Abstract
Coronin3 expression is increased in gastric cancer (GC) tissues and can promote GC invasion and metastasis. However, the mechanisms underlying Coronin3 function in GC remain unclear. In this study, we aimed to explore the interacting molecules essential for the tumor-promoting effects of Coronin3 in GC. Using mass spectrometric analysis, functional studies, and immunohistochemistry, we found that Arp2 interacted with Coronin3, and ectopic expression of Arp2 promoted GC cell migration and invasion, while Arp2 knockdown suppressed whole-cell motility and attenuated the Coronin3-mediated upregulation of cell migration and invasion. In addition, both proteins correlated with the metastatic status of GC patients. Furthermore, survival analyses demonstrated that both Coronin3 and Arp2 correlated with overall GC patient survival, and the combination of Coronin3 and Arp2 most accurately predicted GC patient prognosis. Combined, these data demonstrate that Coronin3 can regulate GC invasion and metastasis through Arp2, and the combination of Coronin3 and Arp2 provides a potential marker for predicting GC prognosis.
Keywords: gastric cancer, cell motility, cytoskeleton, Coronin3, Arp2
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide, with particular prevalence in China and other East Asian countries. In spite of substantial progress in diagnostic and therapeutic strategies, the average 5-y survival rate of GC patients remains poor.1 A main reason for this phenomenon is the frequent occurrence of GC metastasis, which is the primary cause of treatment failure and strongly contributes to the poor prognosis of GC patients.2 Therefore, targeting metastasis-associated molecules is essential for improving therapeutic efficacy in GC patients.
Increased cell motility and decreased cell–cell adhesion are the main characteristics of cancer cells with metastatic potential.3 In most cases, the dynamic remodeling of the actin cytoskeleton is involved in these changes.4 Filament nucleation is a key step during actin network formation and is catalyzed by the Arp2/3 complex, a highly conserved filament nucleator.5 The Arp2/3 complex has 2 activities: actin nucleation and actin filament branching.6 The Arp2/3 complex nucleates the actin filaments that branch from the sides of existing filaments and remains localized at branch junctions to maintain the dendritic structure of the network.6,7 Activation of the Arp2/3 complex depends on its interactions with nucleation-promoting factors (NPFs). These proteins, including SCAR/WASP, myosin I, Abp1, cortactin, and Pan1, regulate the assembly of actin filaments synergistically with the Arp2/3 complex.4,8
Coronins are a family of conserved actin-binding proteins that regulate cell motility in various contexts.9 In particular, Coronin3 (Coronin1C) has been associated with metastasis in several cancer types.10-12 In a previous study, we revealed that Coronin3 overexpression promotes GC metastasis both in vitro and in vivo.13 Metastasis-associated gene analysis indicated that MMP-9 and cathepsin K might play certain roles in the downstream pathways regulated by Coronin3.13 However, it remains unclear whether actin-related proteins are involved in the regulation of GC metastasis by Coronin3.
To address the issues above, we employed mass spectrometry and co-immunoprecipitation (co-IP) assays to confirm the interaction between Coronin3 and Arp2 in GC cells. We also found that alterations in Arp2 expression affected GC cell migration and invasion. Furthermore, Arp2 knockdown antagonized the increased cell motility induced by Coronin3 overexpression. Interestingly, we also revealed the clinical significance of Coronin3 and Arp2 in GC tissues and corresponding lymph nodes. These findings suggested that the interaction between Coronin3 and Arp2 potentially plays a key role in the regulation of GC cell motility.
Results
Expression levels of Coronin3 in normal and gastric cancer tissues
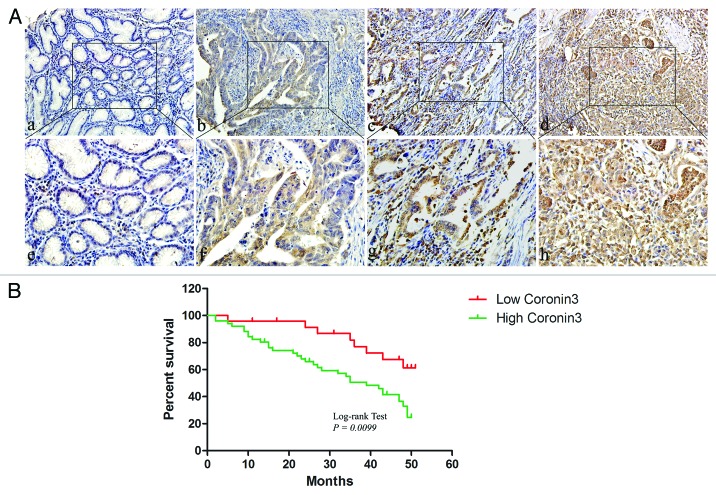
We demonstrated before that Coronin3 was associated with the metastatic status of GC, but its clinical significance in the survival analysis remained unknown.13 To address this issue, we re-evaluated the expression of Coronin3 in a microarray containing 75 GC cases with at least 5-y follow-up information. We found that Coronin3 expression levels differed significantly between normal and GC tissues. Although abundantly expressed in GC tissues, Coronin3 was present at substantially lower levels in normal gastric tissues (Fig. 1A; Table 1). Coronin3 was focally localized to the cytoplasm, and little positive staining for Coronin3 was observed in stromal cells (Fig. 1A). In addition, we found that a relatively low level of Coronin3 expression was observed in highly differentiated cancers, whereas stronger Coronin3 staining was detected in moderately and poorly differentiated gastric cancers (Fig. 1A; Table 1). We also analyzed the expression of Coronin3 in stage I–IV gastric cancers, as classified according the standard TNM system. This analysis revealed that stage III and IV gastric tumors exhibited stronger Coronin3 expression than did gastric tumors of stage I and II (Table 1). Meanwhile, Coronin3 was significantly increased in cases with distant metastases, indicating its association with GC metastases (Table 1).
