SUMMARY
Unique among leukocytes, neutrophils follow daily cycles of release from and migration back into the bone marrow, where they are eliminated. Because removal of dying cells generates homeostatic signals, we explored whether neutrophil elimination triggers circadian events in the steady-state. Here, we report that the homeostatic clearance of neutrophils provides cues that modulate the physiology of the bone marrow. We identify a population of CD62LLO CXCR4HI neutrophils that have “aged” in the circulation and are eliminated at the end of the resting period in mice. Aged neutrophils infiltrate the bone marrow and promote reductions in the size and function of the hematopoietic niche. Modulation of the niche depends on macrophages and activation of cholesterol-sensing nuclear receptors, and is essential for the rhythmic egress of hematopoietic progenitors into the circulation. Our results unveil a process that synchronizes immune and hematopoietic rhythms, and expand the ascribed functions of neutrophils beyond inflammation.
INTRODUCTION
Neutrophils are the most abundant myeloid leukocytes in mammals, and are characterized by a short lifespan of about 12 hours in mice (Basu et al., 2002). This unique feature demands the elimination of large numbers of neutrophils every day, which have been estimated to be in the order of 107 in mice and 1011 in humans (Furze and Rankin, 2008; von Vietinghoff and Ley, 2008). In inflammatory scenarios, neutrophils recruited to injured tissues are eventually cleared by macrophages, a process that contributes to resolving inflammation and restoring homeostasis (Serhan and Savill, 2005; Soehnlein and Lindbom, 2010). In contrast, under non-inflammatory situations, neutrophils are preferentially eliminated in spleen, liver and bone marrow (BM) (Furze and Rankin, 2008; Suratt et al., 2001). Although the mechanisms of neutrophil death and subsequent elimination by phagocytosis have been well characterized in vitro (Fadok et al., 1998; Luo and Loison, 2008; Savill et al., 1989), the properties and fate of circulating neutrophils that are spontaneously cleared from the circulation remain to be elucidated.
Removal of dying cells serves not only to maintain organ size but also generates signals that are essential to maintain homeostasis and immune fitness, as illustrated by the development of autoimmune disease in mice that lack genes required for the elimination of apoptotic cells (Henson and Hume, 2006; Serhan and Savill, 2005). Representative of this group of genes are those coding for Liver X Receptors (LXRα and LXRβ), which are oxysterol-activated transcription factors that sense elevated cellular cholesterol (Calkin and Tontonoz, 2012). LXR receptors are important transcriptional regulators in macrophages that activate or repress gene expression upon engulfment of apoptotic cells (Parzy et al., 2009), including apoptotic neutrophils (Hong et al., 2012). Under steady-state conditions, efficient clearance of neutrophils requires their active extravasation through adhesive pathways similar to those used during inflammation, including endothelial selectins (Ley et al., 2007; Stark et al., 2005). Studies in mice deficient in adhesion receptors established that impaired neutrophil extravasation results in an imbalance in the homeostatic levels of G-CSF and enhanced granulopoiesis, both of which could be normalized by transfer of wild-type apoptotic neutrophils (Stark et al., 2005). These studies demonstrated that phagocytosis of neutrophils was part of a homeostatic loop that controlled their own levels in blood. These studies also suggested that neutrophil clearance might be a significant source of homeostatic signals able to functionally modulate the tissues and organs where they are eliminated.
The BM is not only a major clearance site for dying neutrophils, but also the main hematopoietic organ in adult mammals. Hematopoietic stem and progenitor cells (HSPC) are maintained within the BM in association with different populations of stromal cells that produce CXCL12 (Mercier et al., 2011), a chemokine that controls the migration to and retention of HSPC within the BM through binding to its receptor CXCR4 (Peled et al., 1999; Petit et al., 2002). Physiological regulation of these stromal components modulates HSPC survival and trafficking, and is afforded by several mechanisms including sympathetic innervation (Katayama et al., 2006; Mendez-Ferrer et al., 2008), signals derived from macrophages (Chow et al., 2011; Winkler et al., 2010), hormonal stimulation (Calvi et al., 2003), or cholesterol efflux pathways (Westerterp et al., 2012). Reductions in the number or function of these stromal elements cause the release of HSPC from the BM into the bloodstream (Adams et al., 2007; Mendez-Ferrer et al., 2008; Mendez-Ferrer et al., 2010; Omatsu et al., 2010; Semerad et al., 2005) and into tissues, where they participate in regenerative or immune processes (Laird et al., 2008; Massberg et al., 2007). Notably, the capacity of the BM to host HSPC varies during the day; circadian reductions in the production of CXCL12 in BM during the early resting period of mice correlate with increases of HSPC in the circulation (Mendez-Ferrer et al., 2008). The BM also presents circadian changes in the expression of endothelial selectins and VCAM-1, a process that favors the immigration of circulating leukocytes at night (Scheiermann et al., 2012). Thus, the BM is uniquely characterized by non-overlapping cycles of infiltration of mature leukocytes and emigration of immature/stem cells.
Here, we have hypothesized that the physiological clearance of neutrophils within the BM triggers signals that modulate the hematopoietic niche and promote the ensuing cycles of HSPC release. We report that neutrophils that physiologically age in blood migrate to the BM at the end of the resting period in mice, are engulfed by macrophages, and modulate the size and function of the hematopoietic niche in an LXR-dependent manner. We show that, through this mechanism, neutrophils regulate the homeostatic trafficking pattern of hematopoietic precursors and allow synchronization of immune and hematopoietic rhythms.
