Highlights
-
•
Synaptic activity drives the formation of specific synapses in the retina.
-
•
Neurotransmitter induces the formation of spines in developing cortical neurons.
-
•
Axons are capable of releasing neurotransmitter before synaptic contacts.
-
•
We speculate on the role of early, non-synaptic release in synaptogenesis.
Abstract
The long history of probing the role of neuronal activity in the development of nervous system circuitry has recently taken an interesting turn. Although undoubtedly activity plays a critical part in the maintenance and refinement of synaptic connections, often via competitive mechanisms, evidence is building that it also drives the process of synapse formation itself. Perhaps predictably, this turns out not to be a uniform process. It seems that different circuits, indeed specific synaptic connections, are differentially sensitive to the effects of activity. We examine possible ways in which neurotransmitter may drive synapse formation, and speculate on how the environment of the developing brain may allow a different spatiotemporal range for neuronal activity to operate in the generation of connectivity.
Current Opinion in Neurobiology 2014, 27:47–52
This review comes from a themed issue on Development and regeneration
Edited by Oscar O Marín and Frank F Bradke
For a complete overview see the Issue and the Editorial
Available online 13th March 2014
0959-4388/$ – see front matter, © 2014 The Authors. Published by Elsevier Ltd. All rights reserved.
Introduction
Ever since the classic experiments by Hubel and Wiesel where they demonstrated that visual experience is required to drive the formation of ocular dominance columns in the visual cortex [1,2], the role of neuronal activity in shaping developing circuits has been a subject of intense investigation. Over the years, it has become clear that in many developing systems, from the neuromuscular junction to the visual system, activity plays a key role in the refinement and maintenance of neuronal circuits, often via competitive mechanisms [3–5]. However, it is increasingly evident that neuronal activity, whether spontaneous or driven by sensory experience, is also important for the formation of synapses. In this review, we will focus on the role of activity in synapse formation, recent work on how these effects can be highly cell-type specific, and discuss possible mechanisms that link neurotransmitter release to the process of synaptogenesis.
Activity regulates synapse formation
While it seems that synaptogenesis per se is able to proceed in the absence of neuronal activity [6,7], many studies that have interfered with activity have demonstrated a negative effect on synapse number. Genetic deletion of the Munc18-1 protein, which is critically required for correct assembly of the presynaptic release machinery, leads to complete abolition of neurotransmitter release [7]. Although morphologically normal synapses do develop in this mutant [7], there is a dramatic reduction in synapse number in the cortex [8]. Similarly, mice lacking ChAT, the biosynthetic enzyme for the neurotransmitter acetyl choline (ACh), lose all transmission at the neuromuscular junction and display smaller motor nerve terminals which make fewer synapses [9]. Many other tactics have been employed to interfere with neuronal transmission and the firing of action potentials, including more recently the incorporation of traditional neurotoxins or naturally occurring ion channels that hyperpolarise cells into genetically controlled expression systems to allow spatiotemporal control of neuronal/synaptic activity. For example, expression of the inwardly rectifying potassium channel Kir2.1 results in hyperpolarisation of neurons and hence reduced firing. When this was expressed in hippocampal neurons in culture, fewer synapses were formed on to the silenced cell [10]. However, these effects were likely to be competitive in nature as global silencing of action potential firing with tetrodotoxin, a blocker of sodium channels, rescued these changes. Tetanus toxin prevents neurotransmitter release by cleaving the presynaptic vesicle SNARE protein VAMP2. In the olfactory system, conditional silencing of olfactory sensory neurons (OSNs) was achieved using a cell-type specific promoter to drive either tetanus toxin light chain to prevent neurotransmitter release or Kir2.1 to electrically silence neurons [11]. Interestingly, while blockade of transmitter release had only competitive effects on postsynaptic targeting, global silencing with Kir2.1 led to a delay in axon entry and disorganised OSN targets, the glomeruli [11]. These studies emphasise the notion that differential effects of activity are seen in different contexts and using alternative methods of manipulating activity [12].
