Statistical analysis
All statistical analysis was performed using GraphPad Prism software, version 5 (La Jolla, CA), using the Student t test, Mann-Whitney test, linear regression of values, or log-transformed values, depending on the normality of distribution as appropriate. Mortality was analyzed by the log-rank (Mantel-Cox) test and log-rank test for trend. Significance for all analyses was assumed for P < .05.
Results
Iron overload and placenta growth factor in patients with SCD
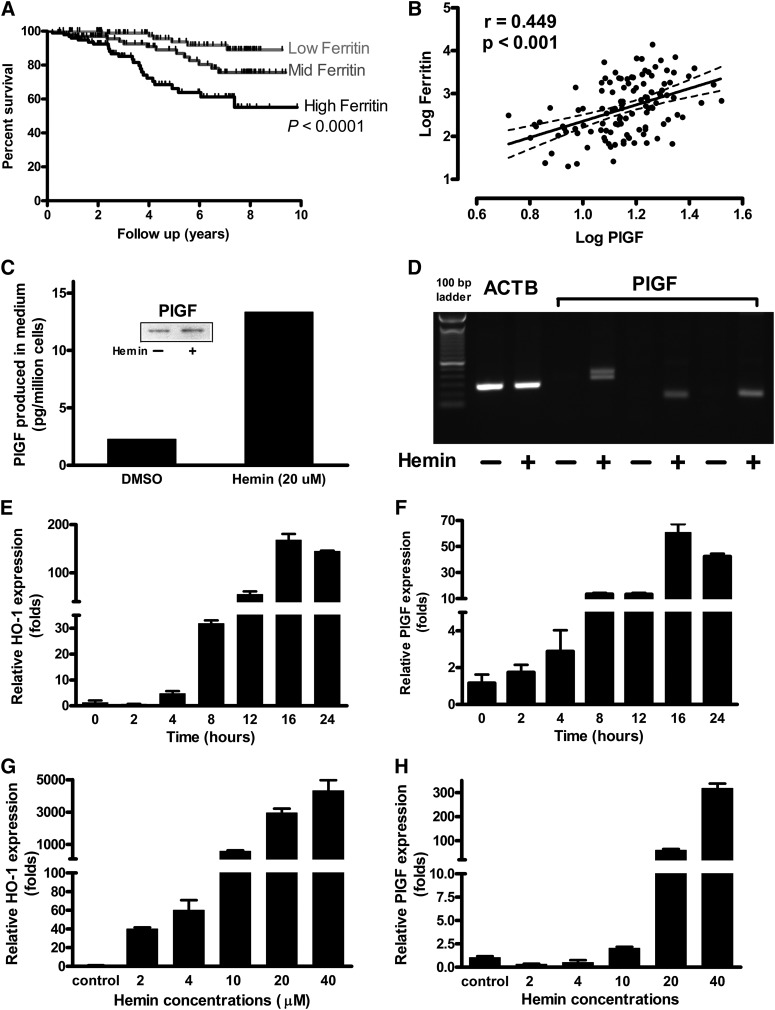
Blood transfusions are rich in heme iron, and patients with inherited hemolytic disorders such as sickle cell anemia who receive repeated transfusions develop iron overload marked by high serum levels of ferritin. We found that this biomarker of iron burden is associated with increased mortality in SCD. Survival data in 279 SCD patients showed significantly higher mortality in the highest ferritin tertile, suggesting a possible adverse effect of iron burden on longevity (P < .0001, log-rank [Mantel-Cox] test; P < .0001, log-rank test for trend; Figure 1A). In these patients with SCD, there is a significant correlation between levels of plasma PlGF protein and serum ferritin (Figure 1B). This led us to hypothesize that iron overload might play a role in regulating expression of PlGF.
Figure 1.
Relationship of iron to PlGF expression and death. (A) Elevated serum ferritin level is associated with higher mortality in SCD by ferritin tertile (n = 279; Kaplan-Meier plot, P < .0001; log-rank [Mantel-Cox] test, P < .0001; log-rank test for trend). (B) PIGF plasma concentration varies as a function of ferritin level, a recognized iron overload marker in SCD patients. (C) K562 cells were incubated with 20 µM hemin for 24 hours, and cells were harvested for immunoblot with anti-PlGF. Equivalent amount of protein from control and tested cell lysate were loaded into 4% to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred onto nitrocellulose membrane, and blotted with antihuman PlGF. PlGF enzyme-linked immunosorbent assay was performed with medium and calculated per million cells. (D) Regular RT-PCR showed that PlGF expression is specifically upregulated by hemin. The oligonucleotides used for PCR span all 7 exons of PlGF mRNA (“Materials and methods”). (E-F) K562 cells were incubated with either indicated concentrations of hemin or same amount of vehicle, and cells were harvested and RNA extracted. cDNA was prepared with use of an Applied Biosciences kit, and quantitative PCR was performed. Time course and concentration dependence of (E,G) heme oxygenase 1 (HO-1) expression as a positive control and (F,H) PlGF expression in response to hemin.