RESULTS
Neutrophil aging and clearance from blood
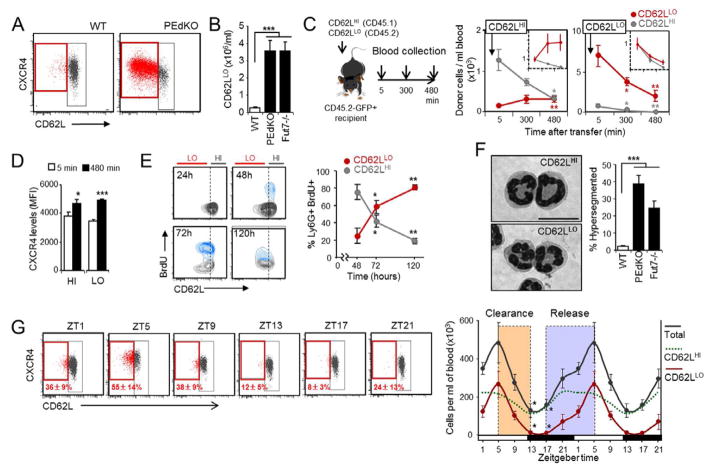
We initially sought to identify neutrophils that have remained the longest in the circulation and are primed for clearance (herein referred to as aged neutrophils). BM-derived neutrophils senesced ex vivo upregulate the expression of CXCR4, the receptor for CXCL12 which mediates their retention within and homing back to the BM (Eash et al., 2010; Eash et al., 2009; Martin et al., 2003; Suratt et al., 2001). In contrast, CD62L levels decrease as neutrophils transit in the circulation (Van Eeden et al., 1997). Using these markers, we detected a small subpopulation of Ly6G+ neutrophils in blood that expresses relatively low levels of CD62L and high levels of CXCR4 (CD62LLO CXCR4HI) (Figure 1A). The CD62LLO CXCR4HI subset of neutrophils was markedly expanded in the blood of mice in which extravasation was prevented by genetic deficiency in endothelial selectins (PEdKO mice) or fucosyltransferase 7 (Fut7−/−), an enzyme required for the synthesis of functional selectin ligands (Frenette et al., 1996; Maly et al., 1996) (Figure 1A–B). Experiments using parabiotic mice showed that the CD62LLO phenotype in these mice was not due to cytokines or other factors present in their plasma (Figure S1A), which suggested that this subset represents physiologically aged neutrophils that remained trapped in the circulation of adhesion-deficient mice. To assess whether the CD62LLO CXCR4HI cells represent bona fide aged neutrophils, we transferred CD45.1+ CD62LHI neutrophils into CD45.2+ wild-type mice. CD62LHI cells gradually converted into CD62LLO cells so that by 8 hours CD62LHI cells had virtually disappeared from blood; in contrast, transfer of CD62LLO cells derived from PEdKO mice did not yield CD62LHI neutrophils and their number progressively declined (Figure 1C). In both cases, transferred neutrophils increased the expression of surface CXCR4 over time (Figure 1D), which confirmed the upregulation of this receptor during aging. To track the fate of endogenously produced neutrophils, we metabolically labeled myeloid precursors by injecting BrdU into wild-type mice in which neutrophil extravasation was prevented by blocking endothelial selectins with antibodies (Figure S1B). Forty-eight hours after labeling the majority of BrdU+ neutrophils that first appeared in blood was CD62LHI and gradually became CD62LLO (Figure 1E). In vivo biotinylation of circulating cells confirmed that newly released, non-biotinylated neutrophils were CD62LHI (Figure S1C). Thus, Ly6G+ CD62LLO cells are a population of physiologically aged neutrophils, while the Ly6G+ CD62LHI subset identifies those recently released from the BM. Importantly, circulating CD62LLO neutrophils did not show evidence of apoptosis (Figure S1D–E) and presented higher surface levels of other receptors involved in cell trafficking, including the integrins CD11b and CD49d (Figure S1F), suggesting an enhanced migratory capacity of these cells. The morphology of aged neutrophils was characterized by a marked nuclear hypersegmentation (Figure 1F) and reduced forward and side scattering properties compared to the CD62LHI subset (Figure S1G), indicating changes in cell size and granularity during aging in blood. In agreement with the increased number of CD62LLO neutrophils, a large fraction of circulating neutrophils was hypersegmented in adhesion-deficient mice (PEdKO and Fut7−/− mice; Figure 1F).
Figure 1. Phenotype and kinetics of aged neutrophils in blood.
(A) Flow cytometric analysis of CD62L and CXCR4 expression in Ly6G+ blood neutrophils. n=5 mice.
(B) Number of CD62LLO neutrophils in the blood of WT, PEdKO and Fut7−/− mice. n=8–18.
(C) Scheme of transfer experiments of CD62LLO CD45.2+ and CD62LHI CD45.1+ neutrophils into GFP+ mice. Graphs show the absolute number of CD62LHI or CD62LLO neutrophils derived from donor cells at different times after transfer. Insets show the relative changes of each population. n=5 mice. Statistics are vs. t = 5 min.
(D) Intensity of CXCR4 expression in CD62LHI and CD62LLO neutrophils 5 and 480 min after transfer. n=5 mice.
(E) Density plots and frequencies of CD62LHI and CD62LLO neutrophils among Ly6G+ BrdU+ cells at different times post-labeling. n=5 mice. Statistics are vs. 48h. See also Figure S1B.
(F) Bright-field micrographs of sorted CD62LHI and CD62LLO blood neutrophils (scale bar, 10 μm), and frequency of hypersegmented neutrophils in blood. n=3–4 mice.
(G) Cytometry plots showing CD62L and CXCR4 expression in blood neutrophils at different times of the day. Indicated also are the mean percentage ± s.e.m. of CD62LLO cells out of total neutrophils at each time. The right graph shows the number of total, CD62LHI and CD62LLO neutrophils at the same times. Colored boxes highlight periods of clearance and release. n=5–9 mice. Statistics are vs. ZT5. Data are shown as mean ± s.e.m. *P<0.05, **P<0.01, ***P<0.001. See also Figure S1.
Leukocyte levels in blood are known to rhythmically oscillate during light-dark cycles (Haus et al., 1983). Absolute neutrophil counts in blood also followed circadian fluctuations, with cycles that suggested a period of active release between zeitgeber times (ZT) 17 and 5 (i.e., 17 and 5 h after initiation of light in a 12h light:12h dark regime), and a period of clearance between ZT5 and 13 (Figure 1G). The levels of CD62LLO CXCR4HI neutrophils in the circulation also followed tight circadian fluctuations, with an acrophase at ZT5 immediately before the clearance phase (Figure 1G). Notably, the observations that at ZT17 virtually all neutrophils are CD62LHI (Figure 1G), and that CD62LLO do not generate CD62LHI cells (Figure 1C), imply that cleared CD62LLO CXCR4HI cells do not return to the circulation.
