Abstract
Dopamine plays a role in the pathophysiology of schizophrenia and addiction. Imaging studies have indicated that striatal dopamine release is increased in schizophrenia, predominantly in the precommissural caudate (preDCA), and blunted in addiction, mostly in the ventral striatum (VST). Therefore we aimed to measure striatal dopamine release in patients with comorbid schizophrenia and substance dependence. We used [11C]raclopride PET and an amphetamine challenge to measure baseline dopamine D2-receptor availability (BPND) and its percent change post-amphetamine (∆BPND, to index amphetamine-induced dopamine release) in striatal subregions in 11 unmedicated, drug-free patients with both schizophrenia and substance dependence and 15 healthy controls. There were no significant group differences in baseline BPND. Linear mixed modeling using ∆BPND as the dependent variable and striatal ROI as a repeated measure indicated a significant main effect of diagnosis, F(1, 24)=8.38, p=.008, with significantly smaller ∆BPND in patients in all striatal subregions (all ps≤.04) except VST. Among patients, change in positive symptoms after amphetamine was significantly associated with ∆BPND in the preDCA (rs=.69, p=.03) and VST (rs=.64, p=.05). In conclusion, patients with comorbid schizophrenia and substance dependence showed significant blunting of striatal dopamine release, in contrast to what has been found in schizophrenia without substance dependence. Despite this blunting, dopamine release was associated with the transient amphetamine-induced positive-symptom change, as observed in schizophrenia. This is the first description of a group of patients with schizophrenia who display low presynaptic dopamine release, yet show a psychotic reaction to increases in D2 stimulation, suggesting abnormal post-synaptic D2 function.
Keywords: PET, dopamine, schizophrenia, striatal, alcohol, drug dependence
Introduction
Dopamine (DA) plays a role in the pathophysiology of schizophrenia and addiction. Imaging studies using PET or SPECT with D2/3 radiotracers and the amphetamine-challenge paradigm measure the change in D2/3 radiotracer binding related to changes in synaptic DA concentration induced by amphetamine, thus providing an indirect measure of stimulated DA release. These studies have shown that, compared to healthy controls, DA release is higher in the striatum in patients with schizophrenia1–4 and is blunted in those with addiction.5–7 With better topographical characterization from use of higher-resolution scanners and better data analysis methods allowing reliable measurements within the striatal subdivisions,8 more recent studies have suggested that in schizophrenia, DA transmission is increased in the precommissural caudate (preDCA) of the associative striatum in particular,9 whereas in addiction, the ventral striatum (VST) appears to be especially affected.6 In summary, DA transmission is altered in opposite directions in schizophrenia and addiction, apparently within discrete striatal subdivisions.
Epidemiological studies have shown that patients with schizophrenia are at greater risk for developing substance-use disorders than the general population. The Epidemiologic Catchment Area Study reported a 4.6-fold increase in the prevalence of any substance abuse in patients with schizophrenia compared to the general population.10 Alcohol is most commonly used, with a lifetime prevalence of abuse or dependence of 33.7% compared to 27.5% for all other drugs. The CATIE trial reported substance use in 60% of patients with schizophrenia,11, 12 and higher rates of homelessness, depression, and severity of psychosis among substance-using patients. In the 544 patients with a substance-use disorder, 87% used alcohol, 44% marijuana, and 36% cocaine. A study examining the temporal relationship between use and symptoms in patients recorded in real time with electronic ambulatory monitoring found that sad mood and psychotic symptoms were associated with later substance use, and that substance use was associated with increased risk of subsequent anxiety and psychotic-symptom onset; these results suggest that while the intent may be to self medicate, the consequences are that substances may exacerbate symptoms.13 Comorbidity occurs early, as substance-use disorders are prevalent in first-episode psychosis patients (up to 50%).14–16 These patients are more likely to show non-adherence to treatment, poor remission rates,17 and higher frequency of suicidal behavior.18
In summary, patients with schizophrenia are often addicted to substances, from early on, and the effects of both factors, the psychotic illness and addiction, are intertwined throughout the stages of the disorders and difficult to disentangle. Few studies have attempted to address how the neurobiology of each disorder may be changed in cases where they co-exist in the same patients. Structural imaging studies have reported inconsistent findings, with some indicating smaller brain volumes in this population compared to schizophrenia without comorbid substance-use disorders,19–21 and others finding no change.22 No studies have assessed parameters of neurotransmission in such patients. Therefore we undertook a study to assess striatal DA dysregulation in patients with both disorders, i.e., schizophrenia and substance dependence. Based on available findings, our working hypothesis was that the striatal DA alterations that have been associated with each disorder, i.e., higher release in the preDCA with schizophrenia and lower release in the VST with addiction, coexist in these comorbid patients. Such a set of alterations could set up a vicious cycle of using drugs to self medicate due to low ventrostriatal dopamine transmission, which in turn may further dysregulate DA functioning in the associative striatum, causing or worsening psychosis. We also hypothesized that DA release in the VST would be negatively related to measures of dependence severity, whereas DA release in the preDCA would be positively associated with severity of psychosis.
