Abstract
Multiple myeloma is a neoplastic disorder of plasma cells characterized by clonal proliferation within the bone marrow. One of the major clinical features of multiple myeloma is the destructive osteolytic bone disease that occurs in the majority of patients. Myeloma bone disease is associated with increased osteoclast activity and suppression of osteoblastogenesis. Bisphosphonates have been the mainstay of treatment for many years; however, their use is limited by their inability to repair existing bone loss. Therefore, research into novel approaches for the treatment of myeloma bone disease is of the utmost importance. This review will discuss the current advances in our understanding of osteoclast stimulation and osteoblast suppression mechanisms in myeloma bone disease and the treatments that are under development to target this destructive and debilitating feature of myeloma.
Myeloma bone disease
Multiple myeloma is a neoplastic disorder of plasma cells in the bone marrow. It is characterized by clonal proliferation within the bone marrow, osteolytic bone disease and secretion of a monoclonal protein in the blood and/or urine of the patient. The accumulation of this protein is often the cause of organ dysfunction such as renal failure in patients with multiple myeloma (Kuehl and Bergsagel, 2002). Myeloma is the second most common of all haematological cancers (10–15%). It has a global incidence of approximately 120 000 cases per year and accounts for around 1% of all cancers (Ludwig et al., 2013). Survival rates have improved in recent years and although myeloma remains incurable, patients are now predicted to have a median survival of approximately 5 years (Bergsagel et al., 2013). The hallmark of myeloma is the osteolytic bone disease that is present in 70–80% of patients (Kyle et al., 2003). It is characterized by the presence of osteolytic lesions accompanied by the suppression of osteoblast differentiation and function (Christoulas et al., 2009). Approximately 20% of patients with myeloma will present with a pathological fracture upon diagnosis, and almost 60% of patients will develop a pathological fracture over the course of their disease (Berenson et al., 1996; Melton et al., 2005). The most common site of fracture is in the spine (55–70% of patients). Other common sites of fracture include the femur, pelvis, ribs, and humerus (Lecouvet et al., 1997).
Factors produced by tumour cells stimulate osteoclasts to resorb bone and inhibit osteoblastic activity. In turn, growth factors released by the increased bone resorption also promote the growth of myeloma cells, creating a vicious cycle of tumour expansion and bone destruction. The biological pathway of the receptor activator of NF-κB (RANK; also known as TNFRSF11A), its ligand (RANKL; also known as TNFSF11) and the soluble decoy receptor osteoprotegerin (OPG; also known as TNFRSF11B), is of major importance for the increased osteoclast activity observed in multiple myeloma (Terpos et al., 2003). The relationship between the Wnt inhibitor Dickkopf 1 (DKK1) expression and osteoblast suppression has also emerged as a critical route to long-term osteoblast suppression in myeloma (Tian et al., 2003).
Bisphosphonates are currently the standard approach for the management of the osteolytic bone disease associated with multiple myeloma (Kyle et al., 2007). Bisphosphonates are pyrophosphate analogues that have high bone affinity and directly inhibit osteoclastic activity (Rogers et al., 2011)., Furthermore, the potential exists for bisphosphonate treatment to reduce tumour burden, either via direct anti-tumour effects or indirect effects on the host microenvironment (Morgan et al., 2011; 2012; Coleman et al., 2012; Clezardin, 2013). Although bisphosphonates have been extremely effective for treating myeloma bone disease, they only reduce the severity of lytic lesions and do not repair existing bone loss (Levy and Roodman, 2009). The demand for additional novel therapeutic targets that prevent and/or repair bone destruction is increasing and would greatly improve treatment for myeloma patients. As bisphosphonates have been extensively reviewed elsewhere, the focus of this review will be novel and emerging targets that may represent the future for treatment of myeloma bone disease.
The RANK/RANKL signalling pathway
The RANK/RANKL/OPG signalling pathway has become known as one of the most important regulatory systems in maintaining the bone remodelling balance (Simonet et al., 1997; Lacey et al., 1998; Kong et al., 1999). RANK is a transmembrane signalling receptor and is part of the TNF receptor family (see Alexander et al., 2013a). It is expressed on the surface of osteoclast precursors. On binding to RANK, RANKL, expressed by bone marrow stromal cells and osteoblasts, induces a signalling cascade that induces osteoclastogenesis and activation of mature osteoclasts. OPG is a soluble member of the TNF superfamily that is secreted by osteoblasts and bone marrow stromal cells (BMSCs). It acts as a decoy receptor for RANKL, blocking the induction of osteoclasts. The ratio of RANKL and OPG has a major impact on the balance of bone resorption and deposition (Vega et al., 2007; Boyce and Xing, 2008) and this signalling pathway is frequently deregulated in myeloma bone disease (Roodman and Dougall, 2008). In the myeloma bone marrow microenvironment, the interaction between BMSCs and myeloma cells results in increased RANKL expression and decreased OPG production, favouring bone resorption (Giuliani et al., 2001; Pearse et al., 2001). Furthermore, circulating levels of OPG and RANKL have been shown to correlate with increased lytic lesions and poor prognosis (Seidel et al., 2001; Terpos et al., 2003). Initial murine studies demonstrated the striking potential for targeting this system in myeloma bone disease (Croucher et al., 2001). A human antibody targeting RANKL has been developed with high affinity and specificity for human RANKL. Upon binding to RANKL, denosumab inhibits RANKL from activating RANK on osteoclasts. Prevention of RANKL–RANK interaction inhibits osteoclast formation, function and survival, which decreases bone resorption and cancer-induced bone destruction (Narayanan, 2013). Denosumab has been successfully examined in phase II and phase III trials in patients with cancer-induced bone disease and those at risk of developing bone metastases (Coleman et al., 2012). These studies found that denosumab increased bone mineral density (BMD) and decreased markers of bone resorption in several different cancers (Lipton et al., 2007; 2008; Fizazi et al., 2009). Several phase III trials comparing denosumab with zoledronic acid reported that denosumab was equal, if not superior, to zoledronic acid in preventing or delaying skeletal-related events in bone metastatic cancer or myeloma (Stopeck et al., 2010; Henry et al., 2011). These data indicate the potential for targeting RANKL for the treatment of myeloma bone disease.
Dickkopf-1
The Wnt signalling pathway plays a major role in myeloma and the associated bone disease. There has been much focus on targeting some of the key molecules in this pathway to treat myeloma bone disease. Dickkopf-1 (DKK1) is one of several inhibitors of the Wnt signalling pathway and acts by binding to LRP5/6, preventing Wnt signalling, and therefore, the translocation of β-catenin to the nucleus (Bafico et al., 2001; Mao et al., 2001). DKK1 has been shown to inhibit osteoblastogenesis in myeloma (Tian et al., 2003; Gunn et al., 2006). Inhibition of Wnt signalling is known to induce bone destruction by increasing osteoclast maturation and decreasing osteoblast function and differentiation (Tian et al., 2003; Hideshima et al., 2007). Myeloma patient sample analysis has also revealed that high serum and bone marrow DKK1 correlates with osteolytic lesions, and that patient serum can block bone morphogenetic protein (BMP)-2-induced osteoblast differentiation in vitro in a DKK1-dependent manner (Tian et al., 2003). It has also been found that patients that are responsive to anti-myeloma treatment have reduced levels of DKK1 in their serum (Heider et al., 2009). A study using a DKK1-DNA vaccine in the murine MOPC-21 myeloma model showed that active vaccination using the DKK1 vaccine protected mice from developing myeloma and also reduced tumour burden in mice with established myeloma (Qian et al., 2012).
