Abstract
Three strictly anaerobic, Gram-positive, non-spore-forming, rod-shaped, motile bacteria, designated strains ACB1T, ACB7T and ACB8, were isolated from human subgingival dental plaque. All strains required yeast extract for growth. Strains ACB1T and ACB8 were able to grow on glucose, lactose, maltose, maltodextrin and raffinose; strain ACB7T grew weakly on sucrose only. The growth temperature range was 30–42 °C with optimum growth at 37 °C. Major metabolic fermentation end products of strain ACB1T were acetate and lactate; the only product of strains ACB7T and ACB8 was acetate. Major fatty acids of strain ACB1T were C14 : 0, C16 : 0, C16 : 1ω7c dimethyl aldehyde (DMA) and C18 : 1ω7c DMA. Major fatty acids of strain ACB7T were C12 : 0, C14 : 0, C16 : 0, C16 : 1ω7c and C16 : 1ω7c DMA. The hydrolysate of the peptidoglycan contained meso-diaminopimelic acid, indicating peptidoglycan type A1γ. Genomic DNA G+C content varied from 42 to 43.3 % between strains. According to 16S rRNA gene sequence phylogeny, strains ACB1T, ACB8 and ACB7T formed two separate branches within the genus Oribacterium, with 98.1–98.6 % sequence similarity to the type strain of the type species, Oribacterium sinus. Predicted DNA–DNA hybridization values between strains ACB1T, ACB8, ACB7T and O. sinusF0268 were <70 %. Based on distinct genotypic and phenotypic characteristics, strains ACB1T and ACB8, and strain ACB7T are considered to represent two distinct species of the genus Oribacterium, for which the names Oribacterium parvum sp. nov. and Oribacterium asaccharolyticum sp. nov. are proposed. The type strains are ACB1T ( = DSM 24637T = HM-481T = ATCC BAA-2638T) and ACB7T ( = DSM 24638T = HM-482T = ATCC BAA-2639T), respectively.
In this study, we report the characterization of three strictly anaerobic strains, designated ACB1T, ACB7T and ACB8, isolated from subgingival plaque obtained from a 25-year-old African American female.
The study protocol was approved by the Institutional Review Board of Northeastern University; informed consent was obtained from the subject. Novel isolates were enriched on liquid basic anaerobic medium (BM) supplemented with l-cysteine–HCl as a reducing agent and isolated in pure culture on agar-BM under an anaerobic atmosphere (2 % H2, 1 % CO2, 97 % N2) (Sizova et al., 2012). The Human Oral Microbiome Database (HOMD) classified the isolated strains as members of oral taxon 108 (Chen et al., 2010). According to preliminary 16S rRNA gene sequence phylogeny, strains ACB1T, ACB7T and ACB8 belong to the genus Oribacterium (Carlier et al., 2004) within the family Lachnospiraceae (Rainey, 2009).
Colony morphology assessment was performed on Wilkins-Chalgren (WC) blood agar and trypticase peptone-yeast extract (TY) agar medium. All media were supplemented with l-cysteine–HCl as a reducing agent (Sizova et al., 2012). Cell morphology was observed with a Leica DMBL light microscope equipped with phase-contrast optics. For electron microscopy, cells grown on TY medium for 24–48 h were collected, fixed as described previously (Ellis, 2006; Sizova et al., 2013) and observed with a Hitachi S4800 scanning electron microscope. Thin sections were stained with uranyl acetate and lead citrate and observed with a JEOL JEM 1010 transmission electron microscope. The Gram reaction was determined using the Difco Gram-stain kit. Resistance to various antibiotics and bile was tested with Oxoid ‘AN-IDENT’ and Remel susceptibility test discs; zones less than 10 mm were considered to indicate resistance. Oxidase, catalase and nitrate reduction activities were tested with Remel reagents. Biochemical reactions and individual carbon source utilization was assessed with API 20A tests and with cultures grown in liquid medium with glucose, lactose, maltose, sucrose, cellobiose, maltodextrin, raffinose or starch supplemented with yeast extract. The effect of temperature was assessed with cultures grown on TY medium. All experiments were conducted under anaerobic conditions; growth was scored as visible turbidity. Fermentation products were determined by HPLC in acidified supernatant of cultures grown on glucose-yeast extract and TY media before and after distillation [Agilent 1200 series HPLC; Poroshell 120 SB-C18 column 2.7 m, 3.0×100 mm with guard column (Agilent Technologies); 10 µM H2SO4 was used as the mobile phase]. Cell biomass grown in trypticase peptone-glucose-yeast extract (TYG) for 48 h was used for the whole-cell fatty acid and peptidoglycan analyses. Fatty acids were methylated, extracted and analysed by GC using the Sherlock Microbial Identification System at Microbial ID. The peptidoglycan structure was analysed in the hydrolysates (4 M HCl, 100 °C, 16 h) according to the method of Rhuland et al. (1955) and by GC/MS analysis after isolation of the peptidoglycan and its hydrolysis (Schumann, 2011) at the Identification Service of the German Collection of Microorganisms and Cell Cultures. 16S rRNA gene sequences were compared with those available from GenBank; phylogenetic analysis was performed as described previously (Sizova et al., 2012). Whole genome sequencing of strains ACB1T and ACB7T was carried out by the Broad Institute of Harvard and MIT, and the data are available at http://www.broadinstitute.org/. The whole genome of strain ACB8 was sequenced by the J. Craig Venter Institute. The genome sequences of strains ACB1T, ACB7T and ACB8 were compared with available genome sequences of other members of the genus Oribacterium. Individual coding sequences were submitted to the Rapid Annotation Subsystem Technology (RAST) server (Aziz et al., 2008) for subsystem annotations. DNA base content (mol% G+C) was calculated from the whole genome sequences. DNA–DNA hybridization (DDH) values were predicted by the Genome-to-Genome Distance calculator 2.0, formula 3, which is available online at http://ggdc.dsmz.de/ (Auch et al., 2010a, b; Meier-Kolthoff et al., 2013).
