Abstract
Child maltreatment often has a negative impact on the development of social behavior and health. The biobehavioral mechanisms through which these adverse outcomes emerge, however, are unclear. To better understand the ways in which early life adversity affect subsequent social behavior, changes in the neuropeptide oxytocin in children (n=73) aged 8.1–11.5 years following a laboratory stressor were examined. Girls with histories of physical abuse have higher levels of urinary oxytocin and lower levels of salivary cortisol following the stressor when compared to controls. Abused and control boys, however, do not differ in their hormonal responses. These data suggest that early adversity may disrupt the development of the stress regulation system in girls by middle childhood.
In addition to the risk of physical injury, children who are the victims of maltreatment are at risk for a number of adverse psychological and behavioral outcomes. For example, such children are at elevated risk for mental health disorders such as anxiety and depression (Cohen, Brown, & Smailes, 2001) and substance abuse problems relative to their peers (Oshri, Tubman & Burnette, 2012; Lansford, Dodge, Pettit & Bates, 2010). They also have lower social status and are perceived as more aggressive and less cooperative (Salzinger, Feldman, Hammer & Rosario, 1993), are more likely to engage in high-risk sexual behaviors that can lead to disease or early pregnancy (Noll, Haralson, Butler & Shenk, 2011), and more frequently perpetrate violent crimes directed toward romantic partners and others as they mature, relative to controls (Kunitz, Levy, McCloskey & Gabriel, 1998). It is not clear, however, which regulatory processes are common across these problems, or how the early experience of maltreatment continues to affect biological processes underlying social behavior.
The neuroendocrine system regulates hormonal changes, which may be associated with the behavioral differences between abused and typically-developing children. This system also regulates phenomena such as pubertal development, aspects of cognition, and stress regulation in humans and other animals. Here, we focus on the effects of the hypothalamic-adrenal-pituitary (HPA) axis and the neuropeptide oxytocin (OT), two elements of the neuroendocrine system that are tied to complex social behaviors and appear to be dysregulated in adults who report having experienced early life stress such as childhood maltreatment (Lupien, McEwen, Gunnar & Heim, 2009; Pierrehumbert et al., 2010).
HPA Axis Functioning and Early Stress
Many advances in understanding children’s responses to life stress have emerged from studies of the HPA axis. This is because the HPA axis functions as the interface between signals from the external environment and internal, individual biology. One of the end products of HPA activity is the production of the hormone cortisol, which increases or decreases adaptively in response to socially relevant stimuli in an individual’s environment (see Gunnar & Quevedo, 2007 for a review). Depending on exposure to early stressors, a complex interplay of hormones can alter developmental trajectories while at the same time predisposing some individuals to certain types of risk later in life (Heim, Young, Newport, Mletzko, Miller & Nemeroff, 2008). In particular, the HPA axis is sensitive and responsive to cues indicating environmental stability or instability. This mechanism helps individuals respond appropriately when confronted with a stressor.
Whether cortisol should be high or low in individuals experiencing early life stress is a question of some debate, with the literature revealing a mixture of findings. These inconsistent results likely reflect differences in individual life circumstances. Heightened responsivity to stress might be adaptive in a constantly changing and/or dangerous environmental context, helping the individual to prepare for repeated challenges. Alternatively, it is possible that less responsivity to stressors is adaptive for individuals living under chronically stressful or unstable conditions. This is because maintaining high levels of vigilance and readiness for fight or flight exacts a physiological cost. Most of the literature is consistent with the idea that a social environment characterized by warm, stable, and responsive relationships is associated with lower HPA axis activity (Korosi & Baram, 2010; Branchi, 2009). Still, individuals with a history of early life stress may show a blunted HPA responses to stressors (Carpenter, Shattuck, Tyrka, Geracioti & Price, 2011; Goldman-Mellor, Hamer & Steptoe, 2012; Lovallo et al., 2012; Ouellet-Morin et al., 2011), necessitating further investigation. In an effort to better understand the associations between the neuroendocrine system and early life experience, we examined how other hormones, such as those involved in affiliative behaviors, may be associated with childhood adversity and behavioral phenotype.
Oxytocin, Stress Regulation, and Social Behavior
When released into the central nervous system, oxytocin (OT) has numerous effects that are relevant to adaptive social behavior, such as the development of trust, affiliation, pair bonding, grooming, maternal-infant attachment and stress buffering (Carter 1998; Snowdon et al., 2010; Gimpl & Fahrenholz, 2001; Insel, 1997). As such, we might expect that individuals who have experienced poor-quality relationships with others, such as maltreated children, to exhibit low levels of oxytocin compared to their peers. A complex relationship between OT and activity of the hypothalamic-pituitary-adrenal (HPA) axis, however, exists. OT binds its G protein-coupled receptor in the paraventricular nucleus of the hypothalamus; this results in activation of a MEK-extracellular signal-regulated kinase (ERK) MAP kinase pathway and produces an anxiolytic, or calming, effect (van den Burg & Newmann, 2001). Indeed, a surge in OT release from the paraventricular nucleus of the hypothalamus is a major component of stress regulation in many organisms, in that this surge acts to buffer the stress response (Jezova et al., 1995). This suppression of a stress response is a critical component of how social relationships are formed and maintained, and is critical to the fitness of sexually reproducing species which must overcome an aversive response to conspecifics, who might otherwise pose a risk of predation, in order to mate (Lukas et al., 2011). In this manner, OT functions in its social role partly by suppressing the stress response and thereby facilitating social motivation and approach to novel others (Carter & Altemus, 1997; Heinrichs & Domes 2008).
