ABSTRACT
Staphylococcus aureus thymidine-dependent small-colony variants (TD-SCVs) are frequently isolated from patients with chronic S. aureus infections after long-term treatment with trimethoprim-sulfamethoxazole (TMP-SMX). While it has been shown that TD-SCVs were associated with mutations in thymidylate synthase (TS; thyA), the impact of such mutations on protein function is lacking. In this study, we showed that mutations in thyA were leading to inactivity of TS proteins, and TS inactivity led to tremendous impact on S. aureus physiology and virulence. Whole DNA microarray analysis of the constructed ΔthyA mutant identified severe alterations compared to the wild type. Important virulence regulators (agr, arlRS, sarA) and major virulence determinants (hla, hlb, sspAB, and geh) were downregulated, while genes important for colonization (fnbA, fnbB, spa, clfB, sdrC, and sdrD) were upregulated. The expression of genes involved in pyrimidine and purine metabolism and nucleotide interconversion changed significantly. NupC was identified as a major nucleoside transporter, which supported growth of the mutant during TMP-SMX exposure by uptake of extracellular thymidine. The ΔthyA mutant was strongly attenuated in virulence models, including a Caenorhabditis elegans killing model and an acute pneumonia mouse model. This study identified inactivation of TS as the molecular basis of clinical TD-SCV and showed that thyA activity has a major role for S. aureus virulence and physiology.
IMPORTANCE
Thymidine-dependent small-colony variants (TD-SCVs) of Staphylococcus aureus carry mutations in the thymidylate synthase (TS) gene (thyA) responsible for de novo synthesis of thymidylate, which is essential for DNA synthesis. TD-SCVs have been isolated from patients treated for long periods with trimethoprim-sulfamethoxazole (TMP-SMX) and are associated with chronic and recurrent infections. In the era of community-associated methicillin-resistant S. aureus, the therapeutic use of TMP-SMX is increasing. Today, the emergence of TD-SCVs is still underestimated due to misidentification in the diagnostic laboratory. This study showed for the first time that mutational inactivation of TS is the molecular basis for the TD-SCV phenotype and that TS inactivation has a strong impact on S. aureus virulence and physiology. Our study helps to understand the clinical nature of TD-SCVs, which emerge frequently once patients are treated with TMP-SMX.
INTRODUCTION
Staphylococcus aureus is a major human pathogen that causes a variety of community-acquired and nosocomial diseases ranging from mild infections, such as skin and soft tissue infections, to life-threatening infections, such as endocarditis, pneumonia, osteomyelitis, and sepsis (1, 2). The emergence of antibiotic-resistant strains (e.g., methicillin [MRSA]- and vancomycin [VRSA]-resistant S. aureus) and especially the rise of community-acquired MRSA (CA-MRSA) and livestock-associated MRSA (LA-MRSA) in the healthy population pose major challenges for the treatment of S. aureus infections (3, 4). In contrast to acute life-threatening infections, S. aureus can also cause highly persistent recurrent infections despite appropriate antibiotic treatment and susceptibility of the pathogen (5). Such persistent S. aureus infections have been associated with a specialized phenotype of S. aureus, the small-colony variants (SCVs) (6–8). Due to their phenotypical characteristics, including slow growth, changed metabolism, and altered expression of virulence factors, SCVs are optimized for long-term persistence and survival within eukaryotic cells, where they are protected against antibiotic therapy and host defense (6, 7, 9–11). The molecular basis for the formation of some clinical SCVs has been elucidated (12). Many clinical S. aureus SCVs have been characterized to be deficient in electron transport (hemin and menadione dependent) (13–15) or to be thymidine dependent (16–18). Also, defects in the stringent stress response with mutations in relA have been reported (19), and phenotype-specific small nonprotein coding RNAs have been described (20). Staphylococcal SCVs have been characterized as a less aggressive and less virulent phenotype with increased cellular adhesion, invasion, and intracellular persistence capacity in cell culture experiments and in animal studies (13, 21). However, most clinical SCV phenotypes are not stable and easily revert back to the normal phenotype (22, 23).
Thymidine-dependent SCVs (TD-SCVs) have been recovered from patients with chronic infections, such as soft tissue infection, bronchitis, peritonitis, and septicemia, and in particular with high prevalence from the airways of cystic fibrosis (CF) patients, often in combination with an isogenic normal phenotype, if the patients were treated with trimethoprim-sulfamethoxazole (TMP-SMX) for extended periods (7, 24, 25). Just recently, it has been shown by Wolter et al. that SCVs were frequently detected in children with CF (24%) and that most of these SCVs (95%) were TD-SCVs. Moreover, the occurrence of TD-SCVs was significantly associated with a worse respiratory function in these children, indicating an important role of TD-SCVs in the pathogenesis of CF lung disease (18). Clinical S. aureus TD-SCVs have been characterized by gross morphological changes, impaired cell separation, and altered transcription patterns for several genes, including important metabolism and virulence genes as well as virulence regulators (26–28). TD-SCVs grow unaffected in the presence of TMP-SMX if extracellular thymidine is available, which is present in purulent airway secretions and infected tissues (29).
TMP-SMX affects the folate pathway by competitive inhibition of dihydropteroate synthase and dihydrofolate reductase, two proteins involved in the synthesis and conversion of tetrahydrofolic acid (THF), which acts as a cofactor for thymidylate synthase (TS; thyA). TS is essential for de novo thymidylate biosynthesis (16, 17) and therefore required for DNA synthesis and bacterial replication. We and others have shown that thymidine dependency of S. aureus SCVs is most probably caused by mutations in thyA (16, 17). Until now, all so-far-characterized clinical TD-SCVs carried nonsynonymous mutations in thyA. However, functional analysis of TS and experimental proof of the association of TMP-SMX to these mutations are still lacking.
In the present work, we showed for the first time that TS proteins of clinical TD-SCVs are inactive due to various mutations in thyA. Site-directed mutants constructed in S. aureus SH1000 and S. aureus JE2 (USA300 background) were strongly altered in morphology and physiology and were significantly attenuated in virulence. We identified NupC as the major transporter of S. aureus for uptake of thymidine into the cell. thyA deletion was associated with decreased virulence in vivo using both an acute mouse pneumonia model and a Caenorhabditis elegans virulence assay.
RESULTS
Clinical TD-SCVs lack thymidylate synthase activity.
thyA is a highly conserved gene in all species and plays a central role in the de novo DNA synthesis (30). In S. aureus, thyA occurs only in two different alleles characterized by A to C exchange at nucleotide position 300, coding either for lysine (as in S. aureus strains COL and 8325-4) or asparagine (as in S. aureus strains MW2 and MU50) at amino acid position 100 in 77 online-available thyA sequences of S. aureus genomes (data not shown). To assess the impact of thyA mutations on the activity of the mature protein, we studied 7 clinical strain pairs comprising S. aureus TD-SCVs and their isogenic normal phenotypes, which were isolated in parallel from the airways of individual CF patients, who were persistently colonized and infected by S. aureus for several years (7). First, we sequenced thyA of these clinical strain pairs to confirm mutations (Table 1). Next, we amplified thyA of these strains, cloned the genes into the expression vector pQE30-Xa, transformed Escherichia coli with this vector, and expressed and purified the proteins (data not shown). Native isolation of mutated proteins was possible for full-length proteins, whereas native isolation of short truncated proteins failed (Table 1). We performed a spectrophotometric thymidylate synthase (TS) activity assay (31) with TS of all purified proteins, including TS of S. aureus 8325-4 and MU50 as a control. In the TS assay, mutated proteins of TD-SCVs did not show any TS activity, while TS of all normal S. aureus strains revealed activities comparable to the control strains 8325-4 and MU50 (Table 1).
TABLE 1 .
Pheno- and genotypical characterization of clinical TD-SCVs and normal strainsd
| Strain | TD | Alteration(s) in nt sequencea | Predicted alteration(s) | Activity of TS (U/mg) |
|---|---|---|---|---|
| 8325-4b | No | Yes (0.3351 ± 0.0186) | ||
| Mu50c | No | Yes (0.3209 ± 0.0314) | ||
| Normal-1c | No | Yes (0.3167 ± 0.0108) | ||
| SCV-1c | Yes | Δ591ACTTCCGCCTT601 | 11-bp deletion → frame shift → stop (aa 229) | NP |
| Normal-2b | No | A914G | PM: Asp → Gly (aa 305)e | Yes (0.3629 ± 0.0570) |
| SCV-2b | Yes | Δ51A | 1-bp deletion → frame shift → stop (aa 19) | NP |
| Normal-3b | No | Yes (0.1978 ± 0.0228) | ||
| SCV-3b | Yes | Δ51A | 1-bp deletion → frame shift → stop (aa 19) | NP |
| Normal-4b | No | G940A | Ala → Thr (aa 314) | Yes (0.3035 ±0.0103) |
| SCV-4b | Yes | Δ247AAT249 | 3-bp deletion (aa 83) → in frame | No |
| Normal-5b | No | G563A, G564T | Trp → Tyr (aa 188) | Yes (0.1665 ± 0.002) |
| SCV-5b | Yes | G563A, T564G | Trp → stop (aa 188) | NP |
| Normal-6b | No | Yes (0.3560 ± 0.0131) | ||
| SCV-6b | Yes | C941A | Ala → Asp (aa 314) | No |
| Normal-7b | No | Yes (0.3594 ± 0.0545) | ||
| SCV-7b | Yes | C705A | Ser → Arg (aa 235) | No |
| Newman WTb | No | ND | ||
| Newman SCVb | Yes | G748T | Glu → stop (aa 249) | NP |
Only nonsynonomous mutations of thyA are shown, which cause changes in the amino acid sequence of TS.