Cells of the three novel three strains were non-spore-forming, highly motile, oval rods, sometimes appearing swollen. Cells of strains ACB1T and ACB8 were 1.2±0.4 µm long (average±sd) and 0.45±0.09 µm wide; cells of strain ACB7T were 1.6±0.6 µm long and 0.50±0.08 µm wide [Fig. 1, Fig. S1 (available in the online Supplementary Material), Table 1]. About 5 % of strain ACB7T cells were curved rods up to 5–7 µm long. Cells appeared Gram-variable after staining, but were structurally Gram-positive (Fig. 1). Three to five RAST-annotated genes associated with synthesis of teichoic and lipoteichoic acids were present in the genome (Table 2). Surprisingly, we detected seven genes associated with synthesis of lipooligosaccharides in strain ACB1T, but not in strain ACB7T, ACB8 or other Oribacteriumstrains with available genomes (Table 2). After 48 h of incubation on TY agar plates at 37 °C, strain ACB1T formed beige, round, convex colonies 1–1.5 mm in diameter. Colonies of strain ACB7T were round, non-pigmented and 0.5 mm in diameter after 48 h and umbonate and 2–3 mm in diameter with irregular wavy edges after 168 h. All three strains were non-haemolytic. After 1–2 days of incubation, strains ACB1T, ACB7T and ACB8 produced a diffusible black pigment in BM and TY liquid media supplemented with l-cysteine–HCl. The pigment was visible as a grainy substance surrounding cells (Fig. S1b). Most cells of strain ACB7T and some cells of strain ACB1T contained intracellular nanometre-sized particles (Fig. 1), probably of ferrous sulfide (Sizova et al., 2013). Upon inspection of the genomes of strains ACB1T, ACB8 and ACB7T, we identified five to six genes putatively annotated as encoding the cysteine desulfurase enzyme EC 2.8.1.7 (Table S1). The activity of these genes probably explains the production of black precipitate, which is presumably FeS (Frazzon & Dean, 2003; Mihara & Esaki, 2002). Similar particles were previously observed in cells of another member of the family Lachnospiraceae, Stomatobaculum longum, which also contained a single gene encoding a cysteine desulfurase enzyme in its genome (Sizova et al., 2013).
Fig. 1.
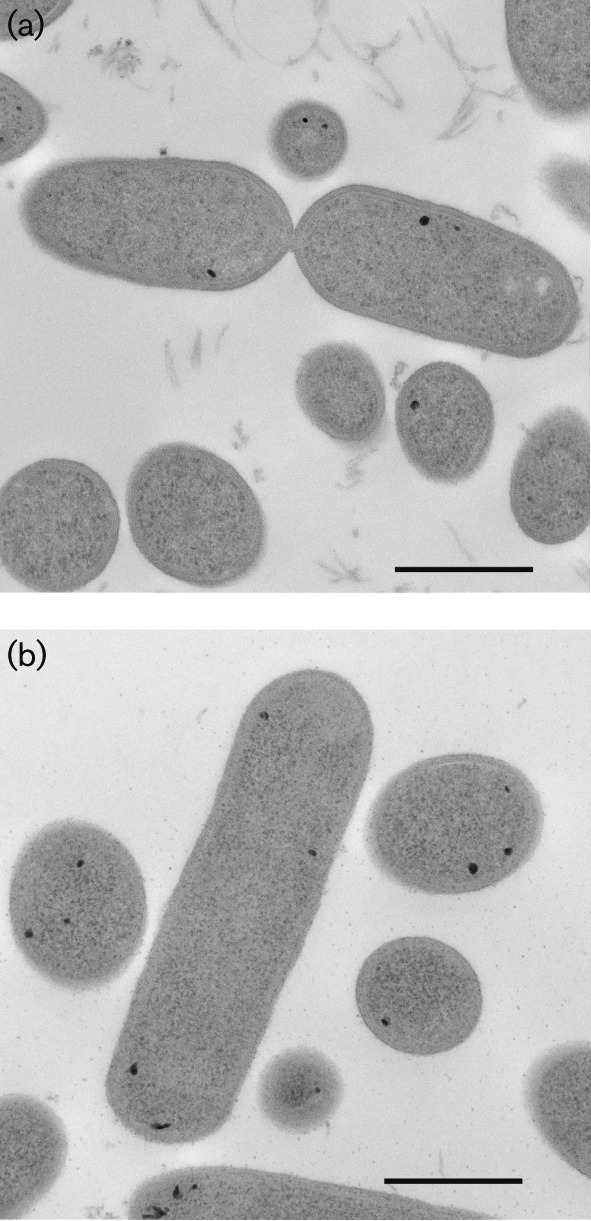
Transmission electron micrograph of cells of strain ACB1T (a) and strain ACB7T (b). General morphology and Gram-positive cell structure of ultrathin sections is shown. Bars, 500 nm.
