Abstract
BACKGROUND:
While epinephrine is the recommended first-line therapy for the reversal of anaphylaxis symptoms, inappropriate use persists because of misunderstandings about proper dosing and administration or misconceptions about its safety. The objective of this review was to evaluate the safety of epinephrine for patients with anaphylaxis, including other emergent conditions, treated in emergency care settings.
METHODS:
A MEDLINE search using PubMed was conducted to identify articles that discuss the dosing, administration, and safety of epinephrine in the emergency setting for anaphylaxis and other conditions.
RESULTS:
Epinephrine is safe for anaphylaxis when given at the correct dose by intramuscular injection. The majority of dosing errors and cardiovascular adverse reactions occur when epinephrine is given intravenously or incorrectly dosed.
CONCLUSION:
Epinephrine by intramuscular injection is a safe therapy for anaphylaxis but training may still be necessary in emergency care settings to minimize drug dosing and administration errors and to allay concerns about its safety.
KEY WORDS: Allergy, Anaphylaxis, Epinephrine, Safety, Cardiovascular side effects
INTRODUCTION
The National Institute of Allergy and Infectious Disease (NIAID) and the Food Allergy Research & Education (FARE; formerly the Food Allergy and Anaphylaxis Network [FAAN]) define anaphylaxis as any serious allergic reaction that is rapid in onset and may cause death. While the treatment approach for anaphylaxis is relatively uniform across age, gender, or comorbidities, confusion still exists about the appropriate indications, timing, dose, and route of administration of epinephrine, or of its safety.[1–7] Epinephrine by the intramuscular (IM) route is the recommended therapy of choice in current guidelines or consensus statements from the American Academy of Allergy, Asthma & Immunology (AAAAI) anaphylaxis guidelines, the World Allergy Organization (WAO) anaphylaxis guidelines, the NIAID/FARE food allergy guidelines, and a newer consensus recommendation (the International Consensus ON [ICON] food allergy) from the International Collaboration in Asthma, Allergy and Immunology (iCAALL) group.[8–11] In a recent study of 1 114 pediatric emergency medicine physicians, 94% correctly identified epinephrine as the treatment of choice for anaphylaxis but only 67% used IM administration, despite the fact that IM administration is the preferred route of administration.[12] Factors associated with proper IM administration were the presence of a residency training program at the institution and a higher volume of anaphylaxis patient cases. It may also be that some healthcare providers in emergency settings are uncomfortable with using epinephrine first, and are concerned with its safety, and additional training may be necessary on proper dosing and administration to ensure that it is given safely.
Why epinephrine first?
An anaphylactic reaction is resulted when mast cells and basophils systemically and abruptly release mediators of inflammation. Epinephrine, a mixed alpha-and beta-adrenergic receptor agonist, treats anaphylaxis symptoms by alleviating allergen-induced inflammatory and physiologic effects.[13] The alpha-adrenergic vasoconstricting effect reverses vasodilation, thus alleviating hypotension and reducing erythema, urticaria and angioedema.[14] Beta-adrenergic receptor agonist activity acts to dilate bronchial airways, increase the force of myocardial muscle contraction (inotropy) and heart rate (chronotropy), which increases cardiac output, and attenuates the severity of IgE-mediated reactions via receptors on mast cells.[13,15,16] Intramuscular administration allows more rapid peak plasma levels compared with subcutaneous (SC) administration in children or adults.[17,18]
Why not antihistamines or corticosteroids first?
