Abstract
BACKGROUND AND PURPOSE
The α1-adrenoceptor family plays a critical role in regulating ocular perfusion by mediating responses to catecholamines. The purpose of the present study was to determine the contribution of individual α1-adrenoceptor subtypes to adrenergic vasoconstriction of retinal arterioles using gene-targeted mice deficient in one of the three adrenoceptor subtypes (α1A-AR−/−, α1B-AR−/− and α1D-AR−/− respectively).
EXPERIMENTAL APPROACH
Using real-time PCR, mRNA expression for individual α1-adrenoceptor subtypes was determined in murine retinal arterioles. To assess the functional relevance of the three α1-adrenoceptor subtypes for mediating vascular responses, retinal vascular preparations from wild-type mice and mice deficient in individual α1-adrenoceptor subtypes were studied in vitro using video microscopy.
KEY RESULTS
Retinal arterioles expressed mRNA for all three α1-adrenoceptor subtypes. In functional studies, arterioles from wild-type mice with intact endothelium responded only negligibly to the α1-adrenoceptor agonist phenylephrine. In endothelium-damaged arterioles from wild-type mice, phenylephrine evoked concentration-dependent constriction that was attenuated by the α1-adrenoceptor blocker prazosin. Strikingly, phenylephrine only minimally constricted endothelium-damaged retinal arterioles from α1B-AR−/− mice, whereas arterioles from α1A-AR−/− and α1D-AR−/− mice constricted similarly to arterioles from wild-type mice. Constriction to U46619 was similar in endothelium-damaged retinal arterioles from all four mouse genotypes.
CONCLUSIONS AND IMPLICATIONS
The present study is the first to demonstrate that α1-adrenoceptor-mediated vasoconstriction in murine retinal arterioles is buffered by the endothelium. When the endothelium is damaged, a vasoconstricting role of the α1B-adrenoceptor subtype is unveiled. Hence, the α1B-adrenoceptor may represent a target to selectively modulate retinal blood flow in ocular diseases associated with endothelial dysfunction.
Introduction
Haemodynamic perturbations in the retina and optic nerve head are considered to play a critical role in the pathogenesis of various ocular diseases, including glaucoma (Grieshaber and Flammer, 2005; Toda and Nakanishi-Toda, 2007) and diabetic retinopathy (Schmetterer and Wolzt, 1999). Hence, signalling pathways involved in the regulation of retinal vascular tone and blood flow appear to be promising therapeutic targets. The discovery of adrenoceptors by Ahlquist (Ahlquist, 1948) more than six decades ago has uncovered the adrenergic pathway as one of the central regulators of organ perfusion that mediates its effects through adrenoceptors located on blood vessels. As transmembrane proteins belonging to the superfamily of GPCRs (see Alexander et al., 2013), adrenoceptors convey responses to the endogenous catecholamines, adrenaline and noradrenaline (Piascik and Perez, 2001). Among adrenoceptors, the α1-adrenoceptor family is critically involved in the regulation of vascular tone and blood flow by mediating the vasoconstrictive effect of catecholamines. This functional role of α1-adrenoceptors was also demonstrated in ocular blood vessels, including retinal arterioles, from different species (Ferrari-Dileo et al., 1990; Ichikawa et al., 2004; Gericke et al., 2011a; Mori et al., 2011). Pharmacological studies and molecular cloning methods have identified three structurally distinct α1-adrenoceptor subtypes designated as α1A, α1B and α1D (Hieble et al., 1995). The expression pattern of individual α1-adrenoceptor subtypes and their involvement in mediating vascular responses to catecholamines differ considerably between individual vascular beds, and, in some vessels, endothelial α1-adrenoceptors may at least partly counteract those on vascular smooth muscle (Guimaraes and Moura, 2001). Hence, the three α1-adrenoceptor subtypes may constitute effective therapeutic targets, allowing a selective modulation of perfusion in various organs, including the eye.
Although the functional roles of individual α1-adrenoceptor subtypes are already well-established in several tissues and vascular beds, there is still a paucity of knowledge regarding the ocular vasculature. Thus, the major goal of the present study was to identify the α1-adrenoceptor subtypes mediating vascular responses in retinal arterioles. In view of increasing doubts about the specificity of commercially available antibodies against murine α1-adrenoceptor subtypes (Jensen et al., 2009b; Pradidarcheep et al., 2009), we refrained from localizing the receptor proteins in retinal arterioles by immunohistochemistry. Instead, we used real-time PCR to quantify mRNA expression levels of all three α1-adrenoceptor subtypes in isolated retinal arterioles. Since highly selective agonists and antagonists are not available for all α1-adrenoceptor subtypes (Zhong and Minneman, 1999; Chen and Minneman, 2005), we employed gene-targeted mice deficient in one of the three subtypes (α1A-AR−/−, α1B-AR−/− and α1D-AR−/−, respectively) to determine the involvement of individual receptor subtypes in retinal vasoconstriction.
Methods
Animals
All experimental procedures involving animals are reported in accordance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). The animal trials were approved by the Animal Care Committee of Rhineland-Palatinate, Germany, and animal care was in accordance with institutional guidelines. Every effort was made to minimize animal suffering and to reduce the number of animals used.
The generation of α1A-AR−/−, α1B-AR−/− and α1D-AR−/− mice has been described previously (Cavalli et al., 1997; Rokosh and Simpson, 2002; Tanoue et al., 2002). Each genotype has been backcrossed with C57BL/6Slc mice for more than eight generations and maintained on a C57BL/6Slc background. Male mice deficient in one of the three α1-adrenoceptor subtypes at the age of 2–5 months and age-matched wild-type controls of the C57BL/6Slc background were used for the experiments. The genotype of each mouse was determined by PCR of DNA isolated from tail biopsies. Mice were housed under standardized conditions (light/dark cycle 12 h, temperature 22 ± 2°C, humidity 55 ± 10%) and had free access to standard mouse chow and tap water.