Allele 1: at nucleotide position 300A; at aa position 100Lys.
Allele 2: at nucleotide position 300C; at aa position 100Asn.
NP, native purification not possible; ND, not done, only thyA sequenced; aa, amino acid; WT, wild type.
PM, point mutation.
ΔthyA mutants exhibited typical features of clinical TD-SCVs.
To study the impact of thyA inactivation in detail, we constructed site-directed ΔthyA deletion mutants in the well-characterized S. aureus strain SH1000 (32) and in S. aureus JE2 (belonging to the clinically important USA300 background), in which thyA was replaced by the erythromycin resistance cassette ermB. The SH1000 ΔthyA mutant was not able to grow in or on thymidine-free media (Mueller-Hinton [MH] agar and/or thymidine-free chemical-defined medium [CDM]) and displayed small, nonpigmented, nonhemolytic colonies on Columbia blood agar compared to those of the wild type and the complemented mutant (Fig. 1A-C, row I). Also, pleomorphic cocci in Gram staining and enlarged cocci with incomplete or multiple cross walls in transmission electron microscopy (TEM) were visible in the mutant (Fig. 1B, row III-IV), in contrast to homogeneous cocci of the wild type with regular cross walls in TEM (Fig. 1A, row III-IV). In the complemented mutant, which expressed wild-type thyA constitutively on a plasmid, all these features were restored, leading to the wild-type phenotype (Fig. 1C, row I-IV). Standard susceptibility testing (E test, agar diffusion) according to CLSI guidelines (33) could not be performed on MH agar due to the lack of thymidine and consequently no growth of the mutant. Therefore, Columbia blood agar was used, on which TMP-SMX did not inhibit the growth of the ΔthyA mutant, whereas the wild type and the complemented mutant showed clear inhibition zones around the disks, although within the inhibition zone tiny SCV-like colonies appeared (Fig. 1, row II). Applying CLSI guidelines for susceptibility testing, the mutant has to be considered resistant, whereas the wild type and the complemented mutant have to be considered susceptible, although all strains showed similar tiny colonies around the disk on Columbia blood agar. Tested on MH agar, the wild type and complemented mutant were TMP-SMX susceptible (MIC of 0.064 µg/ml for both strains). All the described features of the SH1000 ΔthyA mutant and the complemented mutant were also obtained with the JE2 ΔthyA mutant (data not shown).
FIG 1 .
Characteristics and phenotypes of S. aureus SH1000 wild type, ΔthyA mutant, and complemented mutant. Phenotypes of the wild-type strain SH1000 (A), the ΔthyA mutant (B), and the complemented mutant (C) are shown on Columbia blood agar (row I). Susceptibility testing for TMP-SMX was performed on Columbia blood agar (row II). Light microscopy (row III) and transmission electron microscopy (row IV) revealed the typical features of the respective strains.
thyA inactivation caused dramatic changes in growth characteristics apparent after 10 h of incubation in brain heart infusion (BHI) broth, revealing an extended lag phase which was about 3 to 4 h longer than that for the wild type and a significantly reduced final cell density (Fig. 2a). Thus, the generation time of the ΔthyA mutant (98.5 min) during exponential growth phase was about four times longer than that of the wild type (25.2 min). Complementation with an intact thyA (generation time of 30.8 min) and supplementation with thymidine (generation time of 30.7 min) restored the growth defects of the ΔthyA mutant almost to wild-type levels.
FIG 2 .
Growth phase analysis and cocci size of SH1000 wild type, ΔthyA mutant, and complemented mutant. (a) Growth phase analysis. The strains were cultured at 37°C in BHI at 160 rpm in baffled flasks (50 ml cultures in 500-ml flasks). Samples were taken every hour to determine the optical density. For the mutant, growth analysis was also performed in BHI supplemented with 100 µg/ml thymidine. ●, SH1000, WT; ▲, ΔthyA-C, complemented mutant; ■, ΔthyA mutant and thymidine; ♦, ΔthyA mutant. RNA isolation time points for DNA microarray analysis are indicated by black arrows. (b) Size of individual cocci. Five hundred cocci for every phenotype were measured after Gram staining by light microscopy of overnight cultures in BHI and for the mutant also in BHI supplemented with 100 µg/ml thymidine. ●, SH1000, WT; ▲, ΔthyA-C, complemented mutant; ■, ΔthyA mutant and thymidine; ♦, ΔthyA mutant.
Based on Gram stainings (Fig. 1, row III) and verified by TEM (Fig. 1, row IV), cocci of the mutant were markedly enlarged compared to those of the wild-type strain and ranged from 0.7 to 2.3 µm in diameter, with a median diameter of 1.2 µm, corresponding to approximately 3 times the volume of the wild-type cocci, which ranged from 0.6 to 1.2 µm, with a median of 0.8 µm (Fig. 2b). The size of the complemented mutant cocci (0.5 to 1.3 µm, median of 0.8 µm) and the ΔthyA mutant cocci grown in thymidine-rich medium (0.5 to 1.3 µm, median of 0.9 µm) nearly returned to the size of the wild-type cocci (Fig. 2b).
Transcriptional analysis of the ΔthyA mutant revealed dramatic effects on S. aureus global regulation, metabolism, virulence, and stress response.
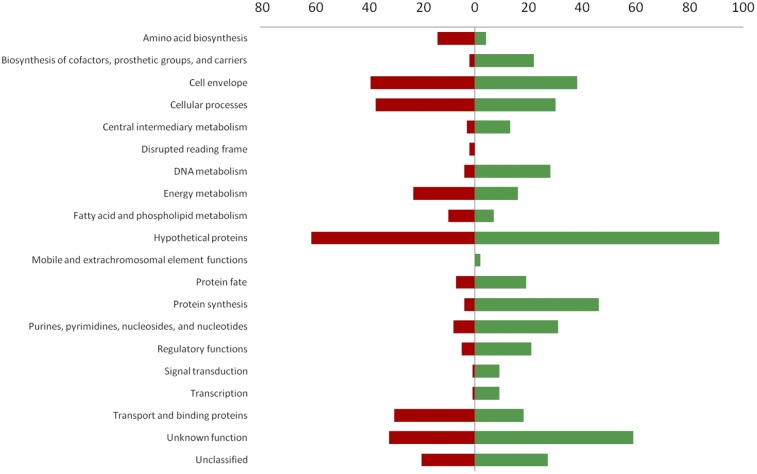
To investigate the molecular effects of thyA mutations on S. aureus physiology, the SH1000 ΔthyA mutant and the corresponding wild-type strain were subjected to whole-genome transcriptional analysis using DNA microarray technology (34). Using stringent statistical filters (fold change of ±2.5 and P value of <0.05), we identified 50, 793, and 647 genes to be significantly regulated at the early exponential, late exponential, and stationary phase of growth, respectively. The most differences became apparent at late exponential phase of growth, in which 490 genes were upregulated, while 303 genes were downregulated (Fig. 3 and Table 2; see also Table S1 in the supplemental material).
FIG 3 .
Differentially regulated genes in SH1000 compared to ΔthyA SH1000. Transcriptional changes were grouped according to the comprehensive microbial resource (CMR) database of the Craig Venter Institute (CVI). Statistical filters (fold change of ±2.5 and P value of <0.05). Transcriptional changes between SH1000 wild type and ΔthyA SH1000 at late exponential phase of growth were analyzed. Red bars indicate downregulation of genes (a total of 303), and green bars indicate upregulation of genes (a total of 490) in the ΔthyA mutant.
TABLE 2 .