Figure 1. The expression of Coronin3 in gastric cancer. (A) Coronin3 expression in normal gastric mucosa and cancers with different grades of differentiation. Little immunoreactivity for Coronin3 was showed in normal gastric tissues (a). An increased intensity of Coronin3 staining was found in gastric cancer cases with high, moderate, and poor differentiation ([b, c, and d] respectively). (a–d) 200×; (e–h), 400×. (B) Kaplan–Meier curve for the correlation between Coronin3 expression and overall survival.
Table 1. The expression of Coronin3 and Arp2 in GC tissues.
| Variable | No. | H-score of Coronin3 | P | H-score of Arp2 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | ||||
| Cancer | 75 | 1 | 23 | 21 | 30 | <0.001* | 7 | 21 | 25 | 22 | <0.001* |
| Normal | 75 | 2 | 35 | 31 | 7 | 36 | 23 | 11 | 5 | ||
| Gender | 0.556 | 0.141 | |||||||||
| Male | 41 | 1 | 13 | 13 | 14 | 4 | 7 | 16 | 14 | ||
| Female | 34 | 0 | 10 | 8 | 16 | 3 | 14 | 9 | 8 | ||
| Age | 0.602 | 0.320 | |||||||||
| ≤60 | 47 | 0 | 14 | 14 | 19 | 5 | 15 | 12 | 15 | ||
| >60 | 28 | 1 | 9 | 7 | 11 | 2 | 6 | 13 | 7 | ||
| Differentiation | 0.028* | 0.001* | |||||||||
| Poor | 40 | 0 | 6 | 13 | 21 | 2 | 8 | 11 | 19 | ||
| Moderate | 28 | 1 | 14 | 5 | 8 | 2 | 10 | 13 | 3 | ||
| High | 7 | 0 | 3 | 3 | 1 | 3 | 3 | 1 | 0 | ||
| TNM stages | 0.014* | <0.001* | |||||||||
| I or II | 23 | 1 | 12 | 3 | 7 | 6 | 5 | 11 | 1 | ||
| III or IV | 52 | 0 | 11 | 18 | 23 | 1 | 16 | 14 | 21 | ||
| Metastases | <0.001* | 0.002* | |||||||||
| 0 | 17 | 1 | 11 | 5 | 0 | 4 | 9 | 3 | 1 | ||
| ≥1 | 58 | 0 | 12 | 16 | 30 | 3 | 12 | 22 | 21 | ||
To better elucidate the clinical significance of Coronin3, we performed further analysis of its correlation with overall survival periods of GC patients. It showed that the overall survival periods of patients with higher expression level of Coronin3 was significantly reduced than that of patients with low Coronin3 expression (Fig. 1B). This phenomenon indicated that Coronin3 might serve as a potential marker in predicting prognosis of GC patients.
Coronin3 interacts with Arp2
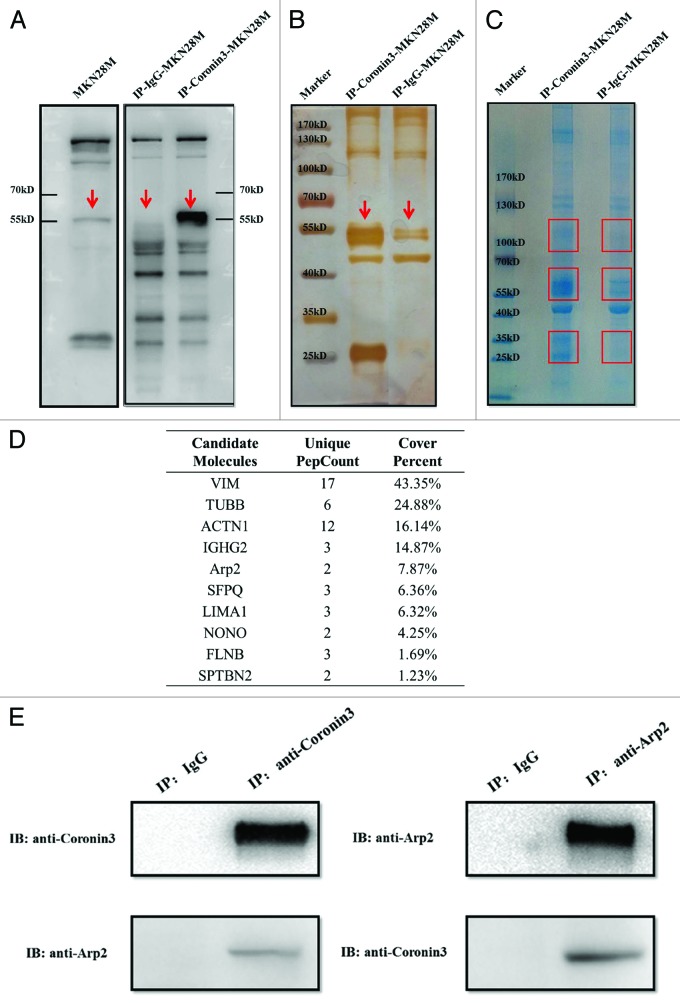
In a previous study, we demonstrated that Coronin3 promotes GC invasion and metastasis; however, precisely how Coronin3 regulated these malignant behaviors remained unclear.13 To clarify the mechanism underlying these functions of Coronin3, we employed MALDI-TOF/TOF analysis to search for its interacting proteins. Co-IP assays were conducted using an anti-Coronin3 antibody in MKN28-M cells with normal mouse IgG as a control. Immunoblotting assays confirmed that Coronin3 was successfully enriched in the immunoprecipitates (Fig. 2A), which were subsequently stained with silver (Fig. 2B) and Coomassie blue G250 (Fig. 2C). We detected 3 bands at approximately 25–35 kDa, 50–60 kDa, and 95–105 kDa in the co-immunoprecipitates of the MKN28-M cells compared with the control (Fig. 2C). The corresponding protein bands were excised from the Coomassie-blue-stained gel and subjected to in-gel tryptic digestion and MALDI-TOF/TOF™ 5800 analysis. The obtained mass spectra were then analyzed using Protein Pilot™ 4.0 software. As depicted in Figure 2D, 10 proteins were identified as Coronin3-interacting proteins. We were particularly interested in actin-related protein 2 (Arp2) because it has been reported to interact with other members of the Coronin family.8 To confirm the results of the MALDI-TOF analysis, we conducted co-IPs of endogenous Coronin3 and Arp2 in MKN28-M cells. In these assays, mutual interactions were observed between Coronin3 and Arp2, revealing that Arp2 was a Coronin3-binding protein (Fig. 2E).