Antihistamines, such as diphenhydramine, have a longer onset of action and longer time to peak activity compared with epinephrine.[19,20] The most compelling evidence against the use of H1 blockers as an initial therapy comes from studies looking at the onset of suppression of histamine-induced cutaneous flares. Time to 50% reduction in histamine-induced flare was 52 minutes for IM diphenhydramine, 80 minutes for oral diphenhydramine, and 101 minutes for oral fexofenadine.[19] We have learned from fatal cases of anaphylaxis that patients are at risk for death in as little as 5 minutes following allergen exposure. [21–23] Since anaphylaxis can involve multiple organ systems and occur in less time than the onset of action of antihistamines, they should not be used as first-line therapy. This is consistent with current recommendations on antihistamines (and corticosteroids for that matter) positioning them as adjunctive therapies.[8–11] There is no evidence that they provide life-saving treatment (i.e. they do not prevent or relieve upper airway obstruction, hypotension, or shock).[24] Antihistamines [IM or intravenous (IV)] are adjunctive therapies and may be tried after epinephrine is administered to help control cutaneous and cardiovascular manifestations, such as itching, flushing, urticaria, angioedema, and nasal and eye symptoms, as well as prevent secondary reactions.[8–11] Similarly, corticosteroids have the potential to prevent recurrent or protracted episodes, but should not be used in place of, or prior to, epinephrine, as they are not helpful for acute symptoms.[8]
It is for these reasons that IM epinephrine is recommended as the treatment of choice for anaphylaxis. Epinephrine may be reserved for patients in shock only or because a clinician is concerned about its safety, when in fact it is indicated and needed to save a patient’s life. The objective of this review was to evaluate the safety of epinephrine for patients with anaphylaxis, including other emergent conditions, treated in emergency care settings.
METHODS
A MEDLINE search using PubMed was conducted without limits to identify articles that discuss the dosing, administration, and safety of epinephrine for anaphylaxis in the emergency setting. The authors prospectively decided that studies discussing the use of epinephrine in the emergency medicine setting for anaphylaxis, or other acute respiratory conditions (e.g., acute asthma, bronchiolitis, croup), with positive or negative results, would be considered for inclusion. Articles that explicitly discussed the use of epinephrine for anesthetic applications or for cardiac arrest from trauma or other non-allergic, non-respiratory causes were excluded. The following terms and their combination were used for the search: “food allergy”, “latex allergy”, “drug allergy”, “seafood allergy”, “anaphylaxis”, “asthma”, “respiratory”, “epinephrine”, “dosing”, “inhaled”, “nebulized”, “emergency department”, “safety”, “cardiovascular”, “side effect”, “intramuscular”, “intravenous”, and “route of administration”. Literature describing studies in both the pediatric and adult populations was reviewed. Descriptive statistics were used to summarize the results of each study.
RESULTS
Search results
To ensure that any report on the use of epinephrine in the emergency setting for anaphylaxis or other acute respiratory condition indexed in MEDLINE was captured, we used the key-terms listed above and the combination of these key terms. Redundancies in the searches were purged until we were confident that all results were unique. All results were used for the systematic summary of epinephrine safety here. Our searches yielded both individual and retrospective case reviews in which epinephrine was used in the emergency setting for anaphylaxis (n=10) and controlled studies in the emergency setting where epinephrine was used for other acute respiratory conditions (n=10).
Epinephrine dosing and administration for anaphylaxis
Once an initial assessment is made and clinicians are confident in their diagnosis of anaphylaxis, appropriate treatment should be initiated with an IM injection of epinephrine [into the anterolateral aspect of the thigh (vastus lateralis)], quickly followed by placement of the patient in a recumbent position, and the provision of supplemental oxygen and/or the administration of IV fluids, if needed based on the severity of symptoms.[8] Please refer to available guidelines cited here for a complete overview of management in the acute and long-term settings, and in patients with comorbid conditions including cardiovascular disease.
Epinephrine is available in different doses and concentrations for delivery by different routes, and for different indications, including anaphylaxis and cardiac arrest. The differences, as well as inconsistent labeling of epinephrine vials, can lead to life-threatening medication errors (Table 1). Labeling of epinephrine concentration is sometimes provided in ratios rather than uniformly as a mass of concentrations or percentages. The availability of epinephrine in both 1:1 000 and 1:10 000 concentrations can lead to incorrect dosing in an acute setting.[5,7] Epinephrine for anaphylaxis is given at doses that are substantially lower than those needed for cardiac arrest (Table 2). If emergency room “crash carts” are stocked only with pre-filled, higher concentration syringes used for IV push during cardiac arrest, there is a potential for inadvertent excessive dosing in an urgent situation.[6]
Table 1.
Factors contributing to dosing errors of epinephrine

Table 2.