Migrating neutrophils modulate the hematopoietic niche
Because aged neutrophils are rhythmically eliminated from the circulation, we investigated their impact on the physiology of organs where clearance preferentially occurs. We chose to study the BM, a major clearance organ for neutrophils (Furze and Rankin, 2008), because daily fluctuations similar to those described here have been reported for the hematopoietic stem cell (HSC) niche (Mendez-Ferrer et al., 2008), and these could be influenced by the rhythmic clearance of neutrophils (Figure 1G). Inside the BM, HSC and progenitor cells (HPC) are maintained in association with various stromal cell populations, which have been functionally shown to provide niches for their survival and retention (Mercier et al., 2011). Depletion of defined cellular niche elements, including CXCL12-abundant reticular cells (CAR cells), nestin+ mesenchymal progenitors or osteoblasts, result in egress of HSPC from the BM (Mendez-Ferrer et al., 2010; Omatsu et al., 2010; Visnjic et al., 2004). In addition, conditional deletion of Cxcl12 from distinct stromal subsets promotes the differential release of HSC or HPC to the periphery (Ding and Morrison, 2013; Greenbaum et al., 2013). Using Cxcl12-Gfp reporter mice (Sugiyama et al., 2006), we found that the number and frequency of CD45/TER119/CD31NEG GFPHI CAR cells underwent changes during the day (Figure 2A), indicating circadian variations in niche size. We thus investigated whether neutrophil clearance influenced the cellular or molecular constituents of the hematopoietic niche in the BM. To this end, we depleted circulating neutrophils by injection of anti-Ly6G antibody before analyzing the BM of Cxcl12-Gfp reporter mice at ZT5. Neutrophil depletion increased the number of CD45/TER119/CD31NEG GFPHI CAR cells (Figure 2B) and osteoblast-enriched CD45/TER119NEG GFPLO cells (Omatsu et al., 2010) (Figure S2A), without changes in total BM cellularity (Figure 2B). The increase in niche cell number was paralleled by elevations in protein and transcript levels of CXCL12 in the BM of neutrophil-depleted mice (Figure 2C). In contrast, neutrophil depletion in Nestin-Gfp mice did not change the number of Nestin+ niche cells (Figure S2A). BrdU-labeling analyses showed that the increase in CXCL12-expressing cells was in part due to elevated proliferation rates (Figure S2B), while there was not upregulation of Cxcl12 transcripts in these cells (Figure S2C). These results reveal that niche size is modulated by neutrophils, with varying effects on different niche components.
Figure 2. Circadian changes of the hematopoietic niche and modulation by neutrophil depletion.
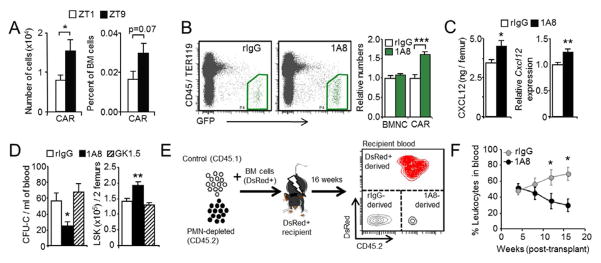
(A) Number and percentage of CAR cells in the BM of Cxcl12-Gfp mice at ZT1 and ZT9. n=8 mice per group.
(B) Representative plots and relative number of total nucleated cells (BMNC) and CAR cells (green regions) in the BM of Cxcl12-Gfp mice depleted (1A8) or not (rIgG) of neutrophils. BM samples were analyzed at ZT5. n=9 mice.
(C) CXCL12 protein and transcript levels in the BM of the groups shown in (A). n=7–9 mice.
(D) CFU-Cs in blood and number of LSK progenitors in femurs of mice treated with antibodies to deplete neutrophils (1A8), T cells (GK1.5) or control rIgG. Blood samples were analyzed at ZT5. n=5–16 mice.
(E) Strategy for the competitive long-term reconstitution assays.
(F) Percentage of blood leukocytes derived from long-term repopulating HSC present in the blood of control (rIgG) or neutrophil-depleted (1A8) donor mice in transplanted mice over sixteen weeks. Data from a representative experiment with 5 mice.
Data are shown as mean ± s.e.m. *P<0.05, **P<0.01. See also Figure S2.
CXCL12 is critical for the retention of HSPC within the BM, and physiologically- or drug-induced reductions in the level of this chemokine correlate with HSPC mobilization (Mendez-Ferrer et al., 2008; Petit et al., 2002). Consistent with this notion, neutrophil-depleted mice presented a ~60% reduction of HPC in blood at ZT5, as determined by colony-forming units in culture (CFU-C; Figure 2D) and flow cytometric analyses of LineageNEG/Sca-1+/cKit+/Thy1LO/Flk2NEG (LSKTF) precursors (Figure S2D). Long-term repopulating HSC, assessed in competitive reconstitution assays over 16 weeks, were also reduced in the blood of neutrophil-depleted mice (Figure 2E–F). These reductions in blood were mirrored by an increase in the number of progenitors in BM (Figure 2D). In contrast, depletion of T cells with an anti-CD4 antibody failed to alter progenitor levels in blood or BM (Figure 2D). Thus, neutrophil depletion alters the distribution of hematopoietic precursors between the BM and the peripheral circulation. Interestingly, neutrophil depletion failed to alter CFU-C numbers in the blood of Fut7−/− mice (Figure S2E), indicating that antibody-treatment was not toxic for hematopoietic precursors and suggesting that active neutrophil trafficking was required for niche modulation.
We next transferred BM-derived neutrophils senesced ex vivo (Martin et al., 2003; Stark et al., 2005) into Cxcl12-Gfp or wild-type mice, at doses similar to those physiologically cleared in vivo (106 cells/mouse; Figure 1G). Analysis of the BM of recipient mice 12 h after transfer (ZT1) revealed mild reductions in the number of CAR cells (Figure 3A), and a modest but significant reduction in CXCL12 protein levels (Figure 3B). This correlated with a 2.4-fold increase in the number of both CFU-C and LSKTF precursors in blood (Figure 3C and S3A), while we did not find changes in the numbers of mature leukocytes (Figure S3B). Importantly, a similar effect was obtained by transfer of blood-derived CD62LLO neutrophils, but not when total blood leukocytes depleted of neutrophils were transferred (Figure 3C).
Figure 3. Modulation of the hematopoietic niche by neutrophil transfer.