Real-time PCR analysis
Expression of individual α1-adrenoceptor genes was determined in isolated retinal arterioles with intact endothelium from wild-type mice (C57BL/6Slc) using real-time PCR. The method of murine retinal arteriole isolation has been described in detail previously (Gericke et al., 2011b). Briefly, after mice had been killed by CO2 inhalation, a midline abdominal and thoracic incision was made. Then, the inferior vena cava was cut, and the left cardiac ventricle was cannulated with a needle that was connected to a silicon tube, and perfused with 10 mL of PBS (Invitrogen, Karlsruhe, Germany) followed by an injection of iron oxide suspension (1% in 20 mL of PBS) to visualize retinal arterioles. After this procedure, the eyes were immediately enucleated and placed in ice-cold PBS. Subsequently, the retina was removed from the eye under a dissecting microscope and retinal arterioles were isolated with fine-point tweezers, transferred into a 1.5 mL tube, and immediately snap frozen. To increase RNA yield, arterioles from seven mice were pooled. Next, vessels were homogenized in lysis buffer (Buffer RLT, QIAGEN, Hilden, Germany) using a homogenizing device (FastPrep; MP Biomedicals, Illkirch, France). After homogenization, total RNA was extracted with the RNeasy Kit (QIAGEN) according to the manufacturer's protocol.
After isolation, mRNA was reverse transcribed with M-MLV reverse transcriptase and random hexamers (Promega, Mannheim, Germany). Quantitative PCR analysis was performed with the ViiA™ 7 system (Applied Biosystems, Darmstadt, Germany). SYBR green was used for the fluorescent detection of DNA generated during the PCR. The PCR was performed in a total volume of 10 µL and 2× SYBR Green master mix (QIAGEN); 2 µL cDNA corresponding to 13 ng RNA was used as template. Published sequences for mouse α1A-adrenoceptor (NM_013461), α1B-adrenoceptor (NM_007416) and α1D-adrenoceptor (NM_013460) were used to design primers for PCR amplification. Primer sequences were α1A-adrenoceptor sense 5′-GCG GTG GAC GTC TTA TGC T-3′ and antisense 5′-TCA CAC CAA TGT ATC GGT CGA-3′; α1B-AR sense 5′-CCT GGT CAT GTA CTG CCG A-3′ and antisense 5′-GAC TCC CGC CTC CAG ATT C-3′; α1D-AR sense 5′-AGT TGG TGA CCG TCT GCA AGT-3′ and antisense 5′-CGC TGT GGT GGG AAC CGG CAG-3′; ß-actin sense 5′-CAC CCG CGA GCA CAG CTT CTT T-3′ and antisense 5′-AAT ACA GCC CGG GGA GCA TC-3′. Standard negative controls were used for the PCR. A control lacking reverse transcriptase was used to check for DNA contamination of the isolated RNA. We also employed a reverse transcription control without RNA to check for contamination of the chemicals used. In both controls, no PCR product was detected, indicating that genomic DNA was absent and the purity of the chemicals high. For positive control, we used RNA isolated from mouse brain. The expression levels of individual α1-adrenoceptor subtype mRNA were normalized to ß-actin using the ΔCt method. Parallelism of standard curves was confirmed.
Measurement of vascular reactivity in retinal arterioles
The method used to measure vascular responses in the murine retina has been described in detail previously (Gericke et al., 2011b). Briefly, after mice had been killed by CO2 inhalation, the eyes were immediately removed together with the retrobulbar tissue and placed in ice-cold Krebs buffer with the following ionic composition (in mM): 118.3 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3 and 11 glucose. Next, the retrobulbar tissue was removed under a dissecting microscope, and the ophthalmic artery and their orbital branches were ligated. To exclude potential influences of the ophthalmic artery on retinal responses the retrobulbar blood vessels were deactivated by immersing the intact eye globe for 10 s into 70% ethanol followed by an intensive wash in cold Krebs buffer. Subsequently, the globe was opened, and the cornea, sclera, uvea and lens were removed. Next, the preparation was placed into an organ chamber filled with cold Krebs buffer, and the ophthalmic artery was cannulated with a glass micropipette and secured with 10–0 nylon monofilament suture.
For experiments requiring a damaged endothelium, retinal arterioles were perfused via the micropipette with Krebs buffer containing 1.0% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS; Sigma-Aldrich Chemie GmbH, Steinheim, Germany) for one minute. Following this procedure, the retina was transferred into another organ chamber filled with fresh cold Krebs buffer, the ophthalmic artery was recannulated and retinal arterioles were perfused with Krebs buffer for about 2 min. For experiments requiring an intact endothelium, retinal arterioles were perfused via the micropipette with Krebs buffer (vehicle) for 3 min before the experiment.
Subsequently, the ophthalmic artery was secured with 10–0 nylon monofilament suture to the micropipette and the retina was placed onto a transparent plastic platform, spread out, and fixed to the bottom with a stainless steel ring. Retinal vessels were pressurized via the micropipette located in the ophthalmic artery using a reservoir filled with Krebs solution to a level corresponding to 50 mmHg and imaged under brightfield conditions with a video camera mounted on an upright microscope. The magnification resulted from a 100× water immersion objective lens (LUMPlanFL, 1.0 NA, Olympus Deutschland GmbH, Hamburg, Germany) and a 2.5× projection on a 0.5′ chip digital camera (TKC1381, JVC Deutschland GmbH, Friedberg, Germany). The spatial resolution of the system was 11 pixel µm−1. Video sequences were captured to a personal computer for analysis of luminal arteriole diameter changes (Figure 1).
Figure 1.

Mouse first-order retinal arteriole with damaged endothelium before (A) and after application of the non-subtype-selective α1-adrenoceptor agonist phenylephrine (B). Red blood cells are visible in the arteriolar lumen.
The organ chamber was continuously perfused with oxygenated and carbonated Krebs buffer at 37°C and pH 7.4. For experiments, we used retinal arterioles of the first and second order depending on which vessel section allowed best focusing of the image. When comparing responses of endothelium-damaged first and second order arterioles to the α1-adrenoceptor agonist phenylephrine in wild-type mice, we did not find any significant differences (data not shown).
Vessels were allowed to equilibrate for 30 min before study. Vascular diameter was stable for at least 10 min at the start of drug application. Drugs were applied cumulatively to the circulating bath solution. All reported concentrations are final molar concentrations in the organ chamber bath. After testing endothelial viability or obtaining a concentration-response curve, the respective drug was washed out by perfusing the organ chamber with fresh Krebs buffer for 10 min.