A reduction in synapse number could be due to impaired synapse formation, but could alternatively be a result of increased rates of synapse elimination or a failure of maintenance. Almost 20 years ago, a study in ferrets mapped the location of a specific subset of synapes in developing and mature visual cortex and found evidence which suggested that activity could itself drive synaptogenesis [13]. With technical advances in imaging it has become possible to distinguish between these possibilities by employing careful timelapse imaging of neurons during the critical period of synaptogenesis. An early study examined turnover of the post-synaptic scaffolding protein, PSD-95, and found that blockade of activity led to a reduction in turnover [14] with a specific effect of antagonising NMDA receptors on inhibition of new cluster generation. More recently, this issue has been carefully addressed in the mouse retina. This system allows elegant genetic control of specific cell types, including presynaptic and postsynaptic partners, coupled with well described anatomical and functional connections. Furthermore, it has long been used to examine activity-dependent effects on circuits in general. In the retina, photoreceptors (rod or cone) connect to bipolar cells which in turn form excitatory synapses onto retinal ganglion cells (RGCs) (Figure 1a). A key study from the Wong laboratory silenced ON bipolar cells by expressing tetanus toxin under the mGluR6 promoter (Grm6-TeNT) [15]. These ON bipolar cells normally project to mono-stratified ON RGCs or to part of the dendritic tree of bi-stratified ON/OFF RGCs. Although the dendrites of the ON RGCs showed normal stratification, there was a 50% reduction in synapse number from ON bipolar cells. Interestingly, bi-stratified ON/OFF RGCs showed a reduction in ON synapses with no effect on OFF connections, suggesting that these two populations of synaptic connections seem to form independently. Live imaging at P9 demonstrated a significant reduction in the rate of synapse formation, with normal levels of elimination, indicating that excitatory synaptic activity from the ON bipolar cell regulates synapse number onto its target specifically via effects on synapse formation.
Figure 1.
Synaptic activity drives the formation of synaptic contacts in the developing retina. (a) Schematic diagram of the retina showing photoreceptors (blue), bipolar cells (pink) and ganglion cells (green). Synapses between bipolar and ganglion cells occur in different layers, broadly divided into ON and OFF layers. (b) Synaptic contacts (yellow) formed between two different types of ON bipolar cells (B6 and B7, pink) and a specific type of ON ganglion cell (G10, green) are modulated by activity. Expressing tetanus toxin in B6 or B7 bipolar cells (middle panel) results in a specific decrease in the number of connections formed by B6 but not by B7 neurons. Conversely, increasing the activity of bipolar cells (bottom panel) causes an increase in the number of B6 synapses, but not those formed by B7 neurons.
Cell-type specific pathways
Taking advantage of the ability to fluorescently label specific subtypes of cells and their connections in the mouse retina, a number of recent studies have demonstrated that the effects of activity are exerted in a highly differential manner [16••,17•,18•]. Detailed mapping of normal developmental changes indicated that the B6 bipolar cell nearly doubles the number of connections with G10 RGCs between P9 and P21, while the B7 bipolar cell maintains a relatively constant number and the RB bipolar cell loses its connections. Silencing bipolar cells with tetanus toxin had completely different effects on each type of bipolar cell: a dramatic reduction in B6-G10 synapses at P21, but no effect on B7-G10 synapses (Figure 1b) and the RB-G10 synapses were correctly eliminated [16••]. Similarly, another study examined the effects of sensory deprivation (dark rearing) on the photoreceptor-to-bipolar cell connection, this time looking at rod versus cone photoreceptors synapsing on to B6,7 or 8 bipolar cells. Once again, they found differential (and complex) effects of sensory deprivation on different connections [18•], which interestingly is reflected in different spatiotemporal patterns of dendritic arbor growth [19•].