Numerous studies also reveal a relationship between OT, stress, and social behavior. For example, male rats treated with OT normalize their levels of corticosterone after exposure to predator scent stress and decrease glucocorticoid receptor expression, whereas high doses of corticosterone cause increases in of plasma oxytocin (Cohen et al., 2010). In rodents, endogenous OT facilitates social approach and also acts to reverse stress-induced social avoidance (Lukas et al., 2011). Similarly, male and female marmosets (Callithrix penicillata) given intranasal OT treatments show enhanced initial partner-seeking behavior and initiate more contact with their partners (huddling); conversely, OT antagonist treatment decreases the frequency of social approach (Smith, 2010). Administration of an oxytocin antagonist may eliminate interest in sexual behavior in female rhesus macaques (Boccia, Goursaud, Bachevalier, Anderson & Pedersen, 2007). In humans, intranasal application of OT inhibits activity of the HPA axis, especially when paired with social support (Clodi et al., 2008; Heinrichs, Baumgartner, Kirschbaum & Ehlert, 2003).
Males and females within the same species, however, often differ with respect to their release of endogenous oxytocin following behavioral paradigms, as well as in their behaviors following exogenous administration. First, females tend to have higher endogenous levels of OT than males, which is both a function of the role of governing steroids (such as estrogen) and also perhaps a reflection of sexually dimorphic responses to challenge (Carter, 2007). Mothers experiencing stressful interactions with their infants show changes in endogenous urinary OT, whereas fathers do not (Feldman, Gordon and Zagoory-Sharon, 2011). Similarly, women in distressed relationships show elevated peripheral OT, whereas men do not (Taylor, Saphire-Bernstein & Seeman, 2010). Women receiving administration of intranasal OT show more negative affect after a stressor, whereas men show less (Kubzansky et al., 2012). This is in contrast to studies that do not use stressors, which tend to show that females show a more robust OT response to such phenomena as empathy (Barraza & Zak, 2009).
Examination of OT changes in the context of stressful events, as opposed to at baseline, is clearly critical to our understanding of how this system operates under ecologically valid conditions between the sexes. Evidence from laboratory animals also suggests that the OT system is highly plastic and dependent upon the social cues encountered in early life (Veenema, 2012), and that higher OT levels may not always translate into positive social outcomes or behaviors.
Effects of Negative Social Relationships on the Oxytocin System
Behavioral research suggests that oxytocin’s actions are context-dependent and can result in negative outcomes. While oxytocin functions in part to facilitate attachment, this may be the case only for those already perceived as being members of one’s own group – for those who are not, oxytocin appears to drive discrimination against outsiders (De Dreu, Greer, Van Kleef, Shalvi & Handgraaf, 2011). In rejection-sensitive individuals with bipolar disorder, oxytocin administration actually decreases the levels of trust exhibited when playing a cooperation game (Bartz et al., 2011). In a similar game, administration of oxytocin reduces the aversion to betrayal in those who tend to fear intimacy and dependence on others (De Dreu, 2012). In the context of parents and children, harsh parenting strategies may moderate the stress-reducing abilities of oxytocin (Bakermans-Kranenburg et al., 2012). Finally, in men who were anxiously attached to their mothers during childhood, intranasal oxytocin seems to facilitate the recollection of negative memories of their rearing, whereas the opposite is true for men who were securely attached (Bartz et al., 2010).
At present it is not clear how such differences emerge, but it is plausible that early life experiences are responsible. While some reports indicate that adverse early experience is associated with reduced central OT (Heim et al., 2009; Meinlschmidt & Heim, 2007), a number of studies indicate a contrasting and seemingly paradoxical finding: comparatively high OT in individuals who have experienced early adversity. This association is observed in both laboratory animals and humans.
Social isolation, for example, results in increased plasma OT levels in prairie voles (Grippo, Cushing & Carter, 2007), as does suffering social defeat in male rats (Ebner, Wotjak, Landgraf & Engelmann, 2000). In humans, individuals who report being in unsatisfying relationships have higher levels of plasma OT (Taylor et al., 2006, Taylor, Saphire-Bernstein & Seeman, 2010), as do those individuals who report high levels of social anxiety (Tabak, McCullough, Szeto, Mendez & McCabe, 2010; Turner, Altemus, Enos, Cooper & McGuinness 1999). Particularly relevant to the present experiment are the observations that adult women who report histories of child sexual abuse have higher OT levels (Parker et al., 2010; Pierrehumbert et al., 2010). These findings raise an intriguing question. If OT is associated with positive emotions such as trust and affiliation, why would adults who have experienced stressful social relationships, especially persistent ones since childhood, have elevated levels of this hormone?
This experiment is aimed at understanding why early stress, such as child maltreatment, might result in high levels of OT.
Central vs. Peripheral Oxytocin
One challenge in translating animal to human studies in this area concerns the measurement of OT. In nonhuman animal studies, OT can be measured centrally – directly from the brain – through cerebrospinal fluid or by sacrificing the animal. In contrast, measurement of oxytocin in humans must be done peripherally and this limitation is currently a controversial subject.
The peripheral and central OT pathways are sometimes considered anatomically and/or functionally separate (Gimpl & Fahrenholz, 2001). Consistent with this view, emotional stressors are associated with changes in intrahypothalamic, or central, OT levels, but not with plasma OT levels (Engelman, Ebner, Landgraf, Holsboer & Wotjak, 1999). For this reason, changes in plasma, urinary, or salivary OT (all of which reflect peripheral measures) are sometimes considered to lack relevance to the study of centrally-mediated behavior.