Protocols
In order to assess endothelial function, responses of retinal arterioles to the endothelium-dependent vasodilator acetylcholine (10−5 M, Sigma-Aldrich) were tested in arterioles that had been preconstricted with the thromboxane mimetic 9,11-dideoxy-9α,11α-methanoepoxy PGF2α (U46619, Cayman Chemical, Ann Arbor, MI, USA) to 30–70% of the initial luminal diameter. U46619 was continuously circulating in the bath solution during these experiments. In a previous study, we already demonstrated that acetylcholine-induced vasodilatation of murine retinal arterioles was reversed to constriction when the endothelium had been removed by CHAPS (Gericke et al., 2011b). Several findings suggest that the vasoconstrictive effect of cholinergic agents is mediated by muscarinic receptors localized on vascular smooth muscle cells or pericytes and is masked by pronounced cholinergic vasodilatation mediated by muscarinic receptors on endothelial cells (Hoste and Andries, 1991; Wu et al., 2003).
Subsequently, concentration–response curves of retinal arterioles from wild-type mice with intact endothelium were obtained for phenylephrine (10−6–10−3 M), a non-subtype-selective α1-adrenoceptor agonist and for the thromboxane mimetic U46619 (10−9–10−6 M). Analogous experiments were conducted in retinal arterioles from wild-type mice with damaged endothelium. After obtaining concentration–response curves in retinal arterioles with damaged endothelium, the same arterioles were incubated with the competitive α1-adrenoceptor antagonist prazosin (10−5 M, Sigma Aldrich) to exclude the possibility of α1-adrenoceptor-independent vasoconstrictive effects to phenylephrine, especially at high drug concentrations. To examine the contribution of individual α1-adrenoceptor subtypes to adrenergic vasoconstriction, we compared concentration–response curves with phenylephrine and U46619 obtained in endothelium-damaged retinal arterioles from α1A-AR−/−, α1B-AR−/− and α1D-AR−/−, and wild-type mice.
Acetylcholine, phenylephrine, prazosin and CHAPS were dissolved and diluted in Krebs buffer. Stock solutions of U46619 were prepared in DMSO, and subsequent dilutions were made in Krebs buffer. After addition to the circulating bath solution, the highest DMSO concentration was 0.003% at 10−6 M of U46619. No changes of retinal arteriole diameter were detected at this DMSO concentration in pilot experiments. The drug and molecular target nomenclature used in this study conforms to BJP's Concise Guide to Pharmacology (Alexander et al., 2013).
Statistical analysis
Data are presented as mean ± SEM, and n represents the number of mice per group. Vasoconstriction responses to phenylephrine and U46619 are presented as % change in luminal arteriole diameter from resting diameter, whereas responses to acetylcholine are presented as % change in luminal diameter from the preconstricted diameter. Statistical significance among concentration–responses was calculated using the Brunner test for non-parametric analysis of longitudinal data. For single-concentration experiments, the Kruskal–Wallis one-way ANOVA, followed by the Dunn's multiple comparison test, was used. Luminal retinal arteriole diameter was compared between the four mouse genotypes using one-way ANOVA. The level of significance α was set at 0.05. Multiple comparisons were tested at a Bonferroni-adjusted α level.
Results
α1-Adrenoceptor mRNA expression in retinal arterioles
Using real-time PCR, we recently demonstrated that murine ophthalmic arteries expressed mRNA for all three α1-adrenoceptor subtypes at similar levels (Gericke et al., 2011a). In the present study, we conducted analogous studies in isolated retinal arterioles with intact endothelium pooled from seven wild-type mice of the same genetic background as those used in the previous study. Similar to ophthalmic arteries, retinal arterioles expressed mRNA for all three α1-adrenoceptor subtypes at similar levels (Figure 2).
Figure 2.
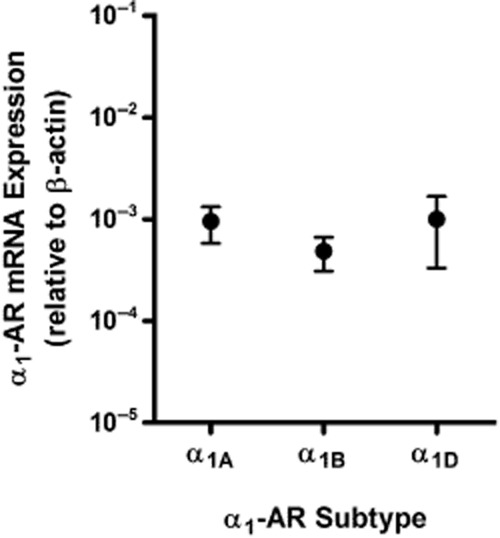
Relative mRNA expression of individual α1-adrenoceptor (α1-AR) subtypes (α1A, α1B and α1D) normalized to β-actin transcripts in retinal arterioles with intact endothelium pooled from seven different wild-type mice. The values are averages of triplicate measurements and expressed as mean ± SEM. mRNA of all three α1-adrenoceptor subtypes was found to be expressed at a similar level.
Responses of retinal arterioles
In endothelium-intact retinal arterioles from wild-type mice (n = 8), the α1-adrenoceptor agonist phenylephrine evoked only negligible vasoconstriction with maximal reduction in luminal diameter of 4 ± 2% at 10−3 M (Figure 3A). In contrast, retinal arterioles with damaged endothelium (n = 6) concentration-dependently constricted in response to phenylephrine (# P < 0.001 intact vs. damaged endothelium), and these responses were markedly attenuated by prazosin (10−5 M), a competitive non-subtype-selective α1-adrenoceptor antagonist (44 ± 4% vs. 16 ± 5% at 10−3 M; n = 6 per concentration; #P < 0.001 before vs. after incubation with prazosin; Figure 3A).
Figure 3.
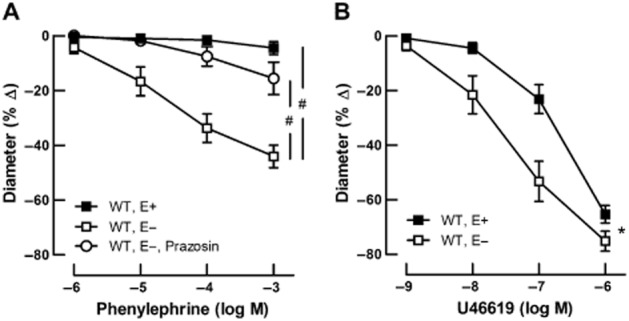
Adrenergic and non-adrenergic responses of retinal arterioles with intact (E+) and damaged (E−) endothelium. Values are expressed as mean ± SEM. (A) The non-subtype-selective α1-adrenoceptor agonist phenylephrine only evoked negligible vasoconstriction in retinal arterioles from wild-type (WT) mice with intact endothelium (n = 8), whereas retinal arterioles with damaged endothelium (n = 6) exhibited pronounced vasoconstriction (#P < 0.001 intact vs. damaged endothelium), which was markedly attenuated after incubation with the α1-adrenoceptor antagonist prazosin (10−5 M, n = 6). #P < 0.001 before versus after incubation with prazosin. (B) In contrast, the non-adrenergic vasoconstrictor U46619 induced a marked concentration-dependent diameter reduction in retinal arterioles from WT mice with intact endothelium (n = 8), which was even enhanced after endothelial damage (n = 6). *P < 0.05 E+ vs. E−.
