Abstract
Background
Patients with ischemic left ventricular dysfunction have higher operative risk with CABG. However, those whose early risk is surpassed by subsequent survival benefit have not been identified.
Objective
To examine the impact of anatomic variables associated with poor prognosis on the effect of coronary artery bypass graft surgery (CABG) in ischemic cardiomyopathy.
Methods
All 1,212 patients in the STICH surgical revascularization trial were included. Patients had coronary artery disease (CAD), ejection fraction (EF) ≤35%, and were randomized to CABG plus medical therapy or optimal medical therapy alone (OMT). This study focused on 3 prognostic factors: presence of 3-vessel CAD; EF below the median (27%); and end-systolic volume index (ESVI) above the median (79 ml/m2). Patients were categorized as having 0–1 or 2–3 of these factors.
Results
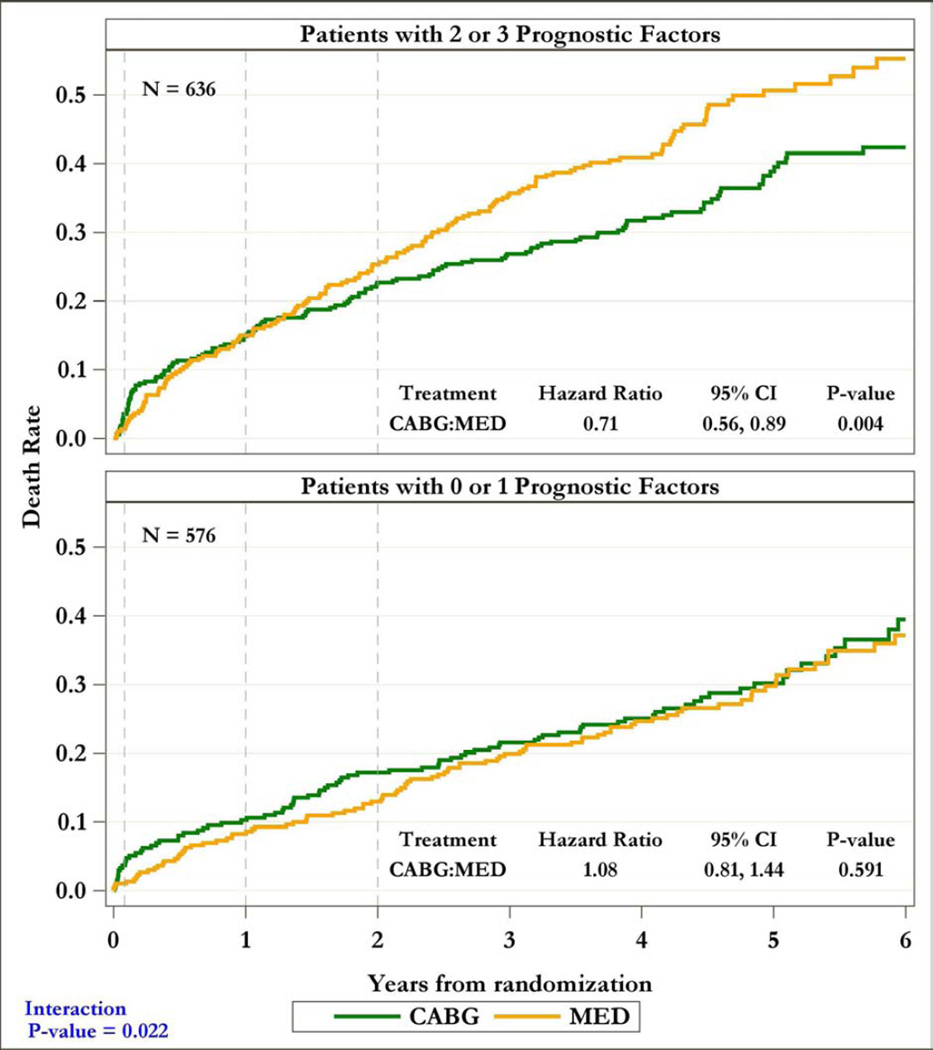
Patients with 2–3 prognostic factors (n= 636) had reduced mortality with CABG, as compared to OMT (HR=0.71, 95% CI=0.56–0.89; p=0.004); CABG had no such effect in patients with 0–1 factors (HR=1.08, 95% CI=0.81–1.44; p=0.591). There was a significant interaction between the number of factors and the effect of CABG on mortality (p=0.022). Although 30-day risk with CABG was higher, a net beneficial effect of CABG over OMT was observed at >2years in patients with 2–3 factors (HR=0.53, 95% CI=0.37–0.75; p#x0003C;0.001), but not in those with 0–1 factors (HR=0.88, 95% CI=0.59–1.31; p=0.535).
Conclusions
Patients with more advanced ischemic cardiomyopathy receive greater benefit from CABG. This supports the indication for surgical revascularization in patients with more extensive CAD and worse myocardial dysfunction and remodeling. (ClinicalTrials.gov number, NCT00023595)
Keywords: coronary artery disease, left ventricular dysfunction, myocardial ischemia, heart failure, outcomes
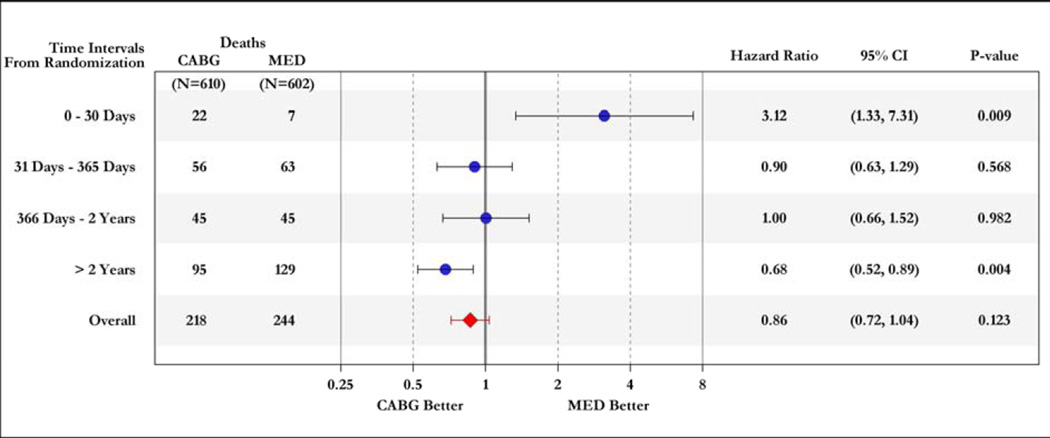
Unlike in any other form of left ventricular (LV) dysfunction, patients with ischemic cardiomyopathy have the potential to improve their prognosis with revascularization. Recent randomized controlled trials have shown that revascularization with coronary artery bypass graft surgery (CABG) is superior to that with percutaneous coronary interventions (PCI) in patients with multi-vessel coronary artery disease (CAD) (1, 2). However, the decision to pursue CABG is usually difficult in ischemic cardiomyopathy patients, particularly because the presence and severity of LV dysfunction impose a higher operative risk (3, 4). The Surgical Treatment of IsChemic Heart failure (STICH) trial recently tested the hypothesis that surgical revascularization with CABG improves the survival of patients with ischemic LV dysfunction compared to optimal medical therapy (OMT) without revascularization. During a median follow-up of 56 months, STICH demonstrated a trend toward better survival with CABG that did not reach statistical significance (p=0.12) (5). Importantly, the treatment effect of CABG over medical therapy occurred in a clear time-dependent pattern, with an early (within 30 days) increased hazard, related to the operative mortality, and a late (≥2 years) survival benefit (Figure 1).