A growing corpus of clinical and behavioral literature, however, suggests that peripheral oxytocin may indeed influence or reflect central processes. In humans, plasma OT is significantly lower in children suffering from autism-spectrum disorders, which is often characterized as a deficit in social understanding (Modahl et al., 1998). Concordant changes in salivary OT between mothers and their young infants are related to affect synchrony and social engagement (Feldman, Gordon & Zagoory-Sharon, 2010). In children, physical touch with a parent releases oxytocin that can be measured in the urine (Wismer-Fries, Ziegler, Kurian, Jacoris & Pollak, 2005). In addition, children allowed to speak with their mothers after a stressful experience show levels of urinary OT comparable to those who interacted in person via touch, whereas children who simply rest alone do not show this effect (Seltzer, Ziegler & Pollak, 2010).
Small amounts of OT can return to the central nervous system once it leaves the posterior pituitary. These small quantities may cross the blood-brain barrier either intact or via metabolites (Burbach et al., 1983; Mens, Witter, & Wimersma Grenadus, 1983). It may be possible that even these very small quantities are sufficient to activate OT receptors, which vary greatly in their density and activity status depending on myriad factors in different species, sexes, and conditions (Donaldson & Young, 2008).
An alternative explanation to OT crossing the blood-brain barrier, however, is perhaps more reasonable. Both the paraventricular (Kozorovitskiy, Hughes, Lee & Gould, 2006) and supraotic (Landgraf & Neumann, 2004) nuclei of the hypothalamus project to numerous regions within the brain as well as to the posterior pituitary from which OT is released peripherally, making it possible that central stimulation to either nucleus may cause release of peptides into both the CNS and the bloodstream (Wotjak et al., 1998). This may account for the consistent success of peripheral measurement techniques without evoking the question of whether OT can cross the blood brain barrier in bioactive quantities. Additional studies are needed to clarify the precise relationship between behavior and peripheral oxytocin measures, of which the present experiment is one.
The Present Study
Here, we test the functioning of the OT system in response to a social stressor among children who have experienced child maltreatment in comparison to controls. We predicted that maltreatment would be associated with increases in OT accompanied by lower levels of salivary cortisol following a social stressor. Support for these hypotheses would be consistent with the idea that the developmental organization of the human stress regulation system may proceed in an adaptive fashion, depending on exposure to different kinds of early life experiences. We also tested to see whether elevated levels of urinary OT would be inversely associated with levels of salivary cortisol, as it is in typically-developing adults (Legros, Chiodera, Geenen, Smitz & von Frenckell, 1984)
METHOD
Participants
Seventy-three children between the ages of 8 and 11.5 years of age participated in this experiment. The sample included 37 children who had experienced verified physical abuse (21 girls, 17 boys; mean age=9.0 yrs., SD=1.2 yrs.). The age range of maltreated children was 8.1–11.4 years. Maltreatment status in these participants was assessed via screening of court and Child Protective Services records (for which parental consent was obtained), as well as parent self-report of their behavior towards their children through the Parent-Child Conflict Tactics Scale (CTS; Straus et al., 1998). We compared the maltreated children to 36 typically-developing peers (18 girls, 18 boys; mean age=9.4 yrs., SD=1.2 yrs.) with similar socio-demographic characteristics (maltreated group 50% non-Caucasian, control group 44% non-Caucasian). The age range of the control children was 8.2–11.5 years. Control children were recruited from the community through school newsletters and were screened for maltreatment as described above. Children recruited from the community who had records indicating investigation or suspicion of maltreatment that was not substantiated, or whose parent reported scores on the CTS that were slightly elevated but not high enough to cross our “maltreatment” threshold were not recruited for this experiment. Participants were also screened for pubertal status at the time of recruitment: only girls who reported they had not yet experienced their first menstrual period and boys who did not yet exceed Tanner stage 3 for pubic hair development were included in this experiment.
The Trier Social Stress Test for Children (TSST-C)
In order to examine the relationship between the activity of the HPA axis and the release of peripheral OT, participants underwent the Trier Social Stress Test for Children (see Gunnar et al., 2009 for description of the child version of this task). As this task is designed to elicit a cortisol response, children rested for 30 min after arrival to reduce cortisol released as a result of any activities taking place beforehand (such as exercise) or nervousness on account of the novel laboratory environment. Participants were also instructed not to eat or drink 30 minutes before arrival at the laboratory as part of an effort to make baseline levels of cortisol as comparable as possible.
The TSST-C paradigm entails a brief (6 minute) preparation period for a speech, followed by a 10 min public speaking challenge that includes both verbal and arithmetic portions. Children complete the task in front of an audience trained to provide no emotional feedback. They then rested by watching a neutral film and did not interact with their accompanying parent until the last urine and saliva samples are collected an hour after termination of the stressor. All participants arrived at 4:00pm. Participants arriving more than thirty minutes early or late for their appointment were asked to reschedule in order to control for any potential circadian variation in hormonal profiles. Stopwatches were used to ensure timely collection of all saliva and urine samples during the course of the study.
Hormone collection and assay
Saliva samples for cortisol analyses were collected after subjects had rested for 30 minutes after arrival at the lab, and then at 0, 15, 30, 45 and 60 minutes after the TSST-C was complete. Saliva was frozen on dry ice after collection and assayed using an enzyme immunoassay for cortisol (Seltzer, Ziegler & Pollak, 2010). 25 μl of each sample was tested in duplicate. Bound cortisol peroxidase was measured by the reaction of the peroxidase enzyme on the substrate tetramethylbenzidine (TMB). Optical density was read using Assay Zap data reduction software at 450 nm using a 4 parameter sigmoid minus curve fit. Standard concentrations range from 3.00 μg/dl to 0.012 μg/dl, with intra- and interassay coefficients at 3.35 and 3.75, respectively.