Heme-bound iron stimulates PlGF expression in human erythroid cell line K562
We treated the K562 human erythroblastoid cell line27,28 with 20 µM of hemin, a principal breakdown product of hemoglobin released during intravascular hemolysis in SCD. Hemin stimulated PlGF release from K562 cells into the supernatant to a significantly greater level than vehicle-treated supernatant (Figure 1C). PCR with multiple primers spanning all exons of PlGF (exons 1-7) on reverse-transcribed cDNA show much higher expression of PlGF in hemin-treated vs vehicle-treated K562 cells (Figure 1D), with exon 4 and 7 bands on gel electrophoresis indicating dominant expression of PlGF isoforms 1 (NM_002632.5) and 2 (NM_001207012.1) (397 and 334 bp, respectively). Nucleotide sequencing confirmed the amplification products to originate from PlGF mRNA. We used quantitative PCR on cDNA from hemin-treated K562 cells to analyze expression of PlGF and a positive control gene known to be induced by hemin, hemoxygenase-1 (HO-1). HO-1 transcripts were significantly higher following hemin treatment compared with vehicle controls (P < .01; Figure 1E,G), and showed the expected concentration-dependent and time-dependent response to hemin. PlGF expression responded in a similar manner to hemin stimulation as HO-1 (P < .01; Figure 1F,H). PlGF mRNA expression was induced by 10 µM hemin, with even greater expression after 20 or 40 µM (Figure 1F). The PlGF transcript was detectable within 2 hours of treatment with 20 µM hemin and reached its maximum after a 16-hour incubation (Figure 1H). These results demonstrate that heme-bound iron is capable of inducing PlGF expression in K562 human erythroid cells.
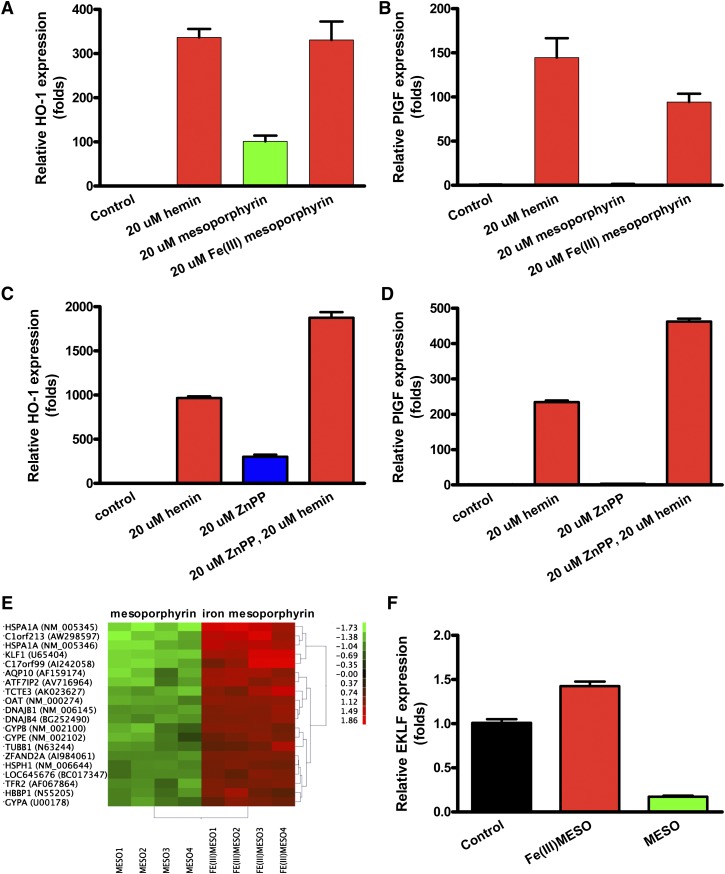
Iron is required for PlGF stimulation
We evaluated the ability of the hemin components protoporphyrin and iron metal ion to induce PlGF expression. Mesoporphyrin stimulated HO-1 mRNA expression both with iron and without iron (P < .0001; Figure 2A), consistent with the published literature. However, mesoporphyrin was able to induce the PlGF transcript only when complexed with iron (Figure 2B). In the absence of iron, mesoporphyrin stimulated HO-1 expression (P < .0001), but was unable to stimulate PlGF expression. Similar studies using hemin (protoporphyrin with iron) vs protoporphyrin alone revealed an identical pattern (data not shown). These results demonstrate that iron is a key factor in PlGF induction. Furthermore, zinc protoporphyrin (ZnPP), a HO-1 enzyme inhibitor, was able to induce HO-1, but not PlGF (Figure 2C-D), indicating that PlGF induction is metal specific.
Figure 2.
Iron is the key factor in promoting PlGF expression. Iron-containing mesoporphyrin, but not mesoporphyrin alone, stimulates PlGF expression in K562 cells. K562 were grown and treated as in Figure 1. (A-B) HO-1 expression and PlGF expression. (C-D) Quantitative PCR results of HO-1 and PlGF expression from K562 cells treated with 20 µM zinc protoporphyrin (ZnPP) or 20 µM hemin or both. Cells were incubated with ZnPP for 30 minutes before hemin was included. (E) Differentially expressed transcripts in the microarray assay. (F) Quantitative PCR of EKLF from K562 cells treated as indicated.
EKLF is regulated by heme-bound iron
To investigate how PlGF expression is regulated, we compared gene expression by microarray assay in K562 cells treated with mesoporphyrin with and without iron, each compared with a dimethylsulfoxide vehicle control. The analysis confirmed increased expression of many genes by iron-mesoporphyrin, such as HO-1 and NAD(P)H dehydrogenase (NM_000903), as previously reported in endothelial cells.29 Interestingly, we found that one of the most highly iron-induced genes was an erythroid developmental transcription factor, EKLF (Figure 2E), known to be crucial to red cell differentiation and globin gene expression.8,18,30-35 These results suggested that EKLF may be involved in the regulation of PlGF expression. Quantitative PCR demonstrated that hemin significantly induced EKLF expression in K562 cells compared with protoporphyrin without iron (P < .001; Figure 2F). Evaluation with Genomatix software online (http://www.genomatix.de/) showed sequences conforming to EKLF consensus binding sites in the PlGF proximal promoter region (1521 bp upstream of the ATG start codon).