Figure 1. Time-varying hazard ratios for all-cause mortality in patients randomized to CABG or OMT in the STICH trial.
OMT= optimal medical therapy; CABG= coronary artery bypass graft surgery.
Several previous studies have shown that, among patients with CAD, the number of vessels with angiographically-detected stenoses, the LV ejection fraction (EF), and the LV end-systolic volume index (ESVI) are associated with prognosis (3, 4, 6–12). However, how these variables should be incorporated into the decision regarding revascularization in patients with ischemic cardiomyopathy is unclear. Hazard ratio analyses of pre-determined subgroups in STICH did not identify any variable with a statistically significant interaction with treatment allocation (see Figure 3 in Reference 5) and the lack of statistical significance on the primary endpoint in STICH has led to the concept that the indication for surgical revascularization in ischemic cardiomyopathy can be safely deferred until medical therapy fails or the patient becomes unstable (13, 14). However, previous analyses did not address whether the time-dependent survival relationship between the 2 treatment arms varies according to baseline risk. Accordingly, the purpose of this study was to examine the impact of key anatomic variables – used in routine clinical practice and known to be associated with prognosis -- on the time-dependent hazard of CABG relative to OMT in patients enrolled in the surgical revascularization hypothesis of the STICH trial. We hypothesized that this analysis could lead to the recognition of a group of patients whose early surgical risk is rapidly surpassed by subsequent survival benefit and in whom, therefore, the indication for CABG is more clearly supported.
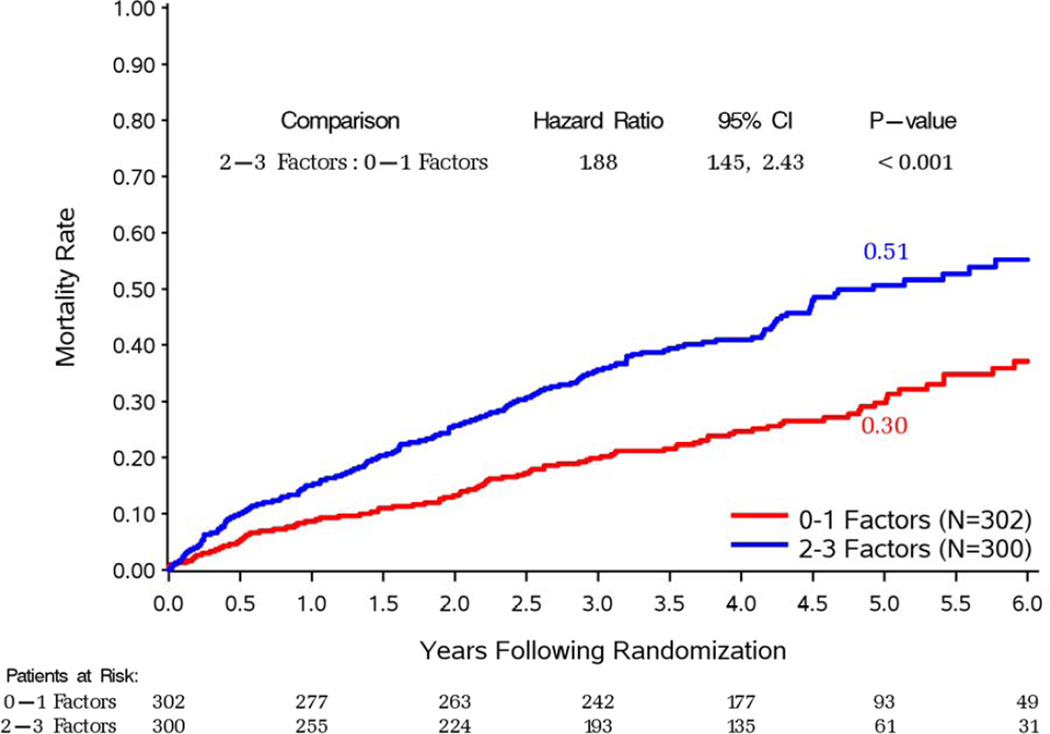
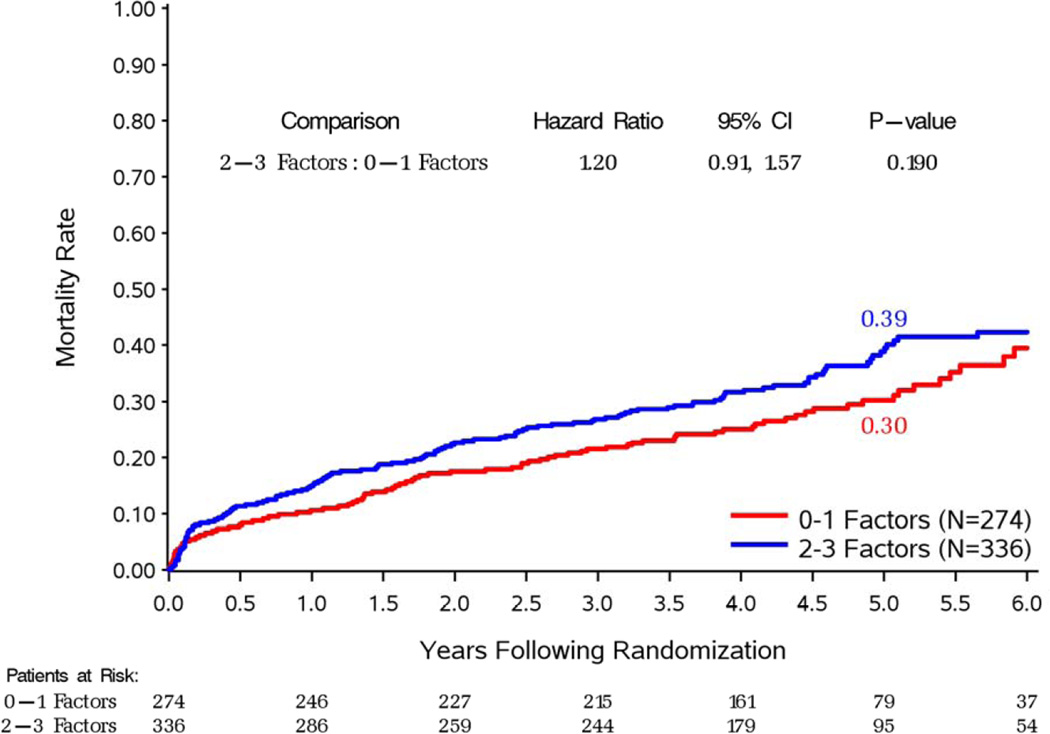
Figure 3. Kaplan-Meier rate estimates of all-cause mortality among patients with 2–3 (top panel) and 0–1 (bottom panel) prognostic factors.
