Abstract
Purpose
To develop a modified ex vivo corneal crosslinking method that increases stromal resistance to enzymatic degradation for use as a carrier for the Boston keratoprosthesis.
Methods
Ex vivo crosslinking of human corneas was performed using Barron® artificial anterior chambers. The corneas were de-epithelialized, pre-treated with riboflavin solution (0.1% riboflavin/20% dextran) and irradiated with ultraviolet A (UVA) light (λ=370nm, irradiance=3mW/cm2) for various durations. The combined effect of UVA and gamma (γ) irradiation was also assessed using the commercially available γ-irradiated corneal donors. The corneas were then trephined and incubated at 37 degrees Celsius with 0.3% collagenase A solution. The time to dissolution of each cornea was compared across treatments.
Results
De-epithelialized corneas (no UV light, no riboflavin) dissolved in 5.8 ± 0.6 hours. Crosslinked corneas demonstrated increased resistance to dissolution, with a time to dissolution of 17.8 +/− 2.6 hours (p < 0.0001). The corneal tissues’ resistance to collagenase increased with longer UVA exposure, reaching a plateau at 30 minutes. Crosslinking both the anterior and posterior corneas did not provide added resistance when compared to crosslinking the anterior corneas only (p>0.05). γ-irradiated corneas dissolved as readily as de-epithelialized controls regardless of whether they were further crosslinked (5.6 ± 1.2 hours) or not (6.1 ± 0.6 hours) (p=0.43)
Conclusions
Collagen crosslinking of the de-epithelialized anterior cornea surface for 30 minutes conferred optimal resistance to in vitro keratolysis by collagenase A.
Keywords: Corneal collagen crosslinking, gamma (γ) irradiation, collagenase, keratolysis
INTRODUCTION
Sterile corneal melting or keratolysis, is a serious and potentially vision-threatening condition that involves the destruction of the stromal extracellular matrix (ECM). Melting may develop following a variety of insults, ranging from autoimmune diseases (rheumatoid arthritis, Stevens-Johnson syndrome, peripheral ulcerative keratitis), chemical burns, atopic disease, infectious keratitis, neurotrophic states, chronic exposure, disruption of local nutrition by intrastromal corneal ring segments, corneal inlays or keratoprosthesis as well as many other iatrogenic sources (mitomcyin C, topical non-steroidal anti-inflammatory eye drops).1–8 The prevention of corneal melting is of particular interest to our group as it was, historically, a common complication that limited the use of early keratoprostheses3 and remains one of the most common causes of keratoprosthesis losses.9
Keratoprostheses are used in patients at high risk of failure following conventional corneal transplantation. The Boston Keratoprosthesis (B-KPro) is the most commonly used keratoprosthesis in North America and elsewhere in the world. It is composed of synthetic materials ([PMMA] and titanium) and requires a donor cornea to function as a carrier for the device.10 Modifications to the B-KPro design and postoperative management have improved the retention rate, but keratolysis remains the most common cause of B-KPro failure. In the most recent report from the Boston Keratoprosthesis Type 1 Study Group, 43% of the B-KPro loss could be attributed to sterile keratolysis.9 Patients with underlying autoimmune conditions such as Stevens-Johnson syndrome (SJS) and ocular mucous membrane pemphigoid have a significantly higher risk of keratolysis following B-KPro.11–13 The prevention of corneal melting after B-KPro implantation has always been a priority as corneal melting may lead to permanent loss of vision or loss of the globe, thus precluding future visual rehabilitation.