The non-adrenergic vasoconstrictor U46619 evoked concentration-dependent constriction in endothelium-intact retinal arterioles (n = 8), with maximal reduction in luminal diameter of 65 ± 3% at 10−6 M (Figure 3B). In arterioles with damaged endothelium (n = 6), responses to U46619 were enhanced, especially at submaximal concentrations. Maximal reduction in luminal diameter to U46619 was 75 ± 4% at 10−6 M (*P < 0.05 intact vs. damaged endothelium; Fig. 3B).
To identify the α1-adrenoceptor subtypes mediating adrenergic vasoconstriction in retinal arterioles with damaged endothelium, we conducted functional studies in wild-type (n = 8), α1A-AR−/−(n = 8), α1B-AR−/− (n = 8) and α1D-AR−/− (n = 9) mice. Baseline luminal arteriole diameters after 30 min of equilibration at 37°C and pH 7.4 were 23 ± 3 µm, 25 ± 2 µm, 19 ± 2 µm, and 18 ± 2 µm in wild-type, α1A-AR−/−, α1B-AR−/−, and α1D-AR−/− mice, respectively, and did not differ between individual mouse genotypes (one-way ANOVA). In each group, the vessels measured were predominantly first order arterioles (6–7 per group).
Endothelial damage was confirmed by testing responses of retinal arterioles preconstricted with U46619 to acetylcholine, which acts as a vasodilator when the endothelium is intact and as a vasoconstrictor when the endothelium is damaged. After endothelial damage, acetylcholine (10−5 M) induced similar diameter reduction in retinal arterioles from all four mouse genotypes that was 22 ± 4%, 25 ± 4%, 21 ± 6%, and 22 ± 4% in wild-type, α1A-AR−/−, α1B-AR−/−, and α1D-AR−/− mice, respectively (Figure 4), indicating that the degree of endothelial damage was similar in all groups.
Figure 4.
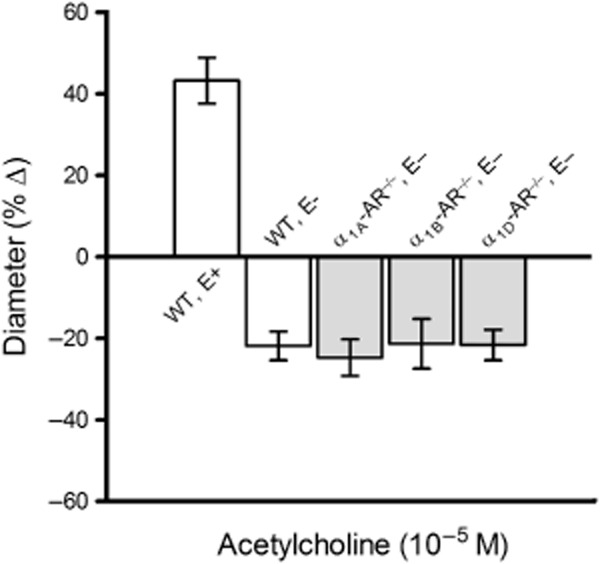
Responses of murine retinal arterioles to acetylcholine (10−5 M). Values are expressed as mean ± SEM (n = 8–9 per group). Arterioles with damaged endothelium (E−) constricted similarly in wild-type (WT), α1A-AR−/−, α1B-AR−/− and α1D-AR−/− mice, suggesting that the extent of endothelial damage did not differ between the four mouse genotypes. To demonstrate the pronounced changes that occurred in acetylcholine-induced vascular reactivity after endothelial damage, we also show responses of retinal arterioles from wild-type mice with intact endothelium (E+).
Phenylephrine induced similar concentration-dependent vasoconstriction of retinal arterioles from wild-type, α1A-AR−/−, and α1D-AR−/− mice. Maximal reduction in luminal diameter was 49 ± 4%, 47 ± 4%, and 41 ± 7% at 10−3 M in wild-type, α1A-AR−/−, and α1D-AR−/− mice respectively. However, in α1B-AR−/− mice, phenylephrine only produced a blunted constrictive effect of 13 ± 5% at 10−3 M that differed significantly from responses in wild-type, α1A-AR−/− and α1D-AR−/− mice (#P < 0.001 α1B-AR−/− vs. all other genotypes; Figure 5A). In contrast, retinal arterioles from all four mouse genotypes displayed concentration-dependent vasoconstriction responses to the non-adrenergic vasoconstrictor U46619 that did not differ significantly between individual groups. Maximal reduction in luminal diameter was 76 ± 3%, 76 ± 3%, 78 ± 5%, and 80 ± 3% at 10−6 M in wild-type, α1A-AR−/−, α1B-AR−/−, and α1D-AR−/− mice respectively (Figure 5B).
Figure 5.
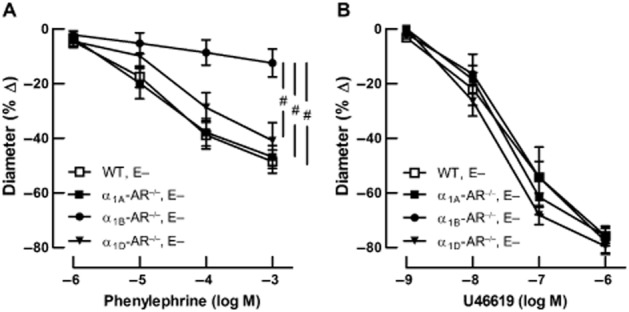
Adrenergic and non-adrenergic responses of retinal arterioles with damaged endothelium (E−). Values are expressed as mean ± SEM (n = 8–9 per substance, concentration and genotype). (A) Retinal arterioles from wild-type, α1A-AR−/− and α1D-AR−/− mice concentration-dependently constricted to the non-subtype-selective α1-adrenoceptor agonist phenylephrine. In contrast, phenylephrine evoked only minor vasoconstriction responses in retinal arterioles from α1B-AR−/− mice. #P < 0.001 α1B-AR−/− versus all other genotypes. (B) In contrast, vascular responses to the non-adrenergic vasoconstrictor U46619 were not affected by deletion of the α1A-, α1B- or α1D-adrenoceptor gene.