Most studies examining activity-dependent effects have used different techniques to silence activity, but a recent study set out to determine whether increasing activity could lead to a corresponding increase in synapse formation [17•]. The Crx−/− mutant mouse displays increased spontaneous firing of RGCs after eye opening (∼P15), due to increased glutamate release from bipolar cells. From this stage onwards, mutants show increasing numbers of bipolar-to-RGC synapses. Live imaging during this period indicates that this increase is due to higher rates of synapse formation, with no impact on elimination rates. Further, there were dramatically different effects in each cell type: B6-G10 synapses showed a doubling in synapse number, while there was no effect on B7/RB-G10 synapses (Figure 1b) [17•].
The finding that activity differentially affects distinct cell types, even when examining different axonal inputs onto the same postsynaptic target, may go some way to explaining why activity blockade spares many synaptic connections. But why, for example, does activity regulate B6 connections onto G10 RGCs, but not those of B7 and RB cells? Activity levels seem also to differentially affect excitatory and inhibitory synapses. In the adult mouse, depriving one eye of vision has recently been found to lead to a reduction in inhibitory synapse formation [20] (and increased rates of loss [21]) whereas excitatory synapses increase [22]. This is perhaps not surprising: excitatory and inhibitory synapses not only release different neurotransmitters with (usually) opposing effects on the postsynaptic cell, but also express different proteins with differing sensitivities to manipulation (e.g. [6]) and show different behaviours during early synapse formation [23]. Clearly, different circuits subserve different roles, even where they converge on the same cells, and there are likely to be key developmental and functional reasons why some need to be regulated by activity and some do not. How and why this occurs will be an area for much future investigation.
What mechanisms could be responsible for activity-driven synapse formation?
Before synapses are formed, axonal growth cones have long been known to release neurotransmitter [24,25], even before synaptic vesicle proteins are expressed [26]. Experiments in Xenopus embryonic spinal cord neurons showed that both evoked and spontaneous neurotransmitter release occurred all along the axons of isolated neurons, including growth cones [25,27]. Indeed, high levels of on-going vesicle cycling have also been shown to exist in young axons and growth cones. In dissociated hippocampal neurons, constitutive vesicle cycling was observed in growing axons [28], as well as in the long thin filopodia of growth cones [29]. More recent work has shown that young axons preferentially undergo spontaneous forms of vesicle cycling, gradually switching towards higher levels of evoked release during development. This switch in vesicle cycling modes was cell autonomous and independent of postsynaptic contact [30•]. Although most studies into the role of activity on synapse and circuit development have focused on action potential firing, a few studies have demonstrated effects of spontaneous release during development, on dendritic spine maintenance [31] and on modulating the dendritic arbor response to the neurotrophin BDNF [32]. Given the high levels of spontaneous, quantal release in developing systems [30•,33] it will be of interest to see if differential roles can be identified for different types of presynaptic transmitter release.
At this early stage in development, dendrites are studded with highly motile protrusions, or filopodia [34]. These filopodia are known to be precursors to dendritic spines [35]. Immature dendrites are also known to express transmitter receptors prior to innervation, such as the NMDA glutamate receptor [36], which undergo constant cycling with the plasma membrane even at these early stages [37]. It is tempting to imagine that the early release of neurotransmitters could exert some kind of effect on these filopodia that might regulate synaptogenesis. Activation of NMDA receptors early in development, in some cases prior to synapse formation, has been observed in different preparations [38–40]. Further, glutamate itself can induce filopodia formation [41] and increase filopodial length [42], while blockade of NMDA receptors or metabotropic glutamate receptors has the reverse effect [42,43]. In the chick retina, glutamate receptor blockade at a specific timepoint in development impaired filopodial extension rates and amounts (although retraction was also affected) [44]. In vivo studies in Xenopus have shown that visual stimulation can increase dendritic branch formation, potentially either a precursor of or a sequel to synapse formation [45,46], which is prevented by antagonising both NMDA and AMPA glutamate receptors [47]. In fact, impairment of filopodial motility (due to deletion of the protein EphB2) is associated with a dramatic reduction in synapse density due to impaired synaptogenesis [48]. Thus it seems that presynaptic activity could operate on dendritic receptors, particularly those of the NMDA subtype, to regulate filopodia and hence have effects on dendritic branching and presumably, synapse formation.
