Abstract
Cancer stem cell-related (CSC) markers have been suggested to have promising potentials as novel types of prognostic and predictive markers in gliomas. However no single CSC-related marker is currently used in clinical decisions. The aim of this study was to investigate the prognostic value of CD133 and nestin separately and in combination using a novel quantitative approach in a well-characterized population-based cohort of glioma patients. The expression of CD133 and nestin was measured by systematic random sampling in stained paraffin sections from 239 glioma patients diagnosed between 2005 and 2009. We found that the expression of CD133 did not correlate with WHO grade, and there was no association with overall survival (OS). The level of nestin correlated positively with WHO grade. In patients with WHO grade II tumors, a high level of nestin was associated with short progression-free survival (PFS) in multivariate analysis. High levels of co-localization were associated with poor PFS in patients with WHO grade II tumors, but not with OS. We conclude that CD133 was not an independent prognostic factor, but a high level of nestin was associated with poor PFS in patients with WHO grade II tumors. The combination of double-immunofluorescence and automated analysis seems to be a feasible and reproducible approach for investigation of the prognostic potential of biomarkers.
Keywords: Glioma, glioblastoma, CD133, nestin, cancer stem cell, population-based
Introduction
The poor prognosis in patients with high-grade gliomas has been explained by the existence of chemo- and radiotherapy-resistant cancer stem cells (CSC) [1,2] possessing the unique ability of tumor initiation, self-renewal and proliferation [3,4]. Two of the most investigated CSC-related markers in gliomas are the membrane marker CD133 [5-10] and the intermediate filament marker nestin [7,11-17]. All though several studies [7-10] report that high expression of CD133 is correlated with higher malignancy grade and poor survival, others report no prognostic value of CD133 [5,6], and the prognostic value remains controversial. Nestin has repeatedly been investigated in gliomas [6,7,12-14,18-22], and a correlation between high expression of nestin and poor survival has been reported [7,13,14,18,19,21]. However, semi-quantitative approaches were used in these studies [6,7,12-14,18-22], and multivariate analyses were seldom performed [6,12,19,20,22].
Despite the promising prognostic results for CD133 and nestin, independently or in combination, none of the proteins are used in neuropathology as prognostic markers. We hypothesized that the use of chromogenic staining and semi-quantitative measurements combined with small patient cohorts could mask clinically important differences in the expression level of both markers. Therefore, the aim of this retrospective study was to thoroughly investigate the clinical value of CD133 and nestin separately and in combination using a well-characterized population-based cohort of glioma patients [23,24] combined with a novel quantitative double-immunofluorescence approach. The prognostic value of each marker, and their co-localization were correlated with the effect of previously identified clinical prognostic factors regarding survival in this population.
We have previously shown that the use of immunofluorescence followed by automated analysis is both feasible, robust and reproducible in gliomas, providing quantitative measurements of protein expression [25]. Although CD133 immunofluorescence has been used in several glioma studies [5,9,26-32] including two studies from our group [5,29] only two studies with cohorts comprising 114 and 44 patients [5,9] contain survival analyses. Using quantitative fluorescence-based measurements and a large glioma cohort, we hypothesized that unique clinically important subpopulations might be identified in the present study.
Methods and materials
Ethics statement
The study was approved by the Regional Committee on Health Research Ethics from the Region of Southern Denmark (Project-ID S2DO9Oo8O) and the Danish Data Protection Agency (J.nr. 2009-41-3070). Use of the tissue was not prohibited by any of the patients according to the Danish Tissue Application Register.
Patients and tissue
In a population-based setup, we identified 433 patients diagnosed with primary gliomas in the period 2005-2009 in the Region of Southern Denmark. Patient characteristics have been thoroughly described previously [23,24]. A total of 239 patients had sufficient available tumor tissue for immunohistochemical analysis. Patient characteristics are summarized in Table 1. Briefly, none of the patients received treatment prior to surgery, mean age at time of diagnosis was 64 years, and median survival was 38 months, 40 months, 13 months and 10 months for WHO grade I, II, III and IV tumors, respectively. Median follow-up was 12 months (0.03-81).
Table 1.
Patient characteristics
| WHO grade II | WHO grade III | WHO grade IV | |
|---|---|---|---|
|
|
|
|
|
| Variable | n (%) | n (%) | n (%) |
| All | 25 (100) | 26 (100) | 185 (100) |
| Status | |||
| Alive | 11 (44) | 6 (23) | 15 (8) |
| Dead | 14 (56) | 20 (77) | 170 (92) |
| Histology | |||
| Astro | 16 (64) | 16 (62) | 185 (100) |
| Oligo/mix | 9 (36) | 10 (38) | - |
| None | - | - | - |
| Age | |||
| < 60 years | 20 (80) | 14 (54) | 56 (30) |
| > 60 years | 5 (20) | 12 (46) | 129 (70) |
| Gender | |||
| Male | 9 (36) | 19 (73) | 106 (67) |
| Female | 16 (64) | 7 (27) | 79 (43) |
| Performance status | |||
| 0-1 | 18 (72) | 20 (77) | 116 (63) |
| 2/2+ | 7 (28) | 6 (23) | 69 (37) |
| Enhancement | |||
| No | 15 (60) | 2 (8) | 0 (0) |
| Yes | 10 (40) | 24 (92) | 177 (96) |
| Missing | - | - | 8 (4) |
| Midline shift | |||
| No | 10 (40) | 10 (38) | 89 (48) |
| Yes | 15 (60) | 16 (62) | 96 (52) |
| Missing | - | - | - |
| Crossing midline | |||
| No | 23 (92) | 22 (85) | 162 (88) |
| Yes | 2 (8) | 4 (15) | 23 (12) |
| Missing | - | - | - |
| Localization | |||
| Frontal | 17 (68) | 16 (62) | 55 (30) |
| Non-frontal | 8 (32) | 10 (38) | 130 (70) |
| Neurologic deficit | |||
| No | 18 (72) | 16 (62) | 99 (54) |
| Yes | 7 (28) | 10 (38) | 86 (46) |
| Headache | |||
| No | 24 (96) | 20 (77) | 136 (74) |
| Yes | 1 (4) | 6 (23) | 49 (26) |
| Epilepsy | |||
| No | 7 (28) | 18 (69) | 144 (78) |
| Yes | 18 (72) | 8 (31) | 41 (22) |
| Mental disturbance | |||
| No | 22 (88) | 17 (65) | 133 (72) |
| Yes | 3 (12) | 9 (35) | 52 (28) |
| Surgery | |||
| Biopsy | 10 (40) | 7 (27) | 13 (7) |
| Partial | 7 (28) | 14 (54) | 113 (61) |
| Total | 8 (32) | 5 (19) | 59 (32) |
| Post-surgical treatment | |||
| No | 24 (96) | 5 (19) | 31 (17) |
| Yes | 1 (4) | 21 (81) | 154 (83) |
| IDH1 status | |||
| Wildtype | 8 (32) | 17 (65) | 181 (98) |
| Mutated | 17 (68) | 9 (35) | 4 (2) |
Pathology
Formalin-fixed paraffin-embedded tissue samples were evaluated by two independent pathologists at the Department of Pathology, Odense University Hospital. If disagreement occurred, the pathologists discussed the case and agreed upon a diagnosis. All samples were classified according to the World Health Organization guidelines 2007 [33].