Figure 2. Identification of molecules interacting with Coronin3. (A) The Coronin3 antigen was successfully enriched by IP. The bands for Coronin3 are indicated with red arrows. (B) The immunoprecipitates were subjected to SDS-PAGE and silver staining to confirm the enrichment of the Coronin3 antigen. The target bands for Coronin3 are indicated with red arrows. (C) The immunoprecipitates were subjected to SDS-PAGE and Coomassie blue G250 staining. The bands that were further subjected to MALDI-TOF analysis are marked with red squares. (D) The candidate Coronin3-interacting molecules. (E) Mutual interactions between endogenous Coronin3 and Arp2. The interaction of Coronin3 and Arp2 was validated by co-IP. Coronin3 and Arp2 were immunoprecipitated separately with their corresponding primary antibodies, and Arp2 and Coronin3 were detected through immunoblotting.
Arp2 regulates the migration and invasion of GC cells
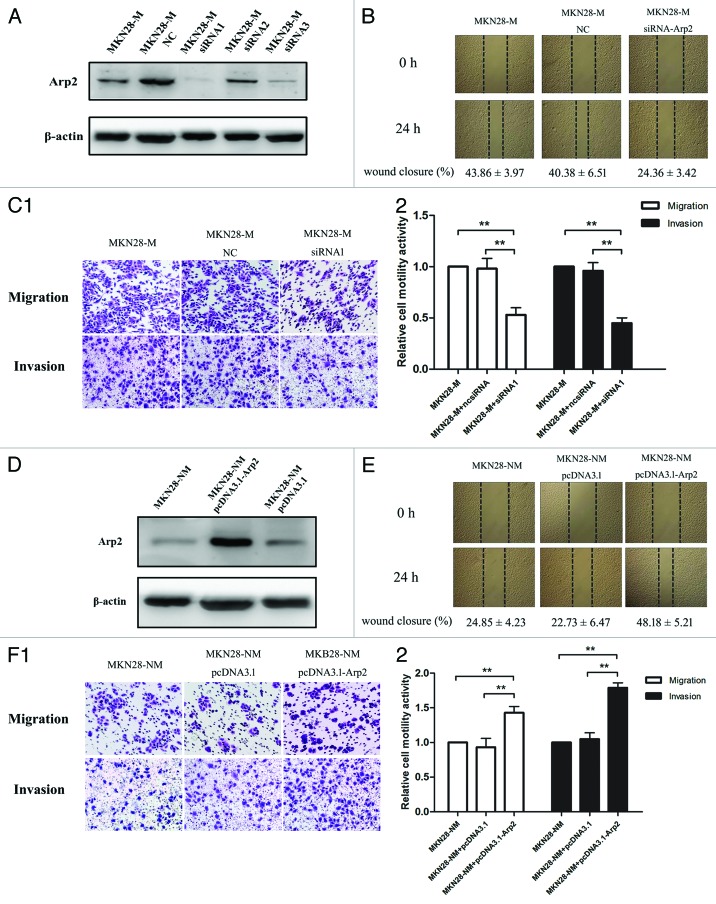
We examined Arp2 expression in multiple cell lines and observed that Arp2 was upregulated in GC cell lines compared with immortalized gastric epithelial cells (GES). In particular, Arp2 expression levels were significantly elevated in MKN28-M cells, a subline of MKN-28 cells with high metastatic potential, compared with that of MKN28-NM cells, which have reduced metastatic potential (Fig. S1). Therefore, the MKN28-M and MKN28-NM lines were employed as cell models to study the regulation of cell motility by Arp2.
Three pairs of siRNAs against Arp2 were synthesized, and their inhibitory effects on Arp2 were confirmed by western blot analysis (Fig. 3A). Based on their individual inhibitory effects, siRNA1 was used to knockdown Arp2 in subsequent experiments. Remarkably, Arp2 silencing prevented cells from migrating into the “empty” zone, leading to reduced scratch closure (Fig. 3B). Boyden chamber assays also demonstrated that Arp2 inhibition suppressed cancer cell invasion and migration (Fig. 3C). In contrast, we transfected Arp2 and control plasmids into MKN28-NM cells, and Arp2 was significantly upregulated in cells with this transfection. The following functional studies showed that ectopic expression of Arp2 promoted the migration and invasion of MKN28-NM cells in vitro (Fig. 3D–F). These data indicated that Arp2 was an essential modulator of cell migration and invasion in GC.
Figure 3. The regulation of cell motility by Arp2 in gastric cancer cells. (A) Three siRNA pairs targeting Arp2 were synthesized, and their inhibitory effects were examined by western blotting. (B) Wound-healing assays showed that Arp2 knockdown decreased the wound closure rate. (C) Arp2 suppression inhibited the migration and invasion of MKN28-M cells. (1) Representative images of the migration and invasion assays; (2) The relative cell motility activities of cells. (D) Western blot analysis for Arp2 following transfection with the Arp2 expression plasmid or empty vector into MKN28-NM cells. (E) Wound-healing assay showed that Arp2 upregulation increased the wound closure rate. (F) Ectopic expression of Apr2 promoted the migration and invasion of MKN28-NM cells. (1) Representative images of the migration and invasion assays; (2) The relative cell motility activities of cells.