Dosing of epinephrine in anaphylaxis compared with cardiac resuscitation

Epinephrine safety in anaphylaxis and other conditions
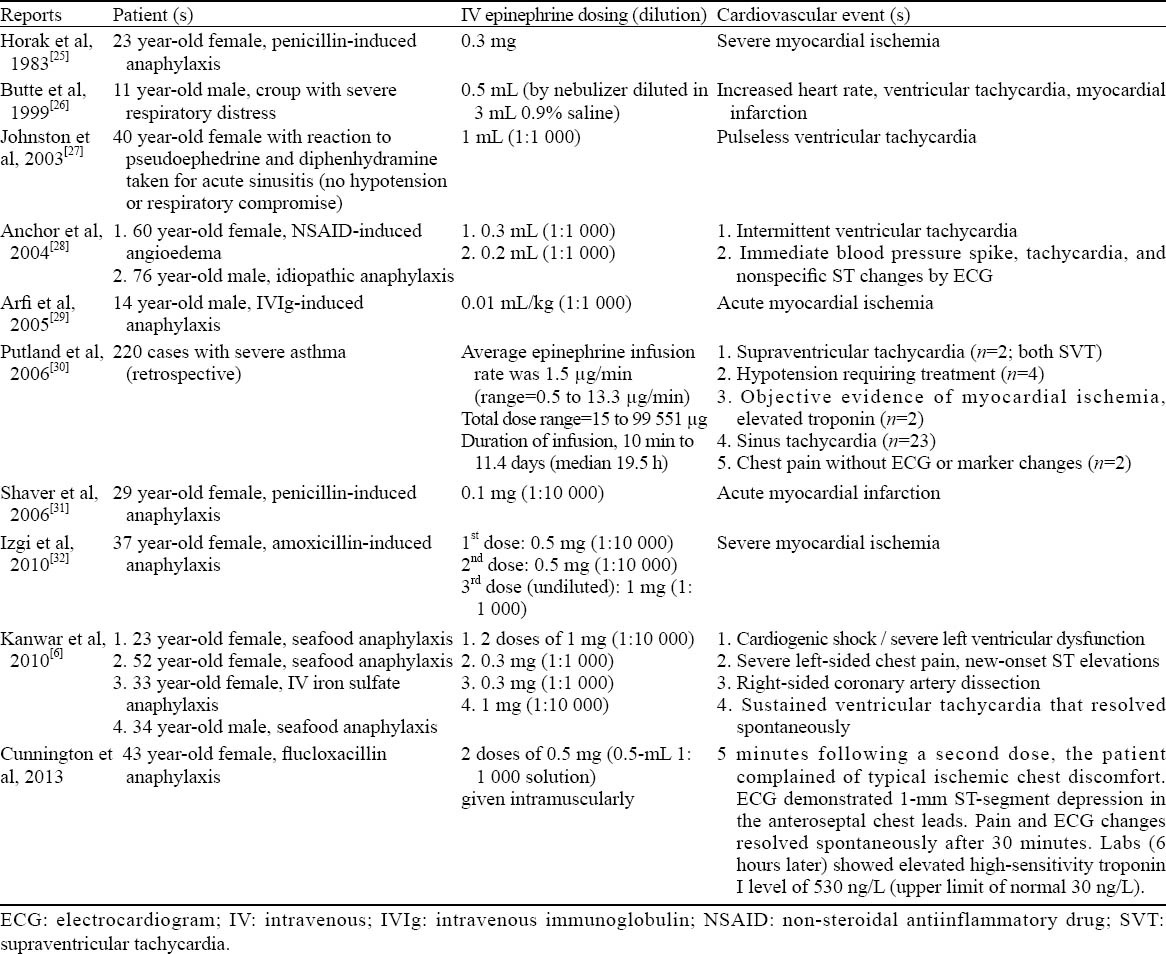
A literature search in which the safety and cardiovascular effects of epinephrine were discussed in the context of anaphylaxis produces ten case-based reports, which are summarized in Table 3.[6,25–33] Although the vast majority of reported cardiovascular adverse events with epinephrine occurred with IV dosing, highlighting the need to proceed with caution if considering epinephrine by this route for anaphylaxis, events have been reported with appropriate IM administration. However, the most serious adverse effects with epinephrine occur with IV administration.[25,31,34] Therefore, the IV route should only be considered in the specific instances discussed below. In some cases, healthcare providers may inappropriately associate epinephrine with a risk for cardiovascular events,[35] which may delay the use of epinephrine in cases where it is clearly indicated.