A commonly used technology in cancer biomarker research is tissue micro arrays (TMAs) [34-36]. However, several groups have shown that TMAs may lead to underestimation of biomarker expression in gliomas [5,37,38]. Accordingly whole slides were used in this study. Moreover; to be included there had to be more than 15 mm2 vital tumor tissues present. Tissue samples were stained routinely with hematoxylin and eosin to define representative tumor regions.
We have previously established chromogenic and fluorescence staining protocols using antibodies against CD133 [5,29,39-41] and nestin [29,39]. In the present study an optimized CD133-nestin immunofluorescence staining (CD133; CD133/1, W6B3C1, Miltenyi Biotec. Nestin; 196908, R&D systems) was established as being suitable for automated analysis by virtue of its high signal-to-noise ratio. Slides were stained on the AutostainerPlus platform (Dako, Glostrup, Denmark). The CSA II Biotin-free Tyramide Signal Amplification System kit (Dako) was used for detection of CD133 and DyLight 650 (1+100 Fisher Scientific) was used for detection of nestin. Using a novel computer-based algorithm, sampling in 1%, 2%, 5% and in 10% of the vital tumor area was performed on a training set consisting of 10 GBMs. In some of the patients, there was a significant difference between sampling in 1% of the tissue compared to sampling in 2%, 5% or 10% of the tissue with regard to the CD133+ area fraction, the CD133+ intensity, the nestin+ area fraction or the nestin intensity (P > 0.05) (Figure S1). Moreover sampling in 1% of the tumor tissue provided a limited number of useable images in some tumors, and sampling in 5% and 10% was time consuming, with no extra benefit. Therefore sampling in 2% was chosen. The effect of fixation time on staining intensity was investigated using spheroids from a GBM-derived so-called CSC line expressing CD133 and nestin [41,42]. Spheroids were fixed for 1 hour, 6 hours, 12 hours, 24 hours and 48 hours, embedded into paraffin, sectioned and stained by the CD133-nestin immunofluorescence staining. The fixation time did not influence the staining intensity of either of CD133 or nestin (P > 0.05 and P > 0.05) (data not shown).
Automated quantitative analysis
The tumor region of interest was identified and delineated on a super image (1.25 magnification) using the Visiopharm Integrator System (Visiopharm, Hoersholm, Denmark). Then sampling in the tumor areas was performed at 40 x magnification. All images were reviewed to ensure that no blurring was present. Major vessels, necroses and normal parenchyma were excluded manually before sampling was performed. Sampling in 2% of the tumor tissue was performed, and all images were analyzed using quantitative image analysis with continuous estimates of staining in the Visiomorph module of the Visiopharm Integrator System. This algorithm is based on the RGB three color model, and for each pixel the intensity of three different colors, red, green, and blue, was defined in the Visiomorf module. The algorithm was made on a training set consisting of 10 GBMs and tested on different glioma subtypes before use.
CD133 and nestin area fractions were initially investigated separately. The area fraction was defined as the measured area with positive staining divided by total sampling area. In addition, the area fraction of co-localization of CD133 and nestin was investigated. Co-localization was defined as CD133 and nestin expression in the same tumor cells, corresponding to expression in the same pixels. The intensities of CD133 and nestin were investigated as well. Since this did not provide additional information in survival analyses, these results are not reported.
Endpoints
Patients were followed until death; patients still alive were censored on February 1, 2012. Overall survival (OS) was defined as time from primary surgery until death or date of censoring. Recurrence was defined as recurrence on MRI, clinical progression or death. Progression-free survival (PFS) was defined as time from primary surgery until date of first recurrence, death or until date of censoring.
Statistics
In the sample fraction study, one-way ANOVA with Bonferroni correction was used, and in the 239 samples comparisons were made using the Kruskall-Wallis test. All variables were investigated in WHO grade II tumors, WHO grade III tumors and in WHO grade IV tumors. CD133, nestin and their co-localization were investigated as continuous variables and as binary variables induced from chosen cut-off values. The univariate relationships between prognostic variables and recurrence and death were illustrated by Kaplan-Meier plots for survival probabilities. The differences between survival functions were compared by the log-rank test. Patients with WHO grade I tumors were not included in the statistical analysis. Patients with WHO grade II, III and IV were investigated separately. Due to a limited number of events in WHO grade II and III tumors only age, performance status (PS) and IDH1 status were included in the multivariate analysis, whereas previously reported significant variables [23] were included in the multivariate analysis for WHO grade IV tumors. All assumptions were tested, and all analyses were carried out using STATA version 11.
