Abstract
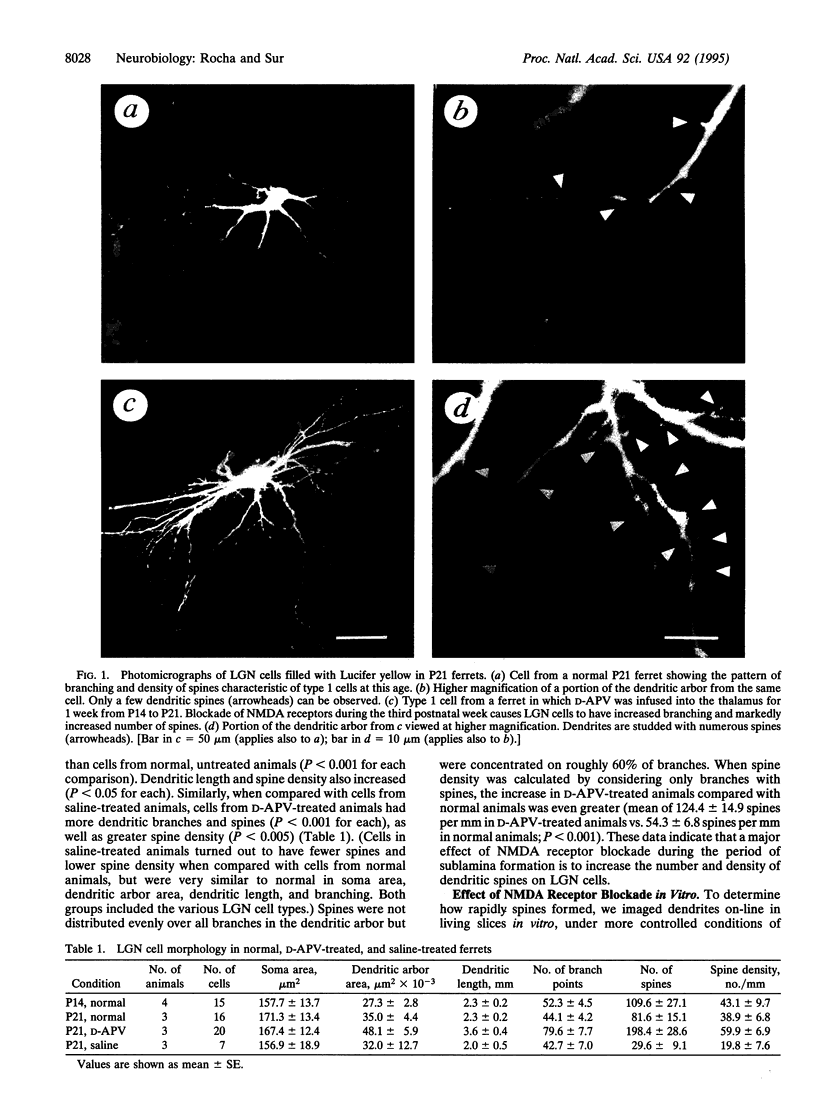
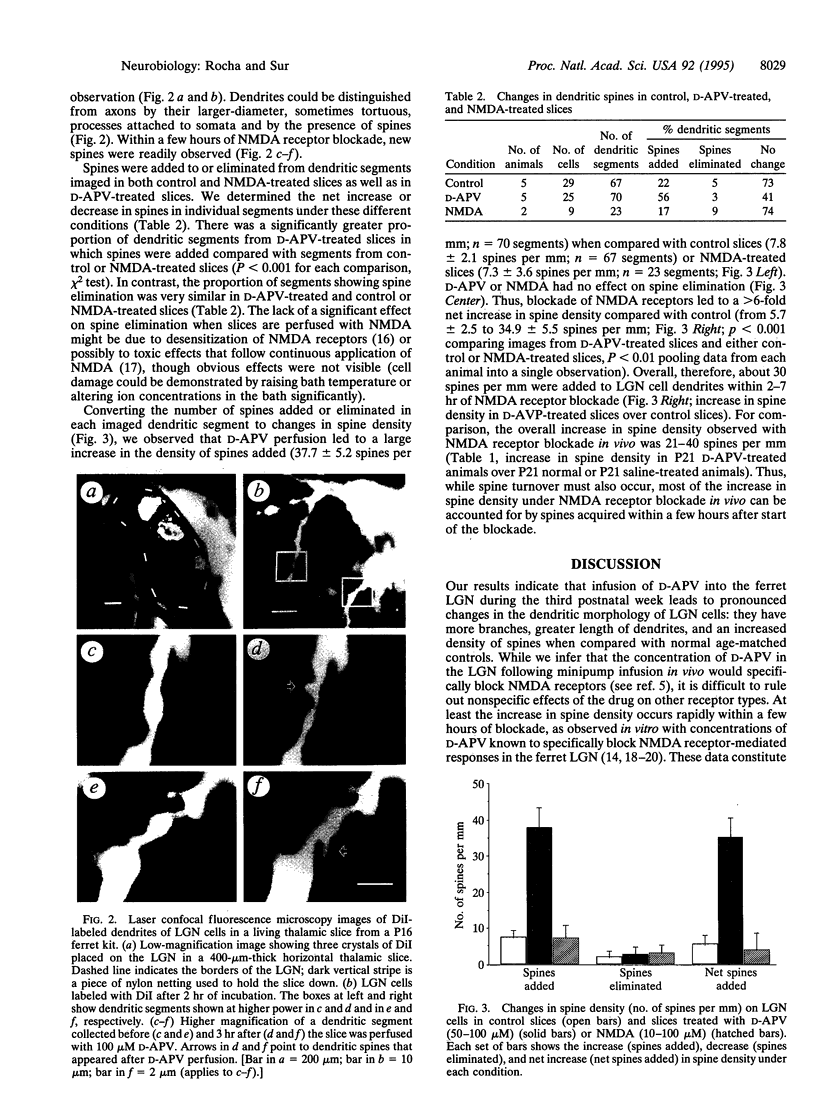
N-Methyl-D-aspartate (NMDA) receptors play an important role in the development of retinal axon arbors in the mammalian lateral geniculate nucleus (LGN). We investigated whether blockade of NMDA receptors in vivo or in vitro affects the dendritic development of LGN neurons during the period that retinogeniculate axons segregate into on-center and off-center sublaminae. Osmotic minipumps containing either the NMDA receptor antagonist D-2-amino-5-phosphonovaleric acid (D-APV) or saline were implanted in ferret kits at postnatal day 14. After 1 week, LGN neurons were intracellularly injected with Lucifer yellow. Infusion of D-APV in vivo led to an increase in the number of branch points and in the density of dendritic spines compared with age-matched normal or saline-treated animals. To examine the time course of spine formation, crystals of 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate were placed in the LGN in brain slices from 14- to 18-day-old ferrets. Labeled LGN cell dendrites were imaged on-line in living slices by confocal microscopy, with slices maintained either in normal perfusion medium or with the addition of D-APV or NMDA to the medium. Addition of D-APV in vitro at doses specific for blocking NMDA receptors led to a > 6-fold net increase in spine density compared with control or NMDA-treated slices. Spines appeared within a few hours of NMDA receptor blockade, indicating a rapid local response by LGN cells in the absence of NMDA receptor activation. Thus, activity-dependent structural changes in postsynaptic cells act together with changes in presynaptic arbors to shape projection patterns and specific retinogeniculate connections.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodnarenko S. R., Chalupa L. M. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993 Jul 8;364(6433):144–146. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Cotman C. W. NMDA receptor regulation of neuronal morphology in cultured hippocampal neurons. Neurosci Lett. 1989 May 8;99(3):268–273. doi: 10.1016/0304-3940(89)90458-8. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol. 1990;52:577–590. doi: 10.1146/annurev.ph.52.030190.003045. [DOI] [PubMed] [Google Scholar]
- Chicurel M. E., Terrian D. M., Potter H. mRNA at the synapse: analysis of a synaptosomal preparation enriched in hippocampal dendritic spines. J Neurosci. 1993 Sep;13(9):4054–4063. doi: 10.1523/JNEUROSCI.13-09-04054.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhmakov I. V., Kiskin N. I., Krishtal O. A. Two types of steady-state desensitization of N-methyl-D-aspartate receptor in isolated hippocampal neurones of rat. J Physiol. 1992 Mar;448:453–472. doi: 10.1113/jphysiol.1992.sp019051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M., Cline H. T., Debski E. Patterned activity, synaptic convergence, and the NMDA receptor in developing visual pathways. Annu Rev Neurosci. 1990;13:129–154. doi: 10.1146/annurev.ne.13.030190.001021. [DOI] [PubMed] [Google Scholar]
