Abstract
BACKGROUND:
This study was undertaken to determine the effect of mesenchymal stem cells (MSCs) engraftment on vascular endothelial cell growth factor (VEGF) in lung tissue, plasma and extravascular lung water at early stage of smoke inhalation injury.
METHODS:
A rabbit smoke inhalation injury model was established using a home-made smoke inhalation injury generator, and rabbits were divided into two groups randomly: a control group (S group, n=32) and a MSCs treatment group (M group, n=32). 10 ml PBS was injected via the ear marginal vein immediately at injury into the S group. Third generation MSCs with a concentration of 1×107/10 ml PBS were injected via the ear marginal vein immediately at injury into the M group. VEGF in peripheral blood and lung tissue were measured at 0 (baseline), 2, 4 and 6 hours after injection respectively and analyzed. The right lungs of rabbits were taken to measure lung water mass fraction.
RESULTS:
In the lung tissue, VEGF decreased gradually in the S group (P<0.05) and significantly decreased in the M group (P<0.05), but it increased more significantly than the values at the corresponding time points (P<0.05). In peripheral blood, VEGF increased gradually in the S group (P<0.05) and markedly increased in the M group (P<0.05), but it decreased more significantly than the values at corresponding time points (P<0.05).
CONCLUSION:
MSCs engraftment to smoke inhalation injury could increase VEGF in lung tissue, decrease VEGF in plasma and reduce extravascular lung water, indicating its protective effect on smoke inhalation injury.
KEY WORDS: Mesenchymal stem cells, Smoke inhalation injury, Vascular endothelial cell growth factor, Extravascular lung water, Rabbit
INTRODUCTION
Pulmonary injury from smoke inhalation is common in burn victims. It significantly contributes to the morbidity and mortality of patients with fire-related injuries, and as a chief contributor to the pathophysiology of smoke inhalation injury, it has been extensively studied.™ Vascular endothelial cell growth factor (VEGF) plays an important role in smoke inhalation injury.[2] Mesenchymal stem cells (MSCs) are multipotential nonhematopoietic progenitor cells capable of differentiating into multiple lineages of the mesenchyme. MSCs have emerged as a promising therapeutic modality for tissue regeneration and repair.[3,4] But there is few report about the effects of MSCs engraftment on VEGF at early stage of somke inhalation injury. We hypothesized that, like other inflammatory cytokines in lung injury, VEGF could increase local and systematic inflammatory response in smoke inhalation injury, while MSCs transplantation could decrease VEGF, contributing to smoke inhalation injury. In this study, we transplanted engraft MSCs into rabbits with smoke inhalation injury, and observed the change of VEGF in lung tissue and plasma.
METHODS
Preparation of MSCs
A total of 64 young healthy New Zealand white rabbits, weighing 2 kg, provided by the Animal Experiment Center of Nanchang University, were enrolled in this study. Three percent pentobarbital sodium (1 ml/ kg) was used for anesthesia via the ear marginal vein. Bone marrow (4 ml) was obtained from bilateral tibial nodules and bilateral iliac crest, and was mixed with the equal amount of PBS for washing. After washing twice and centrifuged at 1500 r/min for 10 minutes, precipitate was discarded and adhered directly for primary culture. The precipitate was mixed with 10% of bovine serum containing DMEM-F12 medium (Hyclone Company) and put into an incubator at 37 °C with 5% CO2 and saturated humidity. After the first 7-8 days when the cells reached 80% fusion, serial subcultivation started according to the proportion of 1:2, and third generation MSCs were digested by trypsin for further use. The cell-specific antigens were detected using flow cytometry and morphological observation to identify MSCs. Lee et al[5] reported that there is no test that can be performed on a single cell to determine whether that cell is an MSC, so in this study we detected CD105, CD44, CD34 and CD45 for identification of MSCs.
Establishment of models with smoke inhalation injury
The procedures were conducted according to the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Animal Care and Use Committee of Medical College of Nanchang University. Models were established by using a home-made smoke inhalation injury generator. The desiccated pine wood chip and kerosene were used to produce smoke. Rabbits inhaled smoke for 10 minutes and repeated at a 2-minute interval. The success of establishing severe smoke inhalation injury was evidenced by clinical manifestations, blood gas analysis and lung histopathological observation.[6]
Sample collection
After establishment of the models, the 64 rabbits were randomly divided into two groups: an injury group (S group, n=32) and a treatment group (M group, n=32). The rabbits in the S group were injected with 10 ml PBS via the ear marginal vein immediately after injury, and served as a control; the rabbits in the M group were injected with PBS containing 1×107/10 ml of third generation MSCs via the ear marginal vein immediately after injury. The lung tissue was collected for preparation of homogenate at pre-injury (baseline) and at 2, 4, and 6 hours post-injury respectively. VEGF concentration in the lung homogenate supernatant was detected by the enzyme-linked immunosorbnent assay (ELISA). The test was performed in accordance with the kit instructions (VEGF quantitative enzyme-linked immunosorbent assay kit Carson offered by Shanghai Senxiong Science and Technology Industrial Co., Ltd.).