Urine was collected after 30m of rest (baseline). Subsequent samples were collected at 30 and 60 minutes post-TSST-C. Urine samples were snap frozen on dry ice immediately after collection and subsequently stored at −80 degrees. A total of 3 samples could not be collected: 1 sample at baseline (for a girl in the control group) and 2 samples at 60-minutes post-test (for one girl in the control group and one girl in the maltreated group). After controlled thawing, urinary samples were subjected to solid phase extraction (SPE) using 1ml SepPak C18 cartridges (Waters, cat# WAT023590). Each column was pretreated with 1ml of methanol and then 1ml of water before application of 1ml of urine. A 10% ACN (acetonitrile) plus 1% TFA (trifluroacetic acid) wash (1ml) was then applied, after which the elutant was collected in a clean tube via application of a final 1ml application of 80/20% ACN solution with 1% TFA to the column. Samples were then dried down in a water bath with air stream and reconstituted in the assay-appropriate buffer supplied in the 96-well ELISA assay kit used (Assay Designs, Cat #901-153). Intra and inter coefficients of variation were determined by a human urine pool and oxytocin standards were used to determine recoveries from the extraction method (intra-assay /inter-assay coefficient of variation = 10.5/24.2, recoveries 92.1 +/− 5.23%, n=8). Creatinine was also collected with each urine sample and analyzed to correct for variation in water content (simple creatinine value divided by peptide concentration) to arrive at pg OT/mg creatinine (Ziegler, Scheffler & Snowdon, 1995).
RESULTS
Although participants were screened to ensure similar pubertal stages, we began by testing whether a relationship existed between the age of participants and the release of either oxytocin or cortisol in response to the TSST-C. Indeed, hormonal data indicated that age was a factor in hormonal outcome measures, with cortisol measures being higher in older children, F(1,69)=5.46, p<0.02; R2=0.06, and oxytocin measures being higher in younger children, F(1,64)=16.63, p<0.01, R2=0.19. Therefore, we included age as a covariate in all subsequent analyses. Next, we examined the possibility that abuse severity or duration might differ between males and females in the maltreatment group. Both sexes, however, had similar levels of maltreatment (t(35)=−1.26, p<0.22).
In order to compare participants by sex and maltreatment status, we conducted a repeated-measures ANOVA to examine hormonal change across time for both cortisol and OT, and applied the Greenhouse-Geisser correction. As is typically the case, hormone values had a skewed distribution, so we log-transformed them; however for ease of interpretation, all accompanying figures are graphed using original units. Raw hormonal values (mean, SE) by sex and maltreatment group are shown in Table 1.
Table 1.
Mean (SE) Values of Unadjusted (Non Log-transformed) Hormonal Measures Across Experimental Time
| Cortisol, ug/dl | Baseline | 0 minutes | 15 minutes | 30 minutes | 45 minutes | 60 minutes | |
|---|---|---|---|---|---|---|---|
| Control girls | 0.08 (0.02) | 0.20 (0.04) | 0.19 (0.04) | 0.13 (0.03) | 0.10 (0.02) | 0.10 (0.02) | |
| Maltreated girls | 0.06 (<0.01) | 0.04 (<0.01) | 0.04 (<0.01) | 0.04 (<0.01) | 0.04 (<0.01) | 0.04 (<0.01) | |
| Control boys | 0.08 (0.02) | 0.08 (0.02) | 0.12 (0.03) | 0.09 (0.02) | 0.07 (0.02) | 0.05 (<0.01) | |
| Maltreated boys | 0.09 (0.01) | 0.09 (0.01) | 0.10 (0.02) | 0.08 (0.02) | 0.08 (0.02) | 0.07 (0.01) | |
| Oxytocin, pg/mg creatinine | Baseline | 30 minutes | 60 minutes | ||||
| Control girls | 12.37 (12.37) | 14.55 (2.80) | 16.28 (5.61) | ||||
| Maltreated girls | 36.10 (8.54) | 109.48 (23.56) | 40.42 (14.34) | ||||
| Control boys | 12.53 (4.35) | 12.43 (4.27) | 10.11 (2.12) | ||||
| Maltreated boys | 13.29 (4.83) | 9.25 (3.65) | 10.50 (3.86) |
Note: Baseline indicates the measure collected after 30 minutes of rest after arrival at the laboratory; the end of the TSST-C is designated as 0 minutes.
Oxytocin
After winsorization of one participant with extremely high urinary OT (a maltreated girl), a repeated measures ANOVA omnibus test revealed a three-way interaction between maltreatment status, sex and time on values for OT (see Table 2), F(2,122) = 3.09, p < .05. This effect led us to evaluate the sexes separately in subsequent analysis.
Table 2.
Repeated Measures ANOVA Results, Effects for Oxytocin.
| Within-subjects | SS num | Df | Error SS | den Df | F | Pr(>F) |
|---|---|---|---|---|---|---|
| Time | 0.67 | 2 | 32.69 | 112 | 1.14 | 0.32 |
| Sex*time | 4.44 | 2 | 32.69 | 112 | 7.58 | <0.01*** |
| Maltreatment Status*time | 0.40 | 2 | 32.69 | 112 | 0.68 | 0.50 |
| Sex*Maltreatment Status *time | 2.92 | 2 | 32.69 | 112 | 3.09 | 0.05* |
| Between-subjects | ||||||
| Sex | 0.44 | 1 | 25.80 | 56 | 0.97 | 0.32 |
| Maltreatment Status | 6.74 | 1 | 25.80 | 56 | 14.63 | <0.01*** |
| Sex*Maltreatment Status | 4.63 | 1 | 25.80 | 56 | 10.06 | <0.01** |
Note:
p <.01,
p<.001,
p<0.0001
Two-way ANOVAs were conducted on OT separately for boys and girls. As shown in Figure 1, maltreated girls released high levels of OT following exposure to the social stressor whereas control girls did not, F(1,34) = 5.30, p <0 02. Neither abused nor control boys showed changes in OT following the stressor, F(1,28) = 0.07, ns.