- Dalva M. B., Ghosh A., Shatz C. J. Independent control of dendritic and axonal form in the developing lateral geniculate nucleus. J Neurosci. 1994 Jun;14(6):3588–3602. doi: 10.1523/JNEUROSCI.14-06-03588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond N. L., Levy W. B. Changes in the numerical density of synaptic contacts with long-term potentiation in the hippocampal dentate gyrus. J Comp Neurol. 1986 Nov 22;253(4):466–475. doi: 10.1002/cne.902530404. [DOI] [PubMed] [Google Scholar]
- Esguerra M., Kwon Y. H., Sur M. Retinogeniculate EPSPs recorded intracellularly in the ferret lateral geniculate nucleus in vitro: role of NMDA receptors. Vis Neurosci. 1992 Jun;8(6):545–555. doi: 10.1017/s0952523800005642. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Stanford L. R., Sherman S. M. Effects of monocular deprivation on the structure-function relationship of individual neurons in the cat's lateral geniculate nucleus. J Neurosci. 1982 Mar;2(3):321–330. doi: 10.1523/JNEUROSCI.02-03-00321.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli L., Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988 Oct 7;242(4875):90–91. doi: 10.1126/science.3175637. [DOI] [PubMed] [Google Scholar]
- Guthrie P. B., Segal M., Kater S. B. Independent regulation of calcium revealed by imaging dendritic spines. Nature. 1991 Nov 7;354(6348):76–80. doi: 10.1038/354076a0. [DOI] [PubMed] [Google Scholar]
- Hahm J. O., Langdon R. B., Sur M. Disruption of retinogeniculate afferent segregation by antagonists to NMDA receptors. Nature. 1991 Jun 13;351(6327):568–570. doi: 10.1038/351568a0. [DOI] [PubMed] [Google Scholar]
- Harris K. M., Stevens J. K. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989 Aug;9(8):2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey T. L., Guillery R. W. An autoradiographic study of retinogeniculate pathways in the cat and the fox. J Comp Neurol. 1974 Jul;156(2):239–253. doi: 10.1002/cne.901560207. [DOI] [PubMed] [Google Scholar]
- Koch C., Zador A. The function of dendritic spines: devices subserving biochemical rather than electrical compartmentalization. J Neurosci. 1993 Feb;13(2):413–422. doi: 10.1523/JNEUROSCI.13-02-00413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau K. C., So K. F., Tay D. APV prevents the elimination of transient dendritic spines on a population of retinal ganglion cells. Brain Res. 1992 Nov 6;595(1):171–174. doi: 10.1016/0006-8993(92)91471-p. [DOI] [PubMed] [Google Scholar]
- Linden D. C., Guillery R. W., Cucchiaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol. 1981 Dec 1;203(2):189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- Mattson M. P. Neurotransmitters in the regulation of neuronal cytoarchitecture. Brain Res. 1988 Apr-Jun;472(2):179–212. doi: 10.1016/0165-0173(88)90020-3. [DOI] [PubMed] [Google Scholar]
- Meister M., Wong R. O., Baylor D. A., Shatz C. J. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991 May 17;252(5008):939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Mooney R., Madison D. V., Shatz C. J. Enhancement of transmission at the developing retinogeniculate synapse. Neuron. 1993 May;10(5):815–825. doi: 10.1016/0896-6273(93)90198-z. [DOI] [PubMed] [Google Scholar]
- Müller W., Connor J. A. Dendritic spines as individual neuronal compartments for synaptic Ca2+ responses. Nature. 1991 Nov 7;354(6348):73–76. doi: 10.1038/354073a0. [DOI] [PubMed] [Google Scholar]