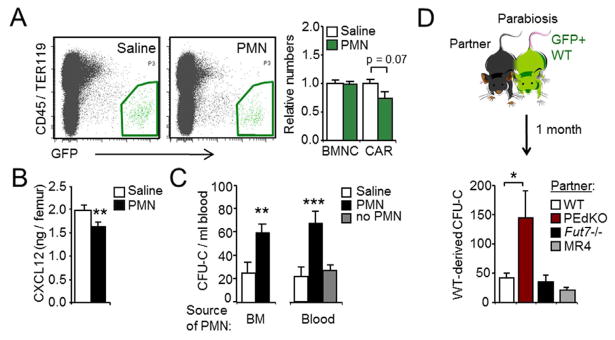
(A) Representative plots and relative number of BMNC and CAR cells (green regions) in the BM of Cxcl12-Gfp mice injected with saline or with neutrophils. BM samples were analyzed at ZT1. n=7–10 mice.
(B) CXCL12 protein levels in WT mice treated as in (A). n=10–14 mice.
(C) CFU-Cs in the blood of mice treated with BM- or blood-derived neutrophils, or blood leukocytes depleted of neutrophils (no PMN). Blood samples were analyzed at ZT1. Note that the baseline levels of CFU-C in blood differ with the neutrophil-depletion experiments (Figure 2D) because samples were collected at different times of the day, ZT5 and ZT1, respectively. n=6–14 mice.
(D) Scheme of parabiosis experiments, and number of blood CFU-C derived from GFP+ WT mice after one month. n=5–12 pairs.
Data are shown as mean ± s.e.m. *P<0.05, **P<0.01, ***P<0.001. See also Figure S3.
To determine whether chronic exposure to elevated numbers of aged neutrophils could also modulate BM niches, we conjoined the circulation of wild-type GFP+ mice and PEdKO mice by parabiosis. After one month, the number of circulating progenitors of wild-type origin (identified by expression of GFP in CFU-C) was elevated 3-fold in the mixed pairs relative to pairs composed of only wild-type animals, whereas we found no changes in circulating CFU-C in the PEdKO partners (Figure 3D and Figure S3C). The elevation in circulating progenitors in the wild-type partner could not be attributed to the presence of mobilizing factors derived from the PEdKO partner (e.g., G-CSF) or by passive overload of the circulation with neutrophils because these changes did not occur when the partners were Fut7−/− mice (Figure 3D), which display neutrophil counts and G-CSF levels in blood similar to PEdKO mice (Figure S3D), but whose neutrophils present impaired migration in a wild-type environment (Figure S2F and (Maly et al., 1996)). These data demonstrate that both acute and chronic elevations in circulating neutrophils promote the release of HPC into blood, while their depletion promotes their retention in the BM.
Neutrophils modulate the hematopoietic niche locally, and reduce its capacity to attract HPC
Migration of neutrophils into the BM is mediated by CXCR4 (Eash et al., 2009; Martin et al., 2003; Suratt et al., 2004). To assess the requirement for CXCR4-mediated homing in niche modulation, we generated mice with myeloid-restricted deficiency in this receptor by crossing LysM-Cre and Cxcr4flox/flox mice (referred herein as MR4 mice; Figure S3E). As expected, MR4-derived neutrophils failed to infiltrate the BM, but not liver or spleen (Figure S2F), and featured an aged-like phenotype characterized by low levels of CD62L, nuclear hypersegmentation and high levels of CD11b (Figure S3F–H). Importantly, despite their marked neutrophilia (Figure S3F), MR4 parabionts were unable to increase the levels of circulating progenitors in their wild-type partners (Figure 3D), suggesting that neutrophils need to infiltrate the BM in order to modulate the hematopoietic niche.
Because the hematopoietic niche is comprised of multiple molecules and cell types that are only partially characterized (Mercier et al., 2011), we decided to assess functional changes in the niche using homing and mobilization assays, which rely on the niche’s capacity to attract or retain HSPC, respectively. Using intravital imaging and flow cytometry, we found that homing of 32D myeloprogenitor cells and LSK progenitors into the calvarial or femoral BM was increased by ~40% in neutrophil-depleted mice (Figure 4A–C). Notably, homed cells exclusively infiltrated areas enriched in CAR cells (Figure 4D and Movie S1), which further supported the functional relevance of the increased CAR cell number after neutrophil depletion (Figure 2B). Mobilization of CFU-C was also increased by 40% in neutrophil-depleted mice treated with the CXCR4 antagonist AMD3100 (Broxmeyer et al., 2005) (Figure 4E). While the increased homing can be readily explained by the elevation in CXCL12-producing cells and chemokine levels in the BM after neutrophil depletion (Figure 2B–C), the enhanced mobilization is likely due to an increased number of “mobilizable” HPC in neutrophil-depleted mice (Figure 2D). Altogether, these results indicate that neutrophils that emigrate from blood in a selectin-and CXCR4-dependent manner modulate the composition and function of the hematopoietic niche under homeostatic conditions.
Figure 4. Neutrophils modulate the capacity of the hematopoietic niche to attract and retain immature leukocytes.
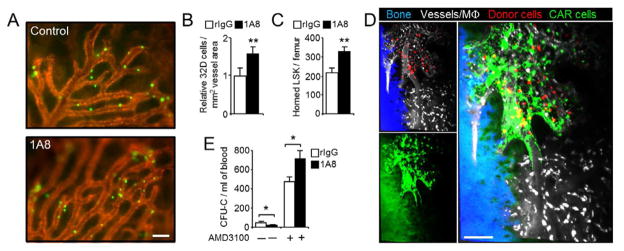
(A) Representative micrographs showing homed 32D cells (green) and BM microvessels (red) in control and 1A8-treated mice. Scale bar, 100 μm.
(B) Quantification of homed cells per vessel area in the BM of control (rIgG) or neutrophil-depleted (1A8) mice. Values were obtained from the experiments illustrated in (A). n =4–5 mice.
(C) Relative number of donor-derived DsRed+ LSK cells that home into the BM of control (rIgG) or neutrophil-depleted (1A8) mice, as determined by flow cytometry. n=5–6 mice.
(D) DsRed+ BM-derived donor cells (red) home exclusively into areas of the BM enriched in CXCL12-producing cells (green; background green on the left corresponds to autofluorescent bone). Vessels and macrophages (white) are visualized with fluorescent dextran, and bone (blue) was imaged by second harmonic generation (See also Movie S1). Small panels on the left are shown merged on the right panel. The micrograph is representative of four Cxcl12-Gfp mice imaged by multiphoton microscopy. Scale bar, 100 μm.
(E) Mobilization of control (rIgG) or neutrophil-depleted mice (1A8) with AMD3100. n=5–6 mice.
Data are shown as mean ± s.e.m. *P<0.05, **P<0.01.