Genes of the purine and pyrimidine metabolism found to be differentially regulated in the ΔthyA mutant compared to SH1000
| Regulation in the ΔthyA mutant | Gene ID | Gene name | Gene annotation | Fold change (ΔthyA mutant versus wild type)a |
Category | |
|---|---|---|---|---|---|---|
| Late exponential phase | Stationary phase | |||||
| Downregulated | SACOL1078 | purL | Phosphoribosylformylglycinamidine synthase II | −23.95 | Purine ribonucleotide biosynthesis | |
| SACOL1079 | purF | Amidophosphoribosyltransferase | −20.85 | |||
| SACOL1080 | purM | Phosphoribosylformylglycinamidine cyclo-ligase | −20.73 | |||
| SACOL1082 | purH | Phosphoribosylaminoimidazolecarboxamide formyltransferase/IMP cyclohydrolase | −11.94 | |||
| SACOL1083 | purD | Phosphoribosylamine—glycine ligase | −10.95 | |||
| SACOL1081 | purN | Phosphoribosylglycinamide formyltransferase | −17.54 | |||
| Upregulated | SACOL0461 | guaA | GMP synthase | 2.57 | Purine ribonucleotide biosynthesis | |
| SACOL1212 | pyrB | Aspartate carbamoyltransferase | 10.77 | 19.03 | Pyrimidine ribonucleotide biosynthesis | |
| SACOL1213 | pyrC | Dihydro-orotase | 9.08 | 15.02 | ||
| SACOL1214 | carA | Carbamoyl-phosphate synthase, small subunit | 10.34 | 15.06 | ||
| SACOL1215 | carB | Carbamoyl-phosphate synthase, large subunit | 9.75 | 11.63 | ||
| SACOL1216 | pyrF | Orotidine 5-phosphate decarboxylase | 8.06 | 7.74 | ||
| SACOL1217 | pyrE | Orotate phosphoribosyltransferase | 9.70 | 7.65 | ||
| SACOL2119 | pyrG | CTP synthase | 12.31 | 6.20 | ||
| SACOL2606 | pyrD | Dihydroorotate dehydrogenase | 8.73 | 11.92 | ||
| SACOL0524 | tmk | Thymidylate kinase | 3.30 | Nucleotide and nucleoside interconversions | ||
| SACOL0603 | Deoxynucleoside kinase family protein | 7.46 | ||||
| SACOL0604 | Deoxynucleoside kinase family protein | 6.68 | ||||
| SACOL1277 | pyrH | Uridylate kinase | 4.84 | |||
| SACOL1509 | ndk | Nucleoside diphosphate kinase | 3.77 | |||
| SACOL1518 | cmk | Cytidylate kinase | 2.96 | 2.80 | ||
| SACOL2111 | tdk | Thymidine kinase | 4.75 | 3.29 | ||
| SACOL0566 | nupC | Nucleoside permease NupC | 6.88 | 5.14 | Nucleosides, purines, and pyrimidines | |
| SACOL1211 | uraA | Uracil permease | 16.43 | 17.81 | ||
All values shown were filtered to be statistically significant with P values of <0.05 and regulated ±2.5.
The array data indicated large differences in global S. aureus regulatory systems, such as the agr (35) and arlRS regulons (36), both members of two-component signal transduction systems, the sarA family of global regulators (37), as well as sigB-dependent regulatory pathways (38), which have been described to be the major determinants of virulence factor regulation in S. aureus. The ΔthyA mutant showed strong downregulation of all components of the major quorum-sensing system (agr), which activates gene expression of extracellular proteins, while it represses the synthesis of cell wall-associated proteins (39). The genes agrA to agrD as well as the major effector molecule RNAIII (hld) were strongly downregulated, indicating inactivity of this system (see Table S1). Accordingly, the genes of the cell wall-associated proteins, including fibronectin-binding proteins A and B (fnbA/B), coagulase (coa), protein A (spa), clumping factor B (clfB), Sdr proteins (sdrC, sdrD), and Sas proteins (sasG, sasH, sasA, sasD) (40, 41), which have been implicated in the colonization capacity of S. aureus, were upregulated, whereas genes of secreted proteins such as alpha-toxin (hla), β-toxin (hlb), serine protease (sspA), and cysteine protease (sspB), as well as the gene encoding lipase (geh), were downregulated (see Table S1).
arlRS, which together with agr regulates capsule expression (42), was also less transcribed in the mutant, which is in line with downregulation of the entire capsule cluster (see Table S1). Furthermore, arlRS also regulates genes involved in autolysis, cell growth, and virulence (36) and has been shown to positively regulate lytRS, a regulator involved in cell growth and cell division (43). However, in the ΔthyA mutant, lytR was upregulated, indicating regulation independent of arlRS. Consistent with this, we found the lytRS-dependent genes lrgA/B and lytM upregulated in the ΔthyA mutant. Since lrgA and lrgB inhibit murein hydrolase activity (44), increased transcription of these genes together with decreased transcription of arlRS could contribute to the impaired cell division in the mutant. In addition and corresponding to the morphological picture of the mutant, genes involved in cell wall growth and division were differentially regulated. isaA and sceD, both described as putative lytic transglycosylases, were upregulated (45), while atl, a peptidoglycan hydrolase (46), was downregulated in the ΔthyA mutant compared to in the wild-type strain (see Table S1).
Five members of the marR family transcriptional regulators were found to be differentially regulated in the ΔthyA mutant. sarS, sarT, sarV, and sarX were upregulated, while sarA was downregulated (47–50). This observed pattern is consistent with the literature, where sarA has been described to repress sarS, sarT, and sarV (51). All these regulators modulate staphylococcal virulence genes directly and/or indirectly.
Another characteristic of clinical TD-SCVs and of the ΔthyA mutant is a decreased pigmentation compared to that of the wild type. In line with this phenotype, the genes crtN/M, which are involved in carotenoid biosynthesis, were downregulated in the mutant (52).
As de novo thymidylate synthesis is blocked in the ΔthyA mutant, we expected major differences in the pyrimidine/purine metabolic pathways compared to those of the wild-type strain. All genes involved in the anabolic pathway of thymidylate, including carA, carB, pyrB, pyrC, pyrD, pyrE, pyrF pyrG, nrd, ndk, and tmk, were particularly upregulated, which indicates a possible lack of end product repression and an attempt of the cell to compensate thymidylate starvation by increased synthesis of precursors (Table 2, Fig. 4).
FIG 4 .
S. aureus pyrimidine metabolic pathway and the transcriptional activities of respective genes in the ΔthyA mutant. Upregulated genes are labeled in green, while unregulated genes are labeled in black. (I) Pyrimidine ribonucleotide biosynthesis pathway; (II) salvage pathways of nucleotides; (III) external sources of nucleosides. Gene identifier and gene names were reported according to S. aureus COL, accession number NC_002951.
In line with this, we observed a strong upregulation of genes involved in uptake and conversion of uracil, a precursor of thymidine. Transcription of uraA, encoding a uracil permease, deoD1, encoding a purine nucleoside phosphorylase, and udk, a uridine kinase, was upregulated in the mutant (Table 2, Fig. 4).
Additionally, we noticed that genes involved in DNA mismatch repair, stress, and SOS response were differentially regulated in the ΔthyA mutant compared to in the wild type, most likely due to thymidylate starvation. The mismatch repair-related genes xseA and xseB, encoding exodeoxyribonuclease VII small and large subunits, pcrA, an ATP-dependent DNA helicase, and recJ, a single-stranded-DNA-specific exonuclease, were upregulated in the mutant. In addition, the sbcCD locus (53), which was described to not only be involved in repression of capsule production (also shown in the ΔthyA mutant) but also function as part of the SOS response in S. aureus, was upregulated. Consistently, the stress response-related chaperone genes groEL and groES were upregulated (54).
To verify the microarray results and to investigate a subset of genes found to be regulated in the ΔthyA mutant in detail under different conditions, we performed quantitative real-time PCRs (qRT-PCR). We analyzed the expression of nupC, carA, pyrB, purl, and hld in JE2 (USA300 wild type), JE2 ΔthyA mutant, JE2 ΔthyA-C mutant (complemented mutant), and JE2 ΔthyA-Cempty mutant (empty control vector) under four different conditions, including BHI, BHI with thymidine, BHI with TMP-SMX, and BHI with thymidine and TMP-SMX (Fig. 5 and 8b). In line with the microarray results, we found upregulation of nupC, carA, and pyrB and downregulation of purL in the ΔthyA mutant. Similar expression patterns were found in the wild type and the complemented mutant under TMP-SMX challenge, indicating the induction of the SCV phenotype. The addition of thymidine restored the expression levels of all investigated genes almost back to wild-type level (Fig. 5 and 8b).
FIG 5 .