Fig 1.
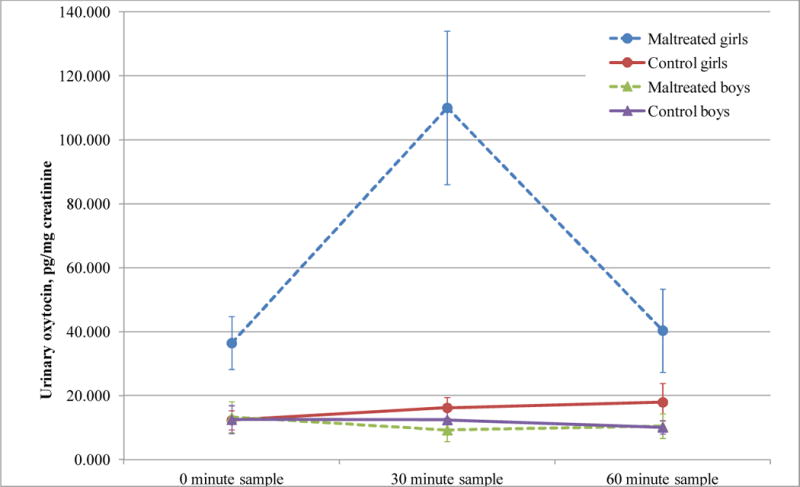
Oxytocin release across the experimental timeframe in children. Oxytocin levels are significantly higher in maltreated girls than in control girls subsequent to the TSST-C. For the maltreated girls, oxytocin at the 30 minute sample is significantly higher than the baseline and 60 minute samples. No significant differences in oxytocin are observed between condition or across time for boys.
Cortisol
A repeated-measures ANOVA for cortisol also revealed a significant three-way interaction effect for maltreatment, sex and time, F(4,244) = 2.25, p < .05, justifying evaluation of the sexes separately (see Table 3).
Table 3.
Repeated Measures ANOVA Results, Effects for Cortisol.
| Within-subjects | SS num | Df | Error SS | den Df | F | Pr(>F) |
|---|---|---|---|---|---|---|
| Time | 0.05 | 4 | 8.51 | 244 | 0.38 | 0.81 |
| Sex*time | 0.38 | 4 | 8.51 | 244 | 2.78 | 0.02* |
| Maltreatment Status*time | 0.65 | 4 | 8.51 | 244 | 4.70 | <0.01** |
| Sex*Maltreatment Status*time | 0.39 | 4 | 8.51 | 244 | 2.46 | 0.05* |
| Between-subjects | ||||||
| Sex | 0.1479 | 1 | 19.46 | 61 | 0.46 | 0.50 |
| Maltreatment Status | 1.5986 | 1 | 19.46 | 61 | 5.00 | 0.02* |
| Sex* Maltreatment Status | 0.7696 | 1 | 19.46 | 61 | 2.41 | 0.12 |
Note:
p <.01,
p<.001
Separate two-way ANOVAs on cortisol values were then conducted for boys and girls. As shown in Figure 2, control girls showed an expected increase in cortisol following the stressor, but abused girls did not, F(1,36) =15.74, p < .001. Both abused and control boys showed the expected increase in cortisol following the stressor, but there were no differences between the two groups of boys, F(1,33) < 1, ns.
Fig 2.
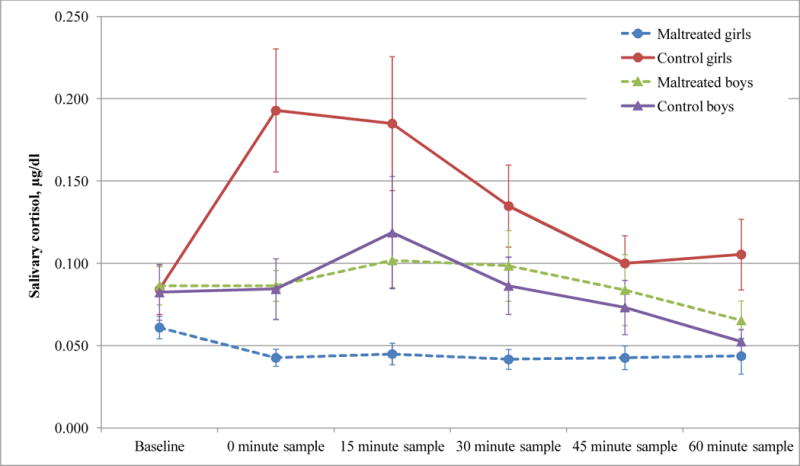
Cortisol levels are significantly lower in maltreated girls than it is in control girls. For the control girls, cortisol increased in response to the TSST-C, whereas maltreated girls show little if any cortisol change in response to the stressor. In contrast, maltreated and control boys show comparable cortisol profiles across time.
Relationship between Cortisol and Oxytocin
We next examined the relation between salivary cortisol and urinary oxytocin following exposure to the stressor. Extant literature suggests that there should be a negative correlation between these two hormones because one of the functions of oxytocin is to suppress or buffer the stress response in order for social approach to take place (Lucas et al., 2011); we had no a priori reason to believe that this relation would be affected by child maltreatment, however. Children’s salivary cortisol responses following the stressor were indeed negatively correlated with their urinary OT responses after completion of the TSST-C, r(64) = −.25, p < .05. Maltreated girls r(19) = −.32, p < .05 maltreated boys r(15) = −.25, p < .05 and their control male r(16) = −.27, p < .05 and female r(16) = −.21, p < .05 counterparts all showed comparable negative relations between the hormones.