BM macrophages attract infiltrating neutrophils and are necessary for niche modulation
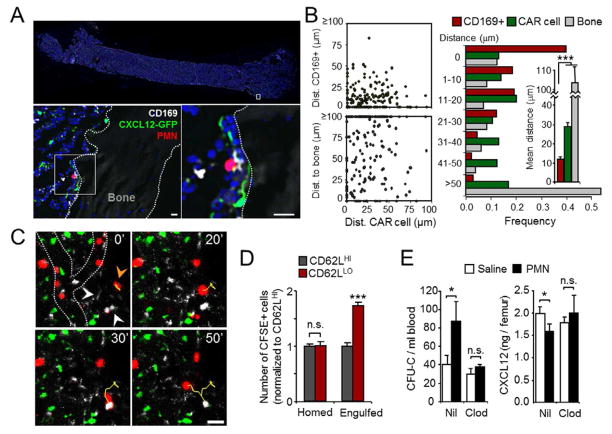
Since the parabiosis experiments (Figure 3D) suggested a requirement for neutrophil migration into the BM for niche modulation, we used high-resolution imaging to investigate possible mechanisms at work within this organ. We performed laser-scanning cytometry to image the localization of homed DsRed+ neutrophils within whole femoral sections of Cxcl12-Gfp recipient mice (Figure 5A). Both BM-derived senescent neutrophils or blood-derived CD62LLO neutrophils migrated to the vicinity of BM-resident macrophages, with 40% of neutrophils found in direct contact with CD169+ macrophages and 78% within 20 μm (Figure 5A–B). In contrast, we could not detect preferential migration near CAR cells or endosteal surfaces (Figure 5B). Multiphoton microscopy of the calvarial BM of live mice injected with DsRed+ neutrophils confirmed that homed neutrophils establish dynamic and frequent interactions with BM-resident macrophages but not CAR cells (Figures 5C and S4A, and Movie S2). This behavior suggested that aged neutrophils that home to the BM may be eventually engulfed by macrophages, which is consistent with previous reports (Furze and Rankin, 2008). Because the relatively low number of events in our sections did not allow us to unequivocally identify neutrophils engulfed by BM macrophages, we set up a flow cytometry-based assay to quantify this process. We transferred purified CD62LLO and CD62LHI neutrophils labeled with CFSE into wild-type mice and 4 hours later monitored the number of fluorescent cells in the Gr1+ F4/80NEG and Gr-1NEG F4/80HI fractions of the BM, which represent homed and macrophage-engulfed neutrophils, respectively (Figure S4B). About 8% of homed neutrophils were associated with macrophages, and 0.4% of BM macrophages had engulfed neutrophils. Notably, although both neutrophil subsets homed equally to the BM, CD62LLO cells were engulfed 73% more efficiently than CD62LHI neutrophils (Figure 5D), indicating preferential phagocytosis of aged neutrophils once they enter the BM. Additional experiments in parabiotic mice set between WT and LysM-GFP mice revealed physiological clearance of circulating neutrophils in the BM, and their engulfment by BM macrophages in the steady-state (Figure S4C).
Figure 5. Intramedullary interactions between macrophages and cleared neutrophils are required for niche modulation.
(A) Whole femoral sections from Cxcl12-Gfp mice injected with DsRed+ aged neutrophils. Scale bars, 10 μm.
(B) Plots showing distances between each homed neutrophil and bone surfaces, CD169+ macrophages or CAR cells, which are quantified in the horizontal bars. Data from 198 homed neutrophils from 3 experiments.
(C) Time-lapse imaging of DsRed+ aged neutrophils (red) in the BM of Cxcl12-Gfp mice, obtained by combined in vivo multiphoton and confocal microscopy (see also Movie S2). Yellow lines depict the trajectory over 50 minutes of one neutrophil (orange arrowhead at t=0) that sequentially moves towards two macrophages (white arrowheads at t=0) but not towards CAR cells (green). Dotted lines in the top left panel show the outline of blood vessels within this region. Scale bar, 10 μm.
(D) Competitive homing and engulfment of CFSE-labeled CD62LHI vs. CD62LLO neutrophils within the BM (see also Figure S4B). n=5 mice.
(E) Blood CFU-C and CXCL12 protein levels in the BM of control (Nil) or macrophage-depleted (Clod) mice, after treatment with saline or 106 neutrophils (see also Figure S4D). n=5 mice.
Data are shown as mean ± s.e.m. *P<0.05, **P<0.01, ***P<0.001. See also Figure S4.
The migration of aged neutrophils towards, and engulfment by resident macrophages raised the possibility that modulation of the BM niches took place through the activity of macrophages rather than by neutrophils acting directly on niche components. To test this possibility, we depleted macrophages by injection of clodronate-loaded liposomes followed by a 10 day recovery period. This regime allowed efficient depletion of macrophages in BM while preserving neutrophil and monocyte numbers (Figure S4D). Under these conditions, transfer of aged neutrophils failed to elicit changes in the levels of CXCL12 protein in BM or CFU-C numbers in blood (Figure 5E), thus confirming that macrophages are required for the niche-modulating effect of neutrophils.
Requirement of LXR receptors for niche modulation
LXR nuclear receptors have been identified as important mediators in the transcriptional modulation of macrophages that engulf apoptotic cells (A. Gonzalez et al., 2009; Hong et al., 2012). Within the BM, we detected expression of the LXR-encoding genes in stromal and CXCL12-producing cells and, among the hematopoietic cells tested, expression was predominant in macrophages (Figure S5A). To determine whether LXR activation might underlie niche modulation during neutrophil clearance, we first analyzed expression of LXR target genes in the BM during light-dark cycles. The transcript levels for Abca1 and Mertk, two LXR target genes involved in cholesterol efflux and phagocytic uptake (Parzy et al., 2009), respectively, showed fluctuations in antiphase with those of circulating neutrophils, with transcript levels increasing as neutrophils disappeared from blood (Figure 6A), and preceding the decline in Cxcl12 transcript levels in BM (Figure S5B). To determine whether LXR activation was sufficient to promote reductions in niche activity and egress of progenitors into blood, we treated mice with the synthetic LXR agonist GW3965 (Collins et al., 2002). This treatment resulted in reduced transcript levels of Cxcl12 and the expected increase in Abca1 transcripts in the BM, and an increase in progenitors in blood at ZT1 (Figure 6B) that was similar to that elicited by neutrophil transfer (Figure 3C). To unambiguously test the requirement of LXRs for the modulatory effect of neutrophils on the niche, we injected aged neutrophils into LXRαβ-deficient mice. Although niche-modulating macrophages were present in these mice (Figure S5C), neutrophil transfer failed to alter blood CFU-C or CXCL12 protein levels in the BM (Figure 6C). In addition, we noted that LXR-deficient mice showed a moderate elevation in the baseline number of circulating progenitors (Figure 6C), raising the possibility that mice with altered progenitor distribution were refractory to modulation through neutrophil clearance. However, mice deficient in Fut7, which display similarly elevated levels of circulating precursors, further increased the numbers of blood CFU-C when injected with wild-type neutrophils (Figure S5D), indicating that the impaired response in LXR-deficient mice was directly related to the absence of these receptors. Interestingly, expression of the LXR-target gene Abca1 was highest in macrophages among all BM cell populations tested (Figure S5E), and the expression of both Abca1 and Mertk was strongly modulated during neutrophil clearance in engulfing macrophages (Figure S5F–I), suggesting a role for macrophage-borne LXR in niche modulation upon neutrophil clearance.