Knockdown of Arp2 attenuated the metastatic potential of GC cells with ectopic Coronin3 expression
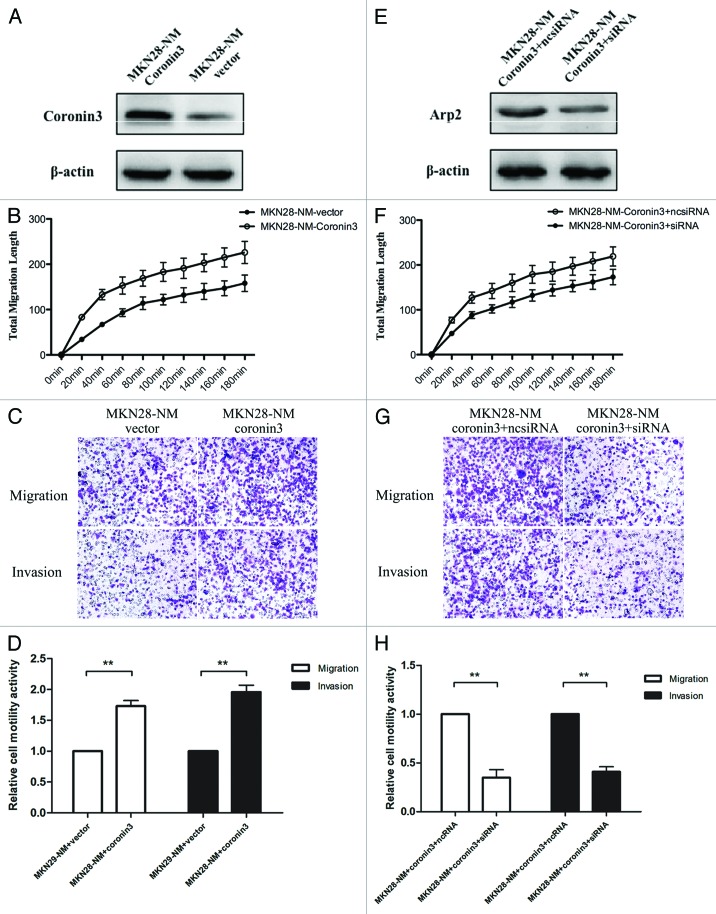
We confirmed that Coronin3 interacted with Arp2 in GC cells with high metastatic potential, but whether this interaction was crucial in regulating cell motility remained unknown. To clarify this aspect, we first developed a subline of MKN-28NM cells that were stably transfected with Coronin3 (Fig. 4A), and then we compared the cell motility by the cellomics and boyden chamber assays. We found that ectopic Arp2 expression increased the total migration length of cells documented by the cellomics instrument (Fig. 4B), and more cells moved to the bottom side of the basal membrane in the boyden chamber (Fig. 4C and D). On this basis, we transfected siRNAs against Arp2 into these cells to disrupt the interaction between Coronin3 and Arp2 (Fig. 4E). The subsequent in vitro cell motility assay showed that Arp2 knockdown antagonized the increased migratory and invasive abilities of cells caused by Coronin3 upregulation (Fig. 4F–H). These data indicated that interaction with Arp2 was essential for the function of Coronin3.
Figure 4. Attenuation of Coronin3-mediated cell migration and invasion by Arp2 inhibition. (A) The establishment of MKN28-NM cells stably transfected with Coronin3. (B) Ectopic expression of Coronin3 increased the total migration of MKN28-NM cells. (C) Representative images of the migration and invasion assays in MKN28-NM cells before and after Coronin3 transfection. (D) Ectopic expression of Coronin3 increased cell motility. (E) Arp2 was knocked down by siRNA in MKN28-NM cells with stable Coronin3 expression. (F) Arp2 knockdown abolished the increased cell migration caused by Coronin3. (G) Representative images of the migration and invasion assays in MKN28-NM-Coronin3 cells before and after Arp2 inhibition. (H) Downregulation of Arp2 suppressed Coronin3-mediated cell motility activities.
Expression levels of Arp2 in normal and GC tissues
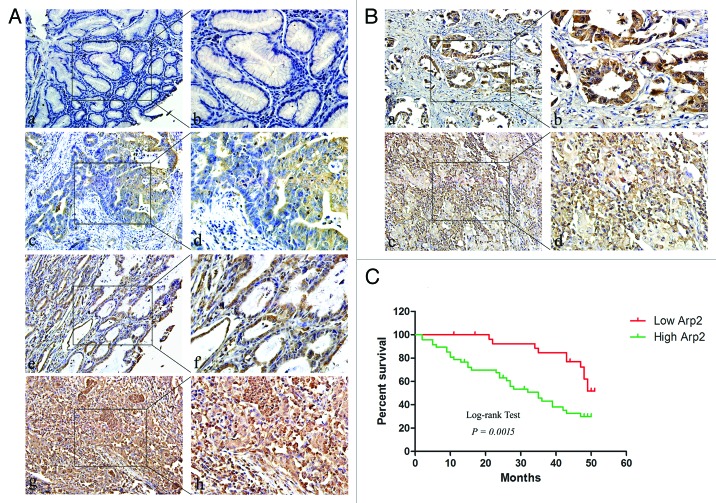
Having identified Arp2 as a protein that can interact with Coronin3, we next performed a tissue-array study to demonstrate the characteristics of Arp2 expression in normal and gastric cancer tissues. Compared with adjacent normal gastric tissues, Arp2 was markedly increased in cancer tissues, and the expression intensity of Arp2 was stronger in tumors with moderate and poor differentiation than in well-differentiated tumors (Fig. 5A; Table 1). Most cases of stage I and II cancers exhibited weak (5/23, 21.7%) or moderate (11/23, 47.8%) Arp2 expression, and the majority of tumors at stage III and IV exhibited moderate (14/52, 26.9%) and strong (21/52, 40.4%) staining (Table 1). Arp2 was not associated with age or gender but was upregulated in cases with metastases (Table 1). We also compared the expression intensity of Arp2 in an additional 40 GC tissues and their related metastatic lymph lodes (Fig. 5B; Table 2). Among the 40 GC cases, 5 primary GC tissue samples (12.5%) showed negative staining, whereas the staining in 14 (35.0%), 17 (42.5%), and 4 (10.0%) samples was scored as weakly, moderately, and strongly positive, respectively. For the related metastatic lymph lode tissues, the staining from 3 (7.5%), 4 (10.0%), 16 (40.0%), and 17 (42.5%) samples was scored as negative, weakly positive, moderately positive, and strongly positive, respectively. The statistical analysis showed that Arp2 was upregulated in the lymph nodes than primary GC tissues.
