Abstract
Anabolic androgenic steroids (AAS) are taken by both sexes to enhance athletic performance and body image, nearly always in conjunction with an exercise regime. Although taken to improve physical attributes, chronic AAS use can promote negative behavior, including anxiety. Few studies have directly compared the impact of AAS use in males versus females or assessed the interaction of exercise and AAS. We show that AAS increase anxiety-like behaviors in female but not male mice and that voluntary exercise accentuates these sex-specific differences. We also show that levels of the anxiogenic peptide corticotrophin releasing factor (CRF) are significantly greater in males, but that AAS selectively increase CRF levels in females, thus abrogating this sex-specific difference. Exercise did not ameliorate AAS-induced anxiety or alter CRF levels in females. Exercise was anxiolytic in males, but this behavioral outcome did not correlate with CRF levels. Brain-derived neurotrophic factor (BDNF) has also been implicated in the expression of anxiety. As with CRF, levels of hippocampal BDNF mRNA were significantly greater in males than females. AAS and exercise were without effect on BDNF mRNA in females. In males, anxiolytic effects of exercise correlated with increased BDNF mRNA, however AAS-induced changes in BDNF mRNA and anxiety did not. In sum, we find that AAS elicit sex-specific differences in anxiety and that voluntary exercise accentuates these differences. In addition, our data suggest that these behavioral outcomes may reflect convergent actions of AAS and exercise on a sexually differentiated CRF signaling system within the extended amygdala.
Keywords: Anabolic androgenic steroids, Anxiety, Corticotrophin releasing factor (CRF or CRH), Sex-specific, Amygdala, Bed nucleus of the stria terminalis, Exercise, Brain derived neurotrophic factor (BDNF), Acoustic startle response, Social interaction
INTRODUCTION
An estimated 10 million Americans illicitly obtain AAS each year to enhance body image and athletic performance. Approximately 4% of adolescents and ~1% of girls use AAS (vandenBerg et al., 2007; Hallberg, 2011; Johnston et al., 2013). Several reports suggest that the mean age for initiation of AAS use is 14–15 years with some reported to use AAS as early as age 8 (Tanner et al., 1995; Bahrke et al., 1998; 2000), although a report published subsequent to the completion of the studies presented here suggests that most users are over 20 when AAS use begins (Pope et al., 2013). Although illegal, a survey of high school students indicates that steroids are easy to obtain (Johnston et al., 2013). AAS may also be surreptitiously added to over-the-counter supplements that are often taken in conjunction with AAS or on their own as performance enhancers (Geyer et al., 2008; Aqai et al., 2013). Thus, AAS use is prevalent and even among adolescents constitutes a significant health risk. Concern with young users is heightened by the fact that adolescence is a time when the brain is primed to be exquisitely sensitive to long-lasting or even permanent effects of steroids (Sato et al., 2008; Cunningham et al., 2013). It is also important to recognize when determining the impact of illicit AAS use on adolescent users that all AAS regimens result in supratherapeutic levels of androgens and their metabolites: by an estimated 10–100× in men and by >1000× in women and in adolescents (Oberlander and Henderson, 2012a).
Although taken to enhance somatic physiology and performance, AAS use is associated with significant changes in behavior, including anxiety, in both human (Su et al., 1993; Cooper et al., 1996; Galligani et al., 1996; Hall et al., 2005) and non-human (for review, Clark and Henderson, 2003; Oberlander and Henderson, 2012a) subjects. Moreover, AAS-dependent anxiety may contribute to other noted behavioral effects of AAS, including increased aggression, hostility and impulsivity (Annitto and Layman, 1980; Pope and Katz, 1988; Burnett and Kleiman, 1994; Cooper et al., 1996; Hall et al., 2005; Pagonis et al., 2006). Studies of AAS in rodent models suggest that chronic exposure to these synthetic steroids has diverse effects on the expression of anxiety-like behaviors that likely depend on the age and sex of the animal, dose and chemical identity of the AAS, and assay performed (for review, Clark and Henderson, 2003; Oberlander and Henderson, 2012a). For example, treatment of adult female mice with a high dose of 17α-methyltestosterone was reported to have no effect on anxiety-like behaviors on the elevated plus maze (EPM), light-dark transitions or defensive behavior tests (Barreto-Estrada et al. 2004), whereas treatment of female mice during adolescence with a mixture of AAS (methandrostenolone, nandrolone decanoate and testosterone cypionate) significantly increased anxiety-like behavior as determined by the acoustic startle response (ASR) and the EPM (Costine et al., 2010; Oberlander and Henderson, 2012b). Beyond fundamental traits such as sex, age and genetic background, environmental factors may also play an important role in AAS actions on anxiety. This hypothesis is supported by studies demonstrating that exposure to a chronic low dose of testosterone propionate increased contextual fear responses only in female mice who had been previously subjected to social isolation (Agis-Balboa et al., 2009).
That the AAS may have significant sex-specific effects on the expression of anxiety-like behaviors is also suggested by the markedly higher prevalence of anxiety and anxiety disorders, in the absence of steroid use, in females compared to males (Cameron and Hill, 1989; Pigott, 1999; for review, Palanza, 2001; Simonds and Whiffen, 2003; Bao and Swaab, 2011; ter Horst et al., 2012) and in notable sex-specific differences in brain regions that are implicated in the expression of anxiety-like behaviors. For example, the amygdala, the intermediate nucleus of the hypothalamus, the bed nucleus of the stria terminalis (BnST), and the human homologue of the medial preoptic area, INAH1, are larger in men than in women, while prefrontal cortices are larger in women than in men (Goldstein et al., 2001; Hamann 2005; Bao and Swaab, 2011). In rodents, it has been well demonstrated that early organizational actions of sex steroids result in sex-specific differences in cell number and critical chemical signaling pathways in these same regions, suggesting that by adolescence, the neural templates that give rise to anxiety are explicitly different in the male versus the female brain (Toufexis, 2007; Forger, 2009; Harada et al., 2009; Hisasue et al., 2010; Bangasser and Valentino, 2012; Gilmore et al., 2012; Valentino et al., 2012, 2013).
Connections among the regions of the brain that govern the expression of anxious states are most often reciprocal loops (McDonald et al., 1999; Dong et al., 2001; Vertes, 2004; Dong and Swanson, 2006), and bidirectional communication among key cortical and subcortical regions are needed for proper regulation of anxiety (Hammack et al., 2009; Kim et al., 2011). Neural circuits in the extended amygdala, including the pathway between the lateral central amygdala (CeA) and the dorsolateral BnST, have been shown to be critical conduits in the expression of generalized anxiety (for review, Shekhar et al., 2005; Walker et al., 2009; Davis et al., 2010). While expression of anxiety arises from the coordinate actions of many signaling systems in these regions, key among them is the peptide corticotropin releasing factor (CRF). Elevated levels of CRF are a hallmark of anxiety and stress disorders in humans (Reul and Holsboer, 2002; Hauger et al., 2006, 2009; Holsboer and Ising, 2008), and central infusion of CRF in rodents stimulates anxiety-like behaviors, as observed in a wide variety of testing paradigms (for review, Bale and Vale, 2004; Davis et al., 2010). The BnST is believed to be the primary site of the anxiogenic action of CRF. Although it has not been studied in the extended amygdala, sex-specific differences in the stress response and in CRF signaling that arise from differential internalization of CRF type 1 receptor have been demonstrated in the locus coeruleus (Bangasser et al., 2010; for review, Bangasser and Valentino, 2012; Valentino et al., 2012, 2013).
An important, but unstudied, environmental determinant in assessing the effects of AAS on anxiety is exercise. Nearly all AAS users follow an exercise regimen (Parkinson and Evans, 2006; Kokkevi et al., 2008; Ip et al., 2011). Physical activity is often found to be anxiolytic in humans (Fox, 1999; Manger and Motta, 2005; Herring et al., 2010; Herring et al., 2012), and voluntary exercise, which is usually accomplished through wheel running, has been shown to influence anxiety-like behaviors in rodents (Binder et al., 2004; Burghardt et al., 2004; Duman et al., 2008; Fox et al., 2008; Salam et al., 2009). Few studies have examined the effect of exercise on anxiety-related peptides like CRF. Voluntary wheel running has been reported to decrease CRF mRNA in the paraventricular nucleus of male mice (Droste et al., 2003, 2006). Gustafsson et al. (2011) found that voluntary exercise augmented CRF mRNA in the CeA of female mice, but that the differences did not attain significance. Overall, the exact impact of exercise on anxiety is dependent upon the duration of exercise and on the specific molecular and or behavioral endpoint examined (for review, Novak et al., 2012). Given the lack of information on sex-specific differences of AAS effects in males versus females under conditions that reflect human use paradigms, the goal of this study was to determine if there are sex-specific differences in the effect of chronic AAS on anxiety-like behaviors and the impact that exercise may have on those AAS actions.
METHODS
Animal Care and Use
80 Female and 96 male C57BL/6J mice (Jackson Laboratories Bar Harbor, ME) were used for these experiments. Standard rodent chow and water were available ad libitum. All mice were maintained in a temperature-controlled facility with a 12/12 hr on/off light cycle with lights on at 0700. On arrival at ~postnatal day (PN) 21, mice were group-housed (4 per cage) in standard cages (L × H × W) 35 cm × 12 cm × 15 cm without enrichment. Animals used to assess the effects of exercise were moved to larger cages on PN24 (vide infra). In all cases, care was taken to minimize the discomfort and the number of animals used, and all procedures were approved by the Dartmouth College Institutional Animal Care and Use Committee and conducted in accordance with guidelines from the National Institutes of Health.
Mice were injected intraperitoneally (i.p.) beginning at PN 24 for 5 days per week for 4 weeks with equal concentrations of a combination of three commonly abused AAS: testosterone cypionate (Sigma; St. Louis, MO), nandrolone decanoate (Sigma) and methandrostenolone (Steraloids; Newport, RI) dissolved in sesame oil at a final concentration of 7.5 mg/kg/day. This treatment period spans adolescence (Laviola et al., 2003), and this dosage reflects a high human abuse regime (for discussion, Costine et al., 2010). Age-matched control subjects were administered the same volume (20–30 µl based on body mass) of sesame oil alone. Behavioral testing commenced ~PN55, and treatment with either AAS or oil was continued during testing. Oil-injected control female mice used for behavioral testing were not restricted by estrous cycle stage. Following cessation of behavioral testing, estrous cycle was determined by daily vaginal lavage (Cooper et al., 1993). As previously reported, AAS treatment imposed an anestrous state characterized by diestrous-like profiles (Blasberg and Clark, 1997; Penatti et al, 2009a, 2011). Following the behavioral tests, all mice were euthanized with CO2 and decapitated. Oil-injected control female mice were euthanized in diestrus for cell biological assays.
Behavioral Testing
Except for initial experiments performed to confirm previously published results from animals housed 4 per cage in standard cages (Costine et al., 2010; Oberlander and Henderson, 2012b), at PN24, mice were transferred to larger cages that measured 45 cm × 20 cm × 23 cm and maintained 2 per cage with either two running wheels (Med Associates, St. Albans, VT) or two plastic igloos; i.e. cages of pair-housed animals had enrichment. All cages were supplied with nestlet material. Mice were maintained under these conditions to avoid possible confounds of social isolation while permitting access of each animal to a running wheel under conditions where we could surmise that both animals in a cage were active (see Voluntary Exercise below). These larger cages were supplied with ~ 4 cm of bedding to allow young mice access to water bottles and chow. All cohorts of mice were subjected to marble burying, elevated plus maze, acoustic startle, and social choice testing (in that order). Behavioral tests of animals maintained under these conditions were repeated in two or three separate cohorts of 8 animals of each sex per each different condition (oil/no exercise; AAS/no exercise; oil/exercise; AAS/exercise). All behavioral tests were performed between 1000 and 1800.
Voluntary Exercise
Each large cage containing 2 mice was equipped with two ENV-044 low profile RF running wheels coupled to a USB interface hub (Med Associates, St. Albans, VT). Running wheel activity was recorded and analyzed for revolutions per 30 min epoch for days 25–30 of the treatment period (prior to behavioral testing) using the Med Associates Wheel Manager software (Med Associates, St. Albans, VT). Although it was not possible to determine which mouse of a given pair was using which wheel during any specific epoch of a given day, analysis of the actogram data for each wheel in a single cage indicated that both wheels showed comparable patterns, suggesting similar activity by the two individual mice in a single cage. Similar results of equal time on a wheel have been reported previously for male mice housed 4/cage (Fox et al., 2008). In all experiments, mice with running wheels are described as “exercising”
Acoustic startle response (ASR)
ASR testing (Koch 1999) was carried out using the MED-ASR-PRO1/ MED-ASR-FPS apparatus and software equipped with a single chamber ENV-264C animal holder (Med Associates; St. Albans, VT, USA) and using testing paradigms effective for both male and female mice on a C57BL/6 background at these ages (Costine et al., 2010; Oberlander and Henderson, 2012b; Maue et al., 2012). Eighty female and 96 male mice were examined for ASR testing. Each cohort of animals was acclimated in the chamber without stimuli for 8 min on two different days. Two days following the second acclimation, ASR testing consisted of 5 minutes of acclimation followed by exposure to the startle stimulus of a 50ms/100 dB burst of white noise (1 ms rise/fall; 10 sec inter-trial interval) for 60 trials. Peak amplitudes of the startle responses were analyzed by linear regression for habituation over the 60 trials. No significant differences in the slopes of the habituation relationship were observed. Therefore, responses corresponding to the first 30 trials were averaged for subsequent analyses of average startle amplitude.