Methods
Human Subjects
This study was approved by the Institutional Review Board of New York State Psychiatric Institute (NYSPI) of Columbia University Medical Center (CUMC). All participants provided written informed consent after the procedures were fully explained to them, and all patient participants were independently assessed for capacity to provide consent by a psychiatrist who was not a member of the research team. Dual-diagnosis (DD) patients were recruited through advertisements, clinician referral, the inpatient and outpatient programs at NYSPI, and the emergency department of CUMC. Healthy controls were recruited through advertisements. Medical screening procedures included a physical examination and history, blood and urine tests, an electrocardiogram, and a structural magnetic resonance imaging (MRI) scan of the brain. Participants were free of significant medical and neurological illnesses and were not pregnant or nursing.
Inclusion criteria for DD patients were: 1) lifetime DSM-IV diagnosis of schizophrenia, schizoaffective or schizophreniform disorder, with the requirement of follow-up assessments of patients with schizophreniform disorder to confirm the diagnosis of schizophrenia; 2) lifetime DSM-IV diagnosis of alcohol, cannabis, and/or cocaine dependence; 3) no history of violent behavior; 4) no antipsychotic treatment for 3 weeks prior to PET scan participation; and 5) a negative urine drug screen prior to PET. Nicotine dependence was permitted for DD patients. Inclusion criteria for healthy controls were: 1) absence of any current or past DSM-IV Axis-I diagnosis, including substance abuse (past but not current nicotine dependence was permitted); and 2) no family history (first-degree) of psychotic illness. Current smokers were excluded from the control group with the aim of having a “clean” comparison group with regard to any kind of substance dependence. Diagnostic status was determined with the Psychiatric Research Interview for Substance and Mental Disorders for DSM-IV (PRISM-IV)23 for DD patients, and with either the Diagnostic Interview for Genetic Studies (DIGS)24 or an abbreviated version of the Structured Clinical Interview for DSM-IV Axis I disorders (SCID-IV)25 for healthy controls. Participant and parental socioeconomic status (SES) were calculated according to Hollingshead.26 Additionally, within one week before participating in PET, DD patients were assessed with the Positive and Negative Syndrome Scale (PANSS).27 Clinical assessments were administered by trained interviewers.
DD patients with current drug use upon study enrollment were offered inpatient admission to assist in fulfilling the abstinence requirements for PET participation. Patients who were psychiatrically stable were permitted to undergo detoxification as outpatients, underwent weekly urine toxicology tests (at minimum), and were scheduled for PET once they tested negative. A urine toxicology test was repeated on PET days to confirm abstinence.
PET Acquisition
All participants underwent two PET scans with an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN) on the same day: at baseline and after receiving amphetamine. [11C]Raclopride was administered as bolus plus constant infusion for 80 minutes as previously described.28 Emission scan data were collected in 3-dimensional mode 40–80 minutes after [11C]raclopride injection as eight frames of 5 minutes each. The second [11C]raclopride injection was initiated 2 minutes after I.V. administration of 0.3 mg/kg of dextro-amphetamine. Venous blood samples were collected 40 minutes after amphetamine administration to measure plasma amphetamine levels.
Image Analysis
All participants received a high-resolution structural MRI scan for coregistration. Image analysis was performed using MEDx (Medical Numerics, MD). The striatum was divided into five anatomical regions of interest (ROIs): ventral striatum, precommissural dorsal caudate (preDCA), precommissural dorsal putamen (preDPU), postcommissural caudate (postCA), and postcommissural putamen (postPU).29 Based on the input each of these regions receives, they have been classified into three functional subdivisions: the ventral, or limbic striatum (VST), the associative striatum (AST, comprising the preDCA, preDPU, and postCA), and the sensorimotor striatum (SMST, comprising the postPU). ROIs were manually drawn on each subject’s MRI and transferred to co-registered PET data. Cerebellum (CER) was used as a reference region to estimate the concentration of free and non-specifically bound [11C]raclopride. Our analyses included the 5 striatal subregions and a composite “whole striatum” region.
Equilibrium analysis was used to derive the outcome measure BPND (binding potential relative to the nondisplaceable compartment in the brain):
BPND reflects dopamine D2/3 receptor availability, as measured by [11C]raclopride. The primary outcome measure for this study was the percent change in BPND from baseline to post-amphetamine scan (∆BPND); this measure reflects the relative reduction in D2/3 receptor availability for [11C]raclopride binding after amphetamine-induced dopamine release and thus is used to index amphetamine-induced DA release.
Cardiovascular and Behavioral Responses to Amphetamine
Blood pressure and heart rate were measured at baseline and at regular intervals after amphetamine (approximately every 2.5 minutes for the first 20 minutes, every 5 minutes from 20–40 minutes, and every 10 minutes from 40–80 minutes). Subjective responses to amphetamine were measured using a simplified version of the Amphetamine Interview Rating Scale (AIRS);30, 31 Supplement Table S1. The area under the curve (AUC) was calculated, relative to baseline, for each cardiovascular measure and AIRS item. The PANSS was used to evaluate behavioral responses to amphetamine. Change scores from pre- to post-amphetamine were calculated for the Positive-Symptom and Negative-Symptom subscales; Supplement Table S2.
