Summary
Sexually dimorphic behaviors, qualitative or quantitative differences in behaviors between the sexes, result from the activity of a sexually differentiated nervous system. Sensory cues and sex hormones control the entire repertoire of sexually dimorphic behaviors, including those commonly thought to be charged with emotion such as courtship and aggression. Recent studies show that these over-arching control mechanisms regulate distinct genes and neurons that in turn specify the display of such behaviors in a modular manner. How such modular control is transformed into cohesive internal states that correspond to sexually dimorphic behavior is poorly understood. We summarize current understanding of the neural circuit control of sexually dimorphic behaviors from several perspectives, including how neural circuits in general, and sexually dimorphic neurons in particular, can generate sex differences in behavior, and how molecular mechanisms and evolutionary constraints shape these behaviors. We propose that emergent themes such as the modular genetic and neural control of dimorphic behavior are broadly applicable to the neural control of other behaviors.
Introduction
Men and women exhibit sex differences in behaviors that immediately enhance reproductive success as well as in tasks that involve higher cognitive function. It is actively debated whether such sex differences are genetically wired or a byproduct of societal influences. While the jury may be out for the underpinnings of these behaviors in humans, research in model organisms leaves little doubt that such manichean distinctions between nature and nurture are simplistic. Indeed research on diverse animals unequivocally demonstrates the importance of both genes and experience on sexually dimorphic behaviors. Nevertheless these studies underscore the primacy of genetically programmed mechanisms that control the development and activation of the neural circuits underlying these behaviors.
Sex-typical displays of behaviors such as mating and aggression are genetically hardwired in the sense that they can be displayed by animals without training. The activation of the underlying neural circuits is controlled by sensory cues as well as by physiological signals such as sex hormones. Such external and internal control mechanisms ensure that these social behaviors are displayed in the appropriate context. Many animals, including mice, secrete pheromones, chemosensory cues that signal social and reproductive status to other members of the species, to initiate social interactions (Karlson and Lüscher, 1959). Sex steroid hormones secreted by the gonads are the critical internal signals that control these behaviors in vertebrates (McEwen, 1981). The identity of the pheromone and hormone-responsive neural circuits that drive specific sexually dimorphic behaviors remains elusive. By contrast, we have significant insight whereby chemosensory input and sex hormones control the development or activation of specific neurons that influence these behaviors (Liberles, 2014; Morris et al., 2004; Touhara and Vosshall, 2009; Wu and Shah, 2011).
Our review discusses the mechanisms that regulate sexually dimorphic behaviors in mammals with a specific focus on mice and the assumption that similar mechanisms are likely to operate in humans. The literature on sexually dimorphic behaviors in other organisms has been reviewed elsewhere (Baum, 2003; Cahill, 2006; Crews and Moore, 2005; Dickson, 2008; Manoli et al., 2006; Moore et al., 2005; Newman et al., 1997; Perkins and Roselli, 2007; Portman, 2007; Wade and Arnold, 2004; Wallen, 2005). We focus largely on sex differences in mating and aggression because the underlying neural pathways have been studied in some detail. We do not list all known cellular or molecular sexual dimorphisms in the nervous system because these have been documented extensively (Cahill, 2006; Cooke et al., 1998; Simerly, 2002; De Vries, 1990). Where instructive, we discuss findings in other model organisms, especially flies, that provide insight into the neurobiological basis of sex differences in behavior.
A framework to understand how the brain can generate sexually dimorphic behaviors
Males and females transform sensory input into sexually dimorphic behaviors, suggesting that such behaviors are generated by neural circuits that differ between the sexes. This insight has led to a highly successful effort to identify anatomical or molecular sex differences in neuronal populations in order to gain an entry-point into the neural circuits underlying gender-typical behaviors (Cachero et al., 2010; Cahill, 2006; Cooke et al., 1998; Jarrell et al., 2012; Liu and Sternberg, 1995; Nottebohm and Arnold, 1976; Raisman and Field, 1971; Simerly, 2002; De Vries, 1990; Yu et al., 2010). How these genes or neurons control neural circuit function is unclear because a neural circuit that controls a sexually dimorphic display has yet to be delineated from sensory input to motor output.
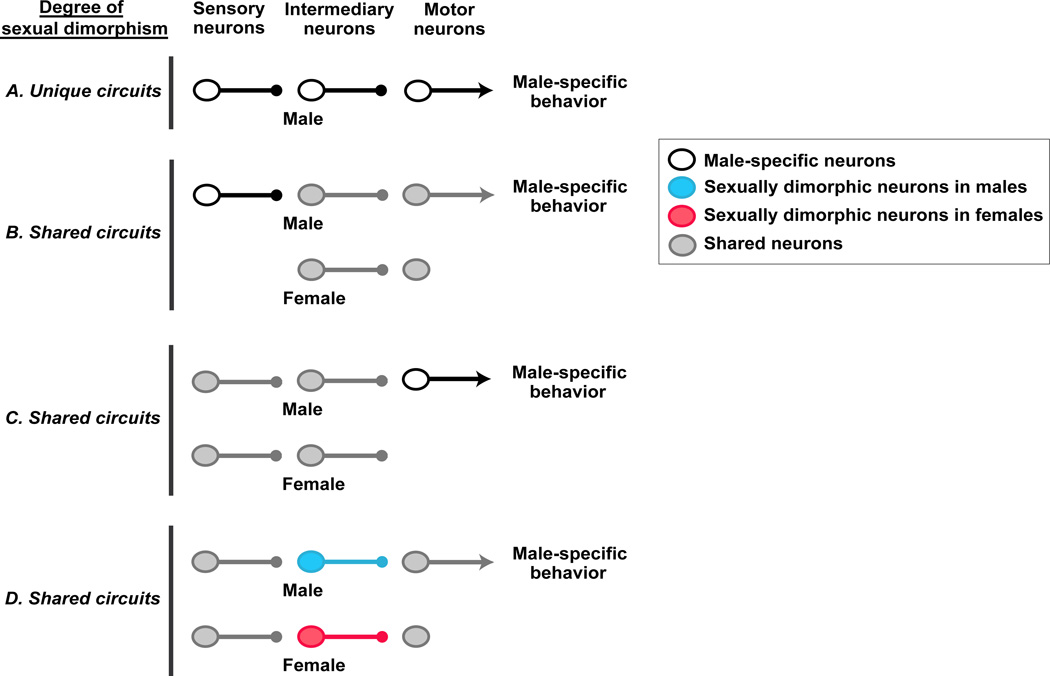
Absent the complete delineation of such a neural circuit, we envision several mutually non-exclusive neural circuit wiring diagrams that enable sexually dimorphic output (Figure 1). In the most extreme case, such a neural circuit is unique to one sex. One example may be the circuit for penile muscles involved in coitus, which are controlled by motor neurons in the spinal nucleus of the bulbo cavernosus (SNB), a population of neurons largely absent in females (Breedlove and Arnold, 1980). Given that wild type females of many species can display some male-type mounting behavior (see later), if the neural pathway controlling these penile muscles is one component of a singular male sexual behavior circuit then at least some neural centers pre-synaptic to the SNB are likely to be shared between the sexes. In this scenario, the gender dimorphism in SNB neurons may represent an example of a shared neural circuit that differs between the sexes at the level of motor neurons. Sex differences at the level of sensory input can also drive sexually dimorphic behaviors. One example of a neural circuit with well-defined sensory sex differences is that underlying female pheromone-elicited chemo tactic flight in the male moth Bombyx mori (Nakagawa et al., 2005; Sakurai et al., 2004; Touhara and Vosshall, 2009). Male but not female moths express chemo receptors for the pheromone mixture emitted by females, and the antennal sensory neurons expressing these receptors project to unique targets in the male antennal lobe. However, the chemo taxis elicited by the presence of the female pheromone engages output pathways that control flight, a behavior shared between the two sexes.
Figure 1. Neural circuits that can generate sexually dimorphic behaviors.
Simplified wiring diagram of some neural circuit configurations that can generate sexually dimorphic behaviors. Although only circuits driving male-specific behaviors are shown for clarity, similar circuits will exist for female-specific behaviors. The axon termini of all neurons except those of motor neurons end in small solid circles to show that they may transmit effectively excitatory, inhibitory, or neuromodulatory output. Termini of male motor neurons are shown as arrows to illustrate stimulation of the muscle groups required for the behavioral display. By contrast, female motor neurons are not shown to have termini to depict lack of activation of the male-specific behavioral program.
(A) The entire neural circuit for generating a male-specific behavior is only present in males.
(B) Sensory neurons unique to males feed into a shared neural circuit to activate a male-typical behavior.
(C) Motor neurons unique to males are regulated by a shared neural circuit to activate a male-typical behavioral response.
(D) Sensory and motor neurons are shared between the sexes but there are sex differences in intermediary neuronal populations. Most sex differences in intermediary neurons appear to be quantitative rather than qualitative in mice; in other words, the comparable neuronal population is shared between the sexes, but it displays cellular or molecular sexual dimorphisms that permit activation of the behavior only in males.
Given that most behaviors are common to the two sexes, males and females probably share many components of a neural circuit that drives a behavior of the opposite sex. For example, biting during inter male aggression, feeding, and maternal retrieval of a wandering pup all entail locomotion and coordinated jaw movements. In such instances, sexually dimorphic behavior is likely to emerge from sex differences in neuronal populations inserted (intermediary neurons in Figure 1) within shared neural circuits. Consistent with this notion, most cell or molecular sex differences in neuronal populations are quantitative rather than all-or-nothing qualitative sexual dimorphisms. We anticipate that real world circuits underlying dimorphic behaviors are likely to be a composite of the wiring diagrams we have discussed here.
A primer on sex determination and sexual differentiation
Whether a sexually dimorphic behavior is innate, in the sense that it can be displayed without prior training, or experiential, animals determine sex early in their development, and sex determination initiates many irreversible sexual differentiation events that influence how the genome and the environment interact to influence gender-specific behaviors. Prior to sex determination, which occurs at mid-gestation in mice, the brain and gonadal primordia are bipotential and can differentiate in a male or female-typical pattern.
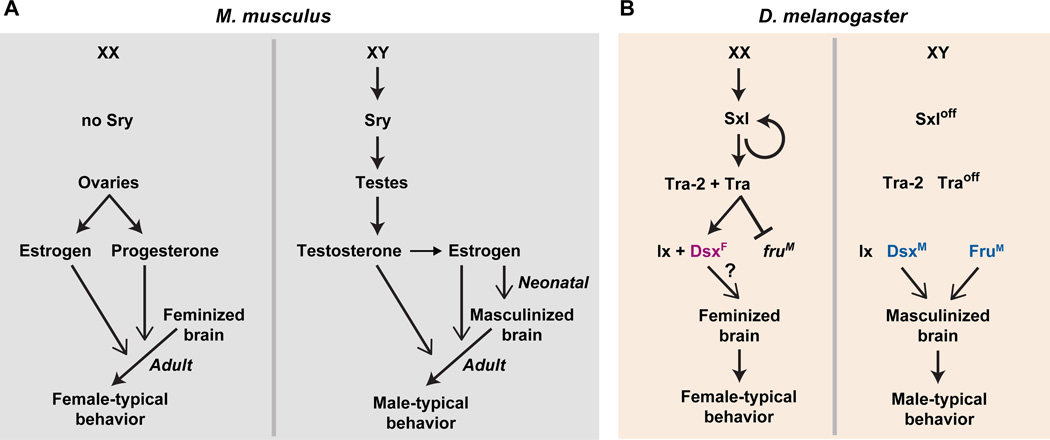
The Y chromosome is determinative for the male sex (Figure 2). A single Y-linked locus, Sry, is necessary and sufficient to masculinize the embryo (Gubbay et al., 1990; Koopman et al., 1991). Sry encodes a transcriptional regulator (but see (Lalli et al., 2003)), and its expression in the bipotential gonads drives their differentiation into testes. Testicular hormones subsequently drive male-pattern sexual differentiation of the body as well as the brain. The embryo is pre-patterned to differentiate as a female in the absence of functional Sry such that the gonads differentiate into ovaries. Moreover, it is the absence of testicular hormones rather than the presence of ovarian hormones that initiates feminization of the body and the brain (Jost, 1983). Thus the default mammalian body plan is female.
Figure 2. Sex determination and sexual differentiation of behavior.
(A) In mice the presence or absence of Sry drives the differentiation of the bipotential gonad into testes or ovaries, respectively. Sex hormones released into the circulation by the gonads act on their cognate receptors to organize the brain during development and to control the activation of sex-typical behaviors in the adult. In males, estrogen organizes the neural substrates for behavior neonatally, and both estrogen and testosterone activate these pathways for male-typical behavior in adults. In the absence of the neonatal organizational effect of estrogen, the default differentiation program of the brain is female although this hormone may be important in adolescence for maturation of the neural substrates underlying female sexual receptivity (not shown) (Bakker et al., 2002; Brock et al., 2011). Both estrogen and progesterone activate the neural circuit underlying this behavior in adult females.