Elevated plus maze (EPM)
The EPM was 45 cm high and arms were 30 cm long. Walls of the closed arms were 15.25 cm high. EPM testing was done according to standard procedures (Lister, 1987; Walf and Frye, 2007). The luminous intensity in the center of the maze and at both ends of the open arms was 3.8 cd, equivalent to the intensity of the home cage during non-testing periods. The mouse was placed in the center of the EPM apparatus facing an open arm, and the time spent in the open and closed arms as well as the number of entries (scored by a keyboard event recorder) during a 5 min epoch were acquired. Assessment of the number of closed arm entries was made as an independent assessment of the effect of wheel running on locomotion. Wheels were locked at least 2 hours prior to behavioral testing to prohibit running in the period preceding the test. Sixty four female and 64 male mice were tested on the EPM.
Social choice paradigm
Tests of social interaction and social novelty preference were made according to Moy et al. (2004) and Crawley (2007). Briefly, cohorts of male or female mice (64 total for each sex) were transferred to a three-compartment arena consisting of two side chambers (24 cm × 33 cm × 39 cm) and a central compartment (19 cm × 33 m × 39 cm). Dividing walls were clear plexiglass. No bedding was added to the compartments, and the arena was thoroughly cleaned with chlorine dioxide (Clidox®) between tests and trials. Testing consisted of three consecutive 5-min sessions. In the first session (acclimation), the test mouse was placed in the center chamber with the doors to the other two chambers closed. Following acclimation, the doors were unblocked and two separate tests were performed. In test 1, an unfamiliar stimulus mouse (male and of comparable age to the test animal) was placed in one side chamber under a wire mesh cup, and an empty identical wire mesh cup was placed on the other side. In test 2, the now familiar stimulus mouse from the first session was placed under the wire mesh cup on the opposite side of the arena, and a second (novel) age-matched male stimulus mouse was placed under the wire mesh cup in the other chamber. As constructed, with no time delay between Test 1 and Test 2, this assay is believed to assess social interaction more than social memory (Moy et al., 2004; Crawley, 2007). Each animal’s movements in tests 1 and 2 were recorded on a video recorder (KODAK Model Z×5), and scoring was done offline using a keyboard event recorder. The number of crosses into each chamber, time spent in each chamber and the number of times the test mouse touched or sniffed the wire mesh cups with the familiar mouse, unfamiliar mouse or empty pencil cup were scored and analyzed. In one experiment for one group of male mice, a slightly modified two-chambered arena was used (no central compartment). A stimulus mouse was placed on one side in a wire mesh cup and an empty wire mesh cup was placed on the other side. The number of crosses the test mouse made, the time spent on each side, and the numbers of times the test mouse touched or sniffed the cup with the stimulus mouse were recorded. In test 2, the now familiar mouse from test 1 was placed on the same side and a novel mouse was placed on the other side. The number of crosses, time spent and touches were recorded as in test 1. Results for the two different arenas for male mice were not statistically different, and the data have been combined. Although the number of crosses was recorded to provide an independent measure of effects on locomotion, they are not reported as this parameter has previously been shown to be independent of a preference for social novelty (Moy et al., 2004)
Marble burying test (MBT)
Cohorts of mice (64 females and 64 males in total) were assessed for marble burying behavior according to Deacon (2006). Mice, like other rodents, engage in digging behavior. This behavior is escalated under conditions of stress and is sensitive to the anxiolytic actions of benzodiazepines (Deacon, 2006). Burying the marbles is a secondary consequence that can be used as an indirect measure of digging and of anxiety-like behavior (Deacon, 2006). Mice were placed for 30 min in a cage filled with 5 cm of bedding and 18 marbles that had been arranged in rows and spaced evenly. Marbles in which greater than two-thirds of the marble was buried were assigned a value of 1 and the number of such marbles scored.
Hanging wire test
The hanging wire test (Crawley, 2000) was performed for 16 female and 8 male mice as a simple assessment of grip strength in all mice tested. Since the ASR is a response of how hard the animal pushed against the accelerometer, strength and mass must be considered in interpreting the startle response. Startle amplitudes were normalized to mass; and grip strength was assessed to determine if there were any differences evident across any of the groups. In brief, the mouse was placed on top of a wire cage lid; the lid was shaken 2 times to prompt the mouse to grab the cage wires and then inverted. The lid remained suspended ~20 cm above a padded surface, and the time until the mouse fell off was recorded. A maximum time of 5 min was allowed for the assay.
Cell and Molecular Biological Assays
Since the effects of AAS observed in the behavioral tests for animals maintained under either 2 per cage or 4 per cage housing conditions were not significantly different, cell biological assays to assess sex-specific differences in baseline levels of CRF peptide and mRNA and the effect of AAS treatment on these molecules were made from brains pooled from animals maintained under the two housing conditions.
Protein extraction and enzyme-linked immunosorbent assay (ELISA)
Following behavioral testing, 300 µm sections were obtained from each mouse brain using an Electron Microscopy Sciences OTS-5000® vibroslicer (Hatfield, PA), and the BnST dorsal to the anterior commissure (Bregma 0.26–0.14mm) was microdissected. This region of the BnST was isolated as neural circuits in the extended amygdala, including the pathway between the lateral central amygdala and the dorsolateral bed nucleus, are critical conduits in the expression of generalized anxiety. Neurons from the central amygdala send a major GABA/CRF projection to the dorsolateral BnST, and the BnST is believed to be the primary site of the anxiogenic action of CRF (for review, Bale and Vale, 2004; Davis et al., 2010; Oberlander and Henderson, 2012a). Prior work from our laboratory has demonstrated that AAS treatment of female mice increases CRF expression in the dlBnST and augments GABAergic inhibition in this region through a CRF-dependent mechanism (Costine et al., 2010; Oberlander and Henderson, 2012b). Protein extraction was performed as described previously (Penatti et al., 2009b). Briefly, tissue was lysed in 100uL of lysis buffer (25mM Tris pH 7.5, 150mM NaCl, 5mM MgCl2, 1% NP-40, 5% glycerol, 0.001% TritonX-100, 1mM PMSF, 2mM NaF, and 1X Complete-mini (Roche, Indianapolis, IN, USA) protease inhibitor cocktail), and protein concentration was determined using a BCA Protein Assay (Pierce, Rockford, IL). Samples were then frozen at −20 °C until tissue from all animals in a cohort had been collected (as noted, for females, brains were collected when animals were in diestrus). Not more than five days later, protein lysates were analyzed using an Enzyme Immuno Assay (EIA) kit (EK-019-06, Phoenix Pharmaceuticals INC., Burlingame, CA) according to the manufacturer’s instructions. Each reaction was analyzed in duplicate on a microtiter plate reader and CRF concentration determined at an absorbance O.D. of 450nm relative to the standard curve. The detection limit for each kit was 0–100ng/mL.
RNA extraction, reverse transcription and quantitative real-time PCR (qRT-PCR)
Real time PCR was performed according to minor modifications of previously described approaches (Penatti et al., 2009a,b; Costine et al., 2010; Oberlander et al., 2012). Briefly, a 300µm slice was made using an Electron Microscopy Sciences OTS-5000® vibroslicer (Hatfield, PA), and tissue corresponding to the CeA was dissected (bilaterally) as the circular nucleus lying dorsal and lateral to the optic tract and medial to the external capsule (−2.2mm Bregma). Tissue corresponding to the hippocampus was dissected from the rest of the block. All tissue was stored in RNAlater® (Ambion Inc., Austin TX, USA) at −20 °C. Total RNA was extracted according to the manufacturers protocol for E.N.Z.A.™ MicroElute™ Total RNA kit (OMEGA bio-tek, Norcross,GA), DNase treated with RNase-Free DNase 1 (OMEGA bio-tek, Norcross,GA), and the concentration of the RNA was determined by measuring the optical density at 260 nm. 50ng of RNA was reverse transcribed using reagents and protocols included in the iScript™ cDNA Synthesis kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR was performed using an AB 7500 Sequence Detection System, and all cDNAs were analyzed in triplicate with TaqMan®Gene Expression Assays (Life Technologies, Foster City, CA). Primers used were Mm01293920_s1 (CRF); Mm01334047_m1 (BDNF) and Hs99999901_s1 (18S rRNA). Samples with reverse transcriptase omitted were used to control for genomic DNA contamination and with template omitted to control for any reagent contamination. All gene expression assays used were demonstrated to amplify with equal efficiencies. Data are expressed normalized to endogenous control as 2−ΔCT values (Livak and Schmittgen, 2001; Peirson et al., 2003).
Statistics
Values for variables are presented as mean ± standard error of the mean. Statistical analyses were run using SPSS 18 and STATView. Repeated measures ANOVA was used to analyze the ASR data. Two-way ANOVA with cohort as a blocking variable was used in analyses of the interaction and overall effects of exercise and AAS. Individual groups were compared using one-way ANOVAs to determine significant differences. Numbers of animals (n) are indicated for all data in figures and/or figure legends. In those figures where data are presented normalized to control groups, statistical analyses were performed on the raw data values. For all data, the alpha level was set at p ≤ 0.05. For data comparisons with p-values greater than alpha (i.e. p ≥ 0.05) but less than 0.1, effect size was calculated as Cohen’s d (Cohen, 1988). In all figures, asterisks indicate statistical significance where * indicates p ≤ 0.05; ** indicates p ≤ 0.01; *** indicates p ≤ 0.001.