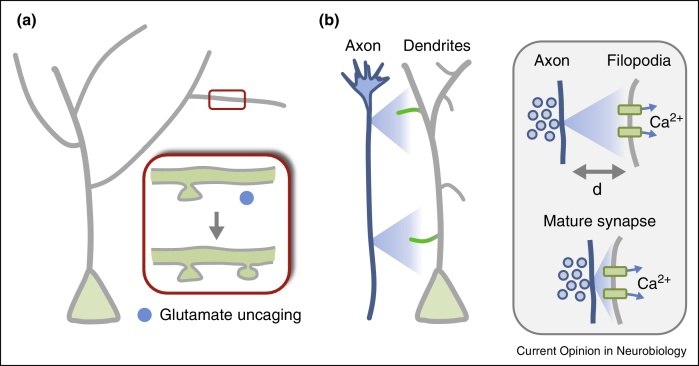
Studies from the field of synaptic plasticity have revealed that high levels of activity (able to induce long-term potentiation of synaptic strength) can induce new spine formation [49] and indeed NMDA-dependent dendritic filopodial growth [50]. It seems intuitive that Hebbian-like plasticity mechanisms could play a role during earlier synapse development [51,52]. A more recent study has now shown that neurotransmitter can directly induce the de novo formation of mature spines during development in cortical neurons (Figure 2a) [53••]. Either focal uncaging of caged glutamate close to a stretch of dendrite (less than 1 μm away) or high frequency stimulation resulted in the local growth of spines very rapidly (within 6 s of the uncaging protocol). This effect was dependent on NMDA receptor activation. Surprisingly, the new spines did not emerge as filopodia, but as mature spines, both structurally and functionally, expressing receptors and channels that allows their rapid integration into the circuit. How these experiments compare to the earlier stages of synapse formation where immature axons have not yet established any synaptic contact, is not yet clear. Also, whether neurotransmitter release could act at a distance, and how far a release site needs to be to activate postsynaptic receptors, remains unknown. Future studies are likely to illuminate further possible mechanisms for activity-driven synaptogenesis.
Figure 2.
The role of the neurotransmitter glutamate in synapse formation. (a) Glutamate can induce the formation of functional postsynaptic spines. The diagram shows a developing neuron in the cortex with a cell body (green) and apical dendrites (gray). The inset shows a zoomed in section of the dendrite (red box), which contains an existing spine (top drawing). Glutamate is uncaged locally with a laser (blue circle), resulting in the emergence of a new postsynaptic spine. Adapted from [53]. (b) Long range communication between neurons in developing circuits. Growing axons release neurotransmitter before they form any synaptic connections (indicated by graded blue signal from axons). Filopodia from neighbouring neurons may be able to sense this neurotransmitter at a distance (indicated by the graded green response in filopodia). Inset: the release of neurotransmitter, such as glutamate, from presynaptic vesicles (blue) clustered along a growing axon could activate distant dendritic receptors (green), such as NMDA receptors, resulting in possible calcium influx and plasticity (top drawing). Such a form of long-range communication, distinct from local synaptic transmission in mature synapses (bottom drawing) could provide information for circuit assembly and synapse formation. The spatial extent of this form of communication (d) may well be larger than the few nanometers of mature synapses and may help instruct postsynaptic filopodia and dendrites during the process of synaptogenesis.
How early can neurotransmitter release influence synapse formation?