(B) The sex of a fruit fly is determined in a cell autonomous manner, with the expression of sex lethal (Sxl) specifying a female differentiation program. Sex-specific splice forms of doublesex (Dsx) and fruitless direct the cell autonomous differentiation of neurons that control sex-typical behaviors. Ix, intersex, and Tra, transformer.
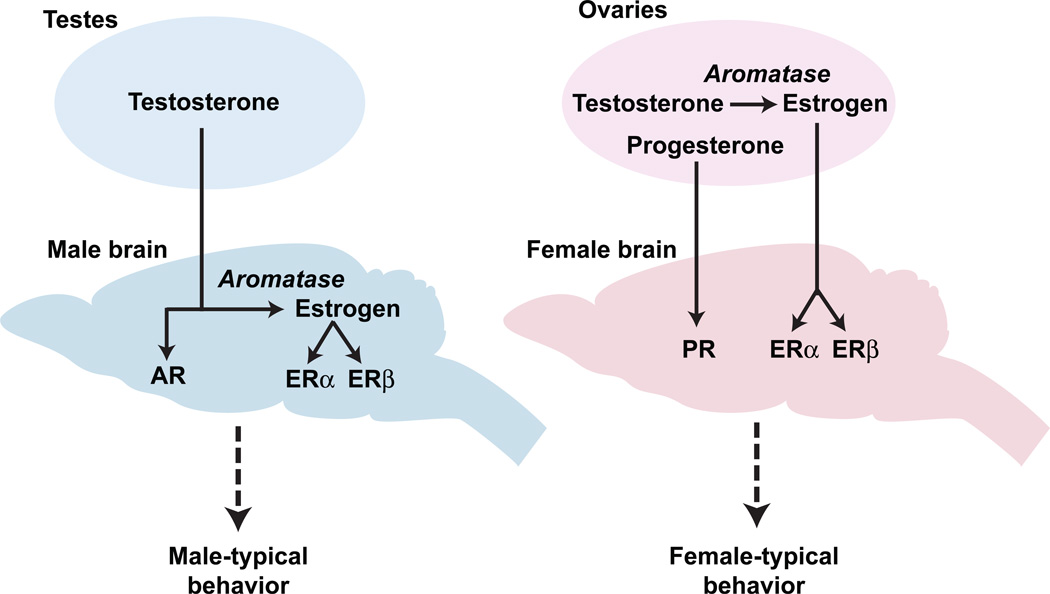
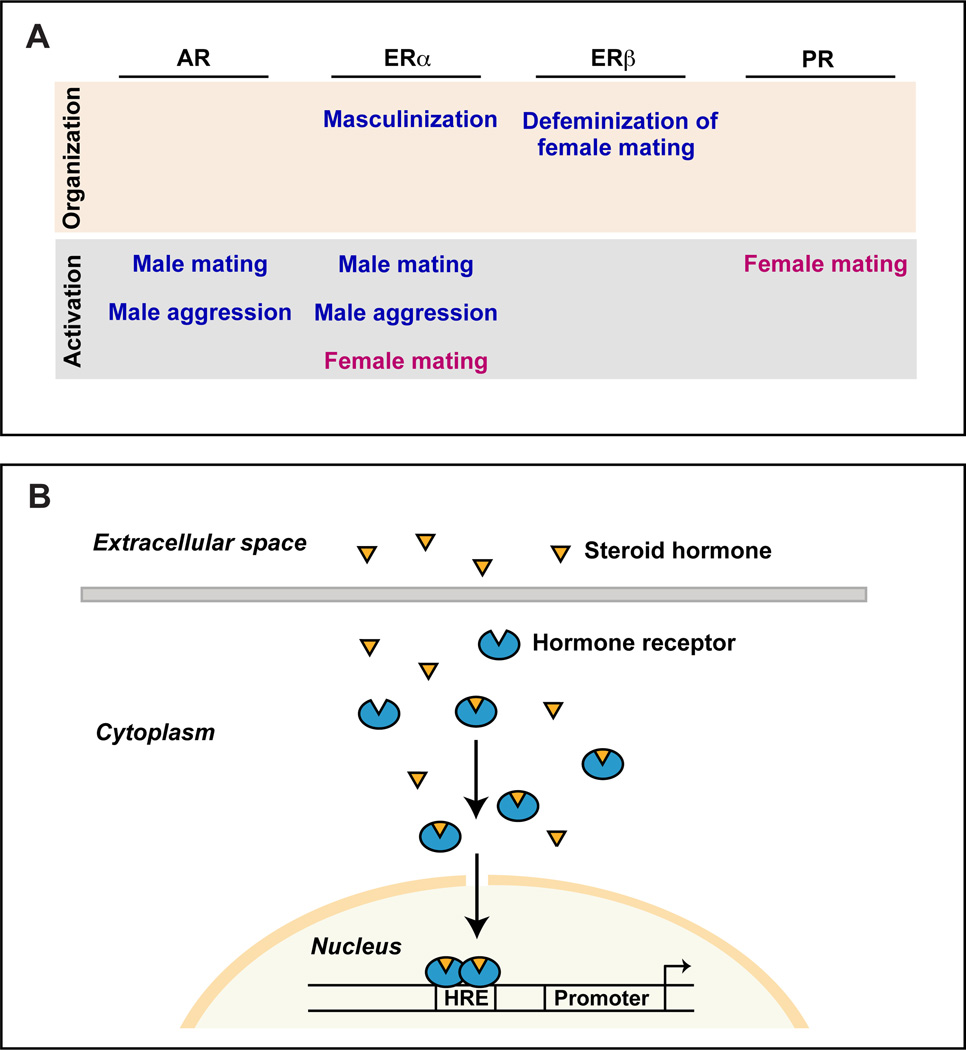
Although early feminization of the body and the brain is independent of the ovarian hormones estrogen and progesterone, sexual maturation and function of various tissues are controlled by these sex steroids. These hormones regulate sexual differentiation and behavior in females via nuclear hormone receptors encoded by distinct but homologous genes, ERα (or Esr1) and ERβ (or Esr2) for estrogen and PR (or Pgr) for progesterone (Figure 3) (Burris et al., 2013). The testes secrete testosterone which masculinizes the external body phenotype and the brain by signaling through its nuclear hormone receptor, the androgen receptor (AR) (Figure 3) (Burris et al., 2013). As we discuss later, many actions of testosterone are mediated subsequent to its conversion into estrogen locally in the brain and activation of signaling via ERα or ERβ.
Figure 3. Sex hormone control of sexually dimorphic behaviors.
Sex hormones produced in the gonads cross the blood-brain barrier and bind to hormone receptors in neurons to regulate sex-typical behaviors. In males, testosterone directs behavior by binding to its receptor AR or it is converted via aromatase into estrogen, which binds to its cognate receptors ERα and ERβ. In females, estrogen and progesterone direct behavior via their cognate receptors ERα and ERβ and PR, respectively.
Sex chromosome linked genetic loci that remain to be identified may also directly control the development and function of neural circuits underlying innate social behaviors (Bonthuis et al., 2012; Carruth et al., 2002; Dewing et al., 2003; Grgurevic et al., 2012; De Vries et al., 2002; reviewed in McCarthy and Arnold, 2011). These loci subtly regulate male mating and aggression in a manner that mirrors the sex chromosome complement rather than circulating sex hormones. Thus, in contrast to the profound influence of sex hormones on sexually dimorphic displays, such genetic loci appear to exert modulatory effects on these behaviors. In addition to such sex chromosome based mechanisms, potentially hormone-independent epigenetically regulated gene expression patterns may also regulate sexual differentiation of the brain. Recent studies have identified many potentially imprinted genes that are transcribed, often in a sexually dimorphic manner, in the developing and adult brain (DeVeale et al., 2012; Gregg et al., 2010a, 2010b). The functional relevance of these loci to sexually dimorphic behaviors is presently unclear, but other imprinted genes have indeed been implicated in the control of sexually dimorphic behaviors, including aggression (Garfield et al., 2011; Lefebvre et al., 1998; Li et al., 1999). In summary, gonadal sex hormones appear to act as the master regulators of sexual differentiation in mammals, and hormone-independent genetic loci may exert modulatory control of sexually dimorphic behaviors.
Evolution of the mechanisms underlying sex determination and sexual differentiation
Despite the near universal nature of gender differences in reproductive behavior in animals – even birds, bees, and fleas do it – one theme emerging from work in different species is that the molecular control of sexual determination and differentiation has evolved rapidly even among closely related species (Cline and Meyer, 1996; Marín and Baker, 1998; Matson and Zarkower, 2012). In contrast to the close conservation of most genetic pathways regulating development, there is little similarity between the pathways controlling sex determination and early sexual differentiation of the brain in flies and mice (Figure 2). Sex lethal, transformer, doublesex, and fruitless in the fly may be analogous to Sry and the genes encoding sex hormone receptors in mice, but they are not encoded by orthologous genes. Doublesex may be an exception in the sense that orthologs have been found in flies, worms, and vertebrates, but whether it performs identical functions across diverse animals is unclear. Sex determination and differentiation do appear operationally similar between flies and mice in that they are controlled by a regulatory cascade, initiated by sex lethal in flies and Sry in mice (Figure 2). However, sex determination and sexual differentiation in the fly are cell autonomous such that each cell undergoes a cell fate decision to become a male or female cell. By contrast, sex determination of the gonads in mice leads to the secretion of sex hormones that act non-cell autonomously elsewhere in the body and the brain to guide sexual differentiation. In contrast to the mouse, the default fly body plan, in the absence of sex lethal but with the appropriate chromosomal complement, appears to be male (Figure 2).
Surprisingly, although the genes involved in sex determination and differentiation have diverged rapidly, some of the same neurotransmitter mechanisms appear to be utilized for reproductive behaviors across multiple species (Asahina et al., 2014; Caldwell et al., 2008; DeVries et al., 1997; Garrison et al., 2012; Winslow et al., 1993; Young et al., 1997). Whether such shared signaling pathways always represent evolutionary conservation or rather reflect, in some cases, convergent evolution of a limited set of inter-neuronal communication signals remains to be determined. In either instance, it will be important to understand the selective advantage of using a particular neurotransmitter to control similar reproductive behavior across species. Similar to the genes regulating sex determination and differentiation, the specific displays of sexually dimorphic behaviors also evolve quickly. This has been well documented in the divergence of the stereotyped male courtship ritual across various drosophilid species (Spieth, 1952). For example, male flies of different drosophilid species, similar to male songbirds of different species, sing a species-specific song to which a female fly of the corresponding species is most attracted (Konishi, 1985; Wheeler et al., 1991). By contrast, male fruit flies utilize chemosensory pathways to recognize conspecific females and to reject potential mates from other species (Fan et al., 2013). Thus there can be rapid divergence of molecular and behavioral reproductive mechanisms between the sexes and across species. Such divergence is to be expected perhaps be cause these mechanisms are subject to sexual selection and are critical to maintain reproductive isolation and continued propagation of individuals within a species.