Fulciniti and colleagues evaluated a DKK1 neutralizing antibody (BHQ880) in myeloma. In vitro BHQ880 increased osteoblast differentiation and in co-culture with BMSCs, myeloma cell growth was also inhibited, suggesting an effect on the bone marrow micro-environment. An in vivo mouse model using BHQ880 led to a significant increase in osteoblast number, serum human osteocalcin level and trabecular bone and also inhibited myeloma cell growth (Fulciniti et al., 2009). A study by Pozzi et al. also confirmed these results using a different neutralizing DKK1 antibody. However, they found that when using myeloma patient samples to study osteoclastogenesis, there was heterogeneity in the response to the DKK1 antibody (Pozzi et al., 2013). A recent report regarding a phase II clinical trial of BHQ880 treatment of patients with smouldering myeloma likely to progress to full myeloma showed bone anabolic activity with treatment of BHQ880. The scale of the increase in bone strength observed was similar to that observed with other approved bone anabolic agents (Munshi et al., 2012). Despite the conflicting roles of Wnt signalling in myeloma tumour growth (Qiang et al., 2005; Edwards et al., 2008), it is clear that blockade of DKK1 is highly effective in treating myeloma bone disease in vivo.
Sclerostin
Sclerostin, a well-characterized Wnt antagonist, is implicated in several bone-related pathological diseases such as sclerosteosis and van Buchem's disease (Balemans et al., 2001; 2002) and now sclerostin in myeloma (Brunetti et al., 2011; Colucci et al., 2011; Terpos et al., 2012a). Sclerostin has been shown to be secreted directly from myeloma cells and is elevated in myeloma patients with advanced bone disease (Brunetti et al., 2011; Gkotzamanidou et al., 2012; Terpos et al., 2012a). Sclerostin binds to LRP5/6 to inhibit the Wnt signalling pathway during bone formation, leading to an increase in osteoclastogenesis via RANKL production and OPG inhibition (van Bezooijen et al., 2005). Sclerostin also decreases the lifespan of osteoblasts by inducing apoptosis (Sutherland et al., 2004). Sclerostin is also involved in BMP signalling. It competes with type I and type II BMP receptors for binding to BMPs. This results in a decrease of BMP signalling and subsequently, down-regulation of BMP-mediated mineralization in osteoblasts (Winkler et al., 2003). Expression of this Wnt inhibitor suggests that myeloma cells can drive the inhibition of osteoblast differentiation and also promote osteoclastogenesis, which leads to progressive bone disease.
Sclerostin monoclonal antibodies have shown promising results in promoting bone formation after pathological and age-related bone loss in rodents (Li et al., 2009; 2010; Agholme et al., 2010; Ominsky et al., 2011; Chen et al., 2013) and in humans (McColm et al., 2013). Inhibiting sclerostin using such an approach may be a simple, non-invasive route to treat bone disease in myeloma patients. Using myeloma cell lines and patient samples, Colucci et al. showed that the addition of an anti-sclerostin mAb prevented the formation of the LRP5/6-sclerostin complex, and partially restored β-catenin translocation into osteoblast nuclei (Colucci et al., 2011).
A human clinical trial in post-menopausal women was conducted using AMG 785 (romosozumab), a humanized mAb that inhibits the activity of sclerostin. AMG 785 resulted in a significant biochemical anabolic effect, which was also translated into statistically significant increases in BMD (Padhi et al., 2011). A recent study also involving post-menopausal women with low bone mass reported that AMG 785 increased BMD and bone formation while decreasing markers of bone resorption (McClung et al., 2014). To date, several studies reported on the effects of AMG 785 on bone disorders with low bone mass. However, studies involving patients with malignant disease have yet to be performed (Gkotzamanidou et al., 2012).
Activin A
Activin A is a member of the TGFβ superfamily (Brosh et al., 1995; Sternberg et al., 1995). Activin binds to the type II receptor (ActRIIA or ActRIIB), which recruits and subsequently phosphorylates the type I receptor (ActRI is also known as the activin receptor-like kinase 4 (ALK4) receptor (Deli et al., 2008). The phosphorylated type I receptor induces phosphorylation of Smads, which subsequently form a complex and are translocated to the nucleus to initiate transcription (Butler et al., 2005). There is evidence that activin A is deregulated in primary bone tumours (Gobbi et al., 2002; Masi et al., 2002) and in bone metastatic tumours (Dowling and Risbridger, 2000; Risbridger et al., 2001) and in myeloma (Vallet et al., 2010). Activin A has been shown to act as a stimulator of osteoclastogenesis in tumour bone disease (Hashimoto et al., 1992; Sugatani et al., 2003; Futakuchi et al., 2009). Furthermore, Galson et al. recently reviewed that IL-3, a potent osteoclast stimulator (Lee et al., 2004), can induce the secretion of activin A from marrow macrophages in the myeloma microenvironment, providing a mechanism for the up-regulation of activin A in myeloma (Galson et al., 2012). In myeloma, it has also been shown that activin A can inhibit osteoblast differentiation. Activin A levels are increased in the bone marrow plasma of myeloma patients with osteolytic disease (Terpos et al., 2012b) and the interaction of myeloma and patient bone marrow stromal cells enhances activin A secretion. Adhesion mediated JNK activation was found to induce the secretion of activin A. This pathway was targeted by two groups using RAP-011, a soluble activin A receptor. In both studies, RAP-011 prevented the development of osteolytic lesions and inhibited tumour growth in myeloma-bearing mice (Chantry et al., 2010; Vallet et al., 2010).
The human version of RAP-011, ACE-011 (also known as sotatercept), is a receptor fusion protein consisting of the extracellular domain of human ActR11A. In myeloma patients a phase II study with ACE-011 reported that patients had reduced bone pain and cancer-induced anaemia (Adulkadyrov et al., 2009). ACE-011 also effectively inhibited markers of bone resorption and stimulated bone formation parameters in post-menopausal women in a double-blind placebo-controlled study (Ruckle et al., 2009). These data indicate that activin A is a promising new approach for reducing the debilitating bone disease that accompanies myeloma.