Discussion
Previous studies reported that α1-adrenoceptors mediate vasoconstriction in retinal arterioles of various species (Ferrari-Dileo et al., 1990; Ichikawa et al., 2004; Mori et al., 2011). The present study is the first to demonstrate that α1-adrenoceptor-mediated vasoconstriction in murine retinal arterioles with damaged endothelium is predominantly mediated by the α1B subtype. This finding is particularly striking, because the α1B-adrenoceptor was shown to contribute only minimally to adrenergic vasoconstriction in other vascular beds tested so far.
Our results, however, suggest that the α1-adrenoceptor-mediated constrictive effect on murine retinal arterioles is largely masked by endothelial mechanisms and becomes relevant only when the endothelium is damaged. Indeed, it is well documented that vascular responses to adrenergic stimuli are modulated by the endothelium and are altered under endothelial impairment (Furchgott, 1984; Miller and Vanhoutte, 1989). For example, removal of the endothelial cell layer markedly enhanced the maximum contractile effect of α1-adrenoceptor agonists in rat aorta (Carrier and White, 1985) and mesenteric arteries (White and Carrier, 1986). Interestingly, it has been shown that α1-adrenoceptor stimulation causes only slight constriction of normal human coronary arteries, but markedly constricts coronary arteries with disrupted endothelium (Vita et al., 1992; Baumgart et al., 1999). A potentiation of α1-adrenoceptor-mediated vasoconstriction was also observed in coronary arteries of anaesthetized dogs after pharmacological blockade of endothelium-dependent relaxation (Jones et al., 1993). Endothelium-dependent attenuation of retinal α1-adrenoceptor-mediated vasoconstriction under physiological conditions appears plausible, as it would confer an advantage by protecting the retina against inappropriate reductions in blood flow during exercise, haemorrhage or stress. Endothelial compensation of α1-adrenoceptor-mediated vasoconstriction is attributed to relaxing factors such as nitric oxide released by endothelial cells (Schmetterer and Polak, 2001). Apart from a basal secretion of endothelium-dependent vasodilators, nitric oxide release in response to increased sheer stress during vascular constriction is considered a possible endothelial mechanism counteracting vasoconstriction (Martin et al., 1986; Tesfamariam and Cohen, 1988; Jones et al., 1993; Sun et al., 2001). Moreover, some studies suggest that activation of α1-adrenoceptors located on endothelium also promotes endothelial release of nitric oxide and hence functionally antagonizes adrenergic vasoconstriction mediated by α1-adrenoceptors on vascular smooth muscle cells (Kaneko and Sunano, 1993; Tuttle and Falcone, 2001). In the present study, responses to the non-adrenergic vasoconstrictor U46619 were also increased after endothelial damage. Hence, apparently even in the absence of an α1-adrenoceptor agonist the endothelium releases factors attenuating vasoconstriction. This may be due to either a tonic release of such factors and/or a release specifically induced by the α1-adrenoceptor agonist and U46619 respectively. Of note, the effect of endothelium removal was greater for phenylephrine than for U46619.
Because in retinal arterioles from α1B-AR−/− mice, phenylpherine-induced vasoconstriction was attenuated to a similar extent as by prazosin in arterioles from wild-type mice, this response is obviously mediated mainly by the α1B-adrenoceptor. The finding that α1-adrenoceptor-mediated vasoconstriction in murine retinal arterioles is predominantly mediated by the α1B subtype is particularly surprising, because in the murine ophthalmic artery, which is directly afferent to the retinal vasculature, the α1A subtype mediates constrictive responses to α1-adrenoceptor stimuli when assessed under similar experimental conditions (Gericke et al., 2011a). Thus, our results demonstrate that smooth muscle cells of the retrobulbar (α1A-adrenoceptor) and the retinal (α1B-adrenoceptor) vasculature are under functional control of different α1-adrenoceptor subtypes. It has previously been reported that distribution of individual α1-adrenoceptor subtypes and their contribution to adrenergic vasoconstriction may vary considerably between various circulatory beds, between different-sized vessels of a given vascular bed and between different species (Rudner et al., 1999; Guimaraes and Moura, 2001; Piascik and Perez, 2001; Docherty, 2010). However, this has not been described for ocular blood vessels so far. Former pharmacological studies making use of subtype-selective ligands and functional studies in gene-targeted mice lacking one or more α1-adrenoceptor subtype have revealed that the α1A-adrenoceptor subtype is substantially involved in adrenergic vasoconstriction of rat and mouse small mesenteric and caudal arteries (Chen et al., 1996; Daly et al., 2002; Hedemann et al., 2004; Hosoda et al., 2005; Marti et al., 2005; Martinez-Salas et al., 2007). In contrast, the α1D-adrenoceptor subtype was found to play a major role in α1-adrenoceptor-mediated constriction of large vessels, such as aorta, iliac and carotid arteries, but was also suggested to mediate vasoconstriction in some blood vessels, for example coronary and femoral small arteries (Chalothorn et al., 2003; Marti et al., 2005; Zacharia et al., 2005; Methven et al., 2009). These findings suggest a major role of α1A- and α1D-adrenoceptors in vasoconstriction and are in line with in vivo studies demonstrating that resting blood pressure is reduced in both α1A-AR−/− and α1D-AR−/− mice, respectively (Rokosh and Simpson, 2002; Tanoue et al., 2002). In contrast, resting blood pressure was reported to be similar in α1B-AR−/− mice and respective wild-type mice, although blood pressure responses to phenylephrine and to noradrenaline were reduced in mice deficient in the α1B-adrenoceptor gene (Cavalli et al., 1997). These findings indicate that the α1B-adrenoceptor plays only a limited role for the maintenance of basal blood pressure, but may be involved in modulating blood pressure changes. In support of this concept, several in vitro studies using subtype-selective antagonists and mice lacking the α1B-adrenoceptor subtype revealed only a minor but significant contribution of the α1B-adrenoceptor to α1-adrenoceptor-mediated vasoconstriction in mouse aorta, carotid, mesenteric and caudal arteries (Cavalli et al., 1997; Daly et al., 2002; Hosoda et al., 2005). Our finding that α1-adrenoceptor-mediated constriction of the murine ophthalmic artery and murine retinal arterioles is mediated by different α1-adrenoceptor subtypes could be regarded as further evidence that the relevance of individual α1-adrenoceptor subtypes for adrenergic vasoconstriction varies considerably depending on which vascular bed is examined. Regulation of retinal circulation was shown to feature some peculiarities that may be due to anatomical, physiological and embryological similarities they share with cerebral vessels (Wong et al., 2001). In contrast to most other blood vessels, the retinal vasculature lacks autonomic innervation (Delaey and Van De Voorde, 2000). Therefore, retinal blood flow regulation might rather be controlled by autacoids released by endothelial cells and retinal tissues as well as humoral factors, such as circulating hormones, including adrenaline (Delaey and Van De Voorde, 2000). Furthermore, to a certain extent, retinal arterioles shield retinal circulation from fluctuations in systemic blood pressure via myogenic autoregulation (Delaey and Van De Voorde, 2000). In this regard, the revealed difference in α1-adrenoceptor-mediated adrenergic vasoconstriction between the murine ophthalmic artery and retinal arterioles may be considered as another peculiarity in the regulation of the retinal circulation.