Pheromonal control of sexually dimorphic behaviors
Pheromonal control of mating and aggression in mice
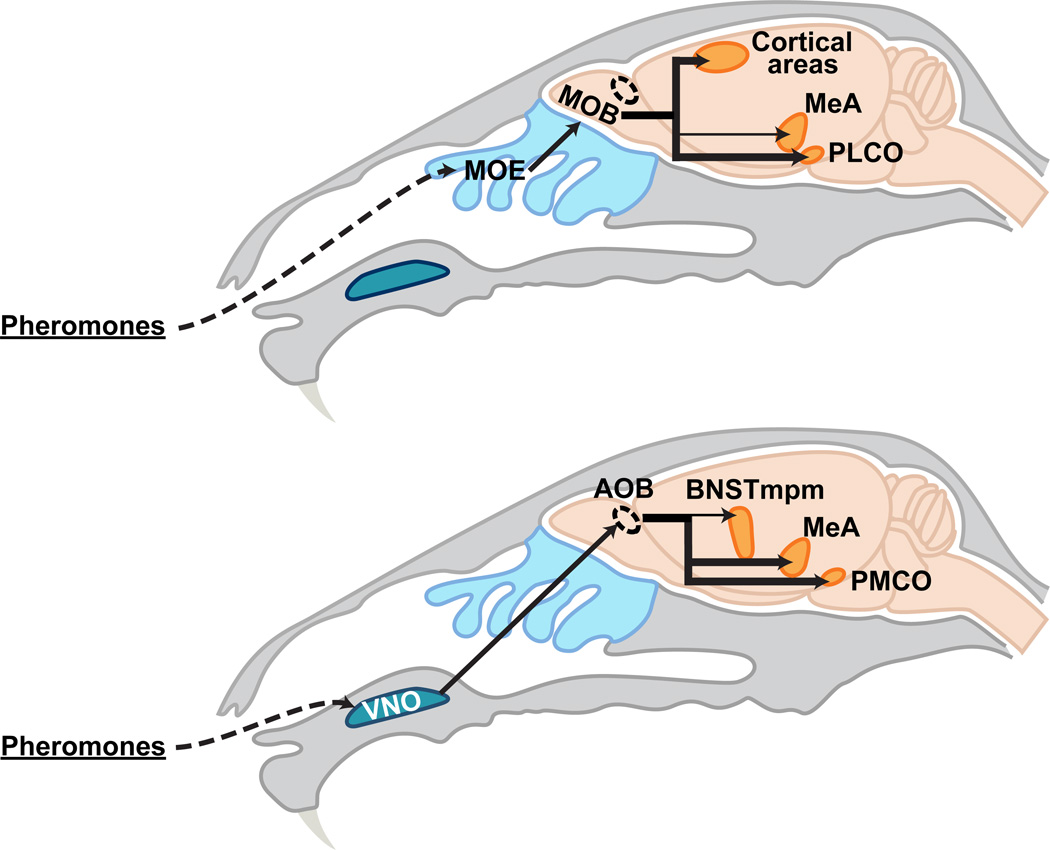
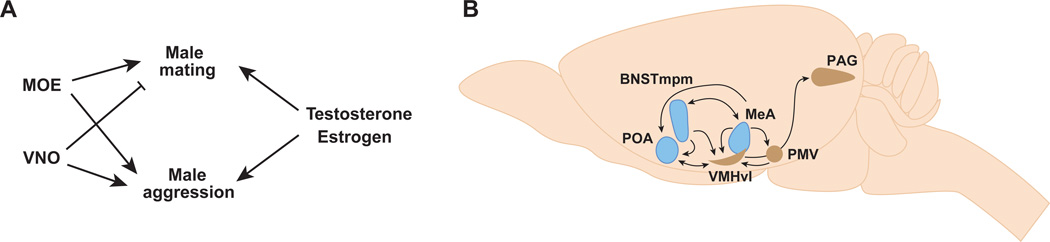
In mice and many other animals, pheromones detected by sensory neurons in the nose are the predominant sensory cues that trigger mating and aggression. Pheromones are recognized by chemosensory neurons located in two sensory epithelia in the nose, the main olfactory epithelium (MOE) and the vomeronasal organ (VNO) (Figure 4) (Zufall and Leinders-Zufall, 2007). Neurons in these two sensory epithelia each express G-protein coupled receptor (GPCR)-type chemo receptors from unrelated gene families, suggesting that they detect distinct chemosensory cues (reviewed in (Liberles, 2014; Touhara and Vosshall, 2009)).
Figure 4. Pheromone sensing pathways.
Schematic representing that pheromone sensing neurons in the main olfactory epithelium and vomeronasal organ activate distinct neural pathways.
Dashed oval represents the AOB. Thin arrows to the MeA and BNSTmpm depict relatively minor projections to these areas from the MOB and AOB, respectively.
Traditional lesioning studies have long implicated pheromonal signaling via the VNO in the control of male mating and aggression (Wysocki and Lepri, 1991). However, genetic studies reveal surprising complexity in the chemosensory control of these behaviors. Male mice with intact MOE but genetically disabled for VNO signaling mate with females essentially normally but exhibit a profound reduction in inter male aggression (Kim et al., 2012; Leypold et al., 2002; Stowers et al., 2002). By contrast, males with an intact VNO but genetically disabled for MOE signaling exhibit a profound reduction in mating and inter male aggression (Mandiyan et al., 2005; Wang et al., 2006; Yoon et al., 2005). Thus, inter male aggression relies on both the MOE and VNO whereas male-typical sexual displays require an intact MOE but not the VNO (Figure 5A).
Figure 5. Sensory and hormonal control of sexually dimorphic behaviors.
(A) Control of male pattern mating and aggression by chemosensory input and sex hormones. (B) Schematic representing extensive interconnections between hypothalamic and amygdalar nuclei that regulate sexually dimorphic behaviors. These areas process pheromonal information, and subsets of adult neurons within each of these regions express sex hormone receptors; neurons within some of these regions (blue) also express aromatase.
PAG, peri-aqueductal gray; PMV, ventral pre-mamillary nucleus; POA, preoptic hypothalamus; VMHvl, ventrolateral component of the ventromedial hypothalamus.
Males and females genetically disabled for VNO signaling exhibit male-pattern sexual behavior with mice of either sex (Kimchi et al., 2007; Leypold et al., 2002; Stowers et al., 2002). These findings suggest that male pheromones detected by the VNO normally inhibit sexual behavior and trigger aggression in males (Figure 5A). However, females normally do not attack male mice, and there appears to be no qualitative sex difference in the expression of the chemoreceptor repertoire in the VNO. It is likely therefore that females do not attack males because of a sexual dimorphism downstream of vomeronasal sensory input in the neural circuit that mediates aggression. Females of many species, including mice, exhibit low frequency male-pattern mating towards conspecific females (Baum et al., 1974; Beach, 1947; Jyotika et al., 2007; Kimchi et al., 2007; Spors and Sobel, 2007). This suggests that the neural circuit for masculine sexual behavior is present in both sexes, but it is normally inhibited by sensory input from the VNO (Figure 5A). One signal that over-rides this sensory inhibition is the male sex hormone testosterone, as adult wild type females supplemented with testosterone display male-pattern mating towards females at male-typical frequencies (Figure 5A) (Edwards and Burge, 1971a). These studies suggest a model in which sex differences in the neural circuit underlying male sexual behavior regulate the probability of displaying this behavior such that VNO sensory input decreases and testosterone increases this probability (Figure 5A).
These genetic studies also show that the VNO and MOE are required for female behaviors, including sexual receptivity and maternal aggression, a behavior that nursing females display toward unfamiliar intruder mice (Fraser and Shah, 2014; Gandelman, 1972; Leypold et al., 2002; Wang and Storm, 2011). Numerous pheromones that regulate sex-typical behaviors or physiology have been identified. The identification of the chemo receptors that recognize these pheromones will permit delineation of the neural circuits that respond to these cues. This should already be feasible in the case of female sexual behavior. ESP1 is a peptidergic pheromone found exclusively in post-pubertal male lacrimal gland secretions (Kimoto et al., 2005), and ESP1 and its chemoreceptor V2Rp-5 are required for the normal, high levels of female sexual receptivity (Haga et al., 2010). These studies now provide a molecular genetic means to trace V2Rp-5 expressing neurons into the central circuits that control female sexual behavior.
Neural circuits that transduce pheromonal cues into sexually dimorphic behaviors
The anatomic segregation of MOE and VNO neurons is maintained in their central projections, which innervate the main olfactory bulb (MOB) and the accessory olfactory bulb (AOB), respectively (Figure 4) (Dulac and Wagner, 2006; Zufall and Leinders-Zufall, 2007). Mitral and tufted cells, projection neurons of the MOB, send axons to olfactory cortical regions associated with learning of general odorants as well as to the posterolateral cortical amygdala (PLCO), a region that may regulate instinctual behaviors (Figure 4) (Kang et al., 2011a; Scalia and Winans, 1975; Shipley and Adamek, 1984; Sosulski et al., 2011). Thus, the MOE pathway may control innate and learned odor-guided behaviors via projections to distinct brain regions.
AOB projection neurons send their axons to the medial amygdala (MeA) and the posteromedial cortical amygdala (PMCO) and, to a lesser degree, to the medial division of the posteromedial bed nucleus of the stria terminalis (BNSTmpm) (Figure 4) (von Campenhausen and Mori, 2000; Scalia and Winans, 1975; Winans and Scalia, 1970). As we discuss later, these regions influence the display of most sexually dimorphic behaviors. Both the VNO and MOE regulate male-pattern sexual displays and inter-male aggression, suggesting cross-talk between or convergence of these two chemosensory circuits. Indeed mapping of the efferent connections of the projection targets of the MOB and AOB reveals such convergence in the BNST and several hypothalamic nuclei (Figure 5B) (Kevetter and Winans, 1981a, 1981b; Licht and Meredith, 1987; Meredith, 1998; Shipley et al., 1996). In addition some, but not all, studies find that the MeA may also receive afferents from the MOB, there by providing a site of convergence of MOE and VNO pathways just one synapse removed from the nose (Kang et al., 2009, 2011b; Licht and Meredith, 1987; Sosulski et al., 2011).
The finding that male and female chemosensory neurons express the same repertoire of pheromone receptors immediately poses the question as to how shared sensory input is used to generate sexually dimorphic output. At least in flies, some pheromone-sensing neurons that elicit distinct behaviors in both sexes in response to the same pheromone project to central circuits that are sexually dimorphic (Datta et al., 2008; Kohl et al., 2013; Ruta et al., 2010). How such sex differences direct sexually dimorphic responses in flies is still an open question. In mice, many sex differences have also been described in central olfactory pathways (Guillamón and Segovia, 1997). How such dimorphic neurons in the mouse brain are connected with pheromone-sensing neurons in the nose is unclear. These sex differences in mice develop under the control of sex hormones, and in adult animals, the central neurons that relay olfactory information are rich in sex hormone receptor expression, thereby affording the potential for sexually dimorphic regulation of the physiology and function of olfactory pathways (Figure 5B). Such convergence of sensory input and hormonal signals in shared neural circuits likely allows the animal to assimilate information about the external world and internal physiological states and to generate sexually dimorphic behaviors.
Hormonal control of sexually dimorphic behaviors
Historical framework for understanding hormonal control of sexually dimorphic behaviors
The influence of gonadal secretions on behavior and physiology has long been appreciated. Studies in birds in the 19th century correlated the presence of male song or sexual displays with the presence of testicular secretions (reviewed in (Fusani, 2008)). Subsequent work in male rodents showed that testosterone restored sexual and aggressive displays in adult castrates (Beeman, 1947; Shapiro, 1937; Stone, 1939). Similarly, ovarian extracts or estrogen and progesterone could elicit estrus and sexual receptivity in castrate female rodents (Allen et al., 1924; Dempsey et al., 1936; Ring, 1944; Wiesner and Mirskaia, 1930). Such studies therefore indicated that adult sex hormones were necessary and sufficient for the display of mating, aggression, and the estrous cycle.
Some of the earliest work elucidating a developmental role for gonadal hormones focused on the neural control of the estrous cycle (Harris, 1937, 1964; Harris and Jacobsohn, 1952; Pfeiffer, 1936; Sawyer et al., 1949). These studies showed that the neonatal hypothalamus was bipotential, and that testosterone irreversibly inhibited the neonatal hypothalamus from supporting normal ovarian function and estrous cyclicity. By contrast, neonatal castration permitted the male hypothalamus to support ovarian function and the estrous cycle in adult life.
These developmental and adult effects of sex hormones on endocrine physiology were subsequently shown to be equally applicable to reproductive behaviors (Arnold, 2009; Phoenix et al., 1959). In mice, neonatal testosterone irreversibly defeminized sexual receptivity and elicited male-typical territorial aggression upon adult provision of testosterone (Bronson and Desjardins, 1969; Edwards, 1968, 1969, 1971). Such work revealed that the testes acted on a bipotential brain during a critical perinatal developmental window to regulate adult behaviors. During this period, the ovaries appeared unnecessary for the subsequent display of female sexual behavior. In accord with these experimental observations, the testes secrete testosterone during this critical period whereas the ovaries are quiescent. The onset and duration of this critical period of sexual differentiation of the brain is species-specific, and in mice it extends from late gestation into the first few days after birth (Corbier et al., 1992; Motelica-Heino et al., 1993; Pang and Tang, 1984). In contrast to the perinatal requirement of gonadal hormones in males, the adult display of gender-typical behaviors requires gonadal hormones in both sexes. The enduring, developmental influence of sex hormones is referred to as their organizational function, whereas the reversible, adult role of sex hormones in the acute regulation of physiology and behavior is referred to as their activational function (Figure 6A)(Phoenix et al., 1959). The notion of distinct developmental, including peri-pubertal, and adult roles of sex hormones is a cornerstone of contemporary understanding of how these steroids control sexually dimorphic behaviors (reviewed in (Arnold, 2009; Schulz et al., 2009)).
Figure 6. Mechanism and function of sex hormone action.
(A) Function of sex hormone receptors in the nervous system during development (organization) and adult life (activation). Not shown is the requirement of estrogen to feminize sexual behavior, which occurs likely via ERα subsequent to the neonatal organizational phase but prior to adulthood (Bakker et al., 2002; Brock et al., 2011).
(B) Schematic illustrating sex hormone action via nuclear hormone receptors. Sex hormones are steroids that can cross the blood-brain barrier and cell membranes to bind their cognate receptors. Hormone-bound receptor translocates to the nucleus, where it can regulate transcription of target genes by directly binding to specific DNA sequences. HRE, hormone response element.