RESULTS
I. Sex-specific effects of chronic AAS treatment on anxiety-like behaviors
Chronic AAS treatment increases anxiety-like behaviors of female but not male mice
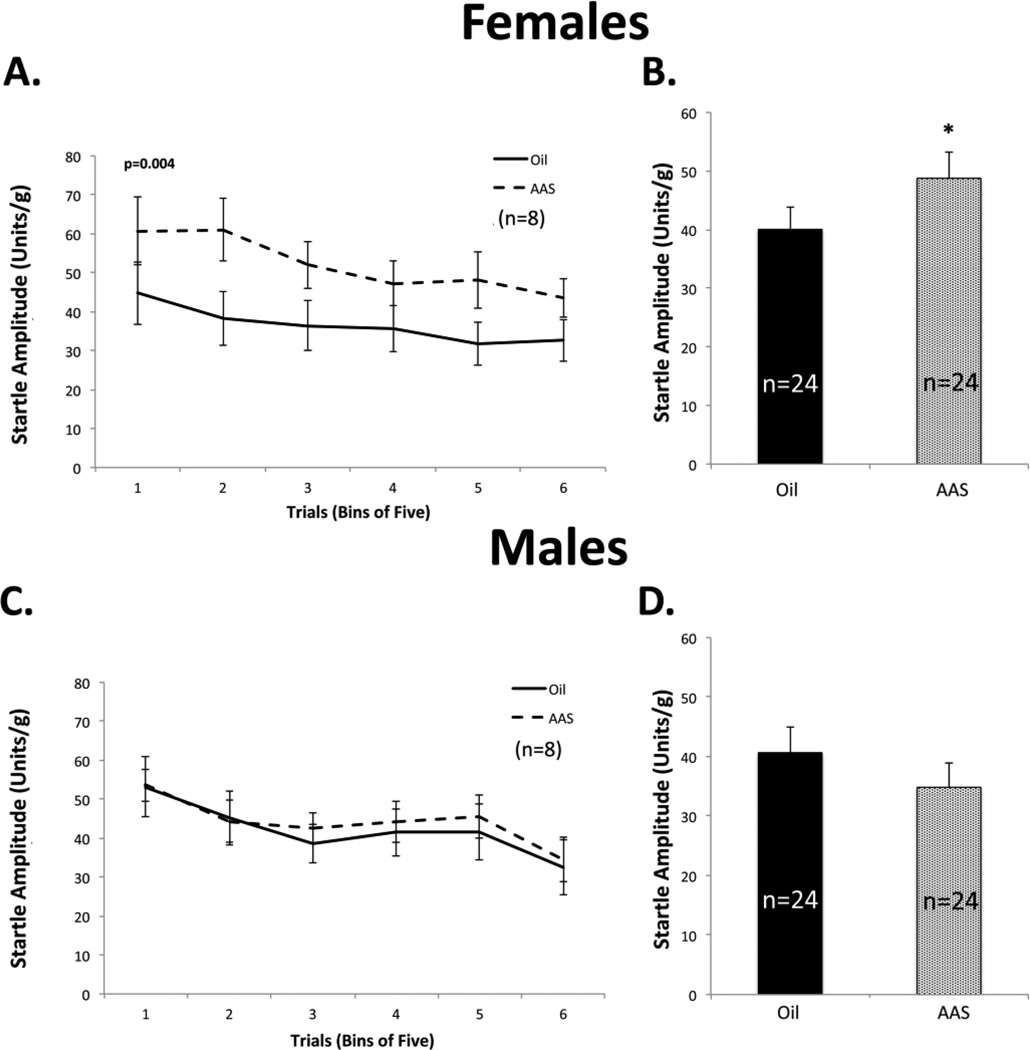
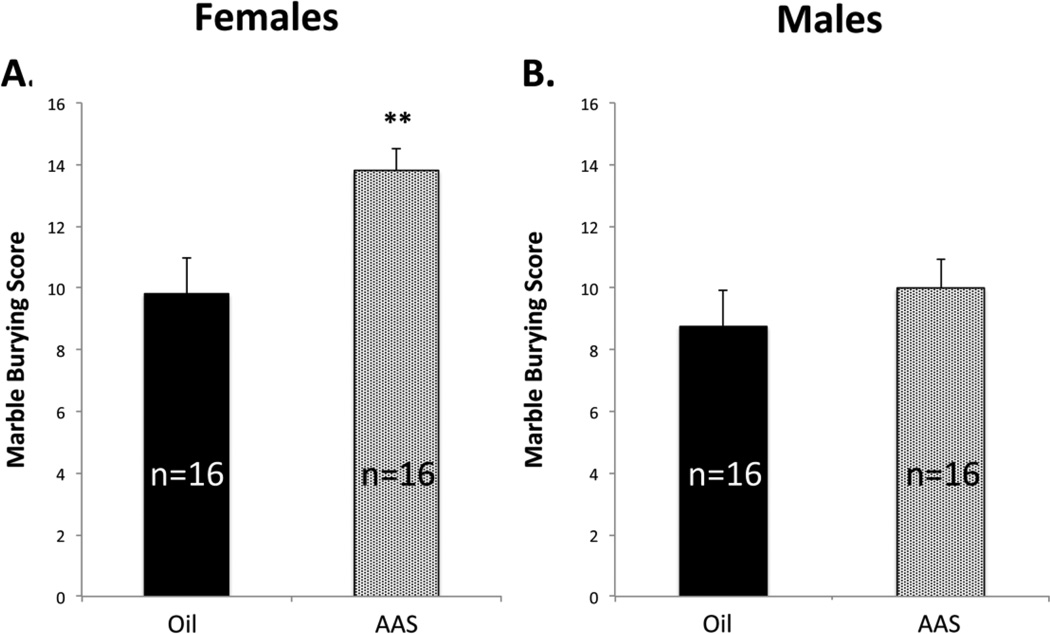
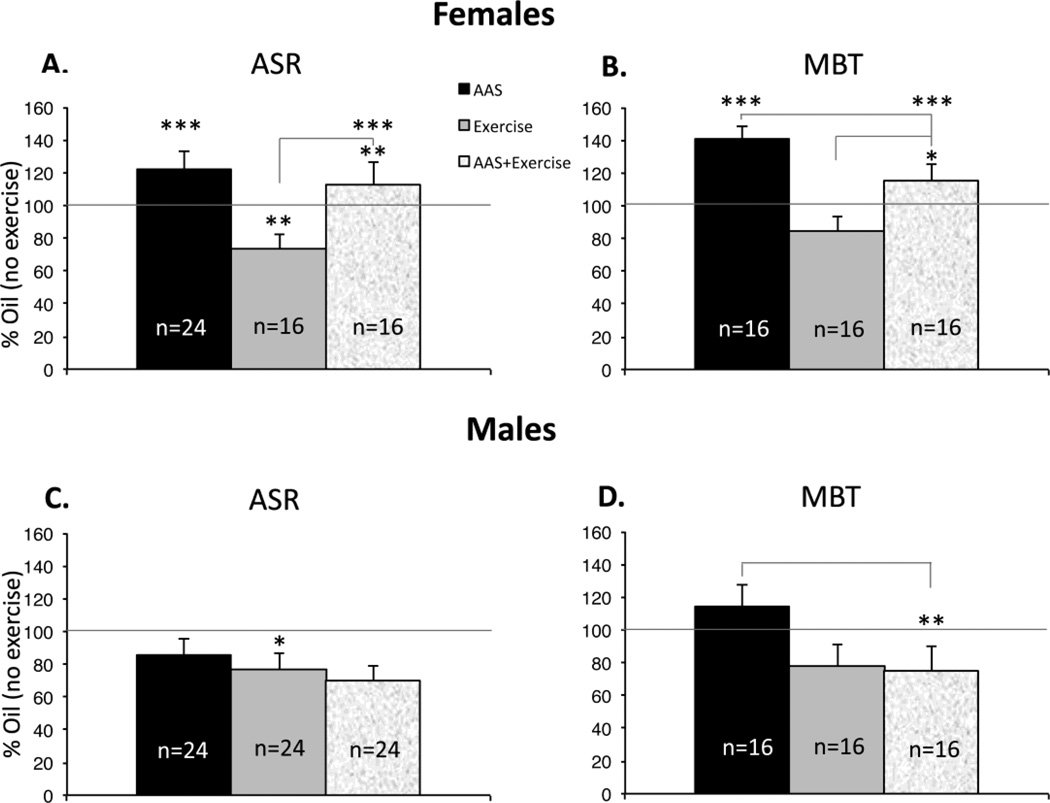
Initial experiments replicated the results of previous studies (Costine et al., 2010; Oberlander and Henderson, 2012b) by confirming that AAS treatment throughout adolescence of female C57BL/6 mice maintained 4/cage in standard mouse housing significantly increased the average startle amplitudes on the ASR [F1,14 = 5.312, p = 0.037]. All subsequent behavioral studies were carried out with multiple cohorts of eight animals for each sex and treatment condition maintained as pairs in larger cages with enrichment. For female mice pair-housed in these larger cages with enrichment, AAS treatment also led to a significant increase in the average amplitude of the ASR [F1,42 = 5.883, p = 0.02] (Figure 1A, B). Anxiety-like behaviors of female mice treated with AAS were also found to be significantly elevated on the marble burying test [F1,28 = 11.00; p = 0.003] (Figure 2A).
Figure 1. AAS effects on startle amplitude.
(A) Representative ASR data from one cohort (n = 8) of female mice maintained in pair-housed conditions with enrichment (igloos) illustrating habituation of the first 30 startle responses (5 responses/bin) and significant AAS-dependent enhancement of the startle response for this cohort (p = 0.004; n = 8 per group). (B) Averaged data for three cohorts of female mice. Oil: oil-injected and AAS: AAS-treated. There was a significant increase in startle with AAS treatment (*p = 0.02). (C) Comparable representative data from a cohort (n = 8) of male mice and (D) averaged data from three cohorts of male mice maintained in pair-housed conditions with enrichment. AAS did not have a significant effect on startle amplitude in males.
Figure 2. AAS effects on marble burying.
Marble burying scores were significantly higher in AAS-treated female (A; **p = 0.003) but not male (B) mice.
When normalized for mass, the average startle amplitudes between oil-injected male and female mice were not significantly different. However, in contrast to female mice, comparable AAS treatment of male mice had no significant effect on startle amplitude with pair-housed animals [F1,42 = 2.625; p = 0.113] (Figure 1C, D) or when all animals were pooled across the different housing conditions (8 cohorts of 8 AAS-treated and 8 oil-injected male mice). Similarly, AAS treatment had no effect on marble burying scores in male mice [F1,28 = 0.916; p = 0.347] (Figure 2B).
Low levels of social interaction may also be symptomatic of anxiety disorders in humans and startle amplitudes in people are correlated with social anxiety (Grillon, 2008). Tests for social interactions have been assessed in mice by using a social choice paradigm, wherein diminished exploration of the novel mouse may reflect an anxiety-related state (Crawley, 2007). Analysis of the parameters measured in this test revealed a significant effect of sex in that oil-injected males spent more time on the novel side than did oil-injected females [F1,28 = 4.313; p = 0.047]. AAS-injected males also spent more time on the familiar side than did AAS-injected females [F1,28 = 7.140; p = 0.012] and made more touches on the familiar side [F1,28 = 13.46; p = 0.001]. However, within a given sex, there was no significant effect of treatment (Table 1A).
Table 1.
Social interaction test parameters.
| Females | Males | |||
|---|---|---|---|---|
| A. Non-Exercising Mice | ||||
| Oil | AAS | Oil | AAS | |
| %Familiar Side | 41.2 ± 2.4 | 37.3 ± 4.0** | 44.3 ± 2.1 | 49.4 ± 2.3 |
| Touches | 23.3 ± 1.9 | 19.8 ± 2.5** | 26.9 ± 1.5 | 27.3 ± 1.3 |
| %Novel Side | 42.4 ± 2.5* | 49.8 ± 3.7 | 49.7 ± 2.5 | 43.1 ± 2.5 |
| Touches | 26.4 ± 2.3 | 24.2 ± 2.6 | 27.9 ± 2.0 | 23.6 ± 1.9 |
| %Center | 16.4 ± 1.9 | 12.9 ± 1.5 | 12.7 ± 1.1 | 15.3 ± 2.7 |
| Females | Males | |||
|---|---|---|---|---|
| B. Exercising Mice | ||||
| Oil | AAS | Oil | AAS | |
| %Familiar Side | 51.9 ± 5.7 | 39.9 ± 3.5 | 46.4 ± 4.8 | 46.6 ± 3.5 |
| Touches | 27.8 ± 3.0 | 22.9 ± 2.9 | 23.0 ± 2.3 | 26.8 ± 2.9 |
| %Novel Side | 33.9 ± 4.7 | 42.9 ± 3.5 | 47.5 ± 4.4 | 46.3 ± 3.5 |
| Touches | 19.4 ± 3.0 | 25.1 ± 2.6 | 26.1 ± 3.2 | 28.5 ± 2.2 |
| %Center | 14.2 ± 2.6 | 17.2 ± 3.1 | 12.3 ± 0.9 | 14.1 ± 2.0 |
(A) Average values for female and male mice in social interaction paradigm for animals housed without access to running wheels (no exercise). Significant differences were evident between the sexes, but there was no significant effect of treatment on these measures. Specifically, between AAS-treated males versus females, there were significant differences on the percent time spent on the familiar side (% Familiar Side; **p = 0.012) and on the number of touches on the familiar side (***p = 0.001). There was also a significant difference on the percent time spent on the novel side (%Novel Side; *p = 0.047) oil-injected males versus females. (B) Average values for female and male mice in social interaction paradigm for animals with access to running wheels (exercise). There was a significant overall effect of sex on the percent time spent on the novel side (% Novel Side; p = 0.043) however no significant differences were evident on pairwise comparisons.
II. Sex-specific effects of chronic AAS treatment on CRF in the extended amygdala
CRF levels in the extended amygdala are sexually differentiated and AAS treatment abrogates that difference
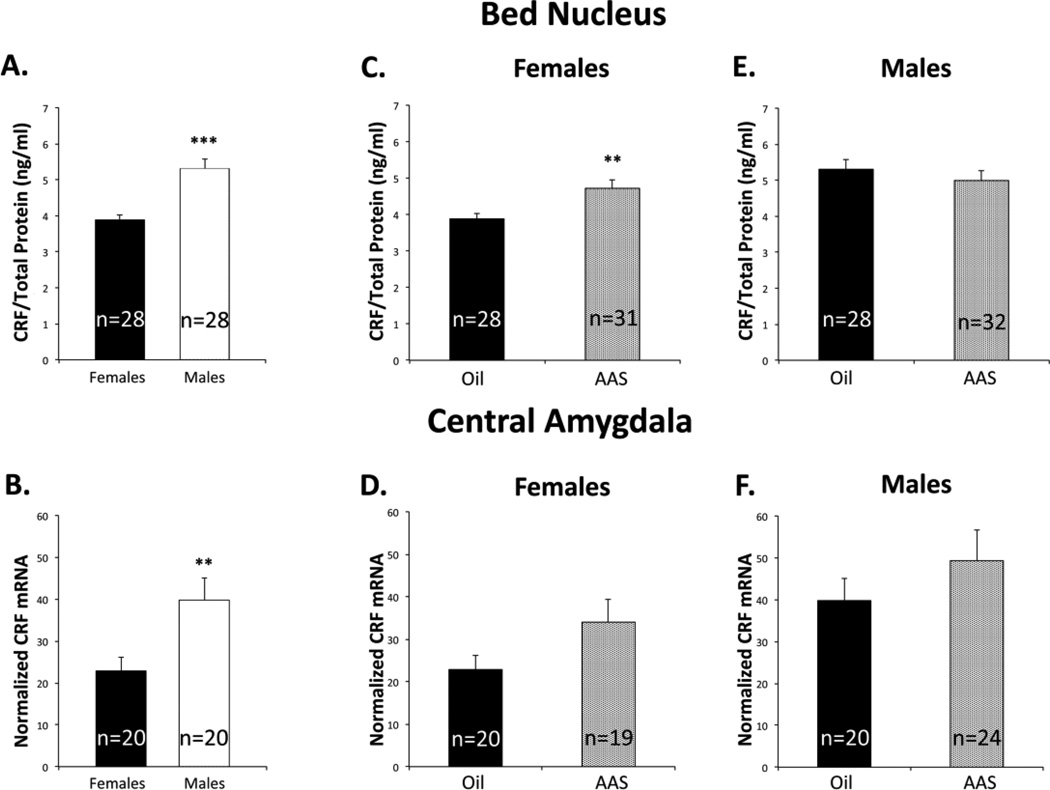
For male and female mice assessed at ~PN65, there was a significant interaction of sex by treatment for CRF peptide levels in the BnST [F1,115 = 12.02; p = 0.02]. Pairwise comparisons indicated that CRF levels were significantly higher in oil-injected male than oil-injected female mice [F1,54 = 21.05; p < 0.0001]. As has been reported previously using immunocytochemistry (Costine et al., 2010), chronic treatment of female mice with AAS throughout adolescence significantly increased CRF peptide (in the current study, as measured by ELISA) in the BnST of female mice [F1,52 = 7.709; p = 0.008] (Figure 3C). No effect of AAS treatment on CRF peptide was observed for male mice (Figure 3E). The AAS-induced increase in CRF in female, but not male, mice abolished the sex-specific difference observed in baseline levels of this peptide. Pairwise comparisons of AAS-treated males versus females indicated no significant difference in the levels of CRF in the BnST [F1,61 = 1.16; p = 0.464].