Our functional findings are in seeming contrast to those of Mori et al., who recently suggested that noradrenaline contracts retinal arterioles with intact endothelium in rats and that this reaction is mediated by α1A- and α1D-adrenoceptors (Mori et al., 2011). Apart from species differences, methodological aspects may account for these differences. The present study was performed in vitro in retinal explants, whereas in the study of Mori et al. experiments on vascular function were conducted in vivo in anaesthetized animals. Under these conditions, the reactivity of retinal vessels may be influenced by multiple factors, including the choice of anaesthetics, duration of anaesthesia, systemic blood pressure, intraocular pressure and the reactivity of retrobulbar vessels. For example, an observed vasoconstriction of retinal arterioles after systemic in vivo application of an adrenoceptor agonist that simultaneously induces an increase in systemic blood pressure does not necessarily reflect a constrictive effect of the agonist mediated by retinal vascular receptors. The vasoconstriction may also result from myogenic autoregulation of retinal arterioles in response to an increase of systemic blood pressure (Ferrari-Dileo et al., 1990). Likewise, an observed reduction in constriction of retinal arterioles to adrenoceptor agonists after systemic in vivo application of an α1-adrenoceptor subtype-specific antagonist is not necessarily attributable to the selective blockade of the respective α1-adrenoceptor subtype in the retinal vasculature. If the respective antagonist simultaneously attenuates the increase in systemic blood pressure in response to adrenoceptor agonists, the reduced responsiveness of retinal arterioles may just as well reflect the effect of myogenic autoregulation to the reduced systemic blood pressure response.
Although we found mRNA of all three α1-adrenoceptor subtypes to be expressed at similar levels in murine retinal arterioles, α1-adrenoceptor-mediated vasoconstriction was predominantly mediated by the α1B-adrenoceptor. Several previous studies on mRNA and protein expression reported that the mRNA expression levels of individual α1-adrenoceptor subtypes were in fairly good agreement with their protein levels or their contribution to adrenergic vasoconstrictor responses (Rudner et al., 1999; Hosoda et al., 2005; Marti et al., 2005; Jensen et al., 2009a). Other studies, including our own in murine ophthalmic arteries (Gericke et al., 2011a), however, demonstrated that the presence of mRNA or even protein of a particular α1-adrenoceptor subtype does not necessarily indicate the participation of that receptor subtype in vasoconstriction (Piascik et al., 1995; Yang et al., 1997; 1998; Hrometz et al., 1999). The results of the present study represent another example of discrepancy between mRNA expression and the particular functional role of α1-adrenoceptor subtypes. However, although our data indicate that the α1A- and α1D-adrenoceptors do not significantly contribute to α1-adrenoceptor-mediated vasoconstricton in murine retinal arterioles despite respective mRNA expression, we cannot exclude the possibility that both receptor subtypes are involved in the regulation of other physiological or pathophysiological processes of the retinal vasculature.
Conclusions
Our study provides evidence that adrenergic vasoconstriction in murine retinal arterioles becomes relevant under conditions of endothelial damage and is mediated by the α1B-adrenoceptor subtype. From a clinical point of view, selective antagonists for this receptor subtype may constitute a therapeutically useful approach to improve retinal perfusion, with minor side effects in pathologies associated with impaired endothelial function, such as diabetic retinopathy, arterial occlusive disease or glaucoma (Cipolla et al., 1996; Nakazawa et al., 2007; Toda and Nakanishi-Toda, 2007; Resch et al., 2009).
Acknowledgments
We are grateful to Professor Paul C. Simpson (Cardiology Section, San Francisco Veterans Affairs Medical Center, and Department of Medicine, Cardiology Division, University of California, San Francisco, CA, USA), Professor Susanna Cotecchia (Department of General and Environmental Physiology, University of Bari, Italy, and Department of Pharmacology and Toxicology, University of Lausanne, Switzerland), Professor Akito Tanoue (Department of Pharmacology, National Research Institute for Child Health and Development, Tokyo, Japan) and Professor Atsushi Sanbe (Department of Pharmacotherapeutics, School of Pharmacy, Iwate Medical University, Iwate, Japan) for making α1A-, α1B- and α1D-AR knockout mice and wild-type controls available for this study. We also thank Ms. Brigitte Ruhland (Department of Internal Medicine II, University Medical Center Regensburg, Germany) for expert technical assistance with real-time PCR experiments. Data are part of the doctoral thesis of TB.
Glossary
- α1A-AR−/− mouse
α1A-adrenoceptor knockout mouse
- α1B-AR−/− mouse
α1B-adrenoceptor knockout mouse
- α1D-AR−/− mouse
α1D-adrenoceptor knockout mouse
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- U46619
9,11-dideoxy-9α,11α-methanoepoxy PGF2α
Author contributions
Tobias Böhmer carried out the functional experiments and wrote the manuscript. Caroline Manicam helped in writing the manuscript. Andreas Steege carried out the PCR. Martin C. Michel contributed to the design of the study protocol and reviewed the manuscript. Norbert Pfeiffer contributed to the design of the study protocol, provided laboratory equipment and work space, and reviewed the manuscript. Adrian Gericke designed the study, provided funding, isolated the retinal arterioles for PCR and assisted in writing the manuscript.
Source of funding
The work was supported by a grant from the Deutsche Ophthalmologische Gesellschaft (DOG).