An important advance in understanding the hormonal control of sex-typical behaviors came about from observations that many developmental and adult effects of testosterone were mimicked by estrogen (Antliff and Young, 1956; Ball, 1937; Bronson and Desjardins, 1968; Edwards and Burge, 1971b; Finney and Erpino, 1976; Gorski and Wagner, 1965; Södersten, 1973; Wallis and Luttge, 1975; Whalen and Nadler, 1963). Given that testosterone or a related androgen is a precursor for estrogen in vivo (Figure 3), Naftolin proposed that circulating testosterone in males is converted into estrogen in specific brain regions via the action of the enzyme aromatase, a proposal referred to as the aromatization hypothesis (MacLusky and Naftolin, 1981; Naftolin et al., 1971a, 1971b). Studies in diverse vertebrates now support such local synthesis of estrogen in the male brain, although the extent of the masculinizing effects of estrogen appears to be species-specific (Amateau et al., 2004; Balthazart and Ball, 1998; Baum, 2003; Finkelstein et al., 2013; Forlano et al., 2006; Holloway and Clayton, 2001; Lephart, 1996; Lieberburg et al., 1979). How is the brain of the female mouse protected from the developmental, masculinizing effects of estrogen? As we noted above, the ovaries are quiescent during this critical period and there is consequently little, if any, estrogen synthesis in female embryos. Moreover in mice and many other species, the embryonic liver-derived a-fetoprotein sequesters circulating estrogen produced by the ovaries (Bakker et al., 2006; MacLusky and Naftolin, 1981). Together, these studies make a compelling case for estrogen in regulating differentiation of the male brain and behavior.
Recent insights into the hormonal control of sexually dimorphic behaviors
Recent genetic studies have provided previously unanticipated insights into the mechanisms whereby sex hormones control dimorphic behaviors (Figure 6A). Females constitutively null for PR or ERα are not sexually receptive, thereby confirming the importance of the cognate hormones in this behavior (Blaustein, 2008; Kudwa and Rissman, 2003; Lydon et al., 1995; Ogawa et al., 1996, 1998; Rissman et al., 1997). By contrast, ERβ is not required for female sexual receptivity, but it appears essential to defeminize this behavior in male mice (Krege et al., 1998; Kudwa and Rissman, 2003; Kudwa et al., 2005; Ogawa et al., 1999). Thus, it appears that the neonatal testosterone surge in male mice defeminizes sexual behavior at least in part subsequent to conversion into estrogen and activation of ERβ.
The evidence that estrogen signaling is required for male behaviors is convincing. Male mice null for aromatase exhibit profound deficits in mating and aggression (Honda et al., 1998; Matsumoto et al., 2003; Toda et al., 2001a, 2001b). Targeted deletions of ERα and ERβ show that ERα and ERβ are required for male sexual behavior whereas only ERα is essential for adult male-typical aggression (Krege et al., 1998; Ogawa et al., 1997, 1999, 2000; Scordalakes and Rissman, 2003; Wersinger et al., 1997). As discussed earlier, estrogen is also sufficient to masculinize the brain during development. Supplementing neonatal females with estrogen suffices for the later display of male pattern territorial behaviors, albeit at lower levels than normally observed in wild-type males (Wu et al., 2009). Thus, neonatal estrogen masculinizes the female as observed by her male-typical aggression toward males. Strikingly, this neonatal estrogen also drives subsequent territorial marking (Wu et al., 2009), which can be displayed without social interactions and may therefore be an objective surrogate for an internal representation of one aspect of masculinity. These masculine behaviors in females neonatally provided with estrogen are abolished upon removal of the ovaries, indicating that they are dependent on gonadal estrogen release (Wu et al., 2009). Thus, estrogen exposure is sufficient to masculinize the brain during development, and adult estrogen permits low intensity male-typical behavioral displays in the adult. More broadly, these studies show that the adult gonads of either sex can support male-type behaviors provided the neonatal mouse has been exposed to estrogen.
In contrast to the masculinization effected by estrogen, testosterone signaling via AR appears essential to scale the intensity of masculine behaviors. Male mice constitutively mutant for AR do not display male-typical mating or aggression (Ohno et al., 1974; Sato et al., 2004). Such males have a normal neonatal testosterone surge, but the testes atrophy subsequently, leading to a loss of circulating testosterone in adults (Sato et al., 2004). Consequently, these males are developmentally masculinized, and the behavioral deficits can be rescued to a large degree with testosterone (or estrogen) in adult life (Olsen, 1992; Rosenfeld et al., 1977; Scordalakes and Rissman, 2004; Wu et al., 2009). Very few cells express AR in the wild type mouse brain at the time of the neonatal surge of testosterone whereas many cells in regions such as the BNSTmpm, POA, and MeA express one or both nuclear ERs and aromatase (Juntti et al., 2010). The adult pattern of sexually dimorphic AR and aromatase expression is established several days after the neonatal testosterone surge, and this masculinization is dependent on estrogen (Juntti et al., 2010; Wu et al., 2009). Taken together, these studies suggest that testosterone signaling via AR is not required to masculinize the neural substrates for behavior, but rather it plays an activational role in male behavioral displays. Strikingly, this hypothesis has been validated in male mice bearing a nervous system-restricted deletion of AR (Juntti et al., 2010; Raskin et al., 2009). Deletion of AR in the brain prior to the perinatal testosterone surge generated male mutants with masculinized genitalia, male-typical testosterone titers, and a loss of AR expression in the adult brain. These mutant males exhibited a male-typical repertoire of mating and territorial aggression, albeit with a reduced frequency or intensity of specific components of these behaviors (Juntti et al., 2010). Thus, testosterone signaling via AR is not required for developmental masculinization of the brain, but it is essential to amplify the adult display of male-typical mating and territoriality.
These genetic studies make a compelling case for the primacy of estrogen in developmental masculinization of the circuits for mating and territorial aggression and for a dual control by testosterone and estrogen signaling in the adult display of these behaviors. A surprising finding from such studies is the circumscribed distribution of aromatase-expressing neurons (Figure 5B), which localize to subsets of cells within a few regions, including the BNSTmpm, MeA, and the preoptic hypothalamus (POA) in mice (Wu et al., 2009). This limited distribution of aromatase-positive neurons suggests that estrogen may signal to a few critical centers to regulate all male typical social behaviors. Alternately, as has been suggested in birds (Balthazart and Ball, 1998; Peterson et al., 2005), estrogen may be distributed widely from such circumscribed sites of synthesis. It is unclear whether such aromatase-expressing neurons function solely as neuroendocrine cells that synthesize estrogen or rather participate directly within the neural circuits that regulate sexually dimorphic behaviors.
Mechanisms whereby sex hormones control sexual differentiation of the nervous system and behavior
The cellular mechanisms activated by sex hormone receptor signaling to drive sexual differentiation are similar, if not identical, to those that control neuronal number, morphology, projection patterns, and gene expression during neural development. Such mechanisms as they relate to sexual differentiation have been thoroughly reviewed elsewhere (Forger and de Vries, 2010; McCarthy and Arnold, 2011; Morris et al., 2004; Simerly, 2002; Toran-Aller and, 1984), and we do not discuss these here. By comparison, we know surprisingly little about the molecular pathways activated by sex steroids to regulate these cellular processes. In addition to binding the nuclear hormone receptors discussed above, sex hormones are also thought to bind transmembrane receptors. However, with the exception of GPR30, a GPCR for estrogen, such transmembrane receptors for other steroid hormones remain to be identified definitively (Burris et al., 2013; Revankar et al., 2005). GPR30 appears not to be required for sexually dimorphic behaviors in mice (Otto et al., 2009; Wang et al., 2008). Many studies also indicate that nuclear receptors for sex hormones can effect rapid changes in cellular function by participating in cytoplasmic or membrane-associated signaling pathways that do not lead to changes in gene expression (Foradori et al., 2008; Henderson, 2007; Lishko et al., 2011; Micevych and Dominguez, 2009; Vasudevan and Pfaff, 2008). This is surprising because the standard view of nuclear hormone receptors has been that they are transcription factors that can bind to consensus DNA sequence elements in the genome to regulate gene expression (Figure 6B) (Burris et al., 2013). These non-transcriptional modes of sex hormone signaling remain poorly understood in the sense that we do not know the specific protein domains of sex hormone receptors involved in these pathways. At least in the case of ERα, genetic studies show that the DNA-binding domain is essential for the normal display of sexually dimorphic behaviors (Jakacka et al., 2002; McDevitt et al., 2007, 2008). These findings suggest that ERα, and perhaps the other nuclear receptors for sex hormones, largely regulates sexually dimorphic behaviors by controlling gene expression. However, no direct transcriptional targets of sex hormone signaling have been identified in the nervous system. In other words, we have yet to learn of a genetic locus whose expression in the nervous system is sexually dimorphic and controlled via occupancy of cis-regulatory DNA sequences by a sex hormone receptor.
We imagine that sex hormones regulate gene expression programs that correspond to their enduring organizational and transient activational roles in the developing and adult animal. The long-term developmental effects of sex hormone signaling are likely reflected in sex differences in morphological features and epigenetic control of gene expression (reviewed in (McCarthy and Nugent, 2013)). Epigenetic programming would permit expression of distinct genes and behaviors upon exposure to the same sex hormone in adult life; for example, such mechanisms may explain why estrogen elicits inter male aggression and sexual receptivity in adult castrate males and females respectively (Edwards and Burge, 1971b). The extent and specificity of epigenetic marks programmed by sex hormones in the brain remain to be determined. In any event, sex hormone receptors have been shown to associate, at least in cell lines, with histone-modifying enzymes that can activate or repress gene expression, and it will important in future studies to identify the genes regulated by this mechanism in the brain (Leader et al., 2006; Rosenfeld et al., 2006).
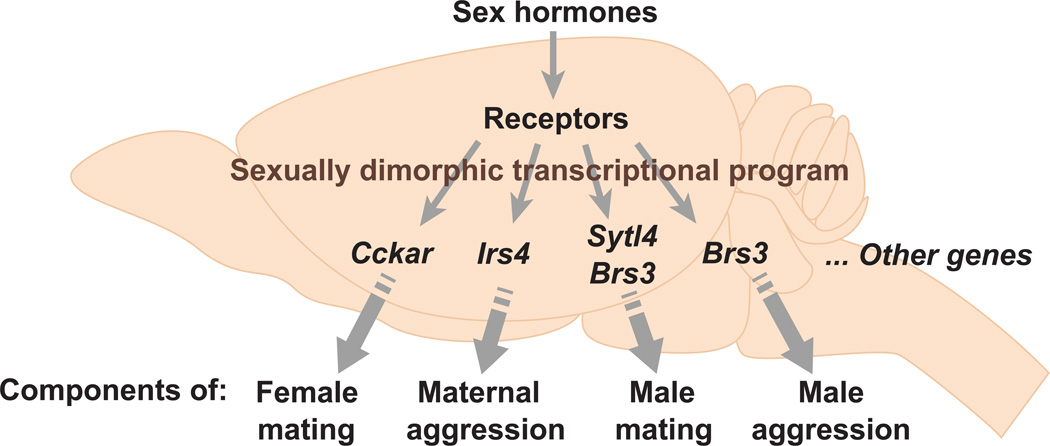
In contrast to the lack of insight on the molecular mechanisms of sex hormone action, many sex differences in gene expression have been identified, and at least some of these are dependent on sex hormones. The nuclear sex hormone receptors and aromatase display some of the most obvious and well characterized differences in gene expression in adult mice (Grgurevic et al., 2012; Shah et al., 2004; Wersinger et al., 1997; Wu et al., 2009; Xu et al., 2012; Yang et al., 2013; Zuloaga et al., 2014). There are many other genes whose expression in the mouse brain is also sexually dimorphic (Dewing et al., 2003, 2006; Edelmann et al., 2007; Gagnidze et al., 2010; Rinn et al., 2004; De Vries, 1990; Wolfe et al., 2005; Xu et al., 2012; Yang et al., 2006). Despite the identification of such dimorphically expressed genes in the brain, there has been no concerted effort to understand whether they influence sexually dimorphic behaviors. A recent study utilized expression profiling to identify many novel sex differences in gene expression in specific centers in the adult mouse hypothalamus, BNST, and MeA (Xu et al., 2012). These gene expression dimorphisms do not correspond to absolute sex differences in cell number, and they often only label a subset of neurons within a brain region. These dimorphic expression patterns are regulated by circulating sex hormones in the adult, although it is unclear whether these genes are direct transcriptional targets of sex hormone receptors. Strikingly, mice singly mutant for these genes exhibited specific deficits in one or a few components of male or female sexual behavior (Brs3, Sytl4, Cckar), inter male aggression (Brs3), or maternal care (Irs4) while maintaining a sex-typical repertoire of other behaviors (Figure 7). Each of these genes is expressed in particular neurons within the hypothalamus and the MeA or elsewhere, implicating one or more neuronal pools in distinct components of sex-typical behaviors. This notion has been validated in the case of Cckar, a GPCR for the neuropeptide cholecystokinin. Cckar is required for the normal high levels of female sexual receptivity (Xu et al., 2012), and it labels a subset of neurons within the VMH that is also required for the display of this behavior (Yang et al., 2013). More generally, the specificity of behavioral deficits observed in mice mutant for such genes is in complete contrast to the global behavioral deficits observed in castrated mice or mice mutant for sex hormone receptors. These findings suggest a model in which individual dimorphic behaviors or components thereof are controlled in a modular manner by genetically separable pathways that are downstream of sex hormone signaling (Figure 7). This genetic modularity in the control of complex sexually dimorphic social behaviors may allow for evolutionary selection of the components of these behaviors that are critical for reproductive success. As we discuss later, such genetic modularity is likely a general principle that underlies other innate behaviors in diverse animals.