Figure 5. Arp2 expression in gastric cancer. (A) Arp2 expression levels in normal and cancerous gastric tissues. Extremely weak positive signals were detected in normal gastric mucosa (a). Moderate Arp2 staining intensity was observed in cancers with high differentiation (c), while substantially stronger signals were detected in moderately and poorly differentiated gastric cancers (e and g). (a, c, e, and g) 200×; (b, d, f, and h) 400×. (B) Arp2 expression levels in primary cancer tissues and corresponding metastatic lymph nodes. The average intensity of Arp2 was detected in a cancerous case with moderate differentiation (a), and an extremely strong signal was observed in the lymph node (c). (a and c) 200×; (b and d) 400×. (C) Kaplan–Meier curve for the correlation between Arp2 expression and overall survival.
Table 2. Expression of Arp2 in gastric cancer and related lymph node metastases.
| Histological type | No. | H-score of Arp2 | P | |||
|---|---|---|---|---|---|---|
| − | + | ++ | +++ | |||
| Primary cancer tissues | 40 | 5 | 14 | 17 | 4 | 0.003* |
| Lymph node metastasis | 40 | 3 | 4 | 16 | 17 | |
Additionally, we compared the localization of Coronin3 and Arp2 in serial sections of GC tissues. The subcellular localization of Coronin3 and Arp2 was similar in GC tissues (Fig. S2), and this phenomenon further supported that Coronin3 and Arp2 were proteins with mutual interactions.
To better elucidate the clinical significance of Arp2, we performed additional analysis of its correlations with overall survival of GC patients. The result demonstrated that the overall survival of patients with higher expression levels of Arp2 was significantly reduced compared with that of patients with low Arp2 expression levels (Fig. 5C).
Clinical significance of Coronin3 and Arp3 in survival analyses of GC patients
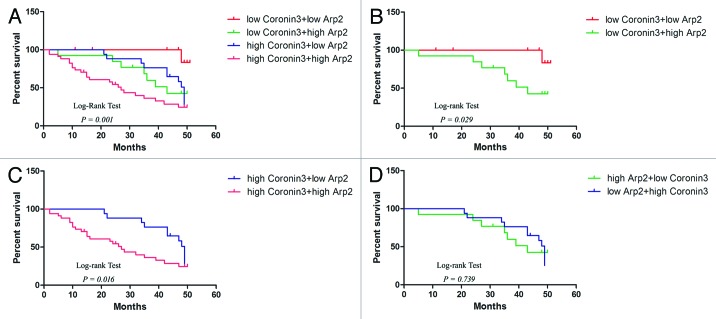
Coronin3 and Arp2 were demonstrated to be associated with over survival periods of GC patients by separate analyses. We next examined their combined value in distinguishing GC patients with different survival periods. In general, the patients were divided into 4 groups: Group 1, low expression of Coronin3 and Arp2; Group 2, low expression of Coronin3 and high expression of Arp2; Group 3, high expression of Coronin3 and low expression of Arp2; Group 4, high expression of Coronin3 and Arp2. Kaplan–Meier analyses depicted distinct survival patterns among the 4 subgroups (Fig. 6A). GC patients with high levels of both Coronin3 and Arp2 had the poorest prognoses (Fig. 6A). In both high-Coronin3 and low-Coronin3 tumors, the patients with tumors with low Arp2 expression achieved longer survival compared with patients with tumors with high Arp2 expression (Fig. 6B and C). Meanwhile, the survival periods of patients with low Coronin3/high Arp2 and high Coronin3/low Arp2 were not significantly different (Fig. 6D).
Figure 6. Kaplan–Meier analyses with a log-rank test of survival. (A) Correlation of the combination of Coronin3 and Arp2 expression with overall survival. (B) Correlation of Arp2 expression with overall survival among patients bearing tumors with low Coronin3 expression. (C) Correlation of Arp2 expression with overall survival among patients bearing tumors with high Coronin3 expression. (D) Survival curves for patients with low Coronin3/high Arp2 and high Coronin3/low Arp2.
Discussion
Coronins are highly conserved F-actin binding proteins. Functional studies have suggested that Coronins play important roles in lamellipodial protrusion, cell motility, and chemotaxis.8,13-15 For example, genetic deletion of Coronin1A in mice results in severe immune system defects, including the failure of T cells to respond to chemokine-induced cell migration.14 Coronin1B, an isoform with ubiquitous expression throughout the body, has been shown to influence both lamellipodial protrusion and whole-cell motility.8,15 Notably, to date, Coronin3 is the only member of the Coronin family to be associated with cancer metastasis. Coronin3 overexpression has been observed in hepatocellular carcinoma (HCC) cell lines with metastatic potential, indicating its use as a novel biomarker for HCC invasion and progression in vivo.11 Ren and colleagues reported for the first time that Coronin3 is highly expressed in GC tissues and metastatic lymph nodes and can regulate the migratory and invasive abilities of GC cells both in vitro and in vivo.13 Based on this study, we explored the connection between Coronin3 expression and overall survival in GC patients. We determined that GC patients with different prognoses could be distinguished by their Coronin3 expression levels and that an increased Coronin3 expression level might indicate a relatively shorter overall survival. Taken together, these findings further validate the clinical significance of Coronin3 in GC and indicate Coronin3 as a potential therapeutic target for GC treatment.