In each panel, study patients are divided according to the treatment arm (CABG or OMT) to which they were randomized.
METHODS
Study Population
STICH is a prospective, multicenter, non-blinded, randomized trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) that recruited 2,136 patients with CAD and LV EF ≤35% between 2002 and 2007. The trial was designed to address two primary hypotheses: 1) CABG combined with OMT improves survival compared to optimal medical therapy alone (surgical revascularization hypothesis), and 2) surgical ventricular reconstruction added to CABG improves survival free of cardiovascular hospitalization compared to CABG alone in patients with significant anterior wall akinesis (surgical ventricular reconstruction hypothesis). The trial design and the results of the 2 primary hypotheses have been reported previously (5, 15, 16). For the purpose of this study, only the 1,212 patients included in the surgical revascularization arm were considered.
All patients had angiographic documentation of CAD amenable to CABG and EF ≤35%. Patients with left main coronary stenosis >50%, cardiogenic shock, myocardial infarction within 3 months, or need for aortic valve surgery were excluded. Patients were randomly assigned to receive CABG with medical therapy or medical therapy alone. PCI was not considered among the revascularization strategies in the STICH protocol. As per the original design of the trial (18), PCI during follow-up was regarded as downstream medical care associated with either treatment strategy, and PCI was performed as a subsequent procedure in only 37 of the 602 (6%) patients randomized to medical therapy alone and in 26 of the 610 (4%) patients randomized to CABG (p=NS). The NHLBI and the ethics committee at each recruiting institution approved the study protocol. All patients provided written informed consent for participation in the trial.
For the purpose of this study, attention was focused on 3 variables known to be prognostically important: 1) presence of 3-vessel CAD (defined as ≥50% stenosis); 2) baseline LV EF; and 3) baseline LV ESVI. These variables were selected prospectively for the purpose of this analysis based on their known prognostic significance. Assessment of coronary anatomy was made by the investigators at each recruiting center and relayed to the Data Coordinating Center at Duke University using specifically designed data collection forms. LV EF and LV ESVI were measured by core laboratories independently funded by NHLBI and blinded to all clinical and outcome information. As previously published, the best available method (based on study quality using a predetermined hierarchical algorithm) was used for LV EF and LV ESVI measurements (19). Patients were divided in a binary fashion according to the presence or absence of 3-vessel disease, LV EF below or above the median, and LV ESVI below or above the median. In addition, each patient was categorized by the number of prognostic factors defined by these variables, namely: 1) presence of 3-vessel CAD; 2) LV EF below the median; and 3) LV ESVI above the median. The presence of 3-vessel CAD was selected based on the results from the CASS (Coronary Artery Surgery Study) trial showing that this variable identifies a population of patients that may preferentially benefit from CABG (19). The thresholds for EF and ESVI were selected post-hoc based on the median values of the STICH population; no multiple looks or comparisons with other thresholds were performed. For the purpose of data analysis, patients were grouped into those having 0–1 or 2–3 prognostic factors.
Follow-up and Outcomes
After enrollment, patients were followed every 4 months for the first year and every 6 months thereafter. Adherence to guideline-directed medical therapy was high throughout the study period, without significant difference between the treatment groups (5). As for the primary STICH trial analysis, the primary outcome was death from any cause and the secondary endpoint was death from cardiovascular causes. Definitions of the trial endpoints have been previously reported (5). An independent clinical events committee adjudicated all endpoints. Median follow-up was 56 months (maximum, 8⅓ years).
Statistical Analyses
Demographic and baseline variables were summarized using means and standard deviations for continuous variables, and by number (n) and percent (%) for categorical variables. Comparisons for continuous and ordinal variables between patient groups were performed by the Wilcoxon rank-sum test. The chi-squared test or Fisher’s exact test compared categorical variables. Event-rate estimates in each patient group were calculated using the Kaplan-Meier method and presented graphically (20). The significance of differences in mortality between patient groups was assessed using the log-rank test (21). Relative risks, expressed as hazard ratios with associated 95% confidence intervals, were derived using the Cox regression model (22). The interaction of prognostic factors and randomized treatment with respect to mortality was also assessed using the Cox model.
To examine the time-dependent nature of the randomized treatment effect, time-varying hazard ratios and 95% confidence intervals comparing CABG vs. medical therapy were calculated for discrete time periods after randomization (i.e., ≤30 days; 31–365 days; 366 days-2 years; and >2 years). These time points were selected prospectively for the purpose of this study. This analysis also was performed separately by patient groups (e.g., patients with 0–1 and 2–3 prognostic factors).