Figure 3. Sex-specific differences in baseline and AAS-induced changes of CRF in the extended amygdala.
(A) Levels of CRF peptide in the BnST were significantly higher in oil-injected male than female mice (***p < 0.0003). (B) Levels of CRF mRNA in the CeA were significantly higher in oil-injected male than female mice (**p < 0.004). (C) AAS treatment significantly increased CRF peptide levels in the BnST (**p = 0.008) in female mice. There was no effect of AAS treatment on (E) CRF peptide in the BnST or (F) CRF mRNA in the CeA in male mice. The data in A and B are the same data for oil-injected animals shown in C–F, but have been shown on separate axes to more clearly illustrate baseline sex-specific differences in CRF in the extended amygdala.
There was also a significant effect of sex with respect to CRF mRNA in the CeA [F1,81 = 7.789; p = 0.007] wherein levels of CRF mRNA in were significantly higher in oil-injected male than oil-injected female mice [F1,40 = 7.037; p = 0.011]. We previously observed that AAS treatment significantly increased CRF mRNA in the CeA of female mice (Costine et al., 2010), however, in the current study, this increase did not attain significance [F1,81 = 3.223; p = 0.076] (Figure 3D). Cohen’s effect size (d = 0.78) suggested a high practical significance. No effect of treatment was evident for CRF mRNA in the CeA of male mice (Figure 3F). As with CRF peptide in the BnST, the overall impact of AAS treatment was to eliminate the sex-specific difference observed in levels of this transcript. Pairwise comparison of AAS-treated male versus female mice indicated no significant difference in the levels of CRF mRNA [F1,41 = 2.56; p = 0.117].
III. Sex-specific interactions of AAS and voluntary exercise
Wheel running as a form of voluntary exercise has been postulated to promote anxiolytic effects in rodents (Binder et al., 2004; Fox et al., 2008; Duman et al., 2008; Salam et al., 2009). To determine if exercise alters the effects of chronic AAS treatment on the expression of anxiety-like behavior, data from oil-injected and AAS-injected male and female mice were compared for animals pair-housed with igloos versus those pair-housed with running wheels.
Chronic AAS treatment significantly alters wheel-running activity in male but not female mice
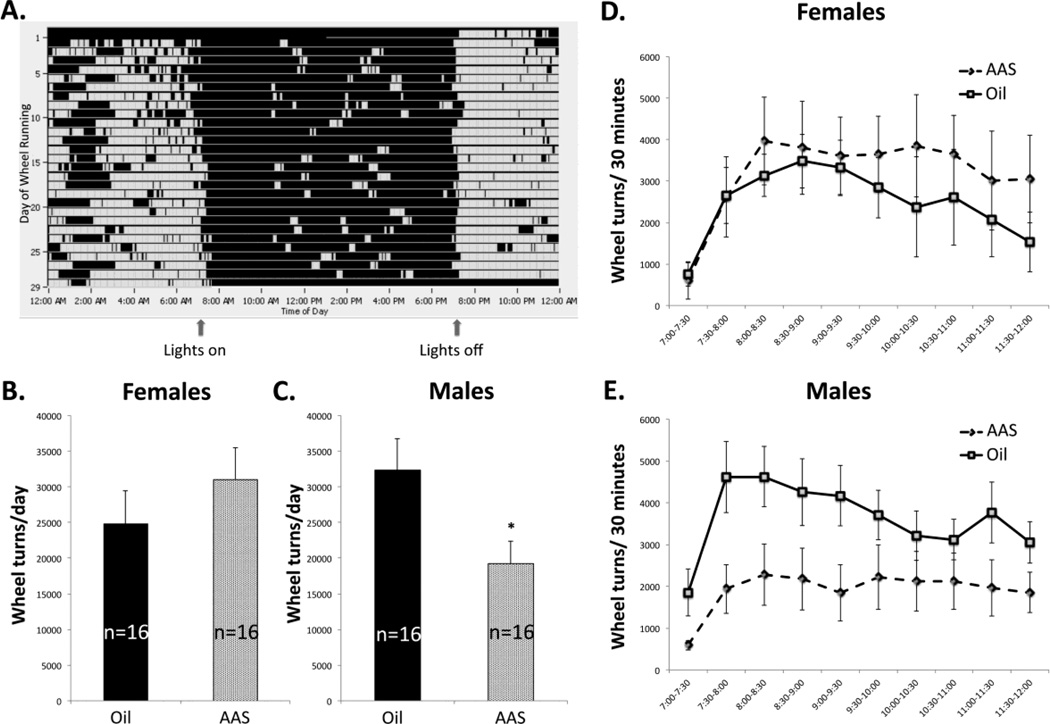
All groups of mice (male or female; AAS-treated or oil-injected) increased wheel running commensurate with lights off (Figure 4A). There was a statistically significant interaction of sex and treatment [F1,60 = 4.972; p = 0.03] for daily running activity. Pairwise comparisons indicated that daily running activity was not significantly different in oil-injected versus AAS-treated females (Figure 4B, D); however, activity was significantly lower in AAS-treated versus oil-injected male mice [F1,28 = 5.414; p = 0.027] (Figure 4C, E).
Figure 4. Running wheel activity in oil- and AAS-treated mice.
A) Representative actogram of daily running activity. Each horizontal line represents a 24 hr period and each grey block represents running activity in 5 min bins over that day. AAS treatment did not change the circadian patterning of running; all groups were most active in the dark cycle. (B) Average data indicating that AAS treatment did not significantly alter the number of wheel rotations in female mice. (C) Average data indicating that AAS treatment significantly decreased average wheel rotations in male mice (*p = 0.027). (D) Representative average running activity for the first 6 hours of the dark cycle (7:00 PM to midnight) binned in 30-minute blocks demonstrating that AAS treatment did not significantly alter running in female mice (same animals as for data in C). (E) Representative average running activity for the first 6 hours of the dark cycle binned in 30-minute blocks demonstrating that AAS treatment significantly decreased average running activity over the period in male mice (same animals as for data in C).
AAS and exercise exert differential effects on body mass in female versus male mice
In females, where AAS-treated and oil-injected mice had comparable levels of activity, there was no significant effect of exercise on body mass. There was however, an overall effect of treatment on body mass [F1,70 = 31.09; p < 0.0001] wherein AAS-treated females weighed significantly more than oil-injected controls in both the non-exercising [F1,42 = 50.98; p < 0.0001] and the exercising groups [F1,28 = 5.764; p =0.023] (Table 2). In males, despite the lower level of activity in AAS-treated male mice, there was an overall effect of exercise on body mass in this sex [F1,83 = 10.77; p = 0.002] wherein males that had access to running wheels weighed less than their counterparts maintained with igloos in both oil-injected [F1,41 = 6.395; p =0.015] and AAS-treated [F1,42 = 4.678; p =0.036] mice (Table 2). There was no effect of AAS treatment on body mass in male mice. Grip strength, as measured by the hanging wire test, was not significantly altered by exercise or AAS treatment in either females or males (Table 2).
Table 2.
AAS effects on body mass and grip strength
| Females | Males | |||
|---|---|---|---|---|
| Mass | Hanging Wire | Mass | Hanging Wire | |
| Oil | 18.1 ± 0.2 | 291.6 ± 8.4 | 23.0 ± 0.6 | 242.9 ± 37.6 |
| AAS | 20.0 ± 0.2*** | 287.3 ± 9.2 | 23.6 ± 0.5 | 226.9 ± 39.9 |
| Oil+Exercise | 18.6 ± 0.2 | 272.8 ± 17.6 | 21.8 ± 0.5*** | 270.3 ± 29.8 |
| AAS+Exercise | 19.5 ± 0.3*** | 263.1 ± 20.9 | 22.5 ± 0.5*** | 293.3 ± 6.8 |
Average values for female and male mice for mass (PN62) and for maximum time that they remained suspended on the hanging wire test. n = 16 animals for each sex for all measurements except the hanging wire test for males (n = 8). AAS-treated females weighed significantly more than oil-injected controls for both exercise and non-exercise groups (***p< 0.0001). In contrast, males that had access to running wheels weighed less than their counterparts maintained with igloos in both oil-injected (p =0.015) and AAS-treated (p =0.036) groups.
Interactions of AAS and exercise on anxiety-like behaviors in female and male mice
Despite the reported anxiolytic effects of exercise, voluntary wheel running did not abrogate the increase in anxiety-like behavior in AAS-treated female mice. Despite access to running wheels, there was still an overall effect of AAS treatment on startle amplitudes [F1,70 = 16.60; p < 0.0001] and on marble burying scores [F1,56 = 13.71; p < 0.0001] in this sex. Pairwise comparisons revealed that ASR amplitudes [F1,28 = 11.52; p = 0.002] (Figure 5A) and marble burying scores [F1,28 = 5.205; p = 0.03] (Figure 5B) were significantly higher for AAS-treated exercising females than their oil-injected counterparts.
Figure 5. Effects of exercise on anxiety-like behaviors.
Data demonstrating average values for AAS-treated animals without access to running wheels (black bar), oil-injected animals with access to running wheels (grey bar) and AAS-injected animals with running wheels (stippled bar) normalized to the values for oil-injected animals without access to running wheels (100%: solid line). (A) ASR amplitudes of female mice were significantly higher with AAS treatment whether female mice had access to running wheels or not (***p < 0.0001; comparison of histogram bar values to the line representing 100% of oil-injected/no exercise). ASR amplitudes were also significantly higher in AAS-treated than oil-injected females who exercised (**p = 0.002; stippled versus grey bars). Exercise was correlated with a significant decrease ASR amplitude for oil-injected female mice (**p = 0.02; grey bar versus line indicating 100%), however the difference reflects a contribution of cohort (p = 0.033). (B) Consistent with results on the ASR, there was a significant effect of AAS treatment on marble burying scores of female mice irrespective of exercise (***p < 0.0001). Marble burying scores were also significantly higher in AAS-treated than oil-injected females who exercised (*p = 0.03; stippled versus grey bars). There was also a significant overall effect of exercise (p = 0.037) on marble burying with exercise significantly decreasing marble burying in AAS-treated female mice (*p = 0.045; black versus stippled bars). (C) For male mice, there was neither a significant effect of treatment nor an interaction of treatment and exercise on the ASR. In males, there was an overall effect of exercise on the ASR (p = 0.005) with significantly lower startle amplitudes with exercise for oil-injected mice (*p = 0.032; grey bar versus 100%). (D) For male mice, there was a significant overall effect of exercise on marble burying (p = 0.015), and scores were significantly lower with exercise for AAS-treated males (**p = 0.006; black versus stippled bars).
Female mice
For female mice, there was a significant effect of exercise on the ASR when cohort was not used as a blocking variable [F1,76 = 5.63; p = 0.02] (Figure 5A). However there was also a significant effect of cohort (p = 0.033), and when it was used as a blocking variable, no overall effect of exercise on the startle response was evident. There was an overall effect of exercise on marble burying in female mice [F1,56 = 4.536; p = 0.037]. This effect could be attributed to lower scores with exercise in the AAS-treated group [AAS versus AAS+Ex; F1,28 = 4.422; p = 0.045] (Figure 5B). Finally, there was no interaction on either test for AAS treatment and exercise. For females in the social choice paradigm, there was no significant interaction between treatment and exercise, however there was a significant overall effect for time spent on the novel side for treatment [F1,56 = 4.793; p = 0.033] and for exercise [F1,56 = 4.211; p = 0.045]. This was because the group of AAS-treated females in toto (exercising and non-exercising) spent more time on the novel side than their oil-injected counterparts and that non-exercising females in toto (AAS-treated and oil-injected) spent more time on the novel side than their exercising counterparts (Table 1B).
Male mice
For male mice as previously discussed, there was no significant effect of AAS treatment on the ASR (Figure 1C, D), nor was there an interaction of AAS treatment and exercise on this test. There was, however, an overall effect of exercise on the ASR [F1,83 = 8.436; p = 0.005], with lower ASR levels in the males from the exercise groups. There was no overall effect of cohort in males. Pairwise comparisons indicated that the decrease in startle amplitudes with exercise in males attained statistical significance in the oil-injected mice [F1,41 = 4.947; p = 0.032], although this change did not attain significance for AAS-treated males [F1,42 = 3.454; p = 0.07] (Figure 5C). For male mice, there was also a significant overall effect of exercise on marble burying score [F1,56 = 23.34; p = 0.015], with the decrease attaining significance in the mice treated with AAS [F1,28 = 8.725; p = 0.006] (Figure 5D). For male mice in the social interaction paradigm, there was no significant effect of exercise on the percent time spent on either the novel side or the familiar side (Table 1B).