Statistical Analysis
Group comparisons on demographic features were performed with independent-samples t-tests or chi-square tests. Within-subject comparisons on scan parameters across scan conditions were performed with paired t-tests, and between-group comparisons on scan parameters, striatal volumes, and AUC values (cardiovascular and AIRS) were performed with independent-samples t-tests. In the case of non-normally distributed data, comparisons were repeated with non-parametric tests (Wilcoxon for paired tests, Mann-Whitney for independent-samples tests); non-parametric test results are included here only if they differed from parametric test results. Group comparisons on our primary PET outcome measures, baseline BPND and ∆BPND, were performed using linear mixed modeling for which diagnosis (DD v. control) and ROI (5 striatal subregions) were treated as fixed effects, and ROI was treated as a repeated-measures variable; between-group planned comparisons were also conducted for each striatal subregion. Cohen’s d was used as an index of effect size; the standard deviation pooled across the 2 groups was used for this calculation. Correlational analyses were performed using Spearman rank correlations, due to non-normal distributions (skew and/or kurtosis) observed for several variables. For exploratory analyses, we used Bonferroni correction for comparisons across 6 ROIs (5 striatal subregions and whole striatum), which resulted in an adjusted p value of .008. All statistical tests were two-tailed.
Results
Demographic and Clinical Characteristics
Twenty-one patients were enrolled to participate in this study; due to technical difficulties or participant withdrawal, only 13 of these 21 completed scanning procedures. However, data from 2 of these patients were excluded from analyses (one due to excessive movement in the scanner, and the other because additional diagnostic information on follow-up suggested that her initial presentation of schizoaffective disorder was due to Lyme disease), resulting in a final patient group of 11. Fifteen healthy adults matched to the patient group for age, sex, ethnicity, and parental SES were included as a comparison group (Table 1). DD patients had significantly lower participant SES compared to controls, t(17.91)=−2.97, p=.008; Table 1.
Table 1.
Sample Characteristics and Scan Parameters
| Sample Characteristic | Controls (n=15) | DD Patients (n=11) | pa | ||
|---|---|---|---|---|---|
| Age | 30.69 ± 7.57 | 29.54 ± 8.62 | 0.72 | ||
| Sex (n) | 4 female, 11 male | 1 female, 10 male | 0.26 | ||
| Ethnicity (n) | 3C, 4AA, 3H, 2As, 3 mixed | 1C, 4AA, 4H, 1As, 1 mixed | 0.45b | ||
| Parental SESc | 40.87 ± 10.87 | 36.36 ± 13.74d | 0.41 | ||
| Participant SESc | 32.07 ± 16.56 | 18.45 ± 5.47 | 0.01 | ||
| Psychosis age of onset | --- | 18.09 ± 4.16 | --- | ||
| Duration of psychotic illness (years) | --- | 11.00 ± 8.73 | --- | ||
| Weeks since antipsychotic medication exposure (median, range)e | --- | 10, 1.71f–313 | --- | ||
| Drugs of dependence or abuse (ng) | --- | 11 cannabis, 8 alcohol, 3 cocaine, 1 otherh | --- | ||
| Age of onset of regular drug use | --- | 16.45 ± 2.46 | --- | ||
| Duration since regular use initiated (years) | --- | 12.90 ± 7.68 | --- | ||
| Weeks since last drug use (median, range)i, j | --- | 4.93, 1.71–15 | --- | ||
| Scan Parameterk | Baseline | Post-amph | Baseline | Post-amph | |
| Injected dose (mCi) | 8.93 ± 2.29 | 8.48 ± 2.42 | 7.59 ± 1.35 | 8.14 ± 1.38 | |
| Injected mass (ug) | 4.30 ± 1.64 | 3.70 ± 1.40 | 4.21 ± 1.77 | 3.63 ± 1.53 | |
| Specific activity (Ci/mmol) | 1287 ± 621 | 1416 ± 755 | 1159 ± 600 | 1430 ± 759 | |
| Amphetamine levell (ng/ml) | --- | 42.53 ± 7.28 | --- | 38.45 ± 9.04 | |
Note. M ± SD unless otherwise noted. AA=African American; As=Asian; C=Caucasian; DD=dual-diagnosis; H=Hispanic; M=mean; post-amph=post-amphetamine; SD=standard deviation; SES=socioeconomic status.
Independent-samples t-tests for continuous variables; chi-square tests for dichotomous variables.
Groups were compared on a dichotomized ethnicity variable (Caucasian v. ethnic minority).
Socioeconomic status was calculated according to Hollingshead, 1975.
Parental SES was missing for 4 patients.
n=9 (2 patients were naïve to antipsychotics).
12 days for one patient, and a minimum of 3 weeks for all others.
Overlapping; see Table S3 for drugs of dependence and/or abuse by subject.
One subject also met criteria for amphetamine, sedative, opioid, and hallucinogenic dependence.
n=10; for 1 patient, duration of drug-free period unclear due to discrepant results between the urine toxicology test (dipstick) conducted on scan day and the quantitative laboratory results of the same urine sample. Results were essentially unchanged when excluding this patient from analyses.
3 patients met criteria for current drug dependence; for 1 of these, duration of drug-free period was unclear (see above footnote); for the other 2 patients, days since last use were 12 (cannabis and alcohol) and 17 (cannabis), respectively. Of the remaining patients, 5 were in early remission and 3 in sustained remission (see Table S3 for details).