All mortality comparisons of the randomized treatment arms were performed according to the intention-to-treat principle (as randomized). Secondary analyses included comparisons by treatment received (e.g., CABG or medical therapy regardless of randomization), and per protocol (e.g., excluding the patients who crossed over to the other treatment arm). All 462 deaths (38.1% of the STICH population) reported at the time of database closure were included in the analysis.
RESULTS
Study Population
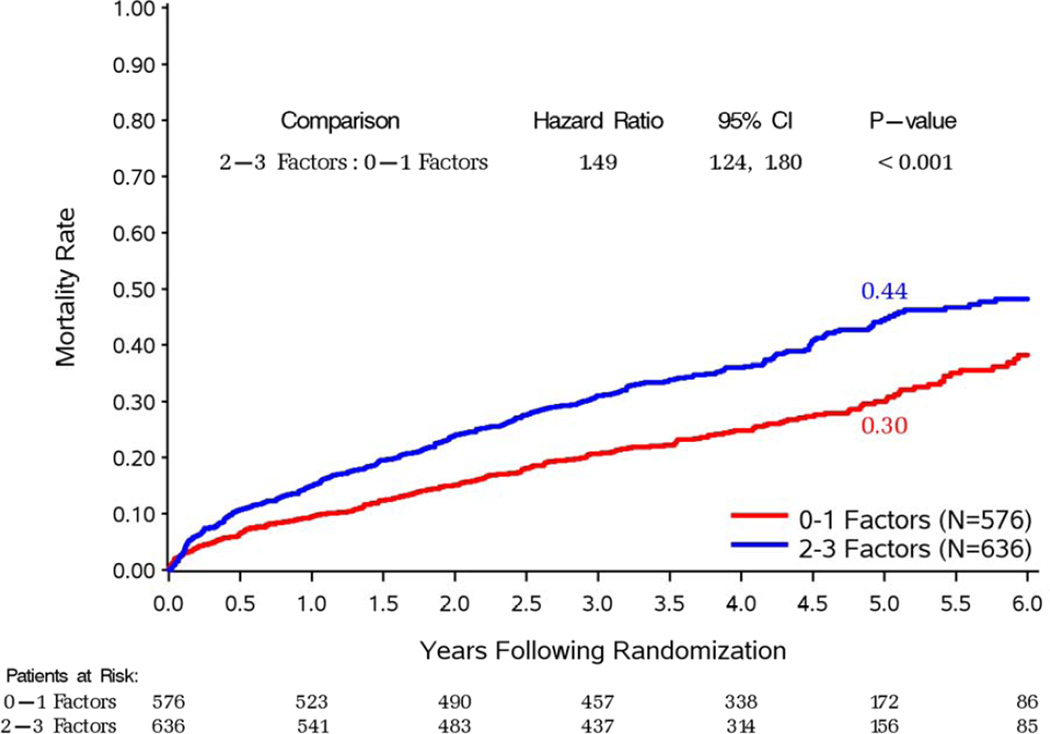
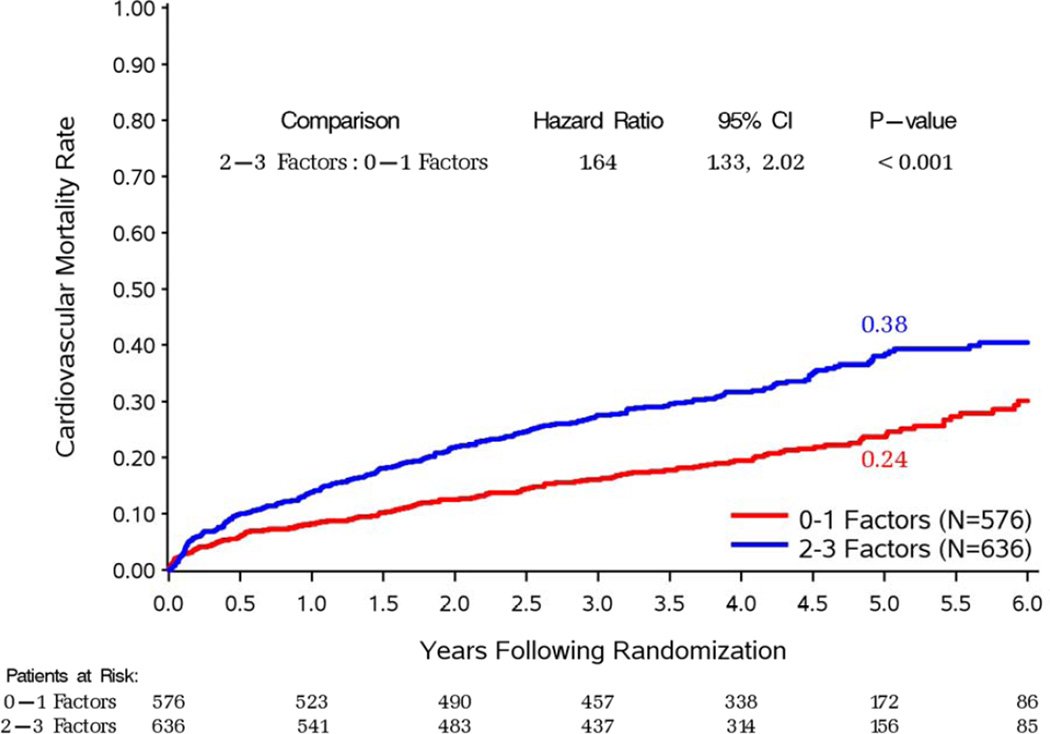
Of the 1,212 patients included in the STICH revascularization hypothesis trial, 734 had 3-vessel CAD. The median LV EF was 26.7% and the median ESVI was 78.6 ml/m2. There were 576 patients with 0–1 prognostic factors and 636 patients with 2–3 prognostic factors, as defined above. Table 1 shows the distribution of baseline characteristics in the patient groups defined according to the number of prognostic variables. As expected, compared to patients with 0–1 prognostic factors, those with 2–3 factors had a greater prevalence of characteristics associated with poor prognosis, including greater proportion of atrial flutter or fibrillation, previous CABG, moderate or severe mitral regurgitation, and more common presentation of advanced heart failure. Accordingly, patients with 2–3 prognostic factors had significantly higher overall and cardiovascular mortality compared to those with 0–1 prognostic factors, when treatment allocation was not considered (Figure 2).