Transcriptional analysis of nupC, carA, pyrB, and purL under different conditions. Wild type (JE2), ΔthyA mutant (JE2 ΔthyA mutant), ΔthyA-C mutant (JE2 ΔthyA pNXR thyA complemented mutant), and ΔthyA-Cempty mutant (JE2 ΔthyA complemented with empty vector pNXR100) were analyzed under different conditions: in BHI, in BHI and thymidine, in BHI and TMP-SMX, and in BHI, TMP-SMX, and thymidine. Quantification of expression of nupC, carA, pyrB, and purL in late exponential growth phase. The data (means ± standard errors of the means [SEM]) were normalized using three internal control genes (gmk, aroE, gyrB) and expressed as n-fold expression relative to the values of the wild-type phenotype. The analysis is based on 3 independent biological replicates analyzed in triplicate.
FIG 8 .
Cytotoxicity and virulence gene regulation under different conditions. Wild type (JE2), ΔthyA mutant (JE2 ΔthyA mutant), ΔthyA-C mutant (JE2 ΔthyA pNXR thyA complemented mutant), and ΔthyA-Cempty (JE2 ΔthyA complemented with empty vector pNXR100) were analyzed under different conditions: in BHI, in BHI and thymidine, in BHI and TMP-SMX, and in BHI, TMP-SMX, and thymidine. (a) The supernatant of the respective strains was used to challenge A549 cells. LDH release was determined to assess cytotoxicity. The data (means ± standard deviations [SD]) were normalized against the positive control (completely lysed cells) and were performed in 3 biological triplicates. *** indicate statistical significant differences (P < 0.0001) tested by unpaired t test. (b) Quantification of expression of hld in late exponential growth phase. The data (means ± SEM) were normalized using three internal control genes (gmk, aroE, gyrB) and presented as n-fold expression relative to the values of the wild-type phenotype. The analysis is based on 3 independent biological replicates analyzed in triplicate.
nupC is responsible for uptake of extracellular thymidine.
Three putative nucleoside transporters were identified in the S. aureus genomes (SACOL0310/0566/0701) (55). Although the function as nucleoside transporters has not been proven experimentally for S. aureus, we assumed that SACOL0566 functions as the primary pyrimidine transporter in S. aureus due to its high homology to nupC from Bacillus subtilis (55). In the microarray analysis, there was a strong upregulation of SACOL0566 (nupC), while the SACOL0310 and SACOL0701 genes were unaffected.
To confirm the biological function of nupC as a pyrimidine transporter, which is responsible for the uptake of extracellular thymidine, we tested a nupC mutant for its ability to use external thymidine for growth under TMP-SMX challenge (56). As expected, the wild-type strain was able to use external thymidine in a disk diffusion experiment, revealing tiny SCV-like colonies around the TMP-SMX disk (Fig. 6a). In contrast, the nupC mutant did not take up external thymidine and was not able to grow around the disk, revealing a clear inhibition zone (Fig. 6b). These results corroborated our hypothesis that nupC is the major transporter for external thymidine and that this transporter is required to bypass the effects of TMP-SMX upon de novo thymidylate biosynthesis in S. aureus.
FIG 6 .

S. aureus wild type (a) and nupC mutant (b) challenged with TMP-SMX in an agar disk diffusion experiment. The black arrow indicates the inhibition zone with tiny SCV-like colonies. Disk with 25 µg TMP-SMX were placed on a Columbia blood agar plate, and respective isolates were incubated for 24 h at 37°C. JE2 (S. aureus USA300 wild type) and NE544 (S. aureus nupC mutant of JE2) were kindly obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program.
S. aureus thyA inactivation caused an attenuated virulence in vivo.
To study the virulence of the SH1000 ΔthyA mutant compared to that of its corresponding wild-type strain and its complemented mutant, we used two different infection models. In the Caenorhabditis elegans killing model, most worms were killed by the wild type and the complemented mutant after 48 h, while the mutant was significantly attenuated and did not kill the worms (Fig. 7a).
FIG 7 .
Virulence of the SH1000 wild type, the ΔthyA mutant, and the complemented mutant. (a) Caenorhabditis elegans infection model. The wild type, ΔthyA mutant, and complemented mutant (ΔthyA-C) were streaked on TA plates, and C. elegans was placed on the plates to assess virulence of the strains by enumeration of surviving nematodes after 24 h, 48 h, 72 h, and 96 h. (b) Acute murine pneumonia model. Cumulative survival rate after infection of C57Bl/6 mice with S. aureus. Mice were infected with 5 × 108 CFU/lung with the SH1000 wild type, the ΔthyA mutant, and the complemented mutant and monitored for mortality over 1 week. Mortality of mice infected with the wild type was significantly greater than that for the ΔthyA mutant and the complemented mutant. Significant differences were detected also between wild type and complemented mutant. The asterisks indicate statistical significance by log rank (Mantel-Cox) test. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (n = 9 mice). (c) Murine lung histology and localization of S. aureus. Mice were infected with 5 × 108 CFU/lung with the SH1000 wild type, the ΔthyA mutant, and the complemented mutant (ΔthyA-C). Lungs recovered after 4 h (A to C, F to H) or 24 h (D, E, I, J) postinfection. The lungs were stained with H&E (A to E) or with a specific antibody against S. aureus strains (red). Counterstaining was performed with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Bars in panels A to E, 200 µm (top) and 50 µm (bottom); bars in panels F to J, 100 µm (top) and 25 µm (bottom).
Furthermore, in an acute murine pneumonia model, mice infected with the wild-type strain were killed within 12 h after challenge, while the survival rate of mice infected with the mutant was significantly longer, with mice killed 84 h postinfection (Fig. 7b). Mice infected with the complemented mutant showed an intermediate survival of 48 h. After 4 h of challenge, histology of the infected lungs revealed that mice infected with the ΔthyA mutant (Fig. 7c) exhibited less severe lesions, hemorrhagic patches, and leukocyte infiltration than mice infected with the wild type and the complemented mutant. After 24 h of survival, the histopathology of the tissue infected with the ΔthyA mutant was less severely affected compared to the tissue infected with the complemented mutant. Immunofluorescence staining revealed that S. aureus was localized mainly in the alveolar septa as single cells for the ΔthyA mutant compared to the complemented mutant (Fig. 7c, panel J), where numerous stained bacteria were visible.
To corroborate the results of the in vivo animal models, we performed an in vitro cytotoxicity assay. Furthermore, we investigated also the impact of different medium composition on the virulence phenotype of S. aureus JE2, JE2 ΔthyA mutant, JE2 ΔthyA-C mutant, and JE2 ΔthyA-Cempty mutant. Lactate dehydrogenase (LDH) release experiments with respiratory epithelial cells (A549) using culture supernatants (SN) of the strains revealed, as expected, a high cytotoxic effect of the wild-type SN after growth in BHI and in BHI with thymidine, while challenge of TMP-SMX significantly decreased cytotoxicity (Fig. 8a). The addition of thymidine almost restored the cytotoxicity in the wild type challenged with TMP-SMX. The same results were obtained with the complemented mutant (Fig. 8a). Both mutants, the JE2 ΔthyA mutant and the JE2 ΔthyA-Cempty mutant complemented with an empty vector, were significantly attenuated in BHI. However, cytotoxicity was restored in both mutants if thymidine was added to the medium with and without TMP-SMX challenge (Fig. 8a). Corresponding to the reduced cytotoxicity of the ΔthyA mutant and the wild type under TMP-SMX challenge, hld expression was found to be significantly reduced (Fig. 8b).
DISCUSSION
For more than a decade, it has been shown that the emergence of S. aureus TD-SCVs is associated with prolonged TMP-SMX treatment (7, 18, 25). Mutations in thyA were identified as a possible cause of the TD-SCV phenotype, but a functional proof was lacking. In this study, we tested for the first time the impact of mutations in thyA of clinical TD-SCVs and isogenic normal strains upon the activity of the protein. Until now, all clinical TD-SCVs carried nonsynonymous mutations in thyA (16, 17). TS activity experiments revealed full activity for TS of all normal strains independent of nonsynonymous point mutations, while TS of all TD-SCVs were found to be inactive, proving that these mutations are responsible for the thymidine-deficient phenotype.
To investigate the impact of TS inactivity on S. aureus physiology in detail, we constructed thyA deletion mutants using both the well-described and widely used laboratory strain SH1000 and the clinically relevant strain JE2 (USA300 background) (57). The site-directed mutants exhibited the typical morphological phenotype of clinical TD-SCVs (27). Morphological changes of S. aureus by antifolate drugs were already described in 1987 by Nishino et al. (58). These authors described enlarged cells with several division planes after treatment with bacteriostatic concentrations of trimethoprim. However, the authors did not culture the strains and therefore did not observe the SCV phenotype.