No significant within- or between-group differences.
40 minutes after amphetamine administration; missing for 1 control.
Six of the 11 DD patients met criteria for schizophrenia, 4 for schizoaffective disorder, and 1 for schizophreniform disorder (with a later confirmed diagnosis of schizophrenia). Their mean age of psychosis onset was 18.09 ± 4.16, and duration of psychotic illness averaged 11.00 ± 8.73 years (Table 1). Two patients were antipsychotic-naïve. DD patients met criteria for current or past cannabis dependence (n=10) or abuse (n=1), alcohol dependence (n=8), and/or cocaine dependence (n=3); Supplement Table S3. Eight DD patients reported current regular tobacco use (all controls were nonsmokers). Patients began using drugs on a regular basis at a mean age of 16.45 ± 2.46, which was, on average, 12.90 ± 7.68 years before study participation.
Striatal Subregion Volumes
There were no significant group differences in any of the ROI volumes. Across the total sample, age was negatively associated with volumes of the preDCA (rs=−.49, p=.011) and whole striatum (rs=−.47, p=.016); however, these associations were not significant after Bonferroni correction.
Scan Parameters
There were no significant differences between conditions (baseline and post-amphetamine) or between groups in injected dose, injected mass, or specific activity of [11C]raclopride (Table 1). Likewise, the groups did not differ significantly in their plasma amphetamine levels.
Group Comparisons on Baseline BPND and ∆BPND
Baseline D2/3 receptor availability
Linear mixed modeling using baseline BPND as the dependent variable and striatal ROI (the 5 subregions) as a repeated measure showed no significant effect of diagnosis [F(1, 24)=1.12, p=.301; Table 2]. There was a significant main effect of ROI [F(4, 24)=317.69, p<.001], and the diagnosis×ROI interaction was also significant [F(4, 24)=2.78, p=.050]. However, as indicated by planned pairwise comparisons of the estimated marginal means derived from the linear mixed model, there were no significant group differences in baseline BPND in any of the striatal subregions (Table 2).
Table 2.
[11C]Raclopride BPND and ΔBPND by Diagnostic Group
| Controls (n=15) |
DD Patients (n=11) |
Group Comparisonsa |
||||||
|---|---|---|---|---|---|---|---|---|
| ROI | Baseline | Post-amph | ΔBPND | Baseline | Post-amph | ΔBPND | p BPND | p ΔBPND |
| VST | 2.52 ± 0.30 | 2.22 ± 0.31 | −12 ± 7% | 2.48 ± 0.36 | 2.28 ± 0.27 | −7 ± 8% | 0.76 | 0.18 |
| AST | 2.90 ± 0.28 | 2.59 ± 0.30 | −11 ± 6% | 2.77 ± 0.35 | 2.65 ± 0.33 | −4 ± 7% | 0.29 | 0.01 |
| preDCA | 2.77 ± 0.31 | 2.51 ± 0.32 | −9 ± 6% | 2.69 ± 0.35 | 2.59 ± 0.33 | −3 ± 8% | 0.57 | 0.04 |
| preDPU | 3.24 ± 0.31 | 2.84 ± 0.35 | −12 ± 6% | 3.06 ± 0.37 | 2.91 ± 0.35 | −5 ± 7% | 0.19 | 0.01 |
| postCA | 2.19 ± 0.27 | 1.95 ± 0.19 | −11 ± 7% | 2.07 ± 0.37 | 1.97 ± 0.37 | −5 ± 7% | 0.35 | 0.04 |
| postPU | 3.45 ± 0.35 | 2.75 ± 0.32 | −20 ± 5% | 3.20 ± 0.43 | 2.80 ± 0.42 | −12 ± 6% | 0.12 | <0.01 |
| STR | 3.02 ± 0.30 | 2.60 ± 0.29 | −14 ± 5% | 2.87 ± 0.37 | 2.66 ± 0.34 | −7 ± 6% | 0.25 | <0.01 |
Note. Mean ± Standard Deviation (observed).
AST=associative striatum; DD=dual-diagnosis; post-amph=post-amphetamine; postCA=postcommissural caudate; postPU= postcommissural putamen; preDCA=precommissural dorsal caudate; preDPU=precommissural dorsal putamen; STR=whole striatum; VST=ventral striatum.
Group comparisons of estimated marginal means derived from linear mixed modeling (see text) with the exception of AST and STR; these composite regions were compared with independent-samples t-tests.
Amphetamine-induced reduction in D2/3 receptor availability
Linear mixed modeling using ∆BPND as the dependent variable and striatal ROI as a repeated measure indicated significant main effects of diagnosis [F(1, 24)=8.38, p=.008; Table 2 and Figure 1a] and ROI [F(4, 24]=46.51, p<.001]; however, the diagnosis×ROI interaction was not significant [F(4, 24)=0.67, p=.618]. Between-group planned comparisons of the estimated marginal means indicated that DD patients had significantly smaller amphetamine-induced ∆BPND in all striatal subregions except for VST (Table 2 and Figure 1a). The effect sizes (Cohen’s d) for group differences in ∆BPND were large for putamen (preDPU=1.15, postPU=1.42) and caudate (preDCA=0.82, postCA=0.85), and moderate for the VST (0.55); Figure 1b.