Table 1. Characteristics that differentiate strains ACB1Tand ACB8 and strain ACB7T from O. sinus AIP 354.02T (Carlier et al., 2004).
Strains: 1, ACB1T; 2, ACB8; 3, ACB7T; 4, O. sinus AIP 354.02T. nd, No data available.
| Characteristic | 1 | 2 | 3 | 4 |
| Cell shape | Short ovoid rods in chains or aggregates, often swollen | Short ovoid rods in chains or aggregates, often swollen | Short ovoid to long curved rods occurring singly, in pairs or chains, often swollen | Ovoid rods occurring singly, in pairs or in chains |
| Cell size (μm) | 1.2±0.4×0.45±0.09 | 1.2±0.4×0.45±0.09 | 1.6±0.6×0.5±0.08 | 1.7–2.2×0.8–1.0 |
| Motility/flagella | +/Sublateral | +/Sublateral | +/Sublateral | +/Laterally inserted |
| Colony shape | Round, convex, beige | Round, convex, beige | Round, transparent, petite | Circular, convex, non-pigmented |
| Colony size after 48 h (mm) | 1.5 | 1.5 | 0.5 | 1.5 |
| Gram-stain | Variable | Variable | Variable | Negative |
| Temperature range (°C) | 30–42 | 30–42 | 30–42 | 37 |
| Assimilation of: | ||||
| d-Glucose | + | + | − | + |
| Lactose | + | + | − | − |
| Sucrose | − | + | + | + |
| Maltose | + | + | − | − |
| Maltodextrin | + | + | − | nd |
| Raffinose | + | + | − | + |
| Indole formation | − | nd | − | + |
| Urease | − | nd | − | nd |
| Oxidase | − | − | − | nd |
| Aesculin hydrolysis | − | nd | + | − |
| Gas formation from TGY medium | + | + | − | + |
| Black pigment on TY medium | + | + | + | nd |
| Resistance to: | ||||
| Kanamycin (1 mg) | − | nd | − | + |
| Erythromycin (60 µg) | − | nd | − | + |
| Metabolic end products* | A, L | A | A | A, L |
| DNA G+C content (mol%) | 42.1 | 42 | 43.3 | 42.4 |
| Peptidoglycan type | A1γ | nd | A1γ | nd |
| Major fatty acid methyl esters | C14 : 0 | nd | C12 : 0 | C14 : 0 |
| C16 : 0 | C14 : 0 | anteiso-C15 : 0 | ||
| C16 : 1ω7c DMA | C16 : 0 | C15 : 0 | ||
| C18 : 1ω7c DMA | C16 : 1ω7c | C16 : 1ω9c | ||
| C16 : 1ω7c DMA | C16 : 0 | |||
| Isolation source | Human subgingival plaque | Human subgingival plaque | Human subgingival plaque | Human maxillary sinus |
A, acetate; L, lactate.
Table 2. Number of genes identified in the biosynthetic pathway from whole genome sequences in different members of the genus Oribacteriumidentified by the RAST server.