The fact that axons release neurotransmitter even before synapsing onto their target dendrites raises the possibility that the process of activity-dependent synapse formation may actually begin very early on, before contacts are made. For this to occur, axons may well relay information at a distance, from a growing axon to a nearby dendrite or filopodium and influence its activity in a meaningful manner (Figure 2b). However, the distance over which neurotransmitter can influence neighbouring neurons during development is unknown. Although transmission is highly localised in mature synapses there are many reasons to believe that this may not be the case early in circuit development. For a start, the expression of transporters that would normally curtail the spread of neurotransmitter from its source is delayed until after synapses have begun to form, increasing the area of influence from a single release site [54,55]. Additionally, developing neurons preferentially express NMDARs containing the NR2B subunit, which has a higher sensitivity to both glutamate and its co-agonist glycine when compared to NR2A-containing receptors that are mainly found in mature synaptic contacts, making developing dendrites highly sensitive to glutamate [56]. Critically, many studies have found that blockade of glutamate transporters during development can induce dramatic synchronous oscillations of neurons which are dependent on NMDA receptor activation [57–59], indicating that extrasynaptic or ‘spillover’ glutamate is present in the developing brain. Indeed, a series of recent studies have compellingly demonstrated the presence of activity-dependent glutamate spillover using outside-out patches or optical sensors, acting via NMDA receptors in developing retinae, which was important for correct spatiotemporal retinal wave structure [60,61•], as well as extracellular ACh associated with cholinergic waves [62•]. Furthermore, there is evidence showing that the neurotransmitters GABA and glutamate can be released in a paracrine fashion and act on postsynaptic receptors in developing systems [63]. Interestingly, this study found that even in neurons prior to synapse formation, ambient GABA (but not glutamate) had tonic activation effects on membrane potentials [63]. GABA is well known to exert multiple trophic actions on neuronal development, including increasing synapse density [64]. Together, these findings suggest that the release of neurotransmitter from a growing axon could, in principle, act at a distance to influence dendritic growth. Such a mechanism could have an important impact on how we think about the spatial domain over which synaptic transmission can operate (long-range, versus direct contact) and the temporal domain (early in development) over which neurotransmitter release can influence dendritic structures.
Studies investigating the role of neuronal activity in the wiring of the brain have mainly focused on the process of circuit refinement that ensues after synapses have formed. More recent findings have shifted the focus of attention to earlier events that occur concurrently with, or immediately preceding, synapse formation and influence the actual process of synaptogenesis itself. The wealth of new molecular and imaging tools to visualise the process of synapse formation will undoubtedly shed light on the critical periods needed for activity-dependent synapse formation and help us understand how and when neurons communicate with each other to form functional synaptic contacts.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Laura C Andreae, Email: laura.andreae@kcl.ac.uk.
Juan Burrone, Email: juan.burrone@kcl.ac.uk.
References
- 1.LeVay S., Wiesel T.N., Hubel D.H. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 2.Wiesel T.N., Hubel D.H. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 3.Huberman A.D., Feller M.B., Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz L.C., Shatz C.J. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 5.Sanes J.R., Lichtman J.W. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 6.Varoqueaux F., Sigler A., Rhee J.S., Brose N., Enk C., Reim K., Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhage M., Maia A.S., Plomp J.J., Brussaard A.B., Heeroma J.H., Vermeer H., Toonen R.F., Hammer R.E., van den Berg T.K., Missler M. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 8.Bouwman J., Maia A.S., Camoletto P.G., Posthuma G., Roubos E.W., Oorschot V.M., Klumperman J., Verhage M. Quantification of synapse formation and maintenance in vivo in the absence of synaptic release. Neuroscience. 2004;126:115–126. doi: 10.1016/j.neuroscience.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Brandon E.P., Lin W., D’Amour K.A., Pizzo D.P., Dominguez B., Sugiura Y., Thode S., Ko C.P., Thal L.J., Gage F.H. Aberrant patterning of neuromuscular synapses in choline acetyltransferase-deficient mice. J Neurosci. 2003;23:539–549. doi: 10.1523/JNEUROSCI.23-02-00539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrone J., O’Byrne M., Murthy V.N. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- 11.Yu C.R., Power J., Barnea G., O’Donnell S., Brown H.E., Osborne J., Axel R., Gogos J.A. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- 12.Bleckert A., Wong R.O. Identifying roles for neurotransmission in circuit assembly: insights gained from multiple model systems and experimental approaches. Bioessays. 2011;33:61–72. doi: 10.1002/bies.201000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalva M.B., Katz L.C. Rearrangements of synaptic connections in visual cortex revealed by laser photostimulation. Science. 1994;265:255–258. doi: 10.1126/science.7912852. [DOI] [PubMed] [Google Scholar]
- 14.Okabe S., Kim H.D., Miwa A., Kuriu T., Okado H. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat Neurosci. 1999;2:804–811. doi: 10.1038/12175. [DOI] [PubMed] [Google Scholar]
- 15.Kerschensteiner D., Morgan J.L., Parker E.D., Lewis R.M., Wong R.O. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Morgan J.L., Soto F., Wong R.O., Kerschensteiner D. Development of cell type-specific connectivity patterns of converging excitatory axons in the retina. Neuron. 2011;71:1014–1021. doi: 10.1016/j.neuron.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; This elegant study takes advantage of previous detailed work characterising distinct patterns of connectivity between bipolar cells and retinal ganglion cells in the developing retina and uses spatiotemporal genetic control of neurotransmitter release to show that even within the same dendritic arbor, different presynaptic inputs are differentially regulated by activity. Additionally, careful time-lapse imaging indicates that where synapses are reduced, this is due to a failure to generate new synapses at sites of existing axo-dendritic contact.
- 17•.Soto F., Ma X., Cecil J.L., Vo B.Q., Culican S.M., Kerschensteiner D. Spontaneous activity promotes synapse formation in a cell-type-dependent manner in the developing retina. J Neurosci. 2012;32:5426–5439. doi: 10.1523/JNEUROSCI.0194-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Unusually, this study investigates the effects of increasing activity, as opposed to inhibiting it, on synapse formation. It uses Crx−/− mutant mice, which display increased glutamate release from bipolar cells and hence increased spontaneous firing of RGCs. It demonstrates that the previously defined ‘activity-sensitive’ connections between B6 bipolar cells and G10 RGCs show corresponding responsiveness to raising activity levels, while B7/RB-G10 connections appear to be bidirectionally activity-insensitive.
- 18•.Dunn F.A., Della Santina L., Parker E.D., Wong R.O. Sensory experience shapes the development of the visual system's first synapse. Neuron. 2013;80:1159–1166. doi: 10.1016/j.neuron.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here the authors examine the effects of sensory deprivation (dark rearing) on another retinal synapse: that between photoreceptors and bipolar cells. They find that, as seen in bipolar-to-RGC contacts, activity regulates synaptic connections differentially, and indeed in a complex manner.
- 19•.Dunn F.A., Wong R.O. Diverse strategies engaged in establishing stereotypic wiring patterns among neurons sharing a common input at the visual system's first synapse. J Neurosci. 2012;32:10306–10317. doi: 10.1523/JNEUROSCI.1581-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Careful characterization of the dendritic arbors of different bipolar cell types in the retina reveals different, type-specific approaches to the formation of connections from photoreceptors and different dendritic shapes.