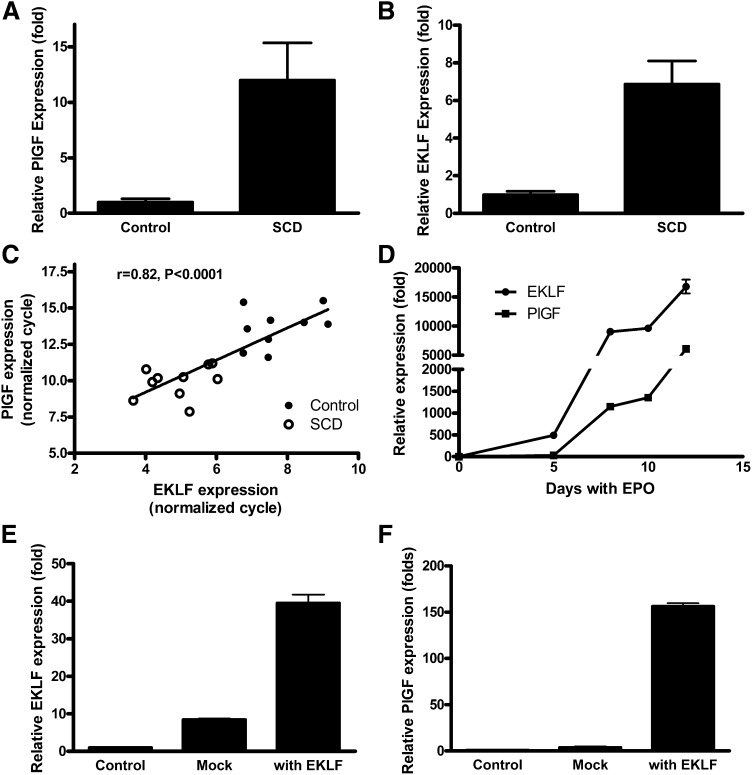
Relationship of EKLF to PlGF expression in human sickle cell blood and in human erythroid cells during differentiation
To investigate the association of EKLF with PlGF expression, we performed quantitative PCR for both factors on cDNA from whole blood of patients with SCD and healthy control subjects. The results revealed that SCD patients have significantly higher level expression of PlGF mRNA compared with healthy controls (P < .0001; Figure 3A), consistent with our previous finding that PlGF protein is higher in SCD plasma than in healthy controls.21 ELKF mRNA expression was also significantly higher (approximately sixfold) in SCD than in control subjects (P < .0001; Figure 3B). The expression of PlGF correlated significantly with EKLF (R = 0.822, P < .0001; Figure 3C). The results are consistent with the higher numbers of erythroid mRNA-containing reticulocytes and nucleated red blood cells in SCD patients than controls and imply a potential role for EKLF in the regulation of PlGF expression.
Figure 3.
PlGF and EKLF are expressed simultaneously at high levels in vivo in patients with SCD and expression of PlGF follows EKLF expression in isolated erythroid cells. (A) PlGF and (B) EKLF expression is significantly higher among SCD patients than healthy controls, and (C) expression of PlGF and EKLF is correlated with each other, indicating that PlGF may be the target of EKLF. (D) Erythroid progenitor cells were cultured by a 2-phase approach and treated with EPO, cells were harvested, and quantitative PCR performed to detect the expression of EKLF and PlGF. The results show that EKLF and PlGF are expressed during erythroid development, with EKLF expression preceding that of PlGF. (E-F) EKLF and PlGF expression in isolated human erythroid progenitor cells. Erythroid progenitor cells were cultured, and then EKLF was transfected by electroporation. Cells were harvested on day 5. Overexpression of EKLF leads to increased PlGF expression in erythroid progenitor cells.
To explore this possibility further, human erythroid progenitor cells were cultured by a 2-phase approach and treated with EPO and iron in the form of holo-transferrin.25,26 Cellular RNA was extracted, and quantitative PCR was performed to assess the sequence of EKLF and PlGF expression during erythroid development. The results demonstrate that EKLF mRNA expression preceded that of PlGF expression during erythroid development, potentially consistent with EKLF induction of PlGF expression (Figure 3D). Transfection of human erythroid progenitor cells with an EKLF expression vector stimulated expression of PlGF mRNA (Figure 3E-F).
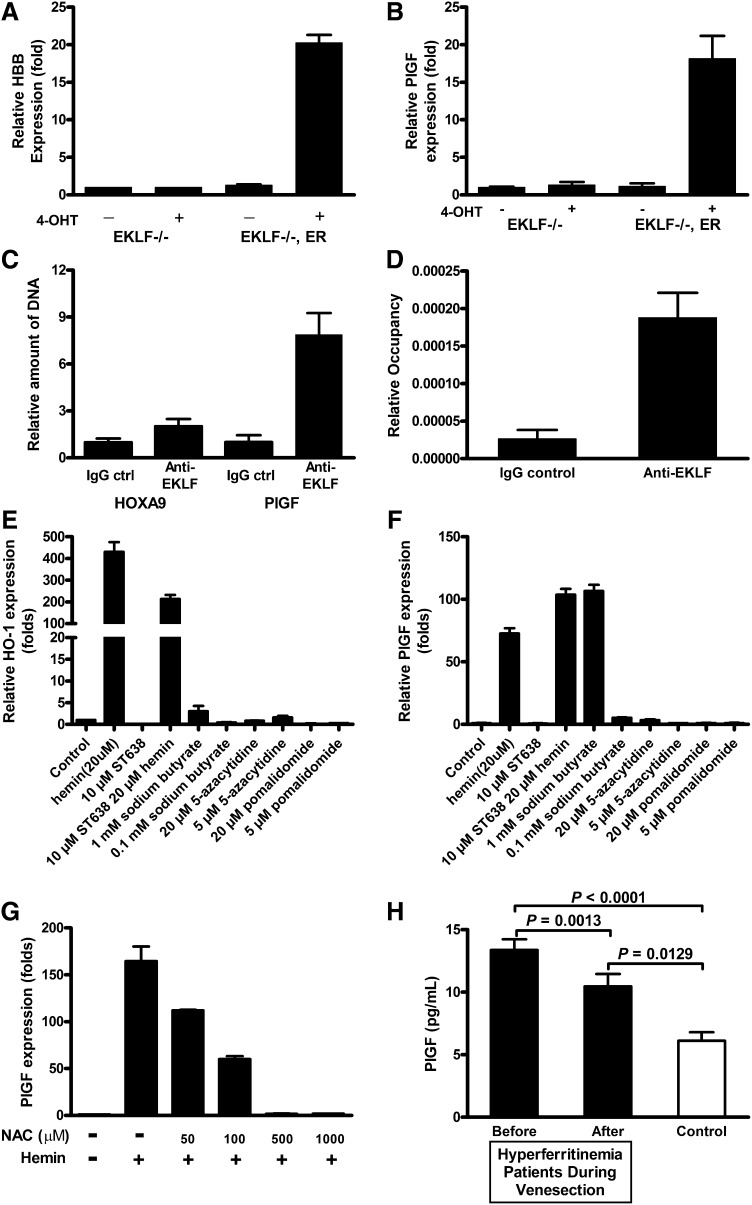
PlGF expression requires EKLF binding to its promoter region
To determine whether EKLF is required for hemin induction of PlGF, we evaluated a murine erythroid cell line genetically deficient in EKLF.30,36 In these EKLF knockout erythroid cells, neither hemin nor 4-hydroxy-tamoxifen (4-OHT) induce mRNA expression of PlGF (Figure 4A-B) or of the known EKLF target β-globin (data not shown). In the same cell line stably transfected with a vector expressing a conditional EKLF-estrogen receptor fusion protein, activation of EKLF activity by 4-OHT induced a β-globin transcript (P < .01; Figure 4A), replicating previously published results, and more importantly, EKLF activated PlGF mRNA expression (P < .01; Figure 4B). This provides strong evidence that EKLF is necessary and sufficient for PlGF expression in these developing murine erythroid cells.
Figure 4.