Strains: 1, ACB1T; 2, ACB8; 3, ACB7T; 4, O. sinus F0268; 5, Oribacterium sp. OT 108 strain F0425; 6, Oribacterium sp. OT 078 strain F0262. OT, oral taxon as defined by HOMD. The number of genes identified for benzoquinones, naphthoquinones, mycolic acids and lipopolysaccharides was zero for all taxa studied.
| Genes responsible for biosynthesis | 1 | 2 | 3 | 4 | 5 | 6 |
| Accession number | NZ_AFZC00000000 | NZ_AJZT00000000 | NZ_AFZD00000000 | NZ_ACKX00000000 | NZ_AFIH00000000 | NZ_ACIQ00000000 |
| Teichoic and lipoteichoic acids | 3 | 4 | 5 | 4 | 5 | 4 |
| Polar lipids | 15 | 19 | 19 | 20 | 19 | 16 |
| Polyamines | 9 | 9 | 13 | 8 | 12 | 11 |
| Diaminopimelic acid | 6 | 6 | 7 | 7 | 7 | 7 |
| Lipooligosaccharides | 7 | 0 | 0 | 0 | 0 | 0 |
The isolated strains grew only under strictly anaerobic conditions. Growth occurred at 30–42 °C, with optimum growth at 37 °C. Isolates ACB1T and ACB7T were susceptible to discs containing 1 mg kanamycin, 5 µg vancomycin, 50 µg metronidazole, 2 U penicillin, 15 mg rifampicin and 15 mg bile but resistant to 10 µg colistin. Catalase, oxidase and urease activities were negative; nitrate reduction was not detected. Gelatin was not liquefied and indole was not produced. Strain ACB7T hydrolysed aesculin while strain ACB1T did not. All strains were able to grow on yeast extract and Bacto proteose peptone No. 3 but not on Casamino acids or trypticase alone. Strain ACB1T produced acid on API 20A media containing glucose, maltose and lactose, but not sucrose, arabinose, cellobiose, mannose, melezitose, raffinose, rhamnose, trehalose, xylose, glycerol, mannitol, salicin or sorbitol. In liquid medium supplemented with yeast extract at 0.5–2.0 g l−1, strains ACB1T and ACB8 weakly fermented glucose, lactose, maltose, maltodextrin and raffinose but not cellobiose or starch; strain ACB8 grew weakly on sucrose. Strain ACB7T did not produce acid on API 20A media, and did not grow in liquid medium with any of the tested carbon sources with the exception of weak growth on sucrose (i.e. OD600 reached ~ 0.1 units after 7–10 days of incubation). No visible biomass was formed in medium with 0.5–2.0 g yeast extract l−1 only; poor growth was observed in liquid medium with 1 g yeast extract l−1 and 0.5 g l-cysteine–HCl l−1. Strains ACB1T and ACB8 produced gas in TY or TGY liquid media. The major metabolic end products of strain ACB1T were acetate and lactate. Acetate was the only end product of strains ACB7T and ACB8.
The whole-cell hydrolysate of strain ACB7T contained meso-diaminopimelic acid (meso-Dpm); meso-Dpm was also detected in strains ACB1T and ACB7T after isolation and hydrolysis of the peptidoglycan. The occurrence of meso-Dpm in the novel strains indicated peptidoglycan type A1γ (or A1γ′; A31 or A32.1) according to http://www.peptidoglycan-types.info. Six and seven RAST-annotated genes associated with diaminopimelic acid synthesis were present in the genome of strains ACB1T and ACB8, and ACB7T, respectively (Table 2).
The genomic DNA G+C content of strains ACB1T, ACB8 and ACB7T was 42.1, 42.0 and 43.3 mol%, respectively. The fatty acid methyl ester profile showed that strain ACB1T contained C14 : 0 (18.6 %), C16 : 0 (24.1 %), C16 : 1ω7c dimethyl aldehyde (DMA) (18.1 %) and C18 : 1ω7c DMA (6.1 %) as major fatty acids, minor amounts of C14 : 0 DMA (4.8 %), C15 : 0 (3.4 %), C16 : 1ω7c (3.3 %) and C16 : 0 DMA (3.7 %), and trace amounts of C14 : 1ω7c DMA (1.65 %), anteiso-C15 : 0 (0.6 %), C15 : 1ω6c (0.3 %), C16 : 0 aldehyde (1.1 %), C16 : 1ω9c (0.2 %), C16 : 1ω5c (0.55 %), C17 : 1ω6c (0.4 %), C17 : 0 (0.15 %), C18 : 1 at 17.254 DMA (0.3 %), C18 : 0 (0.1 %) and C18 : 1ω9c DMA (0.1 %). Strain ACB7T contained C12 : 0 (5.4 %), C14 : 0 (22.4 %), C16 : 0 (15.7 %), C16 : 1ω7c (8.5 %) and C16 : 1ω7c DMA (7.7 %) as major fatty acids, minor amounts of C10 : 0 DMA (4.0 %), C14 : 0 DMA (3.3 %), C15 : 0 (4.2 %) and C18 : 0 (3.1 %), and trace amounts of C11 : 0 DMA (1.8 %), C13 : 0 (1.3 %), C14 : 1ω7c DMA (0.85 %), anteiso-C15 : 0 (0.5 %), C16 : 0 aldehyde (1.2 %), C16 : 0 DMA (1.2 %), C18 : 1ω9c (1.4 %), C18 : 1ω9c DMA (1.1 %) and C18 : 1ω7c DMA (0.8 %).