Figure 1.
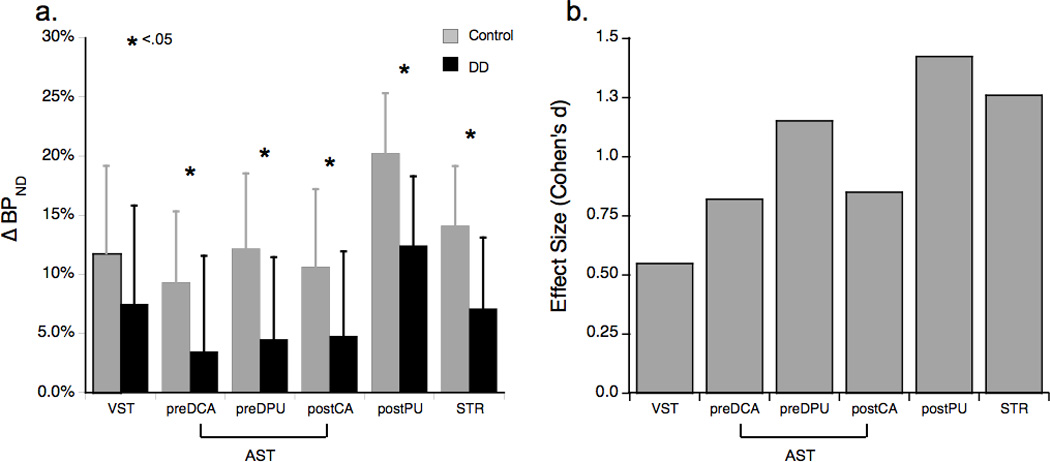
a. ∆BPND for DD (n=11) and control (n=15) groups. *p<.05 per between-group planned comparisons of the estimated marginal means derived from linear mixed modeling (see text) with the exception of STR, which was assessed with an independent-samples t-test.
b. Effect sizes (Cohen’s d) for group differences between DD (n=11) and controls (n=15) in ∆BPND.
DD=dual-diagnosis; see Table 2 for other abbreviations.
Cardiovascular and Behavioral Responses to Amphetamine
The groups did not differ significantly in their mean change (AUC) in systolic or diastolic blood pressure or heart rate following amphetamine administration. Compared to controls, DD patients reported experiencing a greater increase in happiness [t(22)=2.68, p=.014] and energy [t(22)=2.03, p=.054; Mann-Whitney U, p=.032) after amphetamine administration (mean AUC of self-report AIRS ratings; n=9 for patients due to missing data); these differences were not considered significant after Bonferroni correction. Groups did not differ significantly in their amphetamine-related restlessness or anxiety ratings (Supplement Table S1). Among controls but not patients, changes in ratings of energy were positively associated with ∆BPND in the preDPU (rs=.72, p=.002), preDCA (rs=.76, p=.001), VST (rs=.69, p=.005), and whole striatum (rs=.74, p=.002).
Table S2 provides the pre-amphetamine and amphetamine-related change scores for the PANSS Positive- and Negative-Symptom subscales by group. There was a large degree of inter-subject variability in positive-symptom change scores (∆PTOT) among patients, in contrast to controls (DD range=−6.0–10.0; control range=0.0–6.0; Figure 2). For controls, the mean increase in positive symptoms after amphetamine (1.36 ± 1.87, n=14; Table S2) was primarily driven by the excitement item of the Positive-Symptom subscale. As predicted, among DD patients, ∆PTOT (n=10; Figure 2) was significantly associated with ∆BPND in the preDCA (rs=.69, p=.029; Figure 2). This relationship was also observed in the VST (rs=.64, p=.048), although it was not considered significant after multiple-comparisons correction. ∆PTOT was not significantly related to ∆BPND at the level of the whole striatum (rs=.36, p=.306).
Figure 2.

PreDCA ∆BPND and PANSS ∆PTOT (i.e. amphetamine-induced change in PANSS positive symptoms) among DD (n=10) and control (n=14) participants. ∆PTOT could not be calculated for 1 DD patient and 1 control due to missing data. For DD: rs=.69, p=.029. The solid line indicates the least-squares fit for DD patients, and the dashed line marks the “zero” value for PANSS ∆PTOT, i.e. no change in PANSS Positive-Symptom scores from baseline to the post-amphetamine assessment. DD=dual-diagnosis; PANSS=Positive and Negative Syndrome Scale; preDCA=precommissural dorsal caudate.
Correlations with Baseline Symptoms and other Clinical Features
Among the DD patients, there was a significant negative association between age of psychosis onset and ∆BPND in the preDPU (rs=−.77, p=.005), indicating that DD patients who developed psychotic symptoms at an earlier age had greater ∆BPND in the preDPU (or conversely that those with a later age of onset had lower preDPU ∆BPND). There were no other significant associations between psychosis age of onset, duration of psychotic illness, or weeks since antipsychotic medication exposure and baseline BPND or ∆BPND across the striatal subregions. There were also no significant associations between age of onset of regular drug use, years since regular drug use was initiated, or weeks since last drug use and BPND or ∆BPND across the striatal subregions. Baseline symptom severity as measured by the PANSS (using the standard 7-day assessment timeframe) was not significantly associated with baseline BPND or ∆BPND in any striatal subregion.