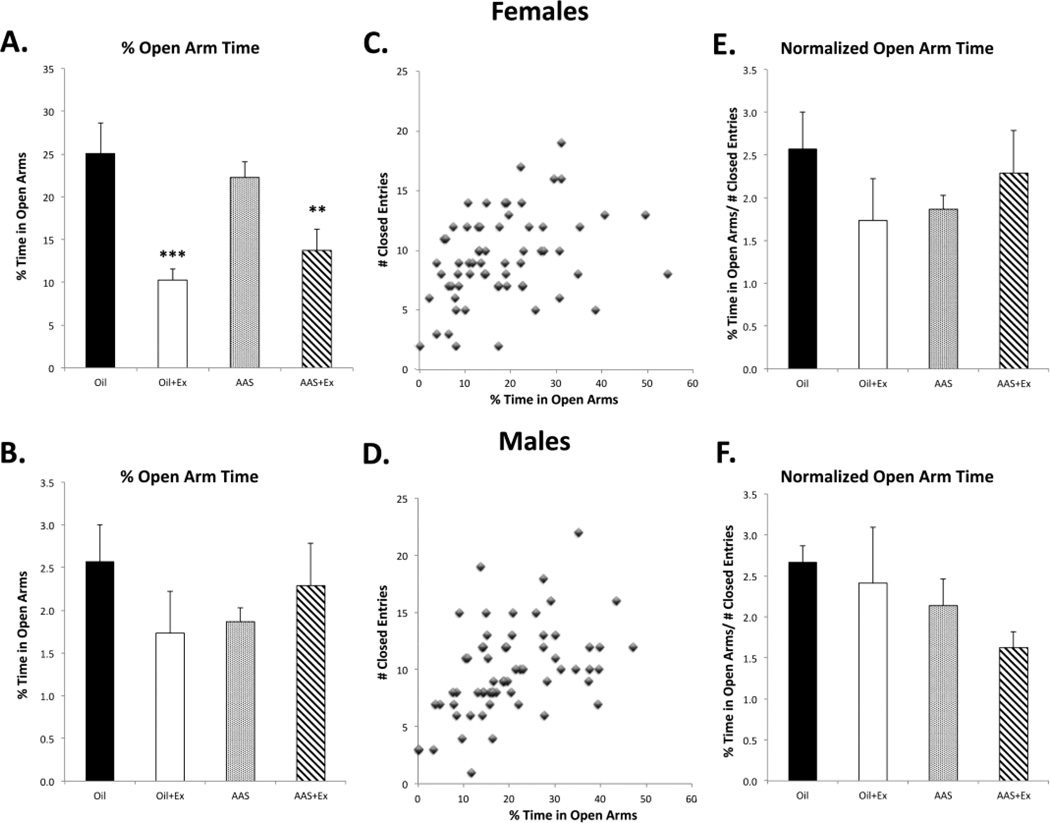
Exercise decreases locomotion on the elevated plus maze in both female and male mice
AAS treatment of female mice maintained 4 per cage without enrichment has been shown to decrease the total time spent on the open arms in the EPM, supporting an anxiogenic action of chronic AAS treatment in this sex (Oberlander and Henderson, 2012b). In the current study, for both oil- and AAS-injected mice, when animals were housed with running wheels, we found that exercise significantly decreased time spent in the open arms in both females [F1,56 = 25.31; p < 0.0001] (Figure 6A) and males [F1,56 = 36.16; p < 0.0001] (Figure 6B). However, exercise also promoted a significant decrease in closed arm entries in both females [F1,56 = 30.52; p < 0.0001] and males [F1,56 = 20.14; p < 0.0001]. Moreover, this decrease in closed arm entries correlated with open arm time in both females (Figure 6C; R = 0.34; p = 0.006) and males (Figure 6D; R = 0.51; p = 0.00002). Fuss et al. (2010) have also reported that wheel running decreased both exploration and overall locomotion in adult male C57BL/6 mice. Normalizing percent open arm time to closed arm entries revealed no significant effect of exercise on percent time spent in the open arms on the EPM in either female or male mice (Figure 6E, F) and suggests that interpretation of the effects of exercise on AAS-induced anxiety as measured on the EPM may be compromised by the overall effect of wheel running on locomotion.
Figure 6. Effects of exercise on locomotion in male and female mice.
Four weeks of voluntary exercise significantly decreased percent time spent in the open arms of the elevated plus maze in (A) female oil-injected (Oil versus Oil+Ex; ***p = 0.0009) and AAS-treated (AAS versus AAS+Ex; **p = 0.01) mice and in (B) male oil-injected (***p < 0.0001) and AAS-treated (*p = 0.028) mice. Time spent in the open arms was directly correlated with the number of closed arm entries (a measure of locomotion) in (C) female (R = 0.34; p = 0.006) and (D) male (R = 0.51; p = 0.00002) mice. Percent time spent in the open arms normalized to the number of closed arm entries to account for the effect of locomotion on open arm time in (E) female and (F) male mice. There was no significant effect of exercise on normalized percent open arm time in either sex.
IV. Interactions of AAS and Exercise on CRF in the Extended Amygdala
Exercise has sex-specific effects on CRF in the extended amygdala
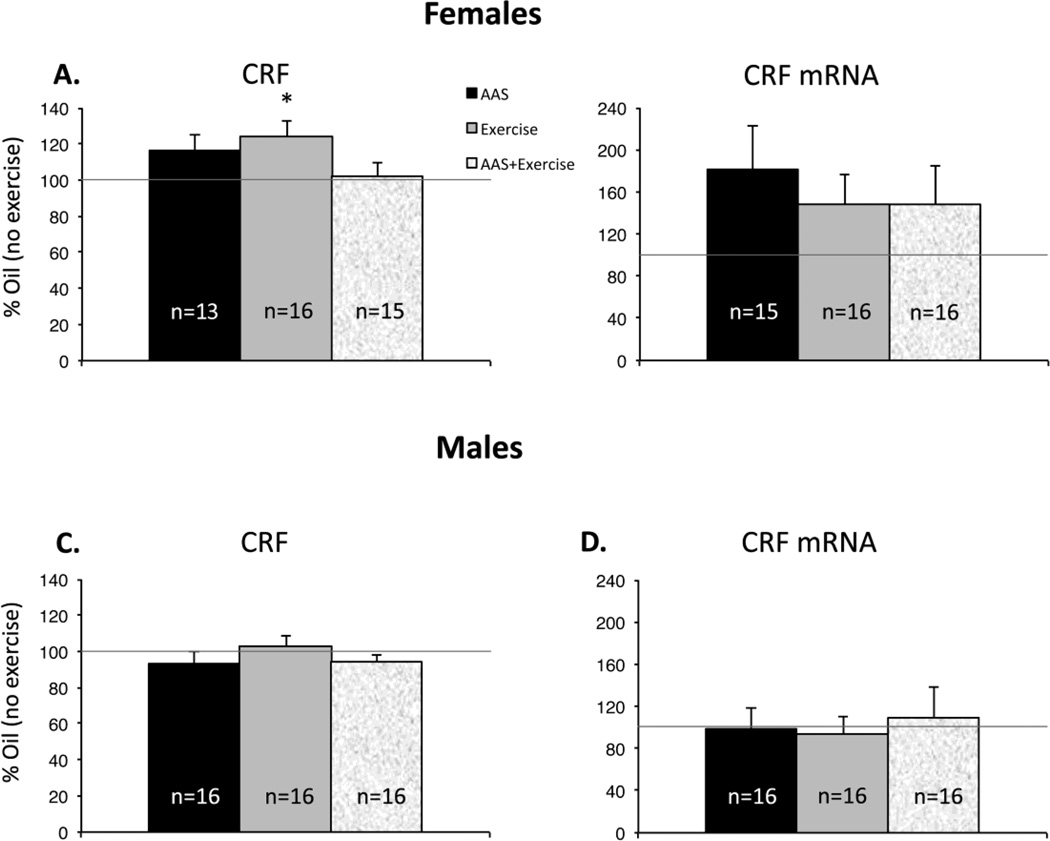
There was a significant interaction of AAS treatment and exercise for female mice [F1,52 = 5.918; p = 0.018] on the levels of CRF peptide in the BnST. Pairwise comparisons revealed that exercise significantly increased CRF peptide in the BnST of oil-injected [F1,28 = 5.498; p = 0.027] but not AAS-treated female mice (Figure 7A). There was no effect of exercise on CRF mRNA in female mice (Figure 7B).
Figure 7. Effects of exercise on CRF levels in the extended amygdala.
Data are displayed as in Figure 6: average values for AAS-treated animals without access to running wheels (black bar), oil-injected animals with access to running wheels (grey bar) and AAS-injected animals with running wheels (stippled bar) normalized to the values for oil-injected animals without access to running wheels (100%: solid line). (A) Levels of CRF peptide in the BnST of female mice were significantly higher in oil-injected animals that had access to running wheels (*p = 0.027; grey bar versus 100%). (B) In non-exercising female mice. (C) Neither treatment nor exercise had an effect on CRF peptide in the BnST of male mice.(D) Neither exercise nor treatment altered CRF mRNA in the CeA in male mice.
For male mice, as discussed, there was no effect of AAS treatment on CRF mRNA or peptide (Figure 3B, D). Moreover there was neither an effect of exercise nor any interaction between AAS treatment and exercise on CRF mRNA or peptide in this sex (Figure 7C, D).
Exercise has sex-specific effects on BDNF in the hippocampus
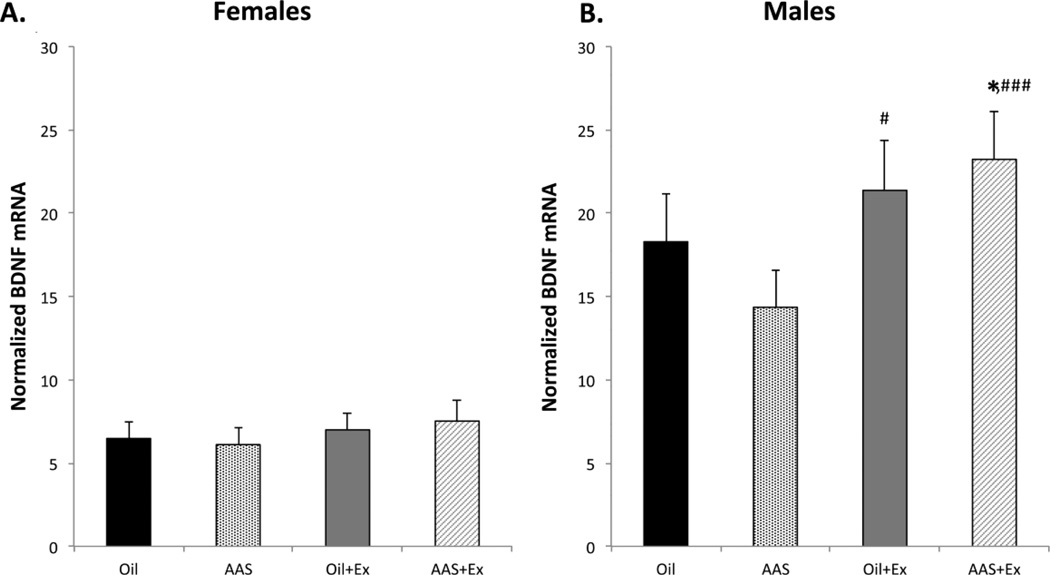
Although neurotransmission within the extended amygdala is central to the expression of generalized anxiety, the full range of anxiety behaviors reflects a broader system that includes bidirectional communication with other key structures, including the hippocampus (Canteras et al., 2010; Kim et al., 2011). Exercise is known to impose significant effects on the expression of anxious states, and brain-BDNF acting in this region has been implicated as a major player in mediating these exercise-induced effects (for review; Pedersen et al., 2009; Zoladz and Pilc, 2010; Novak et al., 2012). As exercise elicited a significant decrease in marble burying scores in AAS-treated males without any corresponding changes in CRF levels, we also examined the effects of AAS and exercise on the levels of BDNF mRNA in the hippocampus.
Hippocampal BDNF mRNA was significantly higher in male than in female mice across all treatment and exercise conditions [F1,119 = 72.86; p < 0.0001]. Neither AAS treatment nor exercise altered BDNF mRNA in female mice (Figure 8A). In male mice, there was a significant interaction of treatment and exercise [F1,55 = 6.447; p = 0.014]. Pairwise comparisons indicated that AAS treatment significantly decreased BDNF mRNA levels in males that did not have access to running wheels [F1,28 = 4.245; p = 0.049], but that exercise significantly increased levels of this transcript in both AAS-treated [F1,27 = 19.89; p < 0.0001] and oil-injected [F1,28 = 4.893; p = 0.035] male mice, thus abolishing the AAS versus oil difference in the exercising group for this sex (Figure 8B).
Figure 8. Effects of exercise on BDNF mRNA expression in the hippocampus.
(A) Neither treatment nor exercise altered BDNF mRNA in the hippocampi of female mice. (B) AAS treatment significantly decreased BDNF mRNA in the hippocampi of male mice (Oil versus AAS; *p = 0.049). Exercise significantly increased BDNF in the hippocampi of oil-injected (Oil versus Oil+Ex; #p = 0.035) and AAS-treated (AAS versus AAS+Ex; ###p < 0.0001) male mice. In both sexes, n = 16 per group.