EphrinB2/EphB4 signalling
The Eph receptors are the largest subgroup of the receptor TK family (Hirai et al., 1987). Bidirectional ephrin–Eph receptor signalling links the negative feedback loop in osteoclast differentiation to positive regulation of osteoblast differentiation, thereby maintaining bone homeostasis (Mundy and Elefteriou, 2006). Reverse signalling between the ligand ephrin B2 (EFNB2) in osteoclasts and its receptor EphB4 in BMSCs and osteoblasts has been found to negatively control osteoclast formation, whereas forward signalling was shown to promote osteoblast differentiation (Zhao et al., 2006). In addition, osteoblast-derived ephrin B2 can promote osteoblastic differentiation, suggesting a paracrine role for ephrin B2/EphB4 signalling in osteoblasts (Allan et al., 2008; Martin et al., 2010; Takyar et al., 2013). Myeloma cells have been shown to down-regulate EphB4 expression in osteoblasts (Bates et al., 2007). As EphB4 forward signalling enhances bone formation, reduction in EphB4 may account for impaired bone formation in myeloma bone disease. A study by Pennisi et al. (2009b) reported decreased ephrinB2 and EphB4 in mesenchymal stem cells (MSCs) from myeloma patients. The peptide ephrinB2-Fc activated EphB4 in MSCs and EphB4-Fc activated ephrinB4 in osteoclasts but not in MSCs. Myeloma bearing mice treated with both peptides demonstrated increased bone volume/tissue volume (BV/TV) and trabecular thickness in bones treated with ephrinB2-Fc or EphB4-Fc. EphB4-Fc and ephrinB2-Fc increased the numbers of osteoblasts, whereas only EphB4-Fc reduced osteoclast numbers. Exploiting this signalling pathway presents a potential mechanism for reducing osteolytic lesions and increasing bone formation in myeloma patients but may be complicated by the multiple receptor : ligand interactions and bidirectional signalling in this family.
Adiponectin
Adiponectin is an adipocyte-derived hormone with implications for the regulation of bone homeostasis. It is almost exclusively secreted by adipocytes, principally those in visceral adipose tissue, although peripheral fat and bone marrow adipocytes also contribute (DiMascio et al., 2007; Swarbrick and Havel, 2008). Adiponectin is also secreted from osteoblasts and BMSCs (Berner et al., 2004). The receptors for adiponectin, Adipo receptor 1 and Adipo receptor 2, have been identified on both osteoblasts and osteoclasts (Berner et al., 2004; Shinoda et al., 2006), suggesting a potential direct influence of this hormone on bone. Adiponectin has been shown to increase osteoblast proliferation and differentiation while inhibiting osteoclastogenesis in vitro (Oshima et al., 2005; Yamaguchi et al., 2007). Studies investigating adiponectin and its effect on bone have been contradictory in cell and murine studies (Oshima et al., 2005; Shinoda et al., 2006; Yamaguchi et al., 2007). In contrast to these contradictory findings in cell culture and animal studies, clinical studies demonstrate that circulating adiponectin concentrations are related to BMD (Lenchik et al., 2003; Jurimae et al., 2005; Richards et al., 2007). These data suggest that there is a strong connection between adiponectin and normal bone homeostasis in humans.
To date, there is little data on the impact that adiponectin could have on the bone disease induced by the presence of myeloma. Fowler et al. demonstrated that a reduction in host-derived adiponectin promoted myeloma development both in a murine model of myeloma and in patients with the non-malignant precursor monoclonal gammopathy of undetermined significance. Adiponectin was also found to be tumour suppressive, inducing myeloma cell apoptosis. The apolipoprotein peptide mimetic L-4F was used for pharmacological enhancement of host-derived adiponectin. L-4F reduced tumour burden, increased survival of myeloma-bearing mice and prevented myeloma bone disease (Fowler et al., 2011). These results indicate that decreased adiponectin is a novel mechanism that promotes myeloma growth and associated bone disease and represents a viable target for myeloma treatment.
Bruton's tyrosine kinase (BTK)
BTK plays a key role in the development and function of normal B-cells through activation of the B-cell antigen receptor signalling pathway on binding to antigens (de Weers et al., 1994). BTK has been implicated in bone resorption by regulating osteoclast differentiation and is expressed in osteoclasts but not in osteoblasts (Lee et al., 2008; Shinohara et al., 2008). BTK is highly expressed in both patients with multiple myeloma and human myeloma cell lines. (Chauhan et al., 2002; Tai et al., 2012; Rushworth et al., 2013). Liu et al. (2013) also identified increased BTK expression in myeloma and in the dexamethasone-resistant myeloma cell line MM1.R. In addition, a single nucleotide polymorphism (SNP) at cDNA position 2062 (T2062C) in the BTK gene was recorded in six out of eight (75%) patients and in U266 cells. This SNP in myeloma cells was not detected in other malignant haematopoietic cells of different lineages suggesting it is myeloma-specific and a potential prognostic indicator.
A potent BTK inhibitor, PCI-32765 (ibrutinib), has been reported to be cytotoxic to myeloma cells via inhibiting the NF-κB pathway and augments the activity of bortezomib and lenalidomide (Rushworth et al., 2013). Recently, Tai and colleagues (Tai et al., 2012) demonstrated PCI-32765 blocked BTK and PLC-γ2 in osteoclasts resulting in diminished osteoclast activity. PCI-32765 also inhibited the secretion of cytokines and chemokines from osteoclasts and BMSC cultures from normal and myeloma patient samples. PCI-32765 treatment significantly inhibits in vivo myeloma cell growth and myeloma cell-induced osteolysis of implanted human bone chips in SCID mice. These data suggest that BTK activation in myeloma mediates osteoclast differentiation and growth of myeloma cells and PCI-32765 merits further investigation as a novel therapeutic for myeloma cells and for myeloma-induced osteolytic bone disease.
Growth factor independence-1 (Gfi1)
Gfi1 is a zinc-finger transcriptional repressor that was originally identified in an in vitro screen for loci where the insertion of the Moloney murine leukaemia virus caused an IL-2-dependent T-cell leukaemia to progress to IL-2-independent growth (Gilks et al., 1993; Grimes et al., 1996; Zweidler-Mckay et al., 1996). A common characteristic of myeloma is the continued suppression of osteoblasts and their function through all stages of the disease. A study by D'Souza et al. (2011) showed that BMSCs from both myeloma-bearing mice and myeloma patients had increased expression of the transcriptional regulator Gfi1. Gfi1 was found to be a novel transcriptional regulator of the critical osteoblast differentiation factor Runx2. Gfi1 induction was blocked by anti-TNF-α and anti-IL-7 antibodies. BMSCs isolated from Gfi1−/− mice were resistant to myeloma-induced osteoblast suppression. In addition, knockdown of Gfi1 using siRNA in BMSCs from myeloma patients significantly restored expression of Runx2 and osteoblast differentiation markers (D'Souza et al., 2011). An abstract presented by Jin et al. demonstrated that Gfi1 binds directly to the Runx2 promoter and that there are multiple Gfi1 sites within the Runx2 promoter. Mutations in the Gfi1 binding site prevents TNF-α-mediated repression of Runx2 indicating that myeloma cell secretion of TNF-α could play a role in regulating Gfi1 expression in myeloma (Jin et al., 2011). These data indicate that Gfi1 could cause long-term suppression of osteoblasts in pathological disease and is a promising novel candidate for targeting myeloma bone disease.