Table 3.
Cardiovascular events reported with epinephrine given for anaphylaxis and other conditions
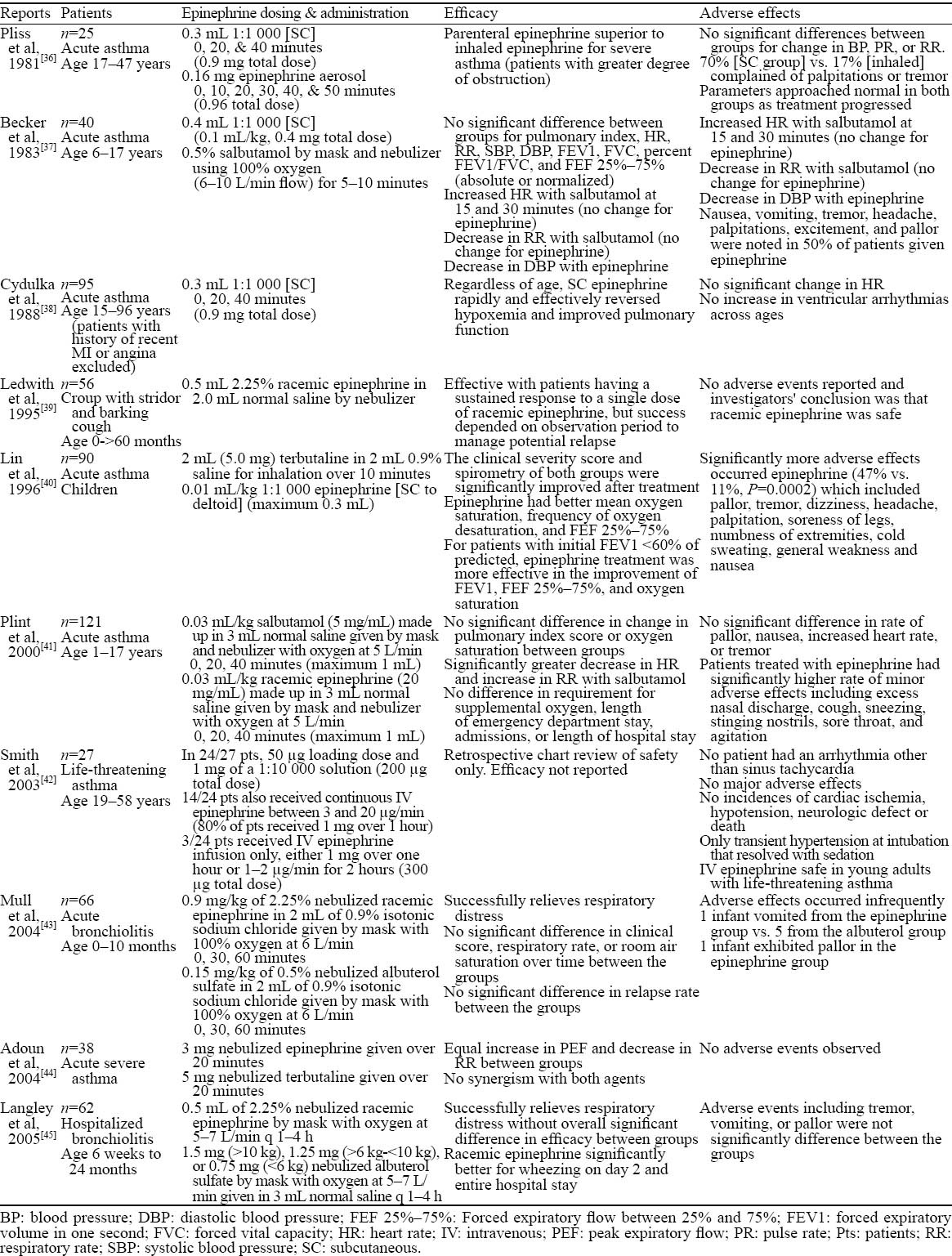
A literature search to identify studies in which the safety of epinephrine was discussed in the context of conditions other than anaphylaxis yielded ten studies which are summarized in Table 4.[36–45] Conditions in which epinephrine was used were described as acute asthma, life-threatening asthma, croup, acute bronchiolitis, and hospitalized bronchiolitis. Administration routes for epinephrine included SC, nebulized aerosol, and IV infusion. Epinephrine dosing was variable and, overall, no serious adverse events were reported. Sinus tachycardia was noted in the study by Smith et al and patients in this study patients received IV epinephrine (3 different dosing strategies). In these 10 studies, epinephrine induced expected side-effects including nausea, dizziness, vomiting, tremor, headache, palpitations, excitement, and pallor, but no life-threatening cardiovascular adverse events were observed. In general, these studies show that epinephrine is safe for patients with urgent medical needs for conditions other than anaphylaxis.