Figure 6. Requirement of LXR receptors during niche modulation.
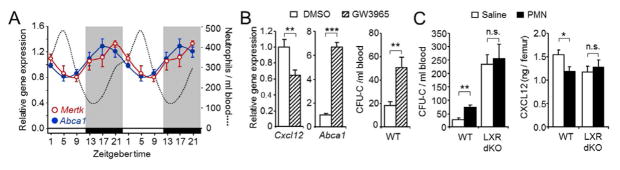
(A) Relative levels of Mertk and Abca1 gene expression at different Zeitgeber times of the day. For reference, the dotted line shows the levels of blood neutrophils. Shaded areas represent periods of darkness. n=4–9 mice per time.
(B) Levels of Cxcl12 and Abca1 transcripts in BM and number of blood CFU-C after treatment with the LXR agonist GW3965, or with vehicle control (DMSO). n=5 mice.
(C) Number of blood CFU-C and CXCL12 protein levels in the BM of WT or LXR-deficient mice (LXR dKO) after treatment with saline or 106 neutrophils. Samples were analyzed at ZT1. n=7–9 mice.
Data are shown as mean ± s.e.m. *P<0.05, **P<0.01, ***P<0.001. See also Figure S5.
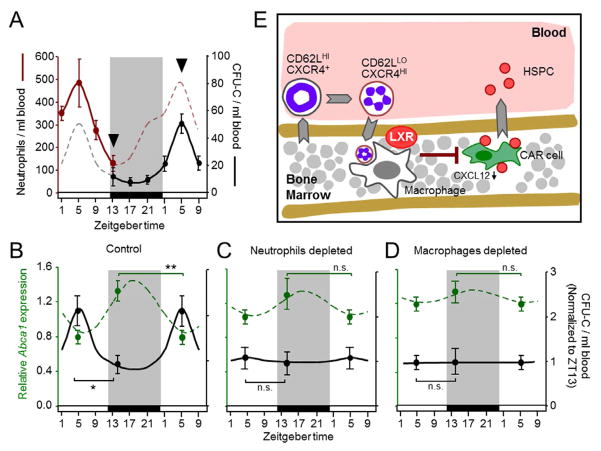
Neutrophil clearance triggers the homeostatic release of HPC
We finally investigated the context in which niche modulation through neutrophil clearance might be physiologically relevant. Given the moderate alterations in hematopoietic niche parameters and in progenitor distribution induced by neutrophil transfer or depletion, we predicted that homeostatic variations in these values, rather than those associated with inflammation, might be influenced by the physiological clearance of aged neutrophils. Particularly fitting with this prediction are the natural circadian variations in CXCL12 levels in the BM which correlate with the rhythmic egress of HSPC into blood (Mendez-Ferrer et al., 2008). Indeed, we found that the disappearance of neutrophils from blood preceded by ~8h the rise of CFU-C levels in the circulation (Figure 7A). To causally connect neutrophil clearance with homeostatic niche fluctuations and progenitor release, we measured changes in the transcription of LXR targets in the BM and the number of circulating progenitors at ZT5 and ZT13, in mice previously depleted of neutrophils or macrophages. Depletion of either cell population blunted the normal rhythms of LXR target gene expression (Abca1) in the BM and of progenitors in blood (Figure 7B–D), confirming that neutrophil clearance underlies the homeostatic rhythmicity in the hematopoietic niche and leads to progenitor release into the circulation.
Figure 7. Circadian changes in the hematopoietic niche and progenitor trafficking are triggered by neutrophil clearance.
(A) Circadian oscillations in the number of neutrophils and CFU-C in the blood of WT mice. Arrowheads highlight the times of highest and lowest levels used for further analyses. Shaded areas represent periods of darkness.
(B–D) Daily fluctuations in the levels of hematopoietic progenitors in blood (black) and Abca1 transcripts in BM (dashed green) at ZT5 and ZT13 in control mice (B), or in mice in which neutrophils (C) or macrophages (D) were depleted. The lines represent the circadian variations of both parameters over a 29h period. Note that the point at ZT5 is repeated for clarity. n=8–14 mice for CFU-C; n=5 for Abca1 transcripts.
(E) Scheme summarizing the sequence of events identified in this study. CD62LHI neutrophils are released into blood and age to become CD62LLO CXCR4HI. At specific times of the day, CD62LLO neutrophils migrate back to the BM where they are phagocytosed by macrophages. Activation of LXR receptors is additionally required to induce reductions in the capacity of the hematopoietic niche to retain HPC, which are released into the bloodstream. All events indicated by arrows occur with circadian periodicity.
Data are shown as mean ± s.e.m. *P<0.05, **P<0.01, ***P<0.001.
DISCUSSION
We have used a combination of surface markers to identify and track the kinetics of a population of aged neutrophils, which we define as those that have accumulated specific phenotypic changes while in the circulation of unperturbed mice, and that are prepared to be cleared from blood. The appearance and elimination from the circulation of these cells followed circadian cycles that preceded those occurring in the hematopoietic niche. Through imaging, pharmacological and genetic approaches we show that the circadian clearance of neutrophils within the BM cause reductions in the size and function of the hematopoietic niche and promote the release of HPC into the periphery (summarized in Figure 7E). Our findings reveal a mechanism by which the immune system sets rhythms in the hematopoietic niche that favor patrolling of peripheral tissues by HPC in the absence of injury.