- Ramoa A. S., Campbell G., Shatz C. J. Dendritic growth and remodeling of cat retinal ganglion cells during fetal and postnatal development. J Neurosci. 1988 Nov;8(11):4239–4261. doi: 10.1523/JNEUROSCI.08-11-04239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa A. S., McCormick D. A. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci. 1994 Apr;14(4):2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoa A. S., McCormick D. A. Enhanced activation of NMDA receptor responses at the immature retinogeniculate synapse. J Neurosci. 1994 Apr;14(4):2098–2105. doi: 10.1523/JNEUROSCI.14-04-02098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid N. A., Cambray-Deakin M. A. N-methyl-D-aspartate effects on the growth, morphology and cytoskeleton of individual neurons in vitro. Brain Res Dev Brain Res. 1992 Jun 19;67(2):301–308. doi: 10.1016/0165-3806(92)90231-k. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. Impulse activity and the patterning of connections during CNS development. Neuron. 1990 Dec;5(6):745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988 Oct 7;242(4875):87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. The prenatal development of the cat's retinogeniculate pathway. J Neurosci. 1983 Mar;3(3):482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D. K., Prusky G. T., O'Leary D. D., Constantine-Paton M. N-methyl-D-aspartate receptor antagonists disrupt the formation of a mammalian neural map. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10593–10597. doi: 10.1073/pnas.89.22.10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J., Stryker M. P. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988 Dec 1;336(6198):468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Stryker M. P., Zahs K. R. On and off sublaminae in the lateral geniculate nucleus of the ferret. J Neurosci. 1983 Oct;3(10):1943–1951. doi: 10.1523/JNEUROSCI.03-10-01943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton J. K., Brunso-Bechtold J. K. A Golgi study of dendritic development in the dorsal lateral geniculate nucleus of normal ferrets. J Comp Neurol. 1991 Jul 1;309(1):71–85. doi: 10.1002/cne.903090106. [DOI] [PubMed] [Google Scholar]
- Sutton J. K., Brunso-Bechtold J. K. Dendritic development in the dorsal lateral geniculate nucleus of ferrets in the postnatal absence of retinal input: a Golgi study. J Neurobiol. 1993 Mar;24(3):317–334. doi: 10.1002/neu.480240305. [DOI] [PubMed] [Google Scholar]
- Torre E. R., Steward O. Demonstration of local protein synthesis within dendrites using a new cell culture system that permits the isolation of living axons and dendrites from their cell bodies. J Neurosci. 1992 Mar;12(3):762–772. doi: 10.1523/JNEUROSCI.12-03-00762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White C. A., Sur M. Membrane and synaptic properties of developing lateral geniculate nucleus neurons during retinogeniculate axon segregation. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9850–9854. doi: 10.1073/pnas.89.20.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. R., Friedlander M. J., Sherman S. M. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proc R Soc Lond B Biol Sci. 1984 Jun 22;221(1225):411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]