Conflict of interest
MCM is an employee of Boehringer Ingelheim. NP has been an investigator in a Boehringer Ingelheim-funded study on the effects of α-blockers on iris function. The other authors report no conflict of interest.
References
- Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart D, Haude M, Gorge G, Liu F, Ge J, Grosse-Eggebrecht C, et al. Augmented alpha-adrenergic constriction of atherosclerotic human coronary arteries. Circulation. 1999;99:2090–2097. doi: 10.1161/01.cir.99.16.2090. [DOI] [PubMed] [Google Scholar]
- Carrier GO, White RE. Enhancement of alpha-1 and alpha-2 adrenergic agonist-induced vasoconstriction by removal of endothelium in rat aorta. J Pharmacol Exp Ther. 1985;232:682–687. [PubMed] [Google Scholar]
- Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, et al. Decreased blood pressure response in mice deficient of the alpha1b-adrenergic receptor. Proc Natl Acad Sci U S A. 1997;94:11589–11594. doi: 10.1073/pnas.94.21.11589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D, McCune DF, Edelmann SE, Tobita K, Keller BB, Lasley RD, et al. Differential cardiovascular regulatory activities of the alpha 1B- and alpha 1D-adrenoceptor subtypes. J Pharmacol Exp Ther. 2003;305:1045–1053. doi: 10.1124/jpet.102.048553. [DOI] [PubMed] [Google Scholar]
- Chen H, Fetscher C, Schafers RF, Wambach G, Philipp T, Michel MC. Effects of noradrenaline and neuropeptide Y on rat mesenteric microvessel contraction. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:314–323. doi: 10.1007/BF00168634. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Minneman KP. Recent progress in alpha1-adrenergic receptor research. Acta Pharmacol Sin. 2005;26:1281–1287. doi: 10.1111/j.1745-7254.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Harker CT, Porter JM. Endothelial function and adrenergic reactivity in human type-II diabetic resistance arteries. J Vasc Surg. 1996;23:940–949. doi: 10.1016/s0741-5214(96)70261-6. [DOI] [PubMed] [Google Scholar]
- Daly CJ, Deighan C, McGee A, Mennie D, Ali Z, McBride M, et al. A knockout approach indicates a minor vasoconstrictor role for vascular alpha1B-adrenoceptors in mouse. Physiol Genomics. 2002;9:85–91. doi: 10.1152/physiolgenomics.00065.2001. [DOI] [PubMed] [Google Scholar]
- Delaey C, Van De Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–256. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci. 2010;67:405–417. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari-Dileo G, Davis EB, Anderson DR. Response of retinal vasculature to phenylephrine. Invest Ophthal Vis Sci. 1990;31:1181–1182. [PubMed] [Google Scholar]
- Furchgott RF. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Gericke A, Kordasz ML, Steege A, Sanbe A, Goloborodko E, Vetter JM, et al. Functional role of alpha1-adrenoceptor subtypes in murine ophthalmic arteries. Invest Ophthal Vis Sci. 2011a;52:4795–4799. doi: 10.1167/iovs.11-7516. [DOI] [PubMed] [Google Scholar]
- Gericke A, Sniatecki JJ, Goloborodko E, Steege A, Zavaritskaya O, Vetter JM, et al. Identification of the muscarinic acetylcholine receptor subtype mediating cholinergic vasodilation in murine retinal arterioles. Invest Ophthal Vis Sci. 2011b;52:7479–7484. doi: 10.1167/iovs.11-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol. 2005;16:79–83. doi: 10.1097/01.icu.0000156134.38495.0b. [DOI] [PubMed] [Google Scholar]
- Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- Hedemann J, Fetscher C, Michel MC. Comparison of noradrenaline and lysosphingolipid-induced vasoconstriction in mouse and rat small mesenteric arteries. Autonomic Autacoid Pharmacol. 2004;24:77–85. doi: 10.1111/j.1474-8673.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, et al. International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- Hosoda C, Tanoue A, Shibano M, Tanaka Y, Hiroyama M, Koshimizu TA, et al. Correlation between vasoconstrictor roles and mRNA expression of alpha1-adrenoceptor subtypes in blood vessels of genetically engineered mice. Br J Pharmacol. 2005;146:456–466. doi: 10.1038/sj.bjp.0706325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste AM, Andries LJ. Contractile responses of isolated bovine retinal microarteries to acetylcholine. Invest Ophthal Vis Sci. 1991;32:1996–2005. [PubMed] [Google Scholar]
- Hrometz SL, Edelmann SE, McCune DF, Olges JR, Hadley RW, Perez DM, et al. Expression of multiple alpha1-adrenoceptors on vascular smooth muscle: correlation with the regulation of contraction. J Pharmacol Exp Ther. 1999;290:452–463. [PubMed] [Google Scholar]
- Ichikawa M, Okada Y, Asai Y, Hara H, Ishii K, Araie M. Effects of topically instilled bunazosin, an alpha1-adrenoceptor antagonist, on constrictions induced by phenylephrine and ET-1 in rabbit retinal arteries. Invest Ophthal Vis Sci. 2004;45:4041–4048. doi: 10.1167/iovs.03-1395. [DOI] [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Laden ME, DeMarco T, Hoopes C, Simpson PC. The alpha-1D Is the predominant alpha-1-adrenergic receptor subtype in human epicardial coronary arteries. J Am Coll Cardiol. 2009a;54:1137–1145. doi: 10.1016/j.jacc.2009.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol. 2009b;379:409–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CJ, DeFily DV, Patterson JL, Chilian WM. Endothelium-dependent relaxation competes with alpha 1- and alpha 2-adrenergic constriction in the canine epicardial coronary microcirculation. Circulation. 1993;87:1264–1274. doi: 10.1161/01.cir.87.4.1264. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Sunano S. Involvement of alpha-adrenoceptors in the endothelium-dependent depression of noradrenaline-induced contraction in rat aorta. Eur J Pharmacol. 1993;240:195–200. doi: 10.1016/0014-2999(93)90898-r. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti D, Miquel R, Ziani K, Gisbert R, Ivorra MD, Anselmi E, et al. Correlation between mRNA levels and functional role of alpha1-adrenoceptor subtypes in arteries: evidence of alpha1L as a functional isoform of the alpha1A-adrenoceptor. Am J Physiol Heart Circ Physiol. 2005;289:H1923–H1932. doi: 10.1152/ajpheart.00288.2005. [DOI] [PubMed] [Google Scholar]