DISCUSSION
Chronic use of supratherapeutic levels of AAS is associated with significant changes in a wide range of complex behaviors and in a multitude of neural signaling mechanisms that give rise to them (for review, Clark and Henderson, 2003; McGinnis, 2004; Wood, 2008; Melloni and Ricci, 2010; Oberlander and Henderson, 2012a). Despite the growing body of data demonstrating the impact of AAS use on the brain and behavior, the majority of studies has not incorporated critical variables such as sex into their designs, and only a handful has assessed the importance of key environmental variables, such as exercise.
Sex-specific differences in AAS-induced anxiety-like behaviors
Anxiogenic effects in rodents treated with AAS have been repeatedly demonstrated (Rocha et al., 2007; Agis-Balboa et al., 2009; Ambar and Chiavegatto, 2009; Costine et al., 2010; Oberlander and Henderson, 2012b). However, AAS exposure does not always promote an increase in anxiety (Bitran et al., 1993; Ågren et al., 1999; Barreto-Estrada et al., 2004; Rojas-Ortiz et al., 2006; Steensland et al., 2005; Frye et al., 2008; Kouvelas et al., 2008). The variability in AAS effects on expression of anxiety-like behaviors is hypothesized to arise from a host of factors, of which sex is likely key (for review, Clark and Henderson, 2003; Clark et al., 2006). However, teasing out the importance of sex has been confounded by the fact that few studies use the same age, species or strain of animal and employ different environmental conditions, behavioral assays, and drugs, including differences in their doses and durations. We show that for animals maintained without access to running wheels, chronic treatment of female C57BL/6 mice throughout adolescence with a mixture of three AAS that are commonly abused in the human population increases anxiety-like behaviors as measured on the ASR (Costine et al., 2010; Oberlander and Henderson, 2012b; current study), the EPM (Oberlander and Henderson, 2012b) and on the marble burying test (current study). In contrast, treatment of male C57BL/6 mice of the same age and with the same AAS paradigm was without effect on these behaviors. Results from the social interaction paradigm also indicated that female mice were more sensitive than male mice to the actions of the AAS in that AAS treatment significantly augmented the time spent on the novel side for female mice, but was without effect in males. However, this sex-specific difference only attained significance when data from both exercising and non-exercising groups were combined. Within a given exercise condition, AAS treatment was without effect on measures of social interaction/social novelty in either sex. Thus, the interpretation of these data is likely to reflect more complex phenomena than simply an anxiogenic or anxiolytic effect.
The basis for the insensitivity to AAS effects on anxiety-like behaviors in these male mice is not known, but is unlikely to reflect an overall insensitivity of gonadally intact male mice to the actions of AAS arising from a “ceiling effect” imposed by endogenous androgens since high doses of AAS diminish testes weight and circulating testosterone (Clark et al., 1997). Similarly, a dearth of androgens, and thus androgen receptor signaling in the brain, is also an unlikely cause as radioimmunoassays of male mice injected with this AAS mixture demonstrate levels of testosterone in brain tissue (likely AAS-derived) that were greater than 8-fold those found in oil-injected controls (Penatti et al., 2009b). Finally, gonadally intact male mice are capable of responding to chronic AAS treatment with increased aggression (Martínez-Sanchis et al., 1998; Ambar and Chiavegatto, 2009; Robinson et al., 2012), as are gonadally intact adolescent male hamsters (for review, Melloni and Ricci, 2010). We instead favor the hypothesis that sex-specific neural templates are established in development such that by adolescence, the circuitry that gives rise to anxiety-like behaviors in females is more sensitive to the effects of these exogenous steroids on the expression of anxiety-like behaviors, than is this circuitry in males. This hypothesis is also supported by studies demonstrating that AAS treatment is far less efficacious in eliciting aggressive behaviors in mutant male mice that have been bereft of androgen receptor signaling since birth, but that acute androgen receptor blockade has only a modest impact on AAS-induced aggression (Robinson et al., 2012).
Sex-specific differences in AAS effects on CRF in the extended amygdala
CRF signaling through the CRF type 1 receptor (CRF1) in the extended amygdala is critical for the expression of sustained anxiety and for the ability of AAS to augment anxiety-like behaviors and alter GABAA receptor-mediated signaling in the extended amygdala in female mice (for review, Oberlander and Henderson, 2012a). Data from the current study suggest that sexually differentiated CRF signaling within the extended amygdala may make an important contribution to the sex-specific effects of chronic AAS treatment on the expression of anxiety-like behaviors. First, levels of CRF peptide in the BnST and CRF mRNA in the CeA were significantly lower in oil-injected female than oil-injected male mice; data that are in agreement with previous studies of these regions of the extended amygdala in rats (Desbonnet et al., 2008; Sterrenburg, 2011). Our data are also consistent with numerous previous reports indicating that the CRF signaling system in the extended amygdala shows both sex-specific and hormone-dependent regulation. Specifically, a sex-specific difference in the number of CRF-expressing cells (as assessed by in situ hybridization) in the CeA of the rat has been reported to arise during development (Viau et al., 2005); levels of CRF mRNA are regulated by endogenous gonadal steroids in the CeA (Lund et al., 2004; Viau et al., 2005); the promoter region of the CRF gene contains response elements for both androgen and estrogen receptors (Bao et al., 2006; Kageyama and Suda, 2010); and mice engineered to be deficient in the expression of both CRF1 and CRF2 show sex-specific differences in the expression of anxiety-like behaviors (Bale et al., 2002). Second, AAS treatment increased CRF levels in the extended amygdala of female mice (Costine et al., 2010; Oberlander and Henderson, 2012b; Figure 3), but had no effect in males. By raising CRF levels in females, AAS treatment nullified the basal sex-specific difference in this critical signaling peptide. These results suggest an interesting parallel with the studies by Bangasser et al. (2010) in which it was shown that exposure to a stressor abolishes a baseline sex-specific difference in CRF1-Gs coupling in the rodent cortex, albeit in this case, by a stress-induced increase in receptor-Gs coupling in males.
How might AAS interact with a sexually differentiated CRF system to elicit anxiety in female but not male mice? Stress-related psychological disorders are twice as prevalent in women than in men and as a general rule of thumb, females show an exaggerated stress response compared to males (for discussion, Iwasaki-Sekino et al., 2009; Valentino et al., 2012). It is interesting to speculate that chronic AAS may function as a “stressor” to which females show a greater response than do males. As noted above, stress has been shown to enhance internalization of CRF1 and desensitization of forebrain neurons to CRF only in male mice, thus rendering females both more sensitive to low levels of CRF and less able to adapt to higher levels of this peptide in stressed states (Bangasser et al., 2010). CRF, in turn, has been shown to influence 5-HT receptor signaling and internalization resulting in sensitization of 5-HT2 receptor signaling and increased anxiety-like behavior (Tan et al., 2004; Magalhaes et al., 2010). 5-HT receptors comprise the major target for the treatment of anxiety disorders (Hammack et al., 2009) and in the medial prefrontal cortex have also been shown to be internalized in a stress- and sex-dependent manner (Goodfellow et al., 2009; Benekareddy et al., 2010). Although the small size of the BnST precludes the kind of direct biochemical assessment that has been made for larger regions of the forebrain, an attractive mechanism for sex-specific actions of AAS on anxiety may entail enhanced CRF signaling in the BnST in female mice (Oberlander and Henderson, 2012b) and a subsequent preferential increase in 5-HT2 receptor-mediated excitation in this region that could then contribute to enhanced anxious states (Hammack et al., 2009).
Sex-specific interactions of exercise and AAS-induced anxiety
Exercise is a fundamental component of the regimes of most individuals who use AAS to enhance performance or body image, whether they are amateur or professional athletes (Wood and Stanton, 2012; Eisenberg et al., 2012; Fitch, 2012; Buckman et al., 2013), and AAS users tend to exercise at a higher rate than non-users (Kokkevi et al., 2008; Ip et al., 2011). Previous studies have focused on the impact of AAS use on voluntary exercise itself. Bronson and colleagues (Bronson, 1996; Bronson et al., 1996) first demonstrated that high doses of a mixture of AAS (testosterone, testosterone cypionate, methyltestosterone and norethandrolone) administered to adult CF-1 mice for 6 months decreased wheel running in both sexes, but the difference attained significance only in females. Chronic exposure to high doses of nandrolone or nandrolone decanoate also reduced wheel running in adult male rats (females were not tested) (McGinnis et al., 2007; Tanehkar et al., 2103). However, the impact of AAS on wheel running was compound-specific. In contrast to nandrolone, testosterone increased wheel running and stanozolol was without effect (McGinnis et al., 2007). Our data also demonstrate that the effects of AAS on wheel running were sex-specific, and that chronic exposure throughout adolescence to the mixture of AAS used in this study (testosterone cypionate, nandrolone decanoate and methandrostenolone) significantly decreased wheel running in male, but not female, C57BL/6 mice.
To our knowledge, the current study and a recent report (Tanehkar et al., 2013) published during the course of our experiments are the first to address the impact of exercise on AAS-induced changes in non-locomotory behaviors. Tanehkar et al. (2013) demonstrated that, despite an exercise-induced increase in hippocampal BDNF (vide infra), wheel running did not alter the deficits in spatial learning elicited in adult male rats by high doses of nandrolone decanoate. In a similar vein, we find that exercise was unable to inhibit the increase in anxiety-like behavior elicited by AAS treatment of female mice and interestingly promoted a significant increase in CRF in the BnST of oil-injected females. Increases in CRF and an excessive drive to exercise are correlated with the pathological anxiety that accompanies anorexia nervosa in women (Hotta et al., 1986; Kaye et al., 1987; Kaye, 2009). It is interesting to speculate that exercise-dependent changes in CRF in the extended amygdala may interact with those elicited by AAS in a markedly different fashion in males and females, and our data underscore that even for healthy women, the combination of AAS and exercise may make them vulnerable to enhanced anxiety in a fashion not seen in their male counterparts.
Although CRF signaling in the extended amygdala is central to the expression of generalized anxiety, the full range of anxiety behaviors reflects a broader system that includes bidirectional communication with other key structures, including the hippocampus (Canteras et al., 2010; Kim et al., 2011). In particular, changes in hippocampal levels of BDNF have been widely accepted as important in promoting beneficial behavioral actions of exercise in both humans and rodents (for review, Zoladz and Pilc, 2010). Studies are however far more variable than common wisdom appears to suggest, and results are dependent on the same range of critical variables, including sex, that are important with respect to AAS effects (for discussion Fuss et al., 2010; Novak et al., 2012). We thus also assessed whether there were sex-specific interactions of AAS and exercise that correlated with the observed impact of these two factors on the expression of anxiety.
Previous studies in male rodents have suggested that, concomitant with its anxiolytic effect, exercise increases BDNF mRNA and protein in the hippocampus (Adlard et al., 2005; Engesser-Cesar, 2007; Fuss et al., 2010; Hopkins and Bucci, 2010; Zajac et al., 2010; Sartori et al., 2011; Abel and Rissman, 2013; Tanehkar et al., 2103). Prior reports in female rodents suggest that exercise either has no effect (Engesser-Cesar et al., 2007; Alvarez-López et al., 2013) or increases hippocampal BDNF (Zajac et al., 2010; Marlatt et al., 2013). Our data indicate a significant sex-specific difference in the baseline levels of hippocampal BDNF mRNA, but that neither AAS treatment nor exercise altered BDNF mRNA levels in female mice. That chronic exposure to supratherapeutic levels of synthetic AAS had no effect on BDNF mRNA in female mice is of interest as BDNF mRNA and protein levels in the hippocampus are regulated by physiological levels of estradiol (for review, Carbone and Handa, 2013). These findings underscore the fact that the prolonged exposure to greatly elevated levels of chemically modified AAS has markedly different molecular, functional and behavioral outcomes than do hormone regimes that mimic physiological conditions (for review, Oberlander at al., 2012). In male mice, we found that AAS treatment decreased the levels of BDNF mRNA and that this AAS-dependent decrease could be reversed by exercise. Our data for males are in agreement with Mastrisciano et al. (2010) who report that AAS treatment decreased hippocampal BDNF in adult male rats but contrast with previous report by Rossbach et al. (2007) and Tanehkar et al. (2013) who found, respectively, either no change or an increase in BDNF with AAS treatment. Although our data from male mice are consistent with other reports that positively correlate exercise-induced increases in BDNF with decreases in anxiety-like behaviors, it is unlikely that hippocampal BDNF is a key modulator of AAS-induced anxiety as AAS did not change the levels of this transcript in female mice, which manifest an AAS-induced increases in anxiety, and, conversely, male mice did not exhibit AAS-induced anxiety, despite the fact that AAS treatment decreased in BDNF mRNA in this sex.
In conclusion, our data demonstrate a clear sex-specific difference in CRF signaling within the extended amygdala and in the effects of AAS on both behavioral and neurochemical correlates of anxiety in C57BL/6 mice. Furthermore, we found that exercise was ineffective in diminishing AAS-induced anxiety or AAS-dependent increases in CRF in female mice, while AAS do not augment anxiety-like behaviors in males and exercise has a modest anxiolytic effects in this sex. This study highlights that behavioral actions of AAS in human cohorts, which are nearly always male, should not be blithely extrapolated to females, and that female athletes who take AAS may be particularly susceptible to changes in neural signaling that promote an increase in the incidence of anxious states.