Detection of water content in the lung
At 6 hours after experiment, 0.5 g right lung tissue was collected and put into an incubator at 80 °C and dried for 72 hours to a constant weigh.
Lung water content = (lung wet quality - lung dry quality) / lung wet quality × 100%.
Statistical analysis
Data were expressed as (mean±SD) and SPSS 15.0 for Windows was used for statistical analysis. Student’s t test was used for the comparison between the two groups. P<0.05 was considered statistically significant.
RESULTS
Isolation, culture and identification of MSCs
Direct adhesion was used to separate MSCs in rabbits. MSCs grew well after passage. Under an invert microscope, MSCs were found to be spindle-shaped, numerous, and closely arranged. Cluster arrangement appeared at the early stage, and MSCs were equally distributed like whirlpool (Figure 1). CD34(-), CD45(-) and CD44(+), and CD105(+) were detected by flow cytometry, and this indicated that the cells were MSCs cultured (Figure 2).
Figure 1.
MSCs of the rabbits (original magnificationxlQQ)
Figure 2.
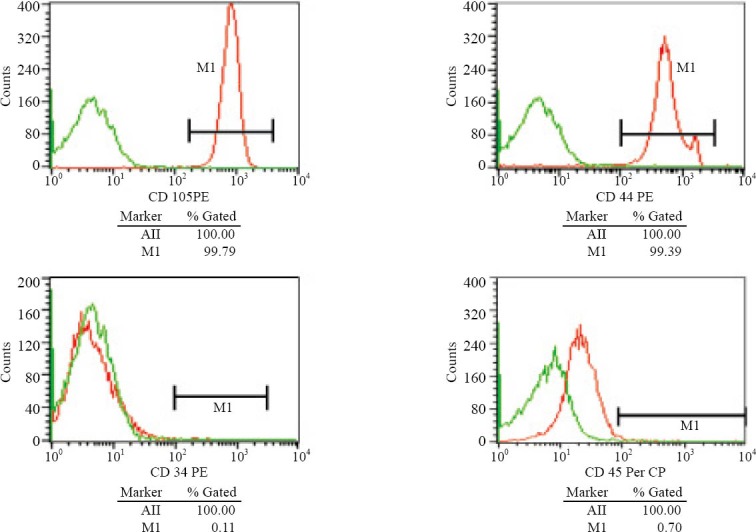
MSCs detected by flow cytometry in rabbits
Changes of VEGF in the lung tissue of rabbits
Compared to the baseline, VEGF in the S group and M group significantly decreased at 2, 4 and 6 hours after injury (P<0.05). VEGF concentration at every time point in the M group increased more significantly than in the S group (P<0.05) (Table 1).
Table 1.
Change and comparison of VEGF at every time point in lung tissue (pg/ml, mean ±SD, n=8)
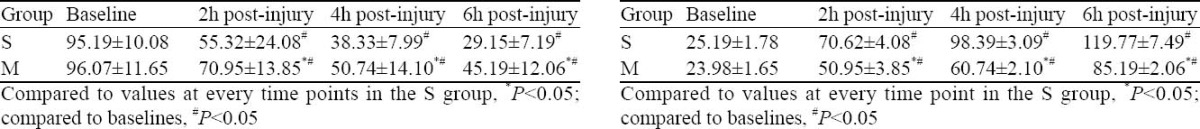
Changes of VEGF of peripheral blood in rabbits
Compared to the baseline, VEGF in the S group and M group increased significantly at 2, 4 and 6 hours after injury (P<0.05). VEGF concentration at every time point in the M group decreased more significantly than in the S group (P<0.05) (Table 2).
Table 2.
Change and comparison of VEGF at every time point in plasma (pg/ml, mean ±SD, n=8)

Changes of VEGF in the lung tissue of rabbits
Compared to the baseline, VEGF in the S group and M group significantly decreased at 2, 4 and 6 hours after injury (P<0.05). VEGF concentration at every time point in the M group increased more significantly than in the S group (P<0.05) (Table 1).
Changes of VEGF of peripheral blood in rabbits
Compared to the baseline, VEGF in the S group and M group increased significantly at 2, 4 and 6 hours after injury (P<0.05). VEGF concentration at every time point in the M group decreased more significantly than in the S group (P<0.05) (Table 2).
Comparison of water content in the lung at 6 hours after injury
Lung water content was lower in the M group than in the S group (59.17%±6.93% vs. 77.43%±6.13%) at 6 hours after injury (P<0.05).