Using a whole-genome microarray comparing the wild type to the ΔthyA mutant, we showed that thyA inactivation had drastic effects upon S. aureus physiology. Specifically, we found downregulation of important virulence regulators (agrA-D, hld, arlRS, and sarA) and genes (capA-P, hla, hlb, sspA, crtNM, and geh) and upregulation of genes important for colonization and invasion (fnbAB, spa, coa, sdrCD, and sasADGH) (26). Many of these virulence factors are important for the acute virulence of S. aureus, such as hemolysins, which cause cytotoxicity and activation of inflammatory responses (59). In line with this transcriptional pattern, the ΔthyA mutant was significantly attenuated in virulence in vivo using a C. elegans killing model and an acute murine pneumonia model. Furthermore, we showed that both inactivation of thyA and TMP-SMX challenge of wild-type strains led to similar phenotypes regarding gene regulation and virulence. Although TD-SCVs are less virulent as the normal S. aureus phenotype in such acute infection models, the emergence of TD-SCVs could be disadvantageous for the course of the disease, as indicated by the study by Wolter et al., who showed that the occurrence of TD-SCVs was associated with worse lung disease in children with CF (18). Moreover, TD-SCVs have been shown to hide intracellularly and are thereby protected against host defense and antibiotic therapy (7, 8, 22).
In addition to changed expression of virulence genes in the ΔthyA mutant, we found major differences in the pyrimidine and purine biosynthetic pathways. While almost all genes involved in de novo synthesis of pyrimidines (e.g., thymidylate) were upregulated, genes of the purine pathways were downregulated. Such results indicate that these pathways are tightly regulated and balanced in the cell. Further studies are warranted to investigate the mechanisms of nucleotide regulation in S. aureus, e.g., deciphering the role of end product repression in these pathways.
For the first time, we showed that NupC functions as the primary thymidine transporter in S. aureus, if de novo thymidylate synthesis was blocked. By challenging an nupC mutant with TMP-SMX, no colonies of the mutant were detected around the TMP-SMX disk on Columbia blood agar, while for the wild type, tiny colonies were observed. This indicated that without the transporter NupC, thymidine cannot be taken up from the extracellular environment into the bacterial cell. Therefore, the TMP-SMX-inhibited thymidylate de novo synthesis could not be compensated by extracellular uptake of thymidine from the environment. The important function of NupC for uptake of extracellular thymidine was also evident in transcriptional analyses. We found significant upregulation of nupC in the ΔthyA mutant as well as in the wild type during TMP-SMX challenge. Therefore, nupC could be a possible target for the development of novel therapeutic compounds, which in combination with TMP-SMX may improve the antibiotic effect of TMP-SMX, especially in cases where extracellular thymidine is available.
The development of SCV phenotypes seems to be a common theme in S. aureus to adapt to different selective environments, e.g., antibacterial substances, host defense, or bacterial interference. In line with this, Hoffman et al. recently reported the selection of SCVs during growth in the presence of Pseudomonas aeruginosa caused by an antistaphylococcal substance (4-hydroxy-2-heptylquinoline-N-oxide) inhibiting the respiration of S. aureus (60). The selected hemin- and menadione-dependent SCVs were resistant against aminoglycosides. Furthermore, pyocyanin, an exotoxin secreted by P. aeruginosa, also acts upon the electron transport chain of S. aureus as reported by Biswas et al. (61), thereby also leading to the selection of SCVs. In the study by Gao et al., the occurrence of SCVs in a patient with bacteremia was described (19). These SCVs were disturbed in stringent response due to point mutations in relA after treatment by different antibiotics, resulting in increased resistance to linezolid (19). In combination with our results, we conclude that point mutations occurring in distinct genes play an important role for the adaptation of S. aureus to selective environments in vivo that facilitate persistence (62).
Interestingly, reports have been published describing thymidine dependency in other species, such as Escherichia coli recovered from blood cultures or Salmonella isolated from stool samples, indicating that thymidine dependency is not restricted to S. aureus but is a general phenomenon if infections are treated by TMP-SMX (63–65).
In our study, we showed that TS was inactive in clinical TD-SCVs, that the constructed ΔthyA mutant resembled morphologically clinical TD-SCVs, and that the expression of important virulence regulators and genes was severely affected in the mutant compared to that of the wild type, explaining the decreased virulence in two acute infection models. Furthermore, we showed that NupC functions as the primary thymidine transporter in S. aureus. In conclusion, our data elucidate the molecular mechanisms underlying TD-SCV phenotypes, which facilitate understanding of the clinical nature and importance of these special SCVs.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains and plasmids used in this study are listed in Table S2 in the supplemental material. S. aureus SH1000, a sigB-positive variant (rsbU+) of S. aureus 8325-4 (32), was used to generate the ΔthyA mutant. Clinical strain pairs of S. aureus, consisting of TD-SCVs and the respective isogenic normal phenotype, were isolated from airway secretions of CF patients. The clonality of the strains was confirmed by spa typing (66).
Media and growth conditions.
For cultivation of S. aureus, tryptic soy agar (TSA; BD, Heidelberg, Germany), Columbia blood agar (BD), Mueller-Hinton agar (heipha Müller GmbH, Eppelheim, Germany), brain heart infusion (BHI) broth (Merck, Darmstadt, Germany), chemical-defined medium, and Luria-Bertani (LB) broth (BD) were used. For cultivation of E. coli, LB broth and agar (BD) were used. Selection for resistance to antibiotics in E. coli or S. aureus was performed with ampicillin (100 µg/ml), erythromycin (2.5 µg/ml), and chloramphenicol (10 µg/ml) (AppliChem GmbH, Darmstadt, Germany).
DNA manipulations.
Manipulations were performed according to standard procedures. S. aureus cells were lysed with lysostaphin (WAK Chemie Medical GmBH, Steinbach/Ts, Germany). Chromosomal DNA was prepared using the PrestoSpin D kit (Molzym GmbH & Co. KG, Bremen, Germany). Plasmid DNA was purified using the Qiagen plasmid kit (Qiagen, Hilden, Germany). DNA fragments were isolated from agarose gels using the Perfectprep gel cleanup kit (Eppendorf AG, Hamburg, Germany). All constructed strains were verified by sequencing analysis of the manipulated DNA regions at Eurofins MWG Operon (Martinsried, Germany).
TS activity assay.
A modification of the method of the spectrophotometric assay of Wahba and Friedkin (31) was used to determine the activity of TS. The assay follows the conversion of N5,N10-methylene tetrahydrofolate (mTHF) to dihydrofolate (DHF) at 340 nm. The reaction buffer consists of 50 mM TES [N-Tris(hydroxymethyl) methyl-2-aminoethanesulfonic acid], 25 mM MgCl2, 1 mM EDTA, and 150 µM β-mercaptoethanol. The assay was performed in a 96-well flat-bottom microtiter plate in a volume of 300 µl. Totals of 250 µM mTHF, 250 µM DHF, and 480 ng protein were used to determine the specific activity.
Construction of a ΔthyA mutant and the complemented mutant in S. aureus SH1000 and JE2. (i) Amplification of thyA.
The upstream and downstream regions as well as the thyA gene from chromosomal DNA of S. aureus strain 8325-4 were amplified by PCR. The erythromycin resistance cassette ermB was amplified from the plasmid pEC4 (14). The oligonucleotide primers are listed in Table S3 in the supplemental material. PCRs were analyzed by agarose gel electrophoresis, and, if indicated, PCR products were purified by Qiagen PCR purification kit (Qiagen GmbH, Hilden, Germany) or gel purified with the Perfectprep gel cleanup kit (Eppendorf AG, Hamburg, Germany).
(ii) Construction of the shuttle vectors for gene replacement and complementation.
The upstream and downstream regions of thyA flanking the erythromycin resistance cassette ermB were cloned into the shuttle vector pBT9, which carries a temperature-sensitive replicon for staphylococci, generating pBT9-thyA::ermB. The thyA gene for complementation was cloned into the E. coli/S. aureus shuttle vector pNXR100 (unpublished, kindly provided by M. Grundmeier), which constitutively expresses the insert, generating pNXR100-thyA.
(iii) Inactivation of thyA.
For construction of a thyA replacement mutant, S. aureus SH1000 (pBT9-thyA::ermB) was cultivated overnight in LB medium in the presence of erythromycin (10 µg/ml) and chloramphenicol (10 µg/ml) with shaking at 30°C. The overnight culture was diluted (1:1,000) in fresh LB medium containing erythromycin and grown at 42°C overnight. This overnight culture was subsequently diluted (1:1,000) in fresh LB medium containing erythromycin and grown at 42°C for 48 h. Finally, this culture was diluted (1:1,000) in LB medium without antibiotics and grown at 42°C for 24 h. Various dilutions from this culture were incubated on Columbia blood agar plates at 37°C for 48 h. Mutants were selected, which appeared normal on Columbia blood agar containing 100 µg/ml thymidine and as SCVs on Columbia blood agar with erythromycin (10 µg/ml) but not with chloramphenicol or on MH agar. Mutants were verified by restriction analysis, PCR amplification, and sequencing. The thyA mutation was transduced to S. aureus USA300 (JE2) as described earlier (37).