HIGHLIGHTS.
Chronic exposure to anabolic steroids increases anxiety in female not male mice
Voluntary exercise accentuates sex-specific differences in anabolic steroid-induced anxiety
Basal levels of CRF are higher in the extended amygdala of male versus female mice
Exposure to anabolic steroids increases CRF in females to male-like levels
Levels of hippocampal BDNF mRNA are sex- and exercise-, but not AAS-dependent
ACKNOWLEDGEMENTS
We thank Drs. David Bucci and Robert Leaton for their valuable input in designing and analyzing the behavioral data, and Drs. Ann Clark and David Bucci for review of the manuscript. We thank Drs. Paul Guyre and Alexandra Howell and Ms. Jane Collins for their input with analyzing the ELISA data, and Drs. Eugene Demidenko, Diane Gilbert-Diamond, Mark McPeek, Christopher Amos and Nadia Penrod for their help with the statistical analyses. We also thank Messrs. David W. McGuire, Eugene E. Thorburn and Todd Bissonnette in the Dartmouth Center for Comparative Medical Research for their assistance with setting up the behavioral experiments and the care of our mice. This work partially fulfills the thesis requirements for MMO.
This work was supported by NIDA R01-DA14137.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abel JL, Rissman EF. Running-induced epigenetic and gene expression changes in the adolescent brain. Int J Dev Neurosci. 2013;31(6):382–390. doi: 10.1016/j.ijdevneu.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26(4):511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Agis-Balboa RC, Pibiri F, Nelson M, Pinna G. Enhanced fear responses in mice treated with anabolic androgenic steroids. Neuroreport. 2009;20(6):617–621. doi: 10.1097/WNR.0b013e32832a2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ågren G, Thiblin I, Tirassa P, Lundeberg T, Stenfors C. Behavioural anxiolytic effects of low-dose anabolic androgenic steroid treatment in rats. Physiol Behav. 1999;66:503–509. doi: 10.1016/s0031-9384(98)00323-0. [DOI] [PubMed] [Google Scholar]
- Alvarez-López MJ, Castro-Freire M, Cosín-Tomás M, Sanchez-Roige S, Lalanza JF, Del Valle J, Párrizas M, Camins A, Pallás M, Escorihuela RM, Kaliman P. Long-term exercise modulates hippocampal gene expression in senescent female mice. J Alzheimers Dis. 2013;33(4):1177–1190. doi: 10.3233/JAD-121264. [DOI] [PubMed] [Google Scholar]
- Ambar G, Chiavegatto S. Anabolic-androgenic steroid treatment induces behavioral disinhibition and downregulation of serotonin receptor messenger RNA in the prefrontal cortex and amygdala of male mice. Genes Brain Behav. 2009;8:161–173. doi: 10.1111/j.1601-183X.2008.00458.x. [DOI] [PubMed] [Google Scholar]
- Annitto WJ, Layman WA. Anabolic steroids and acute schizophrenic episode. J Clin Psychiatry. 1980;41(4):143–144. [PubMed] [Google Scholar]
- Aqai P, Cevik E, Gerssen A, Haasnoot W, Nielen WF. High-throughput bioaffinity mass spectrometry for screening and identification of designer anabolic steroids in dietary supplements. Anal Chem. 2013;85:3255–3262. doi: 10.1021/ac3036052. [DOI] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE, Brower KJ. Anabolic-androgenic steroid abuse and performance-enhancing drugs among adolescents. Child Adolesc Psychiatr Clin N Am. 1998;7(4):821–838. [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE, Kopstein AN, Stephens JA. Risk factors associated with anabolic-androgenic steroid use among adolescents. Sports Med. 2000;29(6):397–405. doi: 10.2165/00007256-200029060-00003. [DOI] [PubMed] [Google Scholar]
- Bale TL, Picetti R, Contarino A, Koob GF, Vale WW, Lee KF. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22(1):193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol. 2012;32(5):709–723. doi: 10.1007/s10571-012-9824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao AM, Fischer DF, Wu YH, Hol EM, Balesar R, Unmehopa UA, Zhou JN, Swaab DF. A direct androgenic involvement in the expression of human corticotropin-releasing hormone. Mol Psychiatry. 2006;11(6):567–576. doi: 10.1038/sj.mp.4001800. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Sexual differentiation of the human brain: relation to gender identity, sexual orientation and neuropsychiatric disorders. Front. Neuroendocrinol. 2011;32(2):214–226. doi: 10.1016/j.yfrne.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Barreto-Estrada JL, Barreto J, Fortis-Santiago Y, Rivera-Ramos I, Fortis-Santiago A, Jorge JC. Modulation of affect after chronic exposure to the anabolic steroid 17alpha-methyltestosterone in adult mice. Behav Neurosci. 2004;118(5):1071–1079. doi: 10.1037/0735-7044.118.5.1071. [DOI] [PubMed] [Google Scholar]
- Benekareddy M, Goodfellow NM, Lambe EK, Vaidya VA. Enhanced function of prefrontal serotonin 5-HT2 receptors in a rat model of psychiatric vulnerability. J Neurosci. 2010;30(36):12138–12150. doi: 10.1523/JNEUROSCI.3245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain Res. 2004;155(2):197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Bitran D, Kellogg CK, Hilvers RJ. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav. 1993;27(4):568–583. doi: 10.1006/hbeh.1993.1041. [DOI] [PubMed] [Google Scholar]
- Blasberg ME, Clark AS. Anabolic-androgenic steroid effects on sexual receptivity in ovariectomized rats. Horm Behav. 1997;32:201–208. doi: 10.1006/hbeh.1997.1422. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Effects of prolonged exposure to anabolic steroid on the behavior of male and female mice. Pharmacol Biochem Behav. 1996;53:329–334. doi: 10.1016/0091-3057(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Nguyen KQ, De La Rosa J. Effect of anabolic steroids on behavior and physiological characteristics of female mice. Physiol Behav. 1996;59:49–55. doi: 10.1016/0031-9384(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Buckman JF, Farris SG, Yusko DA. A national study of substance use behaviors among NCAA male athletes who use banned performance enhancing substances. Drug Alcohol Depend. 2013;131(1–2):50–55. doi: 10.1016/j.drugalcdep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019(1–2):84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Burnett KF, Kleiman ME. Psychological characteristics of adolescent steroid users. Adolescence. 1994;29(113):81–89. [PubMed] [Google Scholar]
- Cameron OG, Hill EM. Women and anxiety. Psychiatr Clin North Am. 1989;12(1):175–186. [PubMed] [Google Scholar]
- Canteras NS, Resstel LB, Bertoglio LJ, Carobrez Ade P, Guimarães FS. Neuroanatomy of anxiety. Curr Top Behav Neurosci. 2010;2:77–96. doi: 10.1007/7854_2009_7. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295–303. doi: 10.1016/j.neuroscience.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, Costine BA, Jones BL, Kelton-Rehkopf MC, Meerts SH, Nutbrown-Greene LL, Penatti CAA, Porter DM, Yang P, Henderson LP. Sex- and age-specific effects of anabolic androgenic steroids on reproductive behaviors and on GABAergic transmission in neuroendocrine control regions. Brain Res. 2006;1126:122–138. doi: 10.1016/j.brainres.2006.08.081. [DOI] [PubMed] [Google Scholar]
- Clark AS, Harrold EV, Fast AS. Anabolic-androgenic steroid effects on the sexual behavior of intact male rats. Horm Behav. 1997;31(1):35–46. doi: 10.1006/hbeh.1997.1355. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27(5):413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Cooper RL, Goldman JM, VandenBergh JG. Monitoring of the Estrous Cycle in the Laboratory Rodent by Vaginal Lavage. In: Heindel JJ, Chapin RE, editors. Methods in Toxicology. New York, NY: Academic Press; 1993. pp. 45–55. [Google Scholar]
- Cooper CJ, Noakes TD, Dunne T, Lambert MI, Rochford K. A high prevalence of abnormal personality traits in chronic users of anabolic-androgenic steroids. Br J Sports Med. 1996;30(3):246–250. doi: 10.1136/bjsm.30.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costine BA, Oberlander JG, Davis MC, Penatti CA, Porter DM, Leaton RN, Henderson LP. Chronic anabolic androgenic steroid exposure alters corticotropin releasing factor expression and anxiety-like behaviors in the female mouse. Psychoneuroendocrinology. 2010;35(10):1473–1485. doi: 10.1016/j.psyneuen.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotying of transgenic and knockout mice. New York, NY USA: Wiley-Liss; 2000. What’s wrong with my mouse? pp. 54–55. [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17(4):448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham RL, Lumia AR, McGinnis MY. Androgenic anabolic steroid exposure during adolescence: Ramifications for brain development and behavior. Horm Behav. 2013;64(2):350–356. doi: 10.1016/j.yhbeh.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic versus sustained fear in rats and humans: role of the extended amygdala in fear versus anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protocols. 2006;1(1):122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci. 2008;26(3–4):259–268. doi: 10.1016/j.ijdevneu.2008.02.004. 2008. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38(1–2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494(1):75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Müller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144(7):3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. J Neuroendocrinol. 2006;18(12):915–925. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ME, Wall M, Neumark-Sztainer D. Muscle-enhancing behaviors among adolescent girls and boys. Pediatrics. 2012;130(6):1019–1026. doi: 10.1542/peds.2012-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Cotman CW. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144(3):1033–1044. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Fitch K. Proscribed drugs at the Olympic Games: permitted use and misuse (doping) by athletes. Clin Med. 2012;2(3):257–260. doi: 10.7861/clinmedicine.12-3-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG. The organizational hypothesis and final common pathways: Sexual differentiation of the spinal cord and peripheral nervous system. Horm Behav. 2009;55(5):605–610. doi: 10.1016/j.yhbeh.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Hammack SE, Falls WA. Exercise is associated with reduction in the anxiogenic effect of mCPP on acoustic startle. Behav Neurosci. 2008;122(4):943–948. doi: 10.1037/0735-7044.122.4.943. [DOI] [PubMed] [Google Scholar]
- Fox K. The influence of physical activity on mental well being. Public Health Nutr. 1999;2(3A):411–418. doi: 10.1017/s1368980099000567. [DOI] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm Behav. 2008;54(5):726–734. doi: 10.1016/j.yhbeh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, Witzemann V, Hellweg R, Gass P. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20(3):364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- Galligani N, Renck A, Hansen S. Personality profile of men using anabolic androgenic steroids. Horm Behav. 1996;30(2):170–175. doi: 10.1006/hbeh.1996.0021. [DOI] [PubMed] [Google Scholar]
- Geyer H, Parr MK, Koehler K, Mareck U, Schänzer W. Nutritional supplements cross-contaminated and faked with doping substances. J Mass Spectrom. 2008;43:892–902. doi: 10.1002/jms.1452. [DOI] [PubMed] [Google Scholar]
- Gilmore RF, Varnum MM, Forger NG. Effects of blocking developmental cell death on sexually dimorphic calbindin cell groups in the preoptic area and bed nucleus of the stria terminalis. Biol Sex Differ. 2012;3:5. doi: 10.1186/2042-6410-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goodfellow NM, Benekareddy M, Vaidya VA, Lambe EK. Layer II/III of the prefrontal cortex: Inhibition by the serotonin 5-HT1A receptor in development and stress. J Neurosci. 2009;29(32):10094–10103. doi: 10.1523/JNEUROSCI.1960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. Psychopharmacology. 2008;199:421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson S, Liang W, Hilke S. Effects of voluntary running in the female mice lateral septum on BDNF and corticotropin-releasing factor receptor 2. Int J Pept. 2011;2011:932361. doi: 10.1155/2011/932361. Epub 2011 Sep 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RC, Hall RC, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46(4):285–290. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- Hallberg M. Impact of anabolic androgenic steroids on neuropeptide systems. Mini Rev Med Chem. 2011;11(5):399–408. doi: 10.2174/138955711795445961. [DOI] [PubMed] [Google Scholar]
- Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005;11(4):288–293. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response o neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiat. 2009;33(8):1309–1320. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Wakatsuki T, Aste N, Yoshimura N, Honda SI. Functional analysis of neurosteroidal oestrogen using gene-disrupted and transgenic mice. J Neuroendocrinol. 2009;21(4):365–369. doi: 10.1111/j.1365-2826.2009.01857.x. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS Neurol Disord Drug Targets. 2006;5(4):453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring MP, O'Connor PJ, Dishman RK. The effect of exercise training on anxiety symptoms among patients: a systematic review. Arch Intern Med. 2010;170(4):321–331. doi: 10.1001/archinternmed.2009.530. [DOI] [PubMed] [Google Scholar]
- Herring MP, Jacob ML, Suveg C, Dishman RK, O'Connor PJ. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21–28. doi: 10.1159/000327898. [DOI] [PubMed] [Google Scholar]