Table 1.
Comparison of Baseline Characteristics for Patients with Different Number of Prognostic Factors According to 3-Vessel Disease, Low LVEF, and High ESVI
| Variable | Patients With 0–1 Prognostic factors (n=576) |
Patients With 2–3 Prognostic Factors (n=636) |
P value |
|---|---|---|---|
| Age (years) (mean±SD) | 60±9 | 60±9 | 0.961 |
| Female (n (%)) | 90 (16%) | 58 (9%) | <0.001 |
| White race (n (%)) | 390 (68%) | 437 (69%) | 0.708 |
| Body mass index (mean±SD) | 27±5 | 27±5 | 0.683 |
| History of previous myocardial infarction (n (%)) | 436 (76%) | 498 (78%) | 0.281 |
| Atrial flutter or fibrillation (n (%)) | 58 (10%) | 95 (15%) | 0.011 |
| Previous CABG (n (%)) | 10 (2%) | 26 (4%) | 0.016 |
| Previous PCI (n (%)) | 68 (12%) | 88 (14%) | 0.292 |
| Advanced angina* (n (%)) | 33 (6%) | 25 (4%) | 0.143 |
| Advanced heart failure† (n (%)) | 179 (31%) | 268 (42%) | <0.001 |
| Three-Vessel CAD (n (%)) | 289 (50%) | 445 (70%) | <0.001 |
| Moderate or severe mitral regurgitation (n (%)) | 83 (14%) | 137 (22%) | 0.001 |
| LV EF (%) (mean±SD) | 34±7 | 23±5 | n/a |
| ESVI‡ (ml/m2) (mean±SD) | 61±16 | 104±30 | n/a |
| EDVI§ (ml/m2) (mean±SD) | 92±24 | 139±37 | n/a |
| Patients randomized to CABG (n (%)) | 274 (48%) | 336 (53%) | 0.067 |
Canadian Cardiac Society Class III or IV
New York Heart Association Functional Class III or IV
End-systolic volume index
End-diastolic volume index
Figure 2. Kaplan-Meier estimates of all-cause (panel A) and cardiovascular (panel B) mortality rates.
In each panel, study patients are divided according to the presence of 0–1 or 2–3 prognostic factors, regardless of treatment allocation.
Effect of Surgical Revascularization on Survival According to Key Anatomic Variables
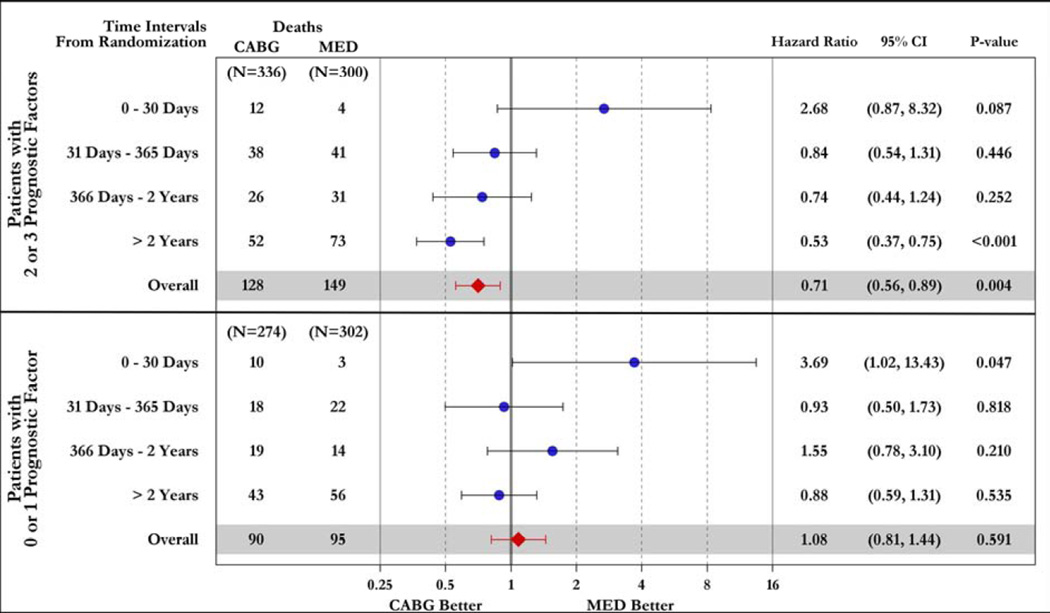
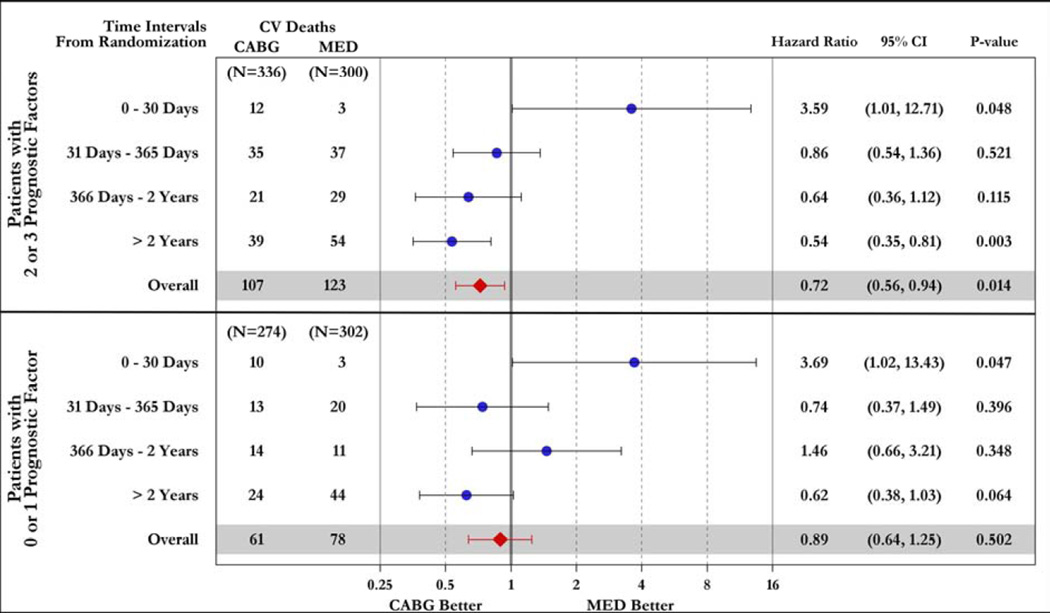
Patients with 3-vessel CAD received a significant benefit with CABG compared to medical therapy alone in terms of overall mortality (p=0.046) and cardiovascular deaths (p=0.030) In contrast, no such an effect was observed among patients without 3-vessel CAD (p=0.906 and p=0.554 for overall and cardiovascular mortality, respectively) (Supplemental Figures 1 and 2). Similarly, patients with LV EF below the median had reduced overall (p=0.021) and cardiovascular (p=0.043) mortality with CABG compared to medical therapy, an effect not found among patients with EF above the median (p=0.970 and p=0.288 for overall and cardiovascular mortality, respectively) (Supplemental Figures 3 and 4). Patients with LV ESVI higher than the median had a marginal reduction in overall mortality (p=0.055) with CABG compared with medical therapy and no significant benefit in terms of cardiovascular mortality (p=0.177), whereas no significant difference was noted between the 2 treatment arms among patients with ESVI below the median (p=0.596 and p=0.064 for overall and cardiovascular mortality, respectively) (Supplemental Figures 5 and 6). Finally, when patients with 2–3 prognostic factors were analyzed as a group, there was a highly significant mortality reduction with CABG compared to that observed with medical therapy alone (HR: 0.71; CI: 0.56–0.89; p=0.004); no such therapeutic effect of CABG was found among patients with 0–1 prognostic factors (HR: 1.08; CI: 0.81–1.44; p=0.591). A statistically significant interaction (p=0.022) was observed between the number of prognostic factors and the treatment effect of CABG on mortality (Figure 3). Analysis of the Kaplan-Meier mortality rates according to treatment allocation among patients with 2–3 prognostic factors revealed that the curves crossed (indicating the elimination of the higher surgical risk) at approximately 6 to 15 months after randomization (Figure 3, top panel). This was also true for patients with only 1 prognostic factor (Supplemental Figures 1, 3, and 5). Patients with 2–3 factors also had reduced cardiovascular mortality with CABG compared to medical therapy (HR: 0.72; CI: 0.56–0.94; p=0.014), with no such effect observed among patients with 0–1 factors (HR: 0.89; CI: 0.64–1.25; p=0.502). Of note, among patients randomized to medical therapy alone, there was a significantly higher mortality in patients with 2–3 prognostic factors compared to those with 0–1 factors (p#x0003C;0.001). However, such a difference was not observed among patients randomized to CABG (p=0.190), hence indicating that the treatment effect of surgical revascularization markedly blunted the higher mortality risk conveyed by the presence of anatomic findings associated with poor prognosis (Figure 4).