Figure 7. Modular genetic control of sexually dimorphic behaviors by sex hormones.
This model proposes that sex hormones control a sexually dimorphic transcriptional program in the nervous system such that individual dimorphically expressed genes control one or a few components of a sex-typical behavior. This model is supported by work showing that genes downstream of sex hormone signaling (Brs3, Cckar, Irs4, Sytl4) are required for the normal display of sexual or aggressive displays (Xu et al., 2012). Many genes downstream of sex hormone signaling still remain to be identified.
What is the function of sexually dimorphic neuronal populations?
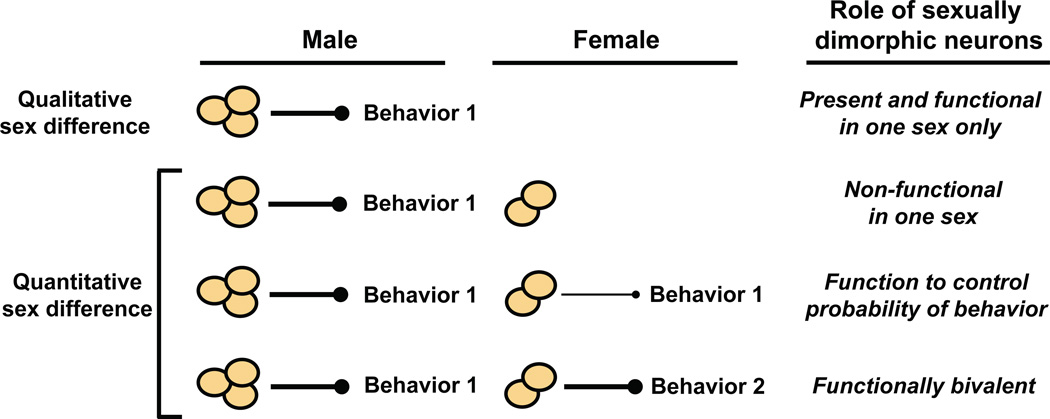
Numerous cell and molecular sex differences have been identified in neurons and other cell types in virtually every sexually reproducing species. In some cases, perhaps especially so in invertebrates, the sex differences are qualitative such that particular neurons are unique to one sex (Figure 8). In such instances, it can be straightforward to determine the function of sexually dimorphic neurons. For example, the male-specific P1 neuronal cluster expresses FruM and appears necessary and sufficient for initiation of singing during courtship (Kimura et al., 2008; Kohatsu et al., 2011; von Philipsborn et al., 2011).
Figure 8. Function of sexually dimorphic neuronal populations.
Neurons present only in one sex (qualitative sex difference) may either activate or inhibit a sexually dimorphic behavior in that sex. More commonly in mice and other vertebrates, a neuronal population is present in both sexes but presents sex differences (quantitative sex difference) in gene expression, cell number, or other cytological feature. In such cases, the neurons may be non-functional in one sex, regulate the probability of displaying a sexually dimorphic behavior, or control the display of different sexually dimorphic behaviors in the two sexes (functionally bivalent).
Functional analysis of sexual dimorphisms is complicated in cases where sex differences represent quantitative rather than qualitative cell or molecular differences in neurons. A quantitative sex difference may represent a dimorphism in the same cell or molecular feature in shared neurons. It is conceivable that smaller subsets of neurons embedded within such shared but quantitatively dimorphic neuronal populations are, in fact, unique to one sex. Such smaller subsets may only become apparent upon careful examination of cellular features such as axon projections or gene expression. It is unclear how quantitative sex differences relate to sexually dimorphic behaviors in the two sexes (De Vries and Boyle, 1998). It is possible that a dimorphic neuronal population is functional in both sexes, and it regulates the probability of displaying the same behavior, thereby leading to a sex difference in this behavior (Figure 8). In the extreme version of this model, the dimorphic neurons in one sex are non-functional, and the probability of displaying the behavior is zero. A quantitative sex difference could also permit the neurons to be functionally bivalent such that they regulate distinct sexually dimorphic behaviors in both sexes.
We recently examined the function of the quantitative sex difference in the number of PR-expressing neurons in the mouse VMHvl (Figure 5B) (Yang et al., 2013). There are more PR-positive neurons in the female VMHvl, and these neurons also express Cckar and ERα (Yang et al., 2013). Similar to PR and ERα, Cckar is also essential for normal female sexual receptivity (Xu et al., 2012). Importantly, PR-expressing VMHvl neurons arise from the same developmental lineage in both sexes (Grgurevic et al., 2012), thereby affording functional characterization of developmentally related dimorphic neurons. We found that genetically targeted ablation of these PR-expressing neurons in the adult female VMHvl led to a profound reduction in female sexual receptivity (Yang et al., 2013). Ablation of the corresponding male neurons resulted in significant reduction in male sexual behavior and aggression. Thus, PR-expressing VMHvl cells constitute a functionally bivalent group of sexually dimorphic neurons (Yang et al., 2013). It will be interesting to test whether all quantitative sex differences in the mouse brain are also functionally bivalent.
Whether a sex difference in neurons is quantitative or qualitative, individual groups of such neurons are dimorphic in multiple dimensions. For example, the PR-expressing VMHvl neurons exhibit sex differences in gene expression, cell density and number within this region, and in their long distance projections (Yang et al., 2013). How each of these molecular and cellular sexually dimorphic features relates to sexually dimorphic behavioral output is unknown, and we anticipate that it will be difficult to address this issue with current approaches.
Which neural circuits underlie mating and aggression?
Decades of rodent lesion or stimulation studies have revealed a limited and overlapping set of sexually dimorphic brain regions that control sexual behavior and aggression in the two sexes (Figure 5B) (Colpaert and Wiepkema, 1976; Commins and Yahr, 1984; Emery and Sachs, 1976; Goy and Phoenix, 1963; Hennessey et al., 1986; Kondo et al., 1990, 1998; Kruk et al., 1979; Lin et al., 2011; Liu et al., 1997; Olivier and Wiepkema, 1974; Pfaff and Sakuma, 1979a, 1979b; Yamanouchi and Arai, 1985). These centers, including the BNST, POA, MeA, and VMH, are interconnected and encompassed largely within the pheromone processing neural pathways that regulate these behaviors (Figure 5B) (reviewed in (Swanson, 2000)). With few exceptions, subsets of adult neurons within these regions also express aromatase or one or more sex hormone receptors in mice (Grgurevic et al., 2012; Shah et al., 2004; Wersinger et al., 1997; Wu et al., 2009; Xu et al., 2012; Yang et al., 2013; Zuloaga et al., 2014). It is presently unclear whether these interconnected pathways are composed of distinct circuits that control each of these behaviors. Alternately, these regions may comprise a single neural circuit controlling both mating and aggression.
In fact, the neurons within these regions (Figure 5B) are molecularly heterogeneous and control diverse behaviors (Choi et al., 2005; Shah et al., 2004; Swanson, 2000; Xu et al., 2012; Yang et al., 2013). How such molecular heterogeneity translates into behavioral pleiotropy is unclear. We have recently examined this issue in the VMHvl and linked a molecularly discrete neuronal population to some, but not all, behaviors controlled by this area (Yang et al., 2013). The VMH is molecularly heterogeneous, and neurons within or adjacent to the VMH regulate female sexual behavior, aggression, defensive reactions to predators, and energy balance in diverse animals, including humans (Goy and Phoenix, 1963; Hess and Akert, 1955; Hetherington and Ranson, 1940; King, 2006; Kow et al., 1985; Kruk et al., 1979; Kurrasch et al., 2007; Lin et al.,2011; Mathews and Edwards, 1977; Musatov et al., 2007, 2006; Olivier and Wiepkema, 1974; Pfaff and Sakuma, 1979a, 1979b; Reeves and Plum, 1969; Robarts and Baum, 2007; Silva et al., 2013; Swaab, 2003; La Vaque and Rodgers, 1975). Experimental lesions or other manipulations that do not target molecularly distinct neurons within the VMH can yield conflicting behavioral phenotypes (see for example (Kow et al., 1985; Pfaff and Sakuma, 1979a, 1979b; La Vaque and Rodgers, 1975)), presumably reflecting nontargeted manipulation of heterogeneous neurons or fibers of passage within this region. Importantly, none of these studies have determined the role of molecularly distinct VMH neurons underlying female sexual behavior or male aggression. To resolve these issues, we used genetic tools to ablate adult PR-expressing VMHvl neurons, which represent ~10%–20% of all cells within the VMH. We found that these neurons are required for the normal display not only of female sexual behavior and male aggression, but also male sexual behavior (Yang et al., 2013). A role of VMH neurons in male sexual behavior was not revealed even with optogenetic manipulation of molecularly unidentified neurons within this region (Lin et al., 2011), illustrating the power of genetic targeting of molecularly identified neuronal subsets within heterogeneous regions such as the VMH (Yang et al., 2013). Importantly, these PR-expressing neurons are not required for other VMH functions such as maintenance of normal body weight. In summary, molecularly distinct VMH neurons appear to underlie the functional diversity of this region.
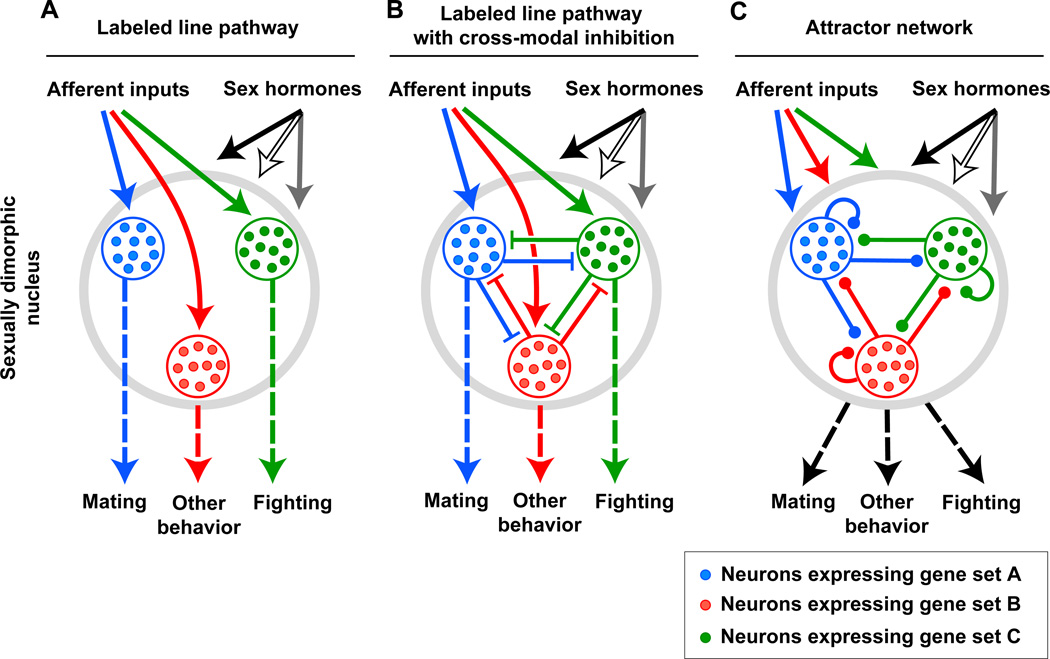
How PR-expressing VMHvl neurons influence both male mating and aggression is unclear. In one scenario, this population consists of molecularly distinct neuronal subsets that sub serve one or the other behavior in a labeled-line manner (Figure 9A). Thus, activation of the appropriate VMHvl subset would elicit mating or fighting. Our observation that PR-positive VMHvl neurons influence both male mating and aggression, but not feeding, is also in accord with Tinbergen’s proposal that these two behaviors are more closely allied to each other than to feeding (Tinbergen, 1951). In his scheme, the reproductive instinct includes mating, aggression, nesting and parental care, but is distinct from the instincts for sleep, feeding, or defense from predators. Tinbergen reasoned that behavioral decisions for instinctual displays are hierarchically organized. In his model, high level decisions would enable an animal to enter a state promoting reproductive rather than, for example, feeding behavior; a lower level decision would subsequently enable an animal to mate, fight, or nurse. He further proposed that such a behavioral hierarchy would be reflected in a hierarchical organization of the underlying neural substrates such that behavioral choices would be made and enforced via cross-modal inhibition of neuronal populations governing comparable decisions at a similar level of the hierarchy (Figure 9B). In the case of the VMHvl, if the PR-positive cells can be further subdivided molecularly into subsets that are dedicated to male mating or fighting, Tinbergen’s model predicts inhibitory interactions between these two populations. However, any such cross-modal inhibition must be quickly reversible because male mice can mate and fight with the appropriate target when presented simultaneously with a male and female conspecific (Leypold et al., 2002). Although not part of Tinbergen’s original proposal, it is tempting to speculate that there are similar cross-modal interactions even for different instincts, a notion supported by the close proximity of PR-positive VMHvl neurons with VMH neurons that regulate feeding and responses to predators.