Macrophage inflammatory protein-1α (MIP-1α)
MIP-1α/chemokine (C-C motif) ligand 3 (CCL3) is a chemokine that is highly expressed by myeloma cells and strongly associated with the development of the osteolytic bone disease. Myeloma cells, both those isolated from the bone marrow of patients with multiple myeloma and myeloma cell lines, express and secrete high concentrations of MIP-1α (Choi et al., 2000; Lentzsch et al., 2003). Indeed, levels of MIP-1α correlate with markers of bone resorption and osteolytic bone disease (Hashimoto et al., 2004). In addition, the primary receptor for MIP-1α, CCR1 (see Alexander et al., 2013b) is also expressed on both myeloma cells, and other cells from the host bone marrow microenvironment, including bone marrow stromal cells, osteoblasts and osteoclasts. MIP-1α is known to play a key role in myeloma cell survival, homing and in osteoclast formation and bone resorption (Choi et al., 2000; Han et al., 2001). A number of in vivo studies using murine models of multiple myeloma have taken different approaches to demonstrate the key role that MIP-1α plays in the pathogenesis of myeloma bone disease. Inhibition of MIP-1α expression in myeloma cells was found to significantly reduce tumour growth and osteoclast number (Oba et al., 2005). A neutralizing antibody to MIP-1α was also found to significantly reduce tumour burden and osteolytic bone disease (Oyayobi et al., 2001). The use of CCR1 antagonists was similarly found to reduce both tumour burden and bone disease (Menu et al., 2006) (Vallet et al., 2011). Taken together, these studies support the development of targeting the MIP-1α/CCR1 axis for the treatment of myeloma bone disease.
B-cell activating factor (BAFF)
BAFF (also know as TNFSF13B) is a member of the TNF superfamily that is expressed by myeloma cells, osteoclasts and bone marrow stromal cells, increased in the serum of patients with myeloma and acts to promote the growth and survival of myeloma cells within the bone marrow microenvironment (Novak et al., 2004; Abe et al., 2006; Tai et al., 2006). Targeting BAFF, using a neutralizing antibody, has proven effective in a murine model of myeloma, with a reduction in tumour burden, an increase in survival and a reduction in osteolytic bone disease (Neri et al., 2007). Initial phase 1 studies using the human anti-BAFF antibody tabalumab have been encouraging, with many patients with previously treated myeloma achieving a partial response or better following treatment with tabalumab (Raje et al., 2012) It will be of interest to see whether targeting BAFF in patients with myeloma reduces bone disease in addition to tumour burden, as suggested by in vivo preclinical models.
Proteasomes
The ubiquitin-proteasome pathway is responsible for the degradation of eukaryotic cellular proteins (Adams, 2002). The degradation of proteins by this pathway is critical for signal transduction, transcriptional regulation, response to stress and control of receptor function (Varshavsky, 1997). This pathway controls the activation of NF-κB (a major transcription factor) by regulating degradation of the NF-κB inhibitor (I-κB; Palombella et al., 1994; 1998). Bortezomib (N-acyl-pseudo dipeptidyl boronic acid) is a dipeptide that binds reversibly to the chymotrypsin-like b5 subunit of the catalytic chamber of the 20S proteasome inhibiting its function (Rajkumar et al., 2005). Myeloma cells secrete a large amount of different proteins, including immunoglobulins, leaving them vulnerable to killing by proteasome inhibition (Meister et al., 2007). Myeloma cells are exquisitely sensitive to proteasome inhibition, leading to tumour cell apoptosis and the fast-tracked approval for the use of proteasome inhibitors in the treatment of patients with multiple myeloma (Lawasut et al., 2012).
Proteasome inhibitors are also known to have direct effects on osteoblasts to promote osteoblast differentiation and bone formation (Garrett et al., 2003). In addition, recent studies have observed direct effects of proteasome inhibitors on osteoclasts, where decreased bone resorption has been shown to correlate with the extent of NF-κB binding (Zavrski et al., 2005). Bortezomib has also been shown to down-regulate TRAF 6, both at the protein and mRNA level (Hongming and Jian, 2009). TRAF 6 is a key signalling mediator between RANK and NF-κB (Darnay et al., 2007). In murine models, an increase in BMD, bone volume, trabecular thickness and bone formation was seen with treatment of bortezomib (Pennisi et al., 2009a) as well as an increase in osteoblast number in myeloma and non-myeloma mice (Deleu et al., 2009). Several studies have indicated that treatment with bortezomib can have bone anabolic effects in human myeloma patients. Clinical studies using bortezomib have demonstrated that levels of alkaline phosphatase and osteocalcin were enhanced and bone lesions were reduced in responders to bortezomib treatment (reviewed in (Zangari et al., 2012). Heider et al. also reported that bortezomib increased osteoblast activity markers, including alkaline phosphatase, in myeloma patients irrespective of level of response (Heider et al., 2006). Bortezomib stimulated osteoblast differentiation and bone formation in bone organ cultures in a BMP-dependent manner but this bone formation was blocked by DKK1, an osteoblast suppressor. However, bortezomib was found to inhibit DKK1 in bone and bone-derived cells (Oyajobi et al., 2007), and in myeloma patients in combination with lenalidomide and dexamethasone (Terpos et al., 2011) giving further weight to the potential bone anabolic capabilities of bortezomib. Although the retrospective analyses from these trials suggest promising results with the treatment of bortezomib, there is a need for prospective trials specifically designed to investigate the effect of bortezomib on myeloma bone disease.
The immune system
Immunomodulatory drugs (IMiDs) are a group of therapeutic agents consisting of thalidomide and its second generation derivatives lenalidomide and the newest member pomalidomide. IMiDs are known to have direct anti-tumour effects via several different mechanisms. In myeloma, IMiDs cause cell cycle arrest (Hideshima et al., 2000), prevent NF-κB activation leading to decreased expression of anti-apoptotic proteins and directly induce caspase-8 activation. It has recently been shown that the anti-myeloma activity of Thalidomide and its derivatives require the protein cereblon to produce the teratogenic effect seen with the use of IMiDs in myeloma cell lines (Ito et al., 2010). As seen in Figure 1, the interaction of myeloma cells with the BM microenvironment enhances myeloma cell growth and survival. IMiDs have been reported to prevent the adhesion of myeloma cells to non-myeloma cells in the BM microenvironment including BMSCs, osteoclasts and immune cells (reviewed in Chang et al., 2013). This would interfere with the vicious cycle of myeloma, reducing myeloma-induced osteoclastogenesis and the resultant growth factors released from bone destruction. A study by Breitkreutz et al. showed that lenalidomide inhibits osteoclast formation and activation by inhibiting key factors, such as PU.1 and pERK, during osteoclastogenesis and also by reducing myeloma burden (Breitkreutz et al., 2008). Lenalidomide decreased RANKL secreted from BMSCs from myeloma patients and in serum RANKL was decreased and OPG increased. In addition, a down-regulation of capthepsin K (which is secreted by osteoclasts and induces matrix degradation during bone resorption) was also observed upon treatment with lenalidomide (Breitkreutz et al., 2008). Pomalidomide (CC-4047) has also been shown to inhibit PU.1 and therefore, osteoclastogenesis (Anderson et al., 2006), further demonstrating the potential for IMiDs to target myeloma bone disease. Another study reported that at a dose that induced apoptosis in myeloma cells, IMiDs also showed an anti-osteoclast effect without affecting osteoblasts (Munemasa et al., 2008). It is increasingly being realized that IMiDs not only kill myeloma cells but have effects on the related bone disease. The results indicate the potential for IMiDs to increase osteoblastogenesis and inhibit osteoclastogenesis which would significantly improve the lytic lesions caused by myeloma.