In whole genomes of strains ACB1T, ACB7T and ACB8 we found no predicted gene sequences with recognizable homology to biosynthesis of lipoquinones, mycolic acids or lipopolysaccharides. Nine to 13 RAST-annotated genes associated with metabolism of polyamines, and 15–19 genes associated with metabolism of polar lipids, were present in the genomes (Table 2).
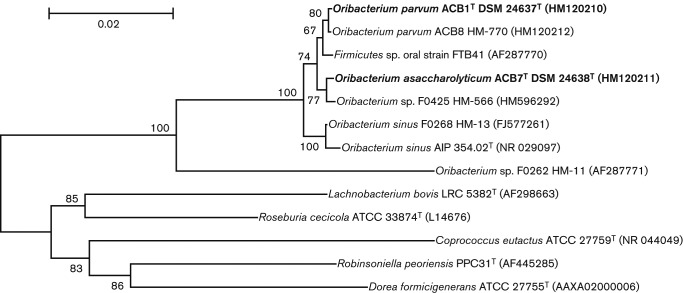
The 16S rRNA gene-based phylogenetic tree showed that strain ACB1T, together with ACB8, and strain ACB7T formed two separate branches within the genus Oribacterium (Fig. 2). The genus Oribacteriumcomprises, at the time of writing, a single recognized species Oribacterium sinus (Carlier et al., 2004; Rainey, 2009). Other known strains (Tables 2, 3 and S1) were reported in GenBank and HOMD (http://www.homd.org/index.php). Strains ACB1T and ACB8 were closely related to each other at 99.7 % 16S rRNA gene sequence similarity and shared 98.5–98.6 % similarity with O. sinus AIP 354.02T (Carlier et al., 2004) and 98.71–98.85 % with O. sinusstrain F0268 (Dewhirst et al., 2010). Strain ACB7T shared 98.1 % similarity with O. sinus AIP 354.02T and 98.7 % with strain F0268 (Table 3). The levels of 16S rRNA gene sequence similarity between strains ACB1T, ACB7T and O. sinus AIP 354.02T were below the 98.7 and 98.65 % cut-off values proposed for species demarcation by Stackebrandt & Ebers (2006) and Kim et al. (2014), respectively. The predicted DDH value between strains ACB1T and ACB8 was 98.2 % (Table 3). The predicted DDH value between strains ACB1T and ACB7T was 22.6 % and between strains ACB8 and ACB7T was 23.1 %, much less than the threshold of 70 %, the widely accepted value of relatedness between different species (Gevers et al., 2005; Tindall et al., 2010; Yarza et al., 2008). Predicted DDH values suggested that strains ACB1T and ACB8 represent the same species, whereas strain ACB7T belongs to a different species. Predicted DDH values between strains ACB1T, ACB8 and O. sinus F0268 and between strain ACB7T and O. sinus F0268 were only 15.6 and 15.7 %, respectively, clearly indicating three separate species (Table 3). Pair-wise comparison of 16S rRNA gene sequence similarities with predicted DDH values of six Oribacteriumstrains revealed that 16S rRNA gene sequence similarity values less than 99.5 % corresponded to DDH values less then 22.3 % (Table 3). The 16S rRNA gene sequence similarity value of 99.5 % between species within a genus is higher than the reported range of 98.2–99.0 % (Kim et al., 2014; Meier-Kolthoff et al., 2013; Stackebrandt & Ebers, 2006; Yarza et al., 2008). However, there are genera such as Brucella, Burkholderia, Bacillus, Brevundimonas, Escherichia, Salmonella and Shigella that contain separate species that share 100 % 16S rRNA gene sequence similarity (Ash et al., 1991; Fukushima et al., 2002; Gee et al., 2004; Gevers et al., 2005; Jaspers & Overmann, 2004).
Fig. 2.
Minimum-evolution phylogenetic tree based on 16S rRNA gene sequence comparisons of strains ACB1Tand ACB8, strain ACB7T, and other members of the genus Oribacterium and the family Lachnospiraceae. Bootstrap values >50 % calculated for 1000 subsets are shown at branch points. Bar, 0.02 substitutions per position.
Table 3. Levels of 16S rRNA gene sequence similarity (above) and predicted DDH values (below) between different members of the genus Oribacterium.
