Abstract
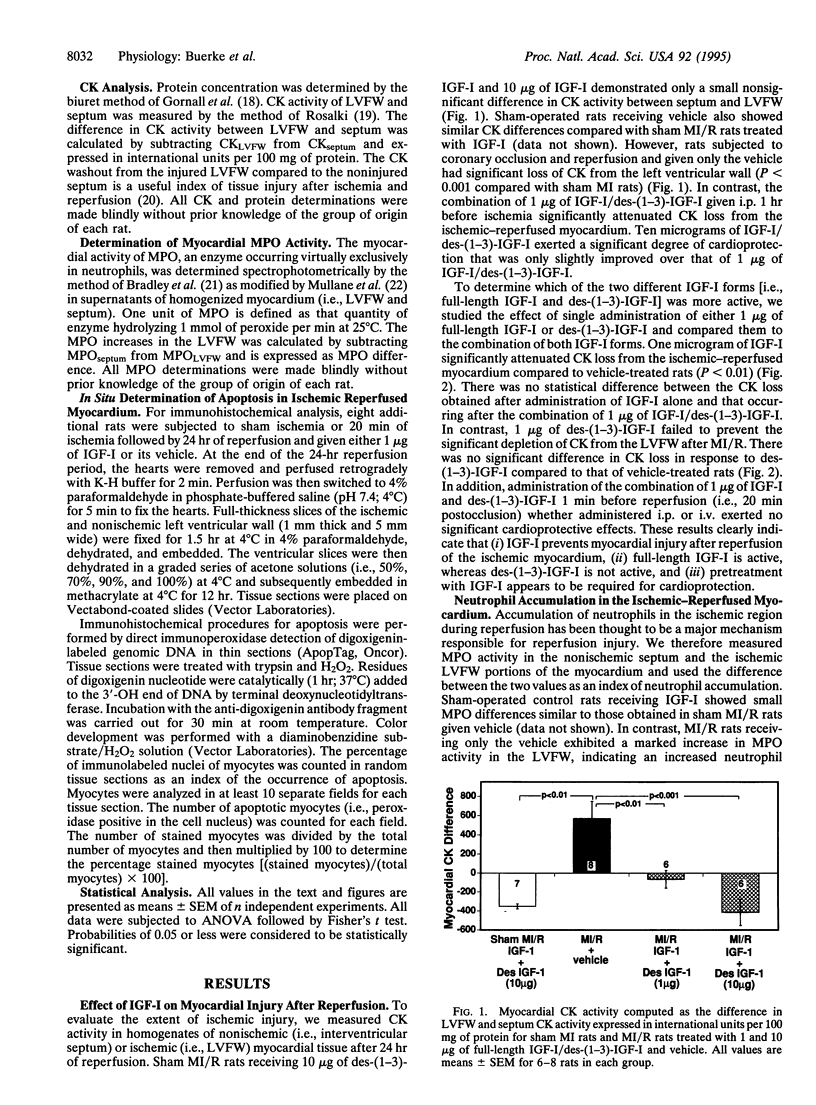
In the present study, the cardioprotective effects of insulin-like growth factor I (IGF-I) were examined in a murine model of myocardial ischemia reperfusion (i.e., 20 min + 24 hr). IGF-I (1-10 micrograms per rat) administered 1 hr prior to ischemia significantly attenuated myocardial injury (i.e., creatine kinase loss) compared to vehicle (P < 0.001). In addition, cardiac myeloperoxidase activity, an index of neutrophil accumulation, in the ischemic area was significantly attenuated by IGF-I (P < 0.001). This protective effect of IGF-I was not observed with des-(1-3)-IGF-I. Immunohistochemical analysis of ischemic-reperfused myocardial tissue demonstrated markedly increased DNA fragmentation due to programmed cell death (i.e., apoptosis) compared to nonischemic myocardium. Furthermore, IGF-I significantly attenuated the incidence of myocyte apoptosis after myocardial ischemia and reperfusion. Therefore, IGF-I appears to be an effective agent for preserving ischemic myocardium from reperfusion injury and protects via two different mechanisms--inhibition of polymorphonuclear leukocyte-induced cardiac necrosis and inhibition of reperfusion-induced apoptosis of cardiac myocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Hart I. K., Coles H. S., Burne J. F., Voyvodic J. T., Richardson W. D., Raff M. C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992 Jul 10;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Buerke M., Weyrich A. S., Lefer A. M. Isolated cardiac myocytes are sensitized by hypoxia-reoxygenation to neutrophil-released mediators. Am J Physiol. 1994 Jan;266(1 Pt 2):H128–H136. doi: 10.1152/ajpheart.1994.266.1.H128. [DOI] [PubMed] [Google Scholar]
- Buerke M., Weyrich A. S., Murohara T., Queen C., Klingbeil C. K., Co M. S., Lefer A. M. Humanized monoclonal antibody DREG-200 directed against I-selectin protects in feline myocardial reperfusion injury. J Pharmacol Exp Ther. 1994 Oct;271(1):134–142. [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Contreras P. C., Steffler C., Vaught J. L. rhIGF-I enhances functional recovery from sciatic crush. Time-course and dose-response study. Ann N Y Acad Sci. 1993 Aug 27;692:314–316. doi: 10.1111/j.1749-6632.1993.tb26245.x. [DOI] [PubMed] [Google Scholar]
- Davenpeck K. L., Gauthier T. W., Lefer A. M. Inhibition of endothelial-derived nitric oxide promotes P-selectin expression and actions in the rat microcirculation. Gastroenterology. 1994 Oct;107(4):1050–1058. doi: 10.1016/0016-5085(94)90229-1. [DOI] [PubMed] [Google Scholar]
- Farb A., Kolodgie F. D., Jenkins M., Virmani R. Myocardial infarct extension during reperfusion after coronary artery occlusion: pathologic evidence. J Am Coll Cardiol. 1993 Apr;21(5):1245–1253. doi: 10.1016/0735-1097(93)90253-w. [DOI] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Gauthier T. W., Davenpeck K. L., Lefer A. M. Nitric oxide attenuates leukocyte-endothelial interaction via P-selectin in splanchnic ischemia-reperfusion. Am J Physiol. 1994 Oct;267(4 Pt 1):G562–G568. doi: 10.1152/ajpgi.1994.267.4.G562. [DOI] [PubMed] [Google Scholar]
- Gluckman P., Klempt N., Guan J., Mallard C., Sirimanne E., Dragunow M., Klempt M., Singh K., Williams C., Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992 Jan 31;182(2):593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- Gottlieb R. A., Burleson K. O., Kloner R. A., Babior B. M., Engler R. L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994 Oct;94(4):1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington E. A., Bennett M. R., Fanidi A., Evan G. I. c-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994 Jul 15;13(14):3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylor J., Singh I., el Nahas A. M. Nitric oxide synthesis inhibitor prevents vasodilation by insulin-like growth factor I. Kidney Int. 1991 Feb;39(2):333–335. doi: 10.1038/ki.1991.42. [DOI] [PubMed] [Google Scholar]