Because of the limited availability of corneal donor grafts in many parts of the world, other tissue options have been explored as carriers for the B-KPro. These include ipsilateral corneal autografts, frozen corneal grafts as well as gamma (γ)-irradiated corneal grafts.14–16 γ-irradiation sterilizes the graft and allows for tissue storage at room temperature for prolonged periods.17 These characteristics have the potential of improving the availability of corneal donors. Further, γ-irradiation results in the devitalization of corneal cells and consequently, in reduced allogenicity of the transplanted tissue.18 In addition to of these interesting characteristics, γ-irradiated corneal tissue has been suggested to be equivalent to fresh tissue when used as a carrier to the B-KPro.16, 19
Corneal collagen crosslinking with riboflavin and ultraviolet A (UVA) light has been shown to increase biomechanical strength,20, 21 decrease tissue permeability22 and increase resistance of the crosslinked cornea to enzymatic degradation.23 The procedure is primarily used to slow or arrest the progression of corneal ectasias and is currently undergoing clinical trials in the United States.24 Corneal crosslinking has also been used to treat recalcitrant, ulcerative corneal infections.6, 25 The corneal collagen crosslinking technique combines the use of riboflavin (B2) and UVA to induce free oxygen radical formation and chemical covalent bonding between the amino groups of collagen fibrils within the stroma.26 Controlled in vitro and ex vivo studies have demonstrated riboflavin/UVA-induced crosslinking between proteoglycan (PG) core proteins (keratocan, lumican, mimecan and decorin) as well as linkages between collagen and PGs.27
In this report, we explore various crosslinking protocols including γ-irradiation alone or followed by crosslinking and compare their effectiveness as pertains to the tissue’s resistance to enzymatic degradation. Ultimately, the use of collagenase-resistant carrier grafts may improve the retention of the B-KPro device.28 Given that carrier corneas can be crosslinked ex vivo prior to B-KPro surgery, the crosslinking treatment options are not limited to those available for in vivo crosslinking of corneas ectasias. For instance, as healthy endothelial cells are not needed to maintain a clear visual axis following B-KPro,15 it becomes permissible to crosslink both the anterior and posterior surfaces of carrier grafts. Moreover, the impact of concurrent gamma-irradiation and crosslinking was examined.
MATERIAL AND METHODS
Reagent preparation
Riboflavin (0.1% or 1 mg/mL) solution was prepared by thoroughly mixing 50 mg of riboflavin 5′-phosphate sodium salt hydrate (Sigma Aldrich, St. Louis, Missouri) and 50 mL of 20% dextran (w/w, Sigma Aldrich, St. Louis, MO). Collagenase A (matrix metalloproteinase 1a or EC 3.4.24.3, 0.3% (3 mg/mL), Sigma Aldrich, St. Louis, MO) was prepared fresh before every experiment using phosphate-buffered saline (PBS). Both solutions were covered in aluminum foil to protect from the light and stored at 4°C until use.
Tissue preparation
Human research corneas were obtained from Tissue Banks International (Baltimore, MD) and North Carolina Eye Bank (Winston-Salem, NC). The corneal tissues were provided in OptiSol® solution and stored at 4°C until use. All experiments were performed within 2 weeks of the donor’s death. Each cornea was fit into a Barron® artificial anterior chamber (Katena Eye Instruments, Denville, NJ) and maintained with balanced salt solution. In addition, corneas were γ-irradiated in accordance with ISO standards for the terminal sterilization of allografts and obtained from Tissue Banks International (TBI VisionGraft, Baltimore, MD).29
Corneal crosslinking
All corneas were de-epithelialized and pre-treated with 0.1% riboflavin solution every 2 minutes for 15 minutes as previously described.30 The corneas were irradiated with UVA light using the VEGA LED-based UV emitter (Costruzione Strumenti Oftalmici, Firenze, Italy) at a wavelength of 370 nm, irradiance of 3 mW/cm2 and distance of 54 mm from the cornea. The UV emitter was calibrated before every experiment. Drops of riboflavin were applied at 5-minute intervals during the irradiation treatment. Consistent with the clinical technique used to treat corneal ectasia in vivo, the anterior surface of the corneas were crosslinked for either 7 ½, 15, 30, 60 or 90 minutes. In addition, other treatment groups included the crosslinking of both the anterior and posterior cornea surfaces for either 15 or 30 minutes (see table 1). Treatment groups are described using the nomenclature AxPy, where x is the duration of crosslinking of the anterior (A) surface of the cornea and y is the duration of crosslinking of the posterior (P) surface of the cornea in minutes. When γ-irradiated corneas were used, crosslinking was applied to the anterior surface of the cornea for 30 minutes. The following groups served as negative controls: 1) untreated corneas, 2) de-epithelialized corneas with application of 0.1% riboflavin solution but no UVA exposure (riboflavin only) and 3) de-epithelialized corneas with UVA exposure without prior instillation of riboflavin (UVA only).
Table 1.