Figure 4. Kaplan-Meier estimates of all-cause mortality rates among patients randomized to optimal medical therapy alone (OMT; top panel) or to coronary artery bypass surgery (CABG; bottom panel).
In each panel, study patients are divided according to the presence of 0–1 or 2–3 prognostic factors.
Time-Dependent Mortality Hazard of CABG vs. Medical Therapy According to Key Anatomic Variables
Patients with 3-vessel CAD, those with LV EF below the median, and those with LV ESVI above the median had a clear time-dependent overall and cardiovascular mortality hazard with surgical revascularization, with an early (within 30 days) higher risk with CABG and a clear benefit at ≥2 years (Supplemental Figures 7–12). This time-dependent hazard was different among the subgroups of patients without these anatomic characteristics, in that their mortality benefit with CABG was not statistically significant at ≥2 years (except for reduced cardiovascular mortality in patients without 3-vessel CAD and in those with LV ESVI above the median) (Supplemental Figures 8 and 12).
Of note, when the anatomic characteristics were combined, patients with 2–3 prognostic factors showed a trend toward higher overall mortality (p=0.087) and statistically significant higher cardiovascular mortality (p=0.048) with CABG within 30 days, and a markedly significant benefit of CABG at ≥2 years in terms of overall mortality (p#x0003C;0.001) and cardiovascular deaths (p=0.003) compared to medical therapy alone. The overall hazard ratio was 0.71 (95% CI: 0.56–0.89; p=0.004) for all-cause mortality and 0.72 (95% CI: 0.56–0.94; p=0.014) for cardiovascular deaths. In contrast, patients with 0–1 prognostic factors had a significantly higher early mortality hazard with CABG compared to medical therapy alone (p=0.047 for both overall and cardiovascular mortality), without survival benefit at any time point (overall hazard ratio 1.08; 95% CI: 0.81–1.44; p=0.591 for all-cause mortality and 0.89; 95% CI: 0.64–1.25; p=0.502 for cardiovascular deaths) (Figure 5). In patients randomized to CABG, the early mortality (within 30 days of randomization) among patients with 2–3 prognostic factors (3.57%) was similar to that in patients with 0–1 prognostic factors (3.65%).
Figure 5. Time-dependent hazard ratios of all-cause (top panel) and cardiovascular (bottom panel) mortality for CABG vs. OMT.
Study patients are divided according to the presence of 2–3 (upper part of each panel) or 0–1 (lower part of each panel) prognostic factors.
Analyses of the data according to treatment received (e.g., CABG or medical therapy regardless of randomization), and per protocol (e.g., excluding the patients who crossed over to the other treatment arm) showed similar results to those of the primary intention-to-treat analysis (Supplemental Figures 13 and 14).
DISCUSSION
The decision whether to proceed with surgical revascularization in a patient with ischemic cardiomyopathy is an increasingly common one, given the rising prevalence of this condition (23). Unfortunately, it is also a difficult one, because of the complexity and variability of the clinical presentations and, until recently, because of the paucity of data from randomized clinical trials. The recently completed STICH trial showed a trend (p=0.12) toward better overall survival with CABG compared to medical therapy alone, and a statistically significant benefit in the secondary endpoints of cardiovascular mortality and death from any cause plus cardiac hospitalization (5). As expected, based on clinical experience, the mortality curves for the 2 treatment arms crossed during the period of follow-up, due to the increased early but decreased late mortality observed with CABG. This increased early risk of death with surgery partly negates the overall benefit of CABG and, most importantly, may be limited to certain subgroups of patients. Identification of those patients whose early surgical risk is clearly offset by the long-term benefit of revascularization could lead to the selection of a specific group of patients in whom deferring surgery may not be the most appropriate choice.