Results
Staining
CD133 and nestin were expressed in all histological subtypes (Figure 1A-G), whereas co-localization was observed mainly in WHO grade III and IV tumors (data not shown).
Figure 1.
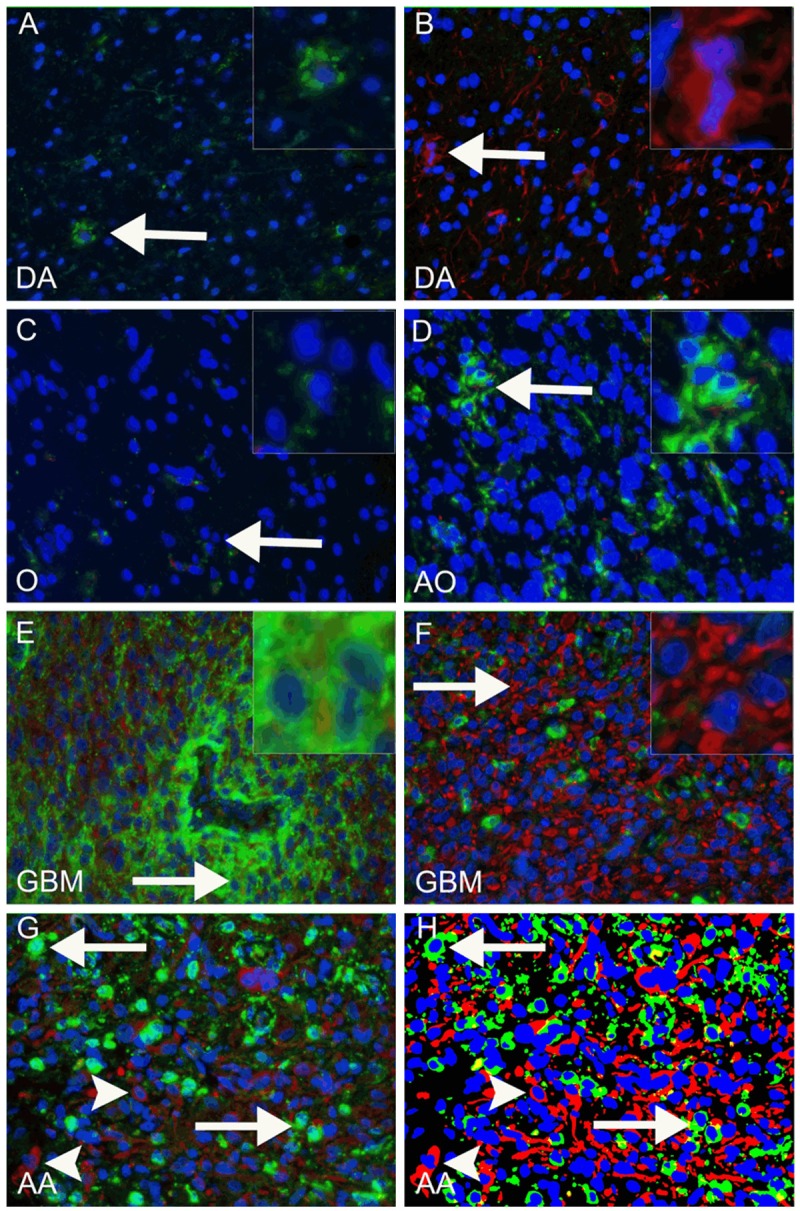
Fluorescence-based staining identifying CD133 and nestin. Examples of CD133-positive areas in diffuse astrocytomas (DA), oligodendrogliomas (O), anaplastic oligodendrogliomas (AO), glioblastomas (GBM) and anaplasticastrocytomas (AA) are shown in (A, C-F) (green, arrows indicate site of inserts). In E a GBM with a CD133 positive peri-vascular niche is shown. Examples of nestin-positive areas are shown in (B and F) (red, arrows indicate site of inserts). When the original fluorescence image (G) was processed and the algorithm applied (H), CD133 positive staining was shown in green, nestin positive staining in red and nuclei in blue. Arrows in G and H indicate CD133 positive cells, arrowheads indicate nestin positive cells.
CD133
The CD133 area fraction did not increase with increasing WHO grade (P = 0.17) (Figure 2A). The distribution of the CD133 area fraction within the histological subtypes did not differ substantially except in patients with pilocytic astrocytomas (PA), oligodendrogliomas (O) and anaplastic oligodendrogliomas (AO) who had a low expression (P = 0.02) (Figure 2B). No significant associations between the CD133 area fraction and OS or PFS were identified for WHO grade II (Figure 2C, 2D), III (Figure 2E, 2F) and IV tumors (Figure 2G, 2H). In multivariate analysis, the CD133 area fraction was not associated with OS or PFS in WHO grade II, and IV tumors (data not shown) but high levels of CD133 was significantly associated with better OS (HR 0.20, 95% CI 0.06-0.71, P = 0.01) in WHO grade III tumors.
Figure 2.