Redefining neutrophil aging and clearance
Although essential to protect against pathogens, the detrimental role that neutrophils can have on vascular integrity explains the need to eliminate and renew the circulating pool of neutrophils with a high frequency. In theory, elimination of undesired cells may be accomplished by intrinsic changes within the cells, by changes in the environment, or both. Our data reveals that neutrophils undergo intrinsic changes during aging that include loss of CD62L and increase of CXCR4 on the cell surface (Figure 1A). While it is unlikely that reductions in CD62L are sufficient to trigger clearance (Van Eeden et al., 1997), increased levels of CXCR4 may facilitate migration into or within the BM. Using BM-derived neutrophils senesced in vitro, previous studies found time-dependent increases in surface CXCR4 that promoted their selective migration into BM (Martin et al., 2003). In contrast, we find that the increased levels of CXCR4 do not confer a BM-homing advantage of CD62LLO over CD62LHI neutrophils (Figure 5D). Once inside the BM, however, CD62LLO neutrophils migrated towards and are preferentially engulfed by macrophages (Figure 5C–D), which are a potential source of CXCL12 (Sanchez-Martin et al., 2011). Thus, although not explored here, age-associated changes in the repertoire of migratory receptors may program neutrophils for migration towards specific areas of phagocytosis rather than to specific tissues. In agreement with this possibility, we find a small but consistent reduction in the surface levels of CD47 in the CD62LLO population (Figure S1F), which may favor phagocytosis of aged neutrophils (Jaiswal et al., 2009). These findings argue that ex vivo senesced BM-derived neutrophils may not faithfully recapitulate the biology of those naturally aged in blood. Use of the simple combination of surface markers reported here to purify or track these cells should allow a better characterization of bona fide aged neutrophils. It is also noteworthy that the profile of CD62L and CD11b in aged neutrophils (Figure S1F) resembles that induced by activating stimuli (Kishimoto et al., 1989), suggesting that aging may be a form of programmed activation that uses signaling pathways common to those elicited during inflammation.
In addition to cell-intrinsic changes, clearance may be induced by environmental factors. This could be accomplished if the vasculature and tissues promoted leukocyte recruitment when the levels of these cells in blood are maximal (i.e., starting at ZT5). This possibility fits with the observation that the levels of homing receptors in the microvasculature display circadian increase from ZT5 to ZT13 and favor leukocyte migration at these times (Scheiermann et al., 2012). Thus, it is likely that both cell-intrinsic and environmental changes cooperate for the efficient clearance of neutrophils during homeostasis.
Neutrophil clearance as a regulator of the hematopoietic niche
In mice with disrupted neutrophil trafficking, transfer and subsequent phagocytosis of wild-type apoptotic neutrophils restores neutrophilia by curbing the production of G-CSF (Stark et al., 2005). Because G-CSF is a prominent HSPC-mobilizing cytokine (Papayannopoulou, 2004), we were initially surprised to find that transfer of aged or apoptotic neutrophils into wild-type animals increased the number of circulating progenitors (Figure 3C). A possible explanation for these unexpected results is the presence of subclinical inflammation in adhesion-deficient mice, likely originating from their susceptibility to recurrent infections (Frenette et al., 1996). In the context of our study, inflammation may cause important deviations from the steady-state which can influence the effect of neutrophils on the hematopoietic niche, including increased levels of mobilizing cytokines (Frenette et al., 1996; Stark et al., 2005), inhibition of neutrophil death (Dibbert et al., 1999), and changes in the migratory pattern of neutrophils (Suratt et al., 2001). Illustrating the changes in niche regulation between the steady-state and inflammation, we have found that while transfer of aged neutrophils into Fut7−/− mice maintained under specific pathogen-free barrier conditions caused increases in blood HPC (Figure S5D), the same treatment triggered reductions in mice housed under non-barrier conditions (M.C-A. and A.H., unpublished observations). In our experiments, the contribution of plasma G-CSF in the homeostatic release of HPC induced by neutrophil clearance was ruled out by the parabiosis experiments (Figures 3D and S3D), and by the inability of G-CSF blockade to alter the circadian pattern of circulating progenitors (data not shown). Thus, whereas systemic elevations in cytokines may trigger massive egress of HPC during inflammation, we propose that mobilizing stimuli are missing in the steady-state and that infiltration of aged neutrophils is sufficient to generate mild but physiologically relevant mobilizing signals within the BM.
We find that macrophages and LXRs are essential components for the modulation of the hematopoietic niche by cleared neutrophils (Figures 5E and 6C). Macrophages have been shown to support the function of the hematopoietic niche through the production of undefined protein factors (Chow et al., 2011; Winkler et al., 2010). Identification of these factors will be important to assess how engulfment of aged neutrophils by macrophages ultimately modulates this niche. Because LXRs mediate important transcriptional and functional changes after phagocytosis of apoptotic cells (A. Gonzalez et al., 2009), we predicted that engulfment of dying neutrophils might result in activation of these nuclear receptors in BM macrophages. Although we find that LXR target genes are robustly modulated in BM macrophages during neutrophil clearance (Figure S5F–H) and that macrophage depletion blunts the rhythmic oscillations of these genes in the BM (Figure 7D), we cannot exclude that LXR receptors in other BM populations are required for the circadian modulation of the hematopoietic niche. In addition, because myeloid-specific expression of cholesterol transporters regulates progenitor mobilization (Westerterp et al., 2012), it will be important to determine whether cholesterol efflux or other cellular processes controlled by LXRs are required for their niche-modulating functions.
Our data demonstrates that the circadian release of HPC in the steady-state is governed by signals originating upon neutrophil clearance. Importantly, physiological mobilization of progenitors also requires adrenergic signals provided by the sympathetic nervous system (Mendez-Ferrer et al., 2008). Although at present we do not know whether and how these signals may integrate, it is possible that they cooperate for the homeostatic mobilization of HPC. Since adrenergic signals have also been shown to modulate the expression of adhesion receptors in the BM microvasculature and to control the circadian influx of leukocytes into the BM (Scheiermann et al., 2012), it is conceivable that they promote neutrophil clearance and the ensuing downstream events reported here. In addition, because CXCL12-producing cells can be regulated by both signals, and recent studies have revealed that distinct Cxcl12-expressing stromal cells control the retention of HSC or HPC (Ding and Morrison, 2013; Greenbaum et al., 2013), it will be important to better define which specific niche cells are targeted during neutrophil clearance.