EKLF is required for PlGF expression, and iron overload is significantly related to mortality. Enforced EKLF expression in EKLF knockout erythroid cells increases (A) hemoglobin and (B) PlGF expression, whereas EKLF knockout erythroid cells are not able to respond in the same manner, suggesting that EKLF is required for PlGF induction. (C-D) Quantitative PCR results from anti-EKLF ChIP. EK-1 cells were treated as described in “Materials and methods.” Immunoprecipitation was carried out with anti-EKLF. After immunoprecipitation, quantitative PCR was performed to determine the amount of DNA precipitated. (E) HO-1 and (F) PlGF expression in K562 with indicated chemicals. ST638 was incubated with K562 for 30 minutes before hemin was included. (G) Hemin-induced PlGF expression was attenuated by NAC treatment. K562 cells were treated with indicated concentrations of NAC 30 minutes before hemin induction. Cells were harvested after 24 hours, RNA was extracted, and quantitative PCR was performed on cDNA preparations. (H) Patients with HH the most common form of iron overload disease, at the time of initial diagnosis have elevated levels of serum ferritin and PlGF (r = 0.241, P = .06, Spearman correlation). When ferritin levels are lowered by phlebotomy therapy, the PlGF concentration significantly decreases (P = .0013).
We established that transcription factor EKLF binds directly to the PlGF promoter directly in the conditional EKLF erythroid cells using a ChIP assay. EKLF expression was induced with 4-OHT, and cross-linked EKLF-DNA complexes were immunoprecipitated with anti-EKLF antibodies. Quantitative PCR on the purified DNA showed that anti-EKLF antibodies precipitated significantly more DNA compared with control immunoglobulin G (7.8-fold; Figure 4C-D). Therefore, the results demonstrated that EKLF is capable of binding directly to the promoter of PlGF in intact erythroid cells. However, the precise mechanism by which hemin promotes EKLF expression and DNA binding is not clear. Sodium butyrate has multiple effects on cultured mammalian cells and inhibits histone deacetylase activity. Our results showed that it is able to increased HO-1 and PlGF expression (Figure 4E-F). This finding may help to explore how hemin promotes PlGF expression. In addition, consistent with a mechanism of PlGF induction involving the oxidative potential of heme-bound iron,37 treatment of K562 cells with the potent antioxidant N-acetyl-cysteine (NAC) reduced hemin-inducible PlGF expression (Figure 4G), and higher concentrations of NAC (>0.5 mM) completely eliminated the PlGF response to hemin.
PlGF is also linked to iron overload in patients without SCD
To investigate whether iron burden generally is associated with dysregulated expression of PlGF, we conducted a natural experiment in an entirely different cohort of patients with comparably high serum ferritin levels, comprised of patients with genotype-proven HH, the most prevalent inherited iron overload disorder. PlGF levels were elevated in this group of 30 iron overloaded patients compared with 10 healthy controls (mean ± standard error of the mean, 13.38 ± 0.84 vs 6.03 ± 0.89 pg/mL; P < .0001, unpaired Student t test; Figure 4H). Intentional reduction of the iron burden and serum ferritin to low-normal levels (median and range, 1377 [965-6571] to 33 ng/mL [14-49]; P < .0001, Wilcoxon matched-pairs signed rank test; data not shown) in these patients by repetitive therapeutic venesection over 1 to 2 years resulted in simultaneous reduction of PlGF plasma levels (13.38 ± 0.84 vs 10.46 ± 0.99 pg/mL; P = .0013, paired Student t test; Figure 4H). This association of genetically high iron burden with high PlGF and the significant decline in PlGF induced by iron removal provides strong independent evidence of a relationship in humans between iron and PlGF that is supported by the results from cell culture and from sickle cell patients.