Figure 9. The relation between molecular heterogeneity in and functional pleiotropy of sexually dimorphic brain regions.
Some models that can be used to relate molecular heterogeneity to functional diversity are shown.
(A) Afferent and sex hormone inputs drive labeled line pathways to control different behaviors. Such a labeled line pathway is a simplified version of a multi-layered feed forward network.
(B) Afferent and sex hormone inputs drive labeled line pathways with cross-modal inhibition to control different behaviors.
(C) The heterogeneous neurons constitute an attractor type network with local and recurrent connections. Afferent and sex hormone inputs in conjunction with local circuits lead to a stable state of the network that elicits behavior.
Molecular heterogeneity may afford a single neural circuit to utilize labeled line, labeled line with cross-modal inhibition, and attractor network pathways at distinct synaptic stations. Alternately, a single neural circuit may consist entirely of one or the other of these pathways.
Both the labeled-line as well as the cross-modal inhibition models rely on PR-expressing VMHvl neuronal subsets that control either mating or aggression (Figure 9A, B). However, it is also possible that the same neurons mediate both male mating and fighting via distinct patterns of neural activity or the release of specific neuro modulators (Figure 9C) (Marder, 2012). In this scenario, the activity of PR-expressing VMHvl output neurons would not only depend on afferent input and hormonal signals but also be contingent on feedback loops or local networks between these cells. Such recurrent connectivity can lead to secondary network dynamics with stable, or attractor, states (Hopfield, 1982, 1984). In this model, the output of the PR-positive VMHvl neurons would depend on the dynamics of external input and local connectivity and promote (or inhibit) the display of a particular behavior when a stable state is achieved. Importantly, such a model does not necessitate invoking molecular heterogeneity within the PR-expressing VMHvl neurons, although if the local network consists of both output neurons and interneurons then these two cell types will be molecularly distinct.
Presumably other models can also be invoked to explain the dual control of mating and aggression by PR-expressing VMHvl neurons. The MeA, BNSTmpm, POA, and other regions implicated in mating and aggression (Figure 5B) are also molecularly heterogeneous and behaviorally pleiotropic. It is possible that one or more of the scenarios we have discussed for the VMHvl (Figure 9) also underlie the functional pleiotropy of these regions. Moreover, whether the entire neural circuit(s) for mating or aggression utilizes one or the other models exclusively is an open question.
A common theme emerging from the studies of mating and aggression in flies and mice is the modular or specialized nature of the function of individual neuronal populations. In other words, distinct neuronal pools appear essential for the performance of different components of the same behavior. Distinct neuronal clusters are also required for the performance of various components of male fly courtship song and copulation (Kim et al., 2013; Kimura et al., 2008; Kohatsu et al., 2011; von Philipsborn et al., 2011). Our studies with PR-expressing VMHvl neurons show that these neurons control male mating and aggression, but sex discrimination, conspecific grooming, ejaculation, and territorial marking are unaffected (Yang et al., 2013). Recent work from Rao and colleagues indicates that sex discrimination and sexual preference are regulated by serotonergic neurons located in the hindbrain (Liu et al., 2011b). Taken together, these studies suggest that different elements of mating and aggression are encoded, to a large degree, by distinct neuronal populations. It is presently unclear how such functionally modular neurons interact to generate a cohesive display of mating or aggression.
Conclusions and future directions
There have been significant recent advances in understanding how the brain generates sexually dimorphic behaviors. Gonadal sex hormones control the overall repertoire of male and female typical behaviors, and genes downstream of sex hormone signaling appear to control specific components or routines of these behaviors (Xu et al., 2012). Most sexual dimorphisms in the brain manifest as hormone-regulated quantitative differences in cell or molecular properties of neurons, and in at least one instance such sexual dimorphisms have been shown to control distinct behaviors in the two sexes (Yang et al., 2013). Coupled with modern neural circuit mapping tools, the recent dramatic advances in systematic gene expression profiling, genetic manipulations, and potential deorphanizing of many pheromone receptors will lead to rapid progress in understanding how sex is represented in the brain and transformed into gender-typical behavior in mammals (Isogai et al., 2011, Luo et al., 2008, Mardis, 2008, Wang et al., 2013 and Xu et al., 2012). We anticipate that such insights into how sex differences in the brain control sexually dimorphic behaviors in health will eventually translate to understanding the startling sex differences in many common neuro-psychiatric illnesses. Indeed, at least some sexually dimorphically expressed genes in the brain appear to be linked with such human disorders that occur in sex-skewed ratios (Xu et al., 2012).
Many of the same sexually dimorphic brain regions have been implicated in the control of both mating and aggression. Most of these regions contain pools of molecularly heterogeneous neurons, and with few exceptions (Yang et al., 2013), it is unclear whether molecular heterogeneity within a region always translates into functional specialization such that different neuronal pools, marked by unique sets of genes, regulate different behaviors. Alternatively, such molecular heterogeneity could be used to construct unique network dynamics that generate different behaviors based on afferent input, hormonal signals, and past experience. A resolution of this issue of heterogeneity in conjunction with anatomical neural circuit mapping will provide insight as to how the same brain regions encode mating and fighting.
Although mating and aggression are innate behaviors such that they can be displayed without prior training, they are nevertheless modified by past experience. For example, repeated defeat in aggressive encounters renders a male mouse more liable to defeat in subsequent encounters (Russo et al., 2012); such a submissive male also marks his territory only sparsely in comparison to naïve or dominant males (Desjardins et al., 1973). Significant progress has been made in understanding the molecular and cellular basis whereby such social defeat modifies reward, stress, and defense pathways in the brain (Russo et al., 2012), and it will be interesting in future studies to extend these analyses to the core circuits (Figure 5B) that mediate mating and fighting. Mating and aggressive displays are often dramatically plastic in some species; for example, submissive male cichlid fish can, within minutes of removal of a dominant male from their vicinity, start behaving like a dominant male (Fernald, 2012). Mated prairie voles exhibit aggression toward unfamiliar intruders, including those of the opposite sex, whereas sexually naïve voles do not typically attack members of the opposite sex (Carter and Getz, 1993; Insel, 1997). We anticipate that work in these model systems will provide insight into the neural substrates for plasticity in otherwise hard-wired behaviors.
Studies in mice reveal a surprising modularity at molecular and cellular levels in the control of mating and aggression. Indeed, gene deletion or genetically targeted functional manipulation of restricted neurons generates very specific deficits in one or more components of sexual or aggressive displays while leaving other components intact. It is tempting to speculate that other components of courtship and territoriality such as birdsong are also controlled in a modular manner by specific neuronal pools and genetic loci. In fact, we anticipate that modular control of diverse behaviors, learned or otherwise, may turn out to be a general organizing principle for the underlying neural circuits. It will be interesting to test whether other behaviors (such as predator-defense) that are also usually thought to be associated with emotional states are encoded by a modular genetic and neural architecture. Recent studies in Peromyscus show that individual parameters of species-specific tunnels built by these mice are controlled in a modular manner by a few genetic loci (Weber et al., 2013). Similarly, different aspects of schooling behavior in stickleback fish also appear to be controlled by distinct genetic loci (Greenwood et al., 2013). Such genetic compartmentalization of behavior presumably affords rapid evolvability of circuits and behavior, in a manner perhaps analogous to exon shuffling that leads to the generation of genes that encode proteins with novel combinations of functional domains.
Acknowledgments
We are grateful to members of the N.M.S. lab, H. Bourne, T. Clandinin, H. Ingraham, D. Julius, and S. Lomvardas for helpful discussions or comments on the manuscript and to E. Unger for help with the artwork. This work was supported by a Genentech Graduate Fellowship (CFY); the Ellison Medical Foundation and NIH grants DP1MH099900, R01NS049488, R01NS083872, R01AA010035 (NMS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen E, Francis BF, Robertson LL, Colgate CE, Johnston CG, Doisy EA, Kountz WB, Gibson HV. The hormone of the ovarian follicle; its localization and action in test animals, and additional points bearing upon the internal secretion of the ovary. Am. J. Anat. 1924;34:133–181. [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Antliff HR, Young WC. Behavioral and tissue responses of male guinea pigs to estrogens and the problem of hormone specificity. Endocrinology. 1956;59:74–82. doi: 10.1210/endo-59-1-74. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, González CR, Eyjólfsdóttir EA, Perona P, Anderson DJ. Tachykinin-Expressing Neurons Control Male-Specific Aggressive Arousal in Drosophila. Cell. 2014;156:221–235. doi: 10.1016/j.cell.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda S-I, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat. Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Ball J. Sex activity of castrated male rats increased by estrin administration. J. Comp. Psychol. 1937;24:135–144. [Google Scholar]
- Balthazart J, Ball GF. New insights into the regulation and function of brain estrogen synthase (aromatase) Trends Neurosci. 1998;21:243–249. doi: 10.1016/s0166-2236(97)01221-6. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Activational and organizational effects of estradiol on male behavioral neuroendocrine function. Scand. J. Psychol. 2003;44:213–220. doi: 10.1111/1467-9450.00338. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Södersten P, Vreeburg JT. Mounting and receptive behavior in the ovariectomized female rat: influence of estradiol, dihydrotestosterone, and genital anesthetization. Horm. Behav. 1974;5:175–190. doi: 10.1016/0018-506x(74)90042-7. [DOI] [PubMed] [Google Scholar]
- Beach FA. Hormones and mating behavior in vertebrates. Recent Prog. Horm. Res. 1947;1:27–63. doi: 10.1016/b978-1-4831-9840-8.50005-3. [DOI] [PubMed] [Google Scholar]
- Beeman EA. The effect of male hormone on aggressive behavior in mice. Physiol. Zool. 1947;20:373–405. doi: 10.1086/physzool.20.4.30151969. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Annu. Rev. Psychol. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Rissman EF. X-chromosome dosage affects male sexual behavior. Horm. Behav. 2012;61:565–572. doi: 10.1016/j.yhbeh.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–566. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31:5574–5578. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson FH, Desjardins C. Aggression in adult mice: modification by neonatal injections of gonadal hormones. Science. 1968;161:705–706. doi: 10.1126/science.161.3842.705. [DOI] [PubMed] [Google Scholar]
- Bronson FH, Desjardins C. Aggressive Behavior and Seminal Vesicle Function in Mice: Differential Sensitivity to Androgen Given Neonatally. Endocrinology. 1969;85:971–974. doi: 10.1210/endo-85-5-971. [DOI] [PubMed] [Google Scholar]
- Burris TP, Solt LA, Wang Y, Crumbley C, Banerjee S, Griffett K, Lundasen T, Hughes T, Kojetin DJ. Nuclear receptors and their selective pharmacologic modulators. Pharmacol. Rev. 2013;65:710–778. doi: 10.1124/pr.112.006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GSXE. Sexual dimorphism in the fly brain. Curr. Biol. CB. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Lee H-J, Macbeth AH, Young WS., 3rd Vasopressin: behavioral roles of an “original” neuropeptide. Prog. Neurobiol. 2008;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Campenhausen H, Mori K. Convergence of segregated pheromonal pathways from the accessory olfactory bulb to the cortex in the mouse. Eur. J. Neurosci. 2000;12:33–46. doi: 10.1046/j.1460-9568.2000.00879.x. [DOI] [PubMed] [Google Scholar]
- Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat. Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Sci. Am. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong H-W, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Cline TW, Meyer BJ. Vive la différence: males vs females in flies vs worms. Annu. Rev. Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Wiepkema PR. Effects of ventromedial hypothalamic lesions on spontaneous intraspecies aggression in male rats. Behav. Biol. 1976;16:117–125. doi: 10.1016/s0091-6773(76)91225-6. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Lesions of the sexually dimorphic area disrupt mating and marking in male gerbils. Brain Res. Bull. 1984;13:185–193. doi: 10.1016/0361-9230(84)90020-0. [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- Corbier P, Edwards DA, Roffi J. The neonatal testosterone surge: a comparative study. Arch. Int. Physiol. Biochim. Biophys. 1992;100:127–131. doi: 10.3109/13813459209035274. [DOI] [PubMed] [Google Scholar]