Table 4.
Safety of epinephrine given for conditions other than anaphylaxis
When is IV dosing of epinephrine in anaphylaxis appropriate?
While guidelines recommend that epinephrine be given via IM injection for the initial treatment of anaphylaxis,[8] IV delivery should be considered in special circumstances such as severely hypotensive patients, patients in cardiac or respiratory arrest, or those who have failed to respond to multiple IM injections of epinephrine. In the event that multiple IM injections of epinephrine and aggressive volume replacement do not alleviate hypotension, IV epinephrine and vasopressors may be used. In such cases, continuous hemodynamic monitoring is recommended.[8]
If IV administration is deemed necessary, the use of a highly diluted solution will best balance efficacy and patient safety.[8] An epinephrine infusion may be prepared by adding 1 mg (1 mL) of 1:1000 dilution of epinephrine to 250 mL of D5W to yield a concentration of 4 µg/ mL. This 1:250 000 solution is infused at a rate of 1 µg/min (15 drops/minute using a micro-drop apparatus [60 drops/min =1 mL =60 mL/h]), titrated to desired hemodynamic response, increasing to a maximum of 10 µg/min for adults and adolescents.[8]
CONCLUSION
It is critically important that any misconceptions regarding the safety and use of epinephrine should be resolved. In this review, we attempt to address two real-world concerns regarding the use of epinephrine in emergency care settings for anaphylaxis: 1) appropriate dosing and administration of epinephrine and 2) epinephrine safety. Consensus recommendations on the use of epinephrine as initial therapy for anaphylaxis are shown in Table 5.[11] Limitations of this evaluation include the inability to account for the level of expertise a particular clinician has with anaphylaxis or other acute respiratory conditions, or the uniqueness of each emergent situation. Furthermore, the literature, in general, on this subject is relatively limited.
Table 5.
Consensus recommendations for the treatment of anaphylaxis in the emergency setting[11]
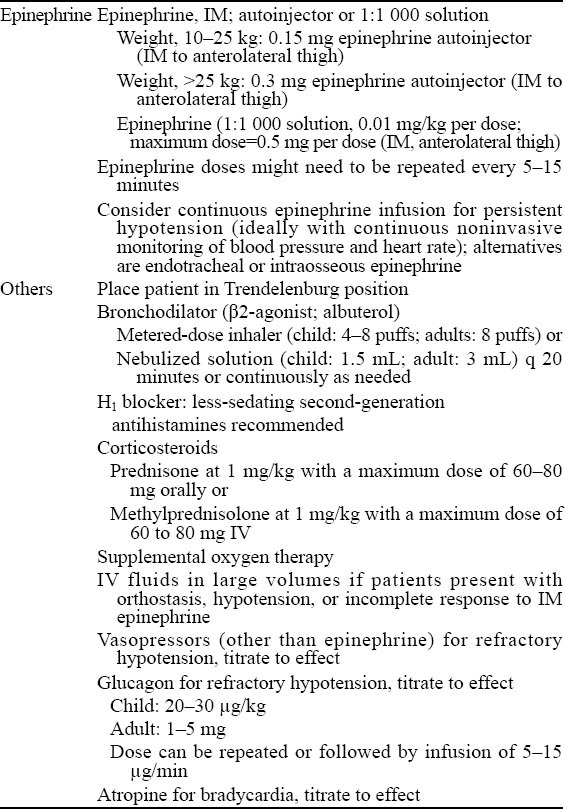
Epinephrine is the therapy of choice for an anaphylactic reaction and, when administered correctly, is safe and effective. Because of the potential for cardiovascular adverse events, IV epinephrine should only be administered for anaphylaxis in profoundly hypotensive patients or patients in cardiac or respiratory arrest who have failed to respond to IV volume replacement and multiple IM doses of epinephrine.