Strains: Oribacterium parvum strains ACB1T and ACB8; O. asaccharolyticum strain ACB7T; O. sinusstrain F0268; O. sinus strain AIP 354.02T; Oribacterium sp. OT 108 strain FTB41; Oribacterium sp. OT 078 strain F0262; Oribacterium sp. OT 108 strain F0425. OT, oral taxon as defined by HOMD. nd, Not determined.
| Strain | Accession no. | Number of bases (top)/Genome size (Mb) (bottom) | ACB1T | ACB7T | ACB8 | AIP 354.02T | F0268 | FTB41 | F0425 |
| 16S rRNA gene sequence similarity (%) | |||||||||
| ACB1T | HM120210 | 1395 | |||||||
| ACB7T | HM120211 | 1436 | 98.9 | ||||||
| ACB8 | HM120212 | 1397 | 99.7 | 99 | |||||
| AIP 354.02T | NR_029097 | 1374 | 98.6 | 98.1 | 98.5 | ||||
| F0268 | FJ577261 | 1497 | 98.9 | 98.7 | 99 | 99.4 | |||
| FTB41 | AF287770 | 1444 | 99.4 | 98.9 | 98.7 | 98.2 | 98.5 | ||
| F0425 | HM596292 | 1497 | 99.3 | 99.5 | 99.4 | 98.2 | 98.6 | 99.2 | |
| F0262 | FJ577249 | 1498 | 93.8 | 93.5 | 93.6 | 93.7 | 93.6 | 93.6 | 93.4 |
| Predicted DDH value (%) | |||||||||
| ACB1T | NZ_AFZC00000000 | 2.47 | |||||||
| ACB7T | NZ_AFZD00000000 | 2.52 | 22.6 | ||||||
| ACB8 | NZ_AJZT00000000 | 2.48 | 98.2 | 23.1 | |||||
| AIP 354.02T | nd | nd | nd | nd | nd | ||||
| F0268 | NZ_ACKX0000000 | 2.71 | 15.6 | 15.7 | 15.6 | nd | |||
| FTB41 | nd | nd | nd | nd | nd | nd | nd | ||
| F0425 | NZ_AFIH00000000 | 2.52 | 23.1 | 80.5 | 23.2 | nd | 15.8 | nd | |
| F0262 | NZ_ACIQ00000000 | 2.68 | 12.9 | 12.9 | 12.9 | nd | 12.9 | nd | 12.9 |
Tables 1–3 summarize physiological and genomic properties that can be used to differentiate strains ACB1T, ACB7T and ACB8 from O. sinus and other members of the genus. The type strain of O. sinus (Carlier et al., 2004) was isolated from sinus pus of a 6-year-old child with bilateral maxillary sinusitis, while strains ACB1T, ACB7T and ACB8 were enriched from a non-infectious subgingival plaque sampled from a generally healthy 25-year-old adult. Cells of O. sinus were 0.8–1.0 µm wide compared with 0.45–0.5 µm for cells of strains ACB1T, ACB8 and ACB7T. In contrast to O. sinus, the three novel strains did not produce indole. Strains ACB1T and ACB8 fermented lactose and maltose while the type strain of O. sinusand strain ACB7T did not. O. sinus and strains ACB1T and ACB8 used glucose and raffinose as carbon sources, while strain ACB7T did not. The only strain that hydrolysed aesculin was ACB7T. Major metabolic end products of O. sinus and strain ACB1T were acetate and lactate; strains ACB7T and ACB8 produced acetate only.
The fatty acid methyl ester profile, spectrum of utilized carbon sources, DNA G+C content, metabolic end products, as well as the number of annotated genes responsible for biosynthesis of teichoic and lipoteichoic acids, polar lipids, polyamines, diaminopimelic acid and lypooligosaccharides distinguish strains ACB1T, ACB8 and ACB7T from O. sinus.
On the basis of physiological, biochemical and molecular properties, we suggest that the strains described in this study represent two novel species, for which we propose the names Oribacterium parvum sp. nov. to accommodate strains ACB1T and ACB8 and Oribacterium asaccharolyticum sp. nov. to accommodate strain ACB7T.
Emended description of Oribacterium (Carlier et al., 2004)
Elongated ovoid rods, about 1.2–2.2 µm long and 0.45–1 µm wide, usually occurring singly, in pairs or, occasionally, in short chains. Motile with laterally inserted flagella. Gram-positive but may appear Gram-negative after staining. Strictly anaerobic. Do not form spores. Weakly fermentative. Major metabolic end products are acetate and lactate or acetate only. Major (>10 %) fatty acids are C14 : 0, C16 : 0 and anteiso-C15 : 0 or C16 : 1ω7c DMA. DNA G+C content is 42.1–43.3 mol%. Phylogenetically related to members of the family Lachnospiraceae. The type species is Oribacterium sinus.
Description of Oribacterium parvum sp. nov.
Oribacterium parvum (par′vum. L. neut. adj. parvum small, little).