DISCUSSION
This experiment reveals that girls who have experienced early life stress in the form of child maltreatment release higher levels of urinary OT following exposure to a social stressor than their peers. Furthermore, although control girls show high levels of cortisol after experiencing a social stressor, maltreated girls had comparatively low levels of cortisol before, during, and after the social stress task. These effects appear to be specific to girls in that no differences were observed between maltreated and control boys with respect to OT or cortisol.
Because maltreated girls often have poor social relationships with others (Cullterton-Sen et al., 2008; Murray-Close et al., 2008), it may seem counter-intuitive that they would show higher levels of OT, a hormone implicated in the maintenance of affiliative behaviors and intimate relationships. These findings, however, in no way refute the bulk of the literature suggesting that higher levels of OT is typically associated with positive social behaviors, nor the literature that high cortisol is typically associated with stress. The fact that maltreated females show an exaggerated OT response and a blunted response to a social stressor is likely to be a special case, and one which may drive adaptive behaviors in this population.
Lower levels of cortisol in response to the laboratory stressor likely reflects the dampening of the HPA in response to repeated stressful events early in life (McEwen, 1998). This is in keeping with a substantial body of literature in humans suggesting this relationship between suppressed HPA functioning and early adversity (e.g., Carpenter et al., 2003; Cicchetti, & Rogosch, 2001; Fries, Hesse, Hellhammer & Hellhammer, 2005; Gunnar & Vazquez, 2001; MacMillian et al, 2009). In other primates, this may be adaptive, as each subsequent stressor provides a measure of stress resistance (Parker et al., 2006). It is possible that similar mechanisms operate in humans.
Moreover, the fact that OT levels are already higher in maltreated girls at baseline indicates that their much higher OT levels after the TSST-C may be indicative not just of dysregulation of the response to acute stress, but to fundamental differences in how their OT system operates. The precise biological mechanisms through which early experience may cause these changes is unknown, but it is possible that epigenetics play a role. In laboratory animals, for example, early life stress is linked to hypomethylation of vasopressin, a peptide closely related to oxytocin (Murgatroyd et al., 2011). If a similar relationship is true of OT, this might explain the combination of high OT and low cortisol in maltreated girls.
It may be the case that this pattern of high OT and low cortisol together is evidence of damage to the social brain. Alternatively, however, it may be adaptive. First, this hormonal pattern may be associated with increased caretaking behaviors within a stressful family environment. Such caretaking in the form of care of infants and young children may constitute preparation for early reproduction. Indeed, early reproduction may be the rule rather than the exception in females exposed to early life stress. In laboratory rats, for example, female pups who receive less licking and grooming from their dams become sexually receptive earlier than their peers (Cameron, Fish & Meaney, 2008). In humans, girls exposed to early adversity also reach puberty sooner than their peers (see Ellis, 2004 for a review). Human females exposed to chronic stressors during childhood might also be expected to experience earlier puberty, sexual behavior and first births, and exhibit lower parental investment in their offspring (Belsky, Steinberg & Draper, 1991). While these behaviors incur social and psychological costs in the modern world, more rapid reproduction and lower-investment parenting strategies can be evolutionarily advantageous for females primed for life in harsh and/or unpredictable environments. In this light, girls developing within abusive family environments may develop biobehavioral adaptations geared toward reproducing early in which the risk of mortality is high, as delaying reproduction in these contexts may carry a greater risk to individual fitness (Ellis, 2004).
Second, is possible that higher OT prompts maltreated females to approach novel individuals, such as unrelated males, earlier than their typically-developing peers. Such behavior, of course, may lead to the precocious sexual behavior and early pregnancy observed in girls with histories of maltreatment (Noll, Haralson, Butler & Shenk, 2011). In laboratory animals, OT indeed facilitates social approach toward novel individuals and suppresses avoidant behaviors (Lukas et al., 2011). Again, while such outcomes carry a social cost in modern human society, early sexual behavior may have been evolutionarily advantageous to our ancestors. Leaving home and seeking out mates earlier than average could reduce the risks of morbidity and mortality at the hands of abusive relatives. Indeed, we might expect children reared in these environments to seek out alternative forms of social support for survival. These relationships might not be stable, but short-term relationships with sex partners may be advantageous in female mammals under some conditions. For example, such relationships can both obscure paternity and maximize contributions from multiple males toward the rearing costs of offspring (Heistermann et al., 2001), particularly when the probability of securing a long-term, high quality mate is low.
With regard to sex differences, it is notable that maltreated boys did not respond to the social stressor in the same way as girls, despite the fact that these boys have not yet gone through puberty and the accompanying exposures to gonadal hormones. Inspection of each child’s data did not reveal any maltreated boy that showed an OT response similar to that of the maltreated girls. While control males showed less of a response to the TSST-C overall than girls in this age range, most male participants responded to the TSST-C with a cortisol increase. Irrespective of their cortisol response, however, OT remained low. This makes it unlikely that failure to release OT in boys can be attributed to an attenuated cortisol response for this particular task. Finally, the maltreated boys and girls in the sample had similar kinds of maltreatment experiences (predominantly physical abuse in the absence of sexual abuse), and both the boys and girls had a similar level of abuse histories.