Figure 1.
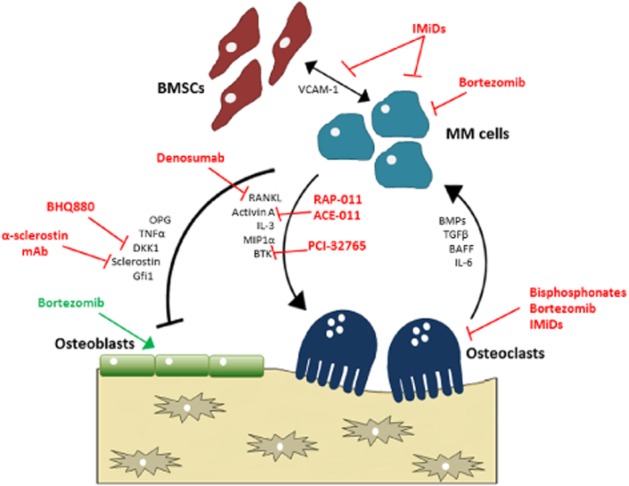
The vicious cycle of myeloma bone disease. The interactions between myeloma cells and cells of the bone marrow microenvironment, including stromal cells, osteoblasts and osteoclasts, promote both tumour growth and osteolytic bone disease. As our understanding of the mechanisms involved in disease pathogenesis increases, novel targets are revealed, which act on distinct components of the cycle to reduce tumour growth and/or bone disease.
Conclusions
The debilitating bone destruction that accompanies myeloma is the result of multiple factors that together contribute to the characteristic bone lesions. The severity of these lesions seen in the majority of patients with myeloma is explained by the vicious cycle of myeloma cell promotion and osteolysis via interaction with multiple cells in the bone marrow microenvironment. The development of novel bone anabolic drugs is essential. Patients with multiple myeloma are now surviving longer due to improved treatments for myeloma tumour burden. They are, however, left with multiple and often incapacitating bone lesions that still require treatment. Bisphosphonates remain the mainstay treatment of myeloma bone disease but come with limitations of their own. A study examining the effects of zoledronic acid and doxorubicin treatment in a breast cancer model highlighted the potential for increased drug potency in combination (Ottewell et al., 2008). It is likely that in order to ultimately eradicate myeloma and the associated bone disease, a combination of agents targeting tumour growth, osteoclastic bone resorption and osteoblastic bone formation are required. It is encouraging that a number of novel pathways and approaches have been identified that appear promising in preclinical studies. The development of novel bone disease targeting drugs that can be used as single treatments or in combination with bisphosphonates and other myeloma drugs will improve the quality of life, and possibly length of life, in myeloma patients.
Acknowledgments
This work was supported by the National Cancer Institute through R01-CA137116, Leukaemia and Lymphoma Research, the Kay Kendall Leukaemia Fund and a Marie Curie Career Integration Grant within the 7th European Community Framework Programme.
Glossary
- BTK
Bruton's tyrosine kinase
- DKK1
dickkopf 1
- Gfi1
growth factor independent-1
- IMiDs
immunomodulatory drugs
- MIP-1α
macrophage inflammatory protein-1α
- OPG
osteoprotegerin (TNFRSF11B)
- RANK
receptor activator of NF-κB (TNFRSF11A)
- RANKL
RANK ligand (TNFSF11)
Conflict of interest
The authors declare no conflict of interest.
References
- Abe M, Kido S, Hiasa M, Nakano A, Oda A, Amou H, et al. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia. 2006;20:1313–1315. doi: 10.1038/sj.leu.2404228. [DOI] [PubMed] [Google Scholar]
- Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7:9–16. doi: 10.1634/theoncologist.7-1-9. [DOI] [PubMed] [Google Scholar]
- Adulkadyrov KSGN, Khuazheva NK, Woolf R, Haltom E, Borgstein NG, Knight R, et al. ACE-011, a soluble activin receptor type IIa IgG-Fc fusion protein, increases hemoglobin (Hb) and improves bone lesions in multiple myeloma patients receiving myelosuppressive chemotherapy:preliminary analysis. Blood. 2009;114:749–750. [Google Scholar]
- Agholme F, Li X, Isaksson H, Ke HZ, Aspenberg P. Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res. 2010;25:2412–2418. doi: 10.1002/jbmr.135. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013a;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan EH, Hausler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170–1181. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- Anderson G, Gries M, Kurihara N, Honjo T, Anderson J, Donnenberg V, et al. Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood. 2006;107:3098–3105. doi: 10.1182/blood-2005-08-3450. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates AL, Mundy GR, Edwards CM. Myeloma cells decrease ephb4 expression in osteoblasts: a novel mechanism for regulation of bone formation in multiple myeloma. J Bone Miner Res. 2007;22(S309) (abstract) [Google Scholar]
- Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San Miguel JF. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood. 2013;121:884–892. doi: 10.1182/blood-2012-05-432203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–849. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev. 2005;16:319–327. doi: 10.1016/j.cytogfr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22:1925–1932. doi: 10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- Brosh N, Sternberg D, Honigwachs-Sha'anani J, Lee BC, Shav-Tal Y, Tzehoval E, et al. The plasmacytoma growth inhibitor restrictin-P is an antagonist of interleukin 6 and interleukin 11. Identification as a stroma-derived activin A. J Biol Chem. 1995;270:29594–29600. doi: 10.1074/jbc.270.49.29594. [DOI] [PubMed] [Google Scholar]
- Brunetti G, Oranger A, Mori G, Specchia G, Rinaldi E, Curci P, et al. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann N Y Acad Sci. 2011;1237:19–23. doi: 10.1111/j.1749-6632.2011.06196.x. [DOI] [PubMed] [Google Scholar]
- Butler CM, Gold EJ, Risbridger GP. Should activin betaC be more than a fading snapshot in the activin/TGFbeta family album? Cytokine Growth Factor Rev. 2005;16:377–385. doi: 10.1016/j.cytogfr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Chang X, Zhu Y, Shi C, Stewart AK. Mechanism of immunomodulatory drugs' action in the treatment of multiple myeloma. Acta Biochim Biophys Sin (Shanghai) 2013;46:240–253. doi: 10.1093/abbs/gmt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantry AD, Heath D, Mulivor AW, Pearsall S, Baud'huin M, Coulton L, et al. Inhibiting activin-A signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. J Bone Miner Res. 2010;25:2633–2646. doi: 10.1002/jbmr.142. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K, et al. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene. 2002;21:1346–1358. doi: 10.1038/sj.onc.1205205. [DOI] [PubMed] [Google Scholar]
- Chen XX, Baum W, Dwyer D, Stock M, Schwabe K, Ke HZ, et al. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann Rheum Dis. 2013;72:1732–1736. doi: 10.1136/annrheumdis-2013-203345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Cruz JC, Craig F, Chung H, Devlin RD, Roodman GD, et al. Macrophage inflammatory protein 1-alpha is a potential osteoclast stimulatory factor in multiple myeloma. Blood. 2000;96:671–675. [PubMed] [Google Scholar]
- Christoulas D, Terpos E, Dimopoulos MA. Pathogenesis and management of myeloma bone disease. Expert Rev Hematol. 2009;2:385–398. doi: 10.1586/ehm.09.36. [DOI] [PubMed] [Google Scholar]