Discussion
In summary, our data show that in erythroid cells, loading of iron bound to heme stimulates expression of EKLF and its binding to the PlGF gene promoter, increasing expression of PlGF mRNA and protein. Analysis of clinical data with SCD, HH, and other disorders with elevated ferritin shows that there is significant correlation between PlGF and iron overload and clinical outcome, providing evidence of the importance of iron burden in regulating PlGF expression in vivo.
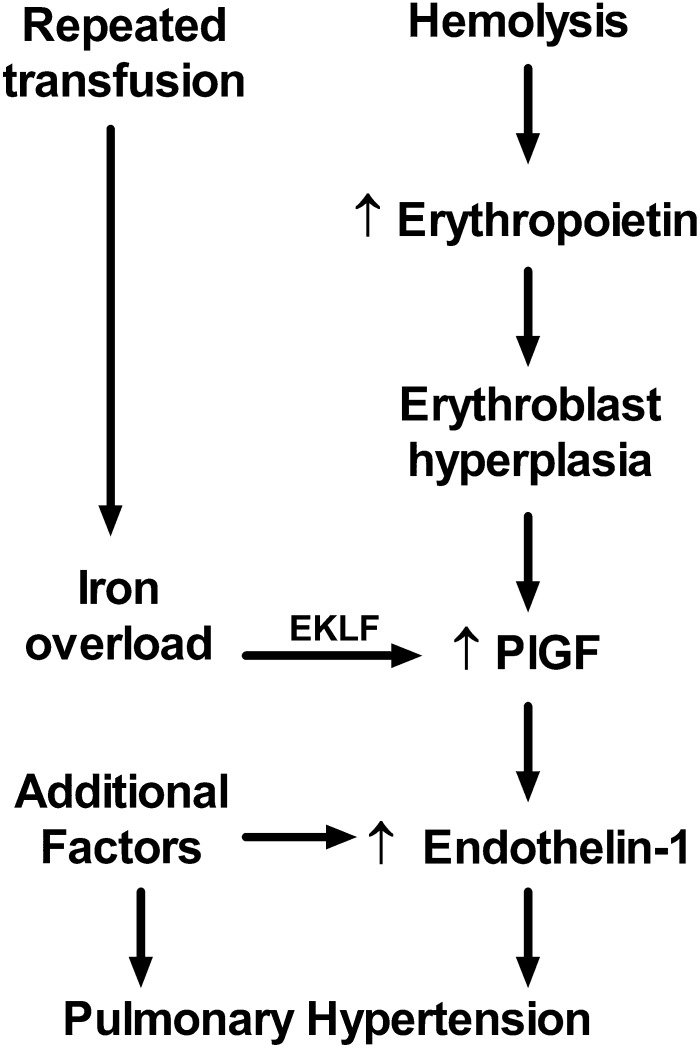
Previously published results demonstrate clearly that PlGF is expressed highly in patients with SCD21,38,39 and stimulates production of the potent vasoconstrictor ET-1 and other factors,40-46 which is then associated in adults with SCD with echocardiographic indicators of elevated pulmonary artery pressure and in mice with histological evidence of pulmonary hypertension.21,41 These prior results combined with our current data indicate for the first time a linkage between iron and expression of EKLF, PlGF, and ET-1 and clinical elevation of pulmonary artery pressure in humans with SCD with increased mortality. Rather than serving as a simple epiphenomenon of frequent transfusions or a source of nonspecific oxidative stress, iron appears to trigger an EKLF/PlGF/ET-1 linear mechanistic pathway to contribute to the development of pulmonary hypertension. This suggests a model describing PlGF expression as a consequence of iron overload that commonly occurs due to repeated blood transfusions in patients with SCD (Figure 5), especially iron complexed with protoporphyrin as hemin, a toxic byproduct of hemolysis now known to cause acute pulmonary vasculopathy in sickle cell mice47 and associated with prevalence of acute complications in children with SCD.48 Chronic hemolysis and severe anemia in SCD promotes EPO production, stimulating erythroblast hyperplasia, which increases the mass of PlGF-producing cells and PlGF expression, consistent with our previously published association of PlGF level with reticulocytosis in patients with SCD.21 Our previous animal model experiments demonstrated unequivocally that PlGF stimulates the expression of ET-1, found to be elevated by many SCD investigators.49-54 ET-1 represents a final pathway common to many forms of clinical pulmonary hypertension, such that its receptor is the target of 3 approved antagonist drugs to treat pulmonary hypertension,55-57 with observed therapeutic responses in sickle cell mice,58,59 and anecdotal clinical responses in SCD patients.60,61 This proposed model of pulmonary vasculopathy promoted by iron burden is of general interest in light of other studies suggesting that iron promotes systemic endothelial dysfunction and atherosclerosis in patients without SCD62,63 and suggests that these pathways each comprise elements of systemic vasculopathy. These pathways are likely to pertain also to β-thalassemia major,64,65 another hemoglobinopathy with transfusion-associated iron overload and prevalent pulmonary hypertension.66
Figure 5.
Proposed model of the iron overload/EKLF/PlGF/ ET-1 pathway. Erythroblast expression of PlGF is affected by erythroblast hyperplasia induced by erythropoietin as a compensatory response to hemolytic anemia. PlGF expression is augmented in some patients by excess iron via EKLF as a consequence of repeated therapeutic transfusion of red blood cells. PlGF induces expression of endothelin-1, which promotes pulmonary artery vasoconstriction and eventual pulmonary hypertension. Additional factors are also involved in dysregulation of endothelin-1 expression, such as expression of inflammatory cytokines, and several more pathways contribute to the development of pulmonary hypertension.