Study Groups
| Study Group | Duration of crosslinking of anterior corneal surface (minutes) | Duration of crosslinking of posterior corneal surface (minutes) |
|---|---|---|
| A7.5 | 7.5 | - |
| A15 | 15 | - |
| A30 | 30 | - |
| A60 | 60 | - |
| A90 | 90 | - |
| A15P15 | 15 | 15 |
| A30P30 | 30 | 30 |
| γ | 0 | 0 |
| γ+A30 | 30 | 0 |
γ = gamma irradiation, A = Anterior corneal surface, P = Posterior corneal surface
Enzymatic degradation
Corneas in each treatment group (N = 5) were trephined into 8.5-mm buttons and incubated with 0.3% collagenase A solution at 37°C on a plate shaker set at 150 rotations-per-minute. The corneas were observed hourly for the first 12 hours, and then every 30 minutes until complete dissolution was achieved. The time to dissolution of the corneal button was recorded and all groups were compared to untreated corneas.
Data analysis
Statistical analysis was performed using Graphpad Instat 3.10. The results were reported as mean ± standard deviation. Normality was tested using the Kolmogorov-Smirnov test, and non-parametric tests were used when indicated. One-way analysis of variance (ANOVA) and Kruskal-Wallis test were used to compare times to complete dissolution between groups. Mann-Whitney U-statistics was used for comparing non-parametric non-matched groups. The tests were performed using a two-tailed p-value of 0.05.
RESULTS
Treatment
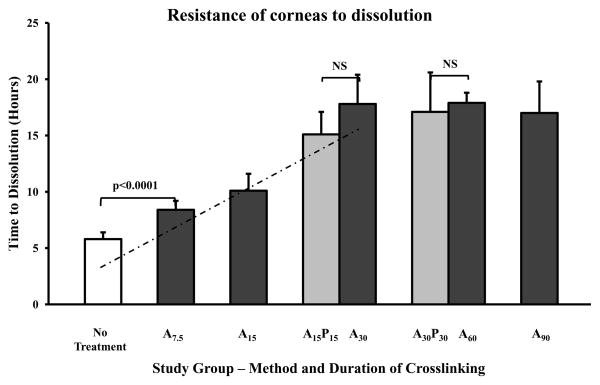
Crosslinked corneas were more resistant to enzymatic degradation when compared to untreated corneas (Figure 1). The resistance of corneas that underwent crosslinking of the anterior surface only reached a plateau after 30 minutes of treatment (p<0.05). Corneas that underwent 15 minutes of crosslinking of the anterior and posterior surfaces (A15P15) were no different than the corneas that underwent 30 minutes of the anterior surface (A30). There was also no significant difference between treatment of both anterior and posterior corneal surfaces for 15 minutes (A15P15, 15 ± 1.5 hours) or 30 minutes (A30P30, 17.9 ± 4.9 hours, p=0.24). There was no difference between untreated, riboflavin only, and UVA only control groups. Untreated corneas dissolved after 5.8 ± 0.6 while corneas crosslinked for 30 minutes (A30) dissolved after 17.8 ± 2.6 hours (p < 0.0001).
Figure 1.
UV crosslinked corneas are relatively resistant to degradation in 0.3% collagenase A. Through 30 minutes of treatment, a linear correlation was demonstrated between the time to dissolution and the duration of crosslinking (dashed line, R2 = 0.98, p<0.05). No increase in resistance was observed when both the anterior and posterior corneas were crosslinked. NS = not significant.