The dynamic branched network comprised of the F-actin cytoskeleton is crucial in protrusion formation during cell migration. Arp2/3, a highly conserved complex with 7 subunits, is the key nucleator for this cytoskeleton network.4-6 Extensive studies have shown that the Arp2/3 complex binds existing actin filaments and initiates the formation of new filaments that branch off the original filaments.16 Recently, an abnormal expression level of Arp2/3 was found in several types of cancer; however, the conclusions of the different studies were not consistent.17-20 The association between Arp2/3 and GC has been reported in at least 3 studies.18-20 All 7 subunits of the Arp2/3 complex were decreased in a qRT-PCR study containing 32 primary cancer tissues.18 In another qRT-PCR analysis, a reduced expression of p41-Arc, a subunit of the Arp2/3 complex, was found in 22 GC tissues and several cell lines.19 In contrast, Zheng et al. conducted an immunohistochemical study demonstrating that both Arp2 and Arp3 are increased in GC tissues.20 In our study, we employed immunohistochemistry to detect Arp2 in GC tissues and observed a remarkable increase in the Arp2 signal. Combined with the reports mentioned above, the expression intensity of Arp2/3 in GC appears to be contrasting at the mRNA and protein levels, perhaps indicating a role for post-transcriptional modulation in the regulation of Arp2/3. As a core subunit of the Arp2/3 complex, the quantity of Arp2 should affect the formation and activity of the Arp2/3 complex, which would ultimately influence the remodeling of the F-actin network and cell motility. To test our hypothesis, we conducted a series of in vitro functional studies and found that Arp2 upregulation promoted the migration and invasion of MKN-28NM cells, while its knockdown suppressed the motility of MKN28-M cells. These findings were in accordance with our hypothesis and suggested a vital role for Arp2 in regulating GC cell motility.
It has been reported that Coronins are Arp2/3-binding proteins.8,15,21,22 Yeast Coronins physically associate with the Arp2/3 complex through binding to the p35 subunit and inhibit the branching of actin filaments.21 Cai et al. performed a series of studies on the function of Coronin1B and its regulation of the F-actin network.8,15 Their findings indicated that Coronin1B coordinates Arp2/3-dependent actin filament nucleation and cofilin-mediated filament “turnover” at the leading edge of migrating fibroblasts.15 In lamellipodia, Coronin1B antagonizes cortactin and remodels Arp2/3-containing actin branches.8 In addition, the authors revealed that the interaction between Coronin1B and the Arp2/3 complex is mediated by phosphorylation of Ser-2 on Coronin1B; PKC was the major kinase in this process.15,23 In our study, Coronin3 was shown to associate with Arp2, and this phenomenon was consistent with previous studies.24 To further demonstrate the role of this interaction in cell motility regulation, we silenced Arp2 in MKN-28NM cells stably transfected with Coronin3. Our results demonstrated that ectopic expression of Coronin3 promoted cancer cell migration and invasion, and this effect was attenuated by Arp2 knockdown. In particular, ectopic expression of Coronin3 did not influence Arp2 expression levels, and neither upregulation nor downregulation of Arp2 affected Coronin3 (data not shown). This fact excluded the possibility that Coronin3 or Arp2 functioned directly through the regulation of their mutual expression. Therefore, these results indicated that Coronin3 function depends on its co-existence with Arp2. Furthermore, these are the first studies to describe that Coronin3 functions in cancer metastasis through coordination with the Arp2/3 complex.
According to previous studies, Coronin1B serves to temper filament nucleation by inhibiting the activity of the Arp2/3 complex in lamellipodia.8,15 In yeast, high concentrations of Coronins inhibit Arp2/3 activity, while low concentrations enhance the binding of filaments to Arp2/3.25 We found that Coronin3 interacts with Arp2 in GC cells; however, its effects on the activity of the Arp2/3 complex remain unclear. Due to the conservation of the Coronins, we propose that Coronin3 might function through Arp2/3 in a similar manner, and this speculation requires further exploration.
Thus far, our series of studies have shown that Coronin3 regulates GC cell migration and invasion via at least 2 pathways. Coronin3 modulates the F-actin network through interacting with Arp2 and also directly affects the expression of MMP-9 and cathepsin K. Therefore, targeting Coronin3 and Arp2 could be a novel therapeutic strategy in the treatment of GC metastases.
Materials and Methods
Cell lines and gastric cancer tissues
The SGC7901, AGS, KATOIII, BGC823, MKN-45, and MKN-28 human gastric cancer cell lines were maintained in our laboratory. Sublines with low (MKN28-NM) and high (MKN28-M) metastatic potential were derived from the MKN28 parental cell line by our laboratory.26 BGC823 cells were maintained in DMEM, and the remaining cell lines were grown in RPMI-1640 supplemented with 10% fetal calf serum (FBS, Gibco). All cells were incubated at 37 °C with 5% CO2 in a humidified incubator. Five gastric cancer tissues were obtained from the Department of Pathology of Xijing Hospital, and serial sections were subsequently generated. Human ethics approval was obtained from Xijing Hospital.
Tissue microarrays
Gastric cancer tissue microarrays (Aomei) included 75 cases of malignant gastric tissues and the corresponding normal tissues. Detailed clinicopathological and follow-up information were provided for all cases. An additional tissue microarray (Aomei) including 40 gastric adenocarcinoma spots and 40 matched lymph lode metastases was also obtained.