Cells are Gram-variable after staining but structurally Gram-positive, short, motile, ovoid rods, 1.2×0.45 µm, sometimes swollen, occurring singly, in chains or as aggregates. Colonies are round, convex, beige, 1.5 mm in diameter and non-haemolytic on WC agar. Black pigment is produced in TY or TGY liquid medium supplemented with l-cysteine–HCl. Yeast extract is required for growth on glucose, lactose, maltose, maltodextrin and raffinose liquid media; gas is produced. Indole is not produced. Gelatin is not liquefied. Aesculin is not hydrolysed. Catalase, oxidase and urease are negative. Nitrate is not reduced. Susceptible to kanamycin, vancomycin, metronidazole, penicillin, rifampicin and bile but resistant to colistin. The DNA G+C content is 42.0–42.1 mol%. The major metabolic end products are acetate and lactate. Growth temperature range is 30–42 °C. Major fatty acids are C14 : 0, C16 : 0, C16 : 1ω7c DMA and C18 : 1ω7c DMA. The peptidoglycan type is A1γ.
The type strain, ACB1T ( = DSM 24637T = HM-481T = ATCC BAA-2638T), was isolated from human subgingival dental plaque. ACB8, isolated from a similar source, is a second strain of the species.
Description of Oribacterium asaccharolyticum sp. nov.
Oribacterium asaccharolyticum [a.sac.cha.ro.ly′ti.cum. Gr. pref. a not; Gr. n. saccharon sugar; N.L. neut. adj. lyticum able to lyse (from Gr. adj. lytikos able to lose); N.L. neut. adj. asaccharolyticum not digesting sugar].
Cells are Gram-variable after staining but structurally Gram-positive, motile rods 1.6×0.5 µm, some cells up to 5–7 µm long, often swollen, occurring singly, in pairs or in chains. Colonies are non-haemolytic, round, non-pigmented and 0.5 mm in diameter after 48 h and umbonate and 2–3 mm in diameter with irregular wavy edges after 168 h. Growth is supported by yeast extract and Bacto proteose peptone. Black pigment is produced in liquid medium supplemented with l-cysteine–HCl; gas is not produced. The lowest concentrations of yeast extract and l-cysteine–HCl required for visible growth are 1 and 0.5 g l−1, respectively. In liquid medium growth is not supported by glucose, maltose, lactose, cellobiose, maltodextrin, raffinose or starch; sucrose supports poor growth. Gelatin is not liquefied. Aesculin is hydrolysed. Catalase, oxidase and urease are negative. Nitrate is not reduced. Susceptible to kanamycin, vancomycin, metronidazole, penicillin, rifampicin and bile but resistant to colistin. The major metabolic end product is acetate. Growth temperature range is 30–42 °C. Major fatty acids are C12 : 0, C14 : 0, C16 : 0, C16 : 1ω7c and C16 : 1ω7c DMA. The peptidoglycan type is A1γ.
The type strain, ACB7T ( = DSM 24638T = HM-482T = ATCC BAA-2639T), was isolated from human subgingival dental plaque. The DNA G+C content of the type strain is 43.3 mol%.
Acknowledgements
This work was supported by NIH grants 1RC1DE020707-01 and 3 R21 DE018026-02S1 to S. S. E., 1U54 AI84844-01 to K. E. N., EF-1002148 to N. P., EU-FP7 Marie Curie International Fellowship PIOF-GA-2009-235470 to M. M. and U54HG004969 to A. M. E. We thank Mr M. Torralba for technical assistance.
Abbreviations:
- DDH
DNA–DNA hybridization
- DMA
dimethyl aldehyde
- HOMD
Human Oral Microbiome Database
- meso-Dpm
meso-diaminopimelic acid
Footnotes
One supplementary figure and one supplementary table are available with the online version of this paper.