Important points related to treatment of the patient with anaphylaxis include:[46] 1) epinephrine is first, antihistamines and corticosteroids are adjunctive therapies, and 2) epinephrine should be administered intramuscularly, and not intravenously in concentrations of greater than 1:10 000, and then only as a last resort.
Footnotes
Funding: We received no independent support for this research study.
Ethical approval: Not needed.
Conflicts of interest: We have no conflicts of interest to report.
Contributors: Wood JP proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Wang J, Sicherer SH, Nowak-Wegrzyn A. Primary care physicians’ approach to food-induced anaphylaxis: a survey. J Allergy Clin Immunol. 2004;114:689–691. doi: 10.1016/j.jaci.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Krugman SD, Chiaramonte DR, Matsui EC. Diagnosis and management of food-induced anaphylaxis: a national survey of pediatricians. Pediatrics. 2006;118:e554–560. doi: 10.1542/peds.2005-2906. [DOI] [PubMed] [Google Scholar]
- 3.Patel BM, Bansal PJ, Tobin MC. Management of anaphylaxis in child care centers: evaluation 6 and 12 months after an intervention program. Ann Allergy Asthma Immunol. 2006;97:813–815. doi: 10.1016/S1081-1206(10)60974-X. [DOI] [PubMed] [Google Scholar]
- 4.Gaeta TJ, Clark S, Pelletier AJ, Camargo CA. National study of US emergency department visits for acute allergic reactions, 1993 to 2004. Ann Allergy Asthma Immunol. 2007;98:360–365. doi: 10.1016/S1081-1206(10)60883-6. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler DW, Carter JJ, Murray LJ, Degnan BA, Dunling CP, Salvador R, et al. The effect of drug concentration expression on epinephrine dosing errors: a randomized trial. Ann Intern Med. 2008;148:11–14. doi: 10.7326/0003-4819-148-1-200801010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Kanwar M, Irvin CB, Frank JJ, Weber K, Rosman H. Confusion about epinephrine dosing leading to iatrogenic overdose: a life-threatening problem with a potential solution. Ann Emerg Med. 2010;55:341–344. doi: 10.1016/j.annemergmed.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Hoyle JD, Davis AT, Putman KK, Trytko JA, Fales WD. Medication dosing errors in pediatric patients treated by emergency medical services. Prehosp Emerg Care. 2012;16:59–66. doi: 10.3109/10903127.2011.614043. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman P, Nicklas RA, Oppenheimer J, Kemp SF, Lang DM, Bernstein DI, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126 doi: 10.1016/j.jaci.2010.06.022. 477-480e471-442. [DOI] [PubMed] [Google Scholar]
- 9.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons FE, Ardusso LR, Bilò BM, El-Gamal YM, Ledford DK, Ring J, et al. World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis. WAO Journal. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. J Allergy Clin Immunol. 2012;129:906–920. doi: 10.1016/j.jaci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Grossman SL, Baumann BM, Garcia Pena BM, Linares MY, Greenberg B, Hernandez-Trujillo VP. Anaphylaxis knowledge and practice preferences of pediatric emergency medicine physicians: a national survey. J Pediatr. 2013;163:841–846. doi: 10.1016/j.jpeds.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Kemp SF, Lockey RF, Simons FE. Epinephrine: the drug of choice for anaphylaxis. A statement of the World Allergy Organization. Allergy. 2008;63:1061–1070. doi: 10.1111/j.1398-9995.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 14.McLean-Tooke AP, Bethune CA, Fay AC, Spickett GP. Adrenaline in the treatment of anaphylaxis: what is the evidence? BMJ. 2003;327:1332–1335. doi: 10.1136/bmj.327.7427.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong LK, Morice AH, Yeo WW, Schleimer RP, Peachell PT. Functional desensitization of beta agonist responses in human lung mast cells. Am J Respir Cell Mol Biol. 1995;13:540–546. doi: 10.1165/ajrcmb.13.5.7576689. [DOI] [PubMed] [Google Scholar]