Growth curve analysis.
For the growth curve analysis, cultures were grown in BHI broth with and without adding thymidine (100 µg/ml) at 37°C on a rotary shaker at 160 rpm. The generation time was calculated by the division of the time interval in minutes by 3.3, multiplied by the logarithm of the difference of the number of bacteria at the end and the beginning of the time interval.
RNA extraction and relative quantitative real-time PCR.
For the RNA extraction from bacterial cultures, a combination of RNAprotect bacteria reagent (Qiagen, Hilden, Germany), RNAiso (Segenetic, Borken, Germany), the RNeasy minikit (Qiagen), and lysing matrix B (MP Biomedicals, Heidelberg, Germany) was used. Cells were mechanically disrupted by a Fast Prep FP120 instrument (MP Biomedicals, Heidelberg, Germany). RNA quality was verified by a Bioanalyzer. For the relative quantitative real-time PCR (qRT-PCR), cDNA was synthesized from 2 µg of RNA using the QuantiTect reverse transcription kit (Qiagen) by following the instructions of the manufacturer. Real-time amplification was conducted using specific primers (see Table S3) and was carried out on an iCycler iQ real-time PCR system (Bio-Rad, Hercules, CA, USA) by using the qPCR EvaGreen Mastermix (Segenetic). The levels of mRNA expression of analyzed genes were normalized against the expression of the DNA gyrase subunit B (gyrB), guanylate kinase (gmk), and shikimate dehydrogenase (aroE) internal control genes, which are housekeeping genes successfully used for qRT-PCR. The transcript quantities are expressed as changes (n-fold) relative to the values of the internal control.
Microarray experiments.
Gene expression profiling was performed using Affymetrix GeneChip S. aureus genome arrays (Affymetrix United Kingdom Ltd., High Wycombe, United Kingdom) containing probe sets for over 3,300 open reading frames based on sequence information from four S. aureus strains. Bacterial strains were grown in BHI broth at 37°C on a rotary shaker at 160 rpm. For each investigated time point (early exponential, late exponential, stationary phase), three biological replicates were analyzed. RNA was isolated at 2 h, 6 h, and 9 h for the wild type and at 4 h, 9 h, and 12 h for the ΔthyA mutant. Total RNA was isolated as described above. RNA quantity and quality were determined by measurement of concentration with absorbance at 260 and 280 nm (NanoDrop 2000c; Thermo, Fisher Scientific, Bonn, Germany) and by means of an Agilent 2100 Bioanalyzer with an RNA 6000 Nano kit and 2100 Expert software (version B.02.07) (all Agilent Technologies Deutschland GmbH, Boeblingen, Germany) at Integrated Functional Genomics (Münster, Germany). Microarray processing was performed as recommended by the manufacturer. For microarray data analysis, the Partek Genomic Suite (Partek Inc., St. Louis, MO, USA) was used. The robust multiarray averaging method was applied for background correction, normalization, and probe summarization. Gene expression differences were determined by applying an analysis of variance (ANOVA). Only genes with a fold change of >2.5 and a P value of ≤0.05 were regarded as significantly changed in expression.
Antibiotic susceptibility testing.
Susceptibility against TMP-SMX was tested by agar diffusion with TMP-SMX disks (A.B. Biodisk, Solna, Sweden) and TMP-SMX E test strips (A.B. Biodisk, Solna, Sweden) on Mueller-Hinton agar or Columbia blood agar for the ΔthyA mutant, which does not grow on media lacking thymidine.
Transmission electron microscopy.
Samples were prepared for ultrastructure analysis as described previously (27). Ultrathin sections were visualized on a transmission electron microscope (Philips EM201) equipped with a digital imaging system (DITABIS, Pforzheim, Germany).
Caenorhabditis elegans spotted-lawn killing assay.
Bristol N2 C. elegans nematodes were maintained at 20°C on nematode growth medium plates seeded with E. coli strain OP50 as a food source. For the killing assay, 35 µl (50 µl for SCVs and E. coli OP50) of 20-h cultures grown in BHI (when indicated supplemented by 10 µg/ml chloramphenicol, 2.5 µg/ml erythromycin, or 100 µg/ml ampicillin) was spotted onto the middle of a tryptic soy agar (TA) plate containing 15 µg/ml polymyxin A/B (diameter, 3.5 cm) with 25 C. elegans individuals preferentially from larval stage 4 or young adults. For the ΔthyA mutant, the cultures were 3× concentrated by centrifugation. After 48 h, the surviving nematodes were transferred to new plates to make sure that progeny did not adulterate the results. The quantity of survivors was counted every 24 h for at least 96 h.
Mice.
C57BL/6NCrlBR male mice 20 to 22 g were obtained from Charles River Laboratories, Italy. Animal studies were conducted according to protocols approved by the San Raffaele Scientific Institute (Milan, Italy) Institutional Animal Care and Use Committee (IACUC) and adhered strictly to the Italian Ministry of Health guidelines for the use and care of experimental animals.
Acute pneumonia mouse model.
Prior to animal experiments, normal strains were grown for 3 h and the ΔthyA mutant for 6 h to reach the exponential growth phase. Subsequently, the bacteria were pelleted by centrifugation (2,700 × g, 15 min) and washed twice with sterile phosphate-buffered saline (PBS), and the optical density (OD) of the bacterial suspension was adjusted by spectrophotometry at 600 nm. The intended number of CFU was extrapolated from a standard growth curve. Appropriate dilutions with sterile PBS were made to prepare the inoculum of 1010 CFU/ml. Mice were anesthetized and the tracheae were directly visualized by a ventral midline incision, exposed, and intubated with a sterile, flexible 22-guage needle attached to a 1-ml syringe. A 50-µl inoculum of 5 × 108 CFU was implanted via the needle into the lung, with both lobes inoculated. After infection, mortality was monitored in one group of mice (n = 9) over 1 week. In the remaining mice, murine lungs were excised, homogenized, and plated onto TSA plates for CFU counting.
Histological examination and immunofluorescence.
Mice were sacrificed by CO2 administration after 4 h and 24 h of infection. Lungs were removed en bloc and fixed in 10% buffered formalin at 4°C for 24 h and processed for paraffin embedding. Longitudinal sections of 5 mm taken at regular intervals were obtained using a microtome from the proximal, medial, and distal lung regions. Sections were stained with hematoxylin and eosin (H&E) according to standard procedures. Immunofluorescence localization of S. aureus was performed in deparaffinized lung sections by indirect immunofluorescence, using a polyclonal rabbit antibody specific for S. aureus, kindly provided by M. Hussain, and Texas Red-labeled goat anti-rabbit IgG. The slides were examined with Axioplan2 (Zeiss, Jena, Germany) with AxioCam provided with the charge-coupled-device (CCD) MRc5 (Zeiss).
LDH release assay.
A549 cells were seeded into 96-well cell culture plates (4 × 104 cells/well). Medium of confluent monolayers was replaced by infection medium, and cells were challenged with 10 µl of sterile S. aureus culture supernatants. After incubation for 8 h, cellular release of LDH was determined using the CytoTox nonradioactive cytotoxicity assay (Promega). Briefly, A549 culture supernatants were incubated for 15 min with an equal amount of substrate mix. After addition of stop solution, absorbance was measured at 490 nm.
Statistical analysis.
Statistical analyses for the in vivo experiments were performed by log rank (Mantel-Cox) test. A P value of ≤0.05 was considered significant.
Microarray data accession number.
The microarray data have been deposited in NCBI’s Gene Expression Omnibus (67) and are accessible through GEO series accession number GSE47406.
SUPPLEMENTAL MATERIAL
Genes of interest found to be differentially regulated in the ΔthyA mutant compared to in SH1000 WT.
Bacterial strains and plasmids used in the study.
Oligonucleotides used in the study.
qRT-PCR of SH1000 wild type and the ΔthyA mutant under TMP-SMX challenge.
ACKNOWLEDGMENTS
We thank M. Rocchi and F. Sanvito (Department of Pathology, San Raffaele Scientific Institute, Milan, Italy) for mouse histopathology and S. Deiwick, D. Kuhn, and E. Leidig (Institute of Medical Microbiology, University Hospital of Münster, Münster, Germany) for excellent technical assistance. We thank Wolfgang Völker (Leibniz-Institute of Atherosclerosis Research, University Hospital of Münster, Münster, Germany) for excellent transmission electron microscopy.
The strains JE2 and NE544 (nupC mutant) were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program, supported under NIAID, NIH contract no. HHSN272200700055C. This project was funded partly by grants of the German Research Foundation (KA 2249/1-3; DFG), the Interdisciplinary Center for Clinical Research (IZKF Münster; Kah2/024/09), the Transregional Collaborative Research Center 34 (C7), the Bundesministerium für Bildung und Forschung (BMBF Medizinische Infektionsgenomik [0315829B]), and the Deutsche Forschungsgemeinschaft (DFG SPP 1316, BE 2546/1-2).