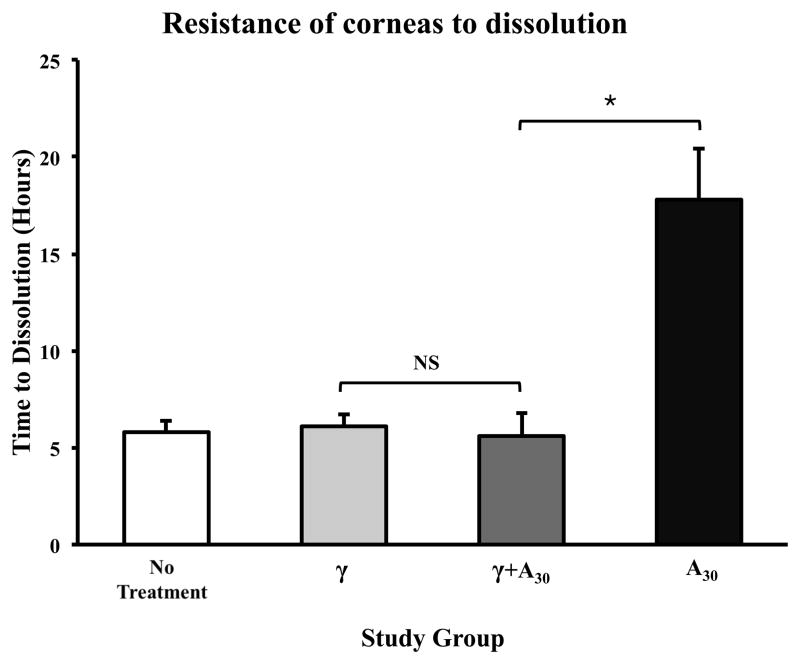
γ-irradiated corneas (without crosslinking) showed similar resistances to degradation as untreated corneas (non-γ-irradiated, not crosslinked) with times to dissolution of 6.1 ± 0.6 hours and 5.8 ± 0.6 hours, respectively (p=0.91) (Figure 2). Interestingly, the time to dissolution of γ-irradiated corneas that were crosslinked for 30 minutes (5.6 ± 1.2 hours) was comparable to both untreated corneas and γ-irradiated corneas that were not crosslinked (p=0.75). In addition, crosslinked γ-irradiated corneas (γ+A30) were less resistant to degradation than non-γ-irradiated corneas that were crosslinked for the same duration (5.6 ± 1.2 hours vs. 17.8 ± 2.6 hours; p<0.0001).
Figure 2.
γ-irradiated corneas with and without subsequent crosslinking were no more resistant to degradation in 0.3% collagenase A than untreated corneas. Crosslinked γ-irradiated corneas where less resistant than non γ-irradiated corneas that were crosslinked for the same duration (30 minutes). Non γ-irradiated, crosslinked corneas were more resistant to degradation than untreated and γ-irradiated corneas. *p<0.05, NS = not significant.
DISCUSSION
In this study, several ex vivo collagen crosslinking protocols were investigated in an effort to maximize the corneal resistance to enzymatic degradation. The clinical relevance of ex vivo collagen crosslinking lies in the potential use of crosslinked corneal donors as carriers for B-KPro or as tectonic grafts in ocular surface diseases with predisposition to corneal melting. Our results demonstrated that collagen crosslinking of the anterior corneal surface exhibited a dose-response increase in the tissue’s resistance to enzymatic degradation. Indeed, a strong linear relationship (R2 = 0.98) existed between the duration of crosslinking and the time to complete dissolution by collagenase. However, this dose-response curve was achieved only for the first 30 minutes of UVA exposure. Longer UVA exposures did not yield additional improvement in the tissues’ resistance to collagenase.
The effects of crosslinking the anterior and posterior corneal surfaces have not been previously reported. While it is not feasible to treat both corneal surfaces during in vivo crosslinking of ectatic corneas, such a treatment is possible in donor tissue destined to act as a carrier for B-KPro or as a corneal patch graft where a viable endothelium is not required. However, crosslinking of the posterior corneal surface did not result in any additional resistance compared to crosslinking of the anterior cornea only.
To our knowledge, this is the first study to compare the collagenase resistance of corneal tissue following γ-irradiation alone, collagen crosslinking alone and the combination of both γ-irradiation and crosslinking. Interestingly, γ-irradiated corneas were no more resistant to enzymatic degradation than untreated corneas. Furthermore, γ-irradiation seemed to offset the effect of crosslinking. Compared to non-γ-irradiated crosslinked corneas, γ-irradiated crosslinked corneas demonstrated significantly less resistance to enzymatic degradation. This is consistent with the existing literature, where γ-irradiation of tendon and bone collagen has been shown to cause a dose-dependent fragmentation of collagen fibers as well as crosslinking between peptides.31, 32 Protein chain degradation and depolymerization appear to be the predominant effects, leading to faster enzymatic degradation as well as decreased mechanical properties.33, 34 For example, the acellular tissue matrix AlloDerm® (LifeCell Corporation, Bridgewater, NJ) was digested at a faster rate by type I collagenase, pepsin, trypsin and proteinase K following γ-irradiation.35 However, to our knowledge, there are no such studies evaluating the effect of γ-irradiation on corneal collagen. Similar to other collagen-based tissues, corneas are also likely to undergo collagen fiber breakdown following γ-irradiation. Therefore, it is hypothesized that the γ-irradiation-induced weakening of corneal collagen was sufficient to annul any further crosslinking effect using riboflavin and UVA.