References
- Ash C., Farrow J. A., Dorsch M., Stackebrandt E., Collins M. D. (1991). Comparative analysis of Bacillus anthracis, Bacillus cereus, and related species on the basis of reverse transcriptase sequencing of 16S rRNA. Int J Syst Bacteriol 41, 343–346 10.1099/00207713-41-3-343 [DOI] [PubMed] [Google Scholar]
- Auch A. F., Klenk H. P., Göker M. (2010a). Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand Genomic Sci 2, 142–148 10.4056/sigs.541628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auch A. F., von Jan M., Klenk H. P., Göker M. (2010b). Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci 2, 117–134 10.4056/sigs.531120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz R. K., Bartels D., Best A. A., DeJongh M., Disz T., Edwards R. A., Formsma K., Gerdes S., Glass E. M. & other authors (2008). The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9, 75 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier J. P., K’ouas G., Bonne I., Lozniewski A., Mory F. (2004). Oribacterium sinus gen. nov., sp. nov., within the family ‘Lachnospiraceae’ (phylum Firmicutes). Int J Syst Evol Microbiol 54, 1611–1615 10.1099/ijs.0.63060-0 [DOI] [PubMed] [Google Scholar]
- Chen T., Yu W. H., Izard J., Baranova O. V., Lakshmanan A., Dewhirst F. E. (2010). The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database: the journal of biological databases and curation 2010, baq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst F. E., Chen T., Izard J., Paster B. J., Tanner A. C., Yu W. H., Lakshmanan A., Wade W. G. (2010). The human oral microbiome. J Bacteriol 192, 5002–5017 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis E. A. (2006). Solutions to the problem of substitution of ERL 4221 for vinyl cyclohexene dioxide in spurr low viscosity embedding formulations. Microsc Today 14, 32–33 [Google Scholar]
- Frazzon J., Dean D. R. (2003). Formation of iron-sulfur clusters in bacteria: an emerging field in bioinorganic chemistry. Curr Opin Chem Biol 7, 166–173 10.1016/S1367-5931(03)00021-8 [DOI] [PubMed] [Google Scholar]
- Fukushima M., Kakinuma K., Kawaguchi R. (2002). Phylogenetic analysis of Salmonella, Shigella, and Escherichia coli strains on the basis of the gyrB gene sequence. J Clin Microbiol 40, 2779–2785 10.1128/JCM.40.8.2779-2785.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee J. E., De B. K., Levett P. N., Whitney A. M., Novak R. T., Popovic T. (2004). Use of 16S rRNA gene sequencing for rapid confirmatory identification of Brucella isolates. J Clin Microbiol 42, 3649–3654 10.1128/JCM.42.8.3649-3654.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D., Cohan F. M., Lawrence J. G., Spratt B. G., Coenye T., Feil E. J., Stackebrandt E., Van de Peer Y., Vandamme P. & other authors (2005). Opinion: Re-evaluating prokaryotic species. Nat Rev Microbiol 3, 733–739 10.1038/nrmicro1236 [DOI] [PubMed] [Google Scholar]
- Jaspers E., Overmann J. (2004). Ecological significance of microdiversity: identical 16S rRNA gene sequences can be found in bacteria with highly divergent genomes and ecophysiologies. Appl Environ Microbiol 70, 4831–4839 10.1128/AEM.70.8.4831-4839.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Oh H. S., Park S. C., Chun J. (2014). Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64, 346–351 10.1099/ijs.0.059774-0 [DOI] [PubMed] [Google Scholar]
- Meier-Kolthoff J. P., Auch A. F., Klenk H. P., Göker M. (2013). Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14, 60 10.1186/1471-2105-14-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara H., Esaki N. (2002). Bacterial cysteine desulfurases: their function and mechanisms. Appl Microbiol Biotechnol 60, 12–23 10.1007/s00253-002-1107-4 [DOI] [PubMed] [Google Scholar]
- Rainey F. A. (2009). Family V. Lachnospiraceae fam. nov. In Bergey’s Manual of Systematic Bacteriology, vol. 3, pp. 921–968 Edited by De Dos P., Garrity G. M., Jones D., Krieg N. R., Ludwig W., Rainey F. A., Schleifer K.-H., Whitman W. B. New York: Springer [Google Scholar]
- Rhuland L. E., Work E., Denman R. F., Hoare D. S. (1955). The behaviour of the isomers of 2,6-diaminopimelic acid on paper chromatograms. J Am Chem Soc 77, 4844–4846 10.1021/ja01623a047 [DOI] [Google Scholar]
- Schumann P. (2011). Peptidoglycan structure. Methods Microbiol 38, 101–129 10.1016/B978-0-12-387730-7.00005-X [DOI] [Google Scholar]
- Sizova M. V., Hohmann T., Hazen A., Paster B. J., Halem S. R., Murphy C. M., Panikov N. S., Epstein S. S. (2012). New approaches for isolation of previously uncultivated oral bacteria. Appl Environ Microbiol 78, 194–203 10.1128/AEM.06813-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizova M. V., Muller P., Panikov N., Mandalakis M., Hohmann T., Hazen A., Fowle W., Prozorov T., Bazylinski D. A., Epstein S. S. (2013). Stomatobaculum longum gen. nov., sp. nov., an obligately anaerobic bacterium from the human oral cavity. Int J Syst Evol Microbiol 63, 1450–1456 10.1099/ijs.0.042812-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackebrandt E., Ebers J. (2006). Taxonomic parameters revisited: tarnished gold standards. Microbiol Today 33, 152–155 [Google Scholar]
- Tindall B. J., Rosselló-Móra R., Busse H. J., Ludwig W., Kämpfer P. (2010). Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol 60, 249–266 10.1099/ijs.0.016949-0 [DOI] [PubMed] [Google Scholar]
- Yarza P., Richter M., Peplies J., Euzeby J., Amann R., Schleifer K. H., Ludwig W., Glöckner F. O., Rosselló-Móra R. (2008). The All-Species Living Tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst Appl Microbiol 31, 241–250 10.1016/j.syapm.2008.07.001 [DOI] [PubMed] [Google Scholar]