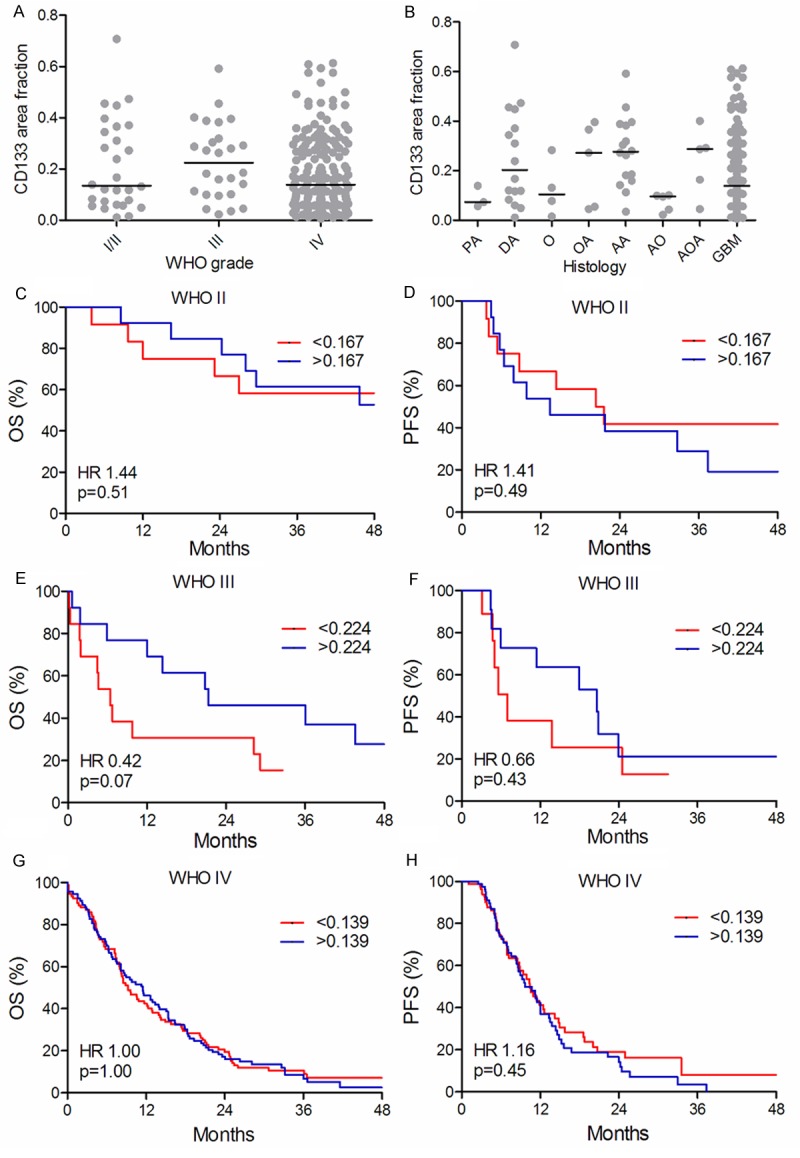
Distribution of CD133 and survival curves. CD133 area fraction in the different WHO grades (A) and in the different histological subtypes (B). The vertical line indicates the median. Overall survival (OS) (C, E, G) and progression-free survival (PFS) (D, F, H) are shown for WHO grade II (n = 25) (C, D), grade III (n = 26) (E, F) and grade IV (n=185) (G, H), respectively. Each WHO grade was dichotomized at the median.
Nestin
The nestin area fraction increased with increasing WHO grade (P < 0.001) (Figure 3A). The distribution of the nestin area fraction was generally low, except in GBMs (Figure 3B). In patients with WHO grade II and IV tumors, the nestin area fraction was not associated with OS (Figure 3C, 3G). In patients with WHO grade III tumors, a high level of nestin was associated with poor OS (Figure 4E), but the prognostic value of the nestin area fraction was not significant in the multivariate analysis (HR 2.24, 95% CI 0.69-7.30, P = 0.18). In WHO grade II tumors, high level of nestin was associated with reduced PFS in both univariate (Figure 3D) and multivariate analysis(HR 7.64, 95% CI 1.90-30.70, P = 0.04). The nestin area fraction was not associated with PFS in WHO grade III and IV tumors in either univariate (Figure 3F, 3H) or multivariate analysis (data not shown).
Figure 3.
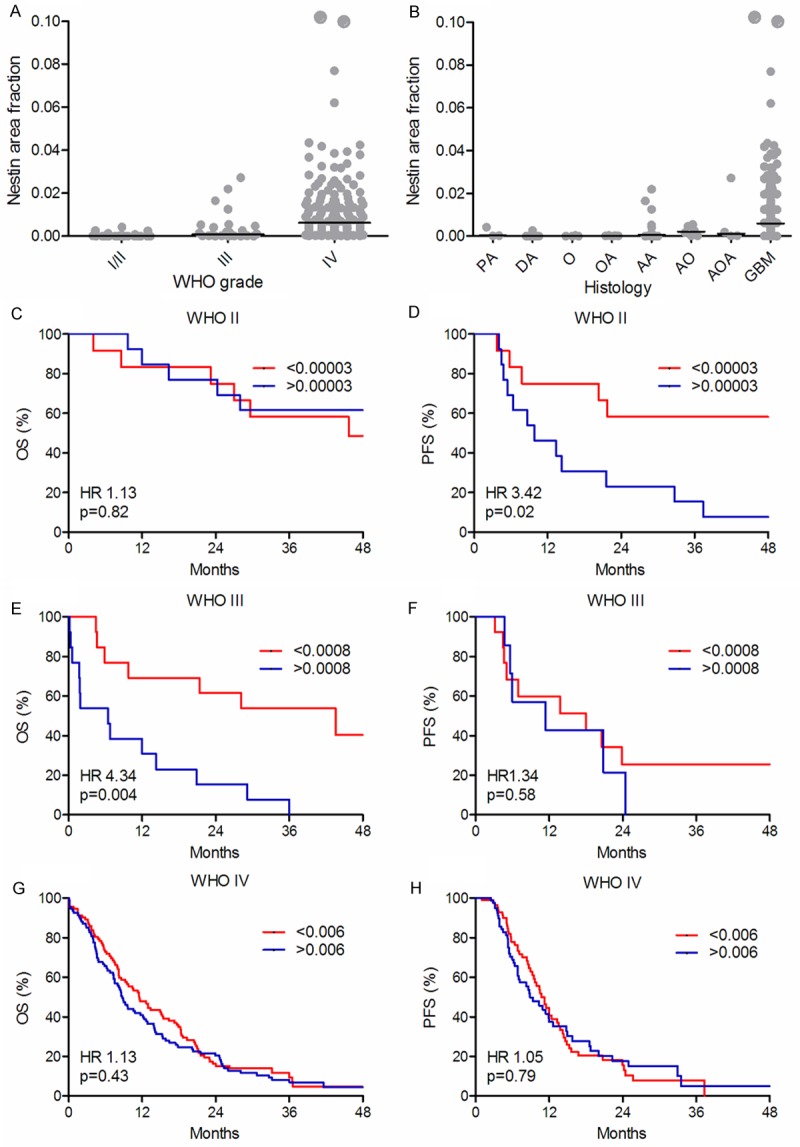
Distribution of nestin and survival curves. Nestin area fraction in the different WHO grades (A) and in the different histological subtypes (B). The vertical line indicates the median. Overall survival (OS) (C, E, G) and progression-free survival (PFS) (D, F, H) are shown for WHO grade II (n = 25) (C, D), grade III (n = 26) (E, F) and grade IV (n = 185) (G, H) tumors, respectively. Each WHO grade was dichotomized at the median.