- 20.Chen J.L., Villa K.L., Cha J.W., So P.T., Kubota Y., Nedivi E. Clustered dynamics of inhibitory synapses and dendritic spines in the adult neocortex. Neuron. 2012;74:361–373. doi: 10.1016/j.neuron.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Versendaal D., Rajendran R., Saiepour M.H., Klooster J., Smit-Rigter L., Sommeijer J.P., De Zeeuw C.I., Hofer S.B., Heimel J.A., Levelt C.N. Elimination of inhibitory synapses is a major component of adult ocular dominance plasticity. Neuron. 2012;74:374–383. doi: 10.1016/j.neuron.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Hofer S.B., Mrsic-Flogel T.D., Bonhoeffer T., Hubener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohmann C., Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Hume R.I., Role L.W., Fischbach G.D. Acetylcholine release from growth cones detected with patches of acetylcholine receptor-rich membranes. Nature. 1983;305:632–634. doi: 10.1038/305632a0. [DOI] [PubMed] [Google Scholar]
- 25.Young S.H., Poo M.M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983;305:634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]
- 26.Taylor J., Docherty M., Gordon-Weeks P.R. GABAergic growth cones: release of endogenous gamma-aminobutyric acid precedes the expression of synaptic vesicle antigens. J Neurochem. 1990;54:1689–1699. doi: 10.1111/j.1471-4159.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 27.Zakharenko S., Chang S., O’Donoghue M., Popov S.V. Neurotransmitter secretion along growing nerve processes: comparison with synaptic vesicle exocytosis. J Cell Biol. 1999;144:507–518. doi: 10.1083/jcb.144.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matteoli M., Takei K., Perin M.S., Sudhof T.C., De Camilli P. Exo-endocytotic recycling of synaptic vesicles in developing processes of cultured hippocampal neurons. J Cell Biol. 1992;117:849–861. doi: 10.1083/jcb.117.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabo S.L., McAllister A.K. Mobility and cycling of synaptic protein-containing vesicles in axonal growth cone filopodia. Nat Neurosci. 2003;6:1264–1269. doi: 10.1038/nn1149. [DOI] [PubMed] [Google Scholar]
- 30•.Andreae L.C., Fredj N.B., Burrone J. Independent vesicle pools underlie different modes of release during neuronal development. J Neurosci. 2012;32:1867–1874. doi: 10.1523/JNEUROSCI.5181-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study builds on previous work examining the development of presynaptic vesicle release. By utilising various genetically encoded reporters of presynaptic function in hippocampal neurons, the authors carefully quantify the relative contributions of different modes of vesicle release during development. They find that early in development, high levels of spontaneous synaptic vesicle cycling from an independent vesicle pool dominate, giving way later on to evoked release. Further, the switch in modes of release is independent of axo-dendritic contact.
- 31.McKinney R.A., Capogna M., Durr R., Gahwiler B.H., Thompson S.M. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- 32.McAllister A.K., Katz L.C., Lo D.C. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 33.Mozhayeva M.G., Sara Y., Liu X., Kavalali E.T. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–665. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dailey M.E., Smith S.J. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziv N.E., Smith S.J. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 36.Lin S.Y., Constantine-Paton M. Suppression of sprouting: an early function of NMDA receptors in the absence of AMPA/kainate receptor activity. J Neurosci. 1998;18:3725–3737. doi: 10.1523/JNEUROSCI.18-10-03725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Washbourne P., Liu X.B., Jones E.G., McAllister A.K. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kilic G., Moran O., Cherubini E. N-methyl-d-aspartate receptor-mediated spontaneous activity in cerebellar granule cells in culture. Neurosci Lett. 1991;130:263–266. doi: 10.1016/0304-3940(91)90411-l. [DOI] [PubMed] [Google Scholar]
- 39.Rossi D.J., Slater N.T. The developmental onset of NMDA receptor-channel activity during neuronal migration. Neuropharmacology. 1993;32:1239–1248. doi: 10.1016/0028-3908(93)90018-x. [DOI] [PubMed] [Google Scholar]
- 40.LoTurco J.J., Mody I., Kriegstein A.R. Differential activation of glutamate receptors by spontaneously released transmitter in slices of neocortex. Neurosci Lett. 1990;114:265–271. doi: 10.1016/0304-3940(90)90574-s. [DOI] [PubMed] [Google Scholar]
- 41.Cornell-Bell A.H., Thomas P.G., Smith S.J. The excitatory neurotransmitter glutamate causes filopodia formation in cultured hippocampal astrocytes. Glia. 1990;3:322–334. doi: 10.1002/glia.440030503. [DOI] [PubMed] [Google Scholar]