- Kjekshus J. K., Sobel B. E. Depressed myocardial creatine phosphokinase activity following experimental myocardial infarction in rabbit. Circ Res. 1970 Sep;27(3):403–414. doi: 10.1161/01.res.27.3.403. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukreja R. C., Hess M. L. The oxygen free radical system: from equations through membrane-protein interactions to cardiovascular injury and protection. Cardiovasc Res. 1992 Jul;26(7):641–655. doi: 10.1093/cvr/26.7.641. [DOI] [PubMed] [Google Scholar]
- LeRoith D., Clemmons D., Nissley P., Rechler M. M. NIH conference. Insulin-like growth factors in health and disease. Ann Intern Med. 1992 May 15;116(10):854–862. doi: 10.7326/0003-4819-116-10-854. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Lefer D. J. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu Rev Pharmacol Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Ma X. L. Cytokines and growth factors in endothelial dysfunction. Crit Care Med. 1993 Feb;21(2 Suppl):S9–14. doi: 10.1097/00003246-199302001-00003. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P., Aoki N., Palladino M. A., Jr Mediation of cardioprotection by transforming growth factor-beta. Science. 1990 Jul 6;249(4964):61–64. doi: 10.1126/science.2164258. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Weyrich A. S., Buerke M. Role of selectins, a new family of adhesion molecules, in ischaemia-reperfusion injury. Cardiovasc Res. 1994 Mar;28(3):289–294. doi: 10.1093/cvr/28.3.289. [DOI] [PubMed] [Google Scholar]
- Lefer D. J., Nakanishi K., Johnston W. E., Vinten-Johansen J. Antineutrophil and myocardial protecting actions of a novel nitric oxide donor after acute myocardial ischemia and reperfusion of dogs. Circulation. 1993 Nov;88(5 Pt 1):2337–2350. doi: 10.1161/01.cir.88.5.2337. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Weyrich A. S., Lefer D. J., Lefer A. M. Diminished basal nitric oxide release after myocardial ischemia and reperfusion promotes neutrophil adherence to coronary endothelium. Circ Res. 1993 Feb;72(2):403–412. doi: 10.1161/01.res.72.2.403. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Kraemer R., Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985 Nov;14(3):157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Murohara T., Buerke M., Lefer A. M. Polymorphonuclear leukocyte-induced vasocontraction and endothelial dysfunction. Role of selectins. Arterioscler Thromb. 1994 Sep;14(9):1509–1519. doi: 10.1161/01.atv.14.9.1509. [DOI] [PubMed] [Google Scholar]
- Noguchi S., Kashihara Y., Ikegami Y., Morimoto K., Miyamoto M., Nakao K. Insulin-like growth factor-I ameliorates transient ischemia-induced acute renal failure in rats. J Pharmacol Exp Ther. 1993 Nov;267(2):919–926. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Rubanyi G. M., Ho E. H., Cantor E. H., Lumma W. C., Botelho L. H. Cytoprotective function of nitric oxide: inactivation of superoxide radicals produced by human leukocytes. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1392–1397. doi: 10.1016/0006-291x(91)92093-y. [DOI] [PubMed] [Google Scholar]
- Siegfried M. R., Carey C., Ma X. L., Lefer A. M. Beneficial effects of SPM-5185, a cysteine-containing NO donor in myocardial ischemia-reperfusion. Am J Physiol. 1992 Sep;263(3 Pt 2):H771–H777. doi: 10.1152/ajpheart.1992.263.3.H771. [DOI] [PubMed] [Google Scholar]
- Tsao P. S., Aoki N., Lefer D. J., Johnson G., 3rd, Lefer A. M. Time course of endothelial dysfunction and myocardial injury during myocardial ischemia and reperfusion in the cat. Circulation. 1990 Oct;82(4):1402–1412. doi: 10.1161/01.cir.82.4.1402. [DOI] [PubMed] [Google Scholar]
- Tsukahara H., Gordienko D. V., Tonshoff B., Gelato M. C., Goligorsky M. S. Direct demonstration of insulin-like growth factor-I-induced nitric oxide production by endothelial cells. Kidney Int. 1994 Feb;45(2):598–604. doi: 10.1038/ki.1994.78. [DOI] [PubMed] [Google Scholar]