Immunohistochemistry
Immunohistochemical staining of Coronin3 and Arp2 was performed as previously described.13 Target protein expression was evaluated according to the ratio of positive cells per specimen, and staining intensity was determined according to a defined histological scoring method. The proportion of positive cells in each specimen was quantitatively evaluated as follows: 0, for 0% staining; 1 for 0.01–25%; 2 for 25.01–50%; 3 for 50.01–75%; and 4 for >75% of the cells examined. Staining intensity was graded as follows: 0, no signal; 1, weak; 2, moderate; and 3, strong. The histological score of Coronin3 or Arp2 expression for each section was computed by the following formula: histological score (H score) = ratio score × intensity score. A total score of 0–12 was calculated and graded as negative (−, score: 0), weak (+, score: 1–4), moderate (++, score: 5–8), or strong (+++, score: 9–12). Scoring with “−” and “+” scoring was regarded as lower expression of the target proteins, while “++” and “+++” represented higher expression of target proteins.
MALDI-TOF/TOF analysis and peptide sequencing
The excised protein bands of interest were destained with 25 mM ammonium bicarbonate/30% acetonitrile followed by incubation with 100% acetonitrile for 15 min. Disulfide bond cleavage was performed with 20 mM dithiothreitol, and carbamidomethylation was obtained with 50 mM iodine acetamide. Gel sections were dehydrated with 100% acetonitrile and dried for ~25 min in a vacuum centrifuge. The dried gel pieces were soaked in 10 ng/μL trypsin/10 mM ammonium bicarbonate and then covered with 10 mM ammonium bicarbonate and incubated at 37 °C overnight. The following day, the samples were sonicated 3 times for 15 min each in 60% acetonitrile/0.1% trifluoroacetic acid, then centrifuged and dried in a SpeedVac. The samples were diluted with 2% acetonitrile/98% H2O/0.1% TFA for MS and MS/MS analyses, which were performed using the AB SCIEX TOF/TOF™ 5800 system (USA). ProteinPilot 4.0™ software was used for protein identification and quantitation as previously described.27
Immunoprecipitation
All immunoprecipitation (IP) experiments were performed as previously described using an IP kit (Pierce).28 Briefly, cells at approximately 80% confluence were washed with ice-cold PBS and then scraped off. Cells were harvested by centrifugation and lysed in RIPA lysis buffer for 30 min. After centrifugation at 12 000 rpm, the supernatant was collected for IP. Lysates were spun, and the supernatants were transferred to another tube and incubated with primary antibody and 40 mL 25% protein A/G agarose slurry. The protein A/G agarose was recovered by centrifugation at 5000 rpm and washed 4 times with ice-cold lysis buffer. Proteins were eluted with loading buffer by boiling for 10 min and subjected to immunoblot analysis.
Western blot analysis
Cells or tissues were solubilized in RIPA buffer (Beyotime) containing protease inhibitors (Roche), and western blot analysis was performed as previously described.13 Primary antibodies against Coronin3, Arp2 (Abcam), and β-actin (Sigma) were used.
Production and transfection of oligonucleotides, plasmids, and lentivirus
Three pairs of siRNAs targeting Arp2 were purchased from RiboBio Co., Ltd. Arp2 expression plasmids were constructed and produced by GeneChem Co., Ltd. Target cells were transfected with the oligonucleotides or plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Stable transfectants overexpressing Coronin3 were generated by lentiviral transduction using a pGCsil-GFP vector (GeneChem Co., Ltd.). A lentiviral vector that expressed green fluorescent protein alone (LV-GFP) was used as a control.
Wound-healing assay
For wound-healing assays, 3 × 105 cells were seeded into 6-well plates. Following a 48 h incubation, a scratch was drawn across the center of the well using a plastic pipette tip to produce a sharp, 1-mm-wide wound area. The cells were washed with fresh, FBS-free medium 3 times and incubated in medium containing 1% FBS for 48 h. Cell movement into the wound area was examined using a phase-contrast microscope.
Cell migration and invasion assay
Cell migration and invasion assays were performed as previously described using transwell plates (8-μm pore size, Corning Costar Corp.) and Matrigel (BD).13 Cells that migrated to the bottom side of the membrane were counted using a microscope, and relative cell motility activity was calculated using the following formula: relative cell motility activity = number of migrated or invaded cells from different treatment groups ÷ number of migrated or invaded cells from the control group.
Total migration length assay
To analyze total migration length, 1 × 104 lentiviral-infected cells were seeded into 96-well plates and incubated in medium containing 10% FBS for 24 h. The cells were then placed into a cellomics instrument (Thermo Fisher). The migrating paths of the cells were automatically recorded, and the total migration length was calculated.
Statistical analyses
Statistical analyses were performed using SPSS 17.0 software. χ2 tests were used to evaluate the significance of the differences in Coronin3 or Arp2 expression frequency between gastric cancer tissues and lymph node metastases. The Kruskal–Wallis H-test and the Mann–Whitney U test were used to analyze the relationship between Coronin3 or Arp2 expression and clinicopathological factors. The association of Coronin3 or Arp2 with overall survival was analyzed by Kaplan–Meier curves. A one-way ANOVA was used for analyzing the results of the migration and invasion assays. Differences were considered significant when P values were less than 0.05.