What might be the physiological significance of hematopoietic niche modulation by neutrophil clearance? Neutrophils are highly migratory leukocytes and are extremely sensitive to changes in the environment (e.g., trauma or infections), and respond to injury by extending their life-span and by losing tropism for the BM (Dibbert et al., 1999; Suratt et al., 2001). Therefore, an attractive possibility is that circulating neutrophils function as sensors of the organism’s status, such that normal migration of dying neutrophils into the BM reports that no injury has occurred. Even in these injury-free conditions, the need to maintain basal levels of extramedullary progenitors endowed with regenerative or immune-surveilling functions (Laird et al., 2008) could be satisfied through clearance-induced mobilization.
In a broader context, we propose that the massive numbers of neutrophils that are cleared every day modulate macrophage activity in tissues outside the BM. Thus, it will be important to understand whether, and how, aged neutrophils dictate the function of other specialized niches and fundamental homeostatic processes throughout the organism.
EXPERIMENTAL PROCEDURES
Mobilization assays
Mice were intravenously injected with the indicated doses and types of cells at ZT13. Blood and tissue samples were harvested at ZT1. For mobilization with AMD3100 (Tocris; Bristol, UK), mice were injected intraperitoneally with a dose of 2.5 mg/kg at ZT5 and samples harvested one hour later. For mobilization with the LXR agonist GW3965, mice were fasted (4 hours prior and during treatment with GW3965) and intraperitoneally injected at ZT13 with 10 mg/kg of the agonist dissolved in a 10% solution of DMSO in saline or vehicle alone, and blood and BM samples were collected at ZT1. For mobilization with clodronate, mice were injected with 250 μl of clodronate-loaded liposomes intravenously at ZT15, and blood was harvested 14 hours later (ZT5). Blood counts were obtained using an Abacus automated counter (Diatron; Holliston, USA).
Protocols for parabiosis experiments, long term reconstitution assays, flow cytometry, homing assays, immunofluorescence experiments, intravital imaging, chemokine quantification and other methods as well as additional details and are provided in the Extended Experimental Procedures section of the Supplemental Information.
Supplementary Material
HIGHLIGHTS.
Neutrophils undergo a process of aging during their lifetime in circulation.
Neutrophil clearance reduces the size and function of the hematopoietic niche.
Macrophages and LXR receptors mediate changes in the niche.
Neutrophil clearance triggers rhythmic release of progenitors during homeostasis.
Acknowledgments
We thank M. Nácher, J. Ogonek, I. Ortega, V. Zorita, M. Leboeuf, J.M. Ligos and the Cellomics, Microscopy and Comparative Medicine Units at CNIC for technical support; J. Garaude, D. Lucas and A. Ramiro for critically reviewing the manuscript; and S. González and D. Sanz for reagents. This study was supported by Deutsche Forschungsgemeinschaft (DFG WE1913/11-2 and SFB914-B8) to C.W.; R01-DK056638 and R01-HL69438 to P.S.F.; grants SAF2008-00057 and SAF2011-29244 to A.C.; Ramón y Cajal Fellowship (RYC-2007-00697) and SAF2009-11037 from MINECO, S2010/BMD-2314 from Comunidad de Madrid, and FP7-People-IRG Program (246655) to A.H.; C.N-A. is funded by a Human Frontiers in Science Program long-term fellowship 00194/2008. M.C-A. is supported by a FPI fellowship from the Spanish Ministry of Economy and Competitivity. The Centro Nacional de Investigaciones Cardiovasculares is supported by the Spanish Ministry of Economy and Competitivity and the Pro-CNIC Foundation. M.C-A performed experiments and wrote the manuscript; L.A.W., C.P. and N.A-G performed experiments and edited the manuscript. C.N-A., R.C., Y.K. and D.Z. performed experiments; N.V.R., L.E.S., C.W., T.N., P.S.F. and A.C. contributed reagents, discussed experiments and helped in editing the manuscript; A.H. conceived the study, performed experiments and wrote the manuscript.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Gonzalez AN, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.v100.3.854. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, et al. Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. J Med Chem. 2002;45:1963–1966. doi: 10.1021/jm0255116. [DOI] [PubMed] [Google Scholar]
- Dibbert B, Weber M, Nikolaizik WH, Vogt P, Schoni MH, Blaser K, Simon HU. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13330–13335. doi: 10.1073/pnas.96.23.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013 doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013 doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E, Lakatua DJ, Swoyer J, Sackett-Lundeen L. Chronobiology in hematology and immunology. Am J Anat. 1983;168:467–517. doi: 10.1002/aja.1001680406. [DOI] [PubMed] [Google Scholar]
- Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Hong C, Kidani Y, NAG, Phung T, Ito A, Rong X, Ericson K, Mikkola H, Beaven SW, Miller LS, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. The Journal of clinical investigation. 2012;122:337–347. doi: 10.1172/JCI58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews Immunology. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Luo HR, Loison F. Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol. 2008;83:288–295. doi: 10.1002/ajh.21078. [DOI] [PubMed] [Google Scholar]
- Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier FE, Ragu C, Scadden DT. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol. 2011;12:49–60. doi: 10.1038/nri3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004;103:1580–1585. doi: 10.1182/blood-2003-05-1595. [DOI] [PubMed] [Google Scholar]
- Parzy E, Miraux S, Franconi JM, Thiaudiere E. In vivo quantification of blood velocity in mouse carotid and pulmonary arteries by ECG-triggered 3D time-resolved magnetic resonance angiography. NMR Biomed. 2009;22:532–537. doi: 10.1002/nbm.1365. [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Sanchez-Martin L, Estecha A, Samaniego R, Sanchez-Ramon S, Vega MA, Sanchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88–97. doi: 10.1182/blood-2009-12-258186. [DOI] [PubMed] [Google Scholar]
- Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic Nerves Govern Circadian Leukocyte Recruitment to Tissues. Immunity. 2012 doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, Levesque JP, Chappel J, Ross FP, Link DC. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nature immunology. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L913–921. doi: 10.1152/ajplung.2001.281.4.L913. [DOI] [PubMed] [Google Scholar]
- Van Eeden SF, Bicknell S, Walker BA, Hogg JC. Polymorphonuclear leukocytes L-selectin expression decreases as they age in circulation. Am J Physiol. 1997;272:H401–408. doi: 10.1152/ajpheart.1997.272.1.H401. [DOI] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. Journal of immunology. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.