- 16.Kay LJ, Peachell PT. Mast cell beta2-adrenoceptors. Chem Immunol Allergy. 2005;87:145–153. doi: 10.1159/000087641. [DOI] [PubMed] [Google Scholar]
- 17.Simons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol. 1998;101:33–37. doi: 10.1016/S0091-6749(98)70190-3. [DOI] [PubMed] [Google Scholar]
- 18.Liu YC, Qi ZW, Guo SG, Wang Z, Yu XZ, Ma S. Role of corticotrophin releasing hormone in cerebral infarction-related gastrointestinal barrier dysfunction. World J Emerg Med. 2011;2:59–65. [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DH, Romero FA, Casale TB. Time-dependent inhibition of histamine-induced cutaneous responses by oral and intramuscular diphenhydramine and oral fexofenadine. Ann Allergy Asthma Immunol. 2008;100:452–456. doi: 10.1016/S1081-1206(10)60470-X. [DOI] [PubMed] [Google Scholar]
- 20.Simons FE. First-aid treatment of anaphylaxis to food: focus on epinephrine. J Allergy Clin Immunol. 2004;113:837–844. doi: 10.1016/j.jaci.2004.01.769. [DOI] [PubMed] [Google Scholar]
- 21.Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144–1150. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 22.Pumphrey RS. Fatal anaphylaxis in the UK 1992-2001. Novartis Found Symp. 2004;257:116–128. discussion 128-132, 157-160, 276-185. [PubMed] [Google Scholar]
- 23.Greenberger PA, Rotskoff BD, Lifschultz B. Fatal anaphylaxis: postmortem findings and associated comorbid diseases. Ann Allergy Asthma Immunol. 2007;98:252–257. doi: 10.1016/S1081-1206(10)60714-4. [DOI] [PubMed] [Google Scholar]
- 24.Sheikh A, Ten Broek V, Brown SG, Simons FE. H1antihistamines for the treatment of anaphylaxis: Cochrane systematic review. Allergy. 2007;62:830–837. doi: 10.1111/j.1398-9995.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 25.Horak A, Raine R, Opie LH, Lloyd EA. Severe myocardial ischaemia induced by intravenous adrenaline. Br Med J (Clin Res Ed) 1983;286:519. doi: 10.1136/bmj.286.6364.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen ZJ, Freeman ML. Management of upper gastrointestinal bleeding emergencies: evidence-based medicine and practical considerations. World J Emerg Med. 2011;2:5–12. doi: 10.5847/wjem.j.1920-8642.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston SL, Unsworth J, Gompels MM. Adrenaline given outside the context of life threatening allergic reactions. BMJ. 2003;326:589–590. doi: 10.1136/bmj.326.7389.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anchor J, Settipane RA. Appropriate use of epinephrine in anaphylaxis. Am J Emerg Med. 2004;22:488–490. doi: 10.1016/j.ajem.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Arfi AM, Kouatli A, Al-Ata J, Arif H, Syed S. Acute myocardial ischemia following accidental intravenous administration of epinephrine in high concentration. Indian Heart J. 2005;57:261–264. [PubMed] [Google Scholar]
- 30.Putland M, Kerr D, Kelly AM. Adverse events associated with the use of intravenous epinephrine in emergency department patients presenting with severe asthma. Ann Emerg Med. 2006;47:559–563. doi: 10.1016/j.annemergmed.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Shaver KJ, Adams C, Weiss SJ. Acute myocardial infarction after administration of low-dose intravenous epinephrine for anaphylaxis. CJEM. 2006;8:289–294. doi: 10.1017/s1481803500013890. [DOI] [PubMed] [Google Scholar]