DISCUSSION
The essence of smoke inhalation injury is acute lung injury (ALI), and the main pathological characteristics of the injury include diffuse alveoli-capillary membrane damage, high permeability, pulmonary edema, lower pulmonary compliance, increased intrapulmonary shunt, significantly decreased pulmonary ventilation and exchange function leading to respiratory distress and anoxemia.[7] As pro-inflammatory cytokines, VEGF, a stronger vascular permeability factor, participates in and mediates inflammatory reaction, formation of pulmonary edema and aggregation of neutrophilic granulocyte in the lung, which play an important role in progress of ALI. Investigations of inhibition and interruption of VEGF showed good prospects for the therapy of ALI.[8,9] In this study, we found that VEGF in the lung homogenate decreased significantly at 2, 4 and 6 hours, but increased significantly in peripheral blood after smoke inhalation injury. The reasons may be that (1) the production of VEGF decreases significantly in lung homogenate because of destruction of type II alveoli after smoke inhalation injury; (2) the high permeability results in VEGF in lung tissue, flowing into peripheral blood along concentration gradient; and (3) the increased VEGF in plasma is relevant to some other factors, which promote VEGF production in peripheral blood such as activated neutrophilic granulocyte. But the underlying mechanisms are not clear.
Human MSCs avoid allorecognition, interfere with dendritic cells and T-cell function, and generate a local immunosuppressive microenvironment by secreting cytokines.[10] It has been shown that the immunomodulatory function of human MSCs is enhanced when the cells are exposed to an inflammatory environment characterized by the presence of elevated local interferon-gamma levels.[11] Other studies showed contradictory findings, reflecting the highly heterogeneous nature of MSC isolates and the considerable differences between isolates generated by many different methods under development.[12]
Mesenchymal stem cells can be activated and mobilized if necessary. However, the efficiency is very low. For instance, damage to muscles heals very slowly. However, if there is a method of activating the mesenchymal stem cells, such wounds would heal faster. Direct injection or placement of the cells into a site in need of repair may be the preferred method of treatment, as vascular delivery suffers from a “pulmonary first pass effect” where intravenously injected cells are sequestered in the lungs.[13] Case reports in orthopedic applications have been published, though the number of patients treated is small and these methods still lack rigorous investigation demonstrating effectiveness. A study[14] presented a series of nine defects in five knees involving surgical transplantation of mesenchymal stem cells with coverage of the treated chondral defects.
In this study, MSCs significantly increased VEGF in the lung tissue whereas decreased VEGF in peripheral blood and extravascular lung water. This may be due to the anti-inflammation and immunomodulation of MSCs i.e. (1) MSCs can adsorb and neutralize a large number of inflammatory factors and depress inflammatory reaction to alleviate type II alveoli and alveolar capillary membrane damage by expressing a great quantity receptor molecules on its surface;[15] (2) MSCs can secrete some bioactive molecules such as VEGF,[16-17] and this on the one hand complements loss of VEGF caused by synthetic inadequacy, on the other hand is against inflammatory factors (for instance TNF-α) and interrupt water fall effect of inflammatory signal in order to create condition for repair and regeneration of type II alveoli and alveolar capillary cells; (3) MSCs inhibit the activation and proliferation of immune cells by secreting inhibitory factor to reduce the production of factors inducing the production of VEGF.[18,19]
VEGF plays an important role in generation and development of smoke inhalation injury. MSCs engraftment shows protective effect on smoke inhalation injury through regulation of the local and systematic VEGF and reduction of lung water content. But its underlying mechanism needs further clarification.
Footnotes
Funding: This study was supported by a grant from the Education Bureau of Jiangxi Province Youth Science Fund (GJJ09432).
Ethical approval: Not needed.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Zhu F proposed the study, and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Toon MH, Maybauer MO, Greenwood JE, Maybauer DM, Fraser JF. Management of acute smoke inhalation injury. Crit Care Resusc. 2010;12:53–61. [PubMed] [Google Scholar]
- 2.Liu W, Jin FG. Advance s in smoke inhalation ALI/ ARDS. Int J Respiration. 2007;27:307–310. [Google Scholar]
- 3.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 4.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Gupta N, Serikov V, Matthay MA. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther. 2009;9:1259–1270. doi: 10.1517/14712590903213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang PM, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, et al. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology. 2010;15:1131–1139. doi: 10.1111/j.1440-1843.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang ZC. Inhalation injury. In: Yang Zongcheng., editor. Burn Therapy. 3rd ed. Beijing: People's Medical Publishing House; 2006. pp. 349–368. [Google Scholar]
- 8.Kosmidou I, Karmpaliotis D, Kirtane AJ, Barron HV, Gibson CM. Vascular endothelial growth factors in pulmonary edema: an update. J Thromb Thrombolysis. 2008;25:259–264. doi: 10.1007/s11239-007-0062-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen YJ, Liu TW, Chen KZ. Proinflammatory effects of vascular endothelial growth factor in acute lung injury. Chin Pediatr Emerg Med. 2006;13:480–481. [Google Scholar]
- 10.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol 2007. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/ multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. Epub 2007 Sep 27. [DOI] [PubMed] [Google Scholar]
- 13.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11:343–353. [PubMed] [Google Scholar]
- 15.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 16.Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 17.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal s tem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 18.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, et al. T cell responses to allogeneic human mesenchymal stem cells : immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 19.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, et al. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]