Figure 4.
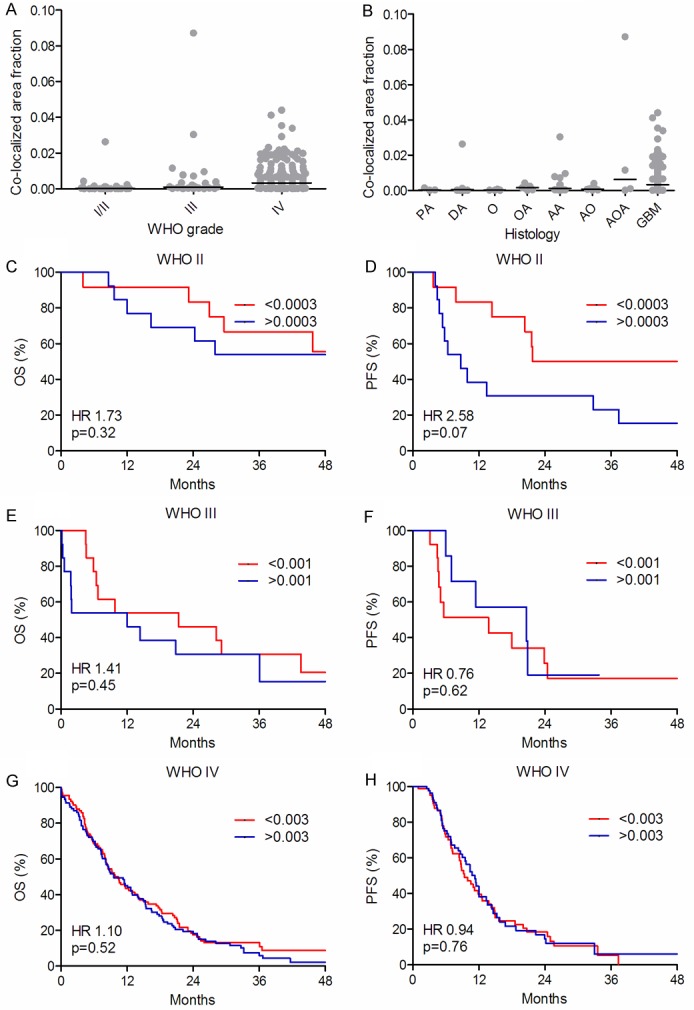
Distribution of co-localization and survival curves. Co-localized area fraction in the different WHO grades (A) and in the different histological subtypes (B). The vertical line indicates the median. Overall survival (OS) (C, E, G) and progression-free survival (PFS) (D, F, H) are shown for WHO grade II (n = 25) (C, D), grade III (n = 26) (E, F) and grade IV (n = 185) (G, H) tumors, respectively. Each WHO grade was dichotomized at the median.
Co-localization
The level of co-localization increased with increasing WHO grades (P = 0.0001) (Figure 4A). Co-localization was generally low, except in AOAs and GBMs (Figure 4B). In univariate analysis, co-localization was not associated with OS (Figure 4C, 4E, 4G) or PFS (Figure 4F, 4H). In patients with WHO grade II tumors a trend toward poorer PFS was observed in patients with high levels of co-localization (Figure 4D). In multivariate analysis high levels of co-localization were associated with poor PFS (HR 4.08, 95% CI 1.31-12.75, p = 0.02) whereas no association with OS was observed in patients with WHO grade II tumors (data not shown). Regarding patients with WHO grade III and IV tumors, co-localization was not associated with OS or PFS (data not shown).
Discussion
Using a fluorescent staining protocol, we investigated the prognostic value of the CSC-related markers CD133 and nestin separately and in combination. Our study contains the largest number of patients, especially GBM’s, as compared to previously published studies [5-10], and survival analyses have been performed for each WHO grade separately, which is an advantage. Moreover, we used systematic random sampling combined with a quantitative immunofluorescence approach.
CD133 was not prognostic in multivariate analysis in our study being thereby in line with previous results from our group [5] and Kim et al. [6]. This contributes to the discussion of to which degree CD133 represents a CSC-related marker. Results in the literature show that different CD133 antibody clones identify not only different epitopes but also epitopes with different glycosylation status thereby highlighting the complexity of this field [40,43]. Pallini et al. [8] showed that patients with GBMs containing more than 2% CD133 positive cells had the poorest survival. We included primary GBMs only, whereas Pallini et al. [8] also included five secondary gliomas. As secondary GBMs contain very few CD133 positive cells [44], it may be speculated that inclusion of both primary and secondary GBMs influences the prognostic estimate. Zeppernick et al. [10] used an even lower cut-point (1%), although they showed that many of the GBMs contained more than 25% CD133 positive cells. As they included both WHO grade II, III and IV tumors as one group it may be speculated whether it is the WHO grade rather than the content of CD133 that causes the poor prognosis. Recently, different groups [44-47] have reported that not only CD133+ cells, but also some CD133- glioma cells possess cancer stem cell characteristics thereby contributing to the complexity of using and interpreting results obtained with CD133.