Footnotes
Citation Kriegeskorte A, Block D, Drescher M, Windmüller N, Mellmann A, Baum C, Neumann C, Lorè NI, Bragonzi A, Liebau E, Hertel P, Seggewiss J, Becker K, Proctor RA, Peters G, Kahl BC. 2014. Inactivation of thyA in Staphylococcus aureus attenuates virulence and has a strong impact on metabolism and virulence gene expression. mBio 5(4):e01447-14. doi:10.1128/mBio.01447-14.
REFERENCES
- 1. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 2. Foster TJ. 2004. The Staphylococcus aureus “superbug.” J. Clin. Invest. 114:1693–1696. 10.1172/JCI23825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying the Pantón-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978–984. 10.3201/eid0908.030089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Köck R, Brakensiek L, Mellmann A, Kipp F, Henderikx M, Harmsen D, Daniels-Haardt I, von Eiff C, Becker K, Hendrix MG, Friedrich AW. 2009. Cross-border comparison of the admission prevalence and clonal structure of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 71:320–326. 10.1016/j.jhin.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 5. Proctor RA, Balwit JM, Vesga O. 1994. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect. Agents Dis. 3:302–312 [PubMed] [Google Scholar]
- 6. Proctor RA, von Eiff C, Kahl BC, Becker K, Mc Namara PJ, Herrmann M, Peters G. 2006. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4:295–305. 10.1038/nrmicro1384 [DOI] [PubMed] [Google Scholar]
- 7. Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, Proctor RA, Peters G. 1998. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177:1023–1029. 10.1086/515238 [DOI] [PubMed] [Google Scholar]
- 8. Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, Peters G, Becker K, Löffler B. 2010. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J. Infect. Dis. 202:1031–1040. 10.1086/656047 [DOI] [PubMed] [Google Scholar]
- 9. von Eiff C, Becker K, Metze D, Lubritz G, Hockmann J, Schwarz T, Peters G. 2001. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with Darier’s disease. Clin. Infect. Dis. 32:1643–1647. 10.1086/320519 [DOI] [PubMed] [Google Scholar]
- 10. Kriegeskorte A, König S, Sander G, Pirkl A, Mahabir E, Proctor RA, von Eiff C, Peters G, Becker K. 2011. Small colony variants of Staphylococcus aureus reveal distinct protein profiles. Proteomics 11:2476–2490. 10.1002/pmic.201000796 [DOI] [PubMed] [Google Scholar]
- 11. von Eiff C, McNamara P, Becker K, Bates D, Lei XH, Ziman M, Bochner BR, Peters G, Proctor RA. 2006. Phenotype microarray profiling of Staphylococcus aureus menD and hemB mutants with the small-colony-variant phenotype. J. Bacteriol. 188:687–693. 10.1128/JB.188.2.687-693.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kahl BC. 2014. Small colony variants (SCVs) of Staphylococcus aureus—a bacterial survival strategy. Infect. Genet. Evol. 21:515–522. 10.1016/j.meegid.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 13. Bates DM, von Eiff C, McNamara PJ, Peters G, Yeaman MR, Bayer AS, Proctor RA. 2003. Staphylococcus aureus menD and hemB mutants are as infective as the parent strains, but the menadione biosynthetic mutant persists within the kidney. J. Infect. Dis. 187:1654–1661. 10.1086/374642 [DOI] [PubMed] [Google Scholar]
- 14. von Eiff C, Heilmann C, Proctor RA, Wolz C, Peters G, Gotz F. 1997. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179:4706–4712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lannergård J, von Eiff C, Sander G, Cordes T, Seggewiss J, Peters G, Proctor RA, Becker K, Hughes D. 2008. Identification of the genetic basis for clinical menadione-auxotrophic small-colony variant isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 52:4017–4022. 10.1128/AAC.00668-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chatterjee I, Kriegeskorte A, Fischer A, Deiwick S, Theimann N, Proctor RA, Peters G, Herrmann M, Kahl BC. 2008. In vivo mutations of thymidylate synthase (encoded by thyA) are responsible for thymidine-dependency in clinical small colony variants (TD-SCVs) of Staphylococcus aureus. J. Bacteriol. 190:834–842. 10.1128/JB.00912-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Besier S, Ludwig A, Ohlsen K, Brade V, Wichelhaus TA. 2007. Molecular analysis of the thymidine-auxotrophic small colony variant phenotype of Staphylococcus aureus. Int. J. Med. Microbiol. 297:217–225. 10.1016/j.ijmm.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 18. Wolter DJ, Emerson JC, McNamara S, Buccat AM, Qin X, Cochrane E, Houston LS, Rogers GB, Marsh P, Prehar K, Pope CE, Blackledge M, Déziel E, Bruce KD, Ramsey BW, Gibson RL, Burns JL, Hoffman LR. 2013. Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin. Infect. Dis. 57:384–391. 10.1093/cid/cit270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao W, Chua K, Davies JK, Newton HJ, Seemann T, Harrison PF, Holmes NE, Rhee HW, Hong JI, Hartland EL, Stinear TP, Howden BP. 2010. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog. 6:e1000944. 10.1371/journal.ppat.1000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abu-Qatouseh LF, Chinni SV, Seggewiss J, Proctor RA, Brosius J, Rozhdestvensky TS, Peters G, von Eiff C, Becker K. 2010. Identification of differentially expressed small non-protein-coding RNAs in Staphylococcus aureus displaying both the normal and the small-colony variant phenotype. J. Mol. Med. 88:565–575. 10.1007/s00109-010-0597-2 [DOI] [PubMed] [Google Scholar]
- 21. Vaudaux P, Francois P, Bisognano C, Kelley WL, Lew DP, Schrenzel J, Proctor RA, McNamara PJ, Peters G, von Eiff C. 2002. Increased expression of clumping factor and fibronectin-binding proteins by hemB mutants of Staphylococcus aureus expressing small colony variant phenotypes. Infect. Immun. 70:5428–5437. 10.1128/IAI.70.10.5428-5437.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, Holzinger D, Roth J, Proctor RA, Becker K, Peters G, Loffler B. 2011. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 3:129–141. 10.1002/emmm.201000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Becker K, Laham NA, Fegeler W, Proctor RA, Peters G, von Eiff C. 2006. Fourier-transform infrared spectroscopic analysis is a powerful tool for studying the dynamic changes in Staphylococcus aureus small-colony variants. J. Clin. Microbiol. 44:3274–3278. 10.1128/JCM.00847-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Besier S, Smaczny C, von Eiff C, Krahl A, Ackermann H, Brade V, Wichelhaus TA. 2007. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J. Clin. Microbiol. 45:168–172. 10.1128/JCM.01510-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilligan PH, Gage PA, Welch DF, Muszynski MJ, Wait KR. 1987. Prevalence of thymidine-dependent Staphylococcus aureus in patients with cystic fibrosis. J. Clin. Microbiol. 25:1258–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kahl BC, Belling G, Becker P, Chatterjee I, Wardecki K, Hilgert K, Cheung AL, Peters G, Herrmann M. 2005. Thymidine-dependent Staphylococcus aureus small colony variants (SCV) are associated with extensive changes in regulator and virulence gene expression profiles. Infect. Immun. 73:4119–4126. 10.1128/IAI.73.7.4119-4126.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kahl BC, Belling G, Reichelt R, Herrmann M, Proctor RA, Peters G. 2003. Thymidine-dependent small-colony variants of Staphylococcus aureus exhibit gross morphological and ultrastructural changes consistent with impaired cell separation. J. Clin. Microbiol. 41:410–413. 10.1128/JCM.41.1.410-413.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chatterjee I, Herrmann M, Proctor RA, Peters G, Kahl BC. 2007. Enhanced post-stationary-phase survival of a clinical thymidine-dependent small-colony variant of Staphylococcus aureus results from lack of a functional tricarboxylic acid cycle. J. Bacteriol. 189:2936–2940. 10.1128/JB.01444-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zander J, Besier S, Saum SH, Dehghani F, Loitsch S, Brade V, Wichelhaus TA. 2008. Influence of dTMP on the phenotypic appearance and intracellular persistence of Staphylococcus aureus. Infect. Immun. 76:1333–1339. 10.1128/IAI.01075-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stryer L. 1995. Biosynthesis of nucleotides. In Stryer L, Biochemistry, p 739–762 W.H. Freeman and Company, London, United Kingdom [Google Scholar]
- 31. Wahba AJ, Friedkin M. 1962. The enzymatic synthesis of thymidylate. I. Early steps in the purification of thymidylate synthetase of Escherichia coli. J. Biol. Chem. 237:3794–3801 [PubMed] [Google Scholar]