Discussion
This is the first description of a group of patients with schizophrenia who display low presynaptic dopamine release, yet show a psychotic reaction to increases in D2 stimulation, suggesting abnormal post-synaptic D2 function. In this small group of patients with comorbid schizophrenia and substance dependence, we observed a generalized blunting of DA release in all striatal subregions, to varying degrees, with putamen most affected and VST least affected. Despite the low range of DA release displayed by DD patients compared to controls and to previously published reports in schizophrenia, DA release was significantly associated with the amphetamine-induced change in positive symptoms, as previously observed in schizophrenia.4 Amphetamine-induced changes in positive symptoms were most strongly associated with ∆BPND in the precommissural caudate and VST, adding to the data linking psychosis more specifically with the associative striatum, as well as the limbic striatum, and confirming a prominent role for these two areas in the neurobiology of psychosis. Quantitatively, the relationship between ∆BPND and change in positive symptoms following amphetamine (∆PTOT) observed in this study is strikingly similar to that observed in patients with schizophrenia.4 When data from the two studies are pooled, analysis of model order suggests that linear regression with a shared slope is more parsimonious than fitting the two studies separately with two different slopes, F(1, 40)=1.15, p=0.71 (∆BPND=1.34 × ∆PTOT + intercept, where the fitted intercept is 13.13 for schizophrenia and 1.73 for DD; Figure 3). That is, a unit difference in ∆PTOT results from 1.34 units of difference in ∆BPND in both studies.
Figure 3.
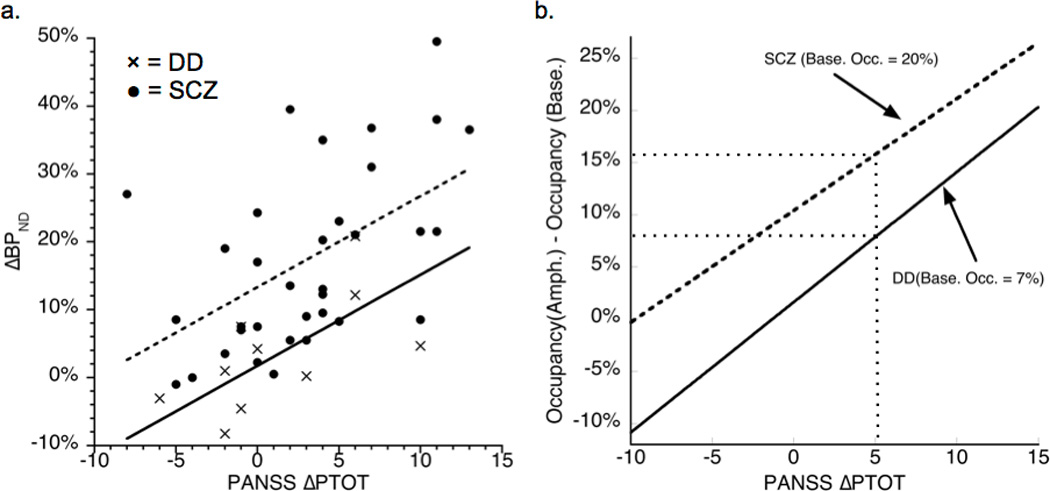
a. The association between ∆BPND and ∆PTOT (i.e. amphetamine-induced change in PANSS positive symptoms) for DD (n=10, data from current study; ∆BPND for preDCA) and schizophrenia (n=34, data from Laruelle et al.;4 ∆BPND for whole striatum). The lines result from a regression with a common slope and study-specific intercepts: ∆BPND = 1.34 × ∆PTOT + 13.13 (schizophrenia, dashed) or ∆BPND = 1.34 × ∆PTOT + 1.73 (DD, solid). The fit demonstrates that the same change in symptoms is associated with a smaller change in tracer binding in DD compared to schizophrenia.
b. The same fits, transformed to the increase in D2 receptor occupancy by DA following amphetamine. Baseline occupancy in DD is assumed to be similar to that previously reported for substance-dependent patients.33 The main qualitative result is that a given increase in symptoms (5 on the Positive-Symptom subscale of the PANSS in this example) results from a smaller increase in the number of receptors being stimulated in DD than in schizophrenia.
Amph.=amphetamine; Base.=baseline; DA=dopamine; DD=dual-diagnosis; Occ.=occupancy; PANSS=Positive and Negative Syndrome Scale; preDCA=precommissural dorsal caudate; SCZ=schizophrenia.
To explore this further, we reframed this correlation in terms of D2 receptor occupancy by DA rather than ∆BPND, assuming that the main effect in ∆BPND is due to competition with DA and using existing information regarding baseline receptor occupancy by DA:
Post-Amphetamine Occupancy – Baseline Occupancy = 1.34 × (1 – Baseline Occupancy) × ∆PTOT + intercept × (1 – Baseline Occupancy); see derivation in Supplement.