The nestin area fraction increased with increasing WHO grade, which is in accordance with previously published studies [7,13,14,19-21]. However, none of these studies investigated the prognostic potential within different WHO grades and only three performed a multivariate analysis [12,19,22].
In patients with WHO grade II tumors, a high level of nestin was associated with shorter PFS. This suggests that a high content of nestin is associated with more aggressive tumors which have a greater potential for recurrence. Since our cohort comprises a limited number of patients with WHO grade II tumors and has a short follow-up period resulting in fewer events in the analyses regarding OS and PFS, this hypothesis needs confirmation in another cohort.
In patients with WHO grade III tumors, a low level of nestin was associated with the longest OS in univariate analysis. However, all patients with a low level of nestin were in good performance status, and they received curatively intended post-surgical treatment, which might be the explanation to the good prognostic estimates. Furthermore, we showed that some patients with AAs had a much higher level of nestin as compared to patients with AOs and AOAs. It has previously been shown that patients with AAs have a poorer prognosis than patients with AOs and AOAs [48] and it may be speculated whether the different histological subtypes may bias our results. The nestin area fraction was not a significant variable in multivariate analysis, indicating that age, performance status and IDH1 status are stronger prognostic variables. The prognostic value of nestin within each histological subtype still needs to be investigated in larger materials.
Previous literature reporting on nestin as a prognostic factor [7,13,14,18-20] include both low-grade and high-grade gliomas. These studies all contain a large percentage of low-grade gliomas, and none of these studies investigated the prognostic potential of nestin within the different WHO grades separately. Although expression of a given markers increases with grade, the translational value is strongly dependent on the prognostic value in each grade separately, at least in the GBM patients. Interestingly, none of the studies performing multivariate analysis took grade into account, although Chinnayan et al. [12] stratified patients based on RPA classes. Moreover, two of the studies [6,12] reporting that nestin is not a prognostic factor contained only GBMs, thereby supporting our results. All together the results may suggest that nestin is an indicator of more aggressive tumor biology in low-grade gliomas but not in high-grade gliomas. However, nestin has been identified in proliferating cells during active angiogenesis [49], and GBMs are indeed angiogenic tumors. Although larger vessels were excluded in the present study, it is possible that detection of nestin-positive endothelial cells may influence the evaluation of the prognostic potential in some of the previous studies.
Only one other group Zhang et al. [50] investigated the co-expression of CD133 and nestin. They showed that patients with high levels of both CD133 and nestin had the poorest survival. However, out of 124 gliomas Zhang et al. included 19 ependymomas, comprising 15 low-grade and 4 high-grade tumors. In multivariate analysis, astrocytomas, oligodendrogliomas and ependymomas were all included as one “glioma group”, which may induce bias since another study suggested nestin to be a strong predictor of poor OS and PFS in patients with low-grade ependymomas but not in malignant ependymomas [51].
We investigated the combined expression of CD133 and nestin using a quantitative approach. Although this is the first study using this approach, new information regarding the association of co-localization with OS or PFS was not obtained.
In the present study, a population-based setup was used, being more representative for the true glioma population. Using this setup, all patients were included despite age and performance status, thereby preventing this type of bias. This may partly explain some of the different results obtained in our study versus previously published studies on CD133 and nestin. Moreover, the traditional semi-quantitative way of scoring is known to be biased by inter- and intra-observer variability [36] as well as having a predominance of scoring at e.g. 0, 100, 200 and 300 [36]. Therefore, the use of immunofluorescence may identify small biological differences as compared to the conventional scoring methods [52]. In the present study, we sampled multiple times with different sampling frequencies in the same tissue slides, when the algorithm was tested. No important differences regarding area fractions or intensity were observed, indicating that the method is both robust and reproducible.
In conclusion, the CD133 area fraction detected by the W6B3C1 clone was without prognostic potential. The level of nestin increased with increasing WHO grade, and a high level of nestin was associated with poor progression-free survival in patients with WHO grade II tumors. Use of quantitative double-immunofluorescence seems to be a feasible, robust and reproducible approach suggestive of great potential in future prognostic studies.