The results of this study demonstrate that patients with more advanced forms of ischemic cardiomyopathy (as expressed by the presence of 3-vessel disease and more severe LV systolic dysfunction and remodeling) are those that receive the greatest benefit from surgical revascularization. Thus, among the 1,212 patients enrolled in the STICH revascularization hypothesis trial, those with ≥50% stenosis in all 3 major coronary arteries, LV EF below the median, and LV ESVI above the median showed a clear time-dependent benefit of CABG compared to medical therapy alone. This resulted in an overall statistically significant benefit of CABG over the entire period of follow-up despite the higher early (within 30 days) mortality with surgery compared to that with medical therapy. Importantly, as the number of prognostic factors defined by these variables increases, the time-dependent pattern of the benefit of CABG over medical therapy (e.g., diminished early surgical risk and enhanced late benefit) becomes more pronounced.
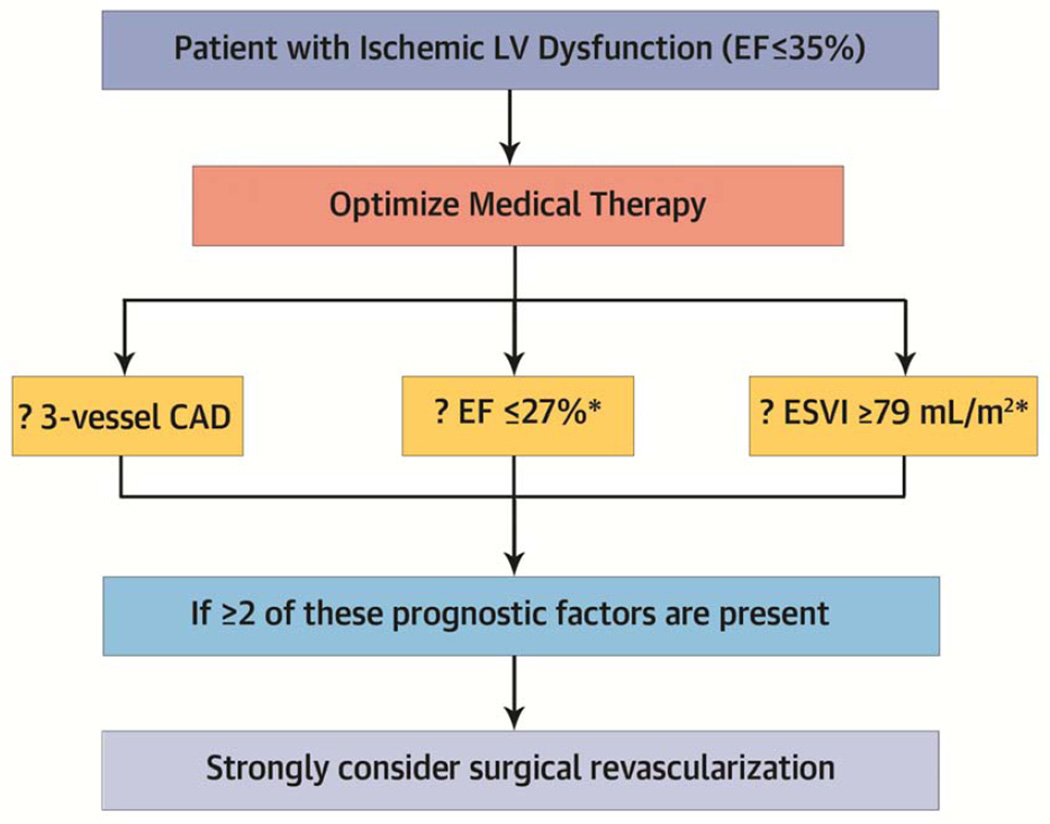
These findings have important clinical implications for patients with ischemic cardiomyopathy that may influence the paradigm used by clinicians for their decision regarding surgical revascularization (Central Illustration). Although more extensive CAD and worse LV dysfunction and remodeling may be intuitively thought to be associated with increased operative mortality – instinctively leading to the avoidance of CABG – our findings do not confirm that view: the early mortality with CABG in patients with 2–3 prognostic factors was similar to that observed among patients with 0–1 prognostic factors. Instead, the present study results indicate that those characteristics are found among patients that derive the greatest benefit from revascularization and hence, are those in whom CABG may not be delayed. Patients with higher number of prognostic factors benefit from surgical revascularization, because their early and late mortality with medical therapy alone is extremely high. Consequently, the risk of CABG in patients with more advanced forms of ischemic cardiomyopathy is counterbalanced by the even higher mortality observed with medical therapy alone. In fact, our findings support the notion that surgical revascularization blunts the higher mortality conveyed by the presence of anatomic prognostic factors when patients are treated with medical therapy alone. As a result, the analysis favors the indication for surgical revascularization in patients who present with worse extent of CAD and more severe LV dysfunction and remodeling. Importantly, although PCI may appear as an alternative for some patients, recent randomized trials have demonstrated the superiority of surgical revascularization in patients with extensive disease or diabetes (1–2). Nevertheless, it must be acknowledged that CABG in STICH was performed by experienced surgeons with a previously documented operative mortality ≤5% in similar patients; hence, these findings may not applicable to centers with a higher surgical death rate.
CENTRAL ILLUSTRATION. Schematic Representation of the Clinical Implications of the Present Study Findings.
*These thresholds are simply the medians of the LV function variables in the present study and have not been validated prospectively in an independent patient population. This algorithm should only be applied conceptually to support the notion that, among patients with ischemic LV systolic dysfunction, the benefit of surgical revascularization is greater when the disease process is more advanced (see text for more detail). CAD= coronary artery disease; EF= ejection fraction; ESVI= end-systolic volume index.