DA depletion studies have suggested that average baseline D2 receptor occupancy by DA is approximately 20% in schizophrenia32 and 7% in substance dependence.33 It is not known which of these might be more reflective of baseline occupancy in DD, but any baseline occupancy in the range of 7 to 20% results in the relationship between amphetamine-induced change in occupancy and ∆PTOT being nearly parallel for both groups, with DD biased downward compared to schizophrenia (Figure 3b). The lower intercept observed for DD compared to schizophrenia suggests that, assuming similar levels of receptor availability, a smaller increase in D2 receptor occupancy by DA in DD compared to schizophrenia leads to a similar magnitude of symptom change. These results suggest that D2 receptors are more sensitive to DA in terms of psychosis exacerbation in DD compared to schizophrenia. Thus our findings suggest that D2 function may be abnormal in this group of patients, such that small variations in agonist stimulation have amplified effects. These may be due to abnormal intracellular signaling events, or alternatively, could be an indirect effect of D2 stimulation on other non-dopaminergic components of the network that may be abnormal in schizophrenia. The exact mechanism of this D2-mediated effect is unclear.
Prior research suggests that the blunted DA release observed among the DD patients is related to their comorbid drug use. The primary drugs of dependence included alcohol and cannabis, and the majority also reported current tobacco use. Previous studies have shown that alcohol dependence is associated with blunted DA release,6, 34 whereas cannabis dependence, at least of moderate severity, is not.35 With regard to tobacco use, there are reports of both decreased D2 receptor availability36 and reduced amphetamine-induced DA release in the striatum of nicotine-dependent subjects.37 A limitation of this study is that we cannot distinguish the respective contributions of any one of these drugs to the overall decrease in DA transmission. For example, most of the DD patients reported current regular cigarette smoking, and given previous findings, this tobacco use may have contributed to our results. However, given that all of the patients used more than one drug and the sample is too small to examine the effect of each drug separately, this study cannot address such questions.
There are no clear mechanisms at this point to explain the decrease in presynaptic DA in either this study or in prior studies reporting a similar decrease in substance-use disorders. One view is that the decrease may precede drug use and predisposes patients to develop addictions, whereas another is that the decrease is a result of chronic substance use (for discussion, see Volkow and colleagues38–40).
In contrast to findings in addiction,6, 34, 36 we did not observe a downregulation of D2 receptors. This may be due to the small sample size and/or the confounding effect of previous antipsychotic exposure, which is known to induce an upregulation of D2.
Our sample size is relatively small, due to the difficulty in recruiting this group of patients. Nevertheless, our results are clear and compelling regarding the magnitude of DA blunting detected in these patients. However, the small sample size limited our ability to confidently characterize some of the clinical correlates of this blunted DA release.
In summary, we observed that patients with comorbid schizophrenia and mixed substance dependence displayed significant blunting of striatal DA release. Despite this blunting, DA release was associated with acute and transient increases in positive symptoms. Overall, these findings suggest that in substance-dependent patients with schizophrenia, hypersensitivity of D2 receptors to dopaminergic stimulation is the predominant dopaminergic alteration, as opposed to excess presynaptic release.41 This hypersensitivity may be related to intrinsic factors within the D2 signaling cascade42 or from the effects of excess D2 signaling on the rest of the circuitry. Translational studies of altered pre- and post-synaptic mechanisms are needed to clarify this issue.
Supplementary Material
Acknowledgments
We thank the research participants of this study, and express gratitude for the expert assistance of Beatriz Alvarez, Rawad Ayoub, Jennifer Bae, Felipe Castillo, John Castrillon, Ray Goetz, Elizabeth Hackett, Deborah Hasin, Olga Kambalov, Olga Medina, Elizabeth Raggi, and Sharon Samet.
This work was supported by a grant from the National Institute on Drug Abuse [R21 DA023039-01] to Anissa Abi-Dargham.