Acknowledgements
We acknowledge the excellent laboratory work done by technicians Helle Wohlleben and Tanja Dreehsen Højgaard. This work was supported by the Danish Medical Research Council, the Region of Southern Denmark, the Research Council at Odense University Hospital, Carl J. Becker’s Foundation, Jacob and Olga Madsen Foundation, Danish Cancer Research Foundation, the Karen A. Tolstrup Foundation, Foundation for Research in Neurology, Foundation o Cancer Research at Copenhagen University, and the Beckett Foundation.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 5.Christensen K, Schroeder HD, Kristensen BW. CD133 identifies perivascular niches in grade II-IV astrocytomas. J Neurooncol. 2008;90:157–170. doi: 10.1007/s11060-008-9648-8. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Lee KH, Kim HS, Moon KS, Jung TY, Jung S, Lee MC. The presence of stem cell marker-expressing cells is not prognostically significant in glioblastomas. Neuropathology. 2011;31:494–502. doi: 10.1111/j.1440-1789.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 7.Ma YH, Mentlein R, Knerlich F, Kruse ML, Mehdorn HM, Held-Feindt J. Expression of stem cell markers in human astrocytomas of different WHO grades. J Neurooncol. 2008;86:31–45. doi: 10.1007/s11060-007-9439-7. [DOI] [PubMed] [Google Scholar]
- 8.Pallini R, Ricci-Vitiani L, Banna GL, Signore M, Lombardi D, Todaro M, Stassi G, Martini M, Maira G, Larocca LM, De MR. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14:8205–8212. doi: 10.1158/1078-0432.CCR-08-0644. [DOI] [PubMed] [Google Scholar]
- 9.Thon N, Damianoff K, Hegermann J, Grau S, Krebs B, Schnell O, Tonn JC, Goldbrunner R. Presence of pluripotent CD133+ cells correlates with malignancy of gliomas. Mol Cell Neurosci. 2010;43:51–59. doi: 10.1016/j.mcn.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 11.Almqvist PM, Mah R, Lendahl U, Jacobsson B, Hendson G. Immunohistochemical detection of nestin in pediatric brain tumors. J Histochem Cytochem. 2002;50:147–158. doi: 10.1177/002215540205000203. [DOI] [PubMed] [Google Scholar]
- 12.Chinnaiyan P, Wang M, Rojiani AM, Tofilon PJ, Chakravarti A, Ang KK, Zhang HZ, Hammond E, Curran W Jr, Mehta MP. The prognostic value of nestin expression in newly diagnosed glioblastoma: report from the Radiation Therapy Oncology Group. Radiat Oncol. 2008;3:32. doi: 10.1186/1748-717X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrmann J, Kolar Z, Mokry J. Nestin as a diagnostic and prognostic marker: immunohistochemical analysis of its expression in different tumours. J Clin Pathol. 2005;58:222–223. doi: 10.1136/jcp.2004.021238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maderna E, Salmaggi A, Calatozzolo C, Limido L, Pollo B. Nestin, PDGFRbeta, CXCL12 and VEGF in glioma patients: different profiles of (pro-angiogenic) molecule expression are related with tumor grade and may provide prognostic information. Cancer Biol Ther. 2007;6:1018–1024. doi: 10.4161/cbt.6.7.4362. [DOI] [PubMed] [Google Scholar]
- 15.Mangiola A, Lama G, Giannitelli C, De BP, Anile C, Lauriola L, La TG, Sabatino G, Maira G, Jhanwar- Uniyal M, Sica G. Stem cell marker nestin and c-Jun NH2-terminal kinases in tumor and peritumor areas of glioblastoma multiforme: possible prognostic implications. Clin Cancer Res. 2007;13:6970–6977. doi: 10.1158/1078-0432.CCR-07-1229. [DOI] [PubMed] [Google Scholar]
- 16.Wei LC, Shi M, Cao R, Chen LW, Chan YS. Nestin small interfering RNA (siRNA) reduces cell growth in cultured astrocytoma cells. Brain Res. 2008;1196:103–112. doi: 10.1016/j.brainres.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 17.Yang XH, Wu QL, Yu XB, Xu CX, Ma BF, Zhang XM, Li SN, Lahn BT, Xiang AP. Nestin expression in different tumours and its relevance to malignant grade. J Clin Pathol. 2008;61:467–473. doi: 10.1136/jcp.2007.047605. [DOI] [PubMed] [Google Scholar]
- 18.Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334–5341. [PubMed] [Google Scholar]
- 19.Strojnik T, Rosland GV, Sakariassen PO, Kavalar R, Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68:133–143. doi: 10.1016/j.surneu.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 20.Wan F, Herold-Mende C, Campos B, Centner FS, Dictus C, Becker N, Devens F, Mogler C, Felsberg J, Grabe N, Reifenberger G, Lichter P, Unterberg A, Bermejo JL, Ahmadi R. Association of stem cell-related markers and survival in astrocytic gliomas. Biomarkers. 2011;16:136–143. doi: 10.3109/1354750X.2010.536256. [DOI] [PubMed] [Google Scholar]
- 21.Arai H, Ikota H, Sugawara K, Nobusawa S, Hirato J, Nakazato Y. Nestin expression in brain tumors: its utility for pathological diagnosis and correlation with the prognosis of high-grade gliomas. Brain Tumor Pathol. 2012;29:160–167. doi: 10.1007/s10014-012-0081-5. [DOI] [PubMed] [Google Scholar]
- 22.Kanamori M, Kumabe T, Sonoda Y, Nishino Y, Watanabe M, Tominaga T. Predictive factors for overall and progression-free survival, and dissemination in oligodendroglial tumors. J Neurooncol. 2009;93:219–228. doi: 10.1007/s11060-008-9762-7. [DOI] [PubMed] [Google Scholar]
- 23.Dahlrot RH, Kristensen BW, Hjelmborg J, Herrstedt J, Hansen S. A population-based study of high-grade gliomas and mutated isocitrate dehydrogenase 1. Int J Clin Exp Pathol. 2013;6:31–40. [PMC free article] [PubMed] [Google Scholar]
- 24.Dahlrot RH, Kristensen BW, Hjelmborg J, Herrstedt J, Hansen S. A population-based study of low-grade gliomas and mutated isocitrate dehydrogenase 1 (IDH1) J Neurooncol. 2013;114:309–317. doi: 10.1007/s11060-013-1186-3. [DOI] [PubMed] [Google Scholar]
- 25.Dahlrot RH, Hansen S, Herrstedt J, Schroder HD, Hjelmborg J, Kristensen BW. Prognostic value of Musashi-1 in gliomas. J Neurooncol. 2013;115:453–461. doi: 10.1007/s11060-013-1246-8. [DOI] [PubMed] [Google Scholar]