Supplementary Material
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by series of grants, including National Natural Science Foundation of China (81201929, 81301804, and 81301883). We would like to thank Zheng Chen, Sijun Hu, and Miaomiao Tian from the Fourth Military Medical University for providing excellent technical assistance in MALDI-TOF/TOF analysis and peptide sequencing.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–95. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rouiller I, Xu XP, Amann KJ, Egile C, Nickell S, Nicastro D, Li R, Pollard TD, Volkmann N, Hanein D. The structural basis of actin filament branching by the Arp2/3 complex. J Cell Biol. 2008;180:887–95. doi: 10.1083/jcb.200709092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper JA, Wear MA, Weaver AM. Arp2/3 complex: advances on the inner workings of a molecular machine. Cell. 2001;107:703–5. doi: 10.1016/S0092-8674(01)00605-5. [DOI] [PubMed] [Google Scholar]
- 7.Kreishman-Deitrick M, Rosen MK. Ignition of a cellular machine. Nat Cell Biol. 2002;4:E31–3. doi: 10.1038/ncb0202-e31. [DOI] [PubMed] [Google Scholar]
- 8.Cai L, Makhov AM, Schafer DA, Bear JE. Coronin 1B antagonizes cortactin and remodels Arp2/3-containing actin branches in lamellipodia. Cell. 2008;134:828–42. doi: 10.1016/j.cell.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Hostos EL. A brief history of the coronin family. Subcell Biochem. 2008;48:31–40. doi: 10.1007/978-0-387-09595-0_4. [DOI] [PubMed] [Google Scholar]
- 10.Thal D, Xavier CP, Rosentreter A, Linder S, Friedrichs B, Waha A, Pietsch T, Stumpf M, Noegel A, Clemen C. Expression of coronin-3 (coronin-1C) in diffuse gliomas is related to malignancy. J Pathol. 2008;214:415–24. doi: 10.1002/path.2308. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Peng CW, Hou JX, Zhang YH, Chen C, Chen LD, Li Y. Coronin-1C is a novel biomarker for hepatocellular carcinoma invasive progression identified by proteomics analysis and clinical validation. J Exp Clin Cancer Res. 2010;29:17. doi: 10.1186/1756-9966-29-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luan SL, Boulanger E, Ye H, Chanudet E, Johnson N, Hamoudi RA, Bacon CM, Liu H, Huang Y, Said J, et al. Primary effusion lymphoma: genomic profiling revealed amplification of SELPLG and CORO1C encoding for proteins important for cell migration. J Pathol. 2010;222:166–79. doi: 10.1002/path.2752. [DOI] [PubMed] [Google Scholar]
- 13.Ren G, Tian Q, An Y, Feng B, Lu Y, Liang J, Li K, Shang Y, Nie Y, Wang X, et al. Coronin 3 promotes gastric cancer metastasis via the up-regulation of MMP-9 and cathepsin K. Mol Cancer. 2012;11:67. doi: 10.1186/1476-4598-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mugnier B, Nal B, Verthuy C, Boyer C, Lam D, Chasson L, Nieoullon V, Chazal G, Guo XJ, He HT, et al. Coronin-1A links cytoskeleton dynamics to TCR alpha beta-induced cell signaling. PLoS One. 2008;3:e3467. doi: 10.1371/journal.pone.0003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai L, Marshall TW, Uetrecht AC, Schafer DA, Bear JE. Coronin 1B coordinates Arp2/3 complex and cofilin activities at the leading edge. Cell. 2007;128:915–29. doi: 10.1016/j.cell.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol. 2013;14:7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- 17.Rauhala HE, Teppo S, Niemelä S, Kallioniemi A. Silencing of the ARP2/3 complex disturbs pancreatic cancer cell migration. Anticancer Res. 2013;33:45–52. [PubMed] [Google Scholar]
- 18.Kaneda A, Kaminishi M, Sugimura T, Ushijima T. Decreased expression of the seven ARP2/3 complex genes in human gastric cancers. Cancer Lett. 2004;212:203–10. doi: 10.1016/j.canlet.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Kaneda A, Kaminishi M, Nakanishi Y, Sugimura T, Ushijima T. Reduced expression of the insulin-induced protein 1 and p41 Arp2/3 complex genes in human gastric cancers. Int J Cancer. 2002;100:57–62. doi: 10.1002/ijc.10464. [DOI] [PubMed] [Google Scholar]
- 20.Zheng HC, Zheng YS, Li XH, Takahashi H, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Arp2/3 overexpression contributed to pathogenesis, growth and invasion of gastric carcinoma. Anticancer Res. 2008;28(4B):2225–32. [PubMed] [Google Scholar]
- 21.Humphries CL, Balcer HI, D’Agostino JL, Winsor B, Drubin DG, Barnes G, Andrews BJ, Goode BL. Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J Cell Biol. 2002;159:993–1004. doi: 10.1083/jcb.200206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai L, Makhov AM, Bear JE. F-actin binding is essential for coronin 1B function in vivo. J Cell Sci. 2007;120:1779–90. doi: 10.1242/jcs.007641. [DOI] [PubMed] [Google Scholar]
- 23.Cai L, Holoweckyj N, Schaller MD, Bear JE. Phosphorylation of coronin 1B by protein kinase C regulates interaction with Arp2/3 and cell motility. J Biol Chem. 2005;280:31913–23. doi: 10.1074/jbc.M504146200. [DOI] [PubMed] [Google Scholar]
- 24.Rosentreter A, Hofmann A, Xavier CP, Stumpf M, Noegel AA, Clemen CS. Coronin 3 involvement in F-actin-dependent processes at the cell cortex. Exp Cell Res. 2007;313:878–95. doi: 10.1016/j.yexcr.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Liu SL, Needham KM, May JR, Nolen BJ. Mechanism of a concentration-dependent switch between activation and inhibition of Arp2/3 complex by coronin. J Biol Chem. 2011;286:17039–46. doi: 10.1074/jbc.M111.219964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, Guo X, Wang B, Gang Y, Zhang Y, et al. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6:e1000879. doi: 10.1371/journal.pgen.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang J, Zhao G, Lin T, Tang S, Xu G, Hu S, Bi Q, Guo C, Sun L, Han S, et al. A peptide derived from phage display library exhibits anti-tumor activity by targeting GRP78 in gastric cancer multidrug resistance cells. Cancer Lett. 2013;339:247–59. doi: 10.1016/j.canlet.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Lu Y, Wang X, Liu Z, Jin B, Chu D, Zhai H, Zhang F, Li K, Ren G, Miranda-Vizuete A, et al. Identification and distribution of thioredoxin-like 2 as the antigen for the monoclonal antibody MC3 specific to colorectal cancer. Proteomics. 2008;8:2220–9. doi: 10.1002/pmic.200700770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.