Our results from HH patients demonstrate a causal effect of iron burden on circulating PlGF level, but pose some interesting complexities. After venesection until attaining normal serum ferritin, mean PlGF level among HH patients did not decline completely to that of normal control subjects. We speculate that PlGF normalization might be incomplete in some patients due to incomplete reduction of total body iron despite normal serum ferritin, because serum ferritin is known to be an imperfect marker of iron burden, or alternatively, due to lagging correction of tissue parenchymal iron as iron is known to load and unload unevenly from tissues. HH patients are not known to develop pulmonary hypertension despite their high level of PlGF in isolation, and this should not be surprising, because like all other human vasculopathies, pulmonary hypertension in patients with SCD appears to involve a cumulative multifactorial burden of high PlGF on a background of hemolytic anemia with scavenging of arginine and nitric oxide, dyslipidemia, circulating inhibitors of nitric oxide synthase, platelet activation, and renal dysfunction.67 Iron-loaded HH patients have been described to have features of endothelial dysfunction,62 which may also be consistent with our conceptualization.
Pulmonary arterial hypertension carries a high mortality, and there are limited options for treatment. Our findings suggest that aggressive use of clinically approved iron chelation drugs should be investigated as adjunctive therapy to reduce the risk of pulmonary hypertension in SCD patients with iron overload.
Acknowledgments
The authors thank the National Heart, Lung and Blood Institute DNA Sequence and Genomics Core Facility for assistance, Drs Qingsong Tang and Keji Zhao for providing HOXA9 and technical advice, and Dr Donald S. Torry (Southern Illinois University School of Medicine) for providing plasmids (PlGF promoter clones, −1521/+34, −698/+34, −1521/−650).
This research was funded by the National Heart, Lung and Blood Institute Division of Intramural Research and National Institutes of Health Clinical Center (grants 1 ZIA HL006162-01, 1 ZIA HL006013-03, and CL002107-12).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood Commentary on this article in this issue.
Authorship
Contribution: X.W. and G.J.K. designed, analyzed, interpreted data, and drafted the manuscript; X.W. performed most of the laboratory assays; H.R. performed human erythroid progenitor cell culture and treatment; L.M. and Y.Y.Y. collected, processed, and retrieved human blood samples and assisted in the research; Y.Y. and N.R. performed microarray assays and analysis; G.J.K., J.G.T., and S.L. performed clinical work; S.L. reviewed/edited the manuscript; M.T. and A.P. directed EKLF knockout cell studies and reviewed/edited the manuscript; and C.T.N. directed human erythroid progenitor cell research, interpreted data, and reviewed/edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gregory J. Kato, Department of Medicine, Division of Hematology-Oncology, and the Heart, Lung, Blood, and Vascular Medicine Institute, University of Pittsburgh, 200 Lothrop St, BST E1240, Pittsburgh, PA 15261; e-mail: katogj@upmc.edu.
References
- 1.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 2.Mehari A, Gladwin MT, Tian X, Machado RF, Kato GJ. Mortality in adults with sickle cell disease and pulmonary hypertension. JAMA. 2012;307(12):1254–1256. doi: 10.1001/jama.2012.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39(1):112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 5.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA. 1991;88(20):9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green CJ, Lichtlen P, Huynh NT, et al. Placenta growth factor gene expression is induced by hypoxia in fibroblasts: a central role for metal transcription factor-1. Cancer Res. 2001;61(6):2696–2703. [PubMed] [Google Scholar]
- 7.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131(3):463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Chang M, Mukherjea D, Gobble RM, Groesch KA, Torry RJ, Torry DS. Glial cell missing 1 regulates placental growth factor (PGF) gene transcription in human trophoblast. Biol Reprod. 2008;78(5):841–851. doi: 10.1095/biolreprod.107.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gobble RM, Groesch KA, Chang M, Torry RJ, Torry DS. Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension. Placenta. 2009;30(10):869–875. doi: 10.1016/j.placenta.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012;44(1):1–9. doi: 10.3858/emm.2012.44.1.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 12.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8(12):942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 13.Hattori K, Heissig B, Wu Y, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8(8):841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8(8):831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 15.Oura H, Bertoncini J, Velasco P, Brown LF, Carmeliet P, Detmar M. A critical role of placental growth factor in the induction of inflammation and edema formation. Blood. 2003;101(2):560–567. doi: 10.1182/blood-2002-05-1516. [DOI] [PubMed] [Google Scholar]
- 16.Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G. PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med. 2008;14(9):973–978. doi: 10.1038/nm.1852. [DOI] [PubMed] [Google Scholar]