The increased resistance of crosslinked corneas to enzymatic degradation found in this study supports previous research done by Spoerl et al.23 Our study involved several modifications. First, the submerged tissue was incubated at a higher temperature (37°C), to optimize the activity of the collagenase enzymes. As well, a higher concentration of collagenase A (0.3%) was employed here to hasten the time to complete degradation. As such, time to dissolution was measured over the course of hours, rather than days.
Corneal melting occurs due to dysregulated and excessive matrix metalloproteinase (MMP) production by epithelial cells and/or inflammatory cells.36 MMPs are zinc-dependent endopeptidases that are capable of degrading virtually all components of the extracellular matrix and basement membrane.37, 38 Increased MMP activity has been shown in the tears of patients with ocular infections, corneal ectasias, and ocular surface diseases at risk of developing sterile corneal ulcers.39–42 Collagenase A, a member of the MMPs family, is recognized for its potency in degrading all forms of collagen and extracellular matrix and was thus selected for use in this study.
A limitation in our study was that all crosslinking experiments were performed using a UVA emitter designed to emit a single irradiation energy (3 mW/cm2). According to the Bunson-Roscoe law of reciprocity, a biological effect is directly proportional to the total energy dose and this dose is a product of the intensity and the duration of exposure.43 While higher irradiation energies can theoretically accelerate the crosslinking and produce enzymatically resistant corneas at a fraction of the time,44 this was not possible with our current instrumentation. Because good clinical outcomes have been described using higher fluence, accelerated CXL (for example, 7 mw/cm2 for 15 minutes),28, 44 future ex vivo CXL studies should include the assessment of higher UVA fluence and the effect of debriding versus leaving the epithelium intact. Indeed, these parameters have the potential benefits of decreasing the duration of UVA exposure and CXL treatment, thus enhancing the feasibility of preoperative ex vivo CXL. Finally, controlled ex vivo experiments likely do not reproduce all of the various factors, enzymes and mechanisms involved in keratolysis in vivo. In this study, the crosslinked corneal buttons were submerged in collagenase A, allowing the tissue to be degraded from the anterior and posterior surfaces. However, in vivo corneal melting appears to occur only from the anterior cornea.31 Nevertheless, crosslinking of donor carrier corneas for B-KPro appears promising, and clinical studies are indicated in patients with high risk of keratolysis, who are also in need of a KPro.
Acknowledgments
Funding: This work was supported in part by the Boston Keratoprosthesis Research Fund (MEEI), Boston, Massachusetts and the National Institutes of Health, National Eye Institute, Bethesda, Maryland (NEI 1K08EY019686 to JBC) and an unrestricted grant to the Department of Ophthalmology, Harvard Medical School, from Research to Prevent Blindness, New York, New York.
Footnotes
Conflict of interest/financial disclosure: The author(s) have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Tuli S, Schultz G, Downer D. Science and strategy for preventing and managing corneal ulceration. Ocul Surf. 2007;5:23–39. doi: 10.1016/s1542-0124(12)70050-2. [DOI] [PubMed] [Google Scholar]
- 2.Knox Cartwright N, Tole D, Georgoudis P, Cook S. Peripheral ulcerative keratitis and corneal melt: a 10-year single center review with historical comparison. Cornea. 2014;33:27–31. doi: 10.1097/ICO.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 3.Harissi-Dagher M, Khan BF, Schaumberg DA, Dohlman CH. Importance of nutrition to corneal grafts when used as a carrier of the Boston Keratoprosthesis. Cornea. 2007;26:564–8. doi: 10.1097/ICO.0b013e318041f0a6. [DOI] [PubMed] [Google Scholar]
- 4.Kugler L, Hill S, Sztipanovits D, et al. Corneal melt of incisions overlying corneal ring segments: case series and literature review. Cornea. 2011;30:968–71. doi: 10.1097/ICO.0b013e3182031ca0. [DOI] [PubMed] [Google Scholar]
- 5.Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye. 2003;17:989–95. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- 6.Tayapad J, Viguilla A, Reyes J. Collagen cross-linking and corneal infections. Curr Opin Ophthalmol. 2013;24:288–90. doi: 10.1097/ICU.0b013e32836229c5. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien TP, Li QJ, Sauerburger F, et al. The role of matrix metalloproteinases in ulcerative keratolysis associated with perioperative diclofenac use. Ophthalmology. 2001;108:656–9. doi: 10.1016/s0161-6420(00)00590-x. [DOI] [PubMed] [Google Scholar]