- 42.Cruz-Martin A., Crespo M., Portera-Cailliau C. Glutamate induces the elongation of early dendritic protrusions via mGluRs in wild type mice, but not in fragile X mice. PLOS ONE. 2012;7:e32446. doi: 10.1371/journal.pone.0032446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Portera-Cailliau C., Pan D.T., Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong W.T., Wong R.O. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nat Neurosci. 2001;4:351–352. doi: 10.1038/85987. [DOI] [PubMed] [Google Scholar]
- 45.Cline H., Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: a review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaughn J.E. Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse. 1989;3:255–285. doi: 10.1002/syn.890030312. [DOI] [PubMed] [Google Scholar]
- 47.Sin W.C., Haas K., Ruthazer E.S., Cline H.T. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- 48.Kayser M.S., Nolt M.J., Dalva M.B. EphB receptors couple dendritic filopodia motility to synapse formation. Neuron. 2008;59:56–69. doi: 10.1016/j.neuron.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engert F., Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 50.Maletic-Savatic M., Malinow R., Svoboda K. Rapid dendritic morphogenesis in CA1 hippocampal dendrites induced by synaptic activity. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 51.Kerschensteiner D. Spontaneous network activity and synaptic development. Neuroscientist. 2013 doi: 10.1177/1073858413510044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohmann C., Kessels H. The developmental stages of synaptic plasticity. J Physiol. 2013 doi: 10.1113/jphysiol.2012.235119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Kwon H.B., Sabatini B.L. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work directly demonstrates the effect of localised repetitive pulses of glutamate, using either 2-photon glutamate uncaging or high-frequency electrical stimulation, on the induction of dendritic spines during development. This induction is dependent on NMDA receptors and new spines are rapidly functional.
- 54.Sykova E., Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–1280. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas C.G., Tian H., Diamond J.S. The relative roles of diffusion and uptake in clearing synaptically released glutamate change during early postnatal development. J Neurosci. 2011;31:4743–4750. doi: 10.1523/JNEUROSCI.5953-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Laurie D.J., Seeburg P.H. Ligand affinities at recombinant N-methyl-d-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 57.Cattani A.A., Bonfardin V.D., Represa A., Ben-Ari Y., Aniksztejn L. Generation of slow network oscillations in the developing rat hippocampus after blockade of glutamate uptake. J Neurophysiol. 2007;98:2324–2330. doi: 10.1152/jn.00378.2007. [DOI] [PubMed] [Google Scholar]
- 58.Demarque M., Villeneuve N., Manent J.B., Becq H., Represa A., Ben-Ari Y., Aniksztejn L. Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J Neurosci. 2004;24:3289–3290. doi: 10.1523/JNEUROSCI.5338-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milh M., Becq H., Villeneuve N., Ben-Ari Y., Aniksztejn L. Inhibition of glutamate transporters results in a “suppression-burst” pattern and partial seizures in the newborn rat. Epilepsia. 2007;48:169–174. doi: 10.1111/j.1528-1167.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- 60.Blankenship A.G., Ford K.J., Johnson J., Seal R.P., Edwards R.H., Copenhagen D.R., Feller M.B. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009;62:230–241. doi: 10.1016/j.neuron.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Firl A., Sack G.S., Newman Z.L., Tani H., Feller M.B. Extrasynaptic glutamate and inhibitory neurotransmission modulate ganglion cell participation during glutamatergic retinal waves. J Neurophysiol. 2013;109:1969–1978. doi: 10.1152/jn.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]; Here an optical sensor of glutamate was used to measure large rises in extrasynaptic glutamate associated with glutamatergic retinal waves.
- 62•.Ford K.J., Felix A.L., Feller M.B. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. J Neurosci. 2012;32:850–863. doi: 10.1523/JNEUROSCI.5309-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study an optical sensor of ACh was able to directly demonstrate cholinergic-wave associated extracellular ACh for the first time.
- 63.Demarque M., Represa A., Becq H., Khalilov I., Ben-Ari Y., Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1060. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- 64.Represa A., Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]