Footnotes
NOTE: Supplementary information is available at Molecular Psychiatry's website.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93(17):9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155(6):761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 3.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94(6):2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46(1):56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 5.Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164(4):622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 6.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 8.Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67(3):231–239. doi: 10.1001/archgenpsychiatry.2010.10. [DOI] [PubMed] [Google Scholar]
- 10.Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- 11.Swartz MS, Wagner HR, Swanson JW, Stroup TS, McEvoy JP, McGee M, et al. Substance use and psychosocial functioning in schizophrenia among new enrollees in the NIMH CATIE study. Psychiatr Serv. 2006;57(8):1110–1116. doi: 10.1176/ps.2006.57.8.1110. [DOI] [PubMed] [Google Scholar]
- 12.Swartz MS, Wagner HR, Swanson JW, Stroup TS, McEvoy JP, Canive JM, et al. Substance use in persons with schizophrenia: baseline prevalence and correlates from the NIMH CATIE study. J Nerv Ment Dis. 2006;194(3):164–172. doi: 10.1097/01.nmd.0000202575.79453.6e. [DOI] [PubMed] [Google Scholar]
- 13.Swendsen J, Ben-Zeev D, Granholm E. Real-time electronic ambulatory monitoring of substance use and symptom expression in schizophrenia. Am J Psychiatry. 2011;168(2):202–209. doi: 10.1176/appi.ajp.2010.10030463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantwell R, Brewin J, Glazebrook C, Dalkin T, Fox R, Medley I, et al. Prevalence of substance misuse in first-episode psychosis. Br J Psychiatry. 1999;174:150–153. doi: 10.1192/bjp.174.2.150. [DOI] [PubMed] [Google Scholar]
- 15.Van Mastrigt S, Addington J, Addington D. Substance misuse at presentation to an early psychosis program. Soc Psychiatry Psychiatr Epidemiol. 2004;39(1):69–72. doi: 10.1007/s00127-004-0713-0. [DOI] [PubMed] [Google Scholar]
- 16.Barnett JH, Werners U, Secher SM, Hill KE, Brazil R, Masson K, et al. Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry. 2007;190:515–520. doi: 10.1192/bjp.bp.106.024448. [DOI] [PubMed] [Google Scholar]
- 17.Lambert M, Conus P, Lubman DI, Wade D, Yuen H, Moritz S, et al. The impact of substance use disorders on clinical outcome in 643 patients with first-episode psychosis. Acta Psychiatr Scand. 2005;112(2):141–148. doi: 10.1111/j.1600-0447.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- 18.Verdoux H, Liraud F, Gonzales B, Assens F, Abalan F, van Os J. Suicidality and substance misuse in first-admitted subjects with psychotic disorder. Acta Psychiatr Scand. 1999;100(5):389–395. doi: 10.1111/j.1600-0447.1999.tb10883.x. [DOI] [PubMed] [Google Scholar]
- 19.Schiffer B, Muller BW, Scherbaum N, Forsting M, Wiltfang J, Leygraf N, et al. Impulsivity-related brain volume deficits in schizophrenia-addiction comorbidity. Brain. 2010;133(10):3093–3103. doi: 10.1093/brain/awq153. [DOI] [PubMed] [Google Scholar]
- 20.Ebdrup BH, Glenthoj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, et al. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. J Psychiatry Neurosci. 2010;35(2):95–104. doi: 10.1503/jpn.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV. Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry. 2003;60(3):245–252. doi: 10.1001/archpsyc.60.3.245. [DOI] [PubMed] [Google Scholar]
- 22.Wobrock T, Sittinger H, Behrendt B, D'Amelio R, Falkai P. Comorbid substance abuse and brain morphology in recent-onset psychosis. Eur Arch Psychiatry Clin Neurosci. 2009;259(1):28–36. doi: 10.1007/s00406-008-0831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasin D, Samet S, Nunes E, Meydan J, Matseoane K, Waxman R. Diagnosis of comorbid psychiatric disorders in substance users assessed with the psychiatric research interview for substance and mental disorders for DSM-IV. Am J Psychiatry. 2006;163(4):689–696. doi: 10.1176/ajp.2006.163.4.689. [DOI] [PubMed] [Google Scholar]
- 24.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic Interview for Genetic Studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Non-patient Edition. New York Biometrics Research: New York State Psychiatric Institute; 1996. [Google Scholar]
- 26.Hollingshead AB. Four Factor Index of Social Status. New Haven, Connecticut: Working paper published by the author; 1975. [Google Scholar]
- 27.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 28.Watabe H, Endres CJ, Breier A, Schmall B, Eckelman WC, Carson RE. Measurement of dopamine release with continuous infusion of [11C]raclopride: Optimization and signal-to-noise considerations. J Nucl Med. 2000;41(3):522–530. [PubMed] [Google Scholar]
- 29.Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 30.van Kammen DP, Murphy DL. Attenuation of the euphoriant and activating effects of d- and l-amphetamine by lithium carbonate treatment. Psychopharmacologia. 1975;44(3):215–224. doi: 10.1007/BF00428897. [DOI] [PubMed] [Google Scholar]
- 31.Laruelle M, Abi-Dargham A, van Dyck CH, Rosenblatt W, Zea-Ponce Y, Zoghbi SS, et al. SPECT imaging of striatal dopamine release after amphetamine challenge. J Nucl Med. 1995;36(7):1182–1190. [PubMed] [Google Scholar]
- 32.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, et al. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry. 2009;166(10):1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, et al. Dopamine release in chronic cannabis users: A [(11)C]raclopride positron emission tomography study. Biol Psychiatry. 2012;71(8):677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;165(4):507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- 37.Busto UE, Redden L, Mayberg H, Kapur S, Houle S, Zawertailo LA. Dopaminergic activity in depressed smokers: a positron emission tomography study. Synapse. 2009;63(8):681–689. doi: 10.1002/syn.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michaelides M, Thanos PK, Kim R, Cho J, Ananth M, Wang GJ, et al. PET imaging predicts future body weight and cocaine preference. Neuroimage. 2012;59(2):1508–1513. doi: 10.1016/j.neuroimage.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: Meta-analysis of imaging studies. Arch Gen Psychiatry. 2012 doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schubert KO, Focking M, Prehn JH, Cotter DR. Hypothesis review: are clathrin-mediated endocytosis and clathrin-dependent membrane and protein trafficking core pathophysiological processes in schizophrenia and bipolar disorder? Mol Psychiatry. 2011:1–13. doi: 10.1038/mp.2011.123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