- 8.Ewing-Chow D, Romanchuk K, Gilmour G, et al. Corneal melting after pterygium removal followed by topical mitomycin C therapy. Can J Ophthalmol. 1992;27:197–9. [PubMed] [Google Scholar]
- 9.Ciolino JB, Belin M, Todani A, et al. Boston Keratoprosthesis Type 1 Study Group. Retention of the Boston keratoprosthesis type 1: multicenter study results. Ophthalmology. 2013;120:1195–200. doi: 10.1016/j.ophtha.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruzat A, Tauber A, Shukla A, et al. Low-cost and readily available tissue carriers for the Boston Keratoprosthesis: A review of possibilities. J Ophthalmol. 2013:1–5. doi: 10.1155/2013/686587. Epub Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaghouti F, Nouri M, Abad JC, et al. Keratoprosthesis: preoperative prognostic categories. Cornea. 2001;20:19–23. doi: 10.1097/00003226-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Utine CA, Tzu JH, Akpek EK. Clinical Features and Prognosis of Boston Type I Keratoprosthesis-associated Corneal Melt. Ocul Immunol Inflamm. 2011;19:413–8. doi: 10.3109/09273948.2011.621580. [DOI] [PubMed] [Google Scholar]
- 13.Palioura S, Kim B, Dohlman CH, Chodosh J. The Boston keratoprosthesis type I in mucous membrane pemphigoid. Cornea. 2013;32:956–61. doi: 10.1097/ICO.0b013e318286fd73. [DOI] [PubMed] [Google Scholar]
- 14.Ament J, Tilahun Y, Mudawi E, Pineda R. Role for ipsilateral autologous corneas as a carrier for the Boston keratoprosthesis: the Africa experience. Arch Ophthalmol. 2010;128:795–7. doi: 10.1001/archophthalmol.2010.79. [DOI] [PubMed] [Google Scholar]
- 15.Robert M-C, Biernacki K, Harissi-Dagher M. Boston keratoprosthesis type 1 surgery: use of frozen versus fresh corneal donor carriers. Cornea. 2012;31:339–45. doi: 10.1097/ICO.0b013e31823e6110. [DOI] [PubMed] [Google Scholar]
- 16.Fadlallah A, Atallah M, Cherfan G, Awwad S, et al. Gamma-irradiated corneas as carriers for the Boston type 1 keratoprosthesis: Advantages and outcomes in a surgical mission setting. Cornea. 2014;33:235–9. doi: 10.1097/ICO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 17.TBI. VisionGraft. [accessed February 14, 2014];Tissue Banks International. 2012 http://www.visiongraft.org.
- 18.Stevenson W, Cheng S, Emami-Naeini P, et al. Gamma-irradiation reduces the allogenicity of donor corneas. Invest Ophthalmol Vis Sci. 2012;53:7151–8. doi: 10.1167/iovs.12-9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akpek E, Aldave A, Aquavella J. The use of precut, γ-irradiated corneal lenticules in Boston type 1 keratoprosthesis implantation. Am J Ophthalmol. 2012;154:495–8. doi: 10.1016/j.ajo.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Wollensak G, Spoerl E, Mazzota C, et al. Interlamelar cohesion after corneal crosslinking using riboflavin and ultraviolet A light. Br J Ophthalmol. 2011;95:876–80. doi: 10.1136/bjo.2010.190843. [DOI] [PubMed] [Google Scholar]
- 21.Lanchares E, del Buey M, Cristobal J, et al. Biomechanical property analysis after corneal collagen cross-linking in realtion to ultraviolet A irradiation time. Graefes Arch Clin Exp Ophthalmol. 2011;249:1223–7. doi: 10.1007/s00417-011-1674-0. [DOI] [PubMed] [Google Scholar]
- 22.Stewart J, Schultz D, Lee O, Trinidad M. Collagen cross-links reduce corneal permeability. Invest Ophthalmol Vis Sci. 2009;50:1606–12. doi: 10.1167/iovs.08-2727. [DOI] [PubMed] [Google Scholar]
- 23.Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29:35–40. doi: 10.1080/02713680490513182. [DOI] [PubMed] [Google Scholar]
- 24.Hersh P, Greenstein S, Fry K. Corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011;37:149–60. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Makdoumi K, Mortensen J, Sorkhabi O, et al. UVA-riboflavin photochemical therapy of bacterial keratitis. Graefes Arch Clin Exp Ophthalmol. 2011;250:95–102. doi: 10.1007/s00417-011-1754-1. [DOI] [PubMed] [Google Scholar]
- 26.Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006;17:356–60. doi: 10.1097/01.icu.0000233954.86723.25. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Conrad A, Conrad G. Effect of ultraviolet-A and riboflavin on the interaction of collagen and proteoglycans during corneal cross-linking. J Biol Chem. 2011;286:13011–22. doi: 10.1074/jbc.M110.169813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanellopoulos A, Asimellis G. Long-term Safety and Efficacy of High-Fluence Collagen Crosslinking of the Vehicle Cornea in Boston Keratoprosthesis Type 1. Cornea. doi: 10.1097/ICO.0000000000000176. in press. [DOI] [PubMed] [Google Scholar]
- 29.TBI. [accessed February 14, 2014];Tissue Banks International Centers of Excellence - National Processing Center. 2010 http://www.tbionline.org/national-processing-center.php.