The question of why only maltreated girls looked different from the other groups with respect to their hormonal release is not entirely clear. Prepubertal sex differences in behavior, however, should be expected given differential constraints on male versus female fitness later in life. Male primates, for example, may benefit in from conflict in terms of greater mating opportunity, whereas females may incur a higher cost of engaging in fight or flight (due to the risks associated with confrontation when gestating, lactating or, caring for vulnerable infants) and may instead be better off forming relationships with others when circumstances permit (Taylor et al., 2000). This experiment may reveal the neuroendocrinological underpinnings of these behaviors, before the influx of gonadal hormones and accompanying pubertal changes.
The precise biological links between early adversity, oxytocin release, and stress in our own species should be the subject of future research. Such work should explore the implications and robustness of these sex differences. Moreover, while evaluation of group differences in hormonal patterns at baseline, in resting states or across a daily circadian rhythm is important, future work should also focus on how children respond to active stressors and social challenges. This is the best way of examining how children who have suffered the effects of early adversity respond to social situations encountered in daily life.
Conclusion
This is the first evidence showing that high OT levels are observed in maltreated female children. While this may be evidence of neurological dysregulation with potentially negative future consequences, it is also possible that it instead represents phenotypic plasticity with respect to human behavior that may be adaptive. If true, OT in this context may be viewed as the proximate mechanism through which social motivation takes place, with intense family conflict in the form of maltreatment priming females for early departure from the kin group. Such relationships, however, may not necessarily be stable, safe, or developmentally appropriate in modern society. Early pregnancy is associated with higher risk of abuse and neglect for resultant children, as well as lower academic achievement, greater risk of mental health disorders, increased rate of incarceration, and increased risk of offspring becoming teenaged parents themselves relative to other children (Ruedginer & Cox, 2012). The findings presented here provide another layer of complexity to the study of developmental plasticity, health, and the evolution of human behavior.
Acknowledgments
We wish to thank the children and their families who participated in this research and acknowledge the assistance of Barbara Roeber for assistance with participant recruitment. This research was supported by a grant to Seth Pollak from the National Institute of Mental Health (MH61285), including a supplementary award issued under the American Recovery and Reinvestment Act (ARRA). Oxytocin analyses made use of facilities supported by grants RR000167 from the Wisconsin National Primate Research Center and 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
References
- Bakermans-Kranemburg MJ, van Ijzendoorn MH, Riem MM, Tops M, Alink LR. Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Social Cognitive and Affective Neuroscience. 2012;7:951–7. doi: 10.1093/scan/nsr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza JA, Zak PJ. Empathy towards strangers triggers oxytocin release and subsequent generosity. Annals of the New York Academy of Sciences. 2009;1167:182–9. doi: 10.1111/j.1749-6632.2009.04504.x. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. Effects of oxytocin on recollections of maternal care and closeness. PNAS. 2010;107:21371–5. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognition and Affective Neuroscience. 2011;6:556–63. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belski L, Steinberg J, Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Development. 1991;62:647–70. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Goursaud AP, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: a new pharmacological tool for the study of social motivation in non-human primates. Hormones and Behavior. 2007;52:344–51. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neuroscience and Biobehavioral Review. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Burbach JP, Bohus B, Kovacs GL, Van Nispen JW, Greven HM, De Wied D. Oxytocin is a precursor of potent behaviourally active neuropeptides. European Journal of Pharmacology. 1983;94:125–131. doi: 10.1016/0014-2999(83)90449-1. [DOI] [PubMed] [Google Scholar]
- Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Hormones and Behavior. 2008;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–7. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (B) 2011;214:367–75. doi: 10.1007/s00213-010-2007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Annals of the New York Academy of Sciences. 1997;15:164–74. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behavioural Brain Research. 2007;176:170–86. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. American Journal of Physiology. Endocrinology and Metabolism. 2008;295:E686–91. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- Cohen P, Brown J, Smailes E. Child abuse and neglect and the development of mental disorders in the general population. Development and Psychopathology. 2001;13:981–99. [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal microinfusion of oxytocin attenuates behavioral response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. Journal of Neuroendocrinology. 2010;22:889–904. doi: 10.1111/j.1365-2826.2010.02003.x. [DOI] [PubMed] [Google Scholar]
- Cullerton-Sen C, Cassidy AR, Murray-Close D, Cicchetti D, Crick NR, Rogosch FA. Childhood maltreatment and the development of relational and physical aggression: the importance of a gender-informed approach. Child Development. 2008;79:1736–51. doi: 10.1111/j.1467-8624.2008.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Dreu CK. Oxytocin modulates the link between adult attachment and cooperation through reduced betrayal aversion. Psychoneuroendocrinology. 2012;37:871–80. doi: 10.1016/j.psyneuen.2011.10.003. [DOI] [PubMed] [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. PNAS. 2011;108:1262–6. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Landgraf R, Engelmann M. A single social defeat experience selectively stimulates the release of oxytocin, but not vasopressin, within the septal brain area of male rats. Brain Research. 2000;872:87–92. doi: 10.1016/s0006-8993(00)02464-1. [DOI] [PubMed] [Google Scholar]