- Crews D, Moore MC. Historical contributions of research on reptiles to behavioral neuroendocrinology. Horm. Behav. 2005;48:384–394. doi: 10.1016/j.yhbeh.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- Dempsey EW, Hertz R, Young WC. The Experimental Induction of Oestrus (sexual Receptivity) in the Normal and Ovariectomized Guinea Pig. Am. J. Physiol. -- Leg. Content. 1936;116:201–209. [Google Scholar]
- Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- DeVeale B, van der Kooy D, Babak T. Critical evaluation of imprinted gene expression by RNA-Seq: a new perspective. PLoS Genet. 2012;8:e1002600. doi: 10.1371/journal.pgen.1002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Young WS, Nelson RJ., 3rd Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. J. Neuroendocrinol. 1997;9:363–368. doi: 10.1046/j.1365-2826.1997.t01-1-00589.x. [DOI] [PubMed] [Google Scholar]
- Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res. 2003;118:82–90. doi: 10.1016/s0169-328x(03)00339-5. [DOI] [PubMed] [Google Scholar]
- Dewing P, Chiang CWK, Sinchak K, Sim H, Fernagut P-O, Kelly S, Chesselet M-F, Micevych PE, Albrecht KH, Harley VR, et al. Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. CB. 2006;16:415–420. doi: 10.1016/j.cub.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu. Rev. Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev. Neurobiol. 2007;67:1371–1381. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. Mice: Fighting by neonatally and rogenized females. Science. 1968;161:1027–1028. doi: 10.1126/science.161.3845.1027. [DOI] [PubMed] [Google Scholar]
- Edwards DA. Early androgen stimulation and aggressive behavior in male and female mice. Physiol. Behav. 1969;4:333–338. [Google Scholar]
- Edwards DA. Neonatal administration of and rostenedione, testosterone or testosterone propionate: Effects on ovulation, sexual receptivity and aggressive behavior in female mice. Physiol. Behav. 1971;6:223–228. doi: 10.1016/0031-9384(71)90030-8. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm. Behav. 1971a;2:49–58. [Google Scholar]
- Edwards DA, Burge KG. Estrogenic arousal of aggressive behavior and masculine sexual behavior in male and female mice. Horm. Behav. 1971b;2:239–245. [Google Scholar]
- Emery DE, Sachs BD. Copulatory behavior in male rats with lesions in the bed nucleus of the stria terminalis. Physiol. Behav. 1976;17:803–806. doi: 10.1016/0031-9384(76)90044-5. [DOI] [PubMed] [Google Scholar]
- Fan P, Manoli DS, Ahmed OM, Chen Y, Agarwal N, Kwong S, Cai AG, Neitz J, Renslo A, Baker BS, et al. Genetic and Neural Mechanisms that Inhibit Drosophila from Mating with Other Species. Cell. 2013;154:89–102. doi: 10.1016/j.cell.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald RD. Social control of the brain. Annu. Rev. Neurosci. 2012;35:133–151. doi: 10.1146/annurev-neuro-062111-150520. [DOI] [PubMed] [Google Scholar]
- Finkelstein JS, Yu EW, Burnett-Bowie S-AM. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013;369:2457. doi: 10.1056/NEJMc1313169. [DOI] [PubMed] [Google Scholar]
- Finney HC, Erpino MJ. Synergistic effect of estradiol benzoate and dihydrotestosterone on aggression in mice. Horm. Behav. 1976;7:391–400. doi: 10.1016/0018-506x(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forger NG, de Vries GJ. Cell death and sexual differentiation of behavior: worms, flies, and mammals. Curr. Opin. Neurobiol. 2010;20:776–783. doi: 10.1016/j.conb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front. Neuroendocrinol. 2006;27:247–274. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Fraser EJ, Shah NM. Complex chemosensory control of female reproductive behaviors. PLoS One. 2014;9:e90368. doi: 10.1371/journal.pone.0090368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusani L. Testosterone control of male courtship in birds. Horm. Behav. 2008;54:227–233. doi: 10.1016/j.yhbeh.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Gagnidze K, Pfaff DW, Mong JA. Gene expression in neuroendocrine cells during the critical period for sexual differentiation of the brain. In: Ivanka Savic., editor. In Progress in Brain Research. Elsevier; 2010. pp. 97–111. [DOI] [PubMed] [Google Scholar]
- Gandelman R. Mice: postpartum aggression elicited by the presence of an intruder. Horm. Behav. 1972;3:23–28. doi: 10.1016/0018-506x(72)90003-7. [DOI] [PubMed] [Google Scholar]
- Garfield AS, Cowley M, Smith FM, Moorwood K, Stewart-Cox JE, Gilroy K, Baker S, Xia J, Dalley JW, Hurst LD, et al. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Nature. 2011;469:534–538. doi: 10.1038/nature09651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison JL, Macosko EZ, Bernstein S, Pokala N, Albrecht DR, Bargmann CI. Oxytocin/vasopressin-related peptides have an ancient role in reproductive behavior. Science. 2012;338:540–543. doi: 10.1126/science.1226201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J. Neurosci. Off. J. Soc. Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Wagner JW. Gonadal activity and sexual differentiation of the hypothalamus. Endocrinology. 1965;76:226–239. doi: 10.1210/endo-76-2-226. [DOI] [PubMed] [Google Scholar]
- Goy RW, Phoenix CH. Hypothalamic regulation of female sexual behaviour; establishment of behavioural oestrus in spayed guinea-pigs following hypothalamic lesions. J. Reprod. Fertil. 1963;5:23–40. doi: 10.1530/jrf.0.0050023. [DOI] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Yoshida K, Peichel CL. Genetic and neural modularity underlie the evolution of schooling behavior in three spine sticklebacks. Curr Biol. 2013;23:1884–1888. doi: 10.1016/j.cub.2013.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010a;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010b;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgurevic N, Büdefeld T, Spanic T, Tobet SA, Majdic G. Evidence that sex chromosome genes affect sexual differentiation of female sexual behavior. Horm. Behav. 2012;61:719–724. doi: 10.1016/j.yhbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Guillamón A, Segovia S. Sex differences in the vomeronasal system. Brain Res. Bull. 1997;44:377–382. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- Harris GW. The Induction of Ovulation in the Rabbit, by Electrical Stimulation of the Hypothalamo-hypophysial Mechanism. Proc. R. Soc. Lond. Ser. B - Biol. Sci. 1937;122:374–394. [Google Scholar]
- Harris GW. Sex hormones, brain development and brain function. Endocrinology. 1964;75:627–648. doi: 10.1210/endo-75-4-627. [DOI] [PubMed] [Google Scholar]
- Harris GW, Jacobsohn D. Functional Grafts of the Anterior Pituitary Gland. Proc. R. Soc. Lond. Ser. B - Biol. Sci. 1952;139:263–276. doi: 10.1098/rspb.1952.0011. [DOI] [PubMed] [Google Scholar]
- Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52:1439–1453. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey AC, Wallen K, Edwards DA. Preoptic lesions increase the display of lordosis by male rats. Brain Res. 1986;370:21–28. doi: 10.1016/0006-8993(86)91100-5. [DOI] [PubMed] [Google Scholar]
- Hess WR, Akert K. Experimental data on role of hypothalamus in mechanism of emotional behavior. AMA Arch. Neurol. Psychiatry. 1955;73:127–129. doi: 10.1001/archneurpsyc.1955.02330080005003. [DOI] [PubMed] [Google Scholar]
- Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat. Rec. 1940;78:149–172. [Google Scholar]
- Holloway CC, Clayton DF. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat. Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, Maeda S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem. Biophys. Res. Commun. 1998;252:445–449. doi: 10.1006/bbrc.1998.9672. [DOI] [PubMed] [Google Scholar]
- Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc. Natl. Acad. Sci. U. S. A. 1982;79:2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ. Neurons with graded response have collective computational properties like those of two-state neurons. Proc. Natl. Acad. Sci. 1984;81:3088–3092. doi: 10.1073/pnas.81.10.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am. J. Psychiatry. 1997;154:726–735. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–245. doi: 10.1038/nature10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER) alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol. Endocrinol. Baltim. Md. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, Thomson JN, Albertson DG, Hall DH, Emmons SW. The connectome of a decision-making neural network. Science. 2012;337:437–444. doi: 10.1126/science.1221762. [DOI] [PubMed] [Google Scholar]
- Jost A. Genetic and hormonal factors in sex differentiation of the brain. Psychoneuroendocrinology. 1983;8:183–193. doi: 10.1016/0306-4530(83)90055-0. [DOI] [PubMed] [Google Scholar]
- Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S-I, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of the Bax gene disrupts sexual behavior and modestly impairs motor function in mice. Dev. Neurobiol. 2007;67:1511–1519. doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the “vomeronasal” amygdala in female mice selectively responds to volatile pheromones from males. Eur. J. Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole-mount, flattened cortex preparation. Chem. Senses. 2011a;36:251–260. doi: 10.1093/chemse/bjq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, McCarthy EA, Cherry JA, Baum MJ. A sex comparison of the anatomy and function of the main olfactory bulb-medial amygdala projection in mice. NeuroScience. 2011b;172:196–204. doi: 10.1016/j.neuroscience.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson P, Lüscher M. Pheromones: a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J. Comp. Neurol. 1981a;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the “olfactory amygdala”. J. Comp. Neurol. 1981b;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Kim S, Ma L, Jensen KL, Kim MM, Bond CT, Adelman JP, Yu CR. Paradoxical contribution of SK3 and GIRK channels to the activation of mouse vomeronasal organ. Nat. Neurosci. 2012;15:1236–1244. doi: 10.1038/nn.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Jan LY, Jan YN. A PDF/NPF Neuropeptide Signaling Circuitry of Male Drosophila melanogaster Controls Rival-Induced Prolonged Mating. Neuron. 2013;80:1190–1205. doi: 10.1016/j.neuron.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- Kimura K-I, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D. Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron. 2008;59:759–769. doi: 10.1016/j.neuron.2008.06.007. [DOI] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol. Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Kohl J, Ostrovsky AD, Frechter S, Jefferis GSXE. A Bidirectional Circuit Switch Reroutes Pheromone Signals in Male and Female Brains. Cell. 2013;155:1610–1623. doi: 10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Shinoda A, Yamanouchi K, Arai Y. Role of septum and preoptic area in regulating masculine and feminine sexual behavior in male rats. Horm. Behav. 1990;24:421–434. doi: 10.1016/0018-506x(90)90019-t. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav. Brain Res. 1998;91:215–222. [PubMed] [Google Scholar]
- Konishi M. Birdsong: From Behavior to Neuron. Annu. Rev. Neurosci. 1985;8:125–170. doi: 10.1146/annurev.ne.08.030185.001013. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Kow LM, Harlan RE, Shivers BD, Pfaff DW. Inhibition of the lordosis reflex in rats by intra hypothalamic infusion of neural excitatory agents: evidence that the hypothalamus contains separate inhibitory and facilitatory elements. Brain Res. 1985;341:26–34. doi: 10.1016/0006-8993(85)91468-4. [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk MR, van der Poel AM, de Vos-Frerichs TP. The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour. 1979;70:292–322. doi: 10.1163/156853979x00106. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Rissman EF. Double oestrogen receptor alpha and beta knockout mice reveal differences in neural oestrogen-mediated progestin receptor induction and female sexual behaviour. J. Neuroendocrinol. 2003;15:978–983. doi: 10.1046/j.1365-2826.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson J-A, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:13624–13634. doi: 10.1523/JNEUROSCI.2858-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli E, Ohe K, Latorre E, Bianchi ME, Sassone-Corsi P. Sexy splicing: regulatory interplays governing sex determination from Drosophila to mammals. J. Cell Sci. 2003;116:441–445. doi: 10.1242/jcs.00249. [DOI] [PubMed] [Google Scholar]
- Leader JE, Wang C, Fu M, Pestell RG. Epigenetic regulation of nuclear steroid receptors. Biochem. Pharmacol. 2006;72:1589–1596. doi: 10.1016/j.bcp.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Surani MA. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat. Genet. 1998;20:163–169. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res. Brain Res. Rev. 1996;22:1–26. [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Keverne EB, Aparicio SA, Ishino F, Barton SC, Surani MA. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science. 1999;284:330–333. doi: 10.1126/science.284.5412.330. [DOI] [PubMed] [Google Scholar]
- Liberles SD. Mammalian Pheromones. Annu. Rev. Physiol. 2014 doi: 10.1146/annurev-physiol-021113-170334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht G, Meredith M. Convergence of main and accessory olfactory pathways onto single neurons in the hamster amygdala. Exp. Brain Res. Exp. Hirnforsch. Expérimentation Cérébrale. 1987;69:7–18. doi: 10.1007/BF00247024. [DOI] [PubMed] [Google Scholar]
- Lieberburg I, Krey LC, McEwen BS. Sex differences in serum testosterone and in exchangeable brain cell nuclear estradiol during the neonatal period in rats. Brain Res. 1979;178:207–212. doi: 10.1016/0006-8993(79)90102-1. [DOI] [PubMed] [Google Scholar]
- Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Liu KS, Sternberg PW. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron. 1995;14:79–89. doi: 10.1016/0896-6273(95)90242-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jiang Y, Si Y, Kim J-Y, Chen Z-F, Rao Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011b;472:95–99. doi: 10.1038/nature09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J. Neurosci. Off. J. Soc. Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat. Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Meissner GW, Baker BS. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci. 2006;29:444–451. doi: 10.1016/j.tins.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín I, Baker BS. The evolutionary dynamics of sex determination. Science. 1998;281:1990–1994. doi: 10.1126/science.281.5385.1990. [DOI] [PubMed] [Google Scholar]