- Hisasue S, Seney ML, Immerman E, Forger NG. Control of cell number in the bed nucleus of the stria terminalis of mice: role of testosterone metabolites and estrogen receptor subtypes. J Sex Med. 2010;7(4 Pt 1):1401–1409. doi: 10.1111/j.1743-6109.2009.01669.x. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety - Evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Bucci DJ. BDNF expression in perirhinal cortex is associated with exercise-induced improvement in object recognition memory. Neurobiol Learn Mem. 2010;94(2):278–284. doi: 10.1016/j.nlm.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta M, Shibasaki T, Masuda A, Imaki T, Demura H, Ling N, Shizume K. The responses of plasma adrenocorticotropin and cortisol to corticotropin-releasing hormone (CRH) and cerebrospinal fluid immunoreactive CRH in anorexia nervosa patients. J Clin Endocrinol Metab. 1986;62(2):319–324. doi: 10.1210/jcem-62-2-319. [DOI] [PubMed] [Google Scholar]
- Ip EJ, Barnett MJ, Tenerowicz MJ, Perry PJ. The Anabolic 500 survey: characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy. 2011;(8):757–766. doi: 10.1592/phco.31.8.757. [DOI] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2012. Volume I: Secondary school students. Ann Arbor: Institute for Social Research, The University of Michigan; 2013. p. 604. [Google Scholar]
- Kageyama K, Suda S. Transcriptional regulation of hypothalamic corticotropin-rleasing fctor gene. Vitam Horm. 2010;82:301–317. doi: 10.1016/S0083-6729(10)82016-3. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10(8):573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Gwirtsman HE, George DT, Ebert MH, Jimerson DC, Tomai TP, Chrousos GP, Gold PW. Elevated cerebrospinal fluid levels of immunoreactive corticotropin-releasing hormone in anorexia nervosa: relation to state of nutrition, adrenal function, and intensity of depression. J Clin Endocrinol Metab. 1987;64(2):203–208. doi: 10.1210/jcem-64-2-203. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kokkevi A, Fotiou A, Chileva A, Nociar A, Miller P. Daily exercise and anabolic steroids use in adolescents: a cross-national European study. Subst Use Misuse. 2008;43(14):2053–2065. doi: 10.1080/10826080802279342. [DOI] [PubMed] [Google Scholar]
- Kouvelas D, Pourzitaki C, Papazisis G, Dagklis T, Dimou K, Kraus MM. Nandrolone abuse decreases anxiety and impairs memory in rats via central androgenic receptors. Int. J Neuropsychopharmacol. 2008;11:925–934. doi: 10.1017/S1461145708008754. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Revs. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurons in the paraventricular nucleus. J Neuroendocrinol. 2004;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Holmes KD, Dale LB, Comps-Agrar L, Lee D, Yadav PN, Drysdale L, Poulter MO, Roth BL, Pin JP, Anisman H, Ferguson SS. CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat Neurosci. 2010;13(5):622–629. doi: 10.1038/nn.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger TA, Motta RW. The impact of an exercise program on posttraumatic stress disorder, anxiety, and depression. Int J Emerg Ment Health. 2005;7(1):49–57. [PubMed] [Google Scholar]
- Marlatt MW, Potter MC, Bayer TA, van Praag H, Lucassen PJ. Prolonged Running, not Fluoxetine Treatment, Increases Neurogenesis, but does not Alter Neuropathology, in the 3×Tg Mouse Model of Alzheimer's Disease. Curr Top Behav Neurosci. 2013;15:313–340. doi: 10.1007/7854_2012_237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Sanchis S, Salvador A, Moya-Albiol L, González-Bono E, Simón VM. Effects of chronic treatment with testosterone propionate on aggression and hormonal levels in intact male mice. Psychoneuroendocrinology. 1998;23(3):275–293. doi: 10.1016/s0306-4530(98)00005-5. [DOI] [PubMed] [Google Scholar]
- Matrisciano F, Modafferi AM, Togna GI, Barone Y, Pinna G, Nicoletti F, Scaccianoce S. Repeated anabolic androgenic steroid treatment causes antidepressant-reversible alterations of the hypothalamic-pituitary-adrenal axis, BDNF levels and behavior. Neuropharmacology. 2010;58(7):1078–1084. doi: 10.1016/j.neuropharm.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Maue RA, Burgess RW, Wang B, Wooley CM, Seburn KL, Vanier MT, Rogers M, Chang CC, Chang T-Y, Harris BT, Graber DJ, Penatti CAA, Porter DM, Szwergold BS, Henderson LP, Ohno-Iwashita Y, Totenhagen J, Trouard T, Borbon I, Erickson RP. A novel mouse model of Niemann-Pick C disease carrying a A1005G-Npc1 mutation comparable to commonly observed human mutations. Hum Mol Genet. 2012;21(4):730–750. doi: 10.1093/hmg/ddr505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis MY. Anabolic androgenic steroids and aggression: studies using animal models. Ann NY Acad Sci. 2004;21036:399–415. doi: 10.1196/annals.1330.024. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann NY Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Lumia AR, Tetel MJ, Molenda-Figueira HA, Possidente B. Effects of anabolic androgenic steroids on the development and expression of running wheel activity and circadian rhythms in male rats. Physiol Behav. 2007;92(5):1010–1018. doi: 10.1016/j.physbeh.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni RH, Jr, Ricci LA. Adolescent exposure to anabolic/androgenic steroids and the neurobiology of offensive aggression: a hypothalamic neural model based on findings in pubertal Syrian hamsters. Horm Behav. 2010;58(1):177–191. doi: 10.1016/j.yhbeh.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36:1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Henderson LP. The Sturm und drang of anabolic steroid use: angst, anxiety, and aggression. Trends Neurosci. 2012a;35(6):382–392. doi: 10.1016/j.tins.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Henderson LP. Corticotropin releasing factor modulation of forebrain GABAergic transmission has a pivotal role in the expression of anabolic steroid-induced anxiety in the female mouse. Neuropsychopharmacology. 2012b;37(6):1483–1499. doi: 10.1038/npp.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Porter DM, Onakomaiya MM, Vithlani M, Moss SJ, Clark AS, Henderson LP. Estrous cycle variations in GABAA receptor phosphorylation enable rapid modulation by anabolic androgenic steroids in the medial preoptic area. Neuroscience. 2012;226:397–410. doi: 10.1016/j.neuroscience.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Porter DM, Penatti CA, Henderson LP. Anabolic androgenic steroid abuse: multiple mechanisms of regulation of GABAergic synapses in neuroendocrine control regions of the rodent forebrain. J Neuroendocrinol. 2012;24(1):202–214. doi: 10.1111/j.1365-2826.2011.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS. Psychiatric side effects induced by supraphysiological doses of combinations of anabolic steroids correlate to the severity of abuse. Eur Psychiatry. 2006;21(8):551–562. doi: 10.1016/j.eurpsy.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25(3):219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Parkinson AB, Evans NA. Anabolic androgenic steroids: a survey of 500 users. Med Sci Sports Exerc. 2006;38(4):644–651. doi: 10.1249/01.mss.0000210194.56834.5d. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94.12:1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31(14):e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Costine BA, Porter DM, Henderson LP. Effects of chronic exposure to an α5-receptor mediated GABAergic transmission and neural signaling in the forebrain of female mice. Neuroscience. 2009a;161:526–537. doi: 10.1016/j.neuroscience.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CA, Porter DM, Henderson LP. Chronic exposure to anabolic androgenic steroids alters neuronal function in the mammalian forebrain via androgen receptor- and estrogen receptor-mediated mechanisms. J Neurosci. 2009b;29:12484–12496. doi: 10.1523/JNEUROSCI.3108-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penatti CAA, Oberlander JG, Davis MC, Porter DM, Henderson LP. Chronic exposure to anabolic androgenic steroids alters activity and synaptic function in neuroendocrine control regions of the female mouse. Neuropharmacology. 2011;61(4):653–664. doi: 10.1016/j.neuropharm.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott TA. Gender differences in the epidemiology and treatment of anxiety disorders. J Clin Psychiatry. 1999;60(18):4–15. [PubMed] [Google Scholar]
- Pope HG, Jr, Kanayama G, Athey A, Ryan E, Hudson JI, Baggish A. Lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: Current best estimates. Am J. Addict. 2013 Sep 20; doi: 10.1111/j.1521-0391.2013.12118.x. (2013) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, Katz DL. Affective and psychotic symptoms associated with anabolic steroid use. Am J Psychiatry. 1988;145(4):487–490. doi: 10.1176/ajp.145.4.487. [DOI] [PubMed] [Google Scholar]
- 117.Reul JMHM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Robinson S, Penatti CA, Clark AS. The role of the androgen receptor in anabolic androgenic steroid-induced aggressive behavior in C57BL/6J and Tfm mice. Horm Behav. 2012;61(1):67–75. doi: 10.1016/j.yhbeh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Rocha VM, Calil CM, Ferreira R, Moura MJCS, Marcondes FK. Influence of anabolic steroid on anxiety levels in sedentary male rats. Stress. 2007;10:326–331. doi: 10.1080/10253890701281344. [DOI] [PubMed] [Google Scholar]
- Rojas-Ortiz YA, Rundle-Gonzalez V, Rivera-Ramos I, Jorge JC. Modulation of elevated plus maze behavior after chronic exposure to the anabolic steroid 17α-methyltestosterone in adult mice. Horm Behav. 2006;49:123–128. doi: 10.1016/j.yhbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Rossbach UL, Steensland P, Nyberg F, Le Grevès P. Nandrolone-induced hippocampal phosphorylation of NMDA receptor subunits and ERKs. Biochem Biophys Res Commun. 2007;357(4):1028–1033. doi: 10.1016/j.bbrc.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009;197(1):31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Sartori CR, Vieira AS, Ferrari EM, Langone F, Tongiorgi E, Parada CA. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–118. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Sato SM, Schulz KM, Sisk CL, Wood RI. Adolescents and androgens, receptors and rewards. Horm Behav. 2008;53(5):647–658. doi: 10.1016/j.yhbeh.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8(4):209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- Simonds VM, Whiffen VE. Are gender differences in depression explained by gender differences in co-morbid anxiety? J Affect Disord. 2003;77(3):197–202. doi: 10.1016/s0165-0327(02)00113-1. [DOI] [PubMed] [Google Scholar]
- Steensland P, Blakely G, Nyberg F, Fahlke C, Pohorecky LA. Anabolic androgenic steroid affects social aggression and fear-related behaviors in male pair-housed rats. Horm Behav. 2005;48:216–224. doi: 10.1016/j.yhbeh.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One. 2011;6(11):e28128. doi: 10.1371/journal.pone.0028128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Pagliaro M, Schmidt PJ, Pickar D, Wolkowitz O, Rubinow DR. Neuropsychiatric effects of anabolic steroids in male normal volunteers. JAMA. 1993;269(21):2760–2764. [PubMed] [Google Scholar]
- Tan H, Zhong P, Yan Z. Corticotropin-releasing factor and acute stress prolongs serotonergic regulation of GABA transmission in prefrontal cortical pyramidal neurons. J Neurosci. 2004;24(21):5000–5008. doi: 10.1523/JNEUROSCI.0143-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanehkar F, Rashidy-Pour A, Vafaei AA, Sameni HR, Haghighi S, Miladi-Gorji H, Motamedi F, Akhavan MM, Bavarsad K. Voluntary exercise does not ameliorate spatial learning and memory deficits induced by chronic administration of nandrolone decanoate in rats. Horm Behav. 2013;63(1):158–165. doi: 10.1016/j.yhbeh.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Tanner SM, Miller DW, Alongi C. Anabolic steroid use by adolescents: prevalence, motives, and knowledge of risks. Clin J Sport Med. 1995;5(2):108–115. doi: 10.1097/00042752-199504000-00007. [DOI] [PubMed] [Google Scholar]
- ter Horst JP, Carobrez AP, van der Mark MH, de Kloet ER, Oitzl MS. Sex differences in fear memory and extinction of mice with forebrain-specific disruption of the mineralocorticoid receptor. Eur J Neurosci. 2012;36(8):3096–3102. doi: 10.1111/j.1460-9568.2012.08237.x. [DOI] [PubMed] [Google Scholar]
- Toufexis D. Region- and sex-specific modulation of anxiety behaviors in the rat. J Neuroendocrinol. 2007;19(6):461–473. doi: 10.1111/j.1365-2826.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62(1):13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, Van Bockstaele EJ. Sex-biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013;83(4):737–745. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vandenBerg P, Neumark-Sztainer D, Cafri G, Wall M. Steroid use among adolescents: longitudinal findings from Project EAT. Pediatrics. 2007;119(3):476–486. doi: 10.1542/peds.2006-2529. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2(2):322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis in anxiety-like versus phasic fear-like responses. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI. Anabolic-androgenic steroid dependence? Insights from animals and humans. Front Neuroendocrinol. 2008;29(4):490–506. doi: 10.1016/j.yfrne.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RI, Stanton SJ. Testosterone and sport: Current perspective. Horm Behav. 2012;61:147–155. doi: 10.1016/j.yhbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac MS, Pang TY, Wong N, Weinrich B, Leang LS, Craig JM, Saffery R, Hannan AJ. Wheel running and environmental enrichment differentially modify exon-specific BDNF expression in the hippocampus of wild-type and pre-motor symptomatic male and female Huntington's disease mice. Hippocampus. 2010;20(5):621–636. doi: 10.1002/hipo.20658. [DOI] [PubMed] [Google Scholar]
- Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61(5):533–541. [PubMed] [Google Scholar]