- Ellis BJ. Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin. 2004;130:920–58. doi: 10.1037/0033-2909.130.6.920. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Holsboer F, Wotjak1 CT. Emotional stress triggers intrahypothalamic but not peripheral release of oxytocin in male rats. Journal of Neuroendocrinology. 1999;11:867–872. doi: 10.1046/j.1365-2826.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Developmental Science. 2011;14:752–61. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Hormones and Behavior. 2010;58:669–76. doi: 10.1016/j.yhbeh.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–6. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiological Review. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Goldman-Mellor S, Hamer M, Steptoe A. Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology. 2012;37:1755–68. doi: 10.1016/j.psyneuen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, Carter CS. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–80. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13:515–38. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Development and Psychopathology. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry. 2009;14:954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Progress in Brain Research. 2008;170:337–50. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–98. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Heistermann M, Ziegler T, van Schaik CP, Launhardt K, Winkler P, Hodges JK. Loss of oestrus, concealed ovulation and paternity confusion in free-ranging Hanuman langurs. Proceedings of the Royal Society, Biological Sciences. 2001;268:2445–51. doi: 10.1098/rspb.2001.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. American Journal of Psychiatry. 1997;156:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Jezova D, Skultetyova L, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. In: Chrousos GP, editor. Stress: Basic mechanisms and clinical implications. Vol. 771. New York, NY: Annals of the New York Academy of Sciences; 1995. pp. 192–203. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. Plasticity of the stress response early in life: mechanisms and significance. Developmental Psychobiology. 2010;52:661–670. doi: 10.1002/dev.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and V1a receptors in the primate prefrontal cortex. Nature Neuroscience. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton AA, Block J, Adler GK. A heartfelt response: Oxytocin effects on response to social stress in men and women. Biological Psychology. 2012;90:1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitz SJ, Levy JE, McCloskey J, Gabriel KR. Alcohol dependence and domestic violence as sequelae of abuse and conduct disorder in childhood. Child Abuse and Neglect. 1998;22:1079–91. doi: 10.1016/s0145-2134(98)00089-1. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Dodge KA, Pettit GS, Bates JE. Does physical abuse in childhood predict substance abuse in adolescence? Child Maltreatment. 2010;15:190–4. doi: 10.1177/1077559509352359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros JJ, Chiodera P, Geenen V, Smitz S, von Frenckell R. Dose-response relationship between plasma oxytocin and cortisol and adrenocorticotropin concentrations during oxytocin infusion in normal men. The Journal of Clinical Endocrinology and Metabolism. 1984;58:105–9. doi: 10.1210/jcem-58-1-105. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biological Psychiatry. 2012;71:344–9. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:2159–68. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;6:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: results of the youth mood project. Biological Psychiatry. 2009;66:62–8. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Society of Biological Psychiatry. 2007;61:1109–1111. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Research. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, Levin H. Plasma oxytocin levels in autistic children. Biological Psychiatry. 1998;43:270–7. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmühl Y, Fischer D, Holsboer F, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature Neuroscience. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Murray-Close D, Han G, Cicchetti D, Crick NR, Rogosch FA. Neuroendocrine regulation and physical and relational aggression: the moderating roles of child maltreatment and gender. Developmental Psychology. 2008;44:1160–76. doi: 10.1037/a0012564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll JG, Haralson KJ, Butler EM, Shenk CE. Childhood maltreatment, psychological dysregulation, and risky sexual behaviors in female adolescents. Journal of Pediatric Psychology. 2011;36:743–52. doi: 10.1093/jpepsy/jsr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A, Tubman JG, Burnette ML. Childhood maltreatment histories, alcohol and other drug use symptoms, and sexual risk behavior in a treatment sample of adolescents. American Journal of Public Health. 2012;102:S250–7. doi: 10.2105/AJPH.2011.300628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers CL, Danese A, Bowes L, Shakoor S, Papadopoulos AS, Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biological Psychiatry. 2011;70:1016–23. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, Schatzberg AF. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Research. 2010;178:359–62. doi: 10.1016/j.psychres.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. PNAS. 2006;103:3003–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrehumbert B, Torrisi R, Laufer D, Halfon O, Ansermet F, Beck Popovic M. Oxytocin response to an experimental psychosocial challenge in adults exposed to traumatic experiences during childhood or adolescence. Neuroscience. 2010;166:168–77. doi: 10.1016/j.neuroscience.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Ruedinger E, Cox JE. Adolescent childbearing: consequences and interventions. Current Opinions in Pediatrics. 2012;24:446–52. doi: 10.1097/MOP.0b013e3283557b89. [DOI] [PubMed] [Google Scholar]
- Salzinger S, Feldman RS, Hammer M, Rosario M. The effects of physical abuse on children’s social relationships. Child Development. 1993;64:169–187. doi: 10.1111/j.1467-8624.1993.tb02902.x. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proceedings of the Royal Society London, Series B. 2010;277:2661–6. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Agmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Hormones and Behavior. 2010;58:614–8. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the Parent-Child Conflict Tactics Scales: development and psychometric data for a national sample of American parents. Child Abuse and Neglect. 1998;22:249–70. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology. 2011;36:115–22. doi: 10.1016/j.psyneuen.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–29. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosomatic Medicine. 2006;68:238–45. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Turner RA, Altemus M, Enos T, Cooper B, Mcguinness T. Preliminary research on plasma oxytocin in normal cycling women : Investigating emotion and interpersonal distress. Psychiatry. 1999;66:97–113. doi: 10.1080/00332747.1999.11024859. [DOI] [PubMed] [Google Scholar]
- van den Burg EH, Neumann ID. Bridging the gap between GPCR activation and behaviour: oxytocin and prolactin signalling in the hypothalamus. Journal of Molecular Neuroscience. 2011;43:200–8. doi: 10.1007/s12031-010-9452-8. [DOI] [PubMed] [Google Scholar]
- Veenema AH. Toward understanding how early-life social experiences alter oxytocin- and vasopressin-regulated social behaviors. Hormones and Behavior. 2012;61:304–312. doi: 10.1016/j.yhbeh.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Wismer-Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. PNAS. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Ziegler TE, Scheffler G, Snowdon CT. The relationship of cortisol levels to social environment and reproductive functioning in female cotton-top tamarins, Saguinus oedipus. Hormones and Behavior. 1995;29:407–24. doi: 10.1006/hbeh.1995.1028. [DOI] [PubMed] [Google Scholar]