- 17.Basu P, Lung TK, Lemsaddek W, et al. EKLF and KLF2 have compensatory roles in embryonic beta-globin gene expression and primitive erythropoiesis. Blood. 2007;110(9):3417–3425. doi: 10.1182/blood-2006-11-057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang CJ, Lemsaddek W, Alhashem YN, et al. Kruppel-like factor 1 (KLF1), KLF2, and Myc control a regulatory network essential for embryonic erythropoiesis. Mol Cell Biol. 2012;32(13):2628–2644. doi: 10.1128/MCB.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alhashem YN, Vinjamur DS, Basu M, Klingmüller U, Gaensler KM, Lloyd JA. Transcription factors KLF1 and KLF2 positively regulate embryonic and fetal beta-globin genes through direct promoter binding. J Biol Chem. 2011;286(28):24819–24827. doi: 10.1074/jbc.M111.247536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Autiero M, Waltenberger J, Communi D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9(7):936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 21.Sundaram N, Tailor A, Mendelsohn L, et al. High levels of placenta growth factor in sickle cell disease promote pulmonary hypertension. Blood. 2010;116(1):109–112. doi: 10.1182/blood-2009-09-244830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tordjman R, Delaire S, Plouët J, et al. Erythroblasts are a source of angiogenic factors. Blood. 2001;97(7):1968–1974. doi: 10.1182/blood.v97.7.1968. [DOI] [PubMed] [Google Scholar]
- 23.Perkins AC, Peterson KR, Stamatoyannopoulos G, Witkowska HE, Orkin SH. Fetal expression of a human Agamma globin transgene rescues globin chain imbalance but not hemolysis in EKLF null mouse embryos. Blood. 2000;95(5):1827–1833. [PubMed] [Google Scholar]
- 24.Perelman N, Selvaraj SK, Batra S, et al. Placenta growth factor activates monocytes and correlates with sickle cell disease severity. Blood. 2003;102(4):1506–1514. doi: 10.1182/blood-2002-11-3422. [DOI] [PubMed] [Google Scholar]
- 25.Fibach E. Techniques for studying stimulation of fetal hemoglobin production in human erythroid cultures. Hemoglobin. 1998;22(5-6):445–458. doi: 10.3109/03630269809071542. [DOI] [PubMed] [Google Scholar]
- 26.Vaisman B, Meyron-Holtz EG, Fibach E, Krichevsky AM, Konijn AM. Ferritin expression in maturing normal human erythroid precursors. Br J Haematol. 2000;110(2):394–401. doi: 10.1046/j.1365-2141.2000.02167.x. [DOI] [PubMed] [Google Scholar]
- 27.Charnay P, Maniatis T. Transcriptional regulation of globin gene expression in the human erythroid cell line K562. Science. 1983;220(4603):1281–1283. doi: 10.1126/science.6574602. [DOI] [PubMed] [Google Scholar]
- 28.Palma JF, Gao X, Lin CH, Wu S, Solomon WB. Iron protoporphyrin IX (hemin) but not tin or zinc protoporphyrin IX can stimulate gene expression in K562 cells from enhancer elements containing binding sites for NF-E2. Blood. 1994;84(4):1288–1297. [PubMed] [Google Scholar]
- 29.Ghosh S, Tan F, Yu T, et al. Global gene expression profiling of endothelium exposed to heme reveals an organ-specific induction of cytoprotective enzymes in sickle cell disease. PLoS ONE. 2011;6(3):e18399. doi: 10.1371/journal.pone.0018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodge D, Coghill E, Keys J, et al. A global role for EKLF in definitive and primitive erythropoiesis. Blood. 2006;107(8):3359–3370. doi: 10.1182/blood-2005-07-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bieker JJ. Putting a finger on the switch. Nat Genet. 2010;42(9):733–734. doi: 10.1038/ng0910-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou D, Liu K, Sun CW, Pawlik KM, Townes TM. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat Genet. 2010;42(9):742–744. doi: 10.1038/ng.637. [DOI] [PubMed] [Google Scholar]
- 33.Pilon AM, Ajay SS, Kumar SA, et al. NISC Comparative Sequencing Center. Genome-wide ChIP-Seq reveals a dramatic shift in the binding of the transcription factor erythroid Kruppel-like factor during erythrocyte differentiation. Blood. 2011;118(17):e139–e148. doi: 10.1182/blood-2011-05-355107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118(8):2044–2054. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tallack MR, Magor GW, Dartigues B, et al. Novel roles for KLF1 in erythropoiesis revealed by mRNA-seq. Genome Res. 2012;22(12):2385–2398. doi: 10.1101/gr.135707.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coghill E, Eccleston S, Fox V, et al. Erythroid Kruppel-like factor (EKLF) coordinates erythroid cell proliferation and hemoglobinization in cell lines derived from EKLF null mice. Blood. 2001;97(6):1861–1868. doi: 10.1182/blood.v97.6.1861. [DOI] [PubMed] [Google Scholar]
- 37.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brittain JE, Hulkower B, Jones SK, et al. Placenta growth factor in sickle cell disease: association with hemolysis and inflammation. Blood. 2010;115(10):2014–2020. doi: 10.1182/blood-2009-04-217950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duits AJ, Rodriguez T, Schnog JJ CURAMA Study Group. Serum levels of angiogenic factors indicate a pro-angiogenic state in adults with sickle cell disease. Br J Haematol. 2006;134(1):116–119. doi: 10.1111/j.1365-2141.2006.06103.x. [DOI] [PubMed] [Google Scholar]
- 40.Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003;102(4):1515–1524. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- 41.Patel N, Gonsalves CS, Malik P, Kalra VK. Placenta growth factor augments endothelin-1 and endothelin-B receptor expression via hypoxia-inducible factor-1 alpha. Blood. 2008;112(3):856–865. doi: 10.1182/blood-2007-12-130567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel N, Gonsalves CS, Yang M, Malik P, Kalra VK. Placenta growth factor induces 5-lipoxygenase-activating protein to increase leukotriene formation in sickle cell disease. Blood. 2009;113(5):1129–1138. doi: 10.1182/blood-2008-07-169821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dallalio G, Means RT., Jr Placental growth factor attenuates suppression of erythroid colony formation by interferon. Transl Res. 2008;152(5):233–238. doi: 10.1016/j.trsl.