- 26.He J, Liu Y, Zhu T, Zhu J, Dimeco F, Vescovi AL, Heth JA, Muraszko KM, Fan X, Lubman DM. CD90 is identified as a candidate marker for cancer stem cells in primary high-grade gliomas using tissue microarrays. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.010744. M111.010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakata S, Campos B, Bageritz J, Bermejo JL, Becker N, Engel F, Acker T, Momma S, Herold-Mende C, Lichter P, Radlwimmer B, Goidts V. LGR5 is a marker of poor prognosis in glioblastoma and is required for survival of brain cancer stem-like cells. Brain Pathol. 2013;23:60–72. doi: 10.1111/j.1750-3639.2012.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, Sun J, Yu SP, Chen C, Liu B, Liu ZF, Ren BC, Ming HL, Yang XJ. Rac1+ cells distributed in accordance with CD 133+ cells in glioblastomas and the elevated invasiveness of CD 133+ glioma cells with higher Rac1 activity. Chin Med J (Engl) 2012;125:4344–4348. [PubMed] [Google Scholar]
- 29.Christensen K, Schroder HD, Kristensen BW. CD133+ niches and single cells in glioblastoma have different phenotypes. J Neurooncol. 2011;104:129–143. doi: 10.1007/s11060-010-0488-y. [DOI] [PubMed] [Google Scholar]
- 30.Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR Jr, Gillespie GY. CD133 is a marker of bioenergetic stress in human glioma. PLoS One. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao XG, Zhang X, Xue XY, Guo G, Wang P, Zhang W, Fei Z, Zhen HN, You SW, Yang H. Brain tumor stem-like cells identified by neural stem cell marker CD15. Transl Oncol. 2009;2:247–257. doi: 10.1593/tlo.09136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beier D, Wischhusen J, Dietmaier W, Hau P, Proescholdt M, Brawanski A, Bogdahn U, Beier CP. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol. 2008;18:370–377. doi: 10.1111/j.1750-3639.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. International Agency for Research on cancer (IARC) 2007. WHO Classification of Tumours of the Central Nervous System. [Google Scholar]
- 34.Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol. 2008;26:5630–5637. doi: 10.1200/JCO.2008.17.3567. [DOI] [PubMed] [Google Scholar]
- 35.Sauter G. Representativity of TMA studies. Methods Mol Biol. 2010;664:27–35. doi: 10.1007/978-1-60761-806-5_3. [DOI] [PubMed] [Google Scholar]
- 36.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 37.Hayry V, Tynninen O, Haapasalo HK, Wolfer J, Paulus W, Hasselblatt M, Sariola H, Paetau A, Sarna S, Niemela M, Wartiovaara K, Nupponen NN. Stem cell protein BMI-1 is an independent marker for poor prognosis in oligodendroglial tumours. Neuropathol Appl Neurobiol. 2008;34:555–563. doi: 10.1111/j.1365-2990.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- 38.Chiesa-Vottero AG, Rybicki LA, Prayson RA. Comparison of proliferation indices in glioblastoma multiforme by whole tissue section vs tissue microarray. Am J Clin Pathol. 2003;120:902–908. doi: 10.1309/8UAU-KFK3-NBDM-VTNU. [DOI] [PubMed] [Google Scholar]
- 39.Christensen K, Aaberg-Jessen C, Andersen C, Goplen D, Bjerkvig R, Kristensen BW. Immunohistochemical expression of stem cell, endothelial cell, and chemosensitivity markers in primary glioma spheroids cultured in serum-containing and serum-free medium. Neurosurgery. 2010;66:933–947. doi: 10.1227/01.NEU.0000368393.45935.46. [DOI] [PubMed] [Google Scholar]
- 40.Hermansen SK, Christensen KG, Jensen SS, Kristensen BW. Inconsistent immunohistochemical expression patterns of four different CD133 antibody clones in glioblastoma. J Histochem Cytochem. 2011;59:391–407. doi: 10.1369/0022155411400867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolenda J, Jensen SS, Aaberg-Jessen C, Christensen K, Andersen C, Brunner N, Kristensen BW. Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J Neurooncol. 2011;103:43–58. doi: 10.1007/s11060-010-0357-8. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 43.Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med. 2008;86:1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn U, Beier CP. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 45.Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, Greve JM, Soriano RH, Gilmour LL, Rivers CS, Modrusan Z, Nacu S, Guerrero S, Edgar KA, Wallin JJ, Lamszus K, Westphal M, Heim S, James CD, VandenBerg SR, Costello JF, Moorefield S, Cowdrey CJ, Prados M, Phillips HS. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 46.Joo KM, Kim SY, Jin X, Song SY, Kong DS, Lee JI, Jeon JW, Kim MH, Kang BG, Jung Y, Jin J, Hong SC, Park WY, Lee DS, Kim H, Nam DH. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest. 2008;88:808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Sakariassen PO, Tsinkalovsky O, Immervoll H, Boe SO, Svendsen A, Prestegarden L, Rosland G, Thorsen F, Stuhr L, Molven A, Bjerkvig R, Enger PO. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122:761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 48.Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- 49.Sugawara K, Kurihara H, Negishi M, Saito N, Nakazato Y, Sasaki T, Takeuchi T. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002;82:345–351. doi: 10.1038/labinvest.3780428. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M, Song T, Yang L, Chen R, Wu L, Yang Z, Fang J. Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. J Exp Clin Cancer Res. 2008;27:85. doi: 10.1186/1756-9966-27-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milde T, Hielscher T, Witt H, Kool M, Mack SC, Deubzer HE, Oehme I, Lodrini M, Benner A, Taylor MD, von Deimling A, Kulozik AE, Pfister SM, Witt O, Korshunov A. Nestin expression identifies ependymoma patients with poor outcome. Brain Pathol. 2012;22:848–860. doi: 10.1111/j.1750-3639.2012.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rao J, Seligson D, Hemstreet GP. Protein expression analysis using quantitative fluorescence image analysis on tissue microarray slides. Biotechniques. 2002;32:924–926. 928–930, 932. doi: 10.2144/02324pt04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