Certain important limitations of our study must be acknowledged. First, these observations are based on a retrospective non-pre-specified analysis of the STICH trial and therefore, do not necessarily have the same level of credibility as those that can be derived from prospectively defined subgroups of patients entered into a clinical trial. Nonetheless, the allocation to CABG or medical therapy alone was prospectively decided by randomization. Accordingly, the number of patients undergoing CABG was approximately half in each of subgroup, ruling out any potential biased association between allocation to surgical revascularization and presence of 3-vessel CAD, lower EF, or higher ESVI. Second, the STICH trial was designed with statistical power to detect differences in overall survival with CABG over medical therapy alone in all CAD patients with EF ≤35%. Therefore, categorizing patients according to those anatomic variables obviously results in a reduced number of patients in each subgroup with a consequently diminished statistical power. Crossover of patients from one treatment arm to the other also may have affected the results of our study, which was based on an intention-to-treat analysis. The impact of crossovers in the STICH trial has been carefully analyzed in a separate study (24). Importantly, however, the “as treated” and “per protocol” analyses (both of which considered crossover patients) showed similar results to those of the primary intention-to-treat analysis. Finally, because the median follow-up of the STICH trial was 56 months, we cannot establish whether the benefit of CABG observed after 2 years in certain subsets of patients extends beyond this period of follow-up. The STICH Extension Study (STICHES) is presently being conducted to confirm the longer-term effects of surgical revascularization in these patients.
It must be emphasized that the LV EF and LV ESVI values used to separate patients into subgroups were determined based on the distribution of these variables in this particular study population. Since these thresholds have not been validated prospectively in an independent patient population, our findings should not be considered as a dogmatic postulate of specific values to be used when making decisions on individual patients. Instead, these results should be applied conceptually to support the notion that, among patients with LV systolic failure due to ischemic heart disease, the benefit of surgical revascularization is greater when the disease process is more advanced.
Taken in conjunction with the neutral results of the myocardial viability (25) and myocardial ischemia (26) substudies, the present study results indicate that, compared to functional imaging, assessment of the anatomical extent of the disease is a better predictor of benefit from CABG in patients with ischemic cardiomyopathy. This concept is consistent with that reported in a recent analysis by the COURAGE trial investigators (27), in which the anatomic burden was a more consistent predictor of outcome than the assessment of the ischemic burden in patients with EF ≥30%. In conclusion, our findings support the indication for surgical revascularization in patients with ischemic cardiomyopathy who present with more extensive CAD and worse myocardial dysfunction and remodeling.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health.
ABBREVIATIONS
- CABG
coronary artery bypass graft surgery
- CAD
coronary artery disease
- EF
ejection fraction
- ESVI
end-systolic volume index
- LV
left ventricular
- OMT
optimal medical therapy
- NHLBI
National Heart, Lung, and Blood Institute
- PCI
percutaneous coronary interventions
- STICH
Surgical Treatment of IsChemic Heart failure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no relationships with industry in reference to this manuscript
REFERENCES
- 1.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 2.Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 3.Herlitz J, Karlson BW, Sjoland H, et al. Long term prognosis after CABG in relation to preoperative left ventricular ejection fraction. Int J Cardiol. 2000;72:163–171. doi: 10.1016/s0167-5273(99)00187-4. [DOI] [PubMed] [Google Scholar]
- 4.Topkara VK, Cheema FH, Kesavaramanujam S, et al. Coronary artery bypass grafting in patients with low ejection fraction. Circulation. 2005;112(suppl I):I344–I350. doi: 10.1161/CIRCULATIONAHA.104.526277. [DOI] [PubMed] [Google Scholar]
- 5.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 7.Dzavik V, Ghali WA, Norris C, et al. Long-term survival in 11,661 patients with multivessel coronary artery disease in the era of stenting: A report from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) investigators. Am Heart J. 2001;142:119–126. doi: 10.1067/mhj.2001.116072. [DOI] [PubMed] [Google Scholar]
- 8.Smith PK, Califf RM, Tuttle RH, et al. Selection of surgical or percutaneous coronary intervention provides differential longevity benefit. Ann Thorac Surg. 2006;82:1420–1429. doi: 10.1016/j.athoracsur.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 9.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular endsystolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 10.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–2357. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 11.Wong M, Staszewsky L, Latini R, et al. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure. J Am Coll Cardiol. 2004;43:2022–2027. doi: 10.1016/j.jacc.2003.12.053. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, Skali H, Anavekar NS, et al. Changes in ventricular size and function in patients with valsartan, captopril, or both after myocardial infarction. Circulation. 2005;111:3411–3419. doi: 10.1161/CIRCULATIONAHA.104.508093. [DOI] [PubMed] [Google Scholar]
- 13.Fang JC. Underestimating medical therapy for coronary artery disease … again. N Engl J Med. 2011;364:1671–1673. doi: 10.1056/NEJMe1103414. [DOI] [PubMed] [Google Scholar]
- 14.Elamm C, Fang JC. The world post-STICH: Is this a “game changer?” A non-invasive cardiologist’s perspective—Revascularization is the treatment of choice only in patients who fail medical therapy. Prog Cardiovasc Dis. 2013;55:466–469. doi: 10.1016/j.pcad.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Velazquez EJ, Lee KL, O'Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–1717. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velazquez EJ, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for IsChemic Heart failure (STICH) trial. J Thor Cardiovasc Surg. 2007;134:1540–1547. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh JK, Velazquez EJ, Menicanti L, et al. Influence of baseline left ventricular function on the clinical outcome of surgical ventricular reconstruction in patients with ischaemic cardiomyopathy. Eur Heart J. 2013;34:39–47. doi: 10.1093/eurheartj/ehs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passamani E, Davis KB, Gillespie MJ, Killip T. A randomized trial of coronary artery bypass surgery. Survival of patients with a low ejection fraction. N Engl J Med. 1985;312:1665–1671. doi: 10.1056/NEJM198506273122603. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 21.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Second Edition. New York, NY: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 22.Cox DR. Regression models and life-tables (with discussion) J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 23.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doenst T, Cleland JGF, Rouleau JL, et al. Influence of crossover on mortality in a randomized study of revascularization in patients with systolic heart failure and coronary artery disease. Circ Heart Fail. 2013;6:443–450. doi: 10.1161/CIRCHEARTFAILURE.112.000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonow RO, Maurer G, Lee KL, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panza JA, Holly TA, Asch FM, et al. Inducible myocardial Ischemia and outcomes in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol. 2013;61:1860–1870. doi: 10.1016/j.jacc.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancini GBJ, Hartigan PM, Shaw LJ, et al. Predicting outcome in the COURAGE trial. Coronary anatomy versus ischemia. J Am Coll Cardiol Intv. 2014;7:195–201. doi: 10.1016/j.jcin.2013.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.