- 32. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457–5467. 10.1128/JB.184.19.5457-5467.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. CLSI 2012. Performance standards for antimicrobial disk susceptibility tests. Approved standards. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 34. Seggewiss J, Becker K, Kotte O, Eisenacher M, Yazdi MR, Fischer A, McNamara P, Al Laham N, Proctor R, Peters G, Heinemann M, von Eiff C. 2006. Reporter metabolite analysis of transcriptional profiles of a Staphylococcus aureus strain with normal phenotype and its isogenic hemB mutant displaying the small-colony-variant phenotype. J. Bacteriol. 188:7765–7777. 10.1128/JB.00774-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Recsei P, Kreiswirth B, O’Reilly M, Schlievert P, Gruss A, Novick RP. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol. Gen. Genet. 202:58–61. 10.1007/BF00330517 [DOI] [PubMed] [Google Scholar]
- 36. Liang X, Zheng L, Landwehr C, Lunsford D, Holmes D, Ji Y. 2005. Global regulation of gene expression by ArlRS, a two-component signal transduction regulatory system of Staphylococcus aureus. J. Bacteriol. 187:5486–5492. 10.1128/JB.187.15.5486-5492.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheung AL, Koomey JM, Butler CA, Projan SJ, Fischetti VA. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. U. S. A. 89:6462–6466. 10.1073/pnas.89.14.6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kullik I, Giachino P. 1997. The alternative sigma factor ςB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch. Microbiol. 167:151–159. 10.1007/s002030050428 [DOI] [PubMed] [Google Scholar]
- 39. Kornblum J, Kreiswirth BN, Projan SJ, Ross H, Novick RP. 1990. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus, p 373–402 In Novick RP, Molecular biology of the staphylococci. VCH, New York, NY. [Google Scholar]
- 40. Roche FM, Meehan M, Foster TJ. 2003. The Staphylococcus aureus surface protein SasG and its homologues promote bacterial adherence to human desquamated nasal epithelial cells. Microbiology 149:2759–2767. 10.1099/mic.0.26412-0 [DOI] [PubMed] [Google Scholar]
- 41. Roche FM, Massey R, Peacock SJ, Day NP, Visai L, Speziale P, Lam A, Pallen M, Foster TJ. 2003. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology 149:643–654. 10.1099/mic.0.25996-0 [DOI] [PubMed] [Google Scholar]
- 42. Luong TT, Lee CY. 2006. The arl locus positively regulates Staphylococcus aureus type 5 capsule via an mgrA-dependent pathway. Microbiology 152:3123–3131. 10.1099/mic.0.29177-0 [DOI] [PubMed] [Google Scholar]
- 43. Brunskill EW, Bayles KW. 1996. Identification of LytSR-regulated genes from Staphylococcus aureus. J. Bacteriol. 178:5810–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ranjit DK, Endres JL, Bayles KW. 2011. Staphylococcus aureus CidA and LrgA proteins exhibit holin-like properties. J. Bacteriol. 193:2468–2476. 10.1128/JB.01545-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stapleton MR, Horsburgh MJ, Hayhurst EJ, Wright L, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. 2007. Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J. Bacteriol. 189:7316–7325. 10.1128/JB.00734-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takahashi J, Komatsuzawa H, Yamada S, Nishida T, Labischinski H, Fujiwara T, Ohara M, Yamagishi J, Sugai M. 2002. Molecular characterization of an atl null mutant of Staphylococcus aureus. Microbiol. Immunol. 46:601–612. 10.1111/j.1348-0421.2002.tb02741.x [DOI] [PubMed] [Google Scholar]
- 47. Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong YQ. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 40:1–9. 10.1016/S0928-8244(03)00309-2 [DOI] [PubMed] [Google Scholar]
- 48. Cheung AL, Nishina KA, Trotonda MP, Tamber S. 2008. The SarA protein family of Staphylococcus aureus. Int. J. Biochem. Cell Biol. 40:355–361. 10.1016/j.biocel.2007.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Manna AC, Bayer MG, Cheung AL. 1998. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J. Bacteriol. 180:3828–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schmidt KA, Manna AC, Cheung AL. 2003. sarT influences sarS expression in Staphylococcus aureus. Infect. Immun. 71:5139–5148. 10.1128/IAI.71.9.5139-5148.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Manna AC, Ingavale SS, Maloney M, van Wamel W, Cheung AL. 2004. Identification of sarV (SA2062), a new transcriptional regulator, is repressed by SarA and MgrA (SA0641) and involved in the regulation of autolysis in Staphylococcus aureus. J. Bacteriol. 186:5267–5280. 10.1128/JB.186.16.5267-5280.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mishra NN, Liu GY, Yeaman MR, Nast CC, Proctor RA, McKinnell J, Bayer AS. 2011. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 55:526–531. 10.1128/AAC.00680-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen Z, Luong TT, Lee CY. 2007. The sbcDC locus mediates repression of type 5 capsule production as part of the SOS response in Staphylococcus aureus. J. Bacteriol. 189:7343–7350. 10.1128/JB.01079-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Laport MS, de Castro AC, Villardo A, Lemos JA, Bastos MC, Giambiagi-deMarval M. 2001. Expression of the major heat shock proteins DnaK and GroEL in Streptococcus pyogenes: a comparison to Enterococcus faecalis and Staphylococcus aureus. Curr. Microbiol. 42:264–268. 10.1007/s002840110215 [DOI] [PubMed] [Google Scholar]
- 55. Saxild HH, Andersen LN, Hammer K. 1996. dra-nupC-pdp operon of Bacillus subtilis: nucleotide sequence, induction by deoxyribonucleosides, and transcriptional regulation by the deor-encoded DeoR repressor protein. J. Bacteriol. 178:424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4(1):e00537-12. 10.1128/mBio.00537-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, Ray SM, Blumberg HM. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42:647–656. 10.1086/499815 [DOI] [PubMed] [Google Scholar]
- 58. Nishino T, Wecke J, Krüger D, Giesbrecht P. 1987. Trimethoprim-induced structural alterations in Staphylococcus aureus and the recovery of bacteria in drug-free medium. J. Antimicrob. Chemother. 19:147–159. 10.1093/jac/19.2.147 [DOI] [PubMed] [Google Scholar]
- 59. Haslinger-Löffler B, Kahl BC, Grundmeier M, Strangfeld K, Wagner B, Fischer U, Cheung AL, Peters G, Schulze-Osthoff K, Sinha B. 2005. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell. Microbiol. 7:1087–1097. 10.1111/j.1462-5822.2005.00533.x [DOI] [PubMed] [Google Scholar]
- 60. Hoffman LR, Déziel E, D’Argenio DA, Lépine F, Emerson J, McNamara S, Gibson RL, Ramsey BW, Miller SI. 2006. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 103:19890–19895. 10.1073/pnas.0606756104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Biswas L, Biswas R, Schlag M, Bertram R, Götz F. 2009. Small-colony variant selection as a survival strategy for Staphylococcus aureus in the presence of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 75:6910–6912. 10.1128/AEM.01211-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, Bruce D, Rubin E, Myers E, Siggia ED, Tomasz A. 2007. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc. Natl. Acad. Sci. U. S. A. 104:9451–9456. 10.1073/pnas.0609839104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sparham PD, Lobban DI, Speller DC. 1978. Isolation of Staphylococcus aureus from sputum in cystic fibrosis. J. Clin. Pathol. 31:913–918. 10.1136/jcp.31.10.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tanner EI, Bullin CH. 1974. Thymidine-dependent Escherichia coli infection and some associated laboratory problems. J. Clin. Pathol. 27:565–568. 10.1136/jcp.27.7.565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCarthy LR, Chmel H, Bell G, Armstrong D. 1977. Thymidine-dependent strain of Salmonella oslo selected by trimethoprim-sulfamethoxazole therapy. Am. J. Clin. Pathol. 68:307–311 [DOI] [PubMed] [Google Scholar]
- 66. Kahl BC, Mellmann A, Deiwick S, Peters G, Harmsen D. 2005. Variation of the polymorphic region X of the protein A gene during persistent airway infection of cystic fibrosis patients reflects two independent mechanisms of genetic change in Staphylococcus aureus. J. Clin. Microbiol. 43:502–505. 10.1128/JCM.43.1.502-505.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Edgar R, Domrachev M, Lash AE. 2002. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30:207–210. 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genes of interest found to be differentially regulated in the ΔthyA mutant compared to in SH1000 WT.
Bacterial strains and plasmids used in the study.
Oligonucleotides used in the study.
qRT-PCR of SH1000 wild type and the ΔthyA mutant under TMP-SMX challenge.