- Martin W, Furchgott RF, Villani GM, Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986;237:529–538. [PubMed] [Google Scholar]
- Martinez-Salas SG, Campos-Peralta JM, Pares-Hipolito J, Gallardo-Ortiz IA, Ibarra M, Villalobos-Molina R. Alpha1A-adrenoceptors predominate in the control of blood pressure in mouse mesenteric vascular bed. Autonomic Autacoid Pharmacol. 2007;27:137–142. doi: 10.1111/j.1474-8673.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methven L, Simpson PC, McGrath JC. Alpha1A/B-knockout mice explain the native alpha1D-adrenoceptor's role in vasoconstriction and show that its location is independent of the other alpha1-subtypes. Br J Pharmacol. 2009;158:1663–1675. doi: 10.1111/j.1476-5381.2009.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, Vanhoutte PM. Role of the endothelium in modulating vascular adrenergic receptor actions. Prog Clin Biol Res. 1989;286:33–39. [PubMed] [Google Scholar]
- Mori A, Hanada M, Sakamoto K, Nakahara T, Ishii K. Noradrenaline contracts rat retinal arterioles via stimulation of alpha(1A)- and alpha(1D)-adrenoceptors. Eur J Pharmacol. 2011;673:65–69. doi: 10.1016/j.ejphar.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Kaneko Y, Mori A, Saito M, Sakamoto K, Nakahara T, et al. Attenuation of nitric oxide- and prostaglandin-independent vasodilation of retinal arterioles induced by acetylcholine in streptozotocin-treated rats. Vascul Pharmacol. 2007;46:153–159. doi: 10.1016/j.vph.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Piascik MT, Perez DM. Alpha1-adrenergic receptors: new insights and directions. J Pharmacol Exp Ther. 2001;298:403–410. [PubMed] [Google Scholar]
- Piascik MT, Guarino RD, Smith MS, Soltis EE, Saussy DL, Jr, Perez DM. The specific contribution of the novel alpha-1D adrenoceptor to the contraction of vascular smooth muscle. J Pharmacol Exp Ther. 1995;275:1583–1589. [PubMed] [Google Scholar]
- Pradidarcheep W, Stallen J, Labruyere WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:397–402. doi: 10.1007/s00210-009-0393-0. [DOI] [PubMed] [Google Scholar]
- Resch H, Garhofer G, Fuchsjager-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87:4–12. doi: 10.1111/j.1755-3768.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Simpson PC. Knockout of the alpha 1A/C-adrenergic receptor subtype: the alpha 1A/C is expressed in resistance arteries and is required to maintain arterial blood pressure. Proc Natl Acad Sci U S A. 2002;99:9474–9479. doi: 10.1073/pnas.132552699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, et al. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation. 1999;100:2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- Schmetterer L, Polak K. Role of nitric oxide in the control of ocular blood flow. Prog Retin Eye Res. 2001;20:823–847. doi: 10.1016/s1350-9462(01)00014-3. [DOI] [PubMed] [Google Scholar]
- Schmetterer L, Wolzt M. Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia. 1999;42:387–405. doi: 10.1007/s001250051171. [DOI] [PubMed] [Google Scholar]
- Sun D, Huang A, Recchia FA, Cui Y, Messina EJ, Koller A, et al. Nitric oxide-mediated arteriolar dilation after endothelial deformation. Am J Physiol Heart Circ Physiol. 2001;280:H714–H721. doi: 10.1152/ajpheart.2001.280.2.H714. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, et al. The alpha(1D)-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B, Cohen RA. Inhibition of adrenergic vasoconstriction by endothelial cell shear stress. Circ Res. 1988;63:720–725. doi: 10.1161/01.res.63.4.720. [DOI] [PubMed] [Google Scholar]
- Toda N, Nakanishi-Toda M. Nitric oxide: ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26:205–238. doi: 10.1016/j.preteyeres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Tuttle JL, Falcone JC. Nitric oxide release during alpha1-adrenoceptor-mediated constriction of arterioles. Am J Physiol Heart Circ Physiol. 2001;281:H873–H881. doi: 10.1152/ajpheart.2001.281.2.H873. [DOI] [PubMed] [Google Scholar]
- Vita JA, Treasure CB, Yeung AC, Vekshtein VI, Fantasia GM, Fish RD, et al. Patients with evidence of coronary endothelial dysfunction as assessed by acetylcholine infusion demonstrate marked increase in sensitivity to constrictor effects of catecholamines. Circulation. 1992;85:1390–1397. doi: 10.1161/01.cir.85.4.1390. [DOI] [PubMed] [Google Scholar]
- White RE, Carrier GO. Alpha 1- and alpha 2-adrenoceptor agonist-induced contraction in rat mesenteric artery upon removal of endothelium. Eur J Pharmacol. 1986;122:349–352. doi: 10.1016/0014-2999(86)90415-2. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Klein BE, Tielsch JM, Hubbard L, Nieto FJ. Retinal microvascular abnormalities and their relationship with hypertension, cardiovascular disease, and mortality. Surv Ophthalmol. 2001;46:59–80. doi: 10.1016/s0039-6257(01)00234-x. [DOI] [PubMed] [Google Scholar]
- Wu DM, Kawamura H, Sakagami K, Kobayashi M, Puro DG. Cholinergic regulation of pericyte-containing retinal microvessels. Am J Physiol Heart Circ Physiol. 2003;284:H2083–H2090. doi: 10.1152/ajpheart.01007.2002. [DOI] [PubMed] [Google Scholar]
- Yang M, Verfurth F, Buscher R, Michel MC. Is alpha1D-adrenoceptor protein detectable in rat tissues? Naunyn Schmiedebergs Arch Pharmacol. 1997;355:438–446. doi: 10.1007/pl00004966. [DOI] [PubMed] [Google Scholar]
- Yang M, Reese J, Cotecchia S, Michel MC. Murine alpha1-adrenoceptor subtypes. I. Radioligand binding studies. J Pharmacol Exp Ther. 1998;286:841–847. [PubMed] [Google Scholar]
- Zacharia J, Hillier C, Tanoue A, Tsujimoto G, Daly CJ, McGrath JC, et al. Evidence for involvement of alpha1D-adrenoceptors in contraction of femoral resistance arteries using knockout mice. Br J Pharmacol. 2005;146:942–951. doi: 10.1038/sj.bjp.0706395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol. 1999;375:261–276. doi: 10.1016/s0014-2999(99)00222-8. [DOI] [PubMed] [Google Scholar]