- 30.Wittig-Silva C, Chan E, Islam F, et al. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: Three-year results. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2013.10.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Cheung D, Perelman N, Tong D, Nimni M. The effect of gamma-irradiation on collagen molecules, isolated alpha-chains, and crosslinked native fibers. J Biomed Mater Res. 1990;24:581–9. doi: 10.1002/jbm.820240505. [DOI] [PubMed] [Google Scholar]
- 32.Salehpour A, Butler D, Proch F, et al. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res. 1995;13:898–906. doi: 10.1002/jor.1100130614. [DOI] [PubMed] [Google Scholar]
- 33.Hara M, Koshimizu N, Yoshida M, et al. Cross-linking and depolymerisation of gamma-irradiated fish gelatin and porcin gelatin studied by SEC-MALLS and SDS-PAGE: a comparative study. J Biomater Sci Polym Ed. 2010;21:877–92. doi: 10.1163/156856209X449452. [DOI] [PubMed] [Google Scholar]
- 34.Burton B, Gaspar A, Josey D, et al. Bone embrittlement and collagen modifications due to high-dose gamma-irradiation sterilization. Bone. 2014;61:71–81. doi: 10.1016/j.bone.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Gouk S, Lim T, Teoh S, Sun W. Alterations of human acellular tissue matrix by gamma irradiation: histology, biomechanical property, stability, in vitro cell repopulation and remodeling. J Biomed Mater Res B Appl Biomater. 2008;84:205–17. doi: 10.1002/jbm.b.30862. [DOI] [PubMed] [Google Scholar]
- 36.Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;290:S12–23. doi: 10.1007/pl00007449. [DOI] [PubMed] [Google Scholar]
- 37.Birkedal-Hansen H, Moore W, Bodden M, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 38.Page-McCaw A, Ewald A, Werb Z. Matrix metalloproteinases and regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakimoto T, Shoji J, Sawa M. Active form of gelatinases in tear fluid in patients with corneal ulcer or ocular burn. Jpn J Ophthalmol. 2003;47:423–6. doi: 10.1016/s0021-5155(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto K. Proteases in bacterial keratitis. Cornea. 2000;19:S160–4. [Google Scholar]
- 41.Balasubramanian S, Mohan S, Pye D, Willcox M. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012;90:e303–9. doi: 10.1111/j.1755-3768.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 42.Arafat S, Suelves A, Spurr-Michaud S, et al. Neutrophil collagenase, gelatinase, and myeloperoxidase in tears of patients with Stevens-Johnson syndrome and ocular cicatricial pemphigoid. Ophthalmology. 2014;121:79–87. doi: 10.1016/j.ophtha.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunsen R, Roscoe H. Photochemical researches - Part V. On the measurement of the chemical action of direct and diffuse sunlight. Proc R Soc London. 1862;12:306–12. [Google Scholar]
- 44.Kanellopoulos A. Long term results of a prospective randomized bilateral eye comparison triall of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross linking for progressive keratoconus. Clinical Ophthalmology. 2012;6:97–101. doi: 10.2147/OPTH.S27170. [DOI] [PMC free article] [PubMed] [Google Scholar]