- Clezardin P. Mechanisms of action of bisphosphonates in oncology: a scientific concept evolving from antiresorptive to anticancer activities. BoneKEy Rep. 2013;2:267. doi: 10.1038/bonekey.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104:1059–1067. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Specchia G, et al. Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J. 2011;1:e27. doi: 10.1038/bcj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croucher PI, Shipman CM, Lippitt JM, Perry M, Asosingh K, Hijzen A, et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–3540. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- Darnay BG, Besse A, Poblenz AT, Lamothe B, Jacoby JJ. TRAFs in RANK signaling. Adv Exp Med Biol. 2007;597:152–159. doi: 10.1007/978-0-387-70630-6_12. [DOI] [PubMed] [Google Scholar]
- Deleu S, Lemaire M, Arts J, Menu E, Van Valckenborgh E, Vande Broek I, et al. Bortezomib alone or in combination with the histone deacetylase inhibitor JNJ-26481585: effect on myeloma bone disease in the 5T2MM murine model of myeloma. Cancer Res. 2009;69:5307–5311. doi: 10.1158/0008-5472.CAN-08-4472. [DOI] [PubMed] [Google Scholar]
- Deli A, Kreidl E, Santifaller S, Trotter B, Seir K, Berger W, et al. Activins and activin antagonists in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1699–1709. doi: 10.3748/wjg.14.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMascio L, Voermans C, Uqoezwa M, Duncan A, Lu D, Wu J, et al. Identification of adiponectin as a novel hemopoietic stem cell growth factor. J Immunol. 2007;178:3511–3520. doi: 10.4049/jimmunol.178.6.3511. [DOI] [PubMed] [Google Scholar]
- Dowling CR, Risbridger GP. The role of inhibins and activins in prostate cancer pathogenesis. Endocr Relat Cancer. 2000;7:243–256. doi: 10.1677/erc.0.0070243. [DOI] [PubMed] [Google Scholar]
- D'Souza S, del Prete D, Jin S, Sun Q, Huston AJ, Kostov FE, et al. Gfi1 expressed in bone marrow stromal cells is a novel osteoblast suppressor in patients with multiple myeloma bone disease. Blood. 2011;118:6871–6880. doi: 10.1182/blood-2011-04-346775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi KLA, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- Fowler JA, Lwin ST, Drake MT, Edwards JR, Kyle RA, Mundy GR, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–5882. doi: 10.1182/blood-2011-01-330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futakuchi M, Nannuru KC, Varney ML, Sadanandam A, Nakao K, Asai K, et al. Transforming growth factor-beta signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci. 2009;100:71–81. doi: 10.1111/j.1349-7006.2008.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galson DL, Silbermann R, Roodman GD. Mechanisms of multiple myeloma bone disease. BoneKEy Rep. 2012;1:135. doi: 10.1038/bonekey.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks CB, Bear SE, Grimes HL, Tsichlis PN. Progression of interleukin-2 (IL-2)-dependent rat T cell lymphoma lines to IL-2-independent growth following activation of a gene (Gfi-1) encoding a novel zinc finger protein. Mol Cell Biol. 1993;13:1759–1768. doi: 10.1128/mcb.13.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98:3527–3533. doi: 10.1182/blood.v98.13.3527. [DOI] [PubMed] [Google Scholar]
- Gkotzamanidou M, Dimopoulos MA, Kastritis E, Christoulas D, Moulopoulos LA, Terpos E. Sclerostin: a possible target for the management of cancer-induced bone disease. Expert Opin Ther Targets. 2012;16:761–769. doi: 10.1517/14728222.2012.697154. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Sangiorgi L, Lenzi L, Casadei R, Canaider S, Strippoli P, et al. Seven BMPs and all their receptors are simultaneously expressed in osteosarcoma cells. Int J Oncol. 2002;20:143–147. [PubMed] [Google Scholar]
- Grimes HL, Chan TO, Zweidler-McKay PA, Tong B, Tsichlis PN. The Gfi-1 proto-oncoprotein contains a novel transcriptional repressor domain, SNAG, and inhibits G1 arrest induced by interleukin-2 withdrawal. Mol Cell Biol. 1996;16:6263–6272. doi: 10.1128/mcb.16.11.6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- Han J-H, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1α is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor κB ligand. Blood. 2001;97:3349–3353. doi: 10.1182/blood.v97.11.3349. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Shoda A, Inoue S, Yamada R, Kondo T, Sakurai T, et al. Functional regulation of osteoblastic cells by the interaction of activin-A with follistatin. J Biol Chem. 1992;267:4999–5004. [PubMed] [Google Scholar]
- Hashimoto T, Abe M, Oshima T, Shibata H, Ozaki S, Inoue D, et al. Ability of myeloma cells to secrete macrophage inflammatory protein (MIP)-1alpha and MIP-1beta correlates with lytic bone lesions in patients with multiple myeloma. Br J Haematol. 2004;125:38–41. doi: 10.1111/j.1365-2141.2004.04864.x. [DOI] [PubMed] [Google Scholar]
- Heider U, Kaiser M, Muller C, Jakob C, Zavrski I, Schulz CO, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77:233–238. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- Heider U, Kaiser M, Mieth M, Lamottke B, Rademacher J, Jakob C, et al. Serum concentrations of DKK-1 decrease in patients with multiple myeloma responding to anti-myeloma treatment. Eur J Haematol. 2009;82:31–38. doi: 10.1111/j.1600-0609.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- Henry DHCL, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7:585–598. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Hongming H, Jian H. Bortezomib inhibits maturation and function of osteoclasts from PBMCs of patients with multiple myeloma by downregulating TRAF6. Leuk Res. 2009;33:115–122. doi: 10.1016/j.leukres.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- Jin SDS, Sun Q, Sammut B, Grimes HL, Roodman DG, Galson DL. The transcription repressor Gfi1 directly interacts with the Runx2 gene in osteoblast precursors to mediate repression by multiple myeloma cells via TNFalpha, leading to blocked osteoblast differentiation. J Bone Miner Res. 2011;26(S1):S180. [Google Scholar]
- Jurimae J, Rembel K, Jurimae T, Rehand M. Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res. 2005;37:297–302. doi: 10.1055/s-2005-861483. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Kuehl WM, Bergsagel PL. Multiple myeloma: evolving genetic events and host interactions. Nat Rev Cancer. 2002;2:175–187. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25:2464–2472. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Lawasut P, Chauhan D, Laubach J, Hayes C, Fabre C, Maglio M, et al. New proteasome inhibitors in myeloma. Curr Hematol Malig Rep. 2012;7:258–266. doi: 10.1007/s11899-012-0141-2. [DOI] [PubMed] [Google Scholar]
- Lecouvet FE, Vande Berg BC, Maldague BE, Michaux L, Laterre E, Michaux JL, et al. Vertebral compression fractures in multiple myeloma. Part I. Distribution and appearance at MR imaging. Radiology. 1997;204:195–199. doi: 10.1148/radiology.204.1.9205246. [DOI] [PubMed] [Google Scholar]
- Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, et al. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103:2308–2315. doi: 10.1182/blood-2003-06-1992. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim T, Jeong D, Kim N, Choi Y. The tec family tyrosine kinase Btk Regulates RANKL-induced osteoclast maturation. J Biol Chem. 2008;283:11526–11534. doi: 10.1074/jbc.M708935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, et al. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–651. doi: 10.1016/s8756-3282(03)00237-0. [DOI] [PubMed] [Google Scholar]
- Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha ) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101:3568–3573. doi: 10.1182/blood-2002-08-2383. [DOI] [PubMed] [Google Scholar]
- Levy J, Roodman GD. The role of bisphosphonates in multiple myeloma. Curr Hematol Malig Rep. 2009;4:108–112. doi: 10.1007/s11899-009-0015-4. [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res. 2009;24:578–588. doi: 10.1359/jbmr.081206. [DOI] [PubMed] [Google Scholar]
- Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M, et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res. 2010;25:2647–2656. doi: 10.1002/jbmr.182. [DOI] [PubMed] [Google Scholar]
- Lipton ASG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- Lipton ASG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res. 2008;14:6690–6696. doi: 10.1158/1078-0432.CCR-07-5234. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dong Y, Jiang QL, Zhang B, Hu AM. Bruton's tyrosine kinase: potential target in human multiple myeloma. Leuk Lymphoma. 2013;55:177–181. doi: 10.3109/10428194.2013.794458. [DOI] [PubMed] [Google Scholar]
- Ludwig H, Miguel JS, Dimopoulos MA, Palumbo A, Garcia Sanz R, Powles R, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia. 2013;28:981–992. doi: 10.1038/leu.2013.293. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Allan EH, Ho PW, Gooi JH, Quinn JM, Gillespie MT, et al. Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol. 2010;658:51–60. doi: 10.1007/978-1-4419-1050-9_6. [DOI] [PubMed] [Google Scholar]
- Masi L, Malentacchi C, Campanacci D, Franchi A. Transforming growth factor-beta isoform and receptor expression in chondrosarcoma of bone. Virchows Arch. 2002;440:491–497. doi: 10.1007/s00428-001-0544-2. [DOI] [PubMed] [Google Scholar]
- McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- McColm J, Hu L, Womack T, Tang CC, Chiang AY. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2013;29:935–943. doi: 10.1002/jbmr.2092. [DOI] [PubMed] [Google Scholar]
- Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, et al. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Kyle RA, Achenbach SJ, Oberg AL, Rajkumar SV. Fracture risk with multiple myeloma: a population-based study. J Bone Miner Res. 2005;20:487–493. doi: 10.1359/JBMR.041131. [DOI] [PubMed] [Google Scholar]
- Menu E, De Leenheer E, De Raeve H, Coulton L, Imanishi T, Miyashita K, et al. Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clin Exp Metastasis. 2006;23:291–300. doi: 10.1007/s10585-006-9038-6. [DOI] [PubMed] [Google Scholar]
- Morgan GJ, Child JA, Gregory WM, Szubert AJ, Cocks K, Bell SE, et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12:743–752. doi: 10.1016/S1470-2045(11)70157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GJ, Davies FE, Gregory WM, Szubert AJ, Bell SE, Drayson MT, et al. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research Council Myeloma IX Trial. Blood. 2012;119:5374–5383. doi: 10.1182/blood-2011-11-392522. [DOI] [PubMed] [Google Scholar]
- Mundy GR, Elefteriou F. Boning up on ephrin signaling. Cell. 2006;126:441–443. doi: 10.1016/j.cell.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Munemasa S, Sakai A, Kuroda Y, Okikawa Y, Katayama Y, Asaoku H, et al. Osteoprogenitor differentiation is not affected by immunomodulatory thalidomide analogs but is promoted by low bortezomib concentration, while both agents suppress osteoclast differentiation. Int J Oncol. 2008;33:129–136. [PubMed] [Google Scholar]
- Munshi NC, Beck JT, Bensinger W, Facon T, Stockerl-Goldstein K, Baz R, et al. Early evidence of anabolic bone activity of BHQ880, a fully human anti-dkk1 neutralizing antibody: results of a Phase 2 study in previously untreated patients with smoldering multiple myeloma at risk for progression. Blood. 2012;120:Abstract 331. [Google Scholar]
- Narayanan P. Denosumab: a comprehensive review. South Asian J Cancer. 2013;2:272–277. doi: 10.4103/2278-330X.119895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri P, Kumar S, Fulciniti MT, Vallet S, Chhetri S, Mukherjee S, et al. Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res. 2007;13:5903–5909. doi: 10.1158/1078-0432.CCR-07-0753. [DOI] [PubMed] [Google Scholar]
- Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004;103:689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- Oba Y, Lee JW, Ehrlich LA, Chung HY, Jelinek DF, Callander NS, et al. MIP-1alpha utilizes both CCR1 and CCR5 to induce osteoclast formation and increase adhesion of myeloma cells to marrow stromal cells. Exp Hematol. 2005;33:272–278. doi: 10.1016/j.exphem.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Ominsky MS, Li C, Li X, Tan HL, Lee E, Barrero M, et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011;26:1012–1021. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, et al. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–526. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- Ottewell PD, Monkkonen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100:1167–1178. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- Oyajobi BO, Garrett IR, Gupta A, Flores A, Esparza J, Munoz S, et al. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: implications for myeloma bone disease. Br J Haematol. 2007;139:434–438. doi: 10.1111/j.1365-2141.2007.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyayobi B, Williams PJ, Pulkrabek D, Franchin G, Sherry B, Mundy GR. In vivo osteoclastogenic effects of the CC chemokine, macrophage inflammatory protein (MIP)-1α. Bone. 2001;27:S81. [Google Scholar]
- Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Palombella VJ, Conner EM, Fuseler JW, Destree A, Davis JM, Laroux FS, et al. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–15676. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, et al. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci U S A. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol. 2009a;84:6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD, Jr, Barlogie B, et al. The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood. 2009b;114:1803–1812. doi: 10.1182/blood-2009-01-201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi S, Fulciniti M, Yan H, Vallet S, Eda H, Patel K, et al. In vivo and in vitro effects of a novel anti-Dkk1 neutralizing antibody in multiple myeloma. Bone. 2013;53:487–496. doi: 10.1016/j.bone.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Zheng Y, Zheng C, Wang L, Qin H, Hong S, et al. Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood. 2012;119:161–169. doi: 10.1182/blood-2011-07-368472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang YW, Walsh K, Yao L, Kedei N, Blumberg PM, Rubin JS, et al. Wnts induce migration and invasion of myeloma plasma cells. Blood. 2005;106:1786–1793. doi: 10.1182/blood-2005-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raje N, Faber EA, Richardson PG, Schiller GJ, Hohl RJ, Cohen AD, et al. Phase 1 study of Tabalumabm a human anti-BAFF antibody and bortezomi in patients with previously-treated multiple myeloma. Blood. 2012;120:Abstract 447. [Google Scholar]
- Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–1523. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- Risbridger GP, Mellor SL, McPherson SJ, Schmitt JF. The contribution of inhibins and activins to malignant prostate disease. Mol Cell Endocrinol. 2001;180:149–153. doi: 10.1016/s0303-7207(01)00497-x. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Crockett JC, Coxon FP, Monkkonen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Roodman GD, Dougall WC. RANK ligand as a therapeutic target for bone metastases and multiple myeloma. Cancer Treat Rev. 2008;34:92–101. doi: 10.1016/j.ctrv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Ruckle J, Jacobs M, Kramer W, Pearsall AE, Kumar R, Underwood KW, et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24:744–752. doi: 10.1359/jbmr.081208. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Bowles KM, Barrera LN, Murray MY, Zaitseva L, MacEwan DJ. BTK inhibitor ibrutinib is cytotoxic to myeloma and potently enhances bortezomib and lenalidomide activities through NF-kappaB. Cell Signal. 2013;25:106–112. doi: 10.1016/j.cellsig.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Seidel C, Hjertner O, Abildgaard N, Heickendorff L, Hjorth M, Westin J, et al. Serum osteoprotegerin levels are reduced in patients with multiple myeloma with lytic bone disease. Blood. 2001;98:2269–2271. doi: 10.1182/blood.v98.7.2269. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Sternberg D, Honigwachs-sha'anani J, Brosh N, Malik Z, Burstein Y, Zipori D. Restrictin-P/stromal activin A, kills its target cells via an apoptotic mechanism. Growth Factors. 1995;12:277–287. doi: 10.3109/08977199509028966. [DOI] [PubMed] [Google Scholar]
- Stopeck ATLA, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- Sugatani T, Alvarez UM, Hruska KA. Activin A stimulates IkappaB-alpha/NFkappaB and RANK expression for osteoclast differentiation, but not AKT survival pathway in osteoclast precursors. J Cell Biochem. 2003;90:59–67. doi: 10.1002/jcb.10613. [DOI] [PubMed] [Google Scholar]
- Sutherland MK, Geoghegan JC, Yu C, Turcott E, Skonier JE, Winkler DG, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87–102. doi: 10.1089/met.2007.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66:6675–6682. doi: 10.1158/0008-5472.CAN-06-0190. [DOI] [PubMed] [Google Scholar]
- Tai YT, Chang BY, Kong SY, Fulciniti M, Yang G, Calle Y, et al. Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma. Blood. 2012;120:1877–1887. doi: 10.1182/blood-2011-12-396853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takyar FM, Tonna S, Ho PW, Crimeen-Irwin B, Baker EK, Martin TJ, et al. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res. 2013;28:912–925. doi: 10.1002/jbmr.1820. [DOI] [PubMed] [Google Scholar]
- Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, et al. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102:1064–1069. doi: 10.1182/blood-2003-02-0380. [DOI] [PubMed] [Google Scholar]
- Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012a;131:1466–1471. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- Terpos E, Kastritis E, Christoulas D, Gkotzamanidou M, Eleutherakis-Papaiakovou E, Kanellias N, et al. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann Oncol. 2012b;23:2681–2686. doi: 10.1093/annonc/mds068. [DOI] [PubMed] [Google Scholar]
- Terpos ECD, Katodritou E, Kastritis E, Papatheodoru A, Kyrtsonis MC, Papanikolaou X, et al. The combination of lenalidomide and dexamethasone (RD) reduces bone resorption in responding patients with relapsed/refractory multiple myeloma (MM) but has no effect on bone formation: results of a retrospective analysis and a prospective study on 205 patients, on behalf of the Greek myeloma study group. Haematologica. 2011;96(Suppl. 1):abstract P-126. [Google Scholar]
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S, et al. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci U S A. 2010;107:5124–5129. doi: 10.1073/pnas.0911929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P, et al. A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25:1174–1181. doi: 10.1038/leu.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. The ubiquitin system. Trends Biochem Sci. 1997;22:383–387. doi: 10.1016/s0968-0004(97)01122-5. [DOI] [PubMed] [Google Scholar]
- Vega D, Maalouf NM, Sakhaee K. CLINICAL Review #: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J Clin Endocrinol Metab. 2007;92:4514–4521. doi: 10.1210/jc.2007-0646. [DOI] [PubMed] [Google Scholar]
- de Weers M, Mensink RG, Kraakman ME, Schuurman RK, Hendriks RW. Mutation analysis of the Bruton's tyrosine kinase gene in X-linked agammaglobulinemia: identification of a mutation which affects the same codon as is altered in immunodeficient xid mice. Hum Mol Genet. 1994;3:161–166. doi: 10.1093/hmg/3.1.161. [DOI] [PubMed] [Google Scholar]
- Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–6276. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Kukita T, Li YJ, Martinez Argueta JG, Saito T, Hanazawa S, et al. Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans. FEMS Immunol Med Microbiol. 2007;49:28–34. doi: 10.1111/j.1574-695X.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- Zangari M, Terpos E, Zhan F, Tricot G. Impact of bortezomib on bone health in myeloma: a review of current evidence. Cancer Treat Rev. 2012;38:968–980. doi: 10.1016/j.ctrv.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Zavrski I, Krebbel H, Wildemann B, Heider U, Kaiser M, Possinger K, et al. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem Biophys Res Commun. 2005;333:200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Zweidler-Mckay PA, Grimes HL, Flubacher MM, Tsichlis PN. Gfi-1 encodes a nuclear zinc finger protein that binds DNA and functions as a transcriptional repressor. Mol Cell Biol. 1996;16:4024–4034. doi: 10.1128/mcb.16.8.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]