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel N, Tahara SM, Malik P, Kalra VK. Involvement of miR-30c and miR-301a in immediate induction of plasminogen activator inhibitor-1 by placental growth factor in human pulmonary endothelial cells. Biochem J. 2011;434(3):473–482. doi: 10.1042/BJ20101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel N, Sundaram N, Yang M, Madigan C, Kalra VK, Malik P. Placenta growth factor (PlGF), a novel inducer of plasminogen activator inhibitor-1 (PAI-1) in sickle cell disease (SCD). J Biol Chem. 2010;285(22):16713–16722. doi: 10.1074/jbc.M110.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel N, Kalra VK. Placenta growth factor-induced early growth response 1 (Egr-1) regulates hypoxia-inducible factor-1alpha (HIF-1alpha) in endothelial cells. J Biol Chem. 2010;285(27):20570–20579. doi: 10.1074/jbc.M110.119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghosh S, Adisa OA, Chappa P, et al. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest. 2013;123(11):4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adisa OA, Hu Y, Ghosh S, Aryee D, Osunkwo I, Ofori-Acquah SF. Association between plasma free haem and incidence of vaso-occlusive episodes and acute chest syndrome in children with sickle cell disease. Br J Haematol. 2013;162(5):702–705. doi: 10.1111/bjh.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatzipantelis ES, Pana ZD, Gombakis N, et al. Endothelial activation and inflammation biomarkers in children and adolescents with sickle cell disease. Int J Hematol. 2013;98(2):158–163. doi: 10.1007/s12185-013-1392-y. [DOI] [PubMed] [Google Scholar]
- 50.Ergul S, Brunson CY, Hutchinson J, et al. Vasoactive factors in sickle cell disease: in vitro evidence for endothelin-1-mediated vasoconstriction. Am J Hematol. 2004;76(3):245–251. doi: 10.1002/ajh.20107. [DOI] [PubMed] [Google Scholar]
- 51.Lapouméroulie C, Benkerrou M, Odièvre MH, Ducrocq R, Brun M, Elion J. Decreased plasma endothelin-1 levels in children with sickle cell disease treated with hydroxyurea. Haematologica. 2005;90(3):401–403. [PubMed] [Google Scholar]
- 52.Rybicki AC, Benjamin LJ. Increased levels of endothelin-1 in plasma of sickle cell anemia patients. Blood. 1998;92(7):2594–2596. [PubMed] [Google Scholar]
- 53.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92(7):2551–2555. [PubMed] [Google Scholar]
- 54.Werdehoff SG, Moore RB, Hoff CJ, Fillingim E, Hackman AM. Elevated plasma endothelin-1 levels in sickle cell anemia: relationships to oxygen saturation and left ventricular hypertrophy. Am J Hematol. 1998;58(3):195–199. doi: 10.1002/(sici)1096-8652(199807)58:3<195::aid-ajh6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 55.Barst RJ. Sitaxsentan: a selective endothelin-A receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2007;8(1):95–109. doi: 10.1517/14656566.8.1.95. [DOI] [PubMed] [Google Scholar]
- 56.Rubin LJ. Endothelin receptor antagonists for the treatment of pulmonary artery hypertension. Life Sci. 2012;91(13-14):517–521. doi: 10.1016/j.lfs.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 57.Anderson JR, Nawarskas JJ. Pharmacotherapeutic management of pulmonary arterial hypertension. Cardiol Rev. 2010;18(3):148–162. doi: 10.1097/CRD.0b013e3181d4e921. [DOI] [PubMed] [Google Scholar]
- 58.Rivera A. Reduced sickle erythrocyte dehydration in vivo by endothelin-1 receptor antagonists. Am J Physiol Cell Physiol. 2007;293(3):C960–C966. doi: 10.1152/ajpcell.00530.2006. [DOI] [PubMed] [Google Scholar]
- 59.Sabaa N, de Franceschi L, Bonnin P, et al. Endothelin receptor antagonism prevents hypoxia-induced mortality and morbidity in a mouse model of sickle-cell disease. J Clin Invest. 2008;118(5):1924–1933. doi: 10.1172/JCI33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minniti CP, Machado RF, Coles WA, Sachdev V, Gladwin MT, Kato GJ. Endothelin receptor antagonists for pulmonary hypertension in adult patients with sickle cell disease. Br J Haematol. 2009;147(5):737–743. doi: 10.1111/j.1365-2141.2009.07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lionnet F, Bachmeyer C, Stankovic K, Tharaux PL, Girot R, Aractingi S. Efficacy of the endothelin receptor blocker bosentan for refractory sickle cell leg ulcers. Br J Haematol. 2008;142(6):991–992. doi: 10.1111/j.1365-2141.2008.07206.x. [DOI] [PubMed] [Google Scholar]
- 62.Gaenzer H, Marschang P, Sturm W, et al. Association between increased iron stores and impaired endothelial function in patients with hereditary hemochromatosis. J Am Coll Cardiol. 2002;40(12):2189–2194. doi: 10.1016/s0735-1097(02)02611-6. [DOI] [PubMed] [Google Scholar]
- 63.Kamanna VS, Ganji SH, Shelkovnikov S, Norris K, Vaziri ND. Iron sucrose promotes endothelial injury and dysfunction and monocyte adhesion/infiltration. Am J Nephrol. 2012;35(2):114–119. doi: 10.1159/000334939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaliasos N, Challa A, Hatzimichael E, et al. Serum adipocytokine and vascular inflammation marker levels in Beta-thalassaemia major patients. Acta Haematol. 2010;124(4):191–196. doi: 10.1159/000320274. [DOI] [PubMed] [Google Scholar]
- 65.Viprakasit V, Kankirawatana S, Akarasereenont P, Durongpisitkul K, Chotewuttakorn S, Tanphaichitr VS. Baseline levels of plasma endothelin-1 (ET-1) and changes during transfusion in thalassemic patients. Am J Hematol. 2002;70(3):260–262. doi: 10.1002/ajh.10129. [DOI] [PubMed] [Google Scholar]
- 66.Derchi G, Galanello R, Bina P, et al. Webthal Pulmonary Arterial Hypertension Group*. Prevalence and risk factors for pulmonary arterial hypertension in a large group of β-thalassemia patients using right heart catheterization: a Webthal study. Circulation. 2014;129(3):338–345. doi: 10.1161/CIRCULATIONAHA.113.002124. [DOI] [PubMed] [Google Scholar]
- 67.Kato GJ, Taylor JG., VI Pleiotropic effects of intravascular haemolysis on vascular homeostasis. Br J Haematol. 2010;148(5):690–701. doi: 10.1111/j.1365-2141.2009.08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]