- 32.Izgi C, Cevik C, Nugent K. Severe myocardial ischemia after concentrated epinephrine use for the treatment of anaphylaxis: Kounis syndrome or epinephrine effect? Heart Lung. 2010;39:160–163. doi: 10.1016/j.hrtlng.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Cunnington C, McDonald JE, Singh RK. Epinephrine-induced myocardial infarction in severe anaphylaxis: is nonselective beta-blockade a contributory factor? Am J Emerg Med. 2013;31:759 e751–752. doi: 10.1016/j.ajem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan TJ. Cardiac disorders in penicillin-induced anaphylaxis. Association with intravenous epinephrine therapy. JAMA. 1982;248:2161–2162. [PubMed] [Google Scholar]
- 35.Barach EM, Nowak RM, Lee TG, Tomlanovich MC. Epinephrine for treatment of anaphylactic shock. JAMA. 1984;251:2118–2122. [PubMed] [Google Scholar]
- 36.Pliss LB, Gallagher EJ. Aerosol vs injected epinephrine in acute asthma. Ann Emerg Med. 1981;10:353–355. doi: 10.1016/s0196-0644(81)80235-1. [DOI] [PubMed] [Google Scholar]
- 37.Becker AB, Nelson NA, Simons FE. Inhaled salbutamol (albuterol) vs injected epinephrine in the treatment of acute asthma in children. J Pediatr. 1983;102:465–469. doi: 10.1016/s0022-3476(83)80679-9. [DOI] [PubMed] [Google Scholar]
- 38.Cydulka R, Davison R, Grammer L, Parker M, Mathews Jt. The use of epinephrine in the treatment of older adult asthmatics. Ann Emerg Med. 1988;17:322–326. doi: 10.1016/s0196-0644(88)80772-8. [DOI] [PubMed] [Google Scholar]
- 39.Ledwith CA, Shea LM, Mauro RD. Safety and efficacy of nebulized racemic epinephrine in conjunction with oral dexamethasone and mist in the outpatient treatment of croup. Ann Emerg Med. 1995;25:331–337. doi: 10.1016/s0196-0644(95)70290-3. [DOI] [PubMed] [Google Scholar]
- 40.Lin YZ, Hsieh KH, Chang LF, Chu CY. Terbutaline nebulization and epinephrine injection in treating acute asthmatic children. Pediatr Allergy Immunol. 1996;7:95–99. doi: 10.1111/j.1399-3038.1996.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 41.Plint AC, Osmond MH, Klassen TP. The efficacy of nebulized racemic epinephrine in children with acute asthma: a randomized, double-blind trial. Acad Emerg Med. 2000;7:1097–1103. doi: 10.1111/j.1553-2712.2000.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 42.Smith D, Riel J, Tilles I, Kino R, Lis J, Hoffman JR. Intravenous epinephrine in life-threatening asthma. Ann Emerg Med. 2003;41:706–711. doi: 10.1067/mem.2003.144. [DOI] [PubMed] [Google Scholar]
- 43.Mull CC, Scarfone RJ, Ferri LR, Carlin T, Salvaggio C, Bechtel KA, et al. A randomized trial of nebulized epinephrine vs albuterol in the emergency department treatment of bronchiolitis. Arch Pediatr Adolesc Med. 2004;158:113–118. doi: 10.1001/archpedi.158.2.113. [DOI] [PubMed] [Google Scholar]
- 44.Adoun M, Frat JP, Dore P, Rouffineau J, Godet C, Robert R. Comparison of nebulized epinephrine and terbutaline in patients with acute severe asthma: a controlled trial. J Crit Care. 2004;19:99–102. doi: 10.1016/j.jcrc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Langley JM, Smith MB, LeBlanc JC, Joudrey H, Ojah CR, Pianosi P. Racemic epinephrine compared to salbutamol in hospitalized young children with bronchiolitis; a randomized controlled clinical trial. BMC Pediatrics. 2005;5:7. doi: 10.1186/1471-2431-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeShazo RD. Anaphylaxis: my “top 10“ list. South Med J. 2007;100:233–234. doi: 10.1097/01.smj.0000254177.31586.69. [DOI] [PubMed] [Google Scholar]
- 47.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, et al. Part. 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729–767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]