- Mardis ER. The impact of next-generation sequencing technology on genetics. Trends Genet. 2008;24:133–141. doi: 10.1016/j.tig.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Mathews D, Edwards DA. The ventromedial nucleus of the hypothalamus and the hormonal arousal of sexual behaviors in the female rat. Horm. Behav. 1977;8:40–51. doi: 10.1016/0018-506x(77)90019-8. [DOI] [PubMed] [Google Scholar]
- Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat. Rev. Genet. 2012;13:163–174. doi: 10.1038/nrg3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Honda S, Harada N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. NeuroEndocrinology. 2003;77:416–424. doi: 10.1159/000071313. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM. Epigenetic contributions to hormonally mediated sexual differentiation of the brain. J. Neuroendocrinol. 2013 doi: 10.1111/jne.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE. Estrogen response element-independent estrogen receptor (ER)-alpha signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERalpha knockout mice. Endocrinology. 2007;148:5288–5294. doi: 10.1210/en.2007-0673. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL, Levine JE. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol. Cell. Endocrinol. 2008;290:24–30. doi: 10.1016/j.mce.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Neural gonadal steroid actions. Science. 1981;211:1303–1311. doi: 10.1126/science.6259728. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain. Cooperation or coincidence? Ann. N. Y. Acad. Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front. Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore FL, Boyd SK, Kelley DB. Historical perspective: Hormonal regulation of behaviors in amphibians. Horm. Behav. 2005;48:373–383. doi: 10.1016/j.yhbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Motelica-Heino I, Edwards DA, Roffi J. Intermale aggression in mice: does hour of castration after birth influence adult behavior? Physiol. Behav. 1993;53:1017–1019. doi: 10.1016/0031-9384(93)90284-m. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of and rostenedione by the diencephalon. J. Clin. Endocrinol. Metab. 1971a;33:368–370. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Petro Z. Aromatization of and rostenedione by limbic system tissue from human foetuses. J. Endocrinol. 1971b;51:795–796. doi: 10.1677/joe.0.0510795. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- Newman SW, Parfitt DB, Kollack-Walker S. Mating-induced c-fos expression patterns complement and supplement observations after lesions in the male Syrian hamster brain. Ann. N. Y. Acad. Sci. 1997;807:239–259. doi: 10.1111/j.1749-6632.1997.tb51924.x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. NeuroEndocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Chester AE, Gustafsson JA, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor beta gene-deficient (betaERKO) male and female mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12887–12892. doi: 10.1073/pnas.96.22.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc. Natl. Acad. Sci. U. S. A. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Geller L, Lai E. Tfm mutation and masculinization versus feminization of the mouse central nervous system. Cell. 1974;3:235–242. doi: 10.1016/0092-8674(74)90137-8. [DOI] [PubMed] [Google Scholar]
- Olivier B, Wiepkema PR. Behaviour changes in mice following electrolytic lesions in the median hypothalamus. Brain Res. 1974;65:521–524. doi: 10.1016/0006-8993(74)90241-8. [DOI] [PubMed] [Google Scholar]
- Olsen K. Genetic influences on sexual behavior differentiation. Handbook of Behavioral Neurobiology. 1992:1–40. [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, et al. GPR30 Does Not Mediate Estrogenic Responses in Reproductive Organs in Mice. Biol. Reprod. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- Pang SF, Tang F. Sex differences in the serum concentrations of testosterone in mice and hamsters during their critical periods of neural sexual differentiation. J. Endocrinol. 1984;100:7–11. doi: 10.1677/joe.0.1000007. [DOI] [PubMed] [Google Scholar]
- Perkins A, Roselli CE. The ram as a model for behavioral neuroendocrinology. Horm. Behav. 2007;52:70–77. doi: 10.1016/j.yhbeh.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc. Biol. Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J. Physiol. 1979a;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J. Physiol. 1979b;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer CA. Sexual differences of the hypophyses and their determination by the gonads. Am. J. Anat. 1936;58:195–225. [Google Scholar]
- Von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Portman DS. Genetic control of sex differences in C. elegans neurobiology and behavior. Adv. Genet. 2007;59:1–37. doi: 10.1016/S0065-2660(07)59001-2. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM. Sexual dimorphism in the preoptic area of the rat. Science. 1971;173:731–733. doi: 10.1126/science.173.3998.731. [DOI] [PubMed] [Google Scholar]
- Raskin K, de Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J. Neurosci. Off. J. Soc. Neurosci. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves AG, Plum F. Hyperphagia, rage, and dementia accompanying a ventromedial hypothalamic neoplasm. Arch. Neurol. 1969;20:616–624. doi: 10.1001/archneur.1969.00480120062005. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Ring JR. The estrogen-progesterone induction of sexual receptivity in the spayed female mouse. Endocrinology. 1944;34:269–275. [Google Scholar]
- Rinn JL, Rozowsky JS, Laurenzi IJ, Petersen PH, Zou K, Zhong W, Gerstein M, Snyder M. Major molecular differences between mammalian sexes are involved in drug metabolism and renal function. Dev. Cell. 2004;6:791–800. doi: 10.1016/j.devcel.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- Robarts DW, Baum MJ. Ventromedial hypothalamic nucleus lesions disrupt olfactory mate recognition and receptivity in female ferrets. Horm. Behav. 2007;51:104–113. doi: 10.1016/j.yhbch.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JM, Daley JD, Ohno S, YoungLai EV. Central aromatization of testosterone in testicular feminized mice. Experientia. 1977;33:1392–1393. doi: 10.1007/BF01920200. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han M-H, Charney DS, Nestler EJ. Neurobiology of resilience. Nat. Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nakagawa T, Mitsuno H, Mori H, Endo Y, Tanoue S, Yasukochi Y, Touhara K, Nishioka T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16653–16658. doi: 10.1073/pnas.0407596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, et al. Brain masculinization requires androgen receptor function. Proc. Natl. Acad. Sci. U. S. A. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer CH, Everett JW, Markee JE. A Neural Factor in the Mechanism by Which Estrogen Induces the Release of Luteinizing Hormone in the Rat. Endocrinology. 1949;44:218–233. doi: 10.1210/endo-44-3-218. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J. Comp. Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression in male mice lacking functional estrogen receptor alpha. Behav. Neurosci. 2003;117:38–45. [PubMed] [Google Scholar]
- Scordalakes EM, Rissman EF. Aggression and arginine vasopressin immunoreactivity regulation by androgen receptor and estrogen receptor alpha. Genes Brain Behav. 2004;3:20–26. doi: 10.1111/j.1601-183x.2004.00036.x. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43:313–319. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shapiro H. Effect of Testosterone Propionate on Mating. Nature. 1937;139:588–589. [Google Scholar]
- Shipley MT, Adamek GD. The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res. Bull. 1984;12:669–688. doi: 10.1016/0361-9230(84)90148-5. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Murphy AZ, Rizvi TA, Ennis M, Behbehani MM. Olfaction and brainstem circuits of reproductive behavior in the rat. Prog. Brain Res. 1996;107:355–377. doi: 10.1016/s0079-6123(08)61876-2. [DOI] [PubMed] [Google Scholar]
- Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, Grinevich V, Canteras NS, Ragozzino D, Gross CT. Independent hypothalamic circuits for social and predator fear. Nat. Neurosci. 2013;16:1731–1733. doi: 10.1038/nn.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Södersten P. Estrogen-activated sexual behavior in male rats. Horm. Behav. 1973;4:247–256. doi: 10.1016/0018-506x(73)90009-3. [DOI] [PubMed] [Google Scholar]
- Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth HT. Mating behavior within the genus Drosophila (Diptera). Bulletin of the AMNH; v. 99, article 7. Mating behavior within Drosophila. 1952 [Google Scholar]
- Spors H, Sobel N. Male behavior by knockout. Neuron. 2007;55:689–693. doi: 10.1016/j.neuron.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Stone CP. Copulatory Activity in Adult Male Rats Following Castration and Injections of Testosterone Propionate. Endocrinology. 1939;24:165–174. [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Swaab D. The Human Hypothalamus: Basic and Clinical Aspects Part I: Nuclei of the Human Hypothalamus. Elsevier; 2003. The ventromedial nucleus (VMN; nucleus of Cajal) pp. 239–242. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The Study of Instinct. Oxford: Clarendon Press; 1951. [Google Scholar]
- Toda K, Okada T, Takeda K, Akira S, Saibara T, Shiraishi M, Onishi S, Shizuta Y. Oestrogen at the neonatal stage is critical for the reproductive ability of male mice as revealed by supplementation with 17beta-oestradiol to aromatase gene (Cyp19) knockout mice. J. Endocrinol. 2001a;168:455–463. doi: 10.1677/joe.0.1680455. [DOI] [PubMed] [Google Scholar]
- Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J. Endocrinol. 2001b;168:217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Gonadal hormones and brain development: implications for the genesis of sexual differentiation. Ann. N. Y. Acad. Sci. 1984;435:101–111. doi: 10.1111/j.1749-6632.1984.tb13743.x. [DOI] [PubMed] [Google Scholar]
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- La Vaque TJ, Rodgers CH. Recovery of mating behavior in the female rat following VMH lesions. Physiol. Behav. 1975;14:59–63. doi: 10.1016/0031-9384(75)90142-0. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front. Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Sex Differences in Neurotransmitter Systems. J. Neuroendocrinol. 1990;2:1–13. doi: 10.1111/j.1365-2826.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav. Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang L-Y, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. Off. J. Soc. Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann. N. Y. Acad. Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wallis CJ, Luttge WG. Maintenance of male sexual behavior by combined treatment with oestrogen and dihydrotestosterone in CD-1 mice. J. Endocrinol. 1975;66:257–262. doi: 10.1677/joe.0.0660257. [DOI] [PubMed] [Google Scholar]
- Wang Z, Storm DR. Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2011;36:772–781. doi: 10.1038/npp.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, et al. GPR30 Contributes to Estrogen-Induced Thymic Atrophy. Mol. Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Sindreu CB, Li V, Nudelman A, Chan GC-K, Storm DR. Pheromone Detection in Male Mice Depends on Signaling through the Type 3 Adenylyl Cyclase in the Main Olfactory Epithelium. J. Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JN, Peterson BK, Hoekstra HE. Discrete genetic modules are responsible for complex burrow evolution in Peromyscus mice. Nature. 2013;493:402–405. doi: 10.1038/nature11816. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm. Behav. 1997;32:176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Nadler RD. Suppression of the development of female mating behavior by estrogen administered in infancy. Science. 1963;141:273–275. doi: 10.1126/science.141.3577.273. [DOI] [PubMed] [Google Scholar]
- Wheeler DA, Kyriacou CP, Greenacre ML, Yu Q, Rutila JE, Rosbash M, Hall JC. Molecular transfer of a species-specific behavior from Drosophila simulans to Drosophila melanogaster. Science. 1991;251:1082–1085. doi: 10.1126/science.1900131. [DOI] [PubMed] [Google Scholar]
- Wiesner BP, Mirskaia L. On the endocrine basis of mating in the mouse. Exp. Physiol. 1930;20:273–279. [Google Scholar]
- Wilhelm D, Palmer S, Koopman P. Sex determination and gonadal development in mammals. Physiol. Rev. 2007;87:1–28. doi: 10.1152/physrev.00009.2006. [DOI] [PubMed] [Google Scholar]
- Winans SS, Scalia F. Amygdaloid nucleus: new afferent input from the vomeronasal organ. Science. 1970;170:330–332. doi: 10.1126/science.170.3955.330. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Dev. Brain Res. 2005;157:34–41. doi: 10.1016/j.devbrainres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Wu MV, Shah NM. Control of masculinization of the brain and behavior. Curr. Opin. Neurobiol. 2011;21:116–123. doi: 10.1016/j.conb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S-I, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki CJ, Lepri JJ. Consequences of removing the vomeronasal organ. J. Steroid Biochem. Mol. Biol. 1991;39:661–669. doi: 10.1016/0960-0760(91)90265-7. [DOI] [PubMed] [Google Scholar]
- Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanouchi K, Arai Y. Presence of a neural mechanism for the expression of female sexual behaviors in the male rat brain. NeuroEndocrinology. 1985;40:393–397. doi: 10.1159/000124104. [DOI] [PubMed] [Google Scholar]
- Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, Unger EK, Wells JA, Shah NM. Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Young LJ, Winslow JT, Nilsen R, Insel TR. Species differences in V1a receptor gene expression in monogamous and nonmonogamous voles: behavioral consequences. Behav. Neurosci. 1997;111:599–605. doi: 10.1037//0735-7044.111.3.599. [DOI] [PubMed] [Google Scholar]
- Yu JY, Kanai MI, Demir E, Jefferis GSXE, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. CB. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T. Mammalian pheromone sensing. Curr. Opin. Neurobiol. 2007;17:483–489. doi: 10.1016/j.conb.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. Estrogen receptor beta expression in the mouse forebrain: Age and sex differences. J. Comp. Neurol. 2014 doi: